From Sharks to Yeasts: Squalene in the Development of Vaccine Adjuvants
Abstract
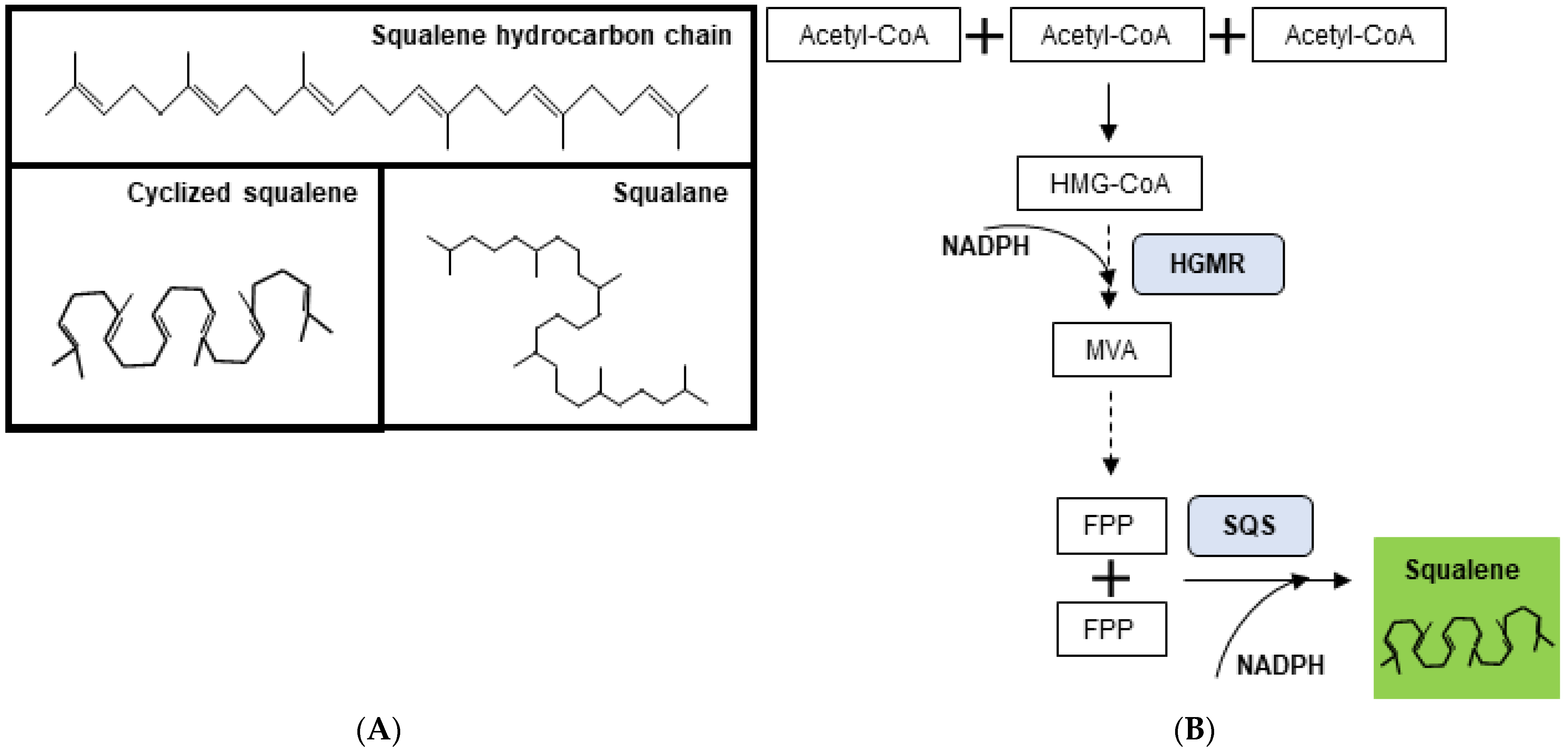
:1. Introduction
2. Squalene-Based Formulations as Vaccine Adjuvants
2.1. MF59 (Novartis)
2.2. AS03 (GSK)
2.3. AF03 (Sanofi)
| Adjuvant | Vaccines | Trade Name | Hemagglutinin (HA) Dose | Possible Mechanism of Action | References |
|---|---|---|---|---|---|
| MF59 (Novartis) Squalene Polysorbate 80 Sorbitan trioleate | A/H1N1 influenza | Focetria® Celtura® | 7.5 μg/0.5 mL 3.75 μg/0.5 mL | Transient increase in local cytokines chemokines Activation of innate immunity independent of TLRs b CD4+ T-cell activation (dependent of extracellular ATP) Cell recruitment (neutrophils, eosinophils, and monocytes) Increase antibody titers in neutrophils in dLNs c IgG Isotype switch | [41,44,45,48] |
| Seasonal influenza | FLUAD® FLUAD Pediatric™ | 15 μg/0.5 mL a 7.5 μg/0.5 mL a | |||
| AS03 (GSK) Squalene Polysorbate 80 α-tocopherol | A/H1N1 influenza | Pandemrix® Arepandix® | 3.75 μg/0.5 mL | Spatio-temporal colocalization with antigen Direct activation of TLRs Transient increase in local cytokines chemokines Increase antibody titers in monocytes in dLNs | [51,62] |
| AF03 (Sanofi) Squalene Polyoxyethylene cetostearyl ether Mannitol Sorbitan oleate | A/H1N1 influenza | Humenza d | Not determined | Cell recruitment to the injection site Immune response mediated by IFN-γ and IL-5 | [60] |
3. Squalene Sources
3.1. Squalene from Shark Liver Oil
3.2. Squalene from Plants
3.3. Microorganisms as Sustainable Sources of Squalene
3.4. Genetic Manipulation and Metabolic Rewiring to Increase Squalene Production
| Source | Squalene (mg/100 g) | References |
|---|---|---|
| Natural oils | ||
| Amaranth | 1040–60,000 | [3,87,88] |
| Olive | 80–1245 | [89,90,91] |
| Hazelnut | 9.3–39.2 | [92] |
| Peanut | 27.4–132.9 | [89,93] |
| Corn | 10–33.8 | [89,94,95] |
| Grape seed | [89,96] | |
| Soybean | 3–22 | [93,94,97] |
| Distillates | ||
| Olive | 10,000–30,000 | [94] |
| Sunflower | 4300–4500 | [98] |
| Soybean | 1800–5500 | [94,99,100] |
| Canola | 3000–3500 | [98] |
| (mg/g DCW) | ||
| Yeast | ||
| S. cerevisiae (wt) | 0.04–1.6 a | [101] |
| S. cerevisiae S. cerevisiae BY4741 | 1.38 b 28.4 | [101] |
| Torulaspora delbrueckii | 0.24 | [32] |
| Aspergilus nidulans | 0.3 | [78] |
| Kluyveromyces lactis | 0.6 mg/109 cells | [102] |
| Saccharomyces uvarum | 14.3 | [103] |
| (Chang et al., 2008) | 70.32 | [42] |
| Archea a | ||
| Halobacterium cutirubrum | 1 | [78] |
| Bacteria/Protists a | ||
| Rubritalea squalenifaciens | 15 | [79] |
| Pseudomonas sp. | 0.10–0.76 | [78] |
| Methylomonas methanolica | 1.16 | |
| Methylococcus capsulatus | 5.5 | |
| Schizochytrium mangrovei | 0.16 | [104] |
| Aurantiochytrium sp. 18 W-13a | 198 | [105] |
| Aurantiochytrium sp. Yonez 5–1 | 317.74 | [76] |
| Aurantiochytrium sp. BR-MP4-A1 | 0.57 | [104] |
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chandran, S.S.; Kealey, J.T.; Reeves, C.D. Microbial Production of Isoprenoids. Process Biochem. 2011, 46, 1703–1710. [Google Scholar] [CrossRef]
- Dairi, T. 1.22—Isoprenoid in Actinomycetes. In Comprehensive Natural Products II; Liu, H.-W., Mander, L., Eds.; Elsevier: Oxford, UK, 2010; pp. 789–814. [Google Scholar]
- Gohil, N.; Bhattacharjee, G.; Khambhati, K.; Braddick, D.; Singh, V. Engineering Strategies in Microorganisms for the Enhanced Production of Squalene: Advances, Challenges and Opportunities. Front. Bioeng. Biotechnol. 2019, 7, 50. [Google Scholar] [CrossRef] [PubMed]
- Micera, M.; Botto, A.; Geddo, F.; Antoniotti, S.; Bertea, C.M.; Levi, R.; Gallo, M.P.; Querio, G. Squalene: More than a Step toward Sterols. Antioxidants 2020, 9, 688. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.C.K.; Ahrens, E.H.; Schreibman, P.H.; Crouse, J.R. Measurement of Squalene in Human Tissues and Plasma: Validation and Application. J. Lipid Res. 1976, 17, 38–45. [Google Scholar] [CrossRef]
- Kohno, Y.; Egawa, Y.; Itoh, S.; Nagaoka, S.I.; Takahashi, M.; Mukai, K. Kinetic Study of Quenching Reaction of Singlet Oxygen and Scavenging Reaction of Free Radical by Squalene in N-Butanol. Biochim. Biophys. Acta-Lipids Lipid Metab. 1995, 1256, 52–56. [Google Scholar] [CrossRef]
- Paramasivan, K.; Rajagopal, K.; Mutturi, S. Studies on Squalene Biosynthesis and the Standardization of Its Extraction Methodology from Saccharomyces Cerevisiae. Appl. Biochem. Biotechnol. 2019, 187, 691–707. [Google Scholar] [CrossRef]
- Tsujimoto, M. About Kuroko-Zame Shark Oil. J. Soc. Chem. Ind. 1916, 9, 953–958. [Google Scholar]
- Cárdeno, A.; Aparicio-Soto, M.; Montserrat-de la Paz, S.; Bermudez, B.; Muriana, F.J.G.; Alarcón-de-la-Lastra, C. Squalene Targets Pro- and Anti-Inflammatory Mediators and Pathways to Modulate over-Activation of Neutrophils, Monocytes and Macrophages. J. Funct. Foods 2015, 14, 779–790. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.-K.; Karadeniz, F. Biological Importance and Applications of Squalene and Squalane. Adv. Food Nutr. Res. 2012, 65, 223–233. [Google Scholar]
- Popa, I.; Băbeanu, N.; Niță, S.; Popa, O. Squalene-Natural Resources and Applications. Farmacia 2014, 62, 840–862. [Google Scholar]
- Sánchez-Fidalgo, S.; Villegas, I.; Rosillo, M.Á.; Aparicio-Soto, M.; de la Lastra, C.A. Dietary Squalene Supplementation Improves DSS-Induced Acute Colitis by Downregulating P38 MAPK and NFkB Signaling Pathways. Mol. Nutr. Food Res. 2015, 59, 284–292. [Google Scholar] [CrossRef] [PubMed]
- Skorkowska-Telichowska, K.; Hasiewicz-Derkacz, K.; Gębarowski, T.; Kulma, A.; Moreira, H.; Kostyn, K.; Gębczak, K.; Szyjka, A.; Wojtasik, W.; Gąsiorowski, K. Emulsions Made of Oils from Seeds of GM Flax Protect V79 Cells against Oxidative Stress. Oxid. Med. Cell. Longev. 2016, 2016, 7510759. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flavio, D.; Romain, B.; Catherine, C.; Franceline, R.; Arnaud, P.; Amandine, G.; Julie, M.; Françoise, G.; Mariana, V.; Patrick, C. Squalene-Based Multidrug Nanoparticles for Improved Mitigation of Uncontrolled Inflammation in Rodents. Sci. Adv. 2021, 6, eaaz5466. [Google Scholar]
- Sobot, D.; Mura, S.; Yesylevskyy, S.O.; Dalbin, L.; Cayre, F.; Bort, G.; Mougin, J.; Desmaële, D.; Lepetre-Mouelhi, S.; Pieters, G.; et al. Conjugation of Squalene to Gemcitabine as Unique Approach Exploiting Endogenous Lipoproteins for Drug Delivery. Nat. Commun. 2017, 8, 15678. [Google Scholar] [CrossRef]
- Nguyen-Contant, P.; Sangster, M.Y.; Topham, D.J. Squalene-Based Influenza Vaccine Adjuvants and Their Impact on the Hemagglutinin-Specific B Cell Response. Pathogens 2021, 10, 355. [Google Scholar] [CrossRef] [PubMed]
- Calabro, S.; Tortoli, M.; Baudner, B.C.; Pacitto, A.; Cortese, M.; O’Hagan, D.T.; De Gregorio, E.; Seubert, A.; Wack, A. Vaccine Adjuvants Alum and MF59 Induce Rapid Recruitment of Neutrophils and Monocytes That Participate in Antigen Transport to Draining Lymph Nodes. Vaccine 2011, 29, 1812–1823. [Google Scholar] [CrossRef]
- Tregoning, J.S.; Russell, R.F.; Kinnear, E. Adjuvanted Influenza Vaccines. Hum. Vaccin. Immunother. 2018, 14, 550–564. [Google Scholar] [CrossRef] [Green Version]
- Ahuja, K.; Singh, S. Squalene Market Size by Source (Shark Liver, VegeTable, Synthetic), by Application (Cosmetic, Supplements, Pharmaceuticals), Industry Analysis Report, Regional Outlook, Application Potential, Price Trends, Competitive Market Share & Forecast, 2015–2022. 2014. Available online: https://www.researchandmarkets.com/reports/4033349/squalene-market-by-source-shark-liver (accessed on 10 January 2022).
- Ducos, L.; Guillonneau, V.; Manach, F.L.; Nouvian, C. Beauty and the Beast: Shark in Our Beauty Creams. 2015. Available online: https://www.bloomassociation.org/en/beauty-and-the-beast-shark-in-our-beauty-creams/ (accessed on 10 January 2022).
- McPhee, D.; Pin, A.; Kizer, L.; Perelman, L. Deriving Renewable Squalane from Sugarcane. Cosmet. Toilet. Mag. 2014, 129, 1–6. [Google Scholar]
- Ciriminna, R.; Pandarus, V.; Béland, F.; Pagliaro, M. Catalytic Hydrogenation of Squalene to Squalane. Org. Process Res. Dev. 2014, 18, 1110–1115. [Google Scholar] [CrossRef]
- Kelly, G.S. Squalene and Its Potential Clinical Uses. Altern. Med. Rev. 1999, 4, 29–36. [Google Scholar]
- Markets and Markets. Squalene Market by Source Type (Animal Source (Shark Liver Oil), VegeTable Source (Olive Oil, Palm Oil, Amaranth Oil), Biosynthetic (GM Yeast]), End-Use Industry (Cosmetics, Food, and Pharmaceuticals), and Region—Global Forecast to 2025; Markets and Markets: Tung Choi St., Mong Kok, Hong Kong, 2020. [Google Scholar]
- Lozano-Grande, M.A.; Gorinstein, S.; Espitia-Rangel, E.; Dávila-Ortiz, G.; Martínez-Ayala, A.L. Plant Sources, Extraction Methods, and Uses of Squalene. Int. J. Agron. 2018, 2018, 1829160. [Google Scholar] [CrossRef]
- Market Reports World. Global Squalene Market Size, Manufacturers, Supply Chain, Sales Channel and Clients, 2021–2027. 2021. Available online: https://rivercountry.newschannelnebraska.com/story/45517817/Global-Squalene (accessed on 10 January 2022).
- Mlakar, S.G.; Turinek, M.; Jakop, M.; Bavec, M.; Bavec, F. Grain Amaranth as an Alternative and Perspective Crop in Temperate Climate. J. Geogr. 2010, 5, 135–145. [Google Scholar]
- Popa, O.; Bəbeanu, N.E.; Popa, I.; Niţə, S.; Dinu-Pârvu, C.E. Methods for Obtaining and Determination of Squalene from Natural Sources. Biomed Res. Int. 2015, 2015, 367202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meadows, A.L.; Hawkins, K.M.; Tsegaye, Y.; Antipov, E.; Kim, Y.; Raetz, L.; Dahl, R.H.; Tai, A.; Mahatdejkul-Meadows, T.; Xu, L.; et al. Rewriting Yeast Central Carbon Metabolism for Industrial Isoprenoid Production. Nature 2016, 537, 694–697. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.-J.; Solbiati, J.O.; Ramamoorthy, G.; Hillerich, B.S.; Seidel, R.D.; Cronan, J.E.; Almo, S.C.; Poulter, C.D. Biosynthesis of Squalene from Farnesyl Diphosphate in Bacteria: Three Steps Catalyzed by Three Enzymes. ACS Cent. Sci. 2015, 1, 77–82. [Google Scholar] [CrossRef] [Green Version]
- Paramasivan, K.; Gupta, N.; Mutturi, S. Adaptive Evolution of Engineered Yeast for Squalene Production Improvement and Its Genome-Wide Analysis. Yeast 2021, 38, 424–437. [Google Scholar] [CrossRef]
- Bhattacharjee, P.; Shukla, V.B.; Singhal, R.S.; Kulkarni, P.R. Studies on Fermentative Production of Squalene. World J. Microbiol. Biotechnol. 2001, 17, 811–816. [Google Scholar] [CrossRef]
- Bomgardner, M. On the Hunt for Alternatives to Shark Squalene for Vaccines. 2020. Available online: https://cen.acs.org/pharmaceuticals/vaccines/hunt-alternatives-shark-squalene-vaccines/98/i47 (accessed on 18 January 2022).
- Fathizadeh, H.; Afshar, S.; Masoudi, M.R.; Gholizadeh, P.; Asgharzadeh, M.; Ganbarov, K.; Köse, Ş.; Yousefi, M.; Kafil, H.S. SARS-CoV-2 (Covid-19) Vaccines Structure, Mechanisms and Effectiveness: A Review. Int. J. Biol. Macromol. 2021, 188, 740–750. [Google Scholar] [CrossRef]
- World Health Organization. Annex 2: WHO Good Manufacturing Practices for Biological Products; WHO: Geneva, Switzerland, 2016. [Google Scholar]
- Vajo, Z.; Balaton, G.; Vajo, P.; Kalabay, L.; Erdman, A.; Torzsa, P. Dose Sparing and the Lack of a Dose-Response Relationship with an Influenza Vaccine in Adult and Elderly Patients—A Randomized, Double-Blind Clinical Trial. Br. J. Clin. Pharmacol. 2017, 83, 1912–1920. [Google Scholar] [CrossRef] [Green Version]
- Dikow, R.; Eckerle, I.; Ksoll-Rudek, D.; Hampel, H.; Schwenger, V.; Zeier, M.; Schnitzler, P.; Sommerer, C. Immunogenicity and Efficacy in Hemodialysis Patients of an AS03(A)-Adjuvanted Vaccine for 2009 Pandemic Influenza A(H1N1): A Nonrandomized Trial. Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 2011, 57, 716–723. [Google Scholar] [CrossRef] [PubMed]
- Siegrist, C.-A.; Aspinall, R. B-Cell Responses to Vaccination at the Extremes of Age. Nat. Rev. Immunol. 2009, 9, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Chilton, P.M.; Hadel, D.M.; To, T.T.; Mitchell, T.C.; Darveau, R.P. Adjuvant Activity of Naturally Occurring Monophosphoryl Lipopolysaccharide Preparations from Mucosa-Associated Bacteria. Infect. Immun. 2013, 81, 3317–3325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kensil, C.R. Saponins as Vaccine Adjuvants. Crit. Rev. Ther. Drug Carrier Syst. 1996, 13, 1–55. [Google Scholar] [PubMed]
- Dupuis, M.; McDonald, D.M.; Ott, G. Distribution of Adjuvant MF59 and Antigen GD2 after Intramuscular Injection in Mice. Vaccine 1999, 18, 434–439. [Google Scholar] [CrossRef]
- Mosca, F.; Tritto, E.; Muzzi, A.; Monaci, E.; Bagnoli, F.; Iavarone, C.; O’Hagan, D.; Rappuoli, R.; De Gregorio, E. Molecular and Cellular Signatures of Human Vaccine Adjuvants. Proc. Natl. Acad. Sci. USA 2008, 105, 10501–10506. [Google Scholar] [CrossRef] [Green Version]
- Awate, S.; Babiuk, L.A.; Mutwiri, G. Mechanisms of Action of Adjuvants. Front. Immunol. 2013, 4, 114. [Google Scholar] [CrossRef] [Green Version]
- Ko, E.-J.; Lee, Y.-T.; Kim, K.-H.; Jung, Y.-J.; Lee, Y.; Denning, T.L.; Kang, S.-M. Effects of MF59 Adjuvant on Induction of Isotype-Switched IgG Antibodies and Protection after Immunization with T-Dependent Influenza Virus Vaccine in the Absence of CD4+ T Cells. J. Virol. 2016, 90, 6976–6988. [Google Scholar] [CrossRef] [Green Version]
- Seubert, A.; Monaci, E.; Pizza, M.; O’Hagan, D.T.; Wack, A. The Adjuvants Aluminum Hydroxide and MF59 Induce Monocyte and Granulocyte Chemoattractants and Enhance Monocyte Differentiation toward Dendritic Cells. J. Immunol. 2008, 180, 5402–5412. [Google Scholar] [CrossRef]
- Kawasaki, T.; Kawai, T. Toll-Like Receptor Signaling Pathways. Front. Immunol. 2014, 5, 461. [Google Scholar] [CrossRef] [Green Version]
- Nakaya, H.I.; Clutterbuck, E.; Kazmin, D.; Wang, L.; Cortese, M.; Bosinger, S.E.; Patel, N.B.; Zak, D.E.; Aderem, A.; Dong, T.; et al. Systems Biology of Immunity to MF59-Adjuvanted versus Nonadjuvanted Trivalent Seasonal Influenza Vaccines in Early Childhood. Proc. Natl. Acad. Sci. USA 2016, 113, 1853–1858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vono, M.; Taccone, M.; Caccin, P.; Gallotta, M.; Donvito, G.; Falzoni, S.; Palmieri, E.; Pallaoro, M.; Rappuoli, R.; Di Virgilio, F.; et al. The Adjuvant MF59 Induces ATP Release from Muscle That Potentiates Response to Vaccination. Proc. Natl. Acad. Sci. USA 2013, 110, 21095–21100. [Google Scholar] [CrossRef] [Green Version]
- Pulendran, B.; Arunachalam, P.S.; O’Hagan, D.T. Emerging Concepts in the Science of Vaccine Adjuvants. Nat. Rev. Drug Discov. 2021, 20, 454–475. [Google Scholar] [CrossRef] [PubMed]
- Wilkins, A.L.; Kazmin, D.; Napolitani, G.; Clutterbuck, E.A.; Pulendran, B.; Siegrist, C.-A.; Pollard, A.J. AS03- and MF59-Adjuvanted Influenza Vaccines in Children. Front. Immunol. 2017, 8, 1760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morel, S.; Didierlaurent, A.; Bourguignon, P.; Delhaye, S.; Baras, B.; Jacob, V.; Planty, C.; Elouahabi, A.; Harvengt, P.; Carlsen, H.; et al. Adjuvant System AS03 Containing α-Tocopherol Modulates Innate Immune Response and Leads to Improved Adaptive Immunity. Vaccine 2011, 29, 2461–2473. [Google Scholar] [CrossRef] [PubMed]
- Givord, C.; Welsby, I.; Detienne, S.; Thomas, S.; Assabban, A.; Lima Silva, V.; Molle, C.; Gineste, R.; Vermeersch, M.; Perez-Morga, D.; et al. Activation of the Endoplasmic Reticulum Stress Sensor IRE1α by the Vaccine Adjuvant AS03 Contributes to Its Immunostimulatory Properties. NPJ Vaccines 2018, 3, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Couch, R.B.; Bayas, J.M.; Caso, C.; Mbawuike, I.N.; López, C.N.; Claeys, C.; El Idrissi, M.; Hervé, C.; Laupèze, B.; Oostvogels, L.; et al. Superior Antigen-Specific CD4+ T-Cell Response with AS03-Adjuvantation of a Trivalent Influenza Vaccine in a Randomised Trial of Adults Aged 65 and Older. BMC Infect. Dis. 2014, 14, 425. [Google Scholar] [CrossRef] [Green Version]
- Howard, L.M.; Hoek, K.L.; Goll, J.B.; Samir, P.; Galassie, A.; Allos, T.M.; Niu, X.; Gordy, L.E.; Creech, C.B.; Prasad, N.; et al. Cell-Based Systems Biology Analysis of Human AS03-Adjuvanted H5N1 Avian Influenza Vaccine Responses: A Phase I Randomized Controlled Trial. PLoS ONE 2017, 12, e0167488. [Google Scholar] [CrossRef]
- Moris, P.; van der Most, R.; Leroux-Roels, I.; Clement, F.; Dramé, M.; Hanon, E.; Leroux-Roels, G.G.; Van Mechelen, M. H5N1 Influenza Vaccine Formulated with AS03 A Induces Strong Cross-Reactive and Polyfunctional CD4 T-Cell Responses. J. Clin. Immunol. 2011, 31, 443–454. [Google Scholar] [CrossRef] [Green Version]
- Klucker, M.-F.; Dalençon, F.; Probeck, P.; Haensler, J. AF03, an Alternative Squalene Emulsion-Based Vaccine Adjuvant Prepared by a Phase Inversion Temperature Method. J. Pharm. Sci. 2012, 101, 4490–4500. [Google Scholar] [CrossRef]
- European Medicines Agency. Humenza (Pandemic Influenza Vaccine—H1N1—Split Virion, Inactivated, Adjuvanted; Withdrawal of the Marketing Authorisation in the European Union: London, UK, 2011. [Google Scholar]
- Allen, J.D.; Ray, S.; Ross, T.M. Split Inactivated COBRA Vaccine Elicits Protective Antibodies against H1N1 and H3N2 Influenza Viruses. PLoS ONE 2018, 13, e0210043. [Google Scholar]
- Darricarrère, N.; Qiu, Y.; Kanekiyo, M.; Creanga, A.; Gillespie, R.A.; Moin, S.M.; Saleh, J.; Sancho, J.; Chou, T.-H.; Zhou, Y.; et al. Broad Neutralization of H1 and H3 Viruses by Adjuvanted Influenza HA Stem Vaccines in Nonhuman Primates. Sci. Transl. Med. 2021, 13, eabe5449. [Google Scholar] [CrossRef]
- European Medicines Agency. Assessment Report: Humenza; European Medicines Agency: London, UK, 2010.
- Goepfert, P.A.; Fu, B.; Chabanon, A.-L.; Bonaparte, M.I.; Davis, M.G.; Essink, B.J.; Frank, I.; Haney, O.; Janosczyk, H.; Keefer, M.C.; et al. Safety and Immunogenicity of SARS-CoV-2 Recombinant Protein Vaccine Formulations in Healthy Adults: Interim Results of a Randomised, Placebo-Controlled, Phase 1–2, Dose-Ranging Study. Lancet. Infect. Dis. 2021, 21, 1257–1270. [Google Scholar] [CrossRef]
- Sarkar, I.; Garg, R.; van Drunen Littel-van den Hurk, S. Selection of Adjuvants for Vaccines Targeting Specific Pathogens. Expert Rev. Vaccines 2019, 18, 505–521. [Google Scholar] [CrossRef]
- Naziri, E.; Mantzouridou, F.; Tsimidou, M.Z. Squalene Resources and Uses Point to the Potential of Biotechnology. Lipid Technol. 2011, 23, 270–273. [Google Scholar] [CrossRef]
- Patel, A.; Mu, L.; Shi, Y.; Rova, U.; Christakopoulos, P.; Matsakas, L. Novel Biorefinery Approach Aimed at Vegetarians Reduces the Dependency on Marine Fish Stocks for Obtaining Squalene and Docosahexaenoic Acid. ACS Sustain. Chem. Eng. 2020, 8, 8803–8813. [Google Scholar] [CrossRef]
- Lean, G.; Gray, L. Hay Festival 2013: Supermodel Lily Cole: Ban Cruel Shark Liver from Make Up. Daily Telegr. 2013. [Google Scholar]
- Sethi, A.; Kaur, T.; Malhotra, S.K.; Gambhir, M.L. Moisturizers: The Slippery Road. Indian J. Dermatol. 2016, 61, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Gullón, P.; Gullón, B.; Astray, G.; Carpena, M.; Fraga-Corral, M.; Prieto, M.A.; Simal-Gandara, J. Valorization of By-Products from Olive Oil Industry and Added-Value Applications for Innovative Functional Foods. Food Res. Int. 2020, 137, 109683. [Google Scholar] [CrossRef]
- León-Camacho, M.; García-González, D.L.; Aparicio, R. A Detailed and Comprehensive Study of Amaranth (Amaranthus Cruentus L.) Oil Fatty Profile. Eur. Food Res. Technol. 2001, 213, 349–355. [Google Scholar] [CrossRef]
- Lozano-Grande, M.A.; Dávila-Ortiz, G.; García-Dávila, J.; Ríos-Cortés, G.; Espitia-Rangel, E.; Martínez-Ayala, A.L. Optimisation of Microwave-Assisted Extraction of Squalene from Amaranthus spp. Seeds. J. Microw. Power Electromagn. Energy 2019, 53, 243–258. [Google Scholar] [CrossRef]
- Kraujalis, P.; Venskutonis, P.R. Supercritical Carbon Dioxide Extraction of Squalene and Tocopherols from Amaranth and Assessment of Extracts Antioxidant Activity. J. Supercrit. Fluids 2013, 80, 78–85. [Google Scholar] [CrossRef]
- Parasuraman, S.; Sujithra, J.; Syamittra, B.; Yeng, W.Y.; Ping, W.Y.; Muralidharan, S.; Raj, P.V.; Dhanaraj, S.A. Evaluation of Sub-Chronic Toxic Effects of Petroleum Ether, a Laboratory Solvent in Sprague-Dawley Rats. J. Basic Clin. Pharm. 2014, 5, 89–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krulj, J.; Brlek, T.; Pezo, L.; Brkljača, J.; Popović, S.; Zeković, Z.; Bodroža Solarov, M. Extraction Methods of Amaranthus sp. Grain Oil Isolation. J. Sci. Food Agric. 2016, 96, 3552–3558. [Google Scholar] [CrossRef]
- Directorate-General for Maritime Affairs and Fisheries. Deep-Sea Fisheries: Increased Protection for Deep-Sea Species; Directorate-General for Maritime Affairs and Fisheries: Brussels, Belgium, 2021.
- Science Business Publishing International SRL. IASMA: New Method for Identifying Oil from Sharks; Science Business Publishing International SRL: Brussels, Belgium, 2010. [Google Scholar]
- Abbattista, R.; Ventura, G.; Calvano, C.D.; Cataldi, T.R.I.; Losito, I. Bioactive Compounds in Waste By-Products from Olive Oil Production: Applications and Structural Characterization by Mass Spectrometry Techniques. Foods 2021, 10, 1236. [Google Scholar] [CrossRef]
- Heggeset, T.M.B.; Ertesvåg, H.; Liu, B.; Ellingsen, T.E.; Vadstein, O.; Aasen, I.M. Lipid and DHA-Production in Aurantiochytrium sp.—Responses to Nitrogen Starvation and Oxygen Limitation Revealed by Analyses of Production Kinetics and Global Transcriptomes. Sci. Rep. 2019, 9, 19470. [Google Scholar] [CrossRef]
- Nakazawa, A.; Kokubun, Y.; Matsuura, H.; Yonezawa, N.; Kose, R.; Yoshida, M.; Tanabe, Y.; Kusuda, E.; Van Thang, D.; Ueda, M.; et al. TLC Screening of Thraustochytrid Strains for Squalene Production. J. Appl. Phycol. 2014, 26, 29–41. [Google Scholar] [CrossRef]
- Chang, M.-H.; Kim, H.-J.; Jahng, K.-Y.; Hong, S.-C. The Isolation and Characterization of Pseudozyma sp. JCC 207, a Novel Producer of Squalene. Appl. Microbiol. Biotechnol. 2008, 78, 963–972. [Google Scholar] [CrossRef]
- Goldberg, I.; Shechter, I. Occurrence of Squalene in Methanol-Grown Bacteria. J. Bacteriol. 1978, 135, 717–720. [Google Scholar] [CrossRef] [Green Version]
- Kasai, H.; Katsuta, A.; Sekiguchi, H.; Matsuda, S.; Adachi, K.; Shindo, K.; Yoon, J.; Yokota, A.; Shizuri, Y. Rubritalea Squalenifaciens sp. nov., a Squalene-Producing Marine Bacterium Belonging to Subdivision 1 of the Phylum “Verrucomicrobia”. Int. J. Syst. Evol. Microbiol. 2007, 57, 1630–1634. [Google Scholar] [CrossRef]
- Espinosa, M.I.; Williams, T.C.; Pretorius, I.S.; Paulsen, I.T. Benchmarking Two Saccharomyces Cerevisiae Laboratory Strains for Growth and Transcriptional Response to Methanol. Synth. Syst. Biotechnol. 2019, 4, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Yao, J.; Liu, L.; Ma, X.; Li, W.; Sun, X.; Wang, Y. Improving Squalene Production by Enhancing the NADPH/NADP+ Ratio, Modifying the Isoprenoid-Feeding Module and Blocking the Menaquinone Pathway in Escherichia Coli. Biotechnol. Biofuels 2019, 12, 68. [Google Scholar] [CrossRef] [PubMed]
- Jandrositz, A.; Turnowsky, F.; Högenauer, G. The Gene Encoding Squalene Epoxidase from Saccharomyces Cerevisiae: Cloning and Characterization. Gene 1991, 107, 155–160. [Google Scholar] [CrossRef]
- Gnamusch, E.; Ryder, N.S.; Paltauf, F. Effect of Squalene on the Structure and Function of Fungal Membranes. J. Dermatolog. Treat. 1992, 3, 9–13. [Google Scholar] [CrossRef]
- Souza, C.M.; Schwabe, T.M.E.; Pichler, H.; Ploier, B.; Leitner, E.; Guan, X.L.; Wenk, M.R.; Riezman, I.; Riezman, H. A STable Yeast Strain Efficiently Producing Cholesterol Instead of Ergosterol Is Functional for Tryptophan Uptake, but Not Weak Organic Acid Resistance. Metab. Eng. 2011, 13, 555–569. [Google Scholar] [CrossRef] [PubMed]
- Furubayashi, M.; Li, L.; Katabami, A.; Saito, K.; Umeno, D. Construction of Carotenoid Biosynthetic Pathways Using Squalene Synthase. FEBS Lett. 2014, 588, 436–442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katabami, A.; Li, L.; Iwasaki, M.; Furubayashi, M.; Saito, K.; Umeno, D. Production of Squalene by Squalene Synthases and Their Truncated Mutants in Escherichia Coli. J. Biosci. Bioeng. 2015, 119, 165–171. [Google Scholar] [CrossRef] [Green Version]
- Lyon, C.K.; Becker, R. Extraction and Refining of Oil from Amaranth Seed. J. Am. Oil Chem. Soc. 1987, 64, 233–236. [Google Scholar] [CrossRef]
- Wejnerowska, G.; Heinrich, P.; Gaca, J. Separation of Squalene and Oil from Amaranthus Seeds by Supercritical Carbon Dioxide. Sep. Purif. Technol. 2013, 110, 39–43. [Google Scholar] [CrossRef]
- Frega, N.; Bocci, F.; Lercker, G. Direct Gas Chromatographic Analysis of the Unsaponifiable Fraction of Different Oils with a Polar Capillary Column. J. Am. Oil Chem. Soc. 1992, 69, 447–450. [Google Scholar] [CrossRef]
- Lanzón, A.; Albi, T.; Cert, A.; Gracián, J. The Hydrocarbon Fraction of Virgin Olive Oil and Changes Resulting from Refining. J. Am. Oil Chem. Soc. 1994, 71, 285–291. [Google Scholar] [CrossRef]
- Grigoriadou, D.; Androulaki, A.; Psomiadou, E.; Tsimidou, M.Z. Solid Phase Extraction in the Analysis of Squalene and Tocopherols in Olive Oil. Food Chem. 2007, 105, 675–680. [Google Scholar] [CrossRef]
- Bada, J.C.; León-Camacho, M.; Prieto, M.; Alonso, L. Characterization of Oils of Hazelnuts from Asturias, Spain. Eur. J. Lipid Sci. Technol. 2004, 106, 294–300. [Google Scholar] [CrossRef]
- Pokkanta, P.; Sookwong, P.; Tanang, M.; Setchaiyan, S.; Boontakham, P.; Mahatheeranont, S. Simultaneous Determination of Tocols, γ-Oryzanols, Phytosterols, Squalene, Cholecalciferol and Phylloquinone in Rice Bran and VegeTable Oil Samples. Food Chem. 2019, 271, 630–638. [Google Scholar] [CrossRef] [PubMed]
- Tuberoso, C.I.G.; Kowalczyk, A.; Sarritzu, E.; Cabras, P. Determination of Antioxidant Compounds and Antioxidant Activity in Commercial Oilseeds for Food Use. Food Chem. 2007, 103, 1494–1501. [Google Scholar] [CrossRef]
- Wen, X.; Zhu, M.; Hu, R.; Zhao, J.; Chen, Z.; Li, J.; Ni, Y. Characterisation of Seed Oils from Different Grape Cultivars Grown in China. J. Food Sci. Technol. 2016, 53, 3129–3136. [Google Scholar] [CrossRef] [PubMed]
- Maguire, L.S.; O’Sullivan, S.M.; Galvin, K.; O’Connor, T.P.; O’Brien, N.M. Fatty Acid Profile, Tocopherol, Squalene and Phytosterol Content of Walnuts, Almonds, Peanuts, Hazelnuts and the Macadamia Nut. Int. J. Food Sci. Nutr. 2004, 55, 171–178. [Google Scholar] [CrossRef]
- Naz, S.; Sherazi, S.T.H.; Talpur, F.N.; Kara, H.; Sirajuddin, S.; Khaskheli, A.R. Chemical Characterization of Canola and Sunflower Oil Deodorizer Distillates. Polish J. Food Nutr. Sci. 2014, 64, 115–120. [Google Scholar] [CrossRef] [Green Version]
- Dumont, M.-J.; Narine, S.S. Characterization of Flax and Soybean Soapstocks, and Soybean Deodorizer Distillate by GC-FID. J. Am. Oil Chem. Soc. 2007, 84, 1101–1105. [Google Scholar] [CrossRef]
- Gunawan, S.; Kasim, N.S.; Ju, Y.-H. Separation and Purification of Squalene from Soybean Oil Deodorizer Distillate. Sep. Purif. Technol. 2008, 60, 128–135. [Google Scholar] [CrossRef]
- Mantzouridou, F.; Tsimidou, M.Z. Observations on Squalene Accumulation in Saccharomyces Cerevisiae Due to the Manipulation of HMG2 and ERG6. FEMS Yeast Res. 2010, 10, 699–707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drozdíková, E.; Garaiová, M.; Csáky, Z.; Obernauerová, M.; Hapala, I. Production of Squalene by Lactose-Fermenting Yeast Kluyveromyces Lactis with Reduced Squalene Epoxidase Activity. Lett. Appl. Microbiol. 2015, 61, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Blagović, B.; Rupvcić, J.; Mesarivc, M.; Georgiú, K.; Marić, V. Lipid Composition of Brewer’s Yeast. Food Technol. Biotechnol. 2001, 39, 175–181. [Google Scholar]
- Li, Q.; Chen, G.-Q.; Fan, K.-W.; Lu, F.-P.; Aki, T.; Jiang, Y. Screening and Characterization of Squalene-Producing Thraustochytrids from Hong Kong Mangroves. J. Agric. Food Chem. 2009, 57, 4267–4272. [Google Scholar] [CrossRef] [PubMed]
- Kaya, K.; Nakazawa, A.; Matsuura, H.; Honda, D.; Inouye, I.; Watanabe, M.M. Thraustochytrid Aurantiochytrium sp. 18W-13a Accummulates High Amounts of Squalene. Biosci. Biotechnol. Biochem. 2011, 75, 2246–2248. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mendes, A.; Azevedo-Silva, J.; Fernandes, J.C. From Sharks to Yeasts: Squalene in the Development of Vaccine Adjuvants. Pharmaceuticals 2022, 15, 265. https://doi.org/10.3390/ph15030265
Mendes A, Azevedo-Silva J, Fernandes JC. From Sharks to Yeasts: Squalene in the Development of Vaccine Adjuvants. Pharmaceuticals. 2022; 15(3):265. https://doi.org/10.3390/ph15030265
Chicago/Turabian StyleMendes, Adélia, João Azevedo-Silva, and João C. Fernandes. 2022. "From Sharks to Yeasts: Squalene in the Development of Vaccine Adjuvants" Pharmaceuticals 15, no. 3: 265. https://doi.org/10.3390/ph15030265
APA StyleMendes, A., Azevedo-Silva, J., & Fernandes, J. C. (2022). From Sharks to Yeasts: Squalene in the Development of Vaccine Adjuvants. Pharmaceuticals, 15(3), 265. https://doi.org/10.3390/ph15030265







