Abstract
Metabolic syndrome is a set of risk factors that consist of abdominal obesity, arterial hypertension, alterations in the lipid profile, and hyperglycemia. The current therapeutic strategy includes polypharmacy, using three or more drugs to control each syndrome component. However, this approach has drawbacks that could lead to therapeutic failure. Multitarget drugs are molecules with the ability to act on different targets simultaneously and are an attractive alternative for treating complex diseases such as metabolic syndrome. Previously, we identified a triamide derivative of 5-aminoanthranilic acid that exhibited hypoglycemic, hypolipemic, and antihypertensive activities simultaneously. In the present study, we report the synthesis and in combo evaluation of new derivatives of anthranilic acid, intending to identify the primary structural factors that improve the activity over metabolic syndrome-related parameters. We found that substitution on position 5, incorporation of 3,4-dimethoxyphenyl substituents, and having a free carboxylic acid group lead to the in vitro inhibition of HMG-CoA reductase, and simultaneously the diminution of the serum levels of glucose, triglycerides, and cholesterol in a diet-induced in vivo model.
1. Introduction
Nowadays, metabolic diseases are one of the most critical health issues worldwide. Metabolic syndrome (MetS) is an assortment of risk factors that cluster abdominal obesity, hypertension, alterations in the lipid profile, and hyperglycemia and is associated with other comorbidities such as prothrombotic state, nonalcoholic fatty liver disease (NAFLD), and reproductive disorders [1,2]. It is estimated that 20–25% of the world’s adult population suffers from MetS, leading to an increased risk of all-cause mortality, especially from cardiovascular diseases [3]. Also, it increases the risk of mortality by infectious agents, as was evidenced during the COVID-19 pandemic [4,5]. Factors that raise the likelihood of developing MetS are genetic background, hypercaloric diet intake, sedentarism, malnutrition, and body habits [2,6,7]. The pathophysiology of MetS consists of complex mechanisms, of which there are still pathways that have not been fully elucidated. Furthermore, it is still under debate whether the individual components of MetS should be treated as distinct pathologies or as manifestations of a common pathogenic mechanism, which is resumed in Figure 1 [8,9]. Of the proposed mechanisms, insulin resistance, neurohormonal activation, and chronic inflammation are the main dysregulated processes involved in the onset and development of MetS and its transition to cardiovascular disease.

Figure 1.
Resumed pathophysiology of metabolic syndrome.
The primary intervention in MetS treatment is lifestyle modification, mainly by increased physical activity and dietary change. The focus of such strategies is weight reduction and the control of metabolic parameters [1,10]. However, a pharmacological intervention for MetS is required in more advanced cases. Currently, there is no single-drug therapy for MetS. Consequently, the current pharmacotherapy focus is on the individual management of each metabolic abnormality and associated comorbidities, resulting in the necessity of polypharmacy, primarily as hypoglycemic and hypotensive drugs, statins for dyslipidemia treatment, and antiplatelet drugs to decrease prothrombotic risk [11]. However, polypharmacy tends to increase the risk of adverse outcomes due to drug–drug interactions or medication errors, prescribing cascade, duplication of therapies, and lack of treatment adherence, alongside an increase in the patient’s financial burden [12,13,14,15].
Indeed, one of the current challenges in Medicinal Chemistry is the development of successful drugs to treat multifactorial diseases such as MetS. The traditional “single-target” approach, which is focused on the development of ligands with high selectivity to a single biological entity (“on-target”), has clear advantages. First, it reduces the probability of undesirable effects resulting from interaction with other unwanted biological targets (“off-targets”). Second, the expected therapeutical results can be explained and modulated if needed [16]. However, the complexity of multifactorial pathologies suggests that a single-target approach may be insufficient. In this challenging scenario, multitarget drugs seem an attractive option: the synergism of the simultaneous modulation of two or more targets is more effective in illnesses where multiple pathways are involved in the onset and progression of the disease [17,18].
A multitarget drug can be defined as a drug that modulates multiple targets simultaneously [19,20,21]. This therapeutic approach offers some advantages over the traditional “singlet-target” approximation. It has been described that they exhibit higher therapeutic effects, simpler administration, less probability of drug−drug interaction, and the reduction of the development of drug resistance [22,23,24]. One of the most used strategies for multitarget drug design is to select a privileged scaffold from natural or synthetic origin, followed by optimization of this initial structure, usually guided by computational tools [25,26,27,28,29,30]. To confirm these in silico predictions, further in vitro and in vivo evaluation is required resulting in authentic in combo studies [31,32,33,34,35,36,37].
In our case, we selected anthranilic acid as the privileged scaffold. Previously, we reported the design, synthesis, and evaluation of compound 1 (Figure 2), a triamide derivative of 5-aminoanthranilic acid, as a potential multitarget drug for managing MetS [38]. The design of this compound was based on anthranilic acid as the initial template, since it is a privileged scaffold included as the core of compounds that have exhibited several bioactivities, including good binding properties against some targets related to metabolic diseases [39,40,41,42,43,44,45]. The incorporation of the appropriate substituents that could increase the affinity against PPAR-α, PPAR-γ, HMG-CoA reductase, and angiotensin-converting enzyme (ACE) was directed through molecular docking.
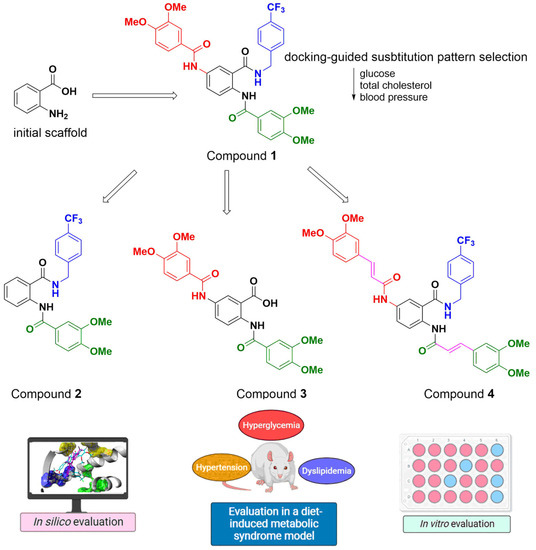
Figure 2.
Modifications of compound 1 evaluated in this work.
Compound 1 simultaneously diminished the glucose, triglyceride, total cholesterol serum levels, and blood pressure in an in vivo diet-induced MetS model [46,47]. This holistic model offers the advantage of providing relevant information on several bioactivities, allowing quick-go or no-go decisions and reducing the number of animals necessary to demonstrate multiple therapeutic effects [48,49].
To identify the structural factors related to the effect of substituted 5-aminoanthranilic acid derivatives over the parameters of MetS, we decided to modify the structure of compound 1, as depicted in Figure 2. These modifications included the simplification of the structure of compound 1 to render compounds 2 and 3, which could improve the physicochemical properties associated with the ADME profile since these compounds would not violate any of Lipinski’s rules; in addition, their bioevaluation would clarify the most relevant structural factors related to their multitarget properties. Additionally, we proposed compound 4 since it has been reported that incorporating ferulic acid-like moieties improves antioxidant and cardioprotective activities, and ferulic acid itself exhibited positive effects in a MetS rodent model [50,51,52,53]. In this work, we present the synthesis, in silico evaluation, and determination of the influence of the administration of these compounds in the in vivo diet-induced MetS model.
2. Results
2.1. Preparation of Compounds 2–4
Scheme 1 illustrates the synthetic route to obtain compounds 2–4 based on our previous work [38].
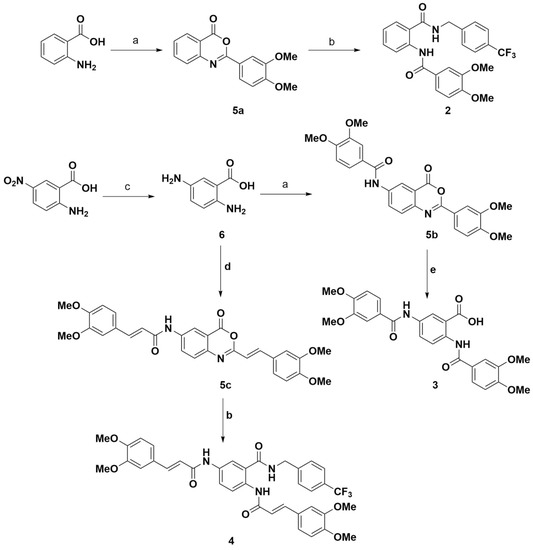
Scheme 1.
Preparation of target compounds 2–4. a. 3,4-dimethoxybenzoyl chloride, DMF, room temperature, overnight; b. 4-(trifluoromethyl)benzylamine, DMF, room temperature, 3 h; c. Sodium hydrosulfite, 80 °C, NaOH 10%; d. 3,4-dimethoxybenzoyl chloride, DMF, room temperature, 24 h; e. NaOH 5%, room temperature, 1 h.
Compound 2 was prepared from 4H-3,1-benzoxazin-4-one derivative 5a, obtained from the reaction of anthranilic acid with 3,4-dimethoxybenzoic acid chloride (94% yield) and the subsequent opening of 5a by treatment with 4-trifluoromethylbenzylamine to obtain compound 2 with a yield of 71%. A similar procedure was carried out to obtain compound 4 from compound 5c, although slightly lower yields were obtained (71% for 5c and 61% for 4). The preparation of compound 3 was initially attempted from the treatment of 5-aminoanthranilic acid with two equivalents of 3,4-dimethoxybenzoic acid chloride, obtaining the targeted disubstituted compound with a small quantity of the mono-substituted derivative. Therefore, we decided to prepare 3 from compound 5b (91% yield) and subsequent treatment with 5% sodium hydroxide solution to open the benzoxazinone ring, a strategy that gave better results in terms of yield (85%) and reaction workup.
2.2. In Silico Studies
Table 1 shows the results of the docking studies carried out on the projected targets. A more negative score value is associated with better binding. As expected, the decrease in molecular size usually leads to poorer binding ability, as seen for compound 2, which displayed the lowest theoretical affinity of the four tested molecules, while compound 4 had the highest affinity. It is important to consider the concept of ligand efficiency (LE), which expresses the sensitivity of affinity to a variation in molecular size [54]. Table 1 displays LE in terms of the score divided by the number of heavy atoms. Based on these values, removing the 4-trifluoromethylbenzylamine fragment leads to more efficient ligands than the initial compound 1a, suggesting that it is not critical for ligand binding. On the other hand, incorporating a vinyl moiety that delivers compound 4 has a detrimental effect on LE. Remarkably, compound 3 exhibited a similar LE to reference ligands. The in silico ADME/Tox profile was predicted using the pkCSM tool. Among the most relevant results, compound 3 would have the safer profile since it is not expected to be an hERG inhibitor, would have the highest tolerated dose, and would have higher metabolic stability than the other three compounds; however, it would possess the lowest intestinal absorption of the series (60% versus >80% of the other compounds). Overall, compound 3 rendered the best balance regarding predicted pharmacodynamic, pharmacokinetic, and toxicological properties.

Table 1.
Results of molecular docking studies.
Figure 3 illustrates the predicted poses of compound 3 in the evaluated targets. The rest of the predicted poses are included as part of the Supplementary Materials. The 3,4-dimethoxybenzoyl groups occupy cavities described as necessary for the binding of the known ligands of these targets.
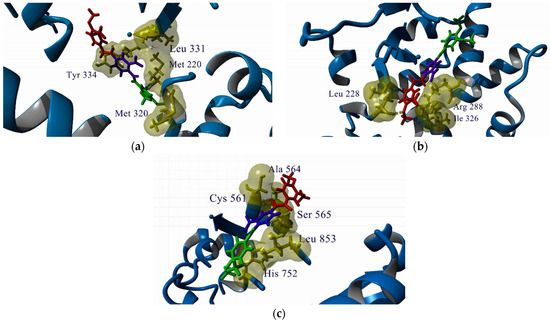
Figure 3.
Predicted poses of compound 3 in the active site or ligand-binding domain (LBD) of the analyzed targets. (a) PPAR-α; (b) PPAR-γ; (c) HMG-CoA reductase. Key residues for ligand binding are labeled. The color code for compound 3 is the same as in Figure 2.
2.3. In Vivo and In Vitro Studies
In the diet-induced model, MetS was generated through 12 weeks of the consumption of a high-fructose high-fat (HFHF) diet per the previous experience in our group [38,46]. The control group received a standard diet during the same period. After this induction phase, the animals of the standard diet were randomly allocated into two groups; one group (C/treated groups) would receive treatment with compounds 2, 3, or 4 for 14 days (10 mg/kg, p.o.), and the other group would receive no treatment (C groups). The same allocation was made for the diet-induced MetS group (the MetS group received no treatment, while MetS/treated groups received compound 2, 3, or 4, 10 mg/kg, p.o. for 14 days), as depicted in Figure 4.
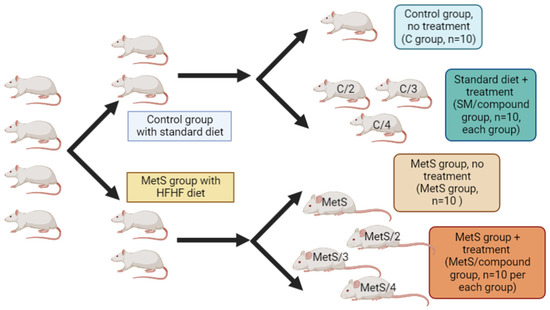
Figure 4.
Experimental design for evaluation of compounds 2–4 in the diet-induced MetS model.
The administration of compound 2 did not affect weight, glucose, cholesterol, or triglyceride levels; no statistical difference was observed compared with the control group (data not shown). The administration of compound 3 reduced body weight, glucose, cholesterol, and triglyceride levels, as seen in Figure 5. The weight and total cholesterol reduction were not significant in the group administered with compound 4. Compound 3 even significantly lowered triglyceride levels, an effect not observed for compound 1 in our previous study. The triglycerides and glucose index was calculated according to the formula TyG = Ln(triglyceride (mg/dL)X glucose (mg/dL)/2 [55]; both compounds 3 and 4 induced a decrease in their value compared to the untreated group.
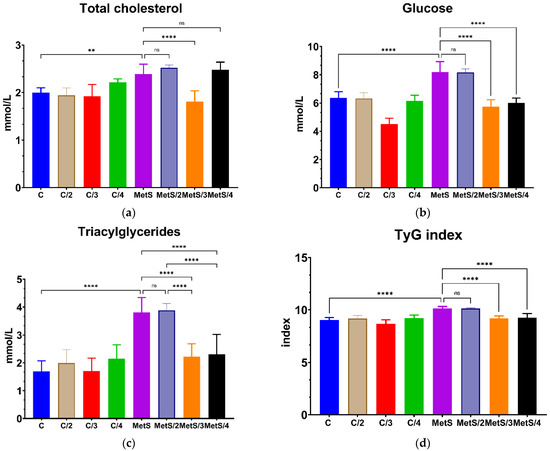
Figure 5.
The effect after 14 days of administration of compounds 2, 3 and 4 on metabolic parameters associated with MetS on the in vivo model. (a) Total cholesterol, (b) Glucose, (c) Triacylglycerides, (d) TyG index. Data are shown as means ± standard deviation (SD). The number of stars represents the level of significance differences between MetS groups and their MetS/treated groups; ns: not statistically significant difference (p > 0.05); ** p < 0.001; **** p < 0.0001.
An initial screening of the in vitro inhibition of HMG-CoA reductase at 20 μM showed that compounds 3 and 4 inhibited the enzymatic activity (94% by compound 3 at 20 μM with an IC50 value of 8.89 ± 0.51 μM, and 46% by compound 4 at 20 μM). Then, we determined the in vitro antioxidant activity based on the determination of the Trolox equivalent antioxidant capacity (TEAC) using the cupric reducing antioxidant capacity (CUPRAC) assay; compound 4 exhibited higher activity (TEAC = 1.33 ± 0.04) than compound 3 (TEAC = 1.01 ± 0.06).
3. Discussion
Several examples of the optimization of an initial privileged scaffold for the discovery of multitarget drugs are extensively described in the literature [56,57,58,59,60,61]. In our case, we selected anthranilic acid as a privileged scaffold. According to our molecular docking-guided process, compound 2 exhibited the lowest theoretical affinity, which was reflected in the in vivo assay, being the only compound that did not demonstrate bioactivity at 10 mg/kg. This result suggests that the substitution in position 5 of the anthranilic acid core is required for in vivo activity. Remarkably, compound 3 simultaneously diminished total cholesterol, triglycerides, and glucose blood levels, showing an advantage over compounds 1 and 4, which improved only two parameters.
To understand the structural factors that could explain these differences in in vivo activity, we compared the binding modes of compounds 1–4. Figure 6 illustrates the predicted poses of compounds 2 and 3 within the LBD of PPAR-α and PPAR-γ.
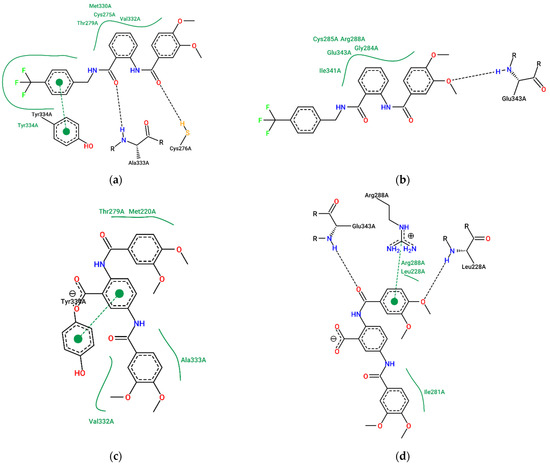
Figure 6.
Comparison of the predicted complexes of compound 1 with (a) PPAR-α and (b) PPAR-γ; complexes of compound 3 with (c) PPAR-α and (d) PPAR-γ. Diagrams generated using the ProteinPlus portal [62].
It has been described that the LBD of PPAR receptors is Y-shaped, meaning that it has three major cavities [63,64,65,66]. Compound 2 can only interact with one of these cavities, the cavity close to Cys 276 in the case of PPAR-α and Cys 285 in PPAR-γ. Meanwhile, compounds 1, 3, and 4 interact with at least two of them: additionally to the cavity occupied by compound 2, the additional aromatic ring occupies the cavity close to Met 220 in PPAR-α and Arg 288 in PPAR-γ. Thus, this is a plausible explanation for the lack of activity of compound 2. Interestingly, the 4-trifluoromethylbenzylamide moiety of compounds 1 and 4 is not required to occupy these cavities, suggesting that it can be removed, and the bioactivity would be maintained as demonstrated by the in vivo experimentation. The dose of 10 mg/kg administrated during this study showed effects on glucose and triglyceride levels similar to those caused by comparable doses of other PPAR agonists in animal models, such as glitazones such as pioglitazone and rosiglitazone (3–10 mg/kg) [67,68] or fibrates such as fenofibrate and bezafibrate (20–50 mg/kg) [69,70].
Compound 3 exhibited the highest effect on total cholesterol levels. Figure 7 depicts the predicted binding mode of compounds 3 and 4 within the active site of HMG-CoA reductase. One of the 3,4-dimethoxyphenyl moieties of compounds 1 and 3 occupies the cavity where the mevalonate binds to HMG-CoA reductase (close to Ser 684 and His 752), while the other 3,4-dimethoxyphenyl moiety occupies the same site as the pyrrole ring of atorvastatin (close to Ser 565 and Cys 561). On the other hand, due to its higher molecular volume, compound 4 cannot bind to the site where mevalonate fits; this could explain the lack of effect of compound 4 in total cholesterol levels.
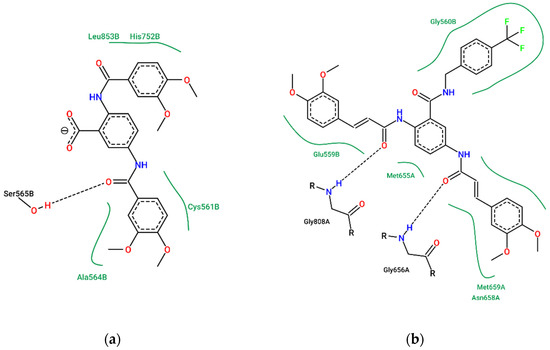
Figure 7.
Comparison of the predicted complexes of (a) compound 3 and (b) compound 4 with HMG-CoA reductase. Diagrams generated using the ProteinPlus portal [62].
In our hands, using a commercial kit to determine the inhibitory activity on HMG-CoA reductase, compound 3 had an IC50 of 8.89 μM. Cao et al. informed that the IC50 values of statins on HMG-CoA reductase activity are reported in the range of several nanomolar to several micromolar levels [71,72]. Also, Mendieta et al. reported IC50 values for α-asarone and simvastatin of 5.86 μM and 6.11μM, respectively, using the same commercial kit [73]. Therefore, compound 3 exhibits similar in vitro HMG-CoA inhibition and in vivo antihypercholesterolemic properties to other hypolipidemic agents. The antihypercholesterolemic activity of both α-asarone (80 mg/kg) [74] and simvastatin (10 mg/kg) [75,76] has been demonstrated in animal models of hypertension and obesity, with results comparable to those obtained with compound 3.
Previously, De las Heras et al. reported that treatment with rosuvastatin reduced plasma cholesterol and triacylglyceride levels in animals fed with a hypercaloric diet, enhancing PPAR-γ expression. PPAR-α agonists such as gemfibrozil, clofibrate, fenofibrate, and fenofibric acid have also been shown to have a similar effect. These compounds substantially decrease plasma TG levels and increase HDL levels. It is appropriate to think that the effect observed in compounds 3 and 4 results from an orchestrated and balanced activation between different PPAR subtypes and, in the case of compound 3, from its HMG-CoA inhibition properties.
The triglyceride glucose (TyG) index is a parameter obtained from fasting triglyceride (TG) and plasma glucose levels. It has been proposed as a surrogate marker of MetS and insulin resistance due to its sensitivity, precision, and specificity compared to the HOMA-IR index and the gold standard euglycemic hyperinsulinemic clamp test [77,78,79]. It has performed better in forecasting the development of diabetes mellitus type II (DMII) than the values of fasting glucose and triglycerides alone [80]. Recently, it has been suggested as a parameter to evaluate the early effects of dietary intervention or antioxidant treatment [81,82]. As seen in Figure 5d, the HFHF diet significantly increases the TyG index value compared to the group with the standard diet, as expected. Due to their effects on glucose and triglyceride levels, both compounds 3 and 4 lowered the TyG index, suggesting they would impact insulin resistance and other factors related to the onset and development of MetS.
An oxidant/antioxidant disparity may influence the development of MetS, since it has been observed that patients suffering from MetS display higher levels of oxidative damage markers along with the reduced activity of antioxidant enzymes [83,84]. Also, there is evidence of the positive effect of antioxidant administration in hypertension and MetS [85,86,87]. Therefore, it would be desirable that a multitarget drug designed for managing MetS would have antioxidant properties, as it was reported for multitarget drugs focused on Alzheimer’s disease [88]. For the estimation of the total antioxidant capacity (TAC), we used a commercial kit based on the cupric reducing antioxidant capacity (CUPRAC) spectrophotometric method. Our results suggest that both compounds 3 and 4 have antioxidant activity similar to ferulic acid (TEAC = 1.20) [89] but lower than more potent antioxidants quercetin or epicatechin gallate (TEAC values above 3.0). Further investigation is needed to demonstrate if the antioxidant activity of these compounds is related to their positive effects in MetS.
4. Materials and Methods
4.1. In Silico Studies
Docking Studies
The molecular structures were built using ACDLabs (Advanced Chemistry Development, Inc., Toronto, ON, Canada), optimized using MMFF//HF 6-31G* in Spartan 10 for Windows (Wavefunction, Inc., Irvine, CA, USA) and saved as mol2format. Then, these files were exported to Molegro Virtual Docker 6.0.1 (Qiagen Bioinformatics, Aarhus, Denmark). The molecular docking studies were carried out in the following targets using the accession codes shown in parenthesis: HMG CoA reductase (PDB ID: 1HWK), PPAR-α (PDB ID: 1I7G), and PPAR-γ (PDB ID: 1I7I). A previously reported procedure was used [26,90]. Briefly, all the co-crystallized ligands and water molecules were deleted from the structures. The ligand-binding domain (LBD) of the nuclear receptors or the active sites of each enzyme were selected as the searching sites under a 15 Å radius sphere. The MolDock Optimizer algorithm was set to 5000 maximum iterations with a simplex evolution population of 5000 and 50 runs for each ligand. Rerank Score was calculated as the criteria for estimating the theoretical binding affinity. Better binding is associated with more negative scores. The co-crystallized ligands were also redocked to their respective receptors to assess the efficacy of this procedure. In all the docking procedures, the RMSD of the co-crystallized ligand pose compared with the original structure was lower than 2.0 Å. The pkCSM online platform [91] was used to predict the in silico ADME/Tox profile. (http://biosig.unimelb.edu.au/pkcsm/, accessed on 10 June 2022).
4.2. Chemistry
All initial materials for the synthesis of compounds 2–4 were purchased from Sigma-Aldrich (St. Louis, MO, USA). Monitoring of the synthetic transformations was carried out using TLC on silica gel 60 on aluminum foils, also from Sigma-Aldrich. Infrared (IR) spectra were acquired in a Perkin Elmer FTIR-670 Plus spectrophotometer in ATR. 1H and 13C NMR spectra were recorded using a JEOL ECA-500 JEOL spectrometer (B0 = 11.75 T) in a DMSO-d6 solution. 1H NMR spectra and 13C NMR were recorded at 500.1599 MHz and 125.7653 MHz, respectively. The FAB-MS analyses were performed on a JEOL Sx102 mass spectrometer. The initial precursor 2,5-diaminobenzoic acid was prepared as previously reported [38].
4.2.1. General Procedure for the Preparation of Benzoxazinone Derivatives 5a–c
Benzoxazinone derivatives were prepared as previously reported. Briefly, to a round bottom flask with magnetic agitation and ice bath, 100 mL of pyridine was added. After that, 26.0 mmol of 3,4-dimethoxybenzoyl chloride or 3,4-dimethoxycinnamoyl chloride was added and stirred until the formation of a yellowish solution. After 10 min, freshly prepared 2,5-diaminoanthranilic acid (1.3 g, 8.7 mmol) was added, and the resulting suspension was stirred for an additional 2 h at 5 °C. Once the reaction was finalized, 250 mL of water was added to the reaction mixture, and a precipitate was immediately formed, which was then filtered, washed with ethanol, and dried to afford the desired product as a yellowish solid.
(E)-3-(3,4-dimethoxyphenyl)-N-(2-((E)-3,4-dimethoxystyryl)-4-oxo-4H-benzo[d][1,3]oxazin-6-yl)acrylamide (5c). IR (ATR, cm−1): 3343 (N-H), 2889 (C=C), 1759 (C=O), 1684 (C=O, amide), 1586 (C=C, aromatic), 1134 (O-CH3). 1H NMR (DMSO-d6, 500 MHz) δ: 10.55 (1H, s, H23), 8.56 (1H, d, J = 2.4 Hz, H9), 7.99 (1H, dd, J = 2.4, 8.8 Hz, H7), 7.62 (1H, d, J = 16.0 Hz, H12), 7.54 (1H, d, J = 8.8 Hz, H6), 7.54 (1H, d, J = 15.5 Hz, H26), 7.40 (1H, d, J = 1.7 Hz, H14), 7.27 (1H, dd, J = 1.7, 8.4 Hz, H18), 7.19 (1H, d, J = 1.7 Hz, H28), 7.17 (1H, dd, J = 1.7, 8.4 Hz, H32), 6.98 (1H, d, J = 8.4 Hz, H31), 6.95 (1H, d, J = 8.4 Hz, H17), 6.87 (1H, d, J = 16.0 Hz, H11), 6.65 (1H, d, J = 15.5 Hz, H25), 3.80 (3H, s, H21), 3.79 (3H, s, H33), 3.76 (6H, s, H22, H34). 13C {1H} NMR (DMSO-d6, 125 MHz) δ: 164.72 (C24), 159.49 (C1), 156.50 (C3), 151.33 (C16), 151.10 (C30), 149.55 (C15), 149.43 (C29), 142,28 (C5), 141.67 (C26), 141.10 (C12), 139.59 (C8), 128.04 (C7, C13), 127.84 (C6), 127.76 (C27), 123.28 (C18), 122.57 (C32), 119.67 (C25), 117.57 (C10), 117.23 (C11), 116.87 (C9), 112.22 (C31), 112.08 (C17), 110.69 (C14), 110.51 (C28), 56.13 (33), 56.07 (34), 55.92 (21), 55.45(22). See Figure 8 for atom numbering. MS (FAB, m/z): 515 (M+ + 1, 100%). Elemental analysis: experimental C, 66.17%: H, 5.18%; N, 5.12%; calculated C, 67.70%; H, 5.09%; N, 5.44%.
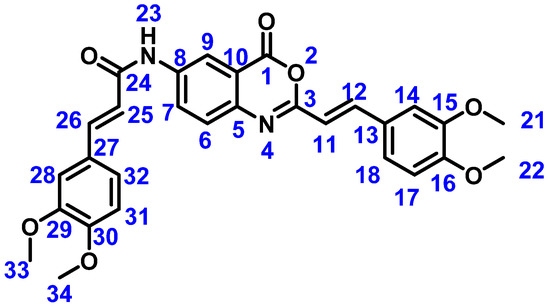
Figure 8.
Structure of novel compound 5c with atom numbering used for NMR characterization.
4.2.2. 2,5-Bis(3,4-dimethoxybenzamido)benzoic Acid (Compound 3)
Method A: In a round-bottom flask, 1.3225 g of 3,4-dimethoxybenzoyl chloride (6.592 mmol) was added to 10 mL of pyridine at room temperature. Next, 0.5 g of 2,5-diaminobenzoic acid (3.2882 mmol) was added to the solution, obtaining a beige -colored suspension. The reaction mixture was stirred for 24 h at room temperature. Afterward, once the reaction was completed, the crude was transferred to a beaker with 20 mL of water and was treated with chloride acid until it reached pH 2, rendering a white precipitate that was filtered and dried under reduced pressure to render the desired product as a white solid in a 35% yield. Method B: Given the low yield obtained from the previous synthesis route for compound 3, an alternative synthesis route was proposed from the basic hydrolysis of the benzoxazinone ring of compound 5a, prepared as previously reported [38]. In a round-bottom flask with 3 mL of DMF, 0.2 g of compound 5a was added, obtaining a milky white suspension. Next, 10 mL of a 5% NaOH solution was slowly added, giving place to a white suspension. The reaction mixture was stirred for 2 h at room temperature, followed by the treatment of the crude with diluted HCl solution until it reached pH 2. The acid treatment of the crude resulted in the formation of a white precipitate that was filtered and dried under reduced pressure to give the desired product as a white solid in an 85% yield. m.p 289–292 °C; IR (ATR, cm−1): 3377 (N-H); 3125.14 (C-H sp2), 2932.72 (C-H sp3), 1654.05 (C=O amide), 1601.18 (C=C aromatic), 1189.74 (C-O); 1H NMR (DMSO-d6, 500 MHz) δ: 12.23 (1H, s, H13), 10.18 (1H, s, H22), 8.62 (1H, d, J = 8.8 Hz, H19), 8.45 (1H, d, J = 2.5 Hz, H16), 7.94 (1H, dd, J = 8.8, 2.5 Hz, H18), 7.58 (1H, dd, J = 8.5, 1.8 Hz, H4), 7.51 (1H, d, J = 1.8 Hz, H6) 7.50 (1H, dd, J = 8.5, 1.8 Hz, H31), 7.45 (1H, d, J = 1.8 Hz, H27), 7.05 (1H, d, J = 8.5 Hz, H3), 7.01 (1H, d, J = 8.5 Hz, H30), 3.80 (6H, s, H10, H35), 3.79 (3H, s, H8), 3.76 (3H, s, H32). 13C{1H} NMR (DMSO-d6, 125 MHz) δ: 170.60 C20), 165.49 (C23), 164.59 (C11), 152.39 (C2), 152.14 (C29), 149.10 (C1), 148.73 (C28), 137.60 (C14), 134.38 (C17), 127.29 (C5), 127.04 (C26), 126.57 (C18), 123.59 (C16), 121.62 (C4), 120.69 (C31), 120.30 (C19), 117.53 (C15), 111.70 (C3), 111.31 (C6, C30), 110.63 (C27), 56.18 (C10), 56.12 (C35), 56.09 (C8), 55.95 (C32). See Figure 9 for atom numbering. MS (FAB, m/z): 481 (M+ + 1, 100%). Elemental analysis: experimental C, 61.18%: H, 5.12%; N, 5.47%; calculated C, 62.50%; H, 5.04%; N, 5.83%.
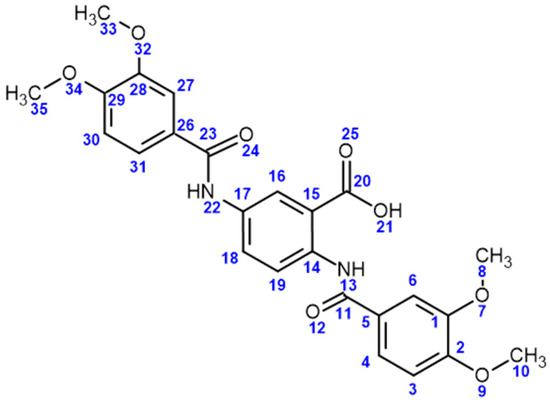
Figure 9.
Structure of compound 3 with atom numbering used for NMR characterization.
4.2.3. (2E,2′E)-N,N′-(2-((4-(Trifluoromethyl)benzyl)carbamoyl)-1,4-phenylene)bis(3-(3,4-dimethoxyphenyl)acrylamide) (Compound 4)
In a round-bottom flask equipped with magnetic agitation, 0.25 g (0.5 mmol) of compound 5c was dissolved in 15 mL of DMF, and 0.7 mmol of 4-trifluorobenzylamine was added. The reaction mixture was stirred at room temperature for 24 h. During the reaction, the amber solution turns into a yellowish suspension after completion. After the reaction was completed, 30 mL of water was added to render a white precipitate that was washed with acetone and subsequently with methanol. Yield: 61%, m.p. >250 °C. IR (ATR, cm−1): 3377 (N-H); 3125.14 (C-H), 2932.72 (C-H), 1654.05 (C=O amide), 1601.18 (C=C aromatic), 1189.74 (C-O); 1H NMR (DMSO-d6, 500 MHz) δ: 10.66 (1H, s, H15), 10.26, (1H, s, H27), 9.33 (1H, t, J = 5.5 Hz, H8), 8.27 (1H, d, J = 8.9 Hz, H6), 8.09 (1H, J < 2 Hz, H3), 7.71 (1H, d, J = 8.9 Hz, H5), 7.67 (2H, d, J = 8.0 Hz, H12) 7.54 (2H, d, J = 8.1 Hz, H11), 7.51 (1H, d, J = 15.0 Hz, H30), 7.48 (1H, d, J = 15.2 Hz, H18), 7.31 (1H, J < 2 Hz, H20), 7.18 (1H, J < 2 Hz, H32), 7.17 (1H, d, J = 8.4 Hz, H24), 7.15 (1H, d, J = 8.3 Hz, H36), 6.98 (1H, d, J = 8.3 Hz, H35), 6.95 (1H, d, J = 8.4 Hz, H23), 6.73 (1H, d, J = 15.2 Hz, H17), 6.68 (1H, d, J = 15.0 Hz, H29), 4.54 (2H, d, J = 5.5 Hz, H9), 3.79 (3H, s, H37), 3.79 (3H, s, H38), 3.76 (3H, s, H25), 3.75 (3H, s, H26). 13C {1H} NMR (DMSO-d6, 125 MHz) δ: 168.80 (C1), 164.41 (C28), 164. 37 (C16), 151.03 (C33), 150.98 (C21), 149.47 (C22), 149.44 (C34), 144.58 (C10), 141.61 (C18), 141.00 (C30), 135.22 (C7), 133.99 (C4), 128.47 (C11), 127.92 (C19), 127.77 (C13), 127.88 (C31), 125.78 (C12), 124.26 (C2) 123.76 (C14), 123.05 (C24), 122.93 (C6), 122.88 (C5), 122.35 (C36), 120.33 (C17), 120.06 (C29), 119.46 (C3), 112.27 (C35), 112.05 (C23), 110.65 (C20), 110.53 (C32), 56.09 (C37, C38), 56.06 (C26), 55.93 (C25), 42.87 (C9). 19F {1H} NMR (DMSO-d6, 470 MHz) δ: 4.32 (F). See Figure 10 for atom numbering. MS (FAB, m/z): 690 (M+ + 1, 100%). Elemental analysis: experimental C, 62.94%: H, 5.11%; N, 5.60%; calculated C, 64.44%; H, 4.97%; N, 6.09%.
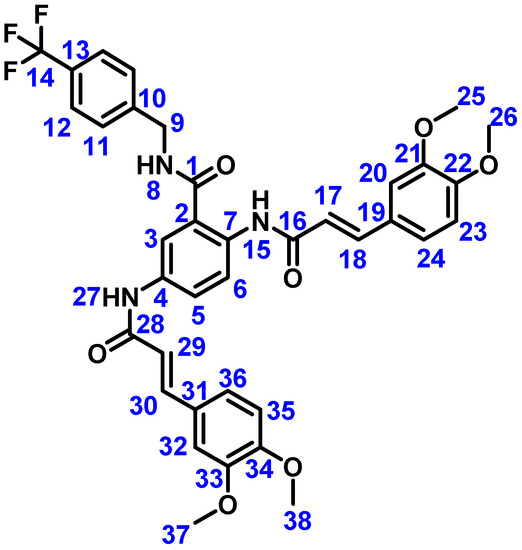
Figure 10.
Structure of compound 4 with atom numbering used for NMR characterization.
4.3. In Vivo Evaluation in Metabolic Syndrome
4.3.1. Animals
For the in vivo experimentation, eighty Sprague–Dawley male rats weighing 250 ± 25 g were housed in acrylic boxes and treated following the recommendations and requirements of the NOM-062-ZOO-1999 (Official Mexican Standard for the Production, Care, and Use of Laboratory Animals). They were kept in a clear-air room maintained on an artificial 12 h light/dark cycle and were given ad libitum access to food and water. The protocol was approved by the Institutional Subcommittee for the Care and Use of Laboratory Animals (SICUAL) with the registration number FMM/SICUAL/006/2017 (date of approval, 30 August 2017).
4.3.2. MetS Induction and Treatment with the Tested Compounds
The animals were randomly allocated to a group (control groups) that was fed on a regular Chow commercial diet (Purina-Rodent Laboratory Chow-5001 3.310 kcal/g) or to a group (MetS groups) that was supplied with a high-fructose and high-fat diet (4.161 kcal/g) for the generation of MetS. From our own experience, MetS is established after twelve weeks with this diet [46]. The weight of each animal was recorded at the beginning of the study and every week after the completion of the induction period. In weeks nine and twelve, the glucose, total cholesterol, and triglyceride levels were monitored in fasting conditions of 12 h, using an Accutrend plus monitor (Roche). Following the MetS induction phase, the control and MetS groups were rearranged into the following groups: (1) control (CM, regular diet + no treatment n = 10); (2) MetS (HFHF diet + no treatment, n = 10); (3) control + compound 2 (C/2 regular diet + treatment with 2, n = 10); (4) MetS + compound 2 (MetS/2, HFHF diet + treatment with 2, n = 10); (5) control + compound 3 (C/3, regular diet + treatment with 1c, n = 10); (6) MetS + compound 3 (MetS/3, HFHF diet + treatment with 3, n = 10); (7) control + compound 4 (C/4, regular diet + treatment with 4, n = 10); (8) MetS + compound 4 (MetS/4, HFHF diet + treatment with 4, n = 10). The treatment stage lasted 14 days, and the animals retained the same diet they had during the induction to exclude the effect of diet change. All the animals received 100 μL of a 1% Kolliphor EL mixture in water with or without the tested compound (10 mg/Kg) via nasogastric. Animals were sacrificed by decapitation. The blood was collected and centrifuged at 1372× g for 15 min at 4 °C. The serum was gathered and stored at −80 °C until the determination of metabolic parameters.
4.3.3. Triacylglycerides, Cholesterol, and Glucose Analysis
The animals’ serum was defrosted, and triacylglyceride, cholesterol, and glucose levels were determined on an Architect c8000 system (Abbott, Wiesbaden, Germany). Statistical analysis was performed using one-way ANOVA, followed by Dunnett’s multiple comparisons tests employing GraphPad Prism version 9.4.1 (GraphPad Software, La Jolla, CA, USA). Differences were considered significant when p ≤ 0.05.
4.4. In Vitro Evaluation of HMG-CoA Reductase Inhibition and Antioxidant Activity
The HMG-CoA Reductase Assay Kit from Sigma-Aldrich (catalog number CS1090, St. Louis, MO, USA) was used to determine the inhibitory properties of compounds 3 and 4 over enzyme activity, using the instructions provided by the fabricant. An initial screening was performed at a final concentration of 20 μM, resulting in a 94% inhibition of enzyme activity by compound 3 and 46% by compound 4. For IC50 determination of compound 3, final concentrations of 2.5, 5.0, 10.0, and 20 μM were employed by triplicate. The Total Antioxidant Capacity Assay Kit from Sigma-Aldrich (catalog number MAK187, St. Louis, MO, USA) was used for the determination of in vitro antioxidant activity following the manufacturer’s instructions by triplicate.
5. Conclusions
In conclusion, we designed compound 3, an anthranilic acid derivative, as a hit in developing new multitarget drugs for the management of MetS. The most relevant findings are summarized in Figure 11. We found that substitution in position 5 is needed for in vivo activity over at least two parameters related to MetS. Removing substituents in the carboxylic group leads to higher in silico ligand efficiency and improves in vivo polypharmacology activity. On the other hand, incorporating a vinyl group besides the 3,4-dimethoxyphenyl group increases antioxidant properties but diminishes the inhibition of HMG-CoA reductase activity, which is reflected in the loss of in vivo activity over cholesterol levels. The accumulated evidence of this work suggests that the underlying mechanisms related to the biological effects of these new compounds are related to numerous pleiotropic actions, including inflammatory response, lipid metabolism, glucose metabolism, and the regulation of oxidative stress. According to this idea, the observed in vivo effects result from an orchestrated and balanced activation between different PPAR subtypes and HMG-CoA inhibitory properties.

Figure 11.
Structural features that modify biological activity on 5-aminoanthranilic acid derivatives.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ph15121461/s1: predicted poses of compounds 2–4 to the studied targets, weight variations during administration of compounds 3 and 4, NMR spectra of compounds 3 and 4.
Author Contributions
Conceptualization, project administration, and funding acquisition, M.A.L.-M. and R.P.-A.; methodology, resources, data curation, supervision, formal analysis, and validation, M.A.L.-M., R.P.-A., C.G.-A., J.R.S., M.d.R.A.-M. and A.A.-C., software, M.M.-A., M.M.-L.; M.F.M.-G., L.M.-R. and M.A.L.-M.; investigation, M.M.-A., E.C.-G.; M.M.-L.; M.F.M.-G., L.M.-R., K.P.-G., L.R.-P. and O.J.Q.-R.; writing—original draft preparation, review, and editing, all authors; visualization, M.M.-A., M.F.M.-G., L.M.-R. and M.A.L.-M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Consejo Nacional de Ciencia y Tecnología (CONACyT), grant number A1-S-39485. This work was submitted in partial fulfillment of the requirements for the PhD degree of E.C.-G. at the Doctorado en Ciencias en Biomedicina y Biotecnología Molecular, Escuela Nacional de Ciencias Biológicas (Instituto Politécnico Nacional, Mexico City 11340, Mexico). E.C.-G. received financial support from CONACyT (CVU:818903). M.M.-A. (register number 31260) and M.M.-L. (register number 31631) also received financial support from CONACyT through project A1-S-39485.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Mexican Official Norm for Animal Care and Handing (NOM-062-ZOO-1999) and approved by the Institutional Subcommittee for the Care and Use of Laboratory Animals (SICUAL) of Universidad La Salle with registration FMM/SICUAL/006/2017OR (approved on 30 August 2017).
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article and Supplementary Materials.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Grundy, S.M. Metabolic syndrome update. Trends Cardiovasc. Med. 2016, 26, 364–373. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Li, X.; Adams, H.; Kubena, K.; Guo, S. Etiology of metabolic syndrome and dietary intervention. Int. J. Mol. Sci. 2019, 20, 128. [Google Scholar] [CrossRef] [PubMed]
- Saklayen, M.G. The Global Epidemic of the Metabolic Syndrome. Curr. Hypertens. Rep. 2018, 20, 12. [Google Scholar] [CrossRef]
- Zuin, M.; Rigatelli, G.; Bilato, C.; Cervellati, C.; Zuliani, G.; Roncon, L. Prognostic Role of Metabolic Syndrome in COVID-19 Patients: A Systematic Review Meta-Analysis. Viruses 2021, 13, 1938. [Google Scholar] [CrossRef]
- Bansal, R.; Gubbi, S.; Muniyappa, R. Metabolic Syndrome and COVID 19: Endocrine-Immune-Vascular Interactions Shapes Clinical Course. Endocrinology 2020, 161, bqaa112. [Google Scholar] [CrossRef] [PubMed]
- Kahn, R.; Buse, J.; Ferrannini, E.; Stern, M. The Metabolic Syndrome: Time for a Critical Appraisal. Diabetes Care 2005, 28, 2289–2304. [Google Scholar] [CrossRef]
- Oladejo, A.O. Overview of the metabolic syndrome; an emerging pandemic of public health significance. Ann. Ibadan Postgrad. Med. 2011, 9, 78–82. [Google Scholar]
- McCracken, E.; Monaghan, M.; Sreenivasan, S. Pathophysiology of the metabolic syndrome. Clin. Dermatol. 2018, 36, 14–20. [Google Scholar] [CrossRef]
- Fahed, G.; Aoun, L.; Zerdan, M.B.; Allam, S.; Zerdan, M.B.; Bouferraa, Y.; Assi, H.I. Metabolic Syndrome: Updates on Pathophysiology and Management in 2021. Int. J. Mol. Sci. 2022, 23, 786. [Google Scholar] [CrossRef] [PubMed]
- Grundy, S.M. Metabolic syndrome: Therapeutic considerations. Handb. Exp. Pharmacol. 2005, 170, 107–133. [Google Scholar]
- Grundy, S.M. Drug therapy of the metabolic syndrome: Minimizing the emerging crisis in polypharmacy. Nat. Rev. Drug Discov. 2006, 5, 295–309. [Google Scholar] [CrossRef] [PubMed]
- Masnoon, N.; Shakib, S.; Kalisch-Ellett, L.; Caughey, G.E. What is polypharmacy? A systematic review of definitions. BMC Geriatr. 2017, 17, 230. [Google Scholar] [CrossRef]
- Davies, L.E.; Spiers, G.; Kingston, A.; Todd, A.; Adamson, J.; Hanratty, B. Adverse Outcomes of Polypharmacy in Older People: Systematic Review of Reviews. J. Am. Med. Dir. Assoc. 2020, 21, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.Z.; Huang, S.T.; Wen, Y.W.; Chen, L.K.; Hsiao, F.Y. Combined Effects of Frailty and Polypharmacy on Health Outcomes in Older Adults: Frailty Outweighs Polypharmacy. J. Am. Med. Dir. Assoc. 2021, 22, 606.e7–606.e18. [Google Scholar] [CrossRef] [PubMed]
- Alwhaibi, M.; Balkhi, B.; Alhawassi, T.M.; Alkofide, H.; Alduhaim, N.; Alabdulali, R.; Drweesh, H.; Sambamoorthi, U. Polypharmacy among patients with diabetes: A cross-sectional retrospective study in a tertiary hospital in Saudi Arabia. BMJ Open 2018, 8, e020852. [Google Scholar] [CrossRef]
- Ramsay, R.R.; Popovic-Nikolic, M.R.; Nikolic, K.; Uliassi, E.; Bolognesi, M.L. A perspective on multi-target drug discovery and design for complex diseases. Clin. Transl. Med. 2018, 7, 3. [Google Scholar] [CrossRef]
- Kabir, A.; Muth, A. Polypharmacology: The science of multi-targeting molecules. Pharmacol. Res. 2022, 176, 106055. [Google Scholar] [CrossRef]
- Zheng, H.; Fridkin, M.; Youdim, M. From Single Target to Multitarget/Network Therapeutics in Alzheimer’s Therapy. Pharmaceuticals 2014, 7, 113–135. [Google Scholar] [CrossRef]
- Bolognesi, M.L.; Cavalli, A. Multitarget Drug Discovery and Polypharmacology. ChemMedChem 2016, 11, 1190–1192. [Google Scholar] [CrossRef]
- Sánchez-Tejeda, J.F.; Sánchez-Ruiz, J.F.; Salazar, J.R.; Loza-Mejía, M.A. A Definition of “Multitargeticity”: Identifying Potential Multitarget and Selective Ligands Through a Vector Analysis. Front. Chem. 2020, 8, 176. [Google Scholar] [CrossRef]
- Talevi, A. Multi-target pharmacology: Possibilities and limitations of the “skeleton key approach” from a medicinal chemist perspective. Front. Pharmacol. 2015, 6, 205. [Google Scholar] [CrossRef] [PubMed]
- Csermely, P.; Ágoston, V.; Pongor, S. The efficiency of multi-target drugs: The network approach might help drug design. Trends Pharmacol. Sci. 2005, 26, 178–182. [Google Scholar] [CrossRef]
- Katselou, M.; Matralis, A.; Kourounakis, A. Multi-Target Drug Design Approaches for Multifactorial Diseases: From Neurodegenerative to Cardiovascular Applications. Curr. Med. Chem. 2014, 21, 2743–2787. [Google Scholar] [CrossRef] [PubMed]
- Prati, F.; Cavalli, A.; Bolognesi, M.L. Navigating the Chemical Space of Multitarget-Directed Ligands: From Hybrids to Fragments in Alzheimer’s Disease. Molecules 2016, 21, 466. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Pei, J.; Lai, L. Computational Multitarget Drug Design. J. Chem. Inf. Model. 2017, 57, 403–412. [Google Scholar] [CrossRef]
- Loza-Mejía, M.; Salazar, J.; Sánchez-Tejeda, J. In Silico Studies on Compounds Derived from Calceolaria: Phenylethanoid Glycosides as Potential Multitarget Inhibitors for the Development of Pesticides. Biomolecules 2018, 8, 121. [Google Scholar] [CrossRef]
- Lavecchia, A.; Cerchia, C. In silico methods to address polypharmacology: Current status, applications and future perspectives. Drug Discov. Today 2016, 21, 288–298. [Google Scholar] [CrossRef] [PubMed]
- Saldívar-González, F.I.; Gómez-García, A.; Chávez-Ponce de León, D.E.; Sánchez-Cruz, N.; Ruiz-Rios, J.; Pilón-Jiménez, B.A.; Medina-Franco, J.L. Inhibitors of DNA Methyltransferases From Natural Sources: A Computational Perspective. Front. Pharmacol. 2018, 9, 1144. [Google Scholar] [CrossRef]
- Speck-Planche, A.; Kleandrova, V.V.; Scotti, M.T. Fragment-based approach for the in silico discovery of multi-target insecticides. Chemom. Intell. Lab. Syst. 2012, 111, 39–45. [Google Scholar] [CrossRef]
- Speck-Planche, A.; Natalia Dias Soeiro Cordeiro, M.; Guilarte-Montero, L.; Yera-Bueno, R. Current Computational Approaches Towards the Rational Design of New Insecticidal Agents. Curr. Comput. Aided-Drug Des. 2011, 7, 304–314. [Google Scholar] [CrossRef]
- Navarrete-Vázquez, G.; Alaniz-Palacios, A.; Hidalgo-Figueroa, S.; González-Acevedo, C.; Ávila-Villarreal, G.; Estrada-Soto, S.; Webster, S.P.; Medina-Franco, J.L.; López-Vallejo, F.; Guerrero-Álvarez, J.; et al. Discovery, synthesis and in combo studies of a tetrazole analogue of clofibric acid as a potent hypoglycemic agent. Bioorg. Med. Chem. Lett. 2013, 23, 3244–3247. [Google Scholar] [CrossRef] [PubMed]
- Colin-Lozano, B.; Torres-Gomez, H.; Hidalgo-Figueroa, S.; Chávez-Silva, F.; Estrada-Soto, S.; Almanza-Pérez, J.C.; Navarrete-Vazquez, G. Synthesis, In Vitro, In Vivo and In Silico Antidiabetic Bioassays of 4-Nitro(thio)phenoxyisobutyric Acids Acting as Unexpected PPARγ Modulators: An In Combo Study. Pharmaceuticals 2022, 15, 102. [Google Scholar] [PubMed]
- Guzmán-Ávila, R.; Flores-Morales, V.; Paoli, P.; Camici, G.; Ramírez-Espinosa, J.J.; Cerón-Romero, L.; Navarrete-Vázquez, G.; Hidalgo-Figueroa, S.; Yolanda Rios, M.; Villalobos-Molina, R.; et al. Ursolic acid derivatives as potential antidiabetic agents: In vitro, in vivo, and in silico studies. Drug Dev. Res. 2018, 79, 70–80. [Google Scholar] [CrossRef]
- Navarrete-Vázquez, G.; Morales-Vilchis, M.G.; Estrada-Soto, S.; Ramírez-Espinosa, J.J.; Hidalgo-Figueroa, S.; Nava-Zuazo, C.; Tlahuext, H.; Leon-Rivera, I.; Medina-Franco, J.L.; López-Vallejo, F.; et al. Synthesis of 2-{2-[(α/β-naphthalen-1-ylsulfonyl)amino]-1,3-thiazol-4-yl} acetamides with 11β-hydroxysteroid dehydrogenase inhibition and in combo antidiabetic activities. Eur. J. Med. Chem. 2014, 74, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Bacci, A.; Corsi, F.; Runfola, M.; Sestito, S.; Piano, I.; Manera, C.; Saccomanni, G.; Gargini, C.; Rapposelli, S. Design, Synthesis, and In Vitro Evaluation of Novel 8-Amino-Quinoline Combined with Natural Antioxidant Acids. Pharmaceuticals 2022, 15, 688. [Google Scholar] [CrossRef] [PubMed]
- Suliman, R.S.; Alghamdi, S.S.; Ali, R.; Aljatli, D.; Aljammaz, N.A.; Huwaizi, S.; Suliman, R.; Kahtani, K.M.; Albadrani, G.M.; Barhoumi, T.; et al. The Role of Myrrh Metabolites in Cancer, Inflammation, and Wound Healing: Prospects for a Multi-Targeted Drug Therapy. Pharmaceuticals 2022, 15, 944. [Google Scholar] [CrossRef]
- Bortolami, M.; Pandolfi, F.; Tudino, V.; Messore, A.; Madia, V.N.; De Vita, D.; Di Santo, R.; Costi, R.; Romeo, I.; Alcaro, S.; et al. Design, Synthesis, and In Vitro, In Silico and In Cellulo Evaluation of New Pyrimidine and Pyridine Amide and Carbamate Derivatives as Multi-Functional Cholinesterase Inhibitors. Pharmaceuticals 2022, 15, 673. [Google Scholar] [CrossRef]
- González-Álvarez, H.; Bravo-Jiménez, A.; Martínez-Arellanes, M.; Gamboa-Osorio, G.O.; Chávez-Gutiérrez, E.; González-Hernández, L.A.; Gallardo-Ignacio, K.; Quintana-Romero, O.J.; Ariza-Castolo, A.; Guerra-Araiza, C.; et al. In Silico-Based Design and In Vivo Evaluation of an Anthranilic Acid Derivative as a Multitarget Drug in a Diet-Induced Metabolic Syndrome Model. Pharmaceuticals 2021, 14, 914. [Google Scholar] [CrossRef]
- Schmidt, J.; Rotter, M.; Weiser, T.; Wittmann, S.; Weizel, L.; Kaiser, A.; Heering, J.; Goebel, T.; Angioni, C.; Wurglics, M.; et al. A Dual Modulator of Farnesoid X Receptor and Soluble Epoxide Hydrolase To Counter Nonalcoholic Steatohepatitis. J. Med. Chem. 2017, 60, 7703–7724. [Google Scholar] [CrossRef]
- Heitel, P.; Faudone, G.; Helmstädter, M.; Schmidt, J.; Kaiser, A.; Tjaden, A.; Schröder, M.; Müller, S.; Schierle, S.; Pollinger, J.; et al. A triple farnesoid X receptor and peroxisome proliferator-activated receptor α/δ activator reverses hepatic fibrosis in diet-induced NASH in mice. Commun. Chem. 2020, 3, 174. [Google Scholar] [CrossRef]
- Merk, D.; Gabler, M.; Gomez, R.C.; Flesch, D.; Hanke, T.; Kaiser, A.; Lamers, C.; Werz, O.; Schneider, G.; Schubert-Zsilavecz, M. Anthranilic acid derivatives as novel ligands for farnesoid X receptor (FXR). Bioorg. Med. Chem. 2014, 22, 2447–2460. [Google Scholar] [CrossRef] [PubMed]
- Levin, J.I.; Chen, J.; Du, M.; Hogan, M.; Kincaid, S.; Nelson, F.C.; Venkatesan, A.M.; Wehr, T.; Zask, A.; DiJoseph, J.; et al. The discovery of anthranilic acid-based MMP inhibitors. Part 2: SAR of the 5-position and P1(1) groups. Bioorg. Med. Chem. Lett. 2001, 11, 2189–2192. [Google Scholar] [CrossRef]
- Han, S.H.; Suh, H.S.; Jo, H.; Oh, Y.; Mishra, N.K.; Han, S.; Kim, H.S.; Jung, Y.H.; Lee, B.M.; Kim, I.S. Synthesis and anti-inflammatory evaluation of N-sulfonyl anthranilic acids via Ir(III)-catalyzed C–H amidation of benzoic acids. Bioorg. Med. Chem. Lett. 2017, 27, 2129–2134. [Google Scholar] [CrossRef] [PubMed]
- Merk, D.; Lamers, C.; Weber, J.; Flesch, D.; Gabler, M.; Proschak, E.; Schubert-Zsilavecz, M. Anthranilic acid derivatives as nuclear receptor modulators—Development of novel PPAR selective and dual PPAR/FXR ligands. Bioorg. Med. Chem. 2015, 23, 499–514. [Google Scholar] [CrossRef] [PubMed]
- El-Azab, A.S.; Abdel-Aziz, A.A.M.; Bua, S.; Nocentini, A.; AlSaif, N.A.; Almehizia, A.A.; Alanazi, M.M.; Hefnawy, M.M.; Supuran, C.T. New anthranilic acid-incorporating N-benzenesulfonamidophthalimides as potent inhibitors of carbonic anhydrases I, II, IX, and XII: Synthesis, in vitro testing, and in silico assessment. Eur. J. Med. Chem. 2019, 181, 111573. [Google Scholar] [CrossRef] [PubMed]
- Espinosa-García, C.; Fuentes-Venado, C.E.; Guerra-Araiza, C.; Segura-Uribe, J.; Chávez-Gutiérrez, E.; Farfán-García, E.D.; Estrada Cruz, N.A.; Pinto-Almazán, R. Sex differences in the performance of cognitive tasks in a murine model of metabolic syndrome. Eur. J. Neurosci. 2020, 52, 2724–2736. [Google Scholar] [CrossRef] [PubMed]
- Panchal, S.K.; Brown, L. Rodent Models for Metabolic Syndrome Research. J. Biomed. Biotechnol. 2011, 2011, 351982. [Google Scholar] [CrossRef]
- Gagnon, M.K.J.; Hausner, S.H.; Marik, J.; Abbey, C.K.; Marshall, J.F.; Sutcliffe, J.L. High-throughput in vivo screening of targeted molecular imaging agents. Proc. Natl. Acad. Sci. USA 2009, 106, 17904–17909. [Google Scholar] [CrossRef]
- Speak, A.O.; Swiatkowska, A.; Karp, N.A.; Arends, M.J.; Adams, D.J.; van der Weyden, L. A high-throughput in vivo screening method in the mouse for identifying regulators of metastatic colonization. Nat. Protoc. 2017, 12, 2465–2477. [Google Scholar] [CrossRef] [PubMed]
- Bumrungpert, A.; Lilitchan, S.; Tuntipopipat, S.; Tirawanchai, N.; Komindr, S. Ferulic Acid Supplementation Improves Lipid Profiles, Oxidative Stress, and Inflammatory Status in Hyperlipidemic Subjects: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Nutrients 2018, 10, 713. [Google Scholar] [CrossRef]
- Senaphan, K.; Kukongviriyapan, U.; Sangartit, W.; Pakdeechote, P.; Pannangpetch, P.; Prachaney, P.; Greenwald, S.; Kukongviriyapan, V. Ferulic Acid Alleviates Changes in a Rat Model of Metabolic Syndrome Induced by High-Carbohydrate, High-Fat Diet. Nutrients 2015, 7, 6446–6464. [Google Scholar] [CrossRef] [PubMed]
- Ardiansyah; Ohsaki, Y.; Shirakawa, H.; Koseki, T.; Komai, M. Novel Effects of a Single Administration of Ferulic Acid on the Regulation of Blood Pressure and the Hepatic Lipid Metabolic Profile in Stroke-Prone Spontaneously Hypertensive Rats. J. Agric. Food Chem. 2008, 56, 2825–2830. [Google Scholar] [CrossRef] [PubMed]
- Pontiki, E.; Peperidou, A.; Fotopoulos, I.; Hadjipavlou-Litina, D. Cinnamate Hybrids: A Unique Family of Compounds with Multiple Biological Activities. Curr. Pharm. Biotechnol. 2018, 19, 1019–1048. [Google Scholar] [CrossRef]
- Kenny, P.W. The nature of ligand efficiency. J. Cheminform. 2019, 11, 8. [Google Scholar] [CrossRef] [PubMed]
- Fuentes-Venado, C.E.; Terán-Pérez, G.; Espinosa-Hernández, V.M.; Martínez-Herrera, E.; Segura-Uribe, J.J.; Mercadillo, R.E.; Pinto-Almazán, R.; Guerra-Araiza, C. Nutritional Status Influences Oxidative Stress and Insulin Resistance in Preschool Children. Metab. Syndr. Relat. Disord. 2021, 19, 513–523. [Google Scholar] [CrossRef]
- Beato, A.; Gori, A.; Boucherle, B.; Peuchmaur, M.; Haudecoeur, R. β-Carboline as a Privileged Scaffold for Multitarget Strategies in Alzheimer’s Disease Therapy. J. Med. Chem. 2021, 64, 1392–1422. [Google Scholar] [CrossRef] [PubMed]
- Kumari, M.; Waseem, M.; Subbarao, N. Discovery of multi-target mur enzymes inhibitors with anti-mycobacterial activity through a Scaffold approach. J. Biomol. Struct. Dyn. 2022. [Google Scholar] [CrossRef] [PubMed]
- Ammazzalorso, A.; Maccallini, C.; Amoia, P.; Amoroso, R. Multitarget PPARγ agonists as innovative modulators of the metabolic syndrome. Eur. J. Med. Chem. 2019, 173, 261–273. [Google Scholar] [CrossRef]
- Koeberle, A.; Werz, O. Multi-target approach for natural products in inflammation. Drug Discov. Today 2014, 19, 1871–1882. [Google Scholar] [CrossRef] [PubMed]
- Colín-Lozano, B.; Estrada-Soto, S.; Chávez-Silva, F.; Gutiérrez-Hernández, A.; Cerón-Romero, L.; Giacoman-Martínez, A.; Almanza-Pérez, J.C.; Hernández-Núñez, E.; Wang, Z.; Xie, X.; et al. Design, Synthesis and in Combo Antidiabetic Bioevaluation of Multitarget Phenylpropanoic Acids. Molecules 2018, 23, 340. [Google Scholar] [CrossRef]
- Lu, C.; Guo, Y.; Yan, J.; Luo, Z.; Luo, H.-B.; Yan, M.; Huang, L.; Li, X. Design, Synthesis, and Evaluation of Multitarget-Directed Resveratrol Derivatives for the Treatment of Alzheimer’s Disease. J. Med. Chem. 2013, 56, 5843–5859. [Google Scholar] [CrossRef] [PubMed]
- Schöning-Stierand, K.; Diedrich, K.; Fährrolfes, R.; Flachsenberg, F.; Meyder, A.; Nittinger, E.; Steinegger, R.; Rarey, M. ProteinsPlus: Interactive analysis of protein–ligand binding interfaces. Nucleic Acids Res. 2020, 48, W48–W53. [Google Scholar] [CrossRef]
- Yamashita, S.; Masuda, D.; Matsuzawa, Y. Pemafibrate, a New Selective PPARα Modulator: Drug Concept and Its Clinical Applications for Dyslipidemia and Metabolic Diseases. Curr. Atheroscler. Rep. 2020, 22, 5. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Takei, K.; Arulmozhiraja, S.; Sladek, V.; Matsuo, N.; Han, S.; Matsuzaka, T.; Sekiya, M.; Tokiwa, T.; Shoji, M.; et al. Molecular association model of PPARα and its new specific and efficient ligand, pemafibrate: Structural basis for SPPARMα. Biochem. Biophys. Res. Commun. 2018, 499, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.A.; Tan, L.; Yang, H.; Im, Y.-G.; Im, Y.J. Structures of PPARγ complexed with lobeglitazone and pioglitazone reveal key determinants for the recognition of antidiabetic drugs. Sci. Rep. 2017, 7, 16837. [Google Scholar] [CrossRef] [PubMed]
- Ohtera, A.; Miyamae, Y.; Yoshida, K.; Maejima, K.; Akita, T.; Kakizuka, A.; Irie, K.; Masuda, S.; Kambe, T.; Nagao, M. Identification of a New Type of Covalent PPARγ Agonist using a Ligand-Linking Strategy. ACS Chem. Biol. 2015, 10, 2794–2804. [Google Scholar] [CrossRef]
- Pickavance, L.C.; Tadayyon, M.; Widdowson, P.S.; Buckingham, R.E.; Wilding, J.P.H. Therapeutic index for rosiglitazone in dietary obese rats: Separation of efficacy and haemodilution. Br. J. Pharmacol. 1999, 128, 1570–1576. [Google Scholar] [CrossRef]
- Collino, M.; Aragno, M.; Castiglia, S.; Miglio, G.; Tomasinelli, C.; Boccuzzi, G.; Thiemermann, C.; Fantozzi, R. Pioglitazone improves lipid and insulin levels in overweight rats on a high cholesterol and fructose diet by decreasing hepatic inflammation. Br. J. Pharmacol. 2010, 160, 1892–1902. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.-F.; Jhao, Y.-T.; Chiu, C.-H.; Sun, L.-H.; Chou, T.-K.; Shiue, C.-Y.; Cheng, C.-Y.; Ma, K.-H. Bezafibrate Exerts Neuroprotective Effects in a Rat Model of Sporadic Alzheimer’s Disease. Pharmaceuticals 2022, 15, 109. [Google Scholar] [CrossRef] [PubMed]
- Holeček, M.; Vodeničarovová, M. Effects of low and high doses of fenofibrate on protein, amino acid, and energy metabolism in rat. Int. J. Exp. Pathol. 2020, 101, 171–182. [Google Scholar] [CrossRef]
- Cao, N.T.; Nguyen, N.A.; Park, C.M.; Cha, G.S.; Park, K.D.; Yun, C.-H. A Novel Statin Compound from Monacolin J Produced Using CYP102A1-Catalyzed Regioselective C-Hydroxylation. Pharmaceuticals 2021, 14, 981. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.Y.; Ryu, S.H.; Park, S.H.; Cha, G.S.; Kim, D.H.; Kim, K.H.; Hong, A.W.; Ahn, T.; Pan, J.G.; Joung, Y.H.; et al. Chimeric cytochromes P450 engineered by domain swapping and random mutagenesis for producing human metabolites of drugs. Biotechnol. Bioeng. 2014, 111, 1313–1322. [Google Scholar] [CrossRef]
- Mendieta, A.; Jiménez, F.; Garduño-Siciliano, L.; Mojica-Villegas, A.; Rosales-Acosta, B.; Villa-Tanaca, L.; Chamorro-Cevallos, G.; Medina-Franco, J.L.; Meurice, N.; Gutiérrez, R.U.; et al. Synthesis and highly potent hypolipidemic activity of alpha-asarone- and fibrate-based 2-acyl and 2-alkyl phenols as HMG-CoA reductase inhibitors. Bioorg. Med. Chem. 2014, 22, 5871–5882. [Google Scholar] [CrossRef]
- Rodríguez-Páez, L.; Juárez-Sanchez, M.; Antúnez-Solís, J.; Baeza, I.; Wong, C. α-Asarone inhibits HMG-CoA reductase, lowers serum LDL-cholesterol levels and reduces biliary CSI in hypercholesterolemic rats. Phytomedicine 2003, 10, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Knight, S.F.; Yuan, J.; Roy, S.; Imig, J.D. Simvastatin and tempol protect against endothelial dysfunction and renal injury in a model of obesity and hypertension. Am. J. Physiol. Physiol. 2009, 298, F86–F94. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Liu, Y.; Zhang, Q.; Ma, B.; Yang, Z.; Liu, L.; Yao, D.; Cui, G.; Sun, J.; Wu, Z. Metabolomic analysis of simvastatin and fenofibrate intervention in high-lipid diet-induced hyperlipidemia rats. Acta Pharmacol. Sin. 2014, 35, 1265–1273. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Han, K.; Park, C.-Y. The triglyceride glucose index is a simple and low-cost marker associated with atherosclerotic cardiovascular disease: A population-based study. BMC Med. 2020, 18, 361. [Google Scholar] [CrossRef]
- Simental-Mendía, L.E.; Rodríguez-Morán, M.; Guerrero-Romero, F. The Product of Fasting Glucose and Triglycerides As Surrogate for Identifying Insulin Resistance in Apparently Healthy Subjects. Metab. Syndr. Relat. Disord. 2008, 6, 299–304. [Google Scholar] [CrossRef]
- Du, T.; Yuan, G.; Zhang, M.; Zhou, X.; Sun, X.; Yu, X. Clinical usefulness of lipid ratios, visceral adiposity indicators, and the triglycerides and glucose index as risk markers of insulin resistance. Cardiovasc. Diabetol. 2014, 13, 146. [Google Scholar] [CrossRef] [PubMed]
- Navarro-González, D.; Sánchez-Íñigo, L.; Pastrana-Delgado, J.; Fernández-Montero, A.; Martinez, J.A. Triglyceride–glucose index (TyG index) in comparison with fasting plasma glucose improved diabetes prediction in patients with normal fasting glucose: The Vascular-Metabolic CUN cohort. Prev. Med. 2016, 86, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Vidal-Ostos, F.; Ramos-Lopez, O.; Blaak, E.E.; Astrup, A.; Martinez, J.A. The triglyceride-glucose index as an adiposity marker and a predictor of fat loss induced by a low-calorie diet. Eur. J. Clin. Investig. 2022, 52, e13674. [Google Scholar] [CrossRef] [PubMed]
- Nunes-Souza, V.; César-Gomes, C.J.; Da Fonseca, L.J.S.; Guedes, G.D.S.; Smaniotto, S.; Rabelo, L.A. Aging Increases Susceptibility to High Fat Diet-Induced Metabolic Syndrome in C57BL/6 Mice: Improvement in Glycemic and Lipid Profile after Antioxidant Therapy. Oxid. Med. Cell. Longev. 2016, 2016, 1987960. [Google Scholar] [CrossRef] [PubMed]
- Monserrat-Mesquida, M.; Quetglas-Llabrés, M.; Capó, X.; Bouzas, C.; Mateos, D.; Pons, A.; Tur, J.A.; Sureda, A. Metabolic Syndrome Is Associated with Oxidative Stress and Proinflammatory State. Antioxidants 2020, 9, 236. [Google Scholar] [CrossRef] [PubMed]
- Yubero-Serrano, E.M.; Delgado-Lista, J.; Peña-Orihuela, P.; Perez-Martinez, P.; Fuentes, F.; Marin, C.; Tunez, I.; Jose Tinahones, F.; Perez-Jimenez, F.; Roche, H.M.; et al. Oxidative stress is associated with the number of components of metabolic syndrome: LIPGENE study. Exp. Mol. Med. 2013, 45, e28. [Google Scholar] [CrossRef]
- Miranda-Rottmann, S.; Aspillaga, A.A.; Pérez, D.D.; Vasquez, L.; Martinez, A.L.F.; Leighton, F. Juice and Phenolic Fractions of the Berry Aristotelia chilensis Inhibit LDL Oxidation in Vitro and Protect Human Endothelial Cells against Oxidative Stress. J. Agric. Food Chem. 2002, 50, 7542–7547. [Google Scholar] [CrossRef]
- Quispe-Fuentes, I.; Vega-Gálvez, A.; Aranda, M. Evaluation of phenolic profiles and antioxidant capacity of maqui (Aristotelia chilensis) berries and their relationships to drying methods. J. Sci. Food Agric. 2018, 98, 4168–4176. [Google Scholar] [CrossRef] [PubMed]
- De la Fuente Muñoz, M.; de la Fuente Fernández, M.; Román-Carmena, M.; Iglesias de la Cruz, M.D.C.; Amor, S.; Martorell, P.; Enrique-López, M.; García-Villalón, A.L.; Inarejos-García, A.M.; Granado, M. Supplementation with Two New Standardized Tea Extracts Prevents the Development of Hypertension in Mice with Metabolic Syndrome. Antioxidants 2022, 11, 1573. [Google Scholar] [CrossRef]
- Pérez-Areales, F.J.; Garrido, M.; Aso, E.; Bartolini, M.; De Simone, A.; Espargaró, A.; Ginex, T.; Sabate, R.; Pérez, B.; Andrisano, V.; et al. Centrally Active Multitarget Anti-Alzheimer Agents Derived from the Antioxidant Lead CR-6. J. Med. Chem. 2020, 63, 9360–9390. [Google Scholar] [CrossRef]
- Apak, R.; Güçlü, K.; Özyürek, M.; Bektaşoğlu, B.; Bener, M. Cupric Ion Reducing Antioxidant Capacity Assay for Antioxidants in Human Serum and for Hydroxyl Radical Scavengers. In Advanced Protocols in Oxidative Stress II. Methods in Molecular Biology; Armstrong, D., Ed.; Humana Press: Totowa, NJ, USA, 2010; pp. 215–239. ISBN 978-1-60761-411-1. [Google Scholar]
- Loza-Mejía, M.A.; Salazar, J.R. Sterols and triterpenoids as potential anti-inflammatories: Molecular docking studies for binding to some enzymes involved in inflammatory pathways. J. Mol. Graph. Model. 2015, 62, 18–25. [Google Scholar] [CrossRef]
- Pires, D.E.V.; Blundell, T.L.; Ascher, D.B. pkCSM: Predicting small-molecule pharmacokinetic and toxicity properties using graph-based signatures. J. Med. Chem. 2015, 58, 4066–4072. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).