Therapeutic Efficacy of Novel HDAC Inhibitors SPA3052 and SPA3074 against Intestinal Inflammation in a Murine Model of Colitis
Abstract
1. Introduction
2. Results
2.1. Body Weight, Colon Shortening, and DAI Score
2.2. Histological Score and Expression of the Tight Junction Protein
2.3. SOCS1 and SOCS3 Protein Expression
2.4. Protein Expression of Inflammation-Associated Mediators
2.5. Protein Expression of Phophorylated Iκbα and IκBα
2.6. Relative mRNA Expression of Pro-Inflammatory Cytokines
3. Discussion
4. Materials and Methods
4.1. Preparation of Chemical Compounds
4.2. Animal Models of DSS-Induced Colitis
4.3. Disease Activity Index and Histological Observation
4.4. Real-Time PCR Analysis
4.5. Protein Extraction and Western Blot Analysis
4.6. Statistical Analysis
5. Conclusion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Neurath, M.F. Current and emerging therapeutic targets for IBD. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Z.; Li, Y.Y. Inflammatory bowel disease: Pathogenesis. World J. Gastroenterol. 2014, 20, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Ventham, N.T.; Kennedy, N.A.; Nimmo, E.R.; Satsangi, J. Beyond gene discovery in inflammatory bowel disease: The emerging role of epigenetics. Gastroenterology 2013, 145, 293–308. [Google Scholar] [CrossRef] [PubMed]
- Khor, B.; Gardet, A.; Xavier, R.J. Genetics and pathogenesis of inflammatory bowel disease. Nature 2011, 474, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Nebbioso, A.; Carafa, V.; Benedetti, R.; Altucci, L. Trials with ‘epigenetic’ drugs: An update. Mol. Oncol. 2012, 6, 657–682. [Google Scholar] [CrossRef]
- Richon, V. Cancer biology: Mechanism of antitumour action of vorinostat (suberoylanilide hydroxamic acid), a novel histone deacetylase inhibitor. Br. J. Cancer 2006, 95, S2–S6. [Google Scholar] [CrossRef]
- Tsaprouni, L.G.; Ito, K.; Powell, J.J.; Adcock, I.M.; Punchard, N. Differential patterns of histone acetylation in inflammatory bowel diseases. J. Inflamm. 2011, 8, 1. [Google Scholar] [CrossRef]
- Schneider, A.; Chatterjee, S.; Bousiges, O.; Selvi, B.R.; Swaminathan, A.; Cassel, R.; Blanc, F.; Kundu, T.K.; Boutillier, A.L. Acetyltransferases (HATs) as targets for neurological therapeutics. Neurotherapeutics 2013, 10, 568–588. [Google Scholar] [CrossRef]
- Wang, Z.; Zang, C.; Rosenfeld, J.A.; Schones, D.E.; Barski, A.; Cuddapah, S.; Cui, K.; Roh, T.Y.; Peng, W.; Zhang, M.Q.; et al. Combinatorial patterns of histone acetylations and methylations in the human genome. Nat. Genet. 2008, 40, 897–903. [Google Scholar] [CrossRef]
- Shakespear, M.R.; Halili, M.A.; Irvine, K.M.; Fairlie, D.P.; Sweet, M.J. Histone deacetylases as regulators of inflammation and immunity. Trends Immunol. 2011, 32, 335–343. [Google Scholar] [CrossRef]
- Kim, D.S.; Kwon, J.E.; Lee, S.H.; Kim, E.K.; Ryu, J.G.; Jung, K.A.; Choi, J.W.; Park, M.J.; Moon, Y.M.; Park, S.H.; et al. Attenuation of Rheumatoid Inflammation by Sodium Butyrate Through Reciprocal Targeting of HDAC2 in Osteoclasts and HDAC8 in T Cells. Front. Immunol. 2018, 9, 1525. [Google Scholar] [CrossRef]
- Shuttleworth, S.J.; Bailey, S.G.; Townsend, P.A. Histone Deacetylase inhibitors: New promise in the treatment of immune and inflammatory diseases. Curr. Drug Targets 2010, 11, 1430–1438. [Google Scholar] [CrossRef]
- Bodas, M.; Mazur, S.; Min, T.; Vij, N. Inhibition of histone-deacetylase activity rescues inflammatory cystic fibrosis lung disease by modulating innate and adaptive immune responses. Respir. Res. 2018, 19, 2. [Google Scholar] [CrossRef]
- Ren, Y.; Su, X.; Kong, L.; Li, M.; Zhao, X.; Yu, N.; Kang, J. Therapeutic effects of histone deacetylase inhibitors in a murine asthma model. Inflamm. Res. 2016, 65, 995–1008. [Google Scholar] [CrossRef]
- Felice, C.; Lewis, A.; Armuzzi, A.; Lindsay, J.O.; Silver, A. Review article: Selective histone deacetylase isoforms as potential therapeutic targets in inflammatory bowel diseases. Aliment. Pharm. 2015, 41, 26–38. [Google Scholar] [CrossRef]
- Grant, S.; Easley, C.; Kirkpatrick, P. Vorinostat. Nat. Rev. Drug Discov. 2007, 6, 21–22. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, C.; Finger, S.N.; Kwajah, S.; Jung, M.; Schwarz, H.; Swanson, N.; Lareu, F.F.; Raghunath, M. Suberoylanilide hydroxamic acid: A potential epigenetic therapeutic agent for lung fibrosis? Eur. Respir. J. 2009, 34, 145–155. [Google Scholar] [CrossRef]
- Ali, M.N.; Choijookhuu, N.; Takagi, H.; Srisowanna, N.; Nguyen Nhat Huynh, M.; Yamaguchi, Y.; Synn Oo, P.; Tin Htwe Kyaw, M.; Sato, K.; Yamaguchi, R.; et al. The HDAC Inhibitor, SAHA, Prevents Colonic Inflammation by Suppressing Pro-inflammatory Cytokines and Chemokines in DSS-induced Colitis. Acta. Histochem. Cytochem. 2018, 51, 33–40. [Google Scholar] [CrossRef]
- Chakrabarti, A.; Oehme, I.; Witt, O.; Oliveira, G.; Sippl, W.; Romier, C.; Pierce, R.J.; Jung, M. HDAC8: A multifaceted target for therapeutic interventions. Trends Pharm. Sci. 2015, 36, 481–492. [Google Scholar] [CrossRef]
- Gao, S.M.; Chen, C.Q.; Wang, L.Y.; Hong, L.L.; Wu, J.B.; Dong, P.H.; Yu, F.J. Histone deacetylases inhibitor sodium butyrate inhibits JAK2/STAT signaling through upregulation of SOCS1 and SOCS3 mediated by HDAC8 inhibition in myeloproliferative neoplasms. Exp. Hematol 2013, 41, 261–270.e264. [Google Scholar] [CrossRef]
- Chen, C.Q.; Yu, K.; Yan, Q.X.; Xing, C.Y.; Chen, Y.; Yan, Z.; Shi, Y.F.; Zhao, K.W.; Gao, S.M. Pure curcumin increases the expression of SOCS1 and SOCS3 in myeloproliferative neoplasms through suppressing class I histone deacetylases. Carcinogenesis 2013, 34, 1442–1449. [Google Scholar] [CrossRef] [PubMed]
- Horino, J.; Fujimoto, M.; Terabe, F.; Serada, S.; Takahashi, T.; Soma, Y.; Tanaka, K.; Chinen, T.; Yoshimura, A.; Nomura, S.; et al. Suppressor of cytokine signaling-1 ameliorates dextran sulfate sodium-induced colitis in mice. Int. Immunol 2008, 20, 753–762. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, A.; Hanada, T.; Mitsuyama, K.; Yoshida, T.; Kamizono, S.; Hoshino, T.; Kubo, M.; Yamashita, A.; Okabe, M.; Takeda, K.; et al. CIS3/SOCS3/SSI3 plays a negative regulatory role in STAT3 activation and intestinal inflammation. J. Exp. Med. 2001, 193, 471–481. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Fossati, G.; Marchetti, C.; Modena, D.; Pozzi, P.; Reznikov, L.L.; Moras, M.L.; Azam, T.; Abbate, A.; Mascagni, P.; et al. Specific inhibition of histone deacetylase 8 reduces gene expression and production of proinflammatory cytokines in vitro and in vivo. J. Biol. Chem. 2015, 290, 2368–2378. [Google Scholar] [CrossRef] [PubMed]
- Jan, J.S.; Chou, Y.C.; Cheng, Y.W.; Chen, C.K.; Huang, W.J.; Hsiao, G. The Novel HDAC8 Inhibitor WK2-16 Attenuates Lipopolysaccharide-Activated Matrix Metalloproteinase-9 Expression in Human Monocytic Cells and Improves Hypercytokinemia In Vivo. Int. J. Mol. Sci. 2017, 18, 1394. [Google Scholar] [CrossRef]
- Di Liddo, R.; Valente, S.; Taurone, S.; Zwergel, C.; Marrocco, B.; Turchetta, R.; Conconi, M.T.; Scarpa, C.; Bertalot, T.; Schrenk, S.; et al. Histone deacetylase inhibitors restore IL-10 expression in lipopolysaccharide-induced cell inflammation and reduce IL-1β and IL-6 production in breast silicone implant in C57BL/6J wild-type murine model. Autoimmunity 2016, 1–11. [Google Scholar] [CrossRef]
- Suzuki, T. Regulation of intestinal epithelial permeability by tight junctions. Cell Mol. Life Sci. 2013, 70, 631–659. [Google Scholar] [CrossRef]
- Duncan, S.A.; Baganizi, D.R.; Sahu, R.; Singh, S.R.; Dennis, V.A. SOCS Proteins as Regulators of Inflammatory Responses Induced by Bacterial Infections: A Review. Front. Microbiol. 2017, 8, 2431. [Google Scholar] [CrossRef]
- Anderson, E.C.; Wong, M.H. Caught in the Akt: Regulation of Wnt signaling in the intestine. Gastroenterology 2010, 139, 718–722. [Google Scholar] [CrossRef]
- Pastore, S.; Mascia, F.; Mariotti, F.; Dattilo, C.; Mariani, V.; Girolomoni, G. ERK1/2 regulates epidermal chemokine expression and skin inflammation. J. Immunol. 2005, 174, 5047–5056. [Google Scholar] [CrossRef]
- Madonna, S.; Scarponi, C.; De Pità, O.; Albanesi, C. Suppressor of cytokine signaling 1 inhibits IFN-gamma inflammatory signaling in human keratinocytes by sustaining ERK1/2 activation. FASEB J. 2008, 22, 3287–3297. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Gaynor, R.B. IkappaB kinases: Key regulators of the NF-kappaB pathway. Trends Biochem. Sci. 2004, 29, 72–79. [Google Scholar] [CrossRef]
- Műzes, G.; Molnár, B.; Tulassay, Z.; Sipos, F. Changes of the cytokine profile in inflammatory bowel diseases. World J. Gastroenterol. 2012, 18, 5848–5861. [Google Scholar] [CrossRef]
- Bamias, G.; Cominelli, F. Role of type 2 immunity in intestinal inflammation. Curr. Opin. Gastroenterol 2015, 31, 471–476. [Google Scholar] [CrossRef]
- Lee, H.S.; Park, S.K.; Park, D.I. Novel treatments for inflammatory bowel disease. Korean J. Intern. Med. 2018, 33, 20–27. [Google Scholar] [CrossRef]
- Chudy-Onwugaje, K.O.; Christian, K.E.; Farraye, F.A.; Cross, R.K. A State-of-the-Art Review of New and Emerging Therapies for the Treatment of IBD. Inflamm. Bowel. Dis. 2019, 25, 820–830. [Google Scholar] [CrossRef]
- Matricon, J.; Barnich, N.; Ardid, D. Immunopathogenesis of inflammatory bowel disease. Self/nonself 2010, 1, 299–309. [Google Scholar] [CrossRef]
- Silva, F.A.; Rodrigues, B.L.; Ayrizono, M.L.; Leal, R.F. The Immunological Basis of Inflammatory Bowel Disease. Gastroenterol. Res. Pract. 2016, 2016, 2097274. [Google Scholar] [CrossRef]
- Do, A.; Reid, R.C.; Lohman, R.J.; Sweet, M.J.; Fairlie, D.P.; Iyer, A. An HDAC6 Inhibitor Confers Protection and Selectively Inhibits B-Cell Infiltration in DSS-Induced Colitis in Mice. J. Pharmacol. Exp. Ther. 2017, 360, 140–151. [Google Scholar] [CrossRef]
- Glauben, R.; Batra, A.; Stroh, T.; Erben, U.; Fedke, I.; Lehr, H.A.; Leoni, F.; Mascagni, P.; Dinarello, C.A.; Zeitz, M.; et al. Histone deacetylases: Novel targets for prevention of colitis-associated cancer in mice. Gut 2008, 57, 613–622. [Google Scholar] [CrossRef]
- Glauben, R.; Batra, A.; Fedke, I.; Zeitz, M.; Lehr, H.A.; Leoni, F.; Mascagni, P.; Fantuzzi, G.; Dinarello, C.A.; Siegmund, B. Histone hyperacetylation is associated with amelioration of experimental colitis in mice. J. Immunol. 2006, 176, 5015–5022. [Google Scholar] [CrossRef]
- Heller, F.; Florian, P.; Bojarski, C.; Richter, J.; Christ, M.; Hillenbrand, B.; Mankertz, J.; Gitter, A.H.; Bürgel, N.; Fromm, M.; et al. Interleukin-13 is the key effector Th2 cytokine in ulcerative colitis that affects epithelial tight junctions, apoptosis, and cell restitution. Gastroenterology 2005, 129, 550–564. [Google Scholar] [CrossRef]
- Pathak, S.; Grillo, A.R.; Scarpa, M.; Brun, P.; D’Incà, R.; Nai, L.; Banerjee, A.; Cavallo, D.; Barzon, L.; Palù, G.; et al. MiR-155 modulates the inflammatory phenotype of intestinal myofibroblasts by targeting SOCS1 in ulcerative colitis. Exp. Mol. Med. 2015, 47, e164. [Google Scholar] [CrossRef] [PubMed]
- Chinen, T.; Kobayashi, T.; Ogata, H.; Takaesu, G.; Takaki, H.; Hashimoto, M.; Yagita, H.; Nawata, H.; Yoshimura, A. Suppressor of cytokine signaling-1 regulates inflammatory bowel disease in which both IFNgamma and IL-4 are involved. Gastroenterology 2006, 130, 373–388. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, A.; Suzuki, M.; Sakaguchi, R.; Hanada, T.; Yasukawa, H. SOCS, Inflammation, and Autoimmunity. Front. Immunol. 2012, 3, 20. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Shan, X.; Qian, J.; Ji, Q.; Wang, L.; Wang, X.; Li, M.; Ding, H.; Liu, Q.; Chen, L.; et al. The suppressor of cytokine signaling SOCS1 promotes apoptosis of intestinal epithelial cells via p53 signaling in Crohn’s disease. Exp. Mol. Pathol. 2016, 101, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.M.; Xu, R.; Huang, X.Y.; Cheng, S.M.; Huang, M.F.; Yue, H.Y.; Wang, X.; Zou, Y.; Lu, A.P.; Liu, D.Y. Curcumin Suppressed Activation of Dendritic Cells via JAK/STAT/SOCS Signal in Mice with Experimental Colitis. Front. Pharm. 2016, 7, 455. [Google Scholar] [CrossRef]
- Cheng, X.; Zhang, X.; Su, J.; Zhang, Y.; Zhou, W.; Zhou, J.; Wang, C.; Liang, H.; Chen, X.; Shi, R.; et al. miR-19b downregulates intestinal SOCS3 to reduce intestinal inflammation in Crohn’s disease. Sci. Rep. 2015, 5, 10397. [Google Scholar] [CrossRef]
- Schmitz, J.; Weissenbach, M.; Haan, S.; Heinrich, P.C.; Schaper, F. SOCS3 exerts its inhibitory function on interleukin-6 signal transduction through the SHP2 recruitment site of gp130. J. Biol. Chem. 2000, 275, 12848–12856. [Google Scholar] [CrossRef]
- Lee, H.; Rezai-Zadeh, N.; Seto, E. Negative regulation of histone deacetylase 8 activity by cyclic AMP-dependent protein kinase A. Mol. Cell Biol. 2004, 24, 765–773. [Google Scholar] [CrossRef]
- Somoza, J.R.; Skene, R.J.; Katz, B.A.; Mol, C.; Ho, J.D.; Jennings, A.J.; Luong, C.; Arvai, A.; Buggy, J.J.; Chi, E.; et al. Structural snapshots of human HDAC8 provide insights into the class I histone deacetylases. Structure 2004, 12, 1325–1334. [Google Scholar] [CrossRef]
- Delghandi, M.P.; Johannessen, M.; Moens, U. The cAMP signalling pathway activates CREB through PKA, p38 and MSK1 in NIH 3T3 cells. Cell. Signal. 2005, 17, 1343–1351. [Google Scholar] [CrossRef]
- Kim, H.P.; Leonard, W.J. CREB/ATF-dependent T cell receptor-induced FoxP3 gene expression: A role for DNA methylation. J. Exp. Med. 2007, 204, 1543–1551. [Google Scholar] [CrossRef]
- Raker, V.K.; Becker, C.; Steinbrink, K. The cAMP Pathway as Therapeutic Target in Autoimmune and Inflammatory Diseases. Front. Immunol 2016, 7, 123. [Google Scholar] [CrossRef]
- Bradbury, N.A. Protein kinase-A-mediated secretion of mucin from human colonic epithelial cells. J. Cell Physiol. 2000, 185, 408–415. [Google Scholar] [CrossRef]
- Dorofeyev, A.E.; Vasilenko, I.V.; Rassokhina, O.A.; Kondratiuk, R.B. Mucosal barrier in ulcerative colitis and Crohn’s disease. Gastroenterol. Res. Pract. 2013, 2013, 431231. [Google Scholar] [CrossRef]
- Impey, S.; Obrietan, K.; Wong, S.T.; Poser, S.; Yano, S.; Wayman, G.; Deloulme, J.C.; Chan, G.; Storm, D.R. Cross talk between ERK and PKA is required for Ca2+ stimulation of CREB-dependent transcription and ERK nuclear translocation. Neuron 1998, 21, 869–883. [Google Scholar] [CrossRef]
- Wen, A.Y.; Sakamoto, K.M.; Miller, L.S. The role of the transcription factor CREB in immune function. J. Immunol. 2010, 185, 6413–6419. [Google Scholar] [CrossRef]
- Maeng, Y.S.; Min, J.K.; Kim, J.H.; Yamagishi, A.; Mochizuki, N.; Kwon, J.Y.; Park, Y.W.; Kim, Y.M.; Kwon, Y.G. ERK is an anti-inflammatory signal that suppresses expression of NF-kappaB-dependent inflammatory genes by inhibiting IKK activity in endothelial cells. Cell. Signal. 2006, 18, 994–1005. [Google Scholar] [CrossRef]
- Hockly, E.; Richon, V.M.; Woodman, B.; Smith, D.L.; Zhou, X.; Rosa, E.; Sathasivam, K.; Ghazi-Noori, S.; Mahal, A.; Lowden, P.A.; et al. Suberoylanilide hydroxamic acid, a histone deacetylase inhibitor, ameliorates motor deficits in a mouse model of Huntington’s disease. Proc. Natl. Acad. Sci. USA 2003, 100, 2041–2046. [Google Scholar] [CrossRef]
- Hunter, R.; Kaschula, C.H.; Parker, I.M.; Caira, M.R.; Richards, P.; Travis, S.; Taute, F.; Qwebani, T. Substituted ajoenes as novel anti-cancer agents. Bioorg. Med. Chem. Lett. 2008, 18, 5277–5279. [Google Scholar] [CrossRef] [PubMed]
- Hrzenjak, A.; Moinfar, F.; Kremser, M.L.; Strohmeier, B.; Petru, E.; Zatloukal, K.; Denk, H. Histone deacetylase inhibitor vorinostat suppresses the growth of uterine sarcomas in vitro and in vivo. Mol. Cancer 2010, 9, 49. [Google Scholar] [CrossRef] [PubMed]
- Simeoli, R.; Mattace Raso, G.; Pirozzi, C.; Lama, A.; Santoro, A.; Russo, R.; Montero-Melendez, T.; Berni Canani, R.; Calignano, A.; Perretti, M.; et al. An orally administered butyrate-releasing derivative reduces neutrophil recruitment and inflammation in dextran sulphate sodium-induced murine colitis. Br. J. Pharm. 2017, 174, 1484–1496. [Google Scholar] [CrossRef] [PubMed]
- Myzak, M.C.; Dashwood, W.M.; Orner, G.A.; Ho, E.; Dashwood, R.H. Sulforaphane inhibits histone deacetylase in vivo and suppresses tumorigenesis in Apc-minus mice. Faseb J. 2006, 20, 506–508. [Google Scholar] [CrossRef]
- Wei, T.T.; Lin, Y.T.; Chen, W.S.; Luo, P.; Lin, Y.C.; Shun, C.T.; Lin, Y.H.; Chen, J.B.; Chen, N.W.; Fang, J.M.; et al. Dual Targeting of 3-Hydroxy-3-methylglutaryl Coenzyme A Reductase and Histone Deacetylase as a Therapy for Colorectal Cancer. EBioMedicine 2016, 10, 124–136. [Google Scholar] [CrossRef][Green Version]
- Cooper, H.S.; Murthy, S.N.; Shah, R.S.; Sedergran, D.J. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab. Invest. 1993, 69, 238–249. [Google Scholar]
- Erben, U.; Loddenkemper, C.; Doerfel, K.; Spieckermann, S.; Haller, D.; Heimesaat, M.M.; Zeitz, M.; Siegmund, B.; Kühl, A.A. A guide to histomorphological evaluation of intestinal inflammation in mouse models. Int. J. Clin. Exp. Pathol. 2014, 7, 4557–4576. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
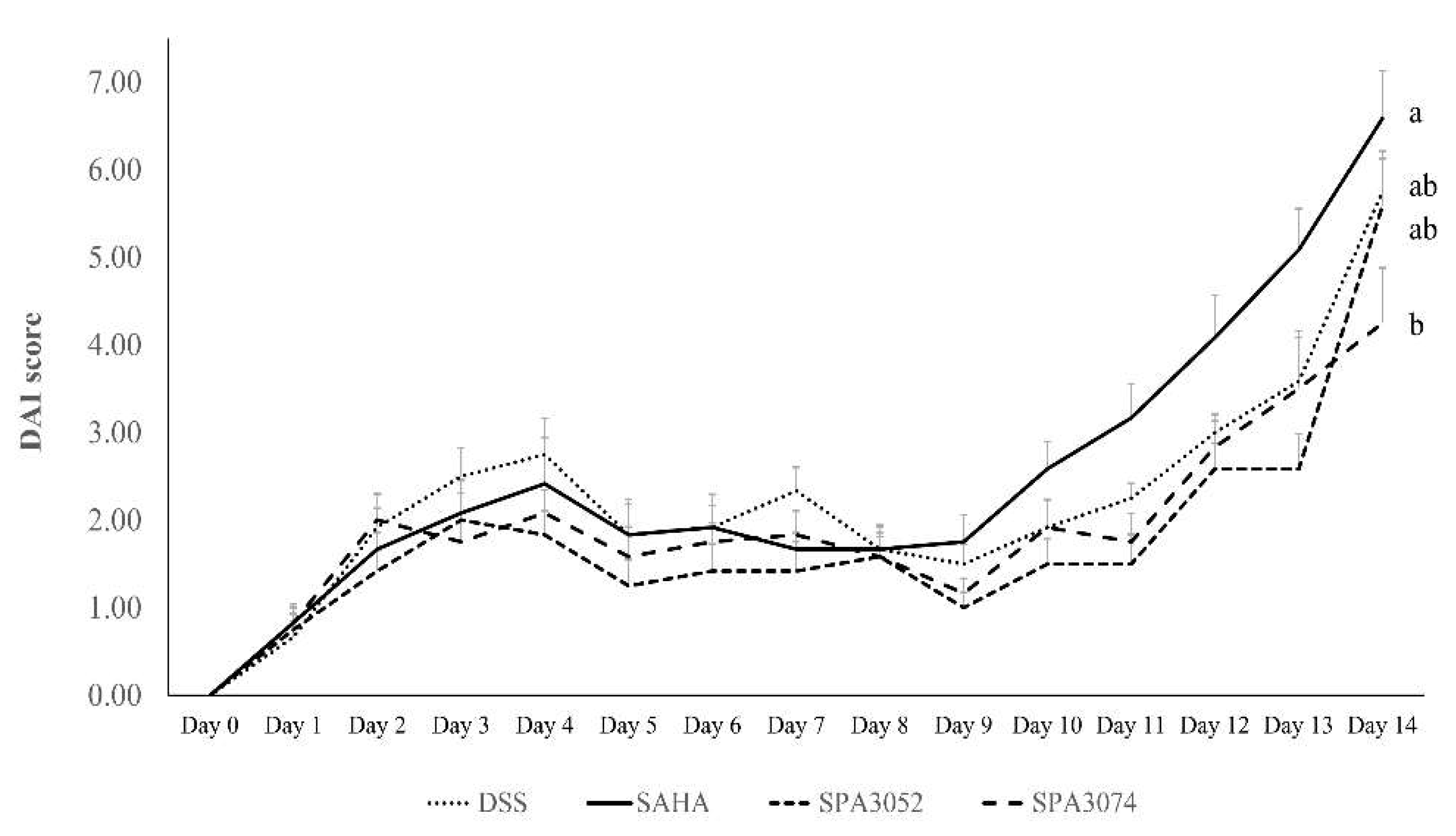
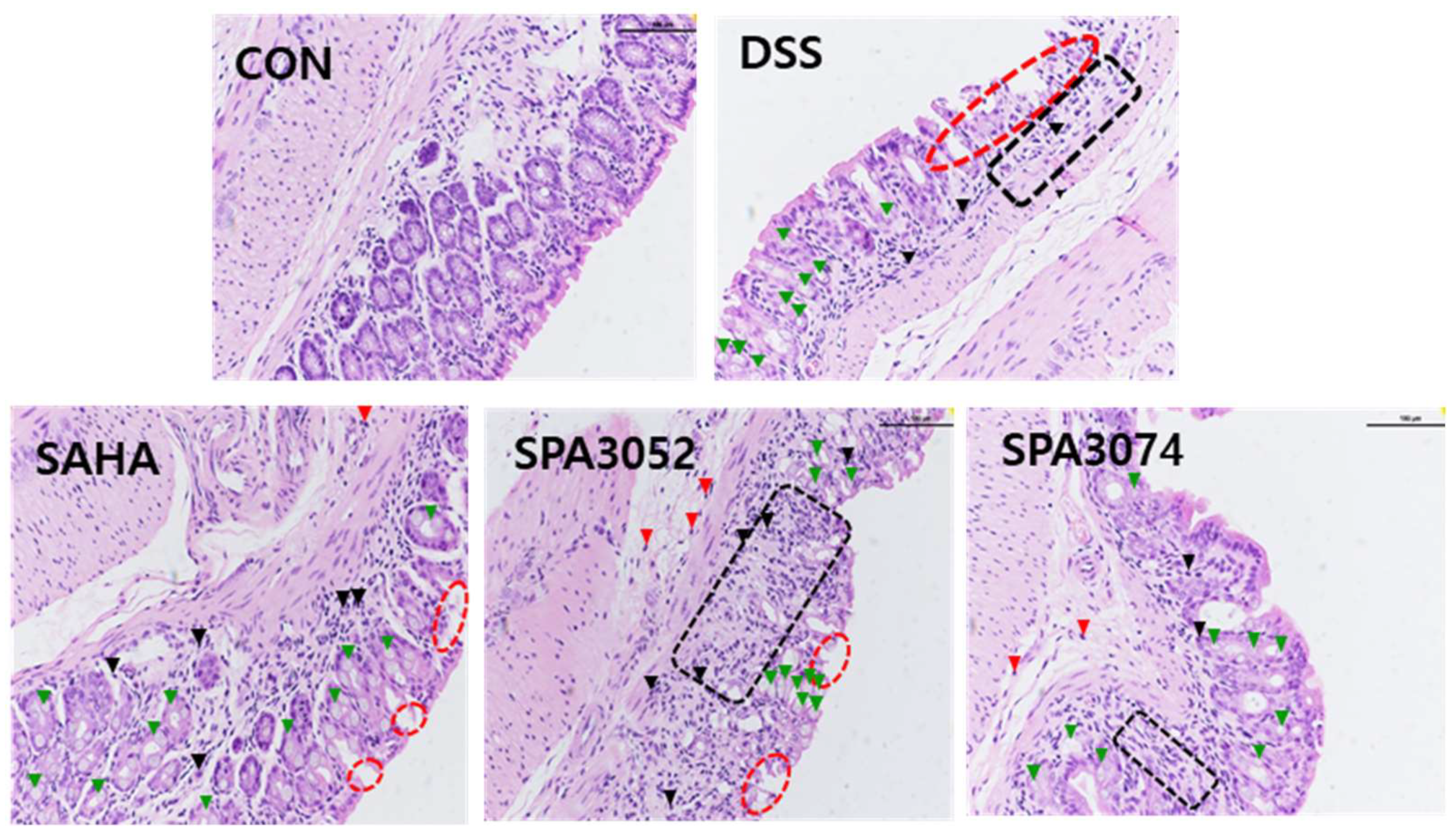



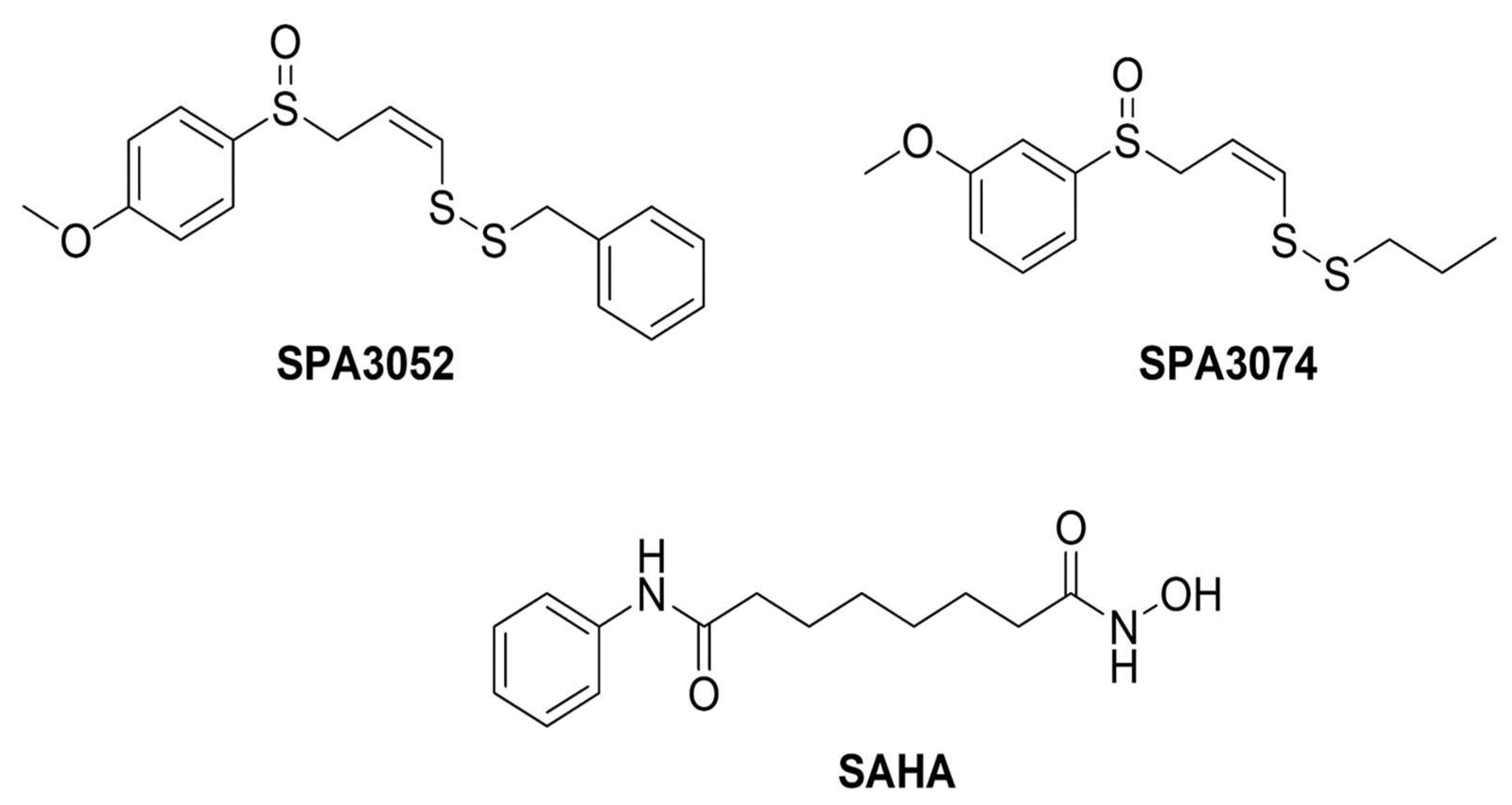
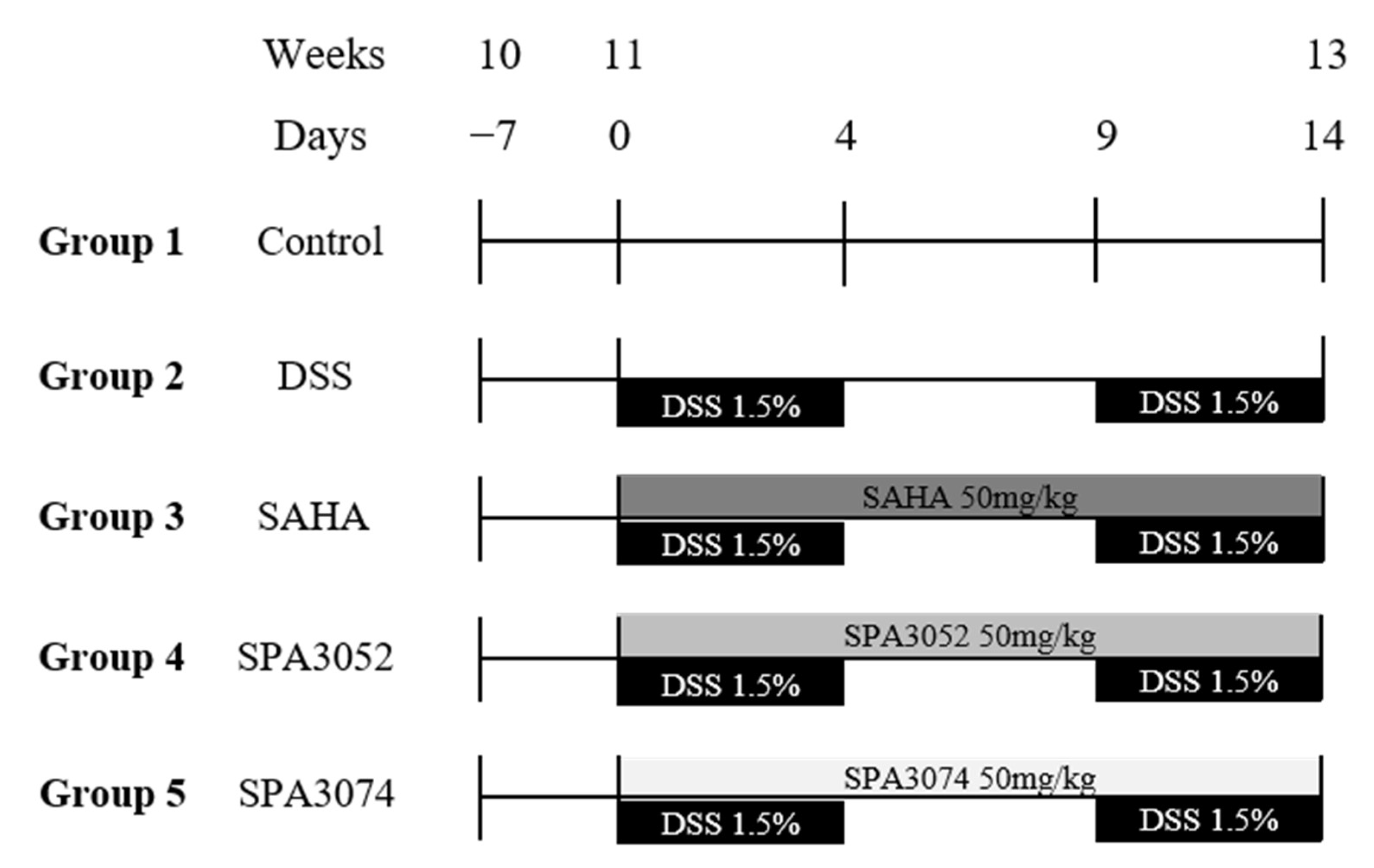
| Group | Final Body Weight | Colon Length |
|---|---|---|
| CON | 25.71 ± 0.88 a | 6.47 ± 0.15 a |
| DSS | 23.97 ± 0.33 b | 5.43 ± 0.19 b |
| SAHA | 22.76 ± 0.31 c | 5.28 ± 0.15 b |
| SPA3052 | 23.96 ± 0.34 b | 5.42 ± 0.18 b |
| SPA3074 | 24.15 ± 0.21 b | 5.46 ± 0.12 b |
| p value | 0.0005 | 0.0002 |
| Group | Inflammatory Cell Infiltrate | Epithelial Changes | Mucosal Architecture | Total Score |
|---|---|---|---|---|
| CON | 0.67 ± 0.667 | 0.00 ± 0.000 | 0.00 ± 0.000 | 0.67 ± 0.667 |
| DSS | 3.60 ± 0.400 ## | 6.60 ± 0.812 ## | 3.40 ± 0.872 # | 13.60 ± 1.077 ### |
| SAHA | 3.20 ± 0.374 | 4.00 ± 1.095 | 2.60 ± 1.077 | 9.80 ± 2.267 |
| SPA3052 | 3.20 ± 0.200 | 5.00 ± 0.894 | 2.80 ± 1.158 | 11.00 ± 1.924 |
| SPA3074 | 3.00 ± 0.000 | 4.00 ± 0.632 * | 3.40 ± 0.872 | 10.40 ± 0.748 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoon, J.-I.; Cho, H.; Jeon, R.; Sung, M.-K. Therapeutic Efficacy of Novel HDAC Inhibitors SPA3052 and SPA3074 against Intestinal Inflammation in a Murine Model of Colitis. Pharmaceuticals 2022, 15, 1515. https://doi.org/10.3390/ph15121515
Yoon J-I, Cho H, Jeon R, Sung M-K. Therapeutic Efficacy of Novel HDAC Inhibitors SPA3052 and SPA3074 against Intestinal Inflammation in a Murine Model of Colitis. Pharmaceuticals. 2022; 15(12):1515. https://doi.org/10.3390/ph15121515
Chicago/Turabian StyleYoon, Ji-In, Hyewon Cho, Raok Jeon, and Mi-Kyung Sung. 2022. "Therapeutic Efficacy of Novel HDAC Inhibitors SPA3052 and SPA3074 against Intestinal Inflammation in a Murine Model of Colitis" Pharmaceuticals 15, no. 12: 1515. https://doi.org/10.3390/ph15121515
APA StyleYoon, J.-I., Cho, H., Jeon, R., & Sung, M.-K. (2022). Therapeutic Efficacy of Novel HDAC Inhibitors SPA3052 and SPA3074 against Intestinal Inflammation in a Murine Model of Colitis. Pharmaceuticals, 15(12), 1515. https://doi.org/10.3390/ph15121515






