Impact of Cabozantinib Exposure on Proteinuria and Muscle Toxicity in Patients with Unresectable Hepatocellular Carcinoma
Abstract
1. Introduction
2. Results
2.1. Patient Features
2.2. Therapeutic Details and Safety
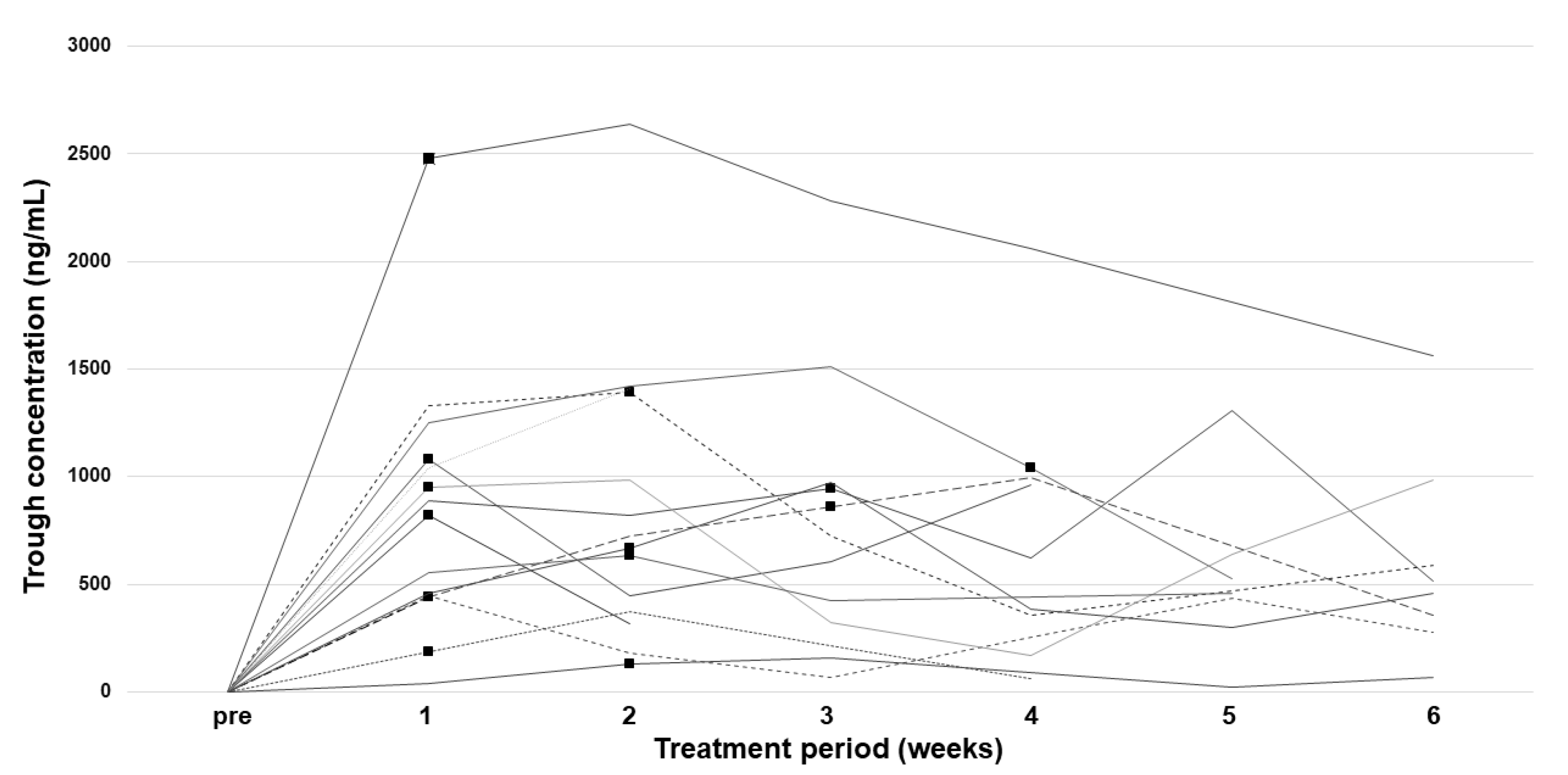
2.3. Pharmacokinetic Analysis
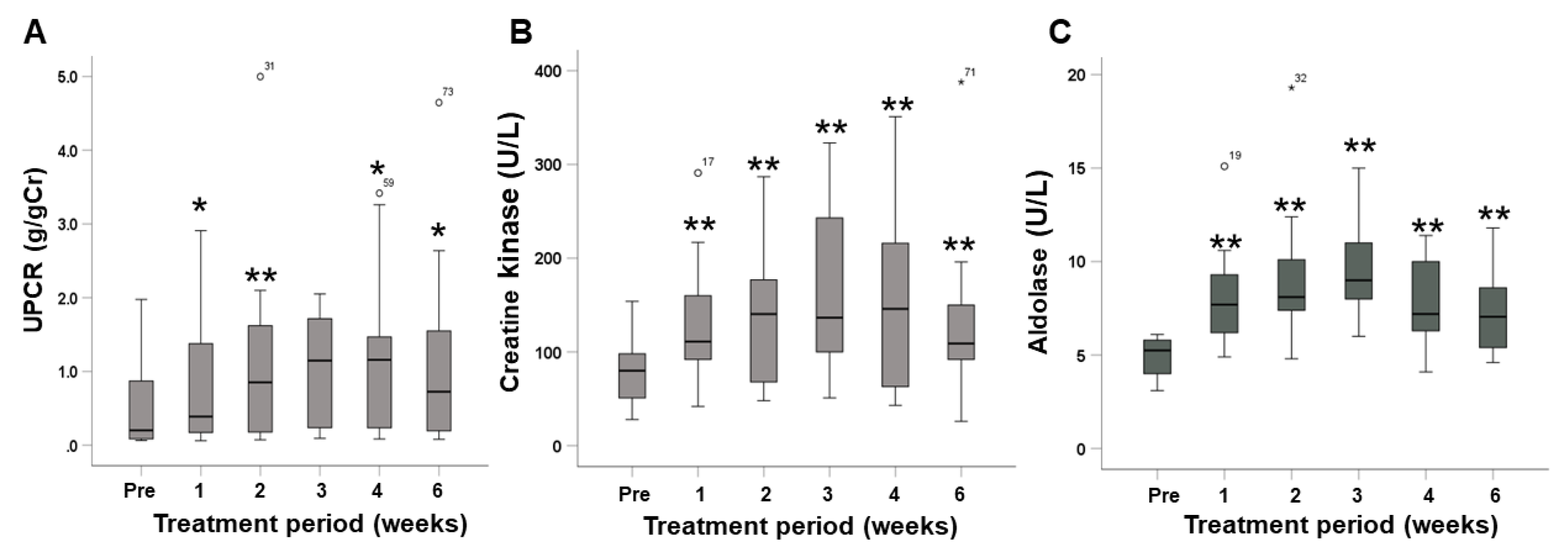
2.4. Changes in Urine Protein and Muscle Enzymes
2.5. Associations between Changes in Urine Protein or Muscle Enzymes and Cabozantinib Exposure
2.6. Receiver Operating Characteristic Curve Analysis
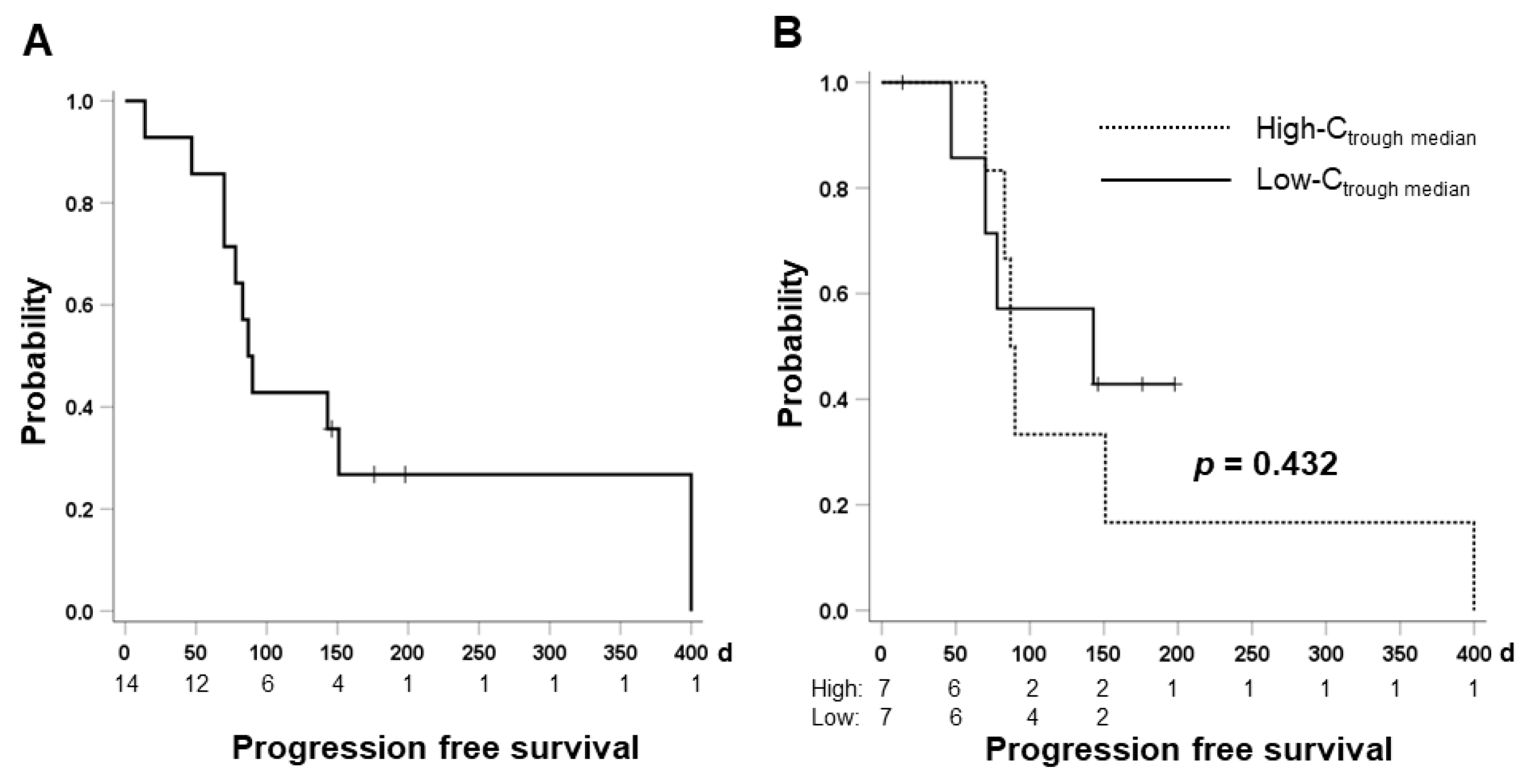
2.7. Therapeutic Effect and Cabozantinib Exposure
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. Pharmacokinetic Analysis of Cabozantinib
4.3. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bruix, J.; Qin, S.; Merle, P.; Granito, A.; Huang, Y.H.; Bodoky, G.; Pracht, M.; Yokosuka, O.; Rosmorduc, O.; Breder, V.; et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017, 389, 56–66. [Google Scholar] [CrossRef]
- Xiang, Q.; Chen, W.; Ren, M.; Wang, J.; Zhang, H.; Deng, D.Y.; Zhang, L.; Shang, C.; Chen, Y. Cabozantinib suppresses tumor growth and metastasis in hepatocellular carcinoma by a dual blockade of VEGFR2 and MET. Clin. Cancer Res. 2014, 20, 2959–2970. [Google Scholar] [CrossRef] [PubMed]
- Abou-Alfa, G.K.; Meyer, T.; Cheng, A.L.; El-Khoueiry, A.B.; Rimassa, L.; Ryoo, B.Y.; Cicin, I.; Merle, P.; Chen, Y.; Park, J.W.; et al. Cabozantinib in Patients with Advanced and Progressing Hepatocellular Carcinoma. N. Engl. J. Med. 2018, 379, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Kudo, M.; Tsuchiya, K.; Kato, N.; Hagihara, A.; Numata, K.; Aikata, H.; Inaba, Y.; Kondo, S.; Motomura, K.; Furuse, J.; et al. Cabozantinib in Japanese patients with advanced hepatocellular carcinoma: A phase 2 multicenter study. J. Gastroenterol. 2021, 56, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Finkelmeier, F.; Scheiner, B.; Leyh, C.; Best, J.; Fründt, T.W.; Czauderna, C.; Beutel, A.; Bettinger, D.; Weiß, J.; Meischl, T.; et al. Cabozantinib in Advanced Hepatocellular Carcinoma: Efficacy and Safety Data from an International Multicenter Real-Life Cohort. Liver Cancer 2021, 10, 360–369. [Google Scholar] [CrossRef] [PubMed]
- Bang, Y.H.; Lee, C.K.; Yoo, C.; Chon, H.J.; Hong, M.; Kang, B.; Kim, H.D.; Park, S.R.; Choi, W.M.; Choi, J.; et al. Real-world efficacy and safety of cabozantinib in Korean patients with advanced hepatocellular carcinoma: A multicenter retrospective analysis. Ther. Adv. Med. Oncol. 2022, 14, 17588359221097934. [Google Scholar] [CrossRef]
- Tovoli, F.; Dadduzio, V.; De Lorenzo, S.; Rimassa, L.; Masi, G.; Iavarone, M.; Marra, F.; Garajova, I.; Brizzi, M.P.; Daniele, B.; et al. Real-Life Clinical Data of Cabozantinib for Unresectable Hepatocellular Carcinoma. Liver Cancer 2021, 10, 370–379. [Google Scholar] [CrossRef]
- Krens, S.D.; van Erp, N.P.; Groenland, S.L.; Moes, D.J.A.R.; Mulder, S.F.; Desar, I.M.E.; van der Hulle, T.; Steeghs, N.; van Herpen, C.M.L. Exposure-response analyses of cabozantinib in patients with metastatic renal cell cancer. BMC Cancer 2022, 22, 228. [Google Scholar] [CrossRef]
- Krens, S.D.; van Boxtel, W.; Uijen, M.J.M.; Jansman, F.G.A.; Desar, I.M.E.; Mulder, S.F.; van Herpen, C.M.L.; van Erp, N.P. Exposure-toxicity relationship of cabozantinib in patients with renal cell cancer and salivary gland cancer. Int. J. Cancer 2022, 150, 308–316. [Google Scholar] [CrossRef]
- Nguyen, L.; Chapel, S.; Tran, B.D.; Lacy, S. Cabozantinib exposure-response analyses of efficacy and safety in patients with advanced hepatocellular carcinoma. J. Pharmacokinet. Pharmacodyn. 2019, 46, 577–589. [Google Scholar] [CrossRef]
- Nguyen, L.; Chapel, S.; Tran, B.D.; Lacy, S. Updated Population Pharmacokinetic Model of Cabozantinib Integrating Various Cancer Types Including Hepatocellular Carcinoma. J. Clin. Pharmacol. 2019, 59, 1551–1561. [Google Scholar] [CrossRef] [PubMed]
- Kanzaki, G.; Puelles, V.G.; Cullen-McEwen, L.A.; Hoy, W.E.; Okabayashi, Y.; Tsuboi, N.; Shimizu, A.; Denton, K.M.; Hughson, M.D.; Yokoo, T.; et al. New insights on glomerular hyperfiltration: A Japanese autopsy study. JCI Insight. 2017, 2, e94334. [Google Scholar] [CrossRef]
- Lacy, S.A.; Miles, D.R.; Nguyen, L.T. Clinical Pharmacokinetics and Pharmacodynamics of Cabozantinib. Clin. Pharmacokinet. 2017, 56, 477–491. [Google Scholar] [CrossRef]
- Kudo, M.; Kawamura, Y.; Hasegawa, K.; Tateishi, R.; Kariyama, K.; Shiina, S.; Toyoda, H.; Imai, Y.; Hiraoka, A.; Ikeda, M.; et al. Management of Hepatocellular Carcinoma in Japan: JSH Consensus Statements and Recommendations 2021 Update. Liver Cancer 2021, 10, 181–223. [Google Scholar] [CrossRef]
- Okubo, H.; Ando, H.; Ishizuka, K.; Morishige, J.I.; Ikejima, K.; Shiina, S.; Nagahara, A. Impact of genetic polymorphisms on the pharmacokinetics and pharmacodynamics of lenvatinib in patients with hepatocellular carcinoma. J. Pharmacol. Sci. 2022, 148, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Bender, B.C.; Schindler, E.; Friberg, L.E. Population pharmacokinetic-pharmacodynamic modelling in oncology: A tool for predicting clinical response. Br. J. Clin. Pharmacol. 2015, 79, 56–71. [Google Scholar] [CrossRef]
- Hiraoka, A.; Kumada, T.; Tada, T.; Hirooka, M.; Kariyama, K.; Tani, J.; Atsukawa, M.; Takaguchi, K.; Itobayashi, E.; Fukunishi, S.; et al. Clinical predictor of urinary protein as adverse event associated with atezolizumab plus bevacizumab treatment for unresectable hepatocellular carcinoma. Oncology 2022, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Shimose, S.; Iwamoto, H.; Niizeki, T.; Shirono, T.; Noda, Y.; Kamachi, N.; Okamura, S.; Nakano, M.; Suga, H.; Kuromatsu, R.; et al. Clinical Significance of Adverse Events for Patients with Unresectable Hepatocellular Carcinoma Treated with Lenvatinib: A Multicenter Retrospective Study. Cancers 2020, 12, 1867. [Google Scholar] [CrossRef] [PubMed]
- Izzedine, H.; Massard, C.; Spano, J.P.; Goldwasser, F.; Khayat, D.; Soria, J.C. VEGF signalling inhibition-induced proteinuria: Mechanisms, significance and management. Eur. J. Cancer 2010, 46, 439–448. [Google Scholar] [CrossRef]
- Sorich, M.J.; Rowland, A.; Kichenadasse, G.; Woodman, R.J.; Mangoni, A.A. Risk factors of proteinuria in renal cell carcinoma patients treated with VEGF inhibitors: A secondary analysis of pooled clinical trial data. Br. J. Cancer 2016, 114, 1313–1317. [Google Scholar] [CrossRef]
- Sarcognato, S.; García-Lezana, T.; Villanueva, A. Mechanisms of action of drugs effective in hepatocellular carcinoma. Clin. Liver Dis. 2019, 14, 62–65. [Google Scholar] [CrossRef] [PubMed]
- Brancaccio, P.; Lippi, G.; Maffulli, N. Biochemical markers of muscular damage. Clin. Chem. Lab. Med. 2010, 48, 757–767. [Google Scholar] [CrossRef] [PubMed]
- Franceschino, A.; Tornaghi, L.; Benemacher, V.; Assouline, S.; Gambacorti-Passerini, C. Alterations in creatine kinase, phosphate and lipid values in patients with chronic myeloid leukemia during treatment with imatinib. Haematologica 2008, 93, 317–318. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Okubo, H.; Morishige, J.; Hasan, N.; Ando, H. Lenvatinib causes reduced expression of carnitine/organic cation transporter 2 and carnitine deficiency in the skeletal muscle of rats. Toxicol. Lett. 2022, 366, 17–25. [Google Scholar] [CrossRef]
- Glass, D.J. PI3 kinase regulation of skeletal muscle hypertrophy and atrophy. Curr. Top. Microbiol. Immunol. 2010, 346, 267–278. [Google Scholar] [CrossRef]

| □ | N = 14 |
|---|---|
| Age, years | 80 (69–91) |
| Sex, male/female | 13/1 |
| Etiology: HCV/NASH/ALC/PBC | 5/5/3/1 |
| Performance status ECOG: 0/1 | 12/2 |
| Body weight, kg | 58 (47–70) |
| Body surface area, m2 | 1.66 (1.45–1.77) |
| Body mass index, kg/m2 | 22.50 (17.26–26.56) |
| BCLC Stage: B/C | 6/8 |
| Treatment line: 2nd/3rd/5th | 6/7/1 |
| Past history of ATZ + BEV: Yes/No | 6/8 |
| Past history of lenvatinib: Yes/No | 13/1 |
| Child-Pugh score: 5/6/7/8 | 6/6/1/1 |
| mALBI grade: 1/2a/2b | 2/8/4 |
| Starting dose: 60/40/20/10 mg | 1/8/4/1 |
| Total bilirubin, mg/dL | 0.8 (0.1–2.3) |
| Albumin, g/dL | 3.6 (3.1–3.4) |
| Prothrombin activity, % | 80 (61–126) |
| eGFR, mL/dL/1.13 m2 | 54.33 (7.08–87.76) |
| Urinary protein-to-creatinine ratio #, g/gCr | 0.202 (0.06–1.98) |
| Creatine kinase, U/L | 80 (28–154) |
| Aldolase, U/L | 5.25 (3.1–6.5) |
| AFP, ng/mL | 22.5 (1–20,200) |
| DCP, mAU/mL | 9780 (20–78,100) |
| □ | Total (N = 14) |
| Median duration of drug exposure, days (range) | 70 (7–380) |
| Median 2-week relative dose intensity, % (range) | 33.3 (16.7–100) |
| Median 4-week relative dose intensity, % (range) | 30.6 (12.5–66.7) |
| Dose reduction rate, % | 100 |
| Median duration of dose reduction, days (range) * | 14 (7–28) |
| Dose interruption rate, % | 79 |
| Median duration of dose interruption, days (range) § | 7 (7–70) |
| □ | Total | Grade 1 | Grade 2 | Grade 3 | Any grade (%) | Grade 3 (%) |
|---|---|---|---|---|---|---|
| Proteinuria | 13 | 3 | 5 | 3 | 85 | 23 |
| Malaise | 14 | 3 | 5 | 2 | 71 | 14 |
| Anorexia | 14 | 4 | 2 | 0 | 42 | 0 |
| Hand and foot syndrome | 14 | 3 | 1 | 0 | 28 | 0 |
| Hypothyroidism | 14 | 2 | 6 | 0 | 57 | 0 |
| Diarrhea | 14 | 1 | 0 | 2 | 21 | 14 |
| Hypertension | 14 | 5 | 2 | 0 | 50 | 0 |
| Increased AST/ALT | 14 | 1 | 1 | 1 | 21 | 7 |
| Increased CK | 14 | 1 | 2 | 0 | 21 | 0 |
| Decreased PLT | 14 | 0 | 2 | 0 | 14 | 0 |
| Ctrough median | Mean ± SD | 755.6 ± 556.1 |
| median (range), ng/mL | 653.5 (68.2–2280.0) | |
| CV, % | 73.6 | |
| Dose-normalized Ctrough median | mean ± SD | 23.8 ±16.9 |
| median (range), ng/mL/mg | 20.6 (5.0–6.1) | |
| CV, % | 71.0 | |
| Maximum Ctrough | mean ± SD | 996.0 ± 611.4 |
| median (range), ng/mL | 929.0 (155.0–2640.0) | |
| CV, % | 61.4 | |
| Dose-normalized maximum Ctrough | mean ± SD | 31.6 ± 18.7 |
| median (range), ng/mL/mg | 25.8 (9.3–70.5) | |
| CV, % | 59.2 |
| Cutoff Value | AUROC (95% CI) | p Value | Sensitivity, % | Specificity, % | PPV, % | NPV, % | |
|---|---|---|---|---|---|---|---|
| Maximum changes in UPCR (g/gCr) | 0.0911 | 0.762 (0.463–1.000) | 0.116 | 100.0 | 66.7 | 50.0 | 60.0 |
| Maximum changes in creatine kinase (mg/dL) | 70.5 | 0.929 (0.776–1.000) | 0.010 | 100.0 | 83.3 | 100.0 | 87.5 |
| Maximum changes in aldolase (mg/dL) | 6.1 | 0.833 (0.600–1.000) | 0.046 | 66.7 | 82.1 | 77.8 | 100.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okubo, H.; Ando, H.; Takasaki, Y.; Nakadera, E.; Fukuo, Y.; Shiina, S.; Ikejima, K. Impact of Cabozantinib Exposure on Proteinuria and Muscle Toxicity in Patients with Unresectable Hepatocellular Carcinoma. Pharmaceuticals 2022, 15, 1460. https://doi.org/10.3390/ph15121460
Okubo H, Ando H, Takasaki Y, Nakadera E, Fukuo Y, Shiina S, Ikejima K. Impact of Cabozantinib Exposure on Proteinuria and Muscle Toxicity in Patients with Unresectable Hepatocellular Carcinoma. Pharmaceuticals. 2022; 15(12):1460. https://doi.org/10.3390/ph15121460
Chicago/Turabian StyleOkubo, Hironao, Hitoshi Ando, Yusuke Takasaki, Eisuke Nakadera, Yuka Fukuo, Shuichiro Shiina, and Kenichi Ikejima. 2022. "Impact of Cabozantinib Exposure on Proteinuria and Muscle Toxicity in Patients with Unresectable Hepatocellular Carcinoma" Pharmaceuticals 15, no. 12: 1460. https://doi.org/10.3390/ph15121460
APA StyleOkubo, H., Ando, H., Takasaki, Y., Nakadera, E., Fukuo, Y., Shiina, S., & Ikejima, K. (2022). Impact of Cabozantinib Exposure on Proteinuria and Muscle Toxicity in Patients with Unresectable Hepatocellular Carcinoma. Pharmaceuticals, 15(12), 1460. https://doi.org/10.3390/ph15121460









