2-Butoxytetrahydrofuran and Palmitic Acid from Holothuria scabra Enhance C. elegans Lifespan and Healthspan via DAF-16/FOXO and SKN-1/NRF2 Signaling Pathways
Abstract
1. Introduction
2. Results
2.1. Effects of Purified of H. scabra Extracts on the Lifespan of C. elegans
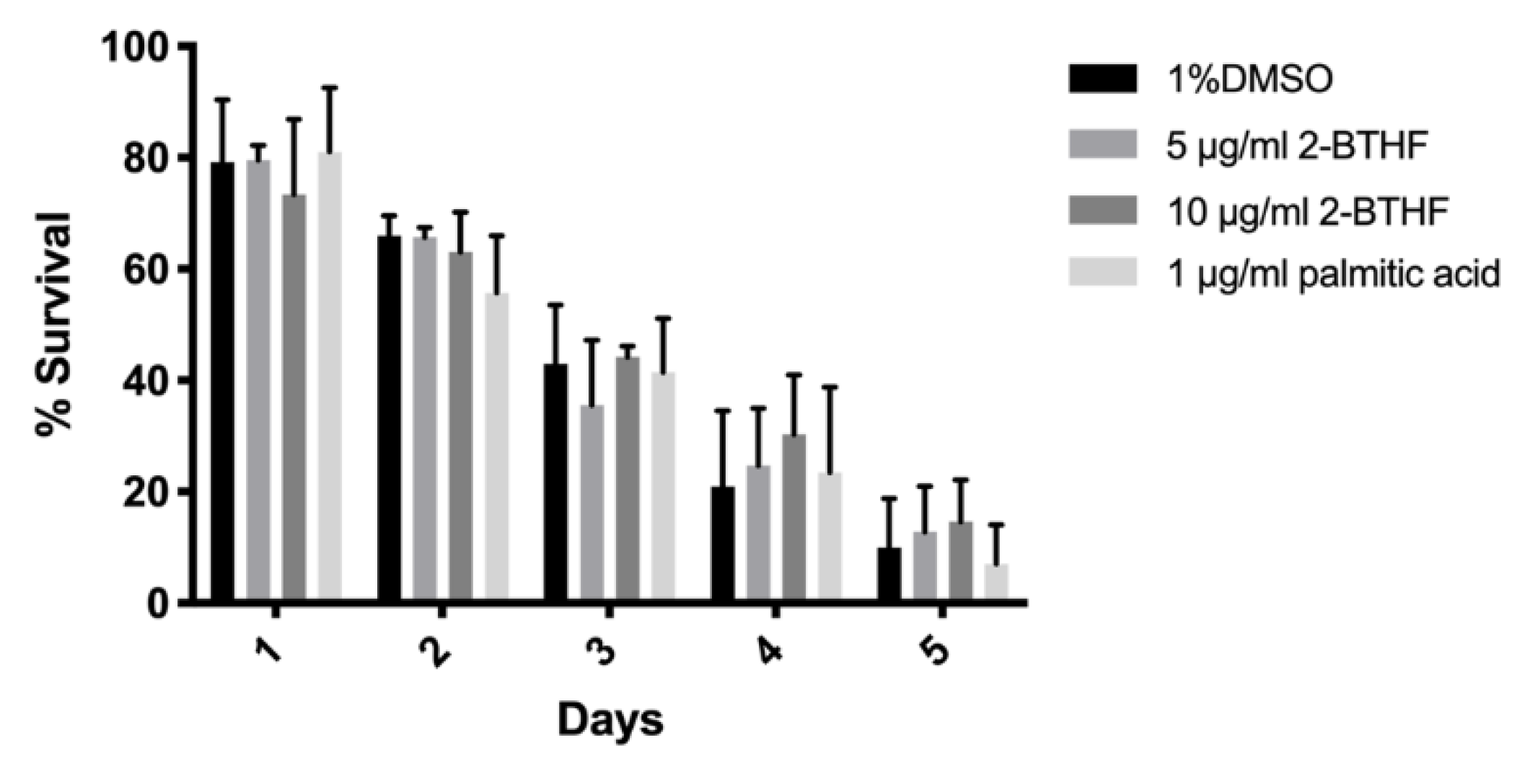
2.2. Effects of Purified H. scabra Compounds on C. elegans’ Resistance to Thermal Stress
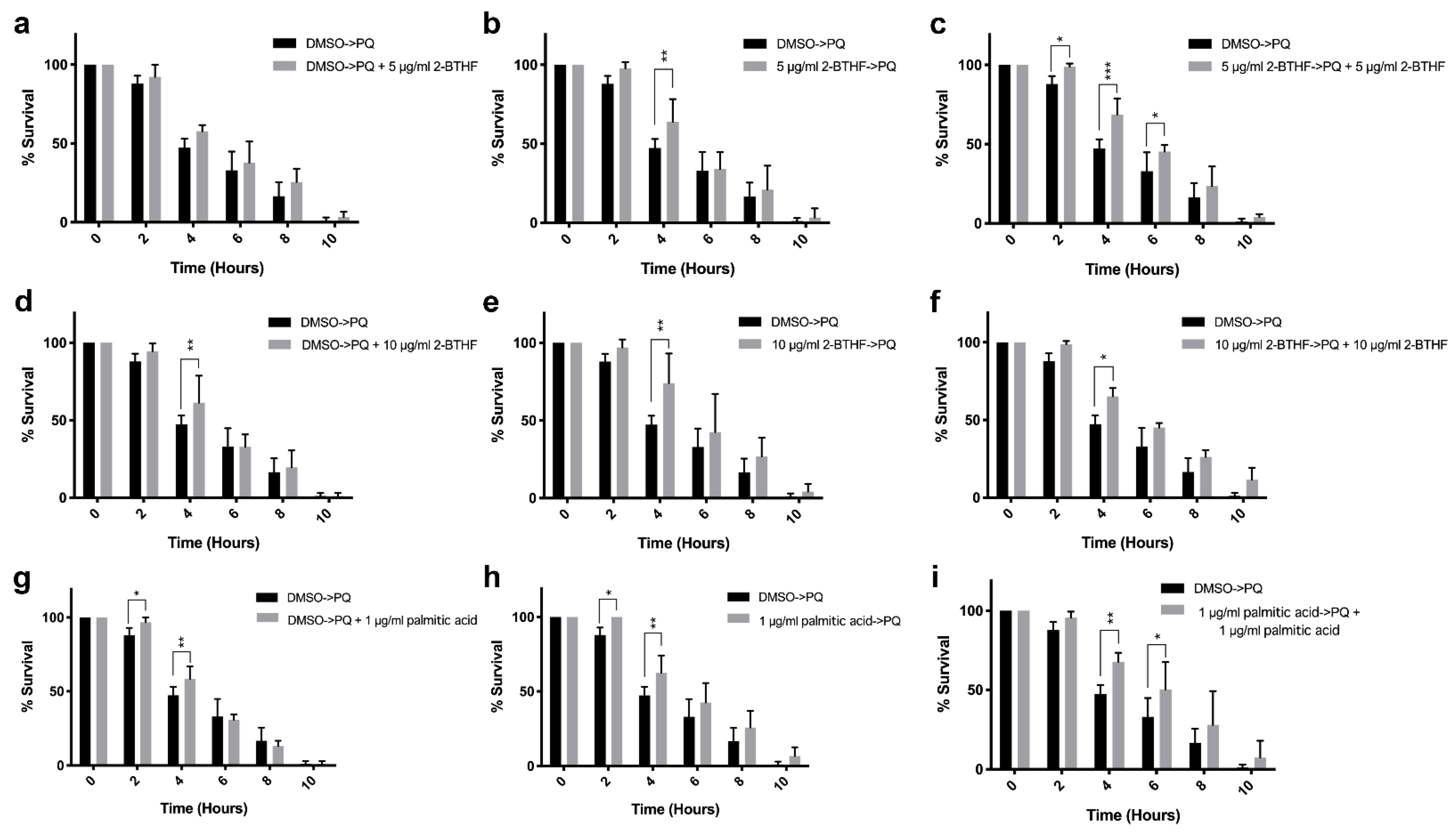
2.3. Effects of Purified H. scabra Compounds on C. elegans’ Resistance to Oxidative Stress
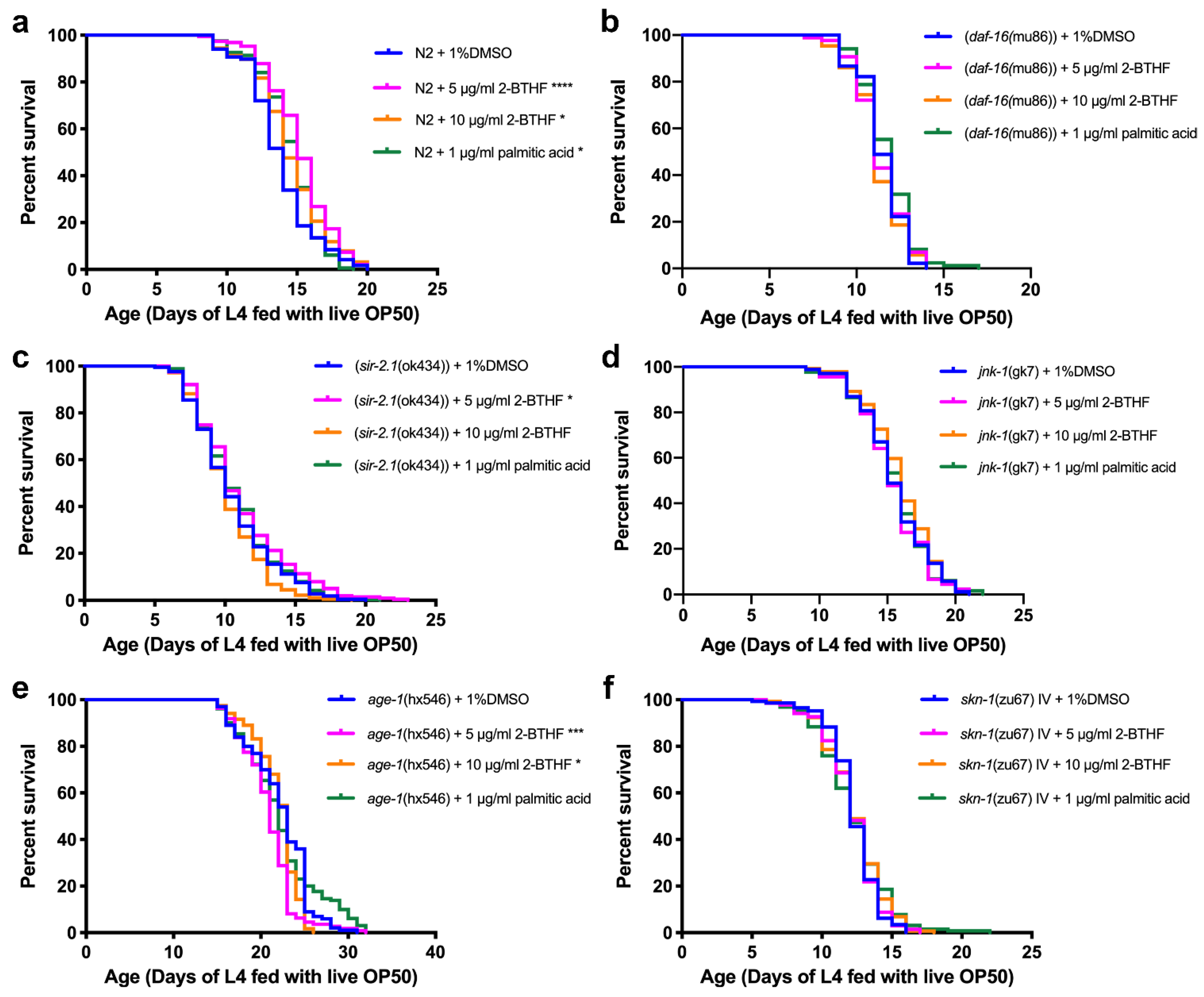
2.4. Effects of Purified H. scabra Compounds on the Pathways That Control Lifespans of C. elegans
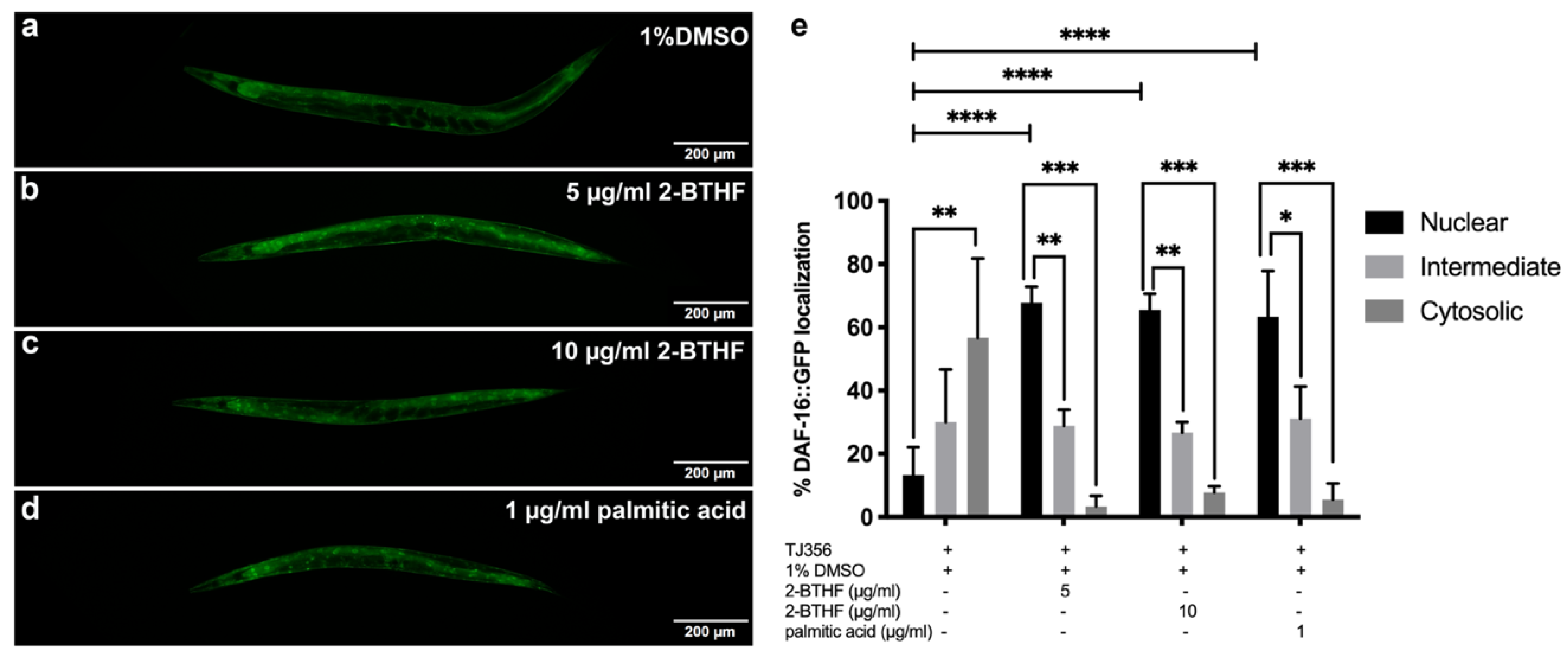
2.5. DAF-16 Translocation
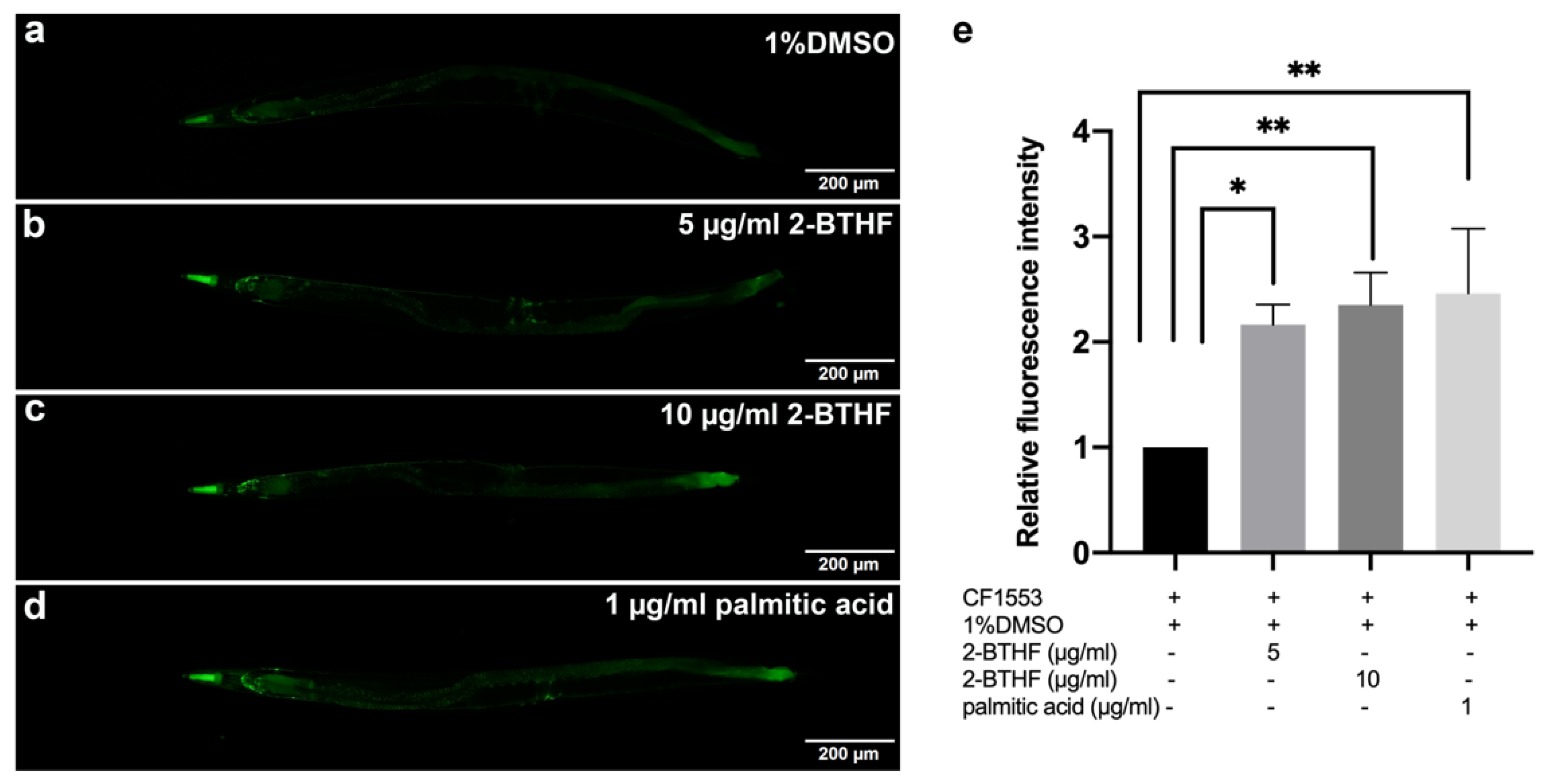
2.6. SOD-3 Expression
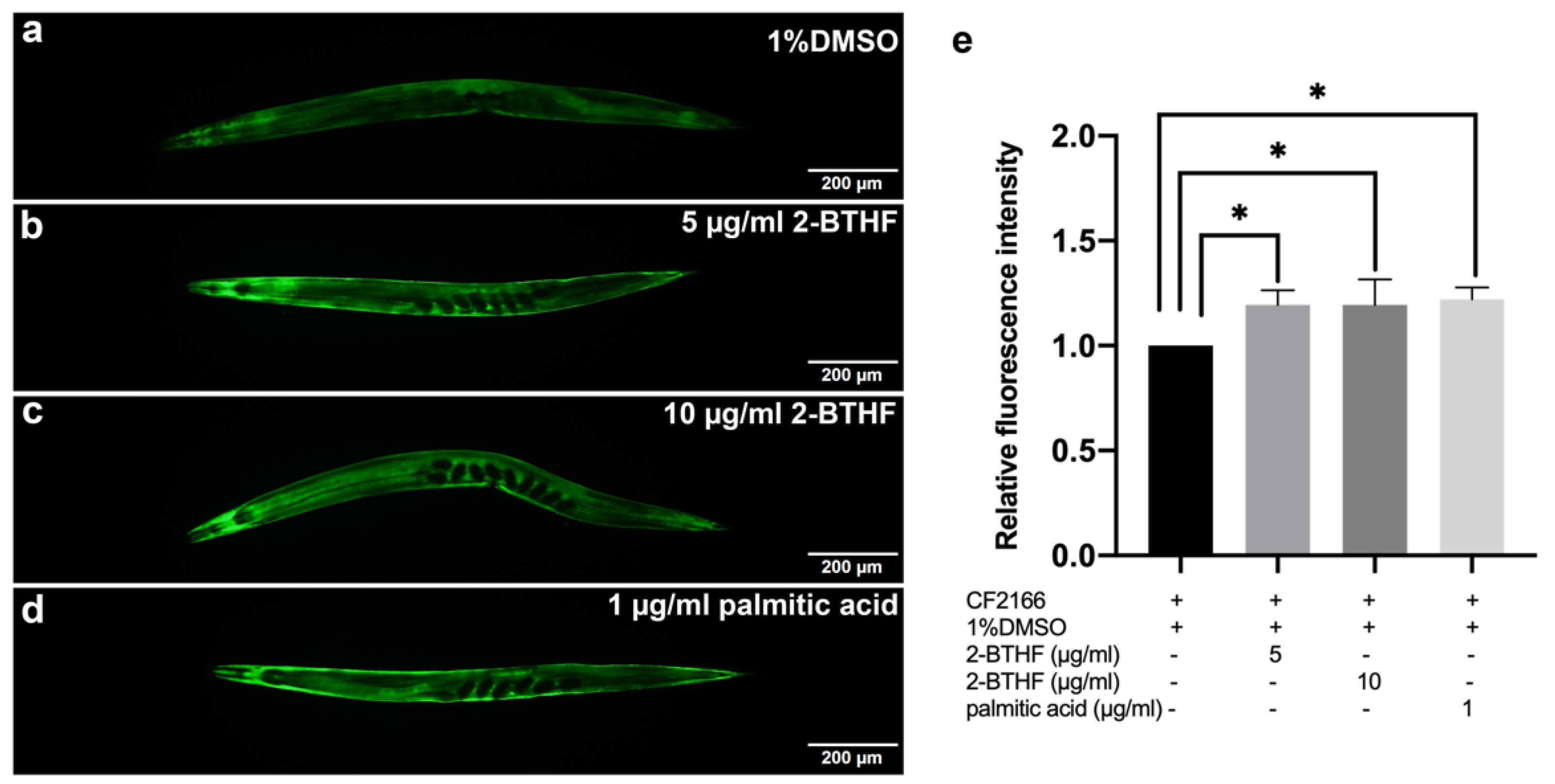
2.7. HSP-16.2 Expression
2.8. GST-4 Expression
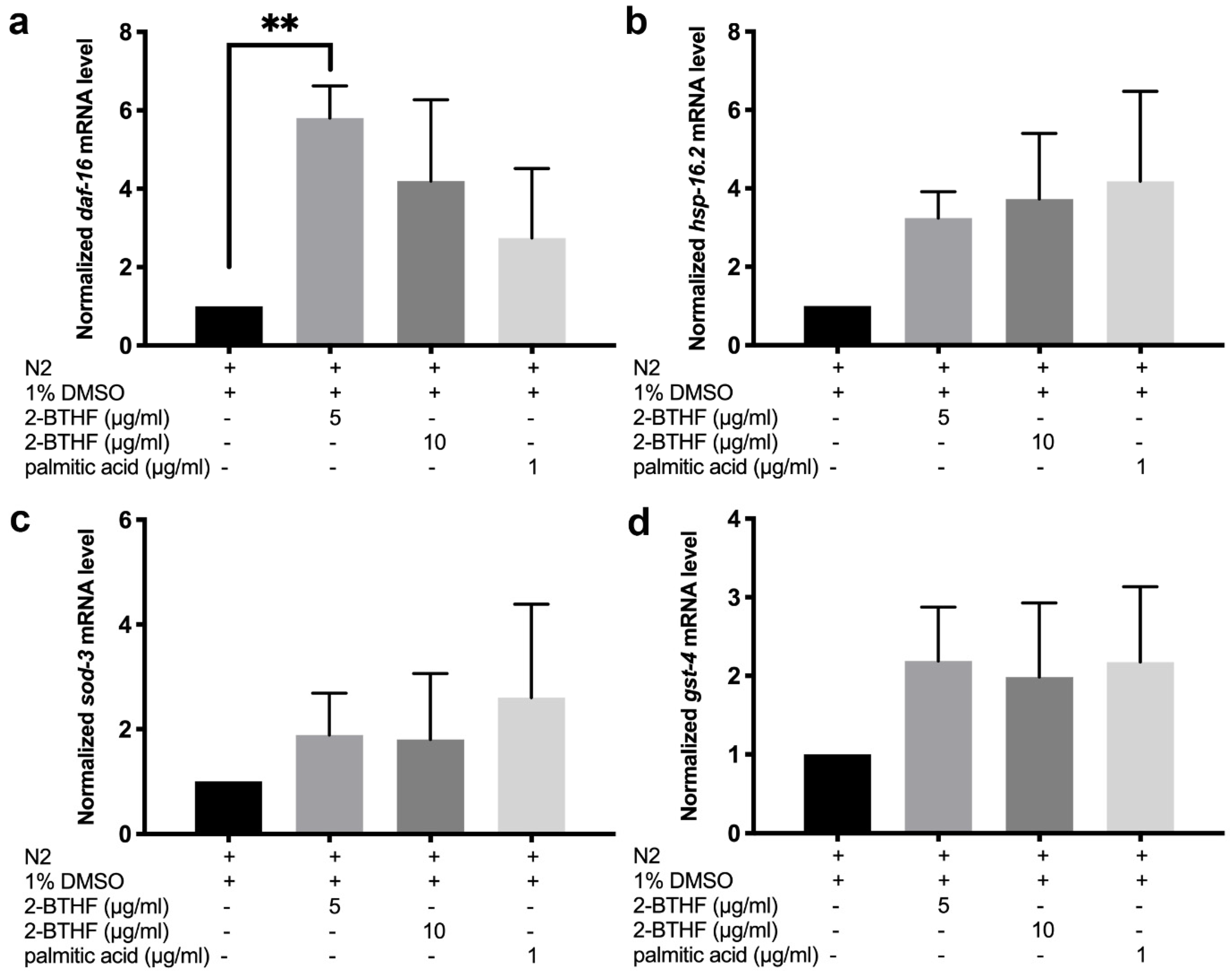
2.9. Expression of mRNA Transcripts
3. Discussion
4. Materials and Methods
4.1. H. scabra Extraction and Purification
4.2. C. elegans Strains and Growth Conditions
4.3. Lifespan Assays
4.4. Stress Resistance Assays
4.5. Lifespan Assays of Mutant Worms
4.6. DAF-16 Translocation and SOD-3, HSP-16.2, and GST-4 Expression in Transgenic Strains of C. elegans
4.7. Gene Expression Analysis by Quantitative RT-PCR
4.8. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Balcombe, N.R.; Sinclair, A. Ageing: Definitions, mechanisms and the magnitude of the problem. Best Pract. Res. Clin. Gastroenterol. 2001, 15, 835–849. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Dan, X.; Babbar, M.; Wei, Y.; Hasselbalch, S.G.; Croteau, D.L.; Bohr, V.A. Ageing as a risk factor for neurodegenerative disease. Nat. Rev. Neurol. 2019, 15, 565–581. [Google Scholar] [CrossRef] [PubMed]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The Hallmarks of Aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef] [PubMed]
- Poljšak, B.; Dahmane, R. Free Radicals and Extrinsic Skin Aging. Dermatol. Res. Pract. 2012, 2012, 135206. [Google Scholar] [CrossRef] [PubMed]
- Raimondi, V.; Ciccarese, F.; Ciminale, V. Oncogenic pathways and the electron transport chain: A dangeROS liaison. Br. J. Cancer 2020, 122, 168–181. [Google Scholar] [CrossRef]
- Pangestuti, R.; Arifin, Z. Medicinal and health benefit effects of functional sea cucumbers. J. Tradit. Complement. Med. 2018, 8, 341–351. [Google Scholar] [CrossRef]
- Bordbar, S.; Anwar, F.; Saari, N. High-Value Components and Bioactives from Sea Cucumbers for Functional Foods—A Review. Mar. Drugs 2011, 9, 1761–1805. [Google Scholar] [CrossRef]
- Jattujan, P.; Chalorak, P.; Siangcham, T.; Sangpairoj, K.; Nobsathian, S.; Poomtong, T.; Sobhon, P.; Meemon, K. Holothuria scabra extracts possess anti-oxidant activity and promote stress resistance and lifespan extension in Caenorhabditis elegans. Exp. Gerontol. 2018, 110, 158–171. [Google Scholar] [CrossRef]
- Kitisin, T.; Suphamungmee, W.; Meemon, K. Saponin-rich extracts from Holothuria leucospilota mediate lifespan extension and stress resistance in Caenorhabditis elegans via daf-16. J. Food Biochem. 2019, 43, e13075. [Google Scholar] [CrossRef]
- Chumphoochai, K.; Chalorak, P.; Suphamungmee, W.; Sobhon, P.; Meemon, K. Saponin-enriched extracts from body wall and Cuvierian tubule of Holothuria leucospilota reduce fat accumulation and suppress lipogenesis in Caenorhabditis elegans. J. Sci. Food Agric. 2019, 99, 4158–4166. [Google Scholar] [CrossRef]
- Pranweerapaiboon, K.; Apisawetakan, S.; Nobsathian, S.; Itharat, A.; Sobhon, P.; Chaithirayanon, K. An ethyl-acetate fraction of Holothuria scabra modulates inflammation in vitro through inhibiting the production of nitric oxide and pro-inflammatory cytokines via NF-kappaB and JNK pathways. Inflammopharmacology 2020, 28, 1027–1037. [Google Scholar] [CrossRef] [PubMed]
- Sangpairoj, K.; Chaithirayanon, K.; Vivithanaporn, P.; Siangcham, T.; Jattujan, P.; Poomtong, T.; Nobsathian, S.; Sobhon, P. Extract of the sea cucumber, Holothuria scabra, induces apoptosis in human glioblastoma cell lines. Funct. Foods Health Dis. 2016, 6, 452–468. [Google Scholar] [CrossRef]
- Pranweerapaiboon, K.; Noonong, K.; Apisawetakan, S.; Sobhon, P.; Chaithirayanon, K. Methanolic Extract from Sea Cucumber, Holothuria scabra, Induces Apoptosis and Suppresses Metastasis of PC3 Prostate Cancer Cells Modulated by MAPK Signaling Pathway. J. Microbiol. Biotechnol. 2021, 31, 775–783. [Google Scholar] [CrossRef] [PubMed]
- Yurasakpong, L.; Apisawetakan, S.; Pranweerapaiboon, K.; Sobhon, P.; Chaithirayanon, K. Holothuria scabra Extract Induces Cell Apoptosis and Suppresses Warburg Effect by Down-Regulating Akt/mTOR/HIF-1 Axis in MDA-MB-231 Breast Cancer Cells. Nutr. Cancer 2021, 73, 1964–1975. [Google Scholar] [CrossRef] [PubMed]
- Chalorak, P.; Jattujan, P.; Nobsathian, S.; Poomtong, T.; Sobhon, P.; Meemon, K. Holothuria scabra extracts exhibit anti-Parkinson potential in C. elegans: A model for anti-Parkinson testing. Nutr. Neurosci. 2018, 21, 427–438. [Google Scholar] [CrossRef]
- Chalorak, P.; Sornkaew, N.; Manohong, P.; Niamnont, N.; Malaiwong, N.; Limboonreung, T.; Sobhon, P.; Aschner, M.; Meemon, K. Diterpene glycosides from Holothuria scabra exert the α-synuclein degradation and neuroprotection against α-synuclein-Mediated neurodegeneration in C. elegans model. J. Ethnopharmacol. 2021, 279, 114347. [Google Scholar] [CrossRef]
- Tangrodchanapong, T.; Sornkaew, N.; Yurasakpong, L.; Niamnont, N.; Nantasenamat, C.; Sobhon, P.; Meemon, K. Beneficial Effects of Cyclic Ether 2-Butoxytetrahydrofuran from Sea Cucumber Holothuria scabra against Abeta Aggregate Toxicity in Transgenic Caenorhabditis elegans and Potential Chemical Interaction. Molecules 2021, 26, 2195. [Google Scholar] [CrossRef]
- Zhang, S.; Li, F.; Zhou, T.; Wang, G.; Li, Z. Caenorhabditis elegans as a Useful Model for Studying Aging Mutations. Front. Endocrinol. 2020, 11, 554994. [Google Scholar] [CrossRef]
- Tissenbaum, H.A. Using C. elegans for aging research. Invertebr. Reprod. Dev. 2015, 59, 59–63. [Google Scholar] [CrossRef]
- Fisher, A.L.; Lithgow, G.J. The nuclear hormone receptor DAF-12 has opposing effects on Caenorhabditis elegans lifespan and regulates genes repressed in multiple long-lived worms. Aging Cell 2006, 5, 127–138. [Google Scholar] [CrossRef]
- Lee, J.; Kwon, G.; Lim, Y.-H. Elucidating the Mechanism of Weissella-dependent Lifespan Extension in Caenorhabditis elegans. Sci. Rep. 2015, 5, 17128. [Google Scholar] [CrossRef] [PubMed]
- Rangsinth, P.; Prasansuklab, A.; Duangjan, C.; Gu, X.; Meemon, K.; Wink, M.; Tencomnao, T. Leaf extract of Caesalpinia mimosoides enhances oxidative stress resistance and prolongs lifespan in Caenorhabditis elegans. BMC Complement. Altern. Med. 2019, 19, 164. [Google Scholar] [CrossRef] [PubMed]
- Guha, S.; Cao, M.; Kane, R.M.; Savino, A.M.; Zou, S.; Dong, Y. The longevity effect of cranberry extract in Caenorhabditis elegans is modulated by daf-16 and osr-1. Age 2013, 35, 1559–1574. [Google Scholar] [CrossRef]
- Oh, S.W.; Mukhopadhyay, A.; Svrzikapa, N.; Jiang, F.; Davis, R.J.; Tissenbaum, H.A. JNK regulates lifespan in Caenorhabditis elegans by modulating nuclear translocation of forkhead transcription factor/DAF-16. Proc. Natl. Acad. Sci. USA 2005, 102, 4494–4499. [Google Scholar] [CrossRef]
- Sun, X.; Chen, W.-D.; Wang, Y.-D. DAF-16/FOXO Transcription Factor in Aging and Longevity. Front. Pharmacol. 2017, 8, 548. [Google Scholar] [CrossRef]
- Uno, M.; Nishida, E. Lifespan-regulating genes in C. elegans. NPJ Aging Mech. Dis. 2016, 2, 16010. [Google Scholar] [CrossRef] [PubMed]
- Berdichevsky, A.; Guarente, L. A Stress Response Pathway Involving Sirtuins, Forkhead and 14-3-3 Proteins. Cell Cycle 2006, 5, 2588–2591. [Google Scholar] [CrossRef]
- Okoro, N.O.; Odiba, A.S.; Osadebe, P.O.; Omeje, E.O.; Liao, G.; Fang, W.; Jin, C.; Wang, B. Bioactive Phytochemicals with Anti-Aging and Lifespan Extending Potentials in Caenorhabditis elegans. Molecules 2021, 26, 7323. [Google Scholar] [CrossRef]
- Zhao, X.; Lu, L.; Qi, Y.; Li, M.; Zhou, L. Emodin extends lifespan of Caenorhabditis elegans through insulin/IGF-1 signaling pathway depending on DAF-16 and SIR-2.1. Biosci. Biotechnol. Biochem. 2017, 81, 1908–1916. [Google Scholar] [CrossRef]
- Wang, B.-Y.; Xu, X.-S.; Cui, Y.-X.; Wang, H.; Liu, G.; Zhao, Z.J.; Ma, J.-F.; Fu, X.-Q. Caenorhabditis elegans eyes absent ortholog EYA-1 Is required for stress resistance. Biochem. Moscow 2014, 79, 653–662. [Google Scholar] [CrossRef]
- Detienne, G.; Van de Walle, P.; De Haes, W.; Schoofs, L.; Temmerman, L. SKN-1-independent transcriptional activation of glutathione S-transferase 4 (GST-4) by EGF signaling. Worm 2016, 5, e1230585. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ullah, F.; Khan, T.A.; Iltaf, J.; Anwar, S.; Khan, M.F.A.; Khan, M.R.; Ullah, S.; Fayyaz ur Rehman, M.; Mustaqeem, M.; Kotwica-Mojzych, K.; et al. Heterocyclic Crown Ethers with Potential Biological and Pharmacological Properties: From Synthesis to Applications. Appl. Sci. 2022, 12, 1102. [Google Scholar] [CrossRef]
- Lipina, C.; Hundal, H.S. Modulation of cellular redox homeostasis by the endocannabinoid system. Open Biol. 2016, 6, 150276. [Google Scholar] [CrossRef] [PubMed]
- Milton, N.G. Anandamide and noladin ether prevent neurotoxicity of the human amyloid-β peptide. Neurosci. Lett. 2002, 332, 127–130. [Google Scholar] [CrossRef]
- Dong, H.; Liu, M.; Wang, L.; Liu, Y.; Lu, X.; Stagos, D.; Lin, X.; Liu, M. Bromophenol Bis (2,3,6-Tribromo-4,5-dihydroxybenzyl) Ether Protects HaCaT Skin Cells from Oxidative Damage via Nrf2-Mediated Pathways. Antioxidants 2021, 10, 1436. [Google Scholar] [CrossRef] [PubMed]
- Haider, K.; Haider, M.R.; Neha, K.; Yar, M.S. Free radical scavengers: An overview on heterocyclic advances and medicinal prospects. Eur. J. Med. Chem. 2020, 204, 112607. [Google Scholar] [CrossRef]
- Nishanthan, G.; Kumara, P.; de Croos, M.; Prasada, D.V.P.; Dissanayake, D.C.T. Effects of processing on proximate and fatty acid compositions of six commercial sea cucumber species of Sri Lanka. J. Food Sci. Technol. 2018, 55, 1933–1941. [Google Scholar] [CrossRef]
- Amine, H.; Benomar, Y.; Taouis, M. Palmitic acid promotes resistin-induced insulin resistance and inflammation in SH-SY5Y human neuroblastoma. Sci. Rep. 2021, 11, 5427. [Google Scholar] [CrossRef]
- Li, J.; Mao, Y.-S.; Chen, F.; Xia, D.-X.; Zhao, T.-Q. Palmitic acid up regulates Gal-3 and induces insulin resistance in macrophages by mediating the balance between KLF4 and NF-kappaB. Exp. Ther. Med. 2021, 22, 1028. [Google Scholar] [CrossRef]
- Mancini, A.; Imperlini, E.; Nigro, E.; Montagnese, C.; Daniele, A.; Orrù, S.; Buono, P. Biological and Nutritional Properties of Palm Oil and Palmitic Acid: Effects on Health. Molecules 2015, 20, 17339–17361. [Google Scholar] [CrossRef]
- Driver, C.J.; Cosopodiotis, G. The effect of dietary fat on longevity of Drosophila melanogaster. Exp. Gerontol. 1979, 14, 95–100. [Google Scholar] [CrossRef]
- Ueda, Y.; Wang, M.-F.; Irei, A.V.; Sarukura, N.; Sakai, T.; Hsu, T.-F. Effect of dietary lipids on longevity and memory in the SAMP8 mice. J. Nutr. Sci. Vitaminol. 2011, 57, 36–41. [Google Scholar] [CrossRef]
- Hidajati, N.; Tukiran, T.; Setiabudi, D.A.; Wardana, A.P. Antioxidant Activity of Palmitic Acid And Pinostrobin From Methanol Extract Of Syzygium litoralle (Myrtaceae). In Proceedings of the International Conference on Science and Technology (ICST 2018), Bali, Indonesia, 18–19 October 2018; Atlantis Press: Berlin/Heidelberg, Germany, 2018; pp. 183–187. [Google Scholar]
- Kumar, P.K.P.; Kumaravel, S.; Lalitha, C. Screening of antioxidant activity, total phenolics and GC-MS study of Vitex negundo. Afr. J. Biochem. Res. 2010, 4, 191–195. [Google Scholar]
- Carta, G.; Murru, E.; Banni, S.; Manca, C. Palmitic Acid: Physiological Role, Metabolism and Nutritional Implications. Front. Physiol. 2017, 8, 902. [Google Scholar] [CrossRef] [PubMed]
- Liao, V.H.; Yu, C.-W.; Chu, Y.-J.; Li, W.-H.; Hsieh, Y.-C.; Wang, T.-T. Curcumin-mediated lifespan extension in Caenorhabditis elegans. Mech. Ageing Dev. 2011, 132, 480–487. [Google Scholar] [CrossRef]
- Schafer, J.C.; Winkelbauer, M.E.; Williams, C.L.; Haycraft, C.J.; Desmond, R.A.; Yoder, B.K. IFTA-2 is a conserved cilia protein involved in pathways regulating longevity and dauer formation in Caenorhabditis elegans. J. Cell Sci. 2006, 119, 4088–4100. [Google Scholar] [CrossRef]
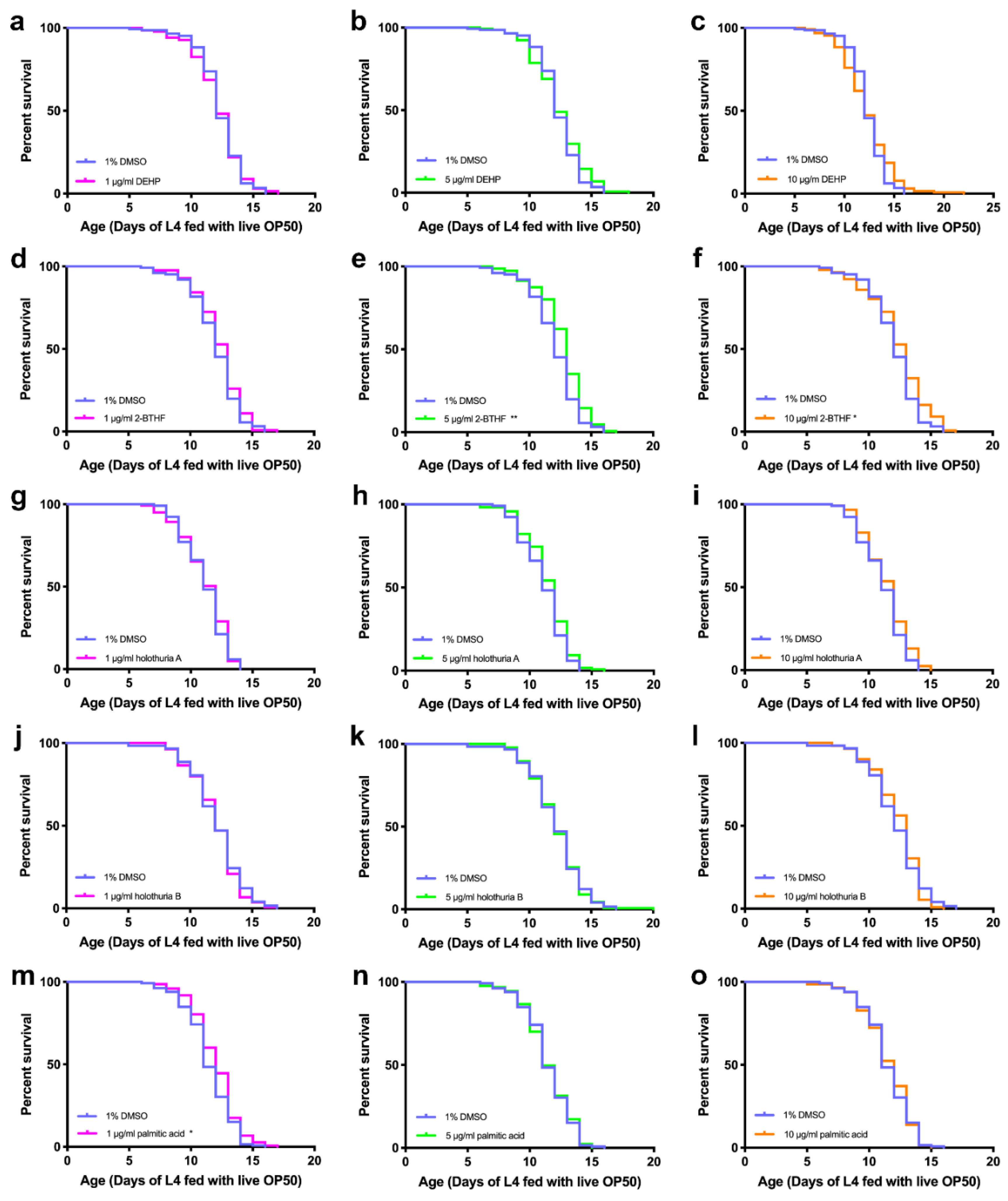

| Compounds | Lifespan (Mean ± SD) | (Number of Worms, Censored) | % Increase Lifespan | p Value (Log-Rank Test) |
|---|---|---|---|---|
| 1% DMSO | 12.28 ± 1.76 | (145, 8) | - | - |
| 1 μg/mL DEHP | 12.18 ± 1.97 | (137, 2) | −0.81 | 0.9737 |
| 5 μg/mL DEHP | 12.36 ± 2.10 | (145, 4) | 0.65 | 0.2603 |
| 10 μg/mL DEHP | 12.29 ± 2.49 | (129, 2) | 0.08 | 0.2695 |
| 1% DMSO | 12.04 ± 1.91 | (126, 2) | - | - |
| 1 μg/mL 2-BTHF | 12.35 ± 1.86 | (127, 9) | 2.57 | 0.1694 |
| 5 μg/mL 2-BTHF | 12.72 ± 1.90 | (151, 2) | 5.65 | 0.0012 ** |
| 10 μg/mL 2-BTHF | 12.37 ± 2.36 | (142, 3) | 2.74 | 0.0192 * |
| 1% DMSO | 11.10 ± 1.70 | (118, 2) | - | - |
| 1 μg/mL holothuria A | 11.13 ± 1.88 | (121, 2) | 0.27 | 0.5654 |
| 5 μg/mL holothuria A | 11.45 ± 1.79 | (118, 1) | 3.15 | 0.0895 |
| 10 μg/mL holothuria A | 11.44 ± 1.77 | (123, 3) | 3.06 | 0.0703 |
| 1% DMSO | 12.12 ± 2.14 | (123, 0) | - | - |
| 1 μg/mL holothuria B | 12.07 ± 1.91 | (134, 0) | −0.41 | 0.5685 |
| 5 μg/mL holothuria B | 12.17 ± 2.00 | (134, 2) | 0.41 | 0.8980 |
| 10 μg/mL holothuria B | 12.30 ± 1.85 | (112, 1) | 1.49 | 0.8498 |
| 1% DMSO | 11.45 ± 1.87 | (132, 4) | - | - |
| 1 μg/mL palmitic acid | 11.99 ± 1.86 | (148, 2) | 4.72 | 0.0207 * |
| 5 μg/mL palmitic acid | 11.46 ± 1.92 | (127, 0) | 0.09 | 0.8550 |
| 10 μg/mL palmitic acid | 11.48 ± 1.96 | (145, 0) | 0.26 | 0.8037 |
| Strains of Worm | Compounds | Lifespan (Mean ± SD) | (Number of Worms, Censored) | % Increase Lifespan | p Value (Log-Rank Test) |
|---|---|---|---|---|---|
| N2 | 1% DMSO | 13.79 ± 2.40 | (118, 0) | - | - |
| 5 μg/mL 2-BTHF | 15.20 ± 2.34 | (190, 0) | 10.22 | <0.0001 **** | |
| 10 μg/mL 2-BTHF | 14.50 ± 2.60 | (126, 0) | 5.15 | 0.0174 * | |
| 1 μg/mL palmitic acid | 14.49 ± 2.11 | (163, 0) | 5.08 | 0.0422 * | |
| CF1038 (daf-16(mu86)) | 1% DMSO | 11.42 ± 1.30 | (90, 0) | - | - |
| 5 μg/mL 2-BTHF | 11.33 ± 1.47 | (86, 0) | −0.79 | 0.9129 | |
| 10 μg/mL 2-BTHF | 11.16 ± 1.49 | (86, 0) | −2.28 | 0.3820 | |
| 1 μg/mL palmitic acid | 11.73 ± 1.48 | (85, 0) | 2.71 | 0.1169 | |
| VC199 (sir-2.1(ok434)) | 1% DMSO | 10.51 ± 2.86 | (215, 0) | - | - |
| 5 μg/mL 2-BTHF | 11.08 ± 3.26 | (203, 0) | 5.42 | 0.0416 * | |
| 10 μg/mL 2-BTHF | 10.13 ± 2.36 | (178, 0) | −3.62 | 0.0799 | |
| 1 μg/mL palmitic acid | 10.81 ± 2.86 | (266, 1) | 2.85 | 0.2841 | |
| VC8 (jnk-1(gk7)) | 1% DMSO | 15.51 ± 2.46 | (176, 2) | - | - |
| 5 μg/mL 2-BTHF | 15.32 ± 2.44 | (92, 0) | −1.23 | 0.5789 | |
| 10 μg/mL 2-BTHF | 15.91 ± 2.43 | (139, 1) | 2.58 | 0.1965 | |
| 1 μg/mL palmitic acid | 15.52 ± 2.49 | (133, 0) | 0.06 | 0.8594 | |
| TJ1052 (age-1(hx546)) | 1% DMSO | 22.16 ± 3.60 | (100, 0) | - | - |
| 5 μg/mL 2-BTHF | 20.87 ± 3.06 | (111, 0) | −5.82 | 0.0002 *** | |
| 10 μg/mL 2-BTHF | 21.96 ± 2.57 | (119, 0) | −0.90 | 0.0136 * | |
| 1 μg/mL palmitic acid | 22.29 ± 4.39 | (130, 0) | 0.59 | 0.6533 | |
| EU1 (skn-1(zu67) IV) | 1% DMSO | 12.28 ± 1.76 | (145, 0) | - | - |
| 5 μg/mL 2-BTHF | 12.18 ± 1.97 | (137, 0) | −0.81 | 0.9737 | |
| 10 μg/mL 2-BTHF | 12.36 ± 2.10 | (145, 0) | 0.65 | 0.2603 | |
| 1 μg/mL palmitic acid | 12.29 ± 2.49 | (129, 0) | 0.08 | 0.2695 |
| Genes | Forward Primers | Reverse Primers |
|---|---|---|
| daf-16 | 5′-CCAGACGGAAGGCTTAAACT-3′ | 5′-ATTCGCATGAAACGAGAATG-3′ |
| hsp-16.2 | 5′-GTCACTTTACCACTATTTCCGT-3′ | 5′-CAATCTCAGAAGACTCAGATGG-3′ |
| sod-3 | 5′-CCAACCAGCGCTGAAATTCAATGG-3′ | 5′-GGAACCGAAGTCGCGCTTAATAGT-3′ |
| gst-4 | 5′- CCCATTTTACAAGTCGATGG-3′ | 5′-CTTCCTCTGCAGTTTTTCCA-3′ |
| act-1 | 5′-ATCGTCACCACCAGCTTTCT-3′ | 5′-CACACCCGCAAATGAGTGAA-3′ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jattujan, P.; Srisirirung, S.; Watcharaporn, W.; Chumphoochai, K.; Kraokaew, P.; Sanguanphun, T.; Prasertsuksri, P.; Thongdechsri, S.; Sobhon, P.; Meemon, K. 2-Butoxytetrahydrofuran and Palmitic Acid from Holothuria scabra Enhance C. elegans Lifespan and Healthspan via DAF-16/FOXO and SKN-1/NRF2 Signaling Pathways. Pharmaceuticals 2022, 15, 1374. https://doi.org/10.3390/ph15111374
Jattujan P, Srisirirung S, Watcharaporn W, Chumphoochai K, Kraokaew P, Sanguanphun T, Prasertsuksri P, Thongdechsri S, Sobhon P, Meemon K. 2-Butoxytetrahydrofuran and Palmitic Acid from Holothuria scabra Enhance C. elegans Lifespan and Healthspan via DAF-16/FOXO and SKN-1/NRF2 Signaling Pathways. Pharmaceuticals. 2022; 15(11):1374. https://doi.org/10.3390/ph15111374
Chicago/Turabian StyleJattujan, Prapaporn, Sirin Srisirirung, Warisra Watcharaporn, Kawita Chumphoochai, Pichnaree Kraokaew, Tanatcha Sanguanphun, Prachayaporn Prasertsuksri, Salinthip Thongdechsri, Prasert Sobhon, and Krai Meemon. 2022. "2-Butoxytetrahydrofuran and Palmitic Acid from Holothuria scabra Enhance C. elegans Lifespan and Healthspan via DAF-16/FOXO and SKN-1/NRF2 Signaling Pathways" Pharmaceuticals 15, no. 11: 1374. https://doi.org/10.3390/ph15111374
APA StyleJattujan, P., Srisirirung, S., Watcharaporn, W., Chumphoochai, K., Kraokaew, P., Sanguanphun, T., Prasertsuksri, P., Thongdechsri, S., Sobhon, P., & Meemon, K. (2022). 2-Butoxytetrahydrofuran and Palmitic Acid from Holothuria scabra Enhance C. elegans Lifespan and Healthspan via DAF-16/FOXO and SKN-1/NRF2 Signaling Pathways. Pharmaceuticals, 15(11), 1374. https://doi.org/10.3390/ph15111374







