“K-Powder” Exposure during Adolescence Elicits Psychiatric Disturbances Associated with Oxidative Stress in Female Rats
Abstract
1. Introduction
2. Materials and Methods
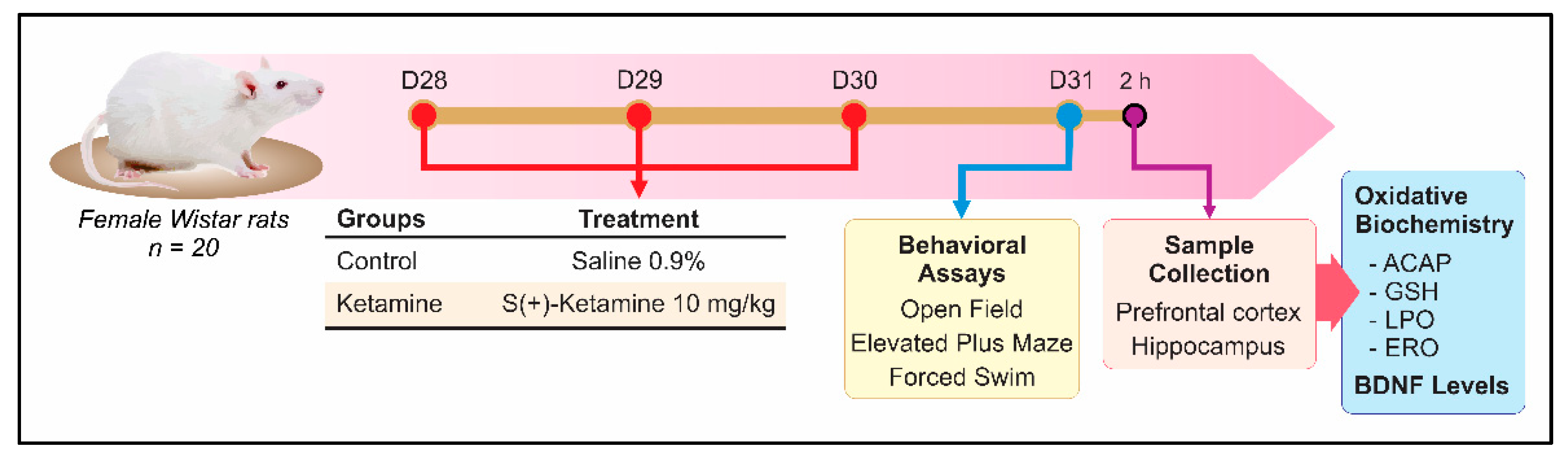
2.1. Animals and Ethical Aspects
2.2. Experimental Design
2.3. Behavioral Assays
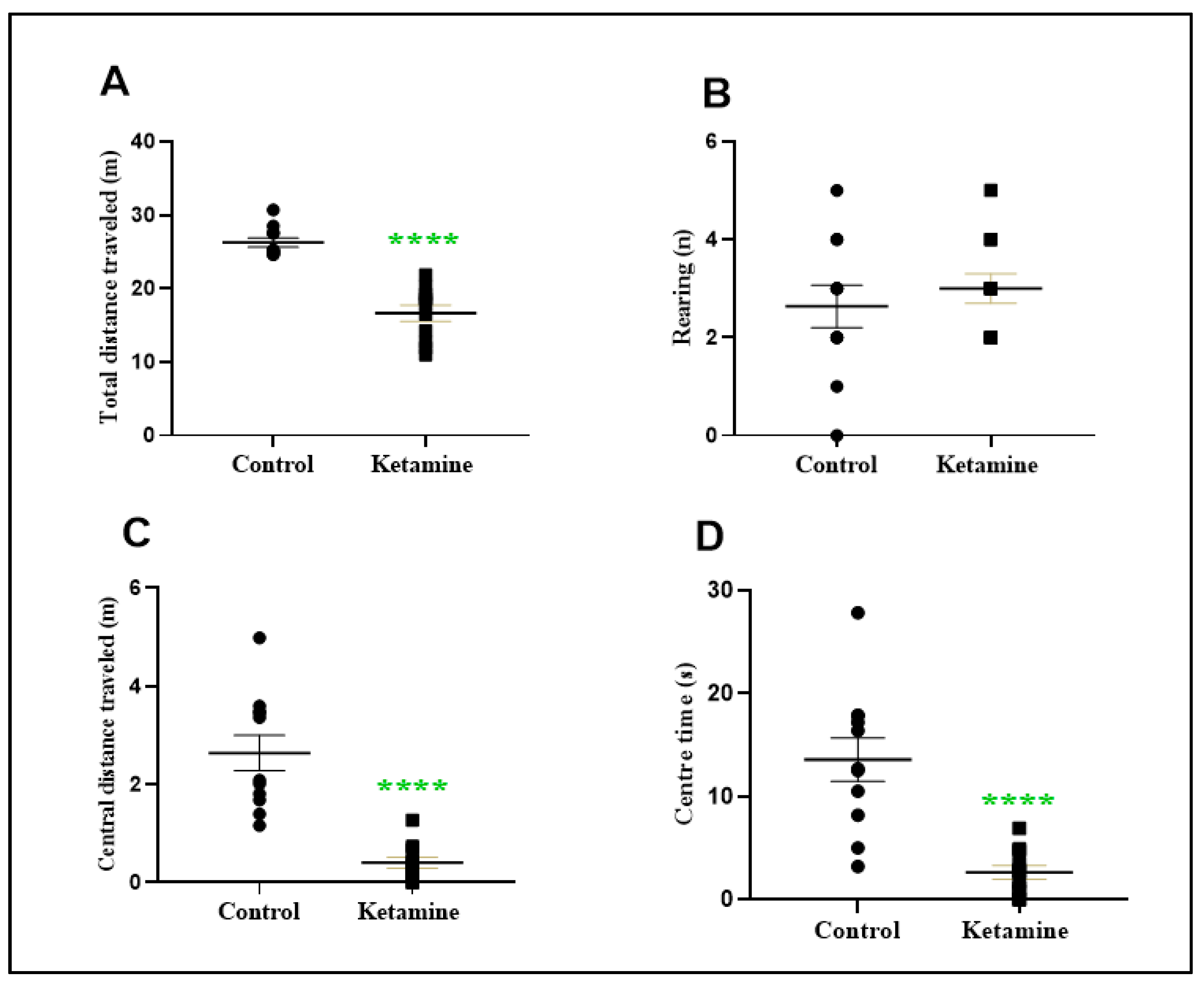
2.3.1. Open Field
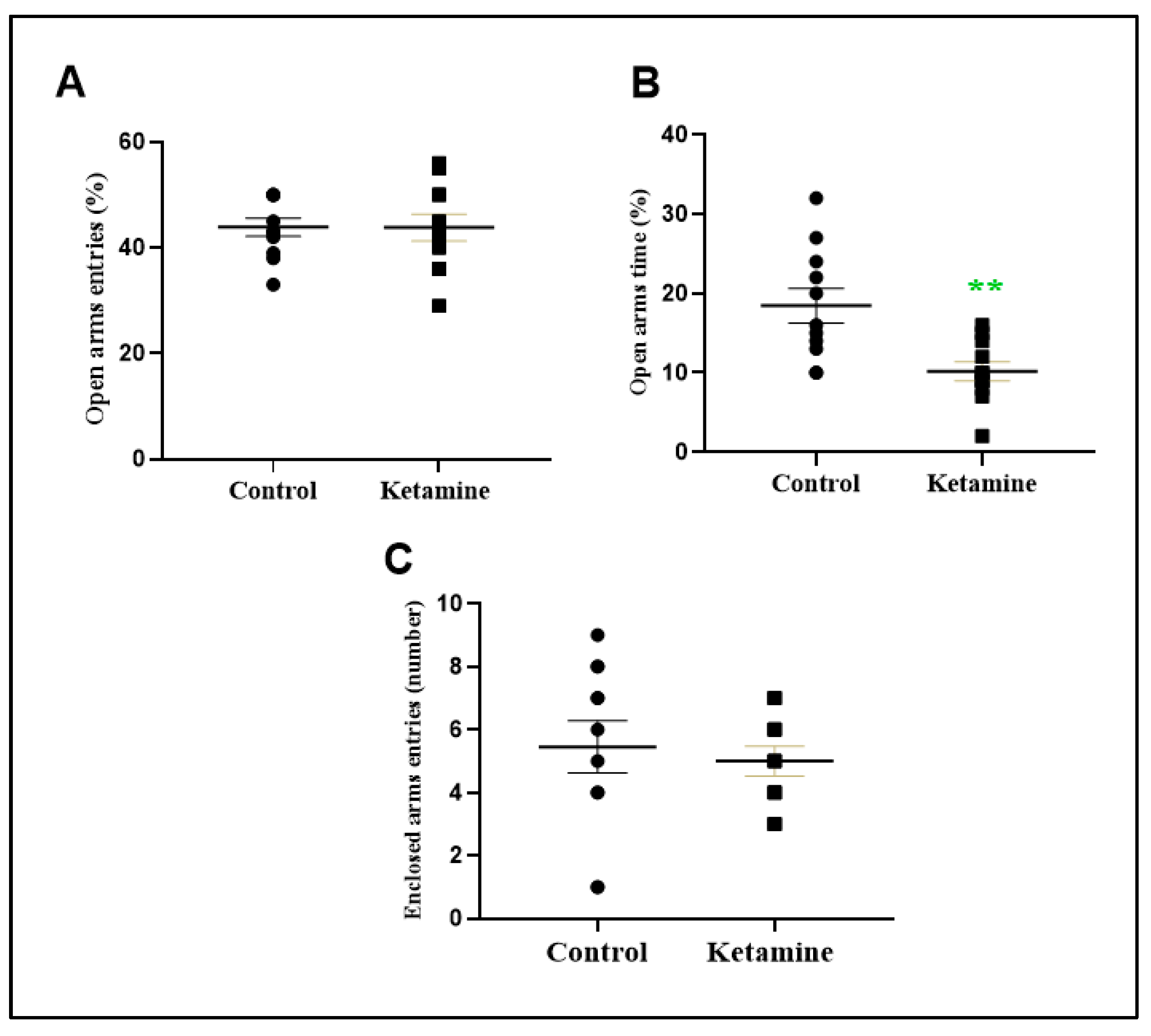
2.3.2. Elevated Plus-Maze
2.3.3. Forced Swimming Test
2.4. Oxidative Biochemistry Assays
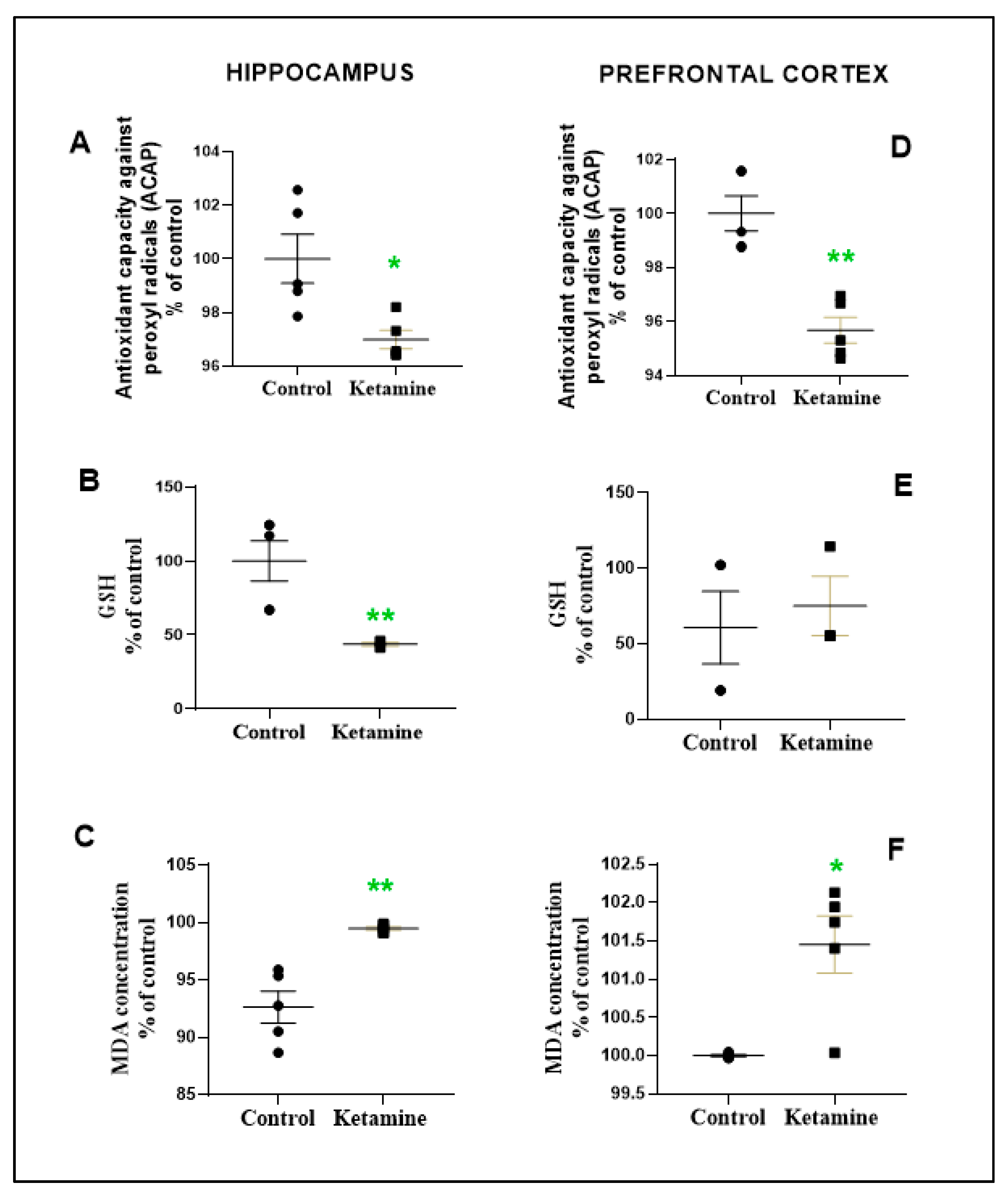
2.4.1. Antioxidant Capacity against Radicals Peroxyl (ACAP)
2.4.2. Reduced Glutathione (GSH)
2.4.3. Lipid Peroxidation (LPO) Levels
2.4.4. Protein Content
2.4.5. Reactive Oxygen Species (ROS)
2.5. Hippocampal Brain-Derived Neurotrophic Factor (BDNF) Levels
2.6. Statistical Analysis
3. Results
3.1. Intermittent Ketamine Exposure during Adolescence Reduces Total and Central Spontaneous Exploratory Behavior in the Early Withdrawal Stage in Female Rats
3.2. Intermittent Ketamine Exposure during Adolescence Induces Psychiatric-like Phenotype Related to Anxiety and Depression in the Early Withdrawal Stage in Female Rats
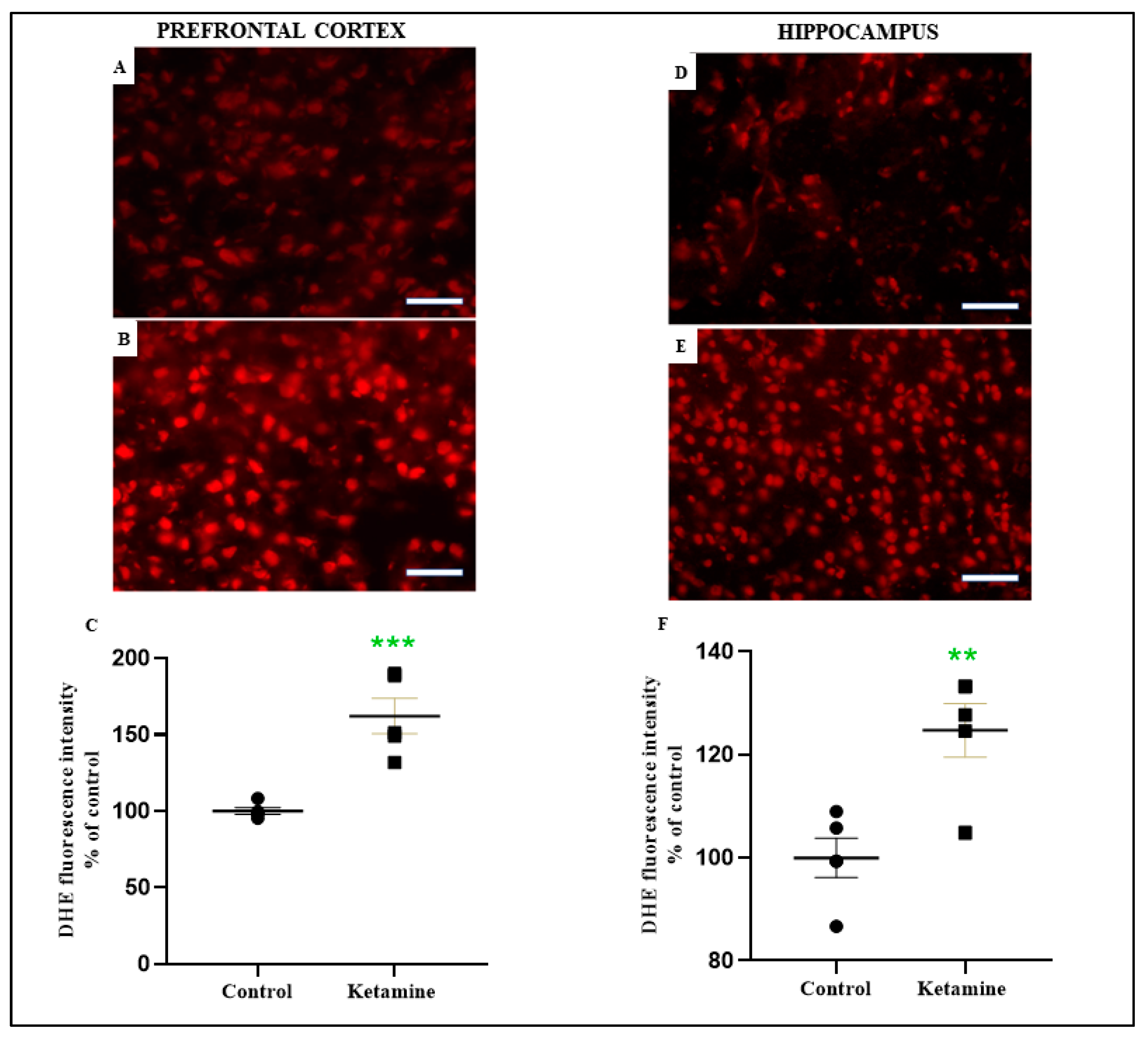
3.3. Emotionality Alteration Induced by Intermittent Ketamine Exposure during Adolescence Was Associated to Overproduction of ROS and Evidence of Oxidative Damage on Prefrontal Cortex and Hippocampus in the Early Withdrawal Stage in Female Rats
3.4. Hippocampal BDNF Levels Were Not Altered in the Early Withdrawal Stage of One Cycle of Ketamine Exposure in Adolescent Female Rats
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maddox, V.H.; Godefroi, E.F.; Parcell, R.F. The Synthesis of Phencyclidine and Other 1-Arylcyclohexylamines. J. Med. Chem. 1965, 8, 230–235. [Google Scholar] [CrossRef]
- Tyler, M.W.; Yourish, H.B.; Ionescu, D.F.; Haggarty, S.J. Classics in Chemical Neuroscience: Ketamine. ACS Chem. Neurosci. 2017, 8, 1122–1134. [Google Scholar] [CrossRef] [PubMed]
- Félix, L.M.; Serafim, C.; Martins, M.J.; Valentim, A.M.; Antunes, L.M.; Matos, M.; Coimbra, A.M. Morphological and Behavioral Responses of Zebrafish after 24 h of Ketamine Embryonic Exposure. Toxicol. Appl. Pharmacol. 2017, 321, 27–36. [Google Scholar] [CrossRef]
- Li, L.; Vlisides, P.E. Ketamine: 50 Years of Modulating the Mind. Front. Hum. Neurosci. 2016, 10, 612. [Google Scholar] [CrossRef] [PubMed]
- Krabseth, H.M.; Tuv, S.S.; Strand, M.C.; Karinen, R.A.; Wiik, E.; Vevelstad, M.S.; Westin, A.A.; Øiestad, E.L.; Vindenes, V. Novel Psychoactive Substances. Tidsskr. Den Nor. Legeforening 2016, 136, 714–717. [Google Scholar] [CrossRef] [PubMed]
- Morgan, C.J.A.; Muetzelfeldt, L.; Curran, H.V. Consequences of Chronic Ketamine Self-Administration upon Neurocognitive Function and Psychological Wellbeing: A 1-Year Longitudinal Study. Addiction 2010, 105, 121–133. [Google Scholar] [CrossRef]
- Domino, E.F.; Chodoff, P.; Corssen, G. Pharmacologic Effects of CI-581, a New Dissociative Anesthetic, in Man. Clin. Pharmacol. Ther. 1965, 6, 279–291. [Google Scholar] [CrossRef]
- Ghasemi, M.; Kazemi, M.H.; Yoosefi, A.; Ghasemi, A.; Paragomi, P.; Amini, H.; Afzali, M.H. Rapid Antidepressant Effects of Repeated Doses of Ketamine Compared with Electroconvulsive Therapy in Hospitalized Patients with Major Depressive Disorder. Psychiatry Res. 2014, 215, 355–361. [Google Scholar] [CrossRef]
- Schnabel, A.; Poepping, D.M.; Kranke, P.; Zahn, P.K.; Pogatzki-Zahn, E.M. Efficacy and Adverse Effects of Ketamine as an Additive for Paediatric Caudal Anaesthesia: A Quantitative Systematic Review of Randomized Controlled Trials. Br. J. Anaesth. 2011, 107, 601–611. [Google Scholar] [CrossRef]
- Fond, G.; Loundou, A.; Rabu, C.; Macgregor, A.; Lançon, C.; Brittner, M.; Micoulaud-Franchi, J.-A.; Richieri, R.; Courtet, P.; Abbar, M.; et al. Ketamine Administration in Depressive Disorders: A Systematic Review and Meta-Analysis. Psychopharmacology 2014, 231, 3663–3676. [Google Scholar] [CrossRef]
- Kishimoto, T.; Chawla, J.M.; Hagi, K.; Zarate, C.A.; Kane, J.M.; Bauer, M.; Correll, C.U. Single-Dose Infusion Ketamine and Non-Ketamine N-Methyl-d-Aspartate Receptor Antagonists for Unipolar and Bipolar Depression: A Meta-Analysis of Efficacy, Safety and Time Trajectories. Psychol. Med. 2016, 46, 1459–1472. [Google Scholar] [CrossRef] [PubMed]
- Beck, K.; Hindley, G.; Borgan, F.; Ginestet, C.; McCutcheon, R.; Brugger, S.; Driesen, N.; Ranganathan, M.; D’Souza, D.C.; Taylor, M.; et al. Association of Ketamine with Psychiatric Symptoms and Implications for Its Therapeutic Use and for Understanding Schizophrenia. JAMA Netw. Open 2020, 3, e204693. [Google Scholar] [CrossRef] [PubMed]
- Dillon, P. Patterns of Use and Harms Associated with Non-Medical Ketamine Use. Drug Alcohol Depend. 2003, 69, 23–28. [Google Scholar] [CrossRef]
- United Nations Office on Drugs and Crime. World Drug Report 2019 (Set of 5 Booklets); United Nations: New York, NY, USA, 2019. [Google Scholar]
- Kalsi, S.S.; Wood, D.M.; Dargan, P.I. The Epidemiology and Patterns of Acute and Chronic Toxicity Associated with Recreational Ketamine Use. Emerg. Health Threat. J. 2011, 4, 7107. [Google Scholar] [CrossRef]
- United Nations Office On Drugs and Crime. World Drug Report 2021. CrimRxiv 2021. [Google Scholar] [CrossRef]
- Ho, J.H.; Dargan, P.I. Arylcyclohexamines: Ketamine, Phencyclidine, and Analogues. In Critical Care Toxicology; Springer: Cham, Germany, 2017; pp. 1439–1484. [Google Scholar] [CrossRef]
- Morgan, C.J.A.; Curran, H.V. Ketamine Use: A Review. Addiction 2011, 107, 27–38. [Google Scholar] [CrossRef]
- Morgan, C.J.A.; Muetzelfeldt, L.; Curran, H.V. Ketamine Use, Cognition and Psychological Wellbeing: A Comparison of Frequent, Infrequent and Ex-Users with Polydrug and Non-Using Controls. Addiction 2009, 104, 77–87. [Google Scholar] [CrossRef]
- Pérez, M.Á.; Morales, C.; Santander, O.; García, F.; Gómez, I.; Peñaloza-Sancho, V.; Fuentealba, P.; Dagnino-Subiabre, A.; Moya, P.R.; Fuenzalida, M. Ketamine-Treatment during Late Adolescence Impairs Inhibitory Synaptic Transmission in the Prefrontal Cortex and Working Memory in Adult Rats. Front. Cell. Neurosci. 2019, 13, 372. [Google Scholar] [CrossRef]
- Maldonado-Devincci, A.M.; Alipour, K.K.; Michael, L.A.; Kirstein, C.L. Repeated Binge Ethanol Administration during Adolescence Enhances Voluntary Sweetened Ethanol Intake in Young Adulthood in Male and Female Rats. Pharmacol. Biochem. Behav. 2010, 96, 476–487. [Google Scholar] [CrossRef]
- Milivojevic, V.; Covault, J. Alcohol Exposure during Late Adolescence Increases Drinking in Adult Wistar Rats, an Effect That Is Not Reduced by Finasteride. Alcohol Alcohol. 2012, 48, 28–38. [Google Scholar] [CrossRef]
- Guerri, C.; Pascual, M. Mechanisms Involved in the Neurotoxic, Cognitive, and Neurobehavioral Effects of Alcohol Consumption during Adolescence. Alcohol 2010, 44, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Crews, F.T.; Braun, C.J.; Hoplight, B.; Switzer, R.C.; Knapp, D.J. Binge Ethanol Consumption Causes Differential Brain Damage in Young Adolescent Rats Compared with Adult Rats. Alcohol. Clin. Exp. Res. 2000, 24, 1712–1723. [Google Scholar] [CrossRef] [PubMed]
- Shibuta, S.; Morita, T.; Kosaka, J.; Kamibayashi, T.; Fujino, Y. Only Extra-High Dose of Ketamine Affects L-Glutamate-Induced Intracellular Ca2+ Elevation and Neurotoxicity. Neurosci. Res. 2015, 98, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.; Patterson, T.A.; Sadovova, N.; Twaddle, N.C.; Doerge, D.R.; Zhang, X.; Fu, X.; Hanig, J.P.; Paule, M.G.; Slikker, W.; et al. Potential Neurotoxicity of Ketamine in the Developing Rat Brain. Toxicol. Sci. 2009, 108, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Hung, C.-C.; Liu, Y.-H.; Huang, C.-C.; Chou, C.-Y.; Chen, C.-M.; Duann, J.-R.; Li, C.-S.R.; Lee, T.S.-H.; Lin, C.-P. Effects of Early Ketamine Exposure on Cerebral Gray Matter Volume and Functional Connectivity. Sci. Rep. 2020, 10, 15488. [Google Scholar] [CrossRef]
- de Carvalho Cartágenes, S.; Fernandes, L.M.P.; Carvalheiro, T.C.V.S.; de Sousa, T.M.; Gomes, A.R.Q.; Monteiro, M.C.; de Oliveira Paraense, R.S.; Crespo-López, M.E.; Lima, R.R.; Fontes-Júnior, E.A.; et al. “Special K” Drug on Adolescent Rats: Oxidative Damage and Neurobehavioral Impairments. Oxidative Med. Cell. Longev. 2019, 2019, 5452727. [Google Scholar] [CrossRef]
- Spear, L.P. Adolescent Brain Development and Animal Models. Ann. N. Y. Acad Sci. 2004, 1021, 23–26. [Google Scholar] [CrossRef]
- Kos, T.; Nikiforuk, A.; Rafa, D.; Popik, P. The Effects of NMDA Receptor Antagonists on Attentional Set-Shifting Task Performance in Mice. Psychopharmacology 2010, 214, 911–921. [Google Scholar] [CrossRef]
- Sigtermans, M.J.; van Hilten, J.J.; Bauer, M.C.R.; Arbous, S.M.; Marinus, J.; Sarton, E.Y.; Dahan, A. Ketamine Produces Effective and Long-Term Pain Relief in Patients with Complex Regional Pain Syndrome Type 1. Pain 2009, 145, 304–311. [Google Scholar] [CrossRef]
- Mion, G.; Villevieille, T. Ketamine Pharmacology: An Update (Pharmacodynamics and Molecular Aspects, Recent Findings). CNS Neurosci. Ther. 2013, 19, 370–380. [Google Scholar] [CrossRef]
- Imbellon, L.; Gouveia, M.; de Morais Filho, G. Comparison of the Effects of Four Subdoses of Dextroketamine to Reduce Pain during Posterior Brachial Plexus Block: A Randomized Double Blind Study. Anesth. Essays Res. 2017, 11, 345. [Google Scholar] [CrossRef] [PubMed]
- Kraeuter, A.-K.; Guest, P.C.; Sarnyai, Z. The Open Field Test for Measuring Locomotor Activity and Anxiety-like Behavior. Methods Mol. Biol. 2018, 1916, 99–103. [Google Scholar] [CrossRef]
- Fernandes, L.M.P.; Cartágenes, S.C.; Barros, M.A.; Carvalheiro, T.C.V.S.; Castro, N.C.F.; Schamne, M.G.; Lima, R.R.; Prediger, R.D.; Monteiro, M.C.; Fontes-Júnior, E.A.; et al. Repeated Cycles of Binge-like Ethanol Exposure Induce Immediate and Delayed Neurobehavioral Changes and Hippocampal Dysfunction in Adolescent Female Rats. Behav. Brain Res. 2018, 350, 99–108. [Google Scholar] [CrossRef] [PubMed]
- do Maia, C.S.F.; de Souza Lucena, G.M.R.; Corrêa, P.B.F.; Serra, R.B.; de Melo Matos, R.W.; da Menezes, F.C.; dos Santos, S.N.; de Sousa, J.B.; da Costa, E.T.; Ferreira, V.M.M. Interference of Ethanol and Methylmercury in the Developing Central Nervous System. NeuroToxicology 2009, 30, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Handley, S.L.; Mithani, S. Effects of Alpha-Adrenoceptor Agonists and Antagonists in a Maze-Exploration Model of? Fear?-Motivated Behaviour. Naunyn-Schmiedeberg’s Arch. Pharmacol. 1984, 327, 1–5. [Google Scholar] [CrossRef]
- Walf, A.A.; Frye, C.A. The Use of the Elevated plus Maze as an Assay of Anxiety-Related Behavior in Rodents. Nat. Protoc. 2007, 2, 322–328. [Google Scholar] [CrossRef]
- Pellow, S.; Chopin, P.; File, S.E.; Briley, M. Validation of Open: Closed Arm Entries in an Elevated Plus-Maze as a Measure of Anxiety in the Rat. J. Neurosci. Methods 1985, 14, 149–167. [Google Scholar] [CrossRef]
- Castro, J.E.; Diessler, S.; Varea, E.; Márquez, C.; Larsen, M.H.; Cordero, M.I.; Sandi, C. Personality Traits in Rats Predict Vulnerability and Resilience to Developing Stress-Induced Depression-like Behaviors, HPA Axis Hyper-Reactivity and Brain Changes in PERK1/2 Activity. Psychoneuroendocrinology 2012, 37, 1209–1223. [Google Scholar] [CrossRef]
- Porsolt, R.D.; Bertin, A.; Jalfre, M. Behavioral Despair in Mice: A Primary Screening Test for Antidepressants. Arch. Int. Pharmacodyn Ther. 1997, 229, 327–336. [Google Scholar]
- Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative Stress and Antioxidant Defense. World Allergy Organ. J. 2012, 5, 9–19. [Google Scholar] [CrossRef]
- Niki, E.; Yoshida, Y.; Saito, Y.; Noguchi, N. Lipid Peroxidation: Mechanisms, Inhibition, and Biological Effects. Biochem. Biophys. Res. Commun. 2005, 338, 668–676. [Google Scholar] [CrossRef] [PubMed]
- Amado, L.L.; Garcia, M.L.; Ramos, P.B.; Freitas, R.F.; Zafalon, B.; Ferreira, J.L.R.; Yunes, J.S.; Monserrat, J.M. A Method to Measure Total Antioxidant Capacity against Peroxyl Radicals in Aquatic Organisms: Application to Evaluate Microcystins Toxicity. Sci. Total Environ. 2009, 407, 2115–2123. [Google Scholar] [CrossRef]
- Bernheim, F.; Bernheim, M.L.C.; Wilbur, K.M. The reaction between thiobarbituric acid and the oxidation products of certain lipides. J. Biol. Chem. 1948, 174, 257–264. [Google Scholar] [CrossRef]
- Halliwell, B.; Whiteman, M. Measuring Reactive Species and Oxidative Damagein Vivoand in Cell Culture: How Should You Do It and What Do the Results Mean? Br. J. Pharmacol. 2004, 142, 231–255. [Google Scholar] [CrossRef] [PubMed]
- Prado, A.F.; Pernomian, L.; Azevedo, A.; Costa, R.A.P.; Rizzi, E.; Ramos, J.; Paes Leme, A.F.; Bendhack, L.M.; Tanus-Santos, J.E.; Gerlach, R.F. Matrix Metalloproteinase-2-Induced Epidermal Growth Factor Receptor Transactivation Impairs Redox Balance in Vascular Smooth Muscle Cells and Facilitates Vascular Contraction. Redox Biol. 2018, 18, 181–190. [Google Scholar] [CrossRef] [PubMed]
- da Ferreira, B.I.S.; da Gomes, N.L.S.; da Coelho, W.L.C.N.P.; da Costa, V.D.; de Carneiro, V.C.S.; Kader, R.L.; Amaro, M.P.; Villar, L.M.; Miyajima, F.; Alves-Leon, S.V.; et al. Validation of a Novel Molecular Assay to the Diagnostic of COVID-19 Based on Real Time PCR with High Resolution Melting. medRxiv 2021. [Google Scholar] [CrossRef] [PubMed]
- Rofael, H.Z.; Abdel-Rahman, M.S. The Role of Ketamine on Plasma Cocaine Pharmacokinetics in Rat. Toxicol. Lett. 2002, 129, 167–176. [Google Scholar] [CrossRef]
- Kleczkowska, P.; Zaremba, M. An Update of Ketamine Illicit Use. In Ketamine Revisited—New Insights into NMDA Inhibitors; IntechOpen: London, UK, 2022. [Google Scholar] [CrossRef]
- Casey, B.J.; Jones, R.M. Neurobiology of the Adolescent Brain and Behavior: Implications for Substance Use Disorders. J. Am. Acad. Child Adolesc. Psychiatry 2010, 49, 1189–1201. [Google Scholar] [CrossRef]
- Dudgeon, P.; Milroy, H.; Walker, R. Working Together: Aboriginal and Torres Strait Islander Mental Health and Wellbeing Principles and Practice; Australian Government, Department Of The Prime Minister And Cabinet: Perth, Australia, 2014. [Google Scholar]
- Ministério Da Saúde. ASSISTÊNCIA PRÉ-NATAL Normas E Manuais Técnicos; Ministério Da Saúde: Brasília, Brasil, 1998. [Google Scholar]
- Ministério Da Saúde. Marco Legal: Saúde, Um Direito de Adolescentes; Ministério Da Saúde: Brasília, Brasil, 2007. [Google Scholar]
- Guerreiro, D.F.; da Navarro, J.A.S.; Góis, C. CLUB DRUGS Um Novo Perfil de Abuso de Substâncias Em Adolescentes E Jovens Adultos. Acta Med. Port. 2011, 24, 739–756. [Google Scholar]
- Jansen, K.L.R.; Darracot-Cankovic, R. The Nonmedical Use of Ketamlne, Part Two: A Review of Problent Use and Dependence. J. Psychoact. Drugs 2001, 33, 151–158. [Google Scholar] [CrossRef]
- Liao, Y.; Tang, J.; Corlett, P.R.; Wang, X.; Yang, M.; Chen, H.; Liu, T.; Chen, X.; Hao, W.; Fletcher, P.C. Reduced Dorsal Prefrontal Gray Matter after Chronic Ketamine Use. Biol. Psychiatry 2011, 69, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Karl, T.; Pabst, R.; von Hörsten, S. Behavioral Phenotyping of Mice in Pharmacological and Toxicological Research. Exp. Toxicol. Pathol. 2003, 55, 69–83. [Google Scholar] [CrossRef] [PubMed]
- Kenna, G.A.; Lewis, D.C. Risk Factors for Alcohol and Other Drug Use by Healthcare Professionals. Subst. Abus. Treat. Prev. Policy 2008, 3, 3. [Google Scholar] [CrossRef]
- Uosukainen, H.; Tacke, U.; Winstock, A.R. Self-Reported Prevalence of Dependence of MDMA Compared to Cocaine, Mephedrone and Ketamine among a Sample of Recreational Poly-Drug Users. Int. J. Drug Policy 2015, 26, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.; Zhang, K.; Pu, Y.; Qu, Y.; Wang, S.; Xiong, Z.; Ren, Q.; Dong, C.; Fujita, Y.; Hashimoto, K. Comparison of Antidepressant and Side Effects in Mice after Intranasal Administration of (R,S)-Ketamine, (R)-Ketamine, and (S)-Ketamine. Pharmacol. Biochem. Behav. 2019, 181, 53–59. [Google Scholar] [CrossRef]
- McDougall, S.A.; Park, G.I.; Ramirez, G.I.; Gomez, V.; Adame, B.C.; Crawford, C.A. Sex-Dependent Changes in Ketamine-Induced Locomotor Activity and Ketamine Pharmacokinetics in Preweanling, Adolescent, and Adult Rats. Eur. Neuropsychopharmacol. 2019, 29, 740–755. [Google Scholar] [CrossRef]
- Crawford, C.A.; Moran, A.E.; Baum, T.J.; Apodaca, M.G.; Montejano, N.R.; Park, G.I.; Gomez, V.; McDougall, S.A. Effects of Monoamine Depletion on the Ketamine-Induced Locomotor Activity of Preweanling, Adolescent, and Adult Rats: Sex and Age Differences. Behav. Brain Res. 2020, 379, 112267. [Google Scholar] [CrossRef]
- Chatterjee, M.; Verma, R.; Ganguly, S.; Palit, G. Neurochemical and Molecular Characterization of Ketamine-Induced Ex-perimental Psychosis Model in Mice. Neuropharmacology 2012, 63, 1161–1171. [Google Scholar] [CrossRef]
- Curran, H.V.; Morgan, C. Cognitive, Dissociative and Psychotogenic Effects of Ketamine in Recreational Users on the Night of Drug Use and 3 Days Later. Addiction 2000, 95, 575–590. [Google Scholar] [CrossRef]
- Weisner, C. Short-Term Alcohol and Drug Treatment Outcomes Predict Long-Term Outcome. Drug Alcohol Depend. 2003, 71, 281–294. [Google Scholar] [CrossRef]
- Mohamed, I.I.; Ahmad, H.E.K.; Hassaan, S.H.; Hassan, S.M. Assessment of Anxiety and Depression among Substance Use Disorder Patients: A Case-Control Study. Middle East Curr. Psychiatry 2020, 27, 22. [Google Scholar] [CrossRef]
- Lim, D.K. Ketamine Associated Psychedelic Effects and Dependence. Singap. Med. J. 2003, 44, 31–34. [Google Scholar]
- Critchlow, D.G. A Case of Ketamine Dependence with Discontinuation Symptoms. Addiction 2006, 101, 1212–1213. [Google Scholar] [CrossRef]
- Liang, H.J.; Tang, K.L.; Chan, F.; Ungvari, G.S.; Tang, W.K. Ketamine Users Have High Rates of Psychosis And/or Depression. J. Addict. Nurs. 2015, 26, 8–13. [Google Scholar] [CrossRef]
- Fan, N.; Xu, K.; Ning, Y.; Rosenheck, R.; Wang, D.; Ke, X.; Ding, Y.; Sun, B.; Zhou, C.; Deng, X.; et al. Profiling the Psychotic, Depressive and Anxiety Symptoms in Chronic Ketamine Users. Psychiatry Res. 2016, 237, 311–315. [Google Scholar] [CrossRef] [PubMed]
- Kuloglu, M.; Atmaca, M.; Tezcan, E.; Ustundag, B.; Bulut, S. Antioxidant Enzyme and Malondialdehyde Levels in Patients with Panic Disorder. Neuropsychobiology 2002, 46, 186–189. [Google Scholar] [CrossRef]
- das Fedoce, A.G.; Ferreira, F.; Bota, R.G.; Bonet-Costa, V.; Sun, P.Y.; Davies, K.J.A. The role of oxidative stress in anxiety disorder: Cause or consequence? Free Radic. Res. 2018, 52, 737–750. [Google Scholar] [CrossRef]
- Bouayed, J.; Rammal, H.; Soulimani, R. Oxidative Stress and Anxiety: Relationship and Cellular Pathways. Oxidative Med. Cell. Longev. 2009, 2, 63–67. [Google Scholar] [CrossRef]
- Geihs, M.A.; Vargas, M.A.; Maciel, F.E.; Vakkuri, O.; Meyer-Rochow, V.B.; Allodi, S.; Nery, L.E.M. Effects of Hypoxia and Reoxygenation on the Antioxidant Defense System of the Locomotor Muscle of the Crab Neohelice Granulata (Decapoda, Varunidae). J. Comp. Physiol. B 2016, 186, 569–579. [Google Scholar] [CrossRef]
- Su, D.; Luo, M.; Liu, H.; Qi, X.; Zeng, Q.; He, S.; Fen, S.; Zhang, J. The Effect of Simulated Digestion on the Composition of Phenolic Compounds and Antioxidant Activities in Lychee Pulp of Different Cultivars. Int. J. Food Sci. Technol. 2019, 54, 3042–3050. [Google Scholar] [CrossRef]
- Liu, Z.; Ren, Z.; Zhang, J.; Chuang, C.-C.; Kandaswamy, E.; Zhou, T.; Zuo, L. Role of ROS and Nutritional Antioxidants in Human Diseases. Front. Physiol. 2018, 9, 477. [Google Scholar] [CrossRef]
- Tsikas, D. Assessment of Lipid Peroxidation by Measuring Malondialdehyde (MDA) and Relatives in Biological Samples: Analytical and Biological Challenges. Anal. Biochem. 2017, 524, 13–30. [Google Scholar] [CrossRef] [PubMed]
- Niki, E. Oxidative Stress and Antioxidants: Distress or Eustress? Free. Radic. Biol. Med. 2018, 124, 564. [Google Scholar] [CrossRef]
- Zhang, Y.; Fang, Y.-C.; Cui, L.-X.; Jiang, Y.-T.; Luo, Y.-S.; Zhang, W.; Yu, D.-X.; Wen, J.; Zhou, T.-T. Zhi-Zi-Chi Decoction Reverses Depressive Behaviors in CUMS Rats by Reducing Oxidative Stress Injury via Regulating GSH/GSSG Pathway. Front. Pharmacol. 2022, 13, 887890. [Google Scholar] [CrossRef] [PubMed]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.D.; Mazur, M.; Telser, J. Free Radicals and Antioxidants in Normal Physiological Functions and Human Disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef]
- Auten, R.L.; Davis, J.M. Oxygen Toxicity and Reactive Oxygen Species: The Devil Is in the Details. Pediatr. Res. 2009, 66, 121–127. [Google Scholar] [CrossRef]
- Salim, S. Oxidative Stress and the Central Nervous System. J. Pharmacol. Exp. Ther. 2016, 360, 201–205. [Google Scholar] [CrossRef]
- Masood, A.; Nadeem, A.; Mustafa, S.J.; O’Donnell, J.M. Reversal of Oxidative Stress-Induced Anxiety by Inhibition of Phosphodiesterase-2 in Mice. J. Pharmacol. Exp. Ther. 2008, 326, 369–379. [Google Scholar] [CrossRef]
- Salim, S.; Asghar, M.; Taneja, M.; Hovatta, I.; Chugh, G.; Vollert, C.; Vu, A. Potential Contribution of Oxidative Stress and Inflammation to Anxiety and Hypertension. Brain Res. 2011, 1404, 63–71. [Google Scholar] [CrossRef]
- Patki, G.; Allam, F.H.; Atrooz, F.; Dao, A.T.; Solanki, N.; Chugh, G.; Asghar, M.; Jafri, F.; Bohat, R.; Alkadhi, K.A.; et al. Grape Powder Intake Prevents Ovariectomy-Induced Anxiety-like Behavior, Memory Impairment and High Blood Pressure in Female Wistar Rats. PLoS ONE 2013, 8, e74522. [Google Scholar] [CrossRef]
- Kobayashi, N.H.C.; Farias, S.V.; Luz, D.A.; Machado-Ferraro, K.M.; da Conceição, B.C.; da Silveira, C.C.M.; Fernandes, L.M.P.; de Cartágenes, S.C.; Ferreira, V.M.M.; Fontes-Júnior, E.A.; et al. Ketamine plus Alcohol: What We Know and What We Can Expect about This. Int. J. Mol. Sci. 2022, 23, 7800. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, S. Oxidative Stress and Antioxidant Defense in Cells. Glob. J. Res. Anal. 2012, 3, 11–14. [Google Scholar] [CrossRef]
- Dyall, S.C.; Balas, L.; Bazan, N.G.; Brenna, J.T.; Chiang, N.; da Costa Souza, F.; Dalli, J.; Durand, T.; Galano, J.-M.; Lein, P.J.; et al. Polyunsaturated Fatty Acids and Fatty Acid-Derived Lipid Mediators: Recent Advances in the Understanding of Their Biosynthesis, Structures, and Functions. Prog. Lipid Res. 2022, 86, 101165. [Google Scholar] [CrossRef] [PubMed]
- Gaschler, M.M.; Stockwell, B.R. Lipid Peroxidation in Cell Death. Biochem. Biophys. Res. Commun. 2017, 482, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Gürbüz, G.; Heinonen, M. LC–MS Investigations on Interactions between Isolated β-Lactoglobulin Peptides and Lipid Oxidation Product Malondialdehyde. Food Chem. 2015, 175, 300–305. [Google Scholar] [CrossRef]
- Romeo, R.D. The Impact of Stress on the Structure of the Adolescent Brain: Implications for Adolescent Mental Health. Brain Res. 2017, 1654, 185–191. [Google Scholar] [CrossRef]
- Wahlstrom, D.; Collins, P.; White, T.; Luciana, M. Developmental Changes in Dopamine Neurotransmission in Adolescence: Behavioral Implications and Issues in Assessment. Brain Cogn. 2010, 72, 146. [Google Scholar] [CrossRef]
- Feinstein, J.S.; Gould, D.; Khalsa, S.S. Amygdala-Driven Apnea and the Chemoreceptive Origin of Anxiety. Biol. Psychol. 2022, 170, 108305. [Google Scholar] [CrossRef]
- Bearden, C.E.; Thompson, P.M.; Avedissian, C.; Klunder, A.D.; Nicoletti, M.; Dierschke, N.; Brambilla, P.; Soares, J.C. Altered Hippocampal Morphology in Unmedicated Patients with Major Depressive Illness. ASN Neuro 2009, 1, AN20090026. [Google Scholar] [CrossRef]
- Chang, B.-J.; Jang, B.-J.; Son, T.G.; Cho, I.-H.; Quan, F.-S.; Choe, N.-H.; Nahm, S.-S.; Lee, J.-H. Ascorbic Acid Ameliorates Oxidative Damage Induced by Maternal Low-Level Lead Exposure in the Hippocampus of Rat Pups during Gestation and Lactation. Food Chem. Toxicol. 2012, 50, 104–108. [Google Scholar] [CrossRef]
- Kim, E.J.; Pellman, B.; Kim, J.J. Stress effects on the hippocampus: A critical review. Learn. Mem. 2015, 22, 411–416. [Google Scholar] [CrossRef] [PubMed]
- Dhikav, V.; Anand, K.S. Hippocampus in health and disease: An overview. Ann. Indian Acad. Neurol. 2012, 15, 239. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, É.R.Q.; Maia, J.G.S.; Fontes-Júnior, E.A.; do Socorro Ferraz Maia, C. Linalool as a Therapeutic and Medicinal Tool in Depression Treatment: A Review. Curr. Neuropharmacol. 2022, 20, 1073–1092. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Song, Y.; Zhang, X.; Zhao, W.; Ma, T.; Liu, Y.; Ma, P.; Zhao, Y.; Zhang, H. Ketamine relieves depression-like behaviors induced by chronic postsurgical pain in rats through anti-inflammatory, anti-oxidant effects and regulating BDNF expression. Psychopharmacology 2020, 237, 1657–1669. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cartágenes, S.d.C.; da Silveira, C.C.S.d.M.; Pinheiro, B.G.; Fernandes, L.M.P.; Farias, S.V.; Kobayashi, N.H.C.; de Souza, P.H.F.S.; Prado, A.F.d.; Ferreira, M.K.M.; Lima, R.R.; et al. “K-Powder” Exposure during Adolescence Elicits Psychiatric Disturbances Associated with Oxidative Stress in Female Rats. Pharmaceuticals 2022, 15, 1373. https://doi.org/10.3390/ph15111373
Cartágenes SdC, da Silveira CCSdM, Pinheiro BG, Fernandes LMP, Farias SV, Kobayashi NHC, de Souza PHFS, Prado AFd, Ferreira MKM, Lima RR, et al. “K-Powder” Exposure during Adolescence Elicits Psychiatric Disturbances Associated with Oxidative Stress in Female Rats. Pharmaceuticals. 2022; 15(11):1373. https://doi.org/10.3390/ph15111373
Chicago/Turabian StyleCartágenes, Sabrina de Carvalho, Cinthia Cristina Sousa de Menezes da Silveira, Bruno Gonçalves Pinheiro, Luanna Melo Pereira Fernandes, Sarah Viana Farias, Natália Harumi Correa Kobayashi, Pablo Henrique Franco Santos de Souza, Alejandro Ferraz do Prado, Maria Karolina Martins Ferreira, Rafael Rodrigues Lima, and et al. 2022. "“K-Powder” Exposure during Adolescence Elicits Psychiatric Disturbances Associated with Oxidative Stress in Female Rats" Pharmaceuticals 15, no. 11: 1373. https://doi.org/10.3390/ph15111373
APA StyleCartágenes, S. d. C., da Silveira, C. C. S. d. M., Pinheiro, B. G., Fernandes, L. M. P., Farias, S. V., Kobayashi, N. H. C., de Souza, P. H. F. S., Prado, A. F. d., Ferreira, M. K. M., Lima, R. R., de Oliveira, E. H. C., de Luna, F. C. F., Burbano, R. M. R., Fontes-Júnior, E. A., & Maia, C. d. S. F. (2022). “K-Powder” Exposure during Adolescence Elicits Psychiatric Disturbances Associated with Oxidative Stress in Female Rats. Pharmaceuticals, 15(11), 1373. https://doi.org/10.3390/ph15111373









