Pimarane Diterpenes from Fungi
Abstract
1. Introduction
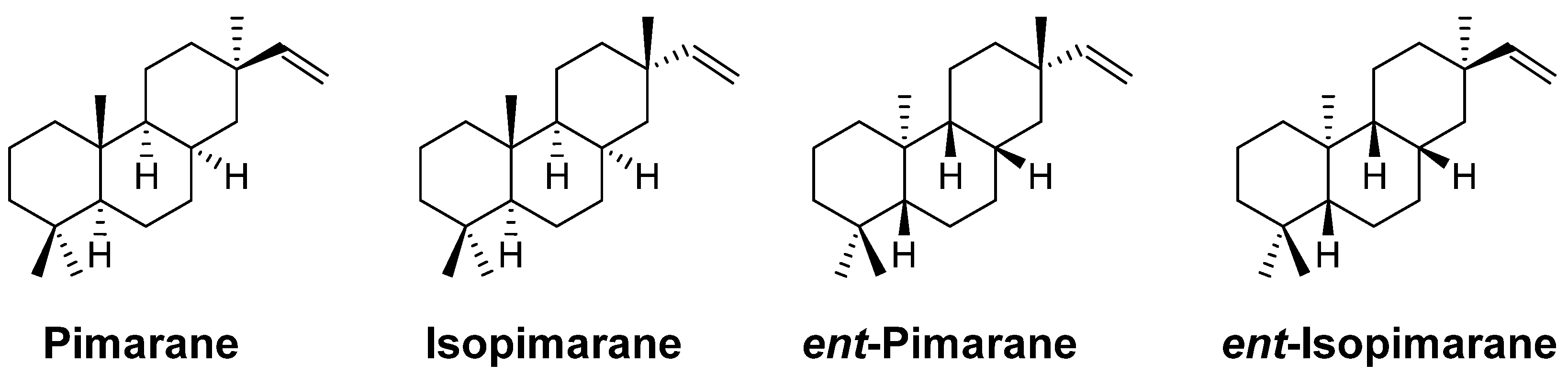
2. Pimarane
3. Isopimarane
4. ent-Pimarane and ent-Isopimarane
5. Pharmacology
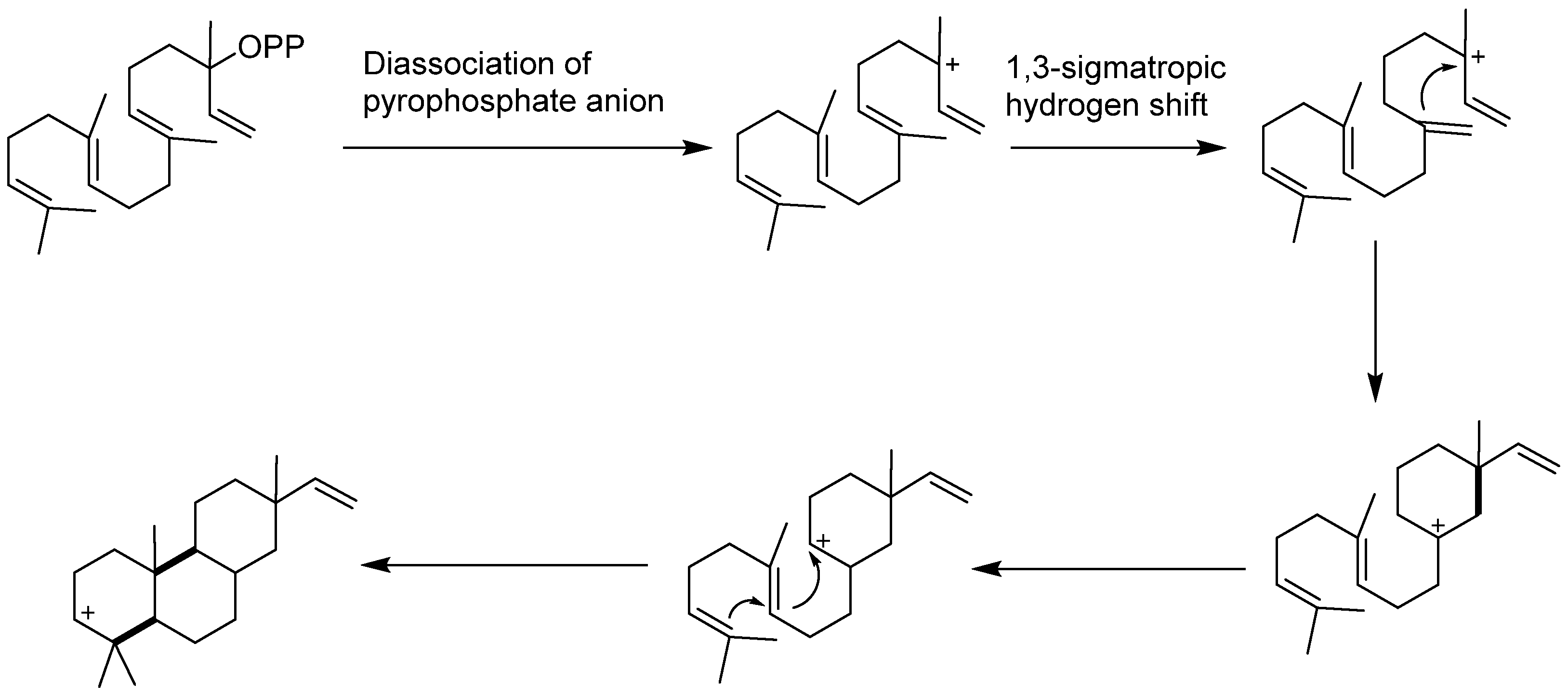
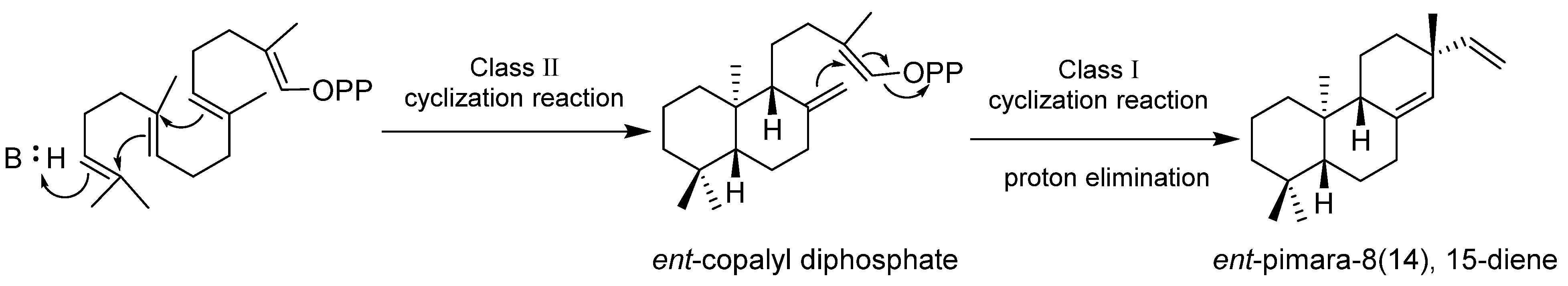
6. Biosynthesis
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Quin, M.B.; Flynn, C.M.; Schmidt-Dannert, C. Traversing the fungal terpenome. Nat. Prod. Rep. 2014, 31, 1449–1473. [Google Scholar] [CrossRef]
- Putter, K.M.; van Deenen, N.; Unland, K.; Prufer, D.; Schulze Gronover, C. Isoprenoid biosynthesis in dandelion latex is enhanced by the overexpression of three key enzymes involved in the mevalonate pathway. BMC Plant Biol. 2017, 17, 88. [Google Scholar] [CrossRef] [PubMed]
- Breitmaier, E. Terpenes: Importance, General Structure, and Biosynthesis. In Terpenes: Flavors, Fragrances, Pharmaca, Pheromones, 1st ed.; Weily-VCH: Weinheim, German, 2006; pp. 1–9. [Google Scholar]
- Zi, J.; Mafu, S.; Peters, R.J. To gibberellins and beyond! Surveying the evolution of (di)terpenoid metabolism. Annu. Rev. Plant Biol. 2014, 65, 259–286. [Google Scholar] [CrossRef] [PubMed]
- Reveglia, P.; Cimmino, A.; Masi, M.; Nocera, P.; Berova, N.; Ellestad, G.; Evidente, A. Pimarane diterpenes: Natural source, stereochemical configuration, and biological activity. Chirality 2018, 30, 1115–1134. [Google Scholar] [CrossRef] [PubMed]
- Cimmino, A.; Masi, M.; Evidente, M.; Superchi, S.; Evidente, A. Fungal phytotoxins with potential herbicidal activity: Chemical and biological characterization. Nat. Prod. Rep. 2015, 32, 1629–1653. [Google Scholar] [CrossRef] [PubMed]
- Evidente, A.; Kornienko, A.; Cimmino, A.; Andolfi, A.; Lefranc, F.; Mathieu, V.; Kiss, R. Fungal metabolites with anticancer activity. Nat. Prod. Rep. 2014, 31, 617–627. [Google Scholar] [CrossRef]
- Hawksworth, D.L.; Lucking, R. Fungal Diversity Revisited: 2.2 to 3.8 Million Species. MicroBiol. Spectr. 2017, 5, 1–17. [Google Scholar] [CrossRef]
- Meng, L.H.; Li, X.M.; Zhang, F.Z.; Wang, Y.N.; Wang, B.G. Talascortenes A-G, Highly Oxygenated Diterpenoid Acids from the Sea-Anemone-Derived Endozoic Fungus Talaromyces scorteus AS-242. J. Nat. Prod. 2020, 83, 2528–2536. [Google Scholar] [CrossRef]
- Niu, S.; Peng, G.; Xia, J.M.; Xie, C.L.; Li, Z.; Yang, X.W. A New Pimarane Diterpenoid from the Botryotinia fuckeliana Fungus Isolated from Deep-Sea Water. Chem. Biodivers. 2019, 16, e1900519. [Google Scholar] [CrossRef]
- Shen, L.; Liu, M.; He, Y.; Al Anbari, W.H.; Li, H.; Lin, S.; Chai, C.; Wang, J.; Hu, Z.; Zhang, Y. Novel Antimicrobial Compounds as Ophiobolin-Type Sesterterpenes and Pimarane-Type Diterpene from Bipolaris Species TJ403-B1. Front. MicroBiol. 2020, 11, 856. [Google Scholar] [CrossRef]
- Zhang, L.Q.; Chen, X.C.; Chen, Z.Q.; Wang, G.M.; Zhu, S.G.; Yang, Y.F.; Chen, K.X.; Liu, X.Y.; Li, Y.M. Eutypenoids A-C: Novel Pimarane Diterpenoids from the Arctic Fungus Eutypella sp. D-1. Mar. Drugs 2016, 14, 44. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.B.; Wang, X.L.; Zhang, Y.X.; Xu, W.H.; Zhang, J.P.; Zhou, X.Y.; Lu, X.L.; Liu, X.Y.; Jiao, B.H. Libertellenones O-S and Eutypellenones A and B, Pimarane Diterpene Derivatives from the Arctic Fungus Eutypella sp. D-1. J. Nat. Prod. 2018, 81, 1553–1560. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Mu, R.; Dou, M.; Yan, D.; Liu, B.; Wei, Q.; Wan, J.; Tang, Y.; Hu, Y. Epigenetic modification in histone deacetylase deletion strain of Calcarisporium arbuscula leads to diverse diterpenoids. Acta Pharm. Sin. B 2018, 8, 687–697. [Google Scholar] [CrossRef]
- Borgschulte, K.; Rebuffat, S.; Trowitzsch-Kienast, W.; Schomburg, D.; Pinon, J.; Bodo, B. Isolation and structure elucidation of hymatoxins B-E and other phytotoxins from Hypoxylon mammatum fungal pathogen of leuce poplars. Tetrahedron 1991, 47, 8351–8360. [Google Scholar] [CrossRef]
- Jossang, A.; Mbeminack, B.; Pinon, J.; Bodo, B. Hymatoxins K and L, Novel Phytotoxins from Hypoxylon mammatum, Fungal Pathogen of Aspens. Nat. Prod. Lett. 1995, 6, 37–42. [Google Scholar] [CrossRef]
- Dettrakul, S.; Kittakoop, P.; Isaka, M.; Nopichai, S.; Suyarnsestakorn, C.; Tanticharoen, M.; Thebtaranonth, Y. Antimycobacterial pimarane diterpenes from the Fungus Diaporthe sp. Bioorganic Med. Chem. Lett. 2003, 13, 1253–1255. [Google Scholar] [CrossRef]
- Zhao, M.; Ruan, Q.; Pan, W.; Tang, Y.; Zhao, Z.; Cui, H. New polyketides and diterpenoid derivatives from the fungus Penicillium sclerotiorum GZU-XW03-2 and their anti-inflammatory activity. Fitoterapia 2020, 143, 104561. [Google Scholar] [CrossRef]
- Cui, H.; Liu, Y.; Ding, M.; Zhang, Z.; Liu, H.; Huang, X.; She, Z. New pyranonaphthazarin and 2-naphthoic acid derivatives from the mangrove endophytic fungus Leptosphaerulina sp. SKS032. PhytoChem. Lett. 2017, 20, 214–217. [Google Scholar] [CrossRef]
- Yoshida, S.; Kito, K.; Ooi, T.; Kanoh, K.; Shizuri, Y.; Kusumi, T. Four Pimarane Diterpenes from Marine Fungus: Chloroform Incorporated in Crystal Lattice for Absolute Configuration Analysis by X-ray. Chem. Lett. 2007, 36, 1386–1387. [Google Scholar] [CrossRef]
- Isaka, M.; Palasarn, S.; Prathumpai, W.; Laksanacharoen, P. Pimarane Diterpenes from the Endophytic Fungus Eutypella sp. BCC 13199. Chem. Pharm. Bull. 2011, 59, 1157–1159. [Google Scholar] [CrossRef]
- Xia, X.; Zhang, J.; Zhang, Y.; Wei, F.; Liu, X.; Jia, A.; Liu, C.; Li, W.; She, Z.; Lin, Y. Pimarane diterpenes from the fungus Epicoccum sp. HS-1 associated with Apostichopus japonicus. Bioorg. Med. Chem. Lett. 2012, 22, 3017–3019. [Google Scholar] [CrossRef]
- Li, X.; Li, X.-D.; Li, X.-M.; Xu, G.-M.; Liu, Y.; Wang, B.-G. Wentinoids A–F, six new isopimarane diterpenoids from Aspergillus wentii SD-310, a deep-sea sediment derived fungus. RSC Adv. 2017, 7, 4387–4394. [Google Scholar] [CrossRef]
- Li, X.; Li, X.-M.; Li, X.-D.; Xu, G.-M.; Liu, Y.; Wang, B.-G. 20-Nor-isopimarane cycloethers from the deep-sea sediment-derived fungus Aspergillus wentii SD-310. RSC Adv. 2016, 6, 75981–75987. [Google Scholar] [CrossRef]
- Miao, F.-P.; Liang, X.-R.; Liu, X.-H.; Ji, N.-Y. Aspewentins A–C, Norditerpenes from a Cryptic Pathway in an Algicolous Strain of Aspergillus wentii. J. Nat. Prod. 2014, 77, 429–432. [Google Scholar] [CrossRef]
- Li, X.D.; Li, X.M.; Li, X.; Xu, G.M.; Liu, Y.; Wang, B.G. Aspewentins D-H, 20-Nor-isopimarane Derivatives from the Deep Sea Sediment-Derived Fungus Aspergillus wentii SD-310. J. Nat. Prod. 2016, 79, 1347–1353. [Google Scholar] [CrossRef]
- Oh, D.C.; Jensen, P.R.; Kauffman, C.A.; Fenical, W. Libertellenones A-D: Induction of cytotoxic diterpenoid biosynthesis by marine microbial competition. Bioorg. Med. Chem. 2005, 13, 5267–5273. [Google Scholar] [CrossRef]
- Tsukada, M.; Fukai, M.; Miki, K.; Shiraishi, T.; Suzuki, T.; Nishio, K.; Sugita, T.; Ishino, M.; Kinoshita, K.; Takahashi, K.; et al. Chemical Constituents of a Marine Fungus, Arthrinium sacchari. J. Nat. Prod. 2011, 74, 1645–1649. [Google Scholar] [CrossRef]
- Lu, X.-L.; Liu, J.-T.; Liu, X.-Y.; Gao, Y.; Zhang, J.; Jiao, B.-H.; Zheng, H. Pimarane diterpenes from the Arctic fungus Eutypella sp. D-1. J. Antibiot. 2014, 67, 171–174. [Google Scholar] [CrossRef]
- Wang, H.; Umeokoli, B.O.; Eze, P.; Heering, C.; Janiak, C.; Müller, W.E.G.; Orfali, R.S.; Hartmann, R.; Dai, H.; Lin, W.; et al. Secondary metabolites of the lichen-associated fungus Apiospora montagnei. Tetrahedron Lett. 2017, 58, 1702–1705. [Google Scholar] [CrossRef]
- Wei, W.; Gao, J.; Shen, Y.; Chu, Y.L.; Xu, Q.; Tan, R.X. Immunosuppressive Diterpenes from Phomopsis sp. S12. Eur. J. Org. Chem. 2014, 2014, 5728–5734. [Google Scholar] [CrossRef]
- Wang, X.; Sun, K.; Wang, B. Bioactive Pimarane Diterpenes from the Arctic Fungus Eutypella sp. D-1. Chem. Biodivers. 2018, 15, e1700501. [Google Scholar] [CrossRef]
- Kildgaard, S.; Subko, K.; Phillips, E.; Goidts, V.; de la Cruz, M.; Díaz, C.; Gotfredsen, C.H.; Andersen, B.; Frisvad, J.C.; Nielsen, K.F.; et al. A Dereplication and Bioguided Discovery Approach to Reveal New Compounds from a Marine-Derived Fungus Stilbella fimetaria. Mar. Drugs 2017, 15, 253. [Google Scholar] [CrossRef]
- Pongcharoen, W.; Rukachaisirikul, V.; Phongpaichit, S.; Rungjindamai, N.; Sakayaroj, J. Pimarane Diterpene and Cytochalasin Derivatives from the Endophytic Fungus Eutypella scoparia PSU-D44. J. Nat. Prod. 2006, 69, 856–858. [Google Scholar] [CrossRef]
- Sun, L.; Li, D.; Tao, M.; Chen, Y.; Dan, F.; Zhang, W. Scopararanes C–G: New Oxygenated Pimarane Diterpenes from the Marine Sediment-Derived Fungus Eutypella scoparia FS26. Mar. Drugs 2012, 10, 539–550. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, L.; Chen, Y.; Li, S.; Tan, G.; Sun, Z.; Pan, Q.; Ye, W.; Li, H.; Zhang, W. Cytotoxic pimarane-type diterpenes from the marine sediment-derived fungus Eutypella sp. FS46. Nat. Prod. Res. 2017, 31, 404–410. [Google Scholar] [CrossRef]
- Klemke, C.; Kehraus, S.; Wright, A.D.; König, G.M. New secondary metabolites from the marine endophytic fungus Apiospora montagnei. J. Nat. Prod. 2004, 67, 1058–1063. [Google Scholar] [CrossRef]
- Hsu, Y.-H.; Nakagawa, M.; Hirota, A.; Shima, S.; Nakayama, M. Structure of Myrocin B, a New Diterpene Antibiotic Produced by Myrothecium verrucaria. Agric. Biol. Chem. 1988, 52, 1305–1307. [Google Scholar] [CrossRef]
- Hsu, Y.H.; Hirota, A.; Shima, S.; Nakagawa, M.; Nozaki, H.; Tada, T.; Nakayama, M. Structure of Myrocin C, a New Diterpene Antibiotic Produced by a Strain of Myrothecium sp. Agric. Biol. Chem. 1987, 51, 3455–3457. [Google Scholar] [CrossRef]
- Evidente, A.; Sparapano, L.; Motta, A.; Giordano, F.; Fierro, O.; Frisullo, S. A phytotoxic pimarane diterpene of Sphaeropsis sapinea f. sp. Cupressi, the pathogen of a canker disease of cypress. PhytoChemistry 1996, 42, 1541–1546. [Google Scholar] [CrossRef]
- Evidente, A.; Sparapano, L.; Fierro, O.; Bruno, G.; Giordano, F.; Motta, A. Sphaeropsidins B and C, phytotoxic pimarane diterpenes from Sphaeropsis sapinea f. sp. Cupressi and Diplodia mutila. PhytoChemistry 1997, 45, 705–713. [Google Scholar] [CrossRef]
- Evidente, A.; Sparapano, L.; Bruno, G.; Motta, A. Sphaeropsidins D and E, two other pimarane diterpenes, produced in vitro by the plant pathogenic fungus Sphaeropsis sapinea f. sp. cupressi. PhytoChemistry 2002, 59, 817–823. [Google Scholar] [CrossRef]
- Kato, H.; Sebe, M.; Nagaki, M.; Eguchi, K.; Kagiyama, I.; Hitora, Y.; Frisvad, J.C.; Williams, R.M.; Tsukamoto, S. Taichunins A–D, Norditerpenes from Aspergillus taichungensis (IBT 19404). J. Nat. Prod. 2019, 82, 1377–1381. [Google Scholar] [CrossRef]
- El-Desoky, A.H.H.; Inada, N.; Maeyama, Y.; Kato, H.; Hitora, Y.; Sebe, M.; Nagaki, M.; Kai, A.; Eguchi, K.; Inazumi, T.; et al. Taichunins E-T, Isopimarane Diterpenes and a 20-nor-Isopimarane, from Aspergillus taichungensis (IBT 19404): Structures and Inhibitory Effects on RANKL-Induced Formation of Multinuclear Osteoclasts. J. Nat. Prod. 2021, 84, 2475–2485. [Google Scholar] [CrossRef]
- Guo, Z.K.; Yan, T.; Guo, Y.; Song, Y.C.; Jiao, R.H.; Tan, R.X.; Ge, H.M. p-Terphenyl and Diterpenoid Metabolites from Endophytic Aspergillus sp. YXf3. J. Nat. Prod. 2012, 75, 15–21. [Google Scholar] [CrossRef]
- Li, Y.-Y.; Hu, Z.-Y.; Lu, C.-H.; Shen, Y.-M. Four New Terpenoids from Xylaria sp. 101. Helv. Chim. Acta 2010, 93, 796–802. [Google Scholar] [CrossRef]
- Isaka, M.; Yangchum, A.; Supothina, S.; Chanthaket, R.; Srikitikulchai, P. Isopimaranes and eremophilanes from the wood-decay fungus Xylaria allantoidea BCC 23163. PhytoChem. Lett. 2014, 8, 59–64. [Google Scholar] [CrossRef]
- Chen, H.-P.; Li, J.; Zhao, Z.-Z.; Li, X.; Liu, S.-L.; Wang, Q.-Y.; Liu, J.-K. Diterpenes with bicyclo [2.2.2]octane moieties from the fungicolous fungus Xylaria longipes HFG101811. Org. Biomol. Chem. 2020, 18, 2410–2415. [Google Scholar] [CrossRef]
- Chen, H.-P.; Zhao, Z.-Z.; Cheng, G.-G.; Zhao, K.; Han, K.-Y.; Zhou, L.; Feng, T.; Li, Z.-H.; Liu, J.-K. Immunosuppressive Nor-isopimarane Diterpenes from Cultures of the Fungicolous Fungus Xylaria longipes HFG1018. J. Nat. Prod. 2020, 83, 401–412. [Google Scholar] [CrossRef]
- Wang, Q.-Y.; Chen, H.-P.; Liu, J.-K. Isopimarane diterpenes from the rice fermentation of the fungicolous fungus Xylaria longipes HFG1018. PhytoChem. Lett. 2021, 45, 100–104. [Google Scholar] [CrossRef]
- Isaka, M.; Srisanoh, U.; Sappan, M.; Kongthong, S.; Srikitikulchai, P. Eremophilane and eudesmane sesquiterpenoids and a pimarane diterpenoid from the wood-decay fungus Xylaria sp. BCC 5484. PhytoChem. Lett. 2012, 5, 78–82. [Google Scholar] [CrossRef]
- Wu, S.H.; He, J.; Li, X.N.; Huang, R.; Song, F.; Chen, Y.W.; Miao, C.P. Guaiane sesquiterpenes and isopimarane diterpenes from an endophytic fungus Xylaria sp. PhytoChemistry 2014, 105, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.H.; Li, Z.H.; Feng, T.; Liu, J.K. Inonotolides A-C, isopimarane diterpenoid lactones from Inonotus sinensis. Fitoterapia 2018, 127, 410–412. [Google Scholar] [CrossRef] [PubMed]
- Findlay, J.A.; Li, G.; Penner, P.E.; Miller, J.D. Novel Diterpenoid Insect Toxins from a Conifer Endophyte. J. Nat. Prod. 1995, 58, 197–200. [Google Scholar] [CrossRef]
- Shiono, Y.; Motoki, S.; Koseki, T.; Murayama, T.; Tojima, M.; Kimura, K. Isopimarane diterpene glycosides, apoptosis inducers, obtained from fruiting bodies of the ascomycete Xylaria polymorpha. PhytoChemistry 2009, 70, 935–939. [Google Scholar] [CrossRef] [PubMed]
- Shiono, Y.; Kikuchi, M.; Koseki, T.; Murayama, T.; Kwon, E.; Aburai, N.; Kimura, K. Isopimarane diterpene glycosides, isolated from endophytic fungus Paraconiothyrium sp. MY-42. PhytoChemistry 2011, 72, 1400–1405. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Zheng, M.; Li, X.-N.; Wei, M.; Zhang, M.; Li, Q.; Zang, Y.; Sun, W.; Wang, J.; Zhu, H.; et al. Hypoxylonoids A−G: Isopimarane diterpene glycosides from Xylaria hypoxylon. PhytoChemistry 2021, 182, 112613. [Google Scholar] [CrossRef]
- Afiyatullov, S.S.; Kalinovsky, A.I.; Kuznetsova, T.A.; Isakov, V.V.; Pivkin, M.V.; Dmitrenok, P.S.; Elyakov, G.B. New Diterpene Glycosides of the Fungus Acremonium striatisporum Isolated from a Sea Cucumber. J. Nat. Prod. 2002, 65, 641–644. [Google Scholar] [CrossRef] [PubMed]
- Cimmino, A.; Andolfi, A.; Zonno, M.C.; Avolio, F.; Santini, A.; Tuzi, A.; Berestetskyi, A.; Vurro, M.; Evidente, A. Chenopodolin: A phytotoxic unrearranged ent-pimaradiene diterpene produced by Phoma chenopodicola, a fungal pathogen for Chenopodium album biocontrol. J. Nat. Prod. 2013, 76, 1291–1297. [Google Scholar] [CrossRef]
- Evidente, M.; Cimmino, A.; Zonno, M.C.; Masi, M.; Berestetskyi, A.; Santoro, E.; Superchi, S.; Vurro, M.; Evidente, A. Phytotoxins produced by Phoma chenopodiicola, a fungal pathogen of Chenopodium album. PhytoChemistry 2015, 117, 482–488. [Google Scholar] [CrossRef]
- Andolfi, A.; Maddau, L.; Basso, S.; Linaldeddu, B.T.; Cimmino, A.; Scanu, B.; Deidda, A.; Tuzi, A.; Evidente, A. Diplopimarane, a 20-nor-ent-Pimarane Produced by the Oak Pathogen Diplodia quercivora. J. Nat. Prod. 2014, 77, 2352–2360. [Google Scholar] [CrossRef]
- Xia, J.-N.; Hu, K.; Su, X.-Z.; Tang, J.-W.; Li, X.-N.; Sun, H.-D.; Puno, P.-T. Discovery of ent-kaurane diterpenoids, characteristic metabolites of Isodon species, from an endophytic fungal strain Geopyxis sp. XY93 inhabiting Isodon parvifolia. Fitoterapia 2022, 158, 105160. [Google Scholar] [CrossRef] [PubMed]
- Bromann, K.; Viljanen, K.; Moreira, V.M.; Yli-Kauhaluoma, J.T.; Ruohonen, L.; Nakari-Setälä, T. Isolation and purification of ent-pimara-8(14),15-diene from engineered Aspergillus nidulans by accelerated solvent extraction combined with HPLC. Anal. Methods 2014, 6, 1227–1234. [Google Scholar] [CrossRef]
- Severiano, M.E.; Simao, M.R.; Porto, T.S.; Martins, C.H.; Veneziani, R.C.; Furtado, N.A.; Arakawa, N.S.; Said, S.; Oliveira, D.C.; Cunha, W.R.; et al. Anticariogenic Properties of ent-Pimarane Diterpenes Obtained by Microbial Transformation. Molecules 2010, 15, 8553–8566. [Google Scholar] [CrossRef] [PubMed]
- Fraga, B.M.; Guillermo, R.; Hernández, M.G.; Chamy, M.C.; Garbarino, J.A. Biotransformation of Two ent-Pimara-9(11),15-diene Derivatives by Gibberella fujikuroi. J. Nat. Prod. 2009, 72, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Holmgren, A. The thioredoxin antioxidant system. Free. Radic. Biol. Med. 2014, 66, 75–87. [Google Scholar] [CrossRef]
- Zhang, W.; Zhu, Y.; Yu, H.; Liu, X.; Jiao, B.; Lu, X. Libertellenone H, a Natural Pimarane Diterpenoid, Inhibits Thioredoxin System and Induces ROS-Mediated Apoptosis in Human Pancreatic Cancer Cells. Molecules 2021, 26, 315. [Google Scholar] [CrossRef]
- Yang, Y.; Yu, G. The analysis of social resource mobilization on new media: A case study of Chinese environmental protection documentary Under the Dome. Telemat. Inform. 2019, 37, 128–136. [Google Scholar] [CrossRef]
- Fan, M.; Xiang, G.; Chen, J.; Gao, J.; Xue, W.; Wang, Y.; Li, W.; Zhou, L.; Jiao, R.; Shen, Y.; et al. Libertellenone M, a diterpene derived from an endophytic fungus Phomopsis sp. S12, protects against DSS-induced colitis via inhibiting both nuclear translocation of NF-κB and NLRP3 inflammasome activation. Int. Immunopharmacol. 2020, 80, 106144. [Google Scholar] [CrossRef]
- Rachner, T.D.; Khosla, S.; Hofbauer, L.C. Osteoporosis: Now and the future. Lancet 2011, 377, 1276–1287. [Google Scholar] [CrossRef]
- Christianson, D.W. Structural and Chemical Biology of Terpenoid Cyclases. Chem. Rev. 2017, 117, 11570–11648. [Google Scholar] [CrossRef]
- Oldfield, E.; Lin, F.Y. Terpene biosynthesis: Modularity rules. Angew. Chem. Int. Ed. Engl. 2012, 51, 1124–1137. [Google Scholar] [CrossRef] [PubMed]
- Faylo, J.L.; Ronnebaum, T.A.; Christianson, D.W. Assembly-Line Catalysis in Bifunctional Terpene Synthases. Acc. Chem. Res. 2021, 54, 3780–3791. [Google Scholar] [CrossRef]
- Zhou, K.; Gao, Y.; Hoy, J.A.; Mann, F.M.; Honzatko, R.B.; Peters, R.J. Insights into Diterpene Cyclization from Structure of Bifunctional Abietadiene Synthase from Abies grandis. J. Biol. Chem. 2012, 287, 6840–6850. [Google Scholar] [CrossRef] [PubMed]
- Morrone, D.; Chambers, J.; Lowry, L.; Kim, G.; Anterola, A.; Bender, K.; Peters, R.J. Gibberellin biosynthesis in bacteria: Separate ent-copalyl diphosphate and ent-kaurene synthases in Bradyrhizobium japonicum. FEBS Lett. 2009, 583, 475–480. [Google Scholar] [CrossRef] [PubMed]
- Hall, D.E.; Zerbe, P.; Jancsik, S.; Quesada, A.L.; Dullat, H.; Madilao, L.L.; Yuen, M.; Bohlmann, J. Evolution of conifer diterpene synthases: Diterpene resin acid biosynthesis in lodgepole pine and jack pine involves monofunctional and bifunctional diterpene synthases. Plant Physiol. 2013, 161, 600–616. [Google Scholar] [CrossRef] [PubMed]
- Toyomasu, T. Recent Advances Regarding Diterpene Cyclase Genes in Higher Plants and Fungi. Biosci. Biotechnol. Biochem. 2008, 72, 1168–1175. [Google Scholar] [CrossRef]
- Peters, R.J.; Carter, O.A.; Zhang, Y.; Matthews, B.W.; Croteau, R.B. Bifunctional Abietadiene Synthase: Mutual Structural Dependence of the Active Sites for Protonation-Initiated and Ionization-Initiated Cyclizations. Biochemistry 2003, 42, 2700–2707. [Google Scholar] [CrossRef]
- Ravn, M.M.; Peters, R.J.; Coates, R.M.; Croteau, R. Mechanism of Abietadiene Synthase Catalysis: Stereochemistry and Stabilization of the Cryptic Pimarenyl Carbocation Intermediates. J. Am. Chem. Soc. 2002, 124, 6998–7006. [Google Scholar] [CrossRef]
- Liu, W.; Feng, X.; Zheng, Y.; Huang, C.-H.; Nakano, C.; Hoshino, T.; Bogue, S.; Ko, T.P.; Chen, C.-C.; Cui, Y.; et al. Structure, function and inhibition of ent-kaurene synthase from Bradyrhizobium japonicum. Sci. Rep. 2014, 4, 6214. [Google Scholar] [CrossRef]
- Bromann, K.; Toivari, M.; Viljanen, K.; Vuoristo, A.; Ruohonen, L.; Nakari-Setala, T. Identification and characterization of a novel diterpene gene cluster in Aspergillus nidulans. PLoS ONE 2012, 7, e35450. [Google Scholar]

| Compound | Fungal Species | Bioactivity | Reference |
|---|---|---|---|
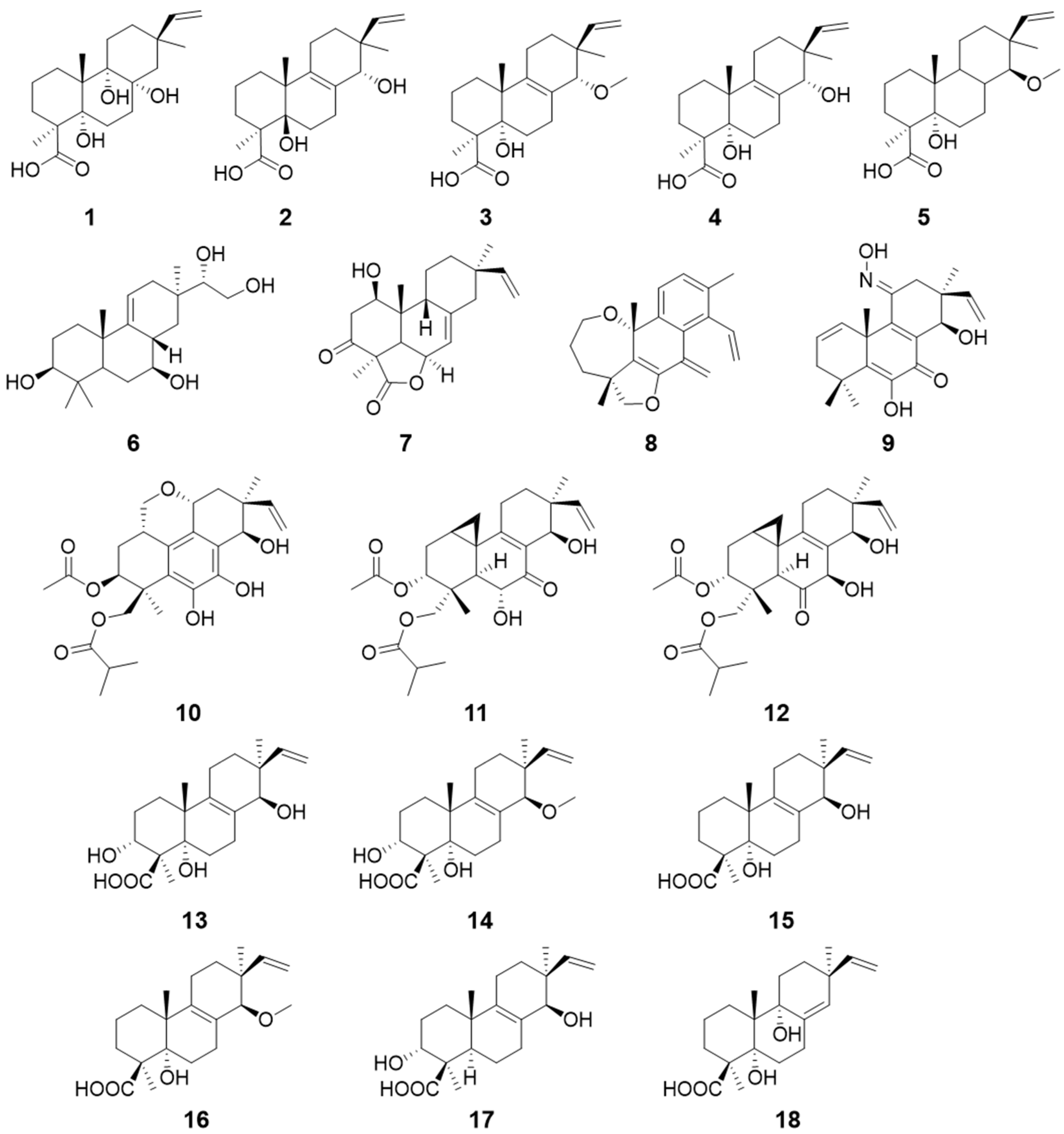
| Talascortenes C–G (1–5) | Talaromyces scorteus | Antimicrobial activity | [9] |
| Botryopimrane A (6) | Botryotinia fuckeliana | / | [10] |
| 1β-hydroxy momilactone A (7) | Bipolaris sp. | / | [11] |
| Euypenoids A–C (8–10) | Eutypella sp. | Immunosuppressive activity | [12] |
| Libertellenones R–S (11–12) | Eutypella sp. | / | [13] |
| Calcarisporic acids E–J (13–18) | Calcarisporium arbuscula | / | [14] |
| Hymatoxins A–E (19–23) | Hypoxylon mammatum | Phytotoxic activity | [15] |
| Hymatoxins K (24) and L (25) | Hypoxylon mammatum Xylaria allantoidea | Phytotoxic activity | [16,47] |
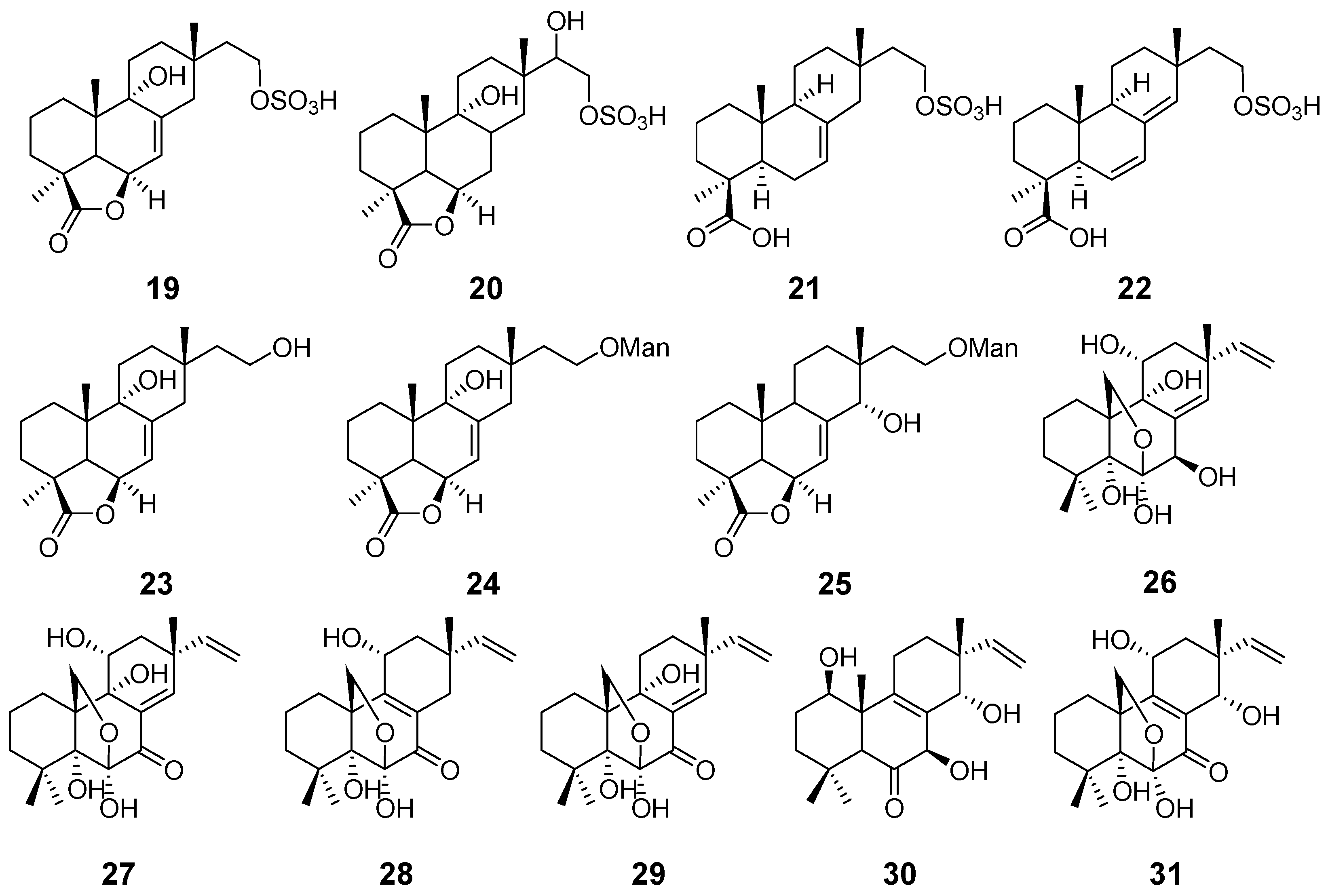
| Diaporthein A (26) | Diaporthe sp. | Antimycobacterial activity | [17] |
| Diaporthein B (27) | Diaporthe sp. Leptosphaerulina sp. Epicoccum sp. | [17,19,22] | |
| Diporthein C (28) | Penicillium sclerotiorum | / | [18] |
| Deoxydiportherin A (29) | Cryptosphaeria eunomi | / | [20] |
| Eutypellones A (30) and B (31) | Eutypella sp. | Cytotoxic activity | [21] |
| Apsergilones A (32) and B (33) | Epicoccum sp. | Cytotoxic activity | [22] |
| Apsergilone C (34) | Epicoccum sp. and Aspergillus wentii | [22,23] | |
| Wentinoid A (35) | Aspergillus wentii | Antimycobacterial activity | [23] |
| Wentinoids B–F (36–40) | / | ||
| Asprethers A–E (41–45) | Aspergillus wentii | Cytotoxic activity | [24] |
| Aspewentins A–C (46–48) | Aspergillus wentii | Inhibitory activity against marine planktons | [25] |
| Aspewentins D–H (49–53) | Aspergillus wentii | Antimycobacterial activity | [26] |
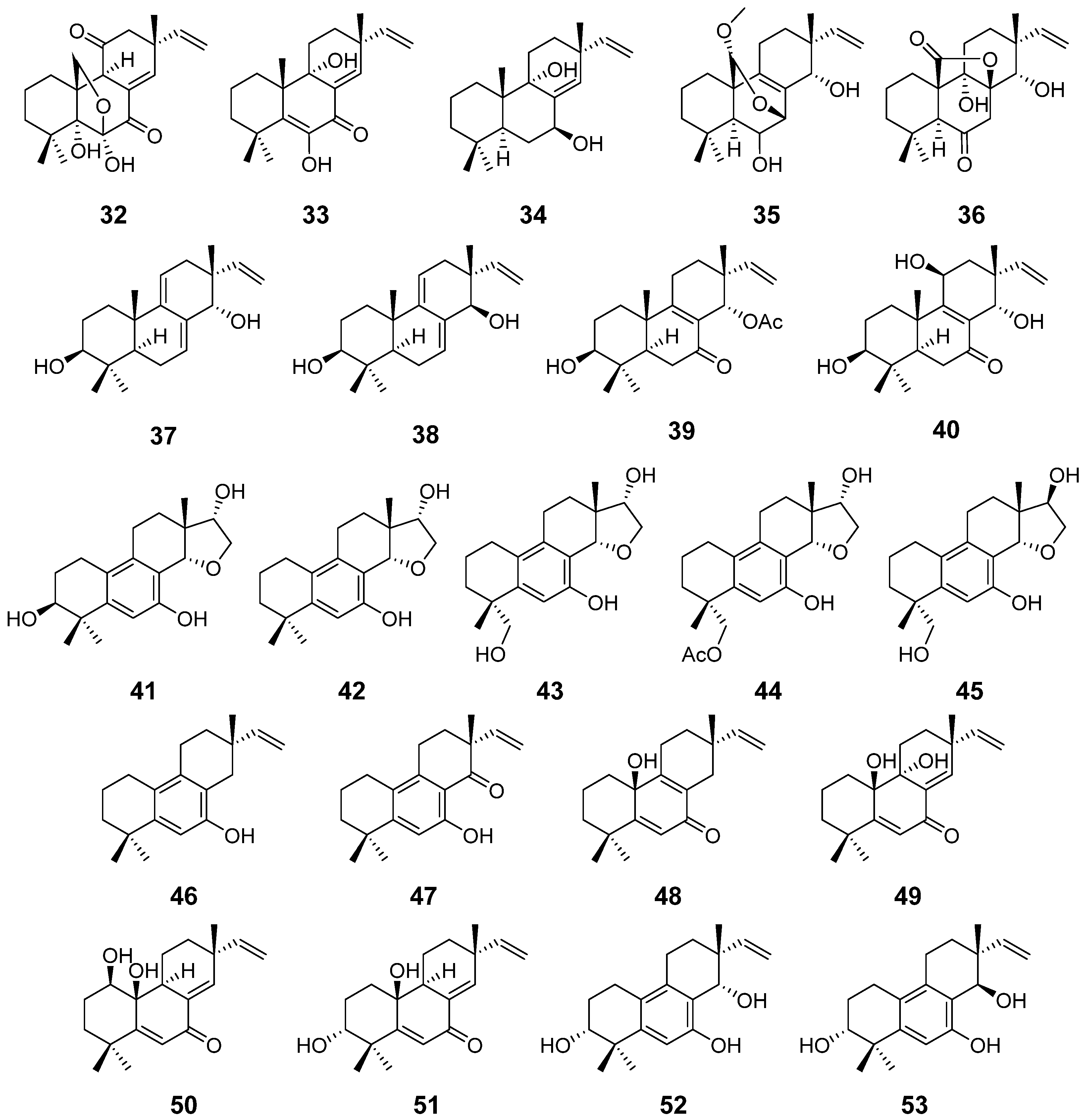
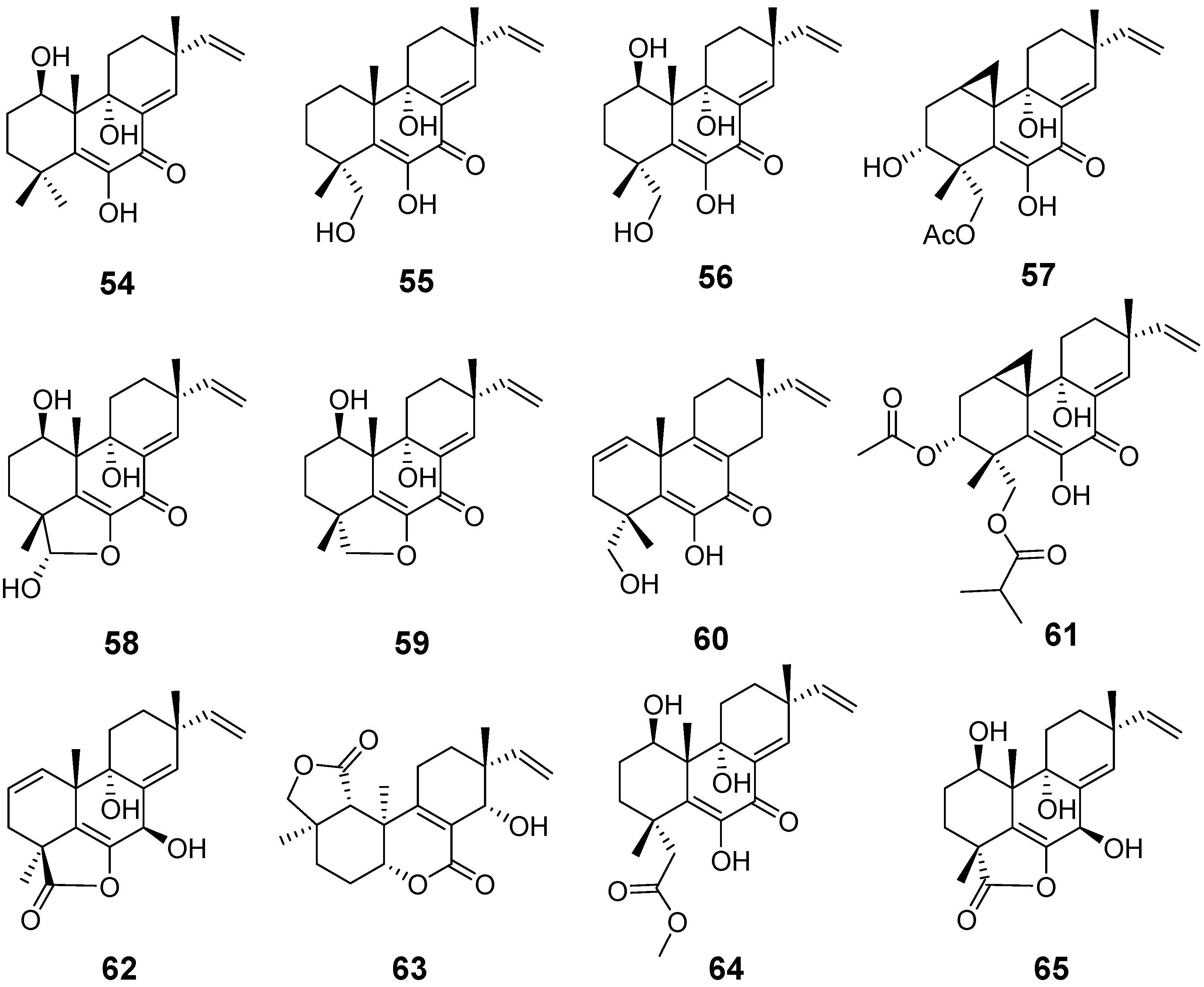
| Libertellenones A (54), B (55), and D (57) | Libertella sp. | cytotoxic activity | [27] |
| Libertellenone C (56) | Libertella sp. Arthrinium sacchari | cytotoxic activity and antiproliferative activity | [27,28] |
| Libertellenones E (58) and F (59) | Arthrinium sacchari | Antiproliferation | [28] |
| Libertellenone G (60) | Eutypella sp. | antibacterial activity | [29] |
| Libertellenone H (61) | Cytotoxic activity | ||
| Libertellenone G (62) and L (63) | Apiospora montagnei | / | [30] |
| Libertellenone J (64) | Phomopsis sp. | anti-inflammatory activity | [31] |
| Libertellenone K (65) | / | ||
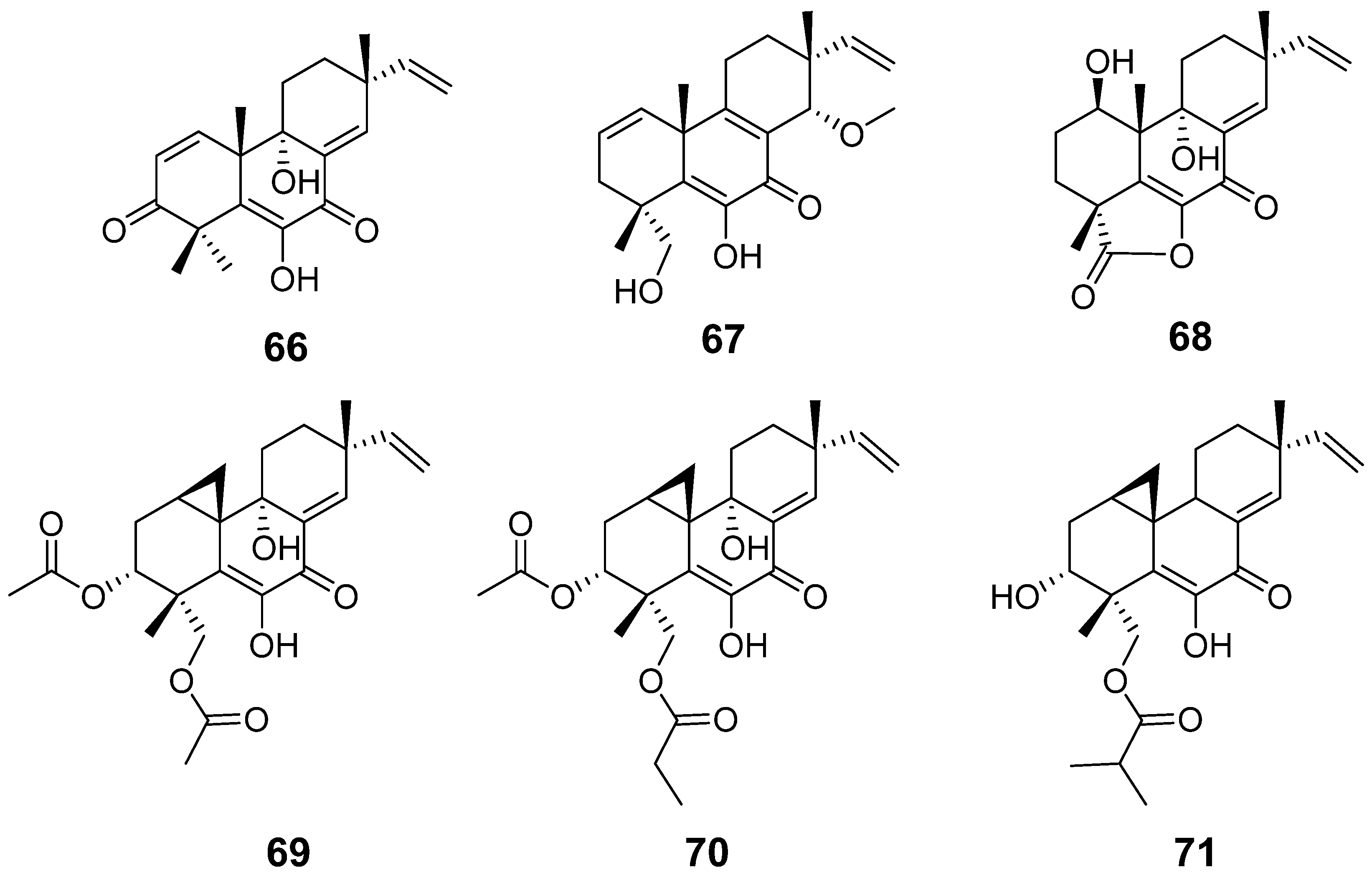
| Libertellenone M (66) | Eutypella sp. | Cytotoxic activity | [32] |
| Libertellenone N (67) | |||
| Libertellenone M (68) | Stilbella fimetaria | Cytotoxic activity | [33] |
| Libertellenones O–P (69–71) | Eutypella sp | Cytotoxic activity | [13] |
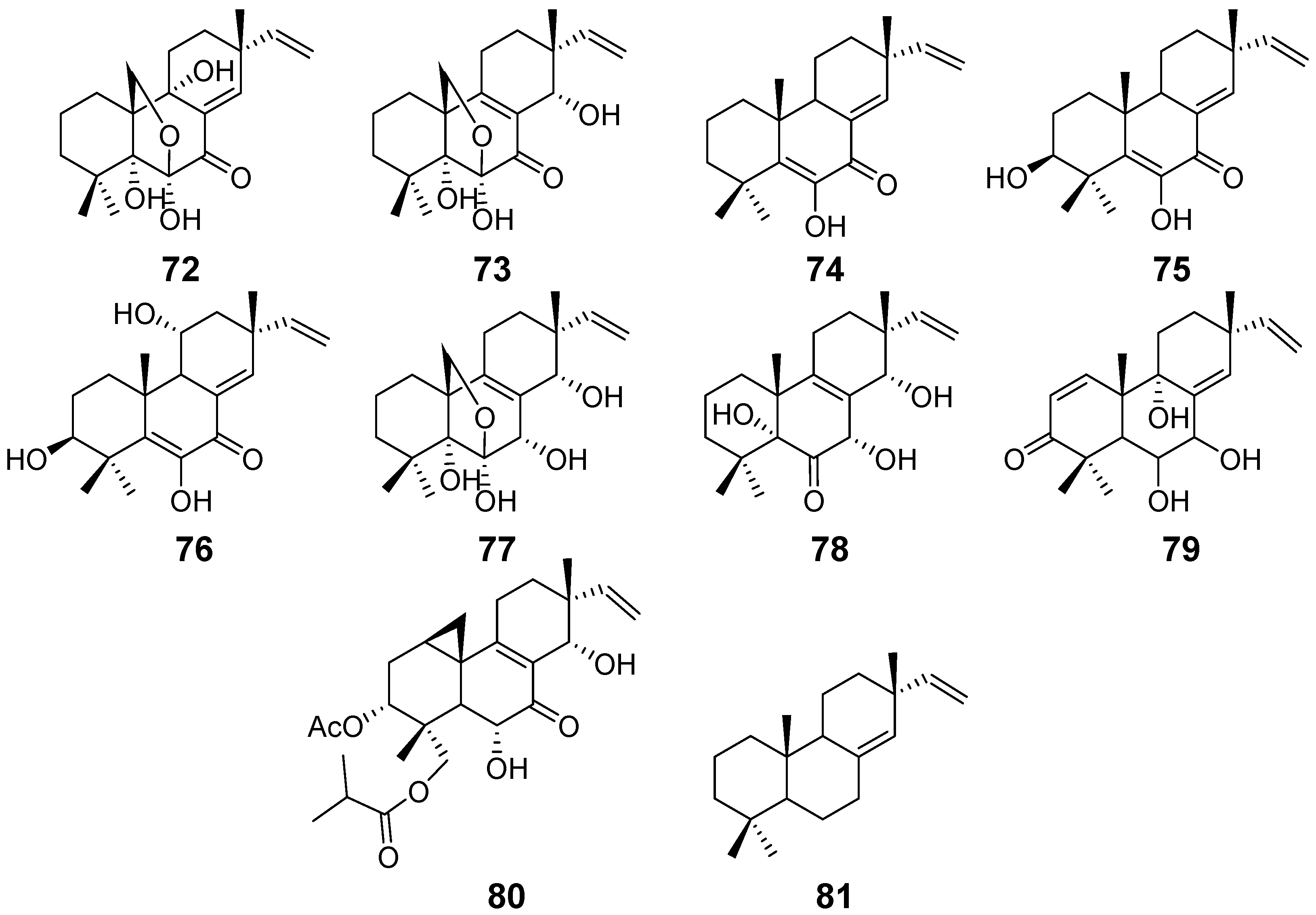
| Scopararanes A–B (72–73) | Eutypella sccparia | / | [34] |
| Scopararanes C–E (74–76), and G (78) | Eutypella sccparia | Cytotoxic activity | [35] |
| Scopararanes F (77) | / | ||
| Scopararane H (79) | Eutypella sp. | / | [36] |
| Scopararane I (80) | Cytotoxic activity | ||
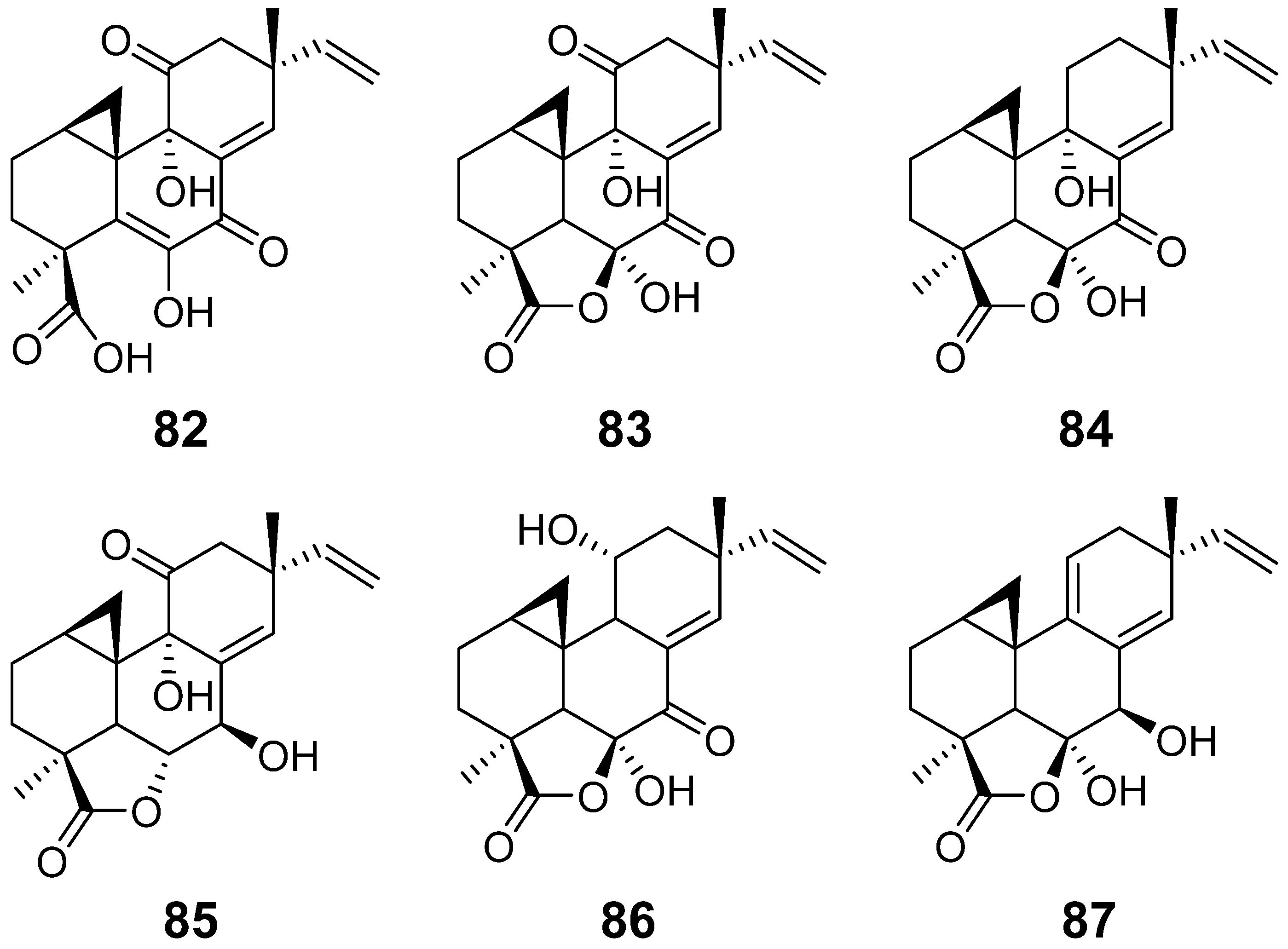
| Myrocin A (82) | Apiospora montagnei. | / | [37] |
| Myrocin B (83) | Myrothecium verrucaria | antimicrobial activity | [38] |
| Myrocin C (84) | Myrothecium sp. | antimicrobial activity | [39] |
| Myrocin D (85) | Arthrinium sacchari | / | [28] |
| Myrocin E (86) | Phomopsis sp. | / | [31] |
| Myrocin F (87) | Stilbella fimetaria | Cytotoxic activity | [33] |
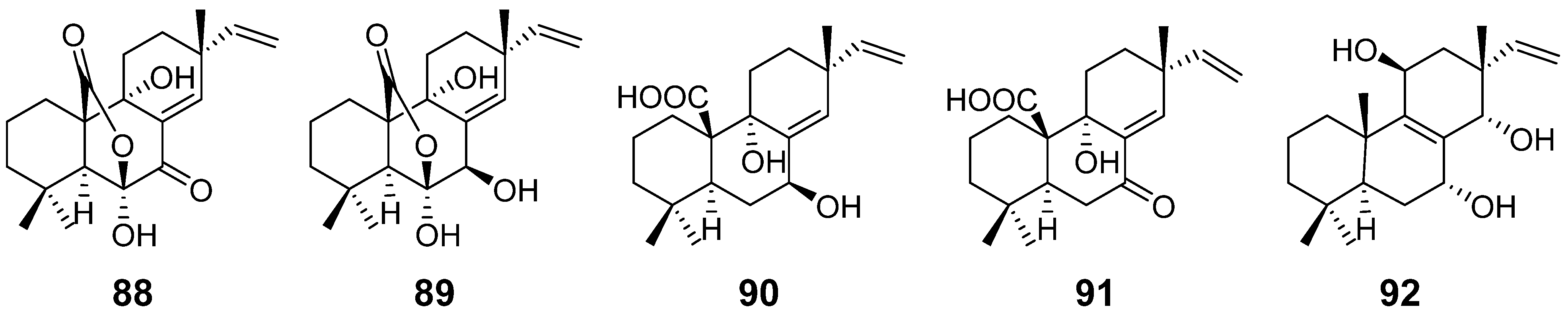
| Sphaeropsidins A–B (88–89) | Sphaeropsis sapinea | phytotoxicity | [40] |
| Sphaeropsidin C (90) | Diplodia mutila | [41] | |
| Sphaeropsidin D (91) | Sphaeropsis sapinea | phytotoxicity | [42] |
| Sphaeropsidin E (92) | / | ||
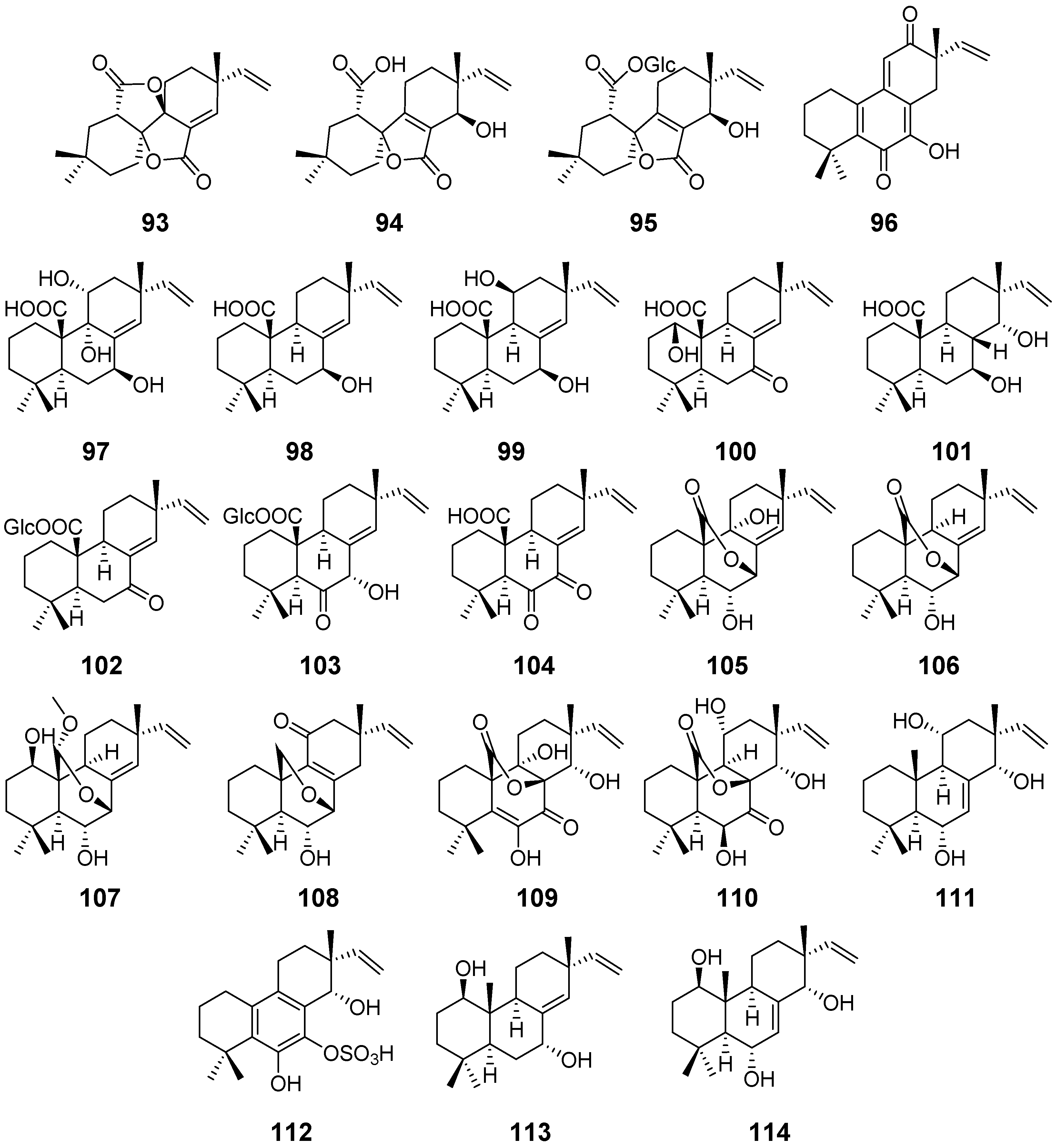
| Taichunin A (93) | Aspergillus taichungensis | Cytotoxic activity | [43] |
| Taichunins B–D (94–96) | / | ||
| Taichunins E (97), F (98), H–J (100–102), L–M (104–105), and O–T (107–112) | Aspergillus taichungensis | / | [44] |
| Taichunin G (99) | Inhibitory Effects on RANKL-Induced Formation of Multinuclear Osteoclasts | ||
| Taichunin K (103) | |||
| Taichunin N (106) | |||
| 1β, 7α-dihydroxysandaracopimar-8(14), 15-diene19 (113) | / | ||
| Apsergiloid D (114) | Aspergillus sp. | / | [45] |
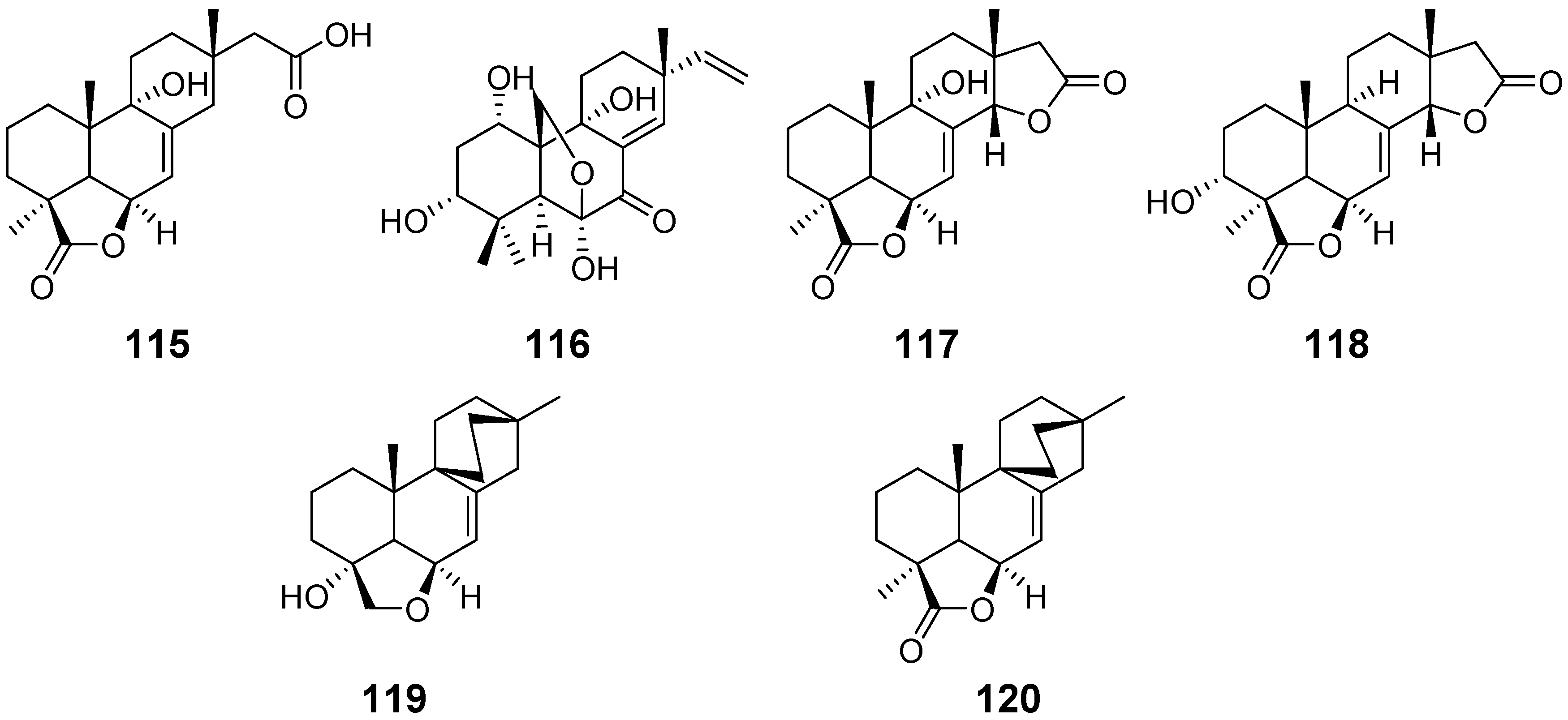
| Xylarenolide (115) | Xylaria sp. Xylaria allantoidea | / | [46,47] |
| Xylallantins A–C (116–118) | Xylaria allantoidea | / | [47] |
| Xylarilongipin A (119) | Xylaria longipes | Immunosuppressive activity | [48] |
| Xylarilongipin B (120) | / | ||
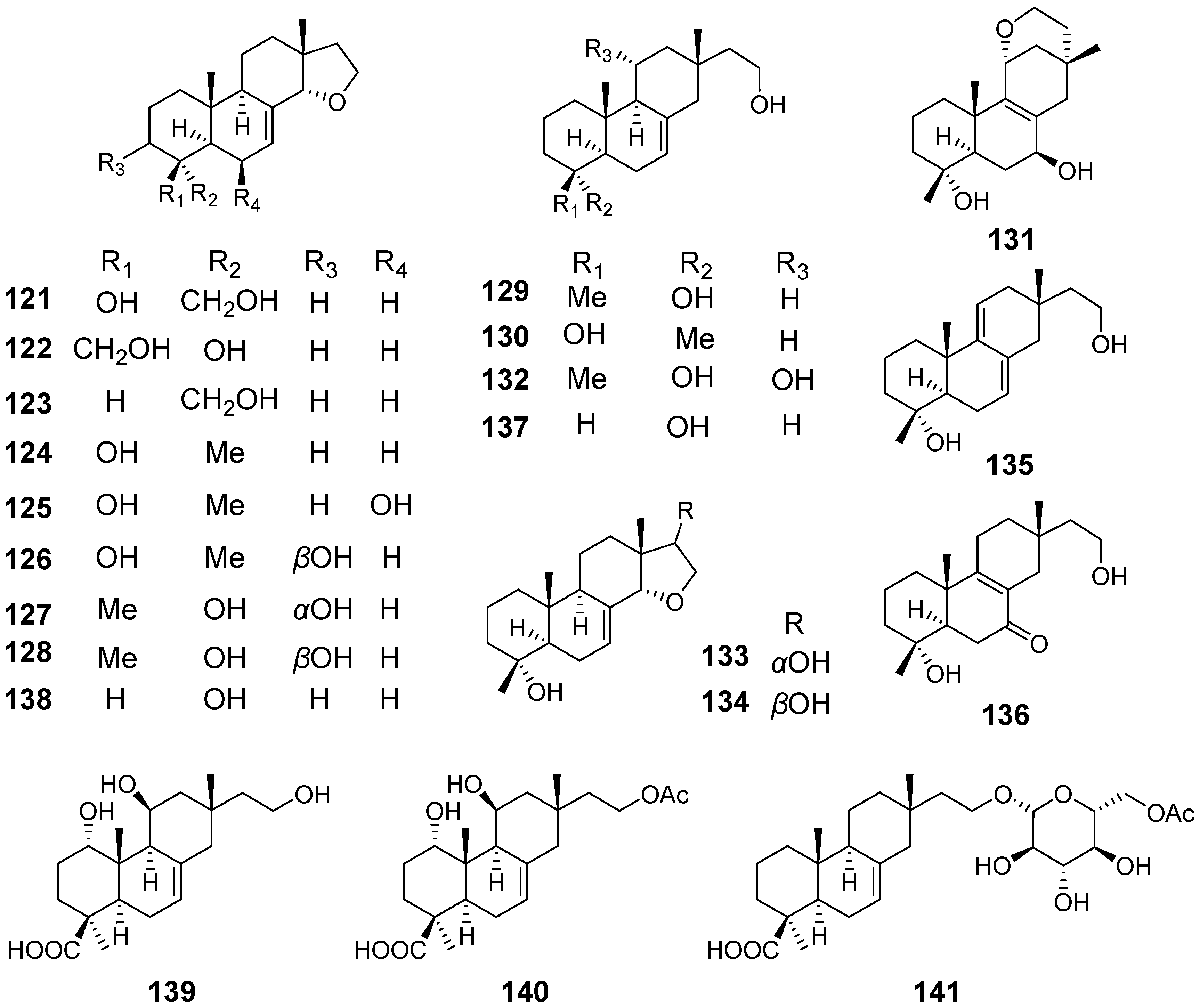
| Xylarinorditerpenes A (121), F–H (126–128), J–M (130–133), O (135), and (136) | Xylaria longipes | / | [49] |
| Xylarinorditerpenes B–E (122–125), I (129), N (134), Q (137), and R (138) | Immunosuppressive activity | ||
| Xylongoic acids A–C (139–141) | Xylaria longipes | / | [50] |
| Compound 142 | Xylaria sp. | / | [51] |
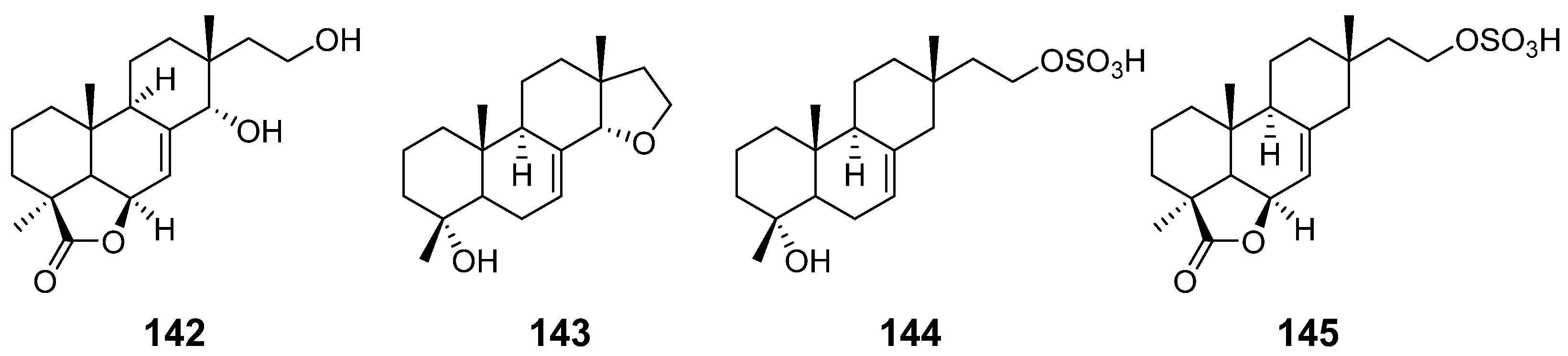
| 14α,16-epoxy-18-norisopimar-7-en-4α-ol (143), 16-O-sulfo-18-norisopimar-7-en-4α,16-diol (144), and 9-deoxy-hymatoxin A (145) | Xylaria sp. | Antifungal activity | [52] |
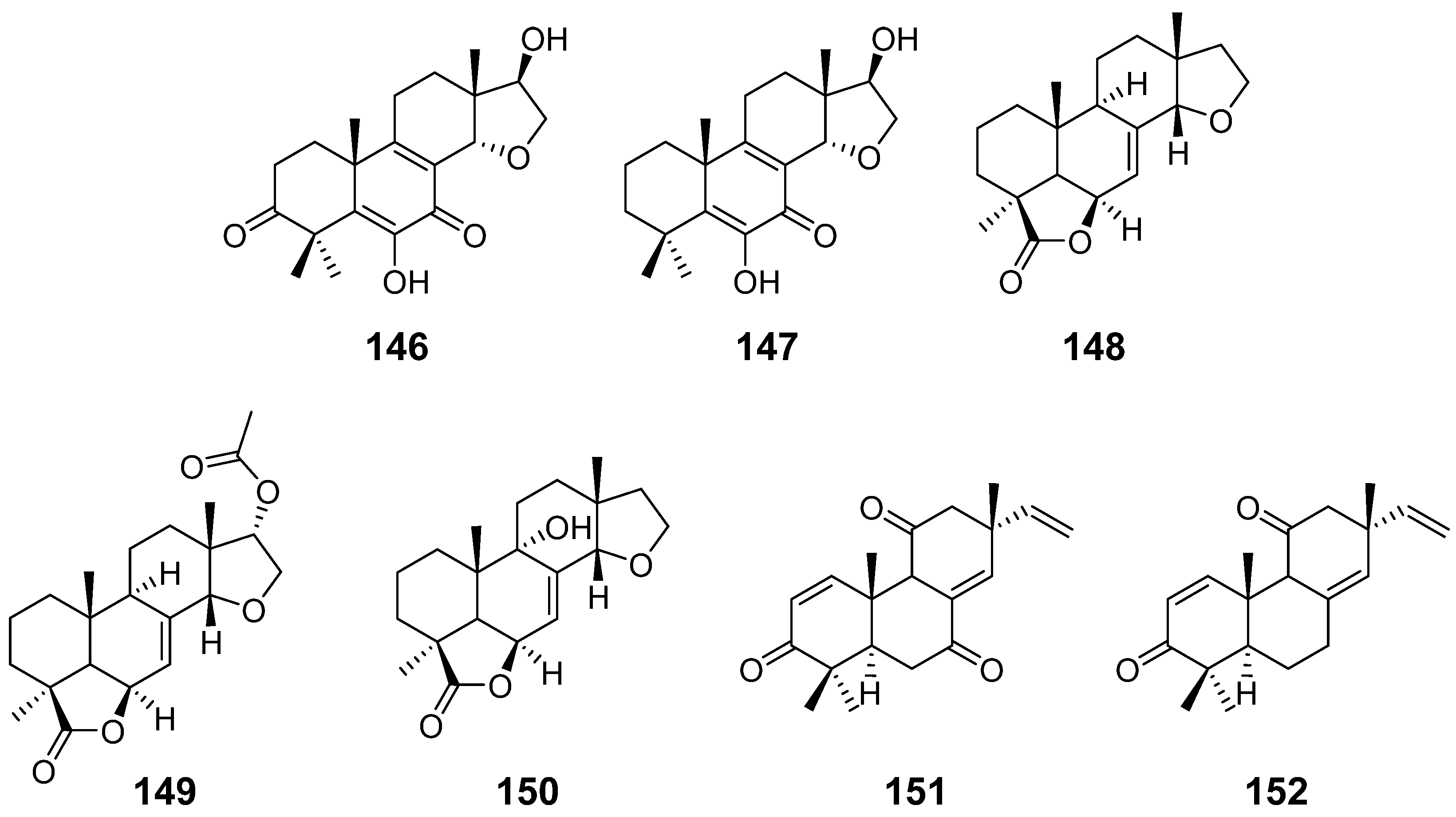
| Calcarisporic acid K (146) and L (147) | Calcarisporium arbuscula | / | [14] |
| Inonotolides A–C (148–150) | Inonotus sinensis | / | [53] |
| 9α-hydroxy-l, 8(14), 15-isopimaratriene-3, 7, 1l-trione (151) and 9α-hydroxy-l, 8(14), 15-isopimaratriene-3, 11-dione (152) | Hormononema dermatioides Phyllosticta sp. | insect toxicity | [54] |
| 16-α-D-mannopyranosyloxyisopimar-7-en-19-oic acid (153), 15-hydroxy-16-α-D-mannopyranosyloxyisopimar-7-en-19-oic acid (154), and 16-α-D-glucopyranosyloxyisopimar-7-en-19-oic acid (155) | Xylaria polymorpha | inhibitory activity against tumour cell lines | [55] |
| Compound 156 and 159–161 | Paraconiothyrium sp. | / | [56] |
| Compound 157 and 158 | Cytotoxic activity | ||
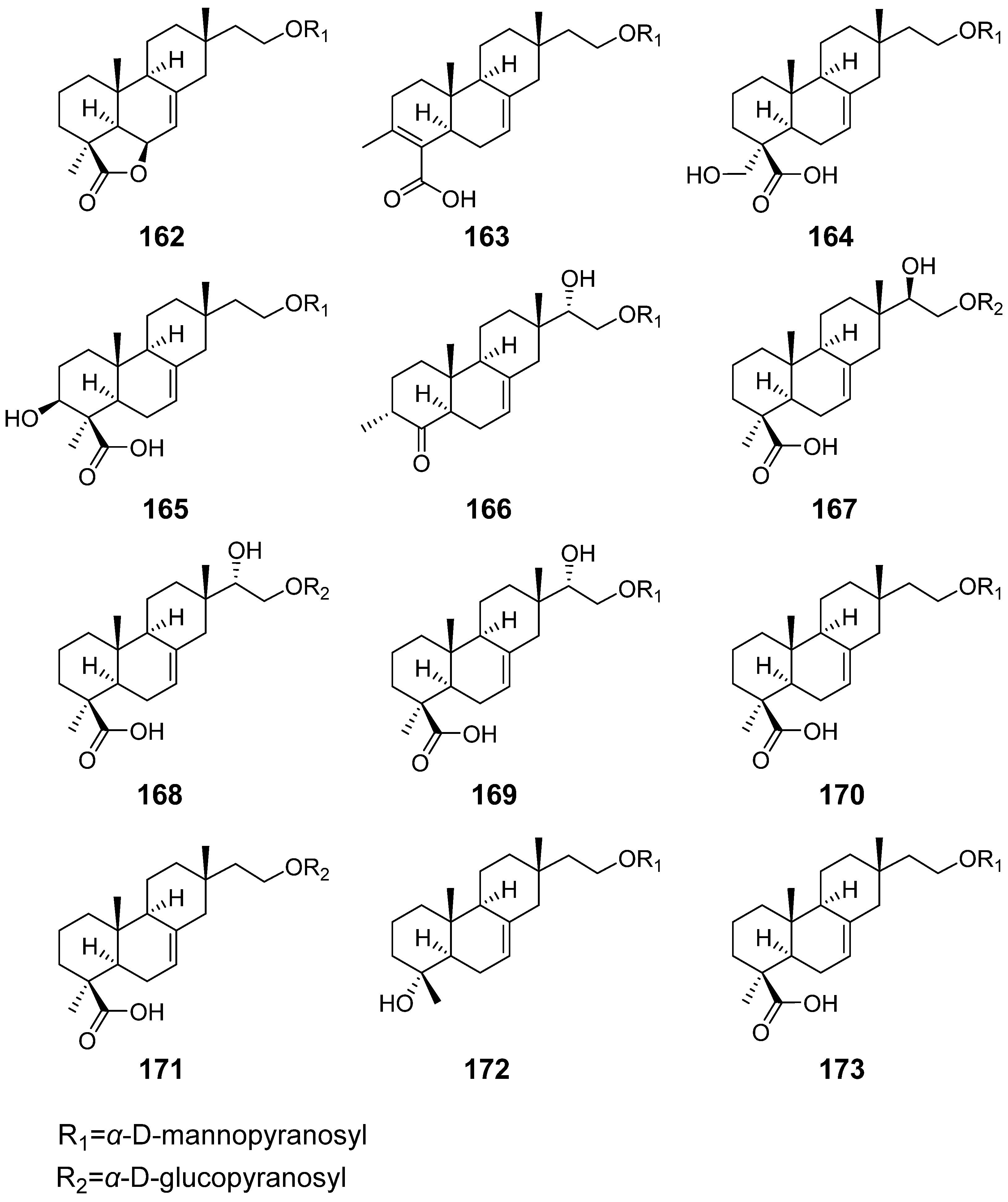
| Hypoxylonoids A–G (162–168) | Xylaria hypoxylon | / | [57] |
| Compound 169–173 | |||
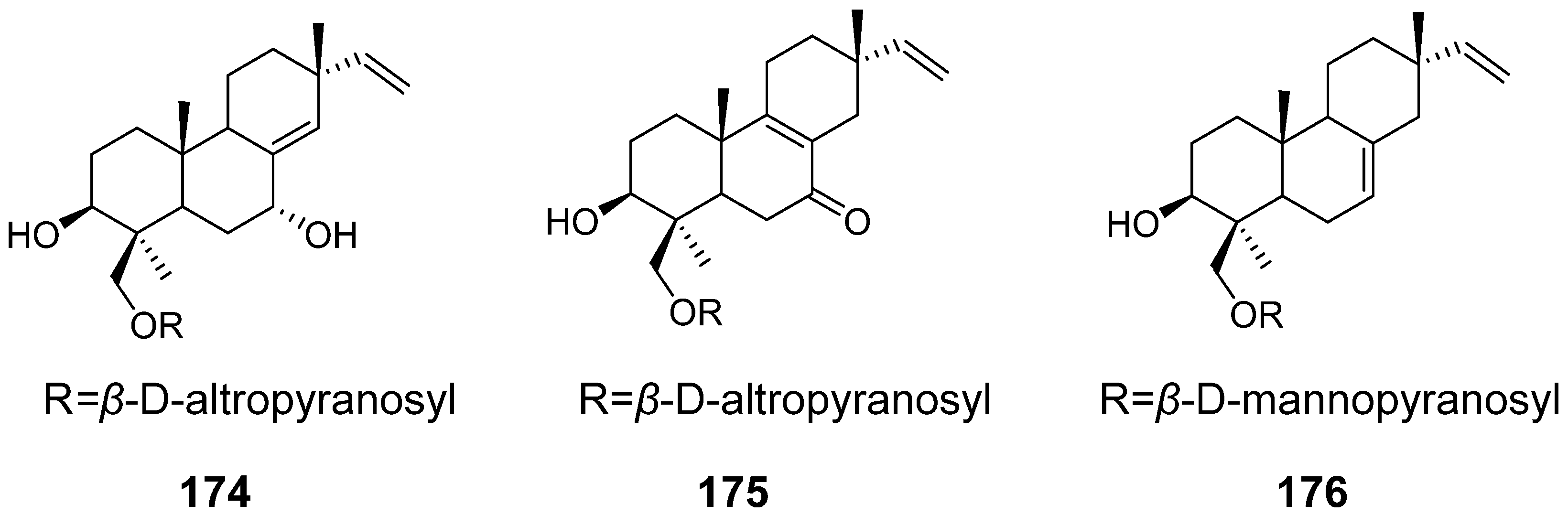
| Virescenosides O–Q (174–176) | Acremonium striatisporum | Cytotoxic activity | [58] |
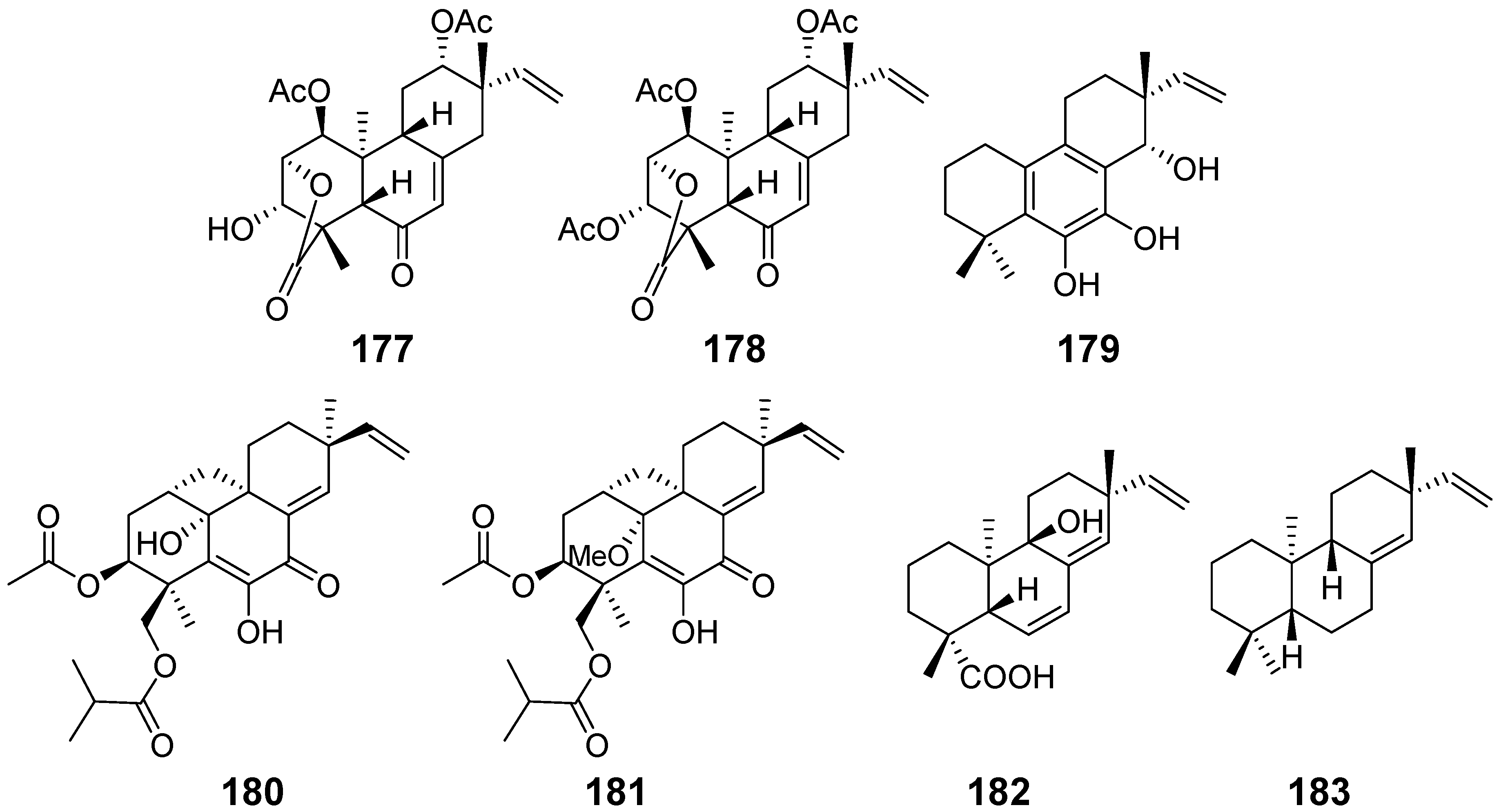
| Chenopodolin (177) | Phoma chenopodiicola | phytotoxic activity | [59] |
| chenopodolin B (178) | Phoma chenopodiicola | phytotoxic activity | [60] |
| Diplopimarane (179) | Diplodia quercivora | phytotoxic activity, zootoxicity, antifungal activity | [61] |
| Eutypellenones A (180) and B (181) | Eutypella sp. | anti-inflammatory activity, cytotoxicity | [13] |
| Isogeopyxin B (182) | Geopyxis sp. | / | [62] |
| ent-Pimara-8(14), 15-diene (183) | Aspergillus nidulans | antioxidant activity | [63] |
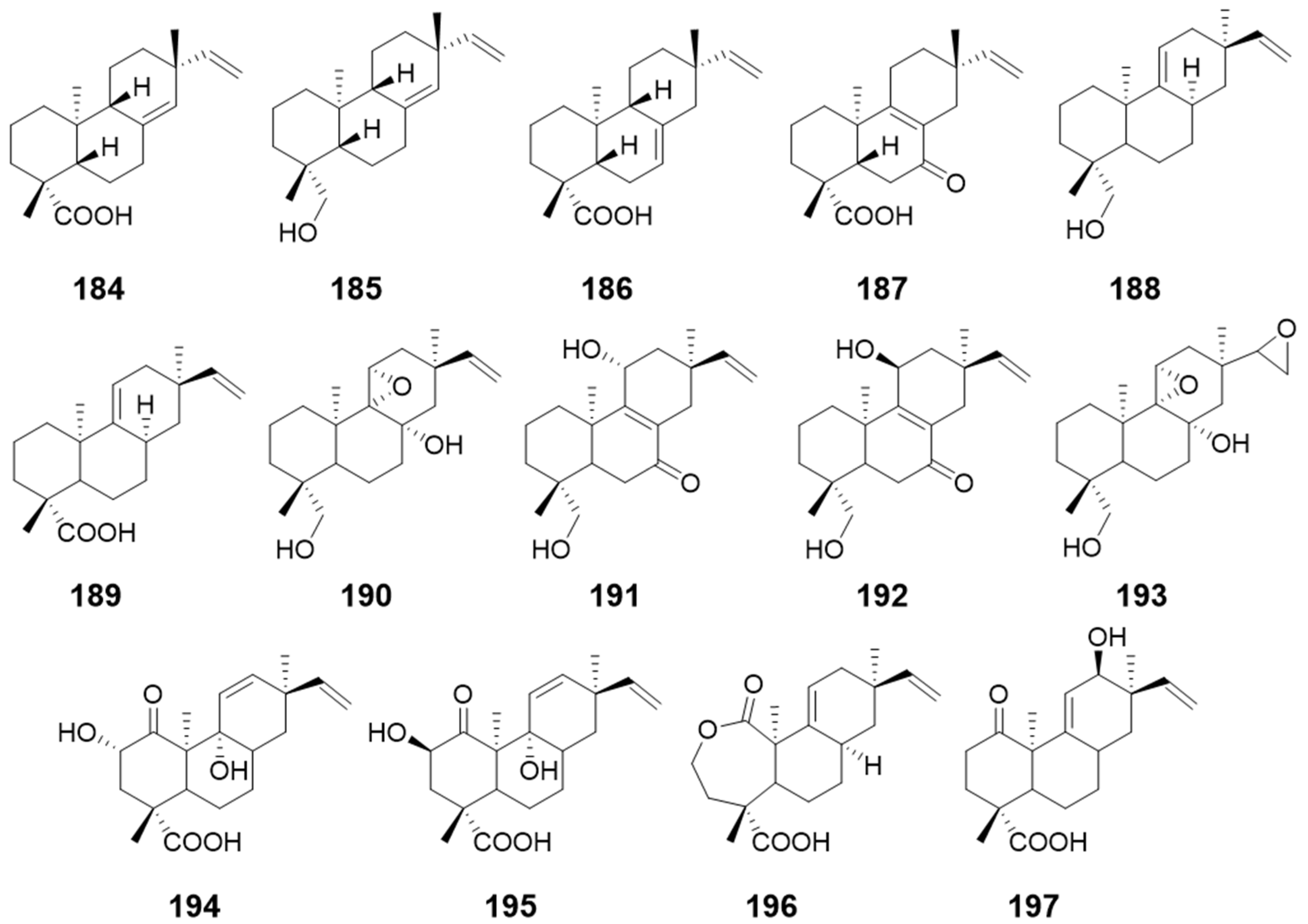
| compounds 184, 185, and 186 | Glomerella cingulateMucor rouxii | antibacterial activity | [64] |
| ent-8(14),15-pimaradien-19-ol (187) | / | ||
| 9-hydroxy-13-epi-ent-pimara-9(11),15-diene (188) and 13-epi-ent-pimara-9 (11),15-diene-19-oic acid (189) | / | / | [65] |
| Compounds 190–193 | Gibberella fujikuroi | / | |
| Compounds 194–197 | Gibberella fujikuroi | / |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ye, K.; Ai, H.-l. Pimarane Diterpenes from Fungi. Pharmaceuticals 2022, 15, 1291. https://doi.org/10.3390/ph15101291
Ye K, Ai H-l. Pimarane Diterpenes from Fungi. Pharmaceuticals. 2022; 15(10):1291. https://doi.org/10.3390/ph15101291
Chicago/Turabian StyleYe, Ke, and Hong-lian Ai. 2022. "Pimarane Diterpenes from Fungi" Pharmaceuticals 15, no. 10: 1291. https://doi.org/10.3390/ph15101291
APA StyleYe, K., & Ai, H.-l. (2022). Pimarane Diterpenes from Fungi. Pharmaceuticals, 15(10), 1291. https://doi.org/10.3390/ph15101291







