Engineered-Skin of Single Dermal Layer Containing Printed Hybrid Gelatin-Polyvinyl Alcohol Bioink via 3D-Bioprinting: In Vitro Assessment under Submerged vs. Air-Lifting Models
Abstract
1. Introduction
2. Results
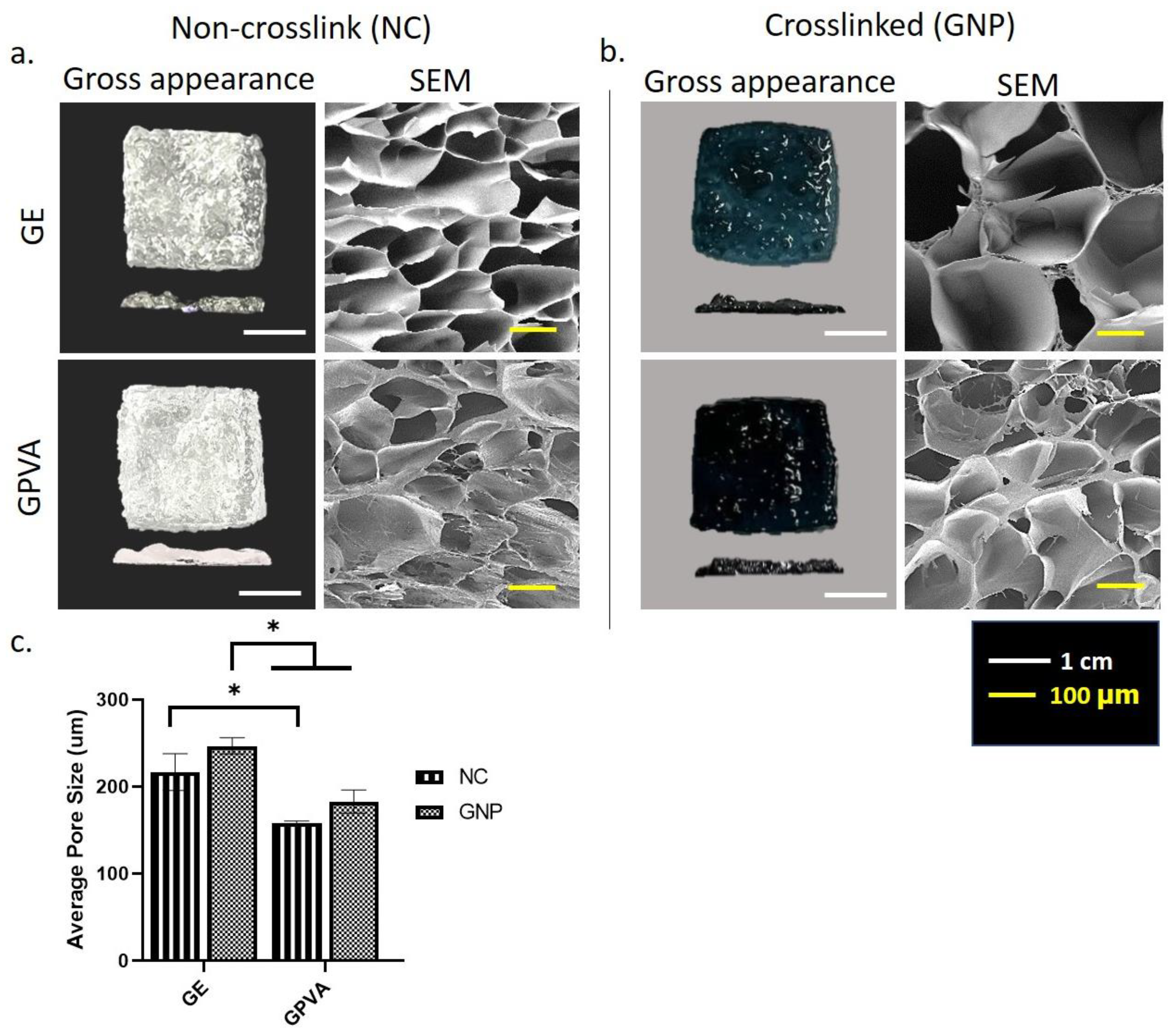
2.1. Gross Appearance of Single Dermal Layer 3D In Vitro Skin Model
2.2. D-Microporous Characterisation Using Scanning Electron Microscopy
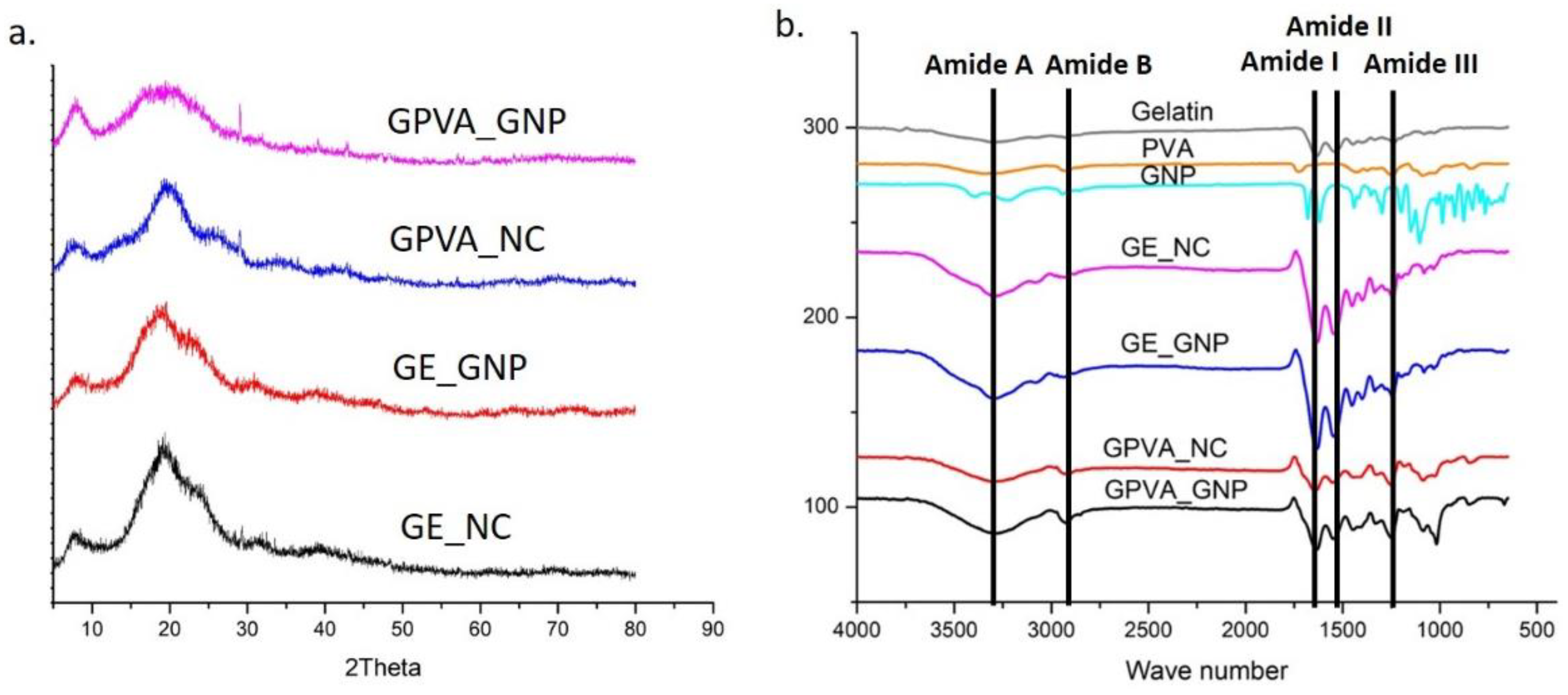
2.3. Chemical Characterisation of 3D-Printed Hydrogel
2.4. D In-Vitro Skin Equivalent for Cytotoxicity Testing and Cell Morphology
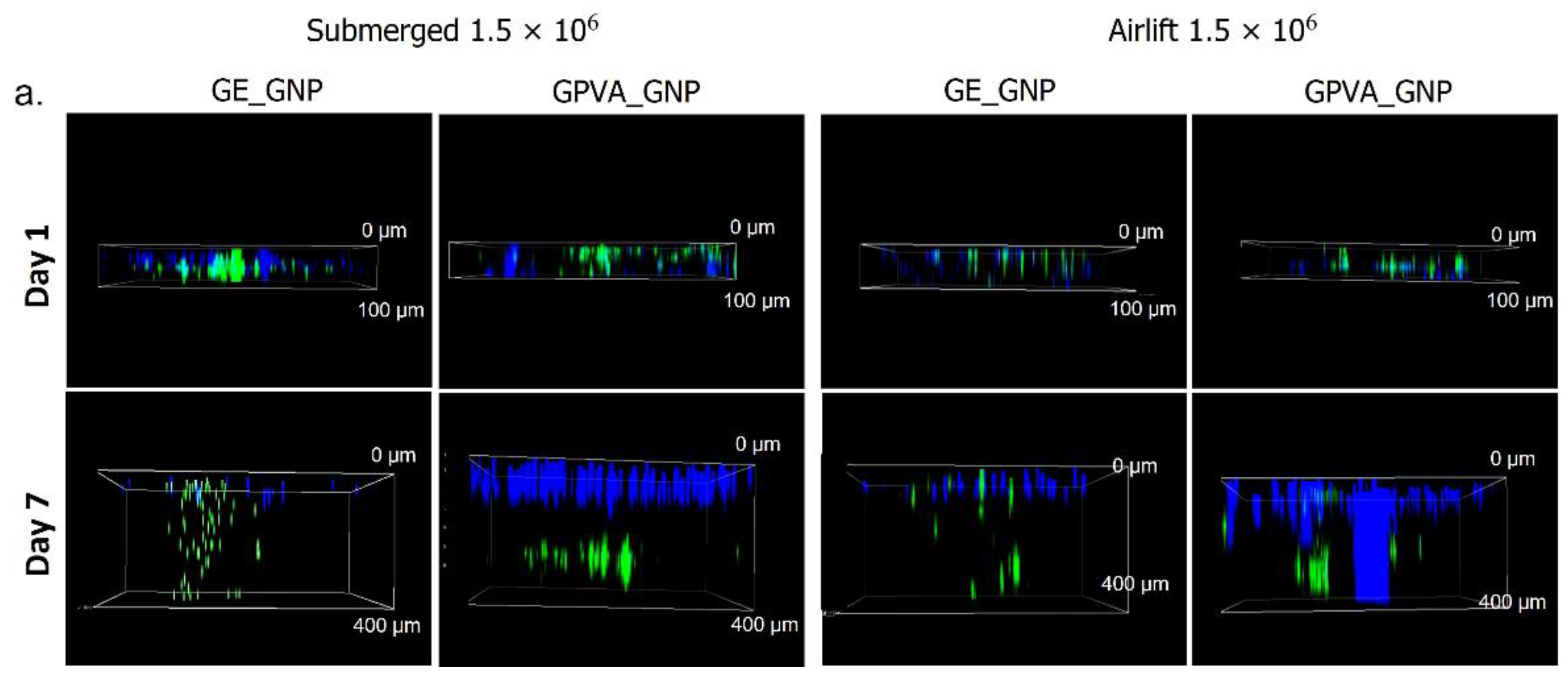
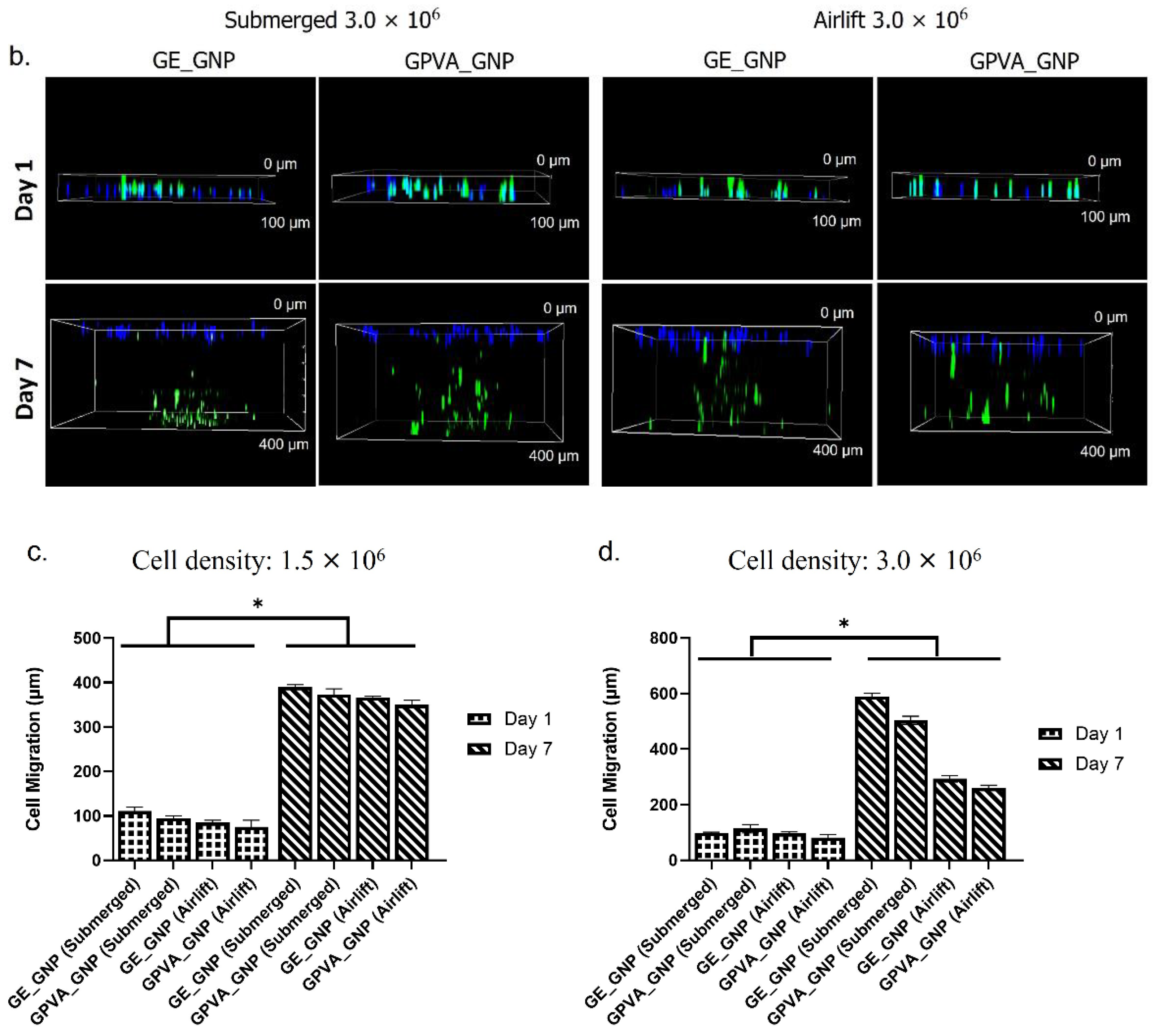
2.5. D In-Vitro Skin Equivalent Cell Migration Assessment
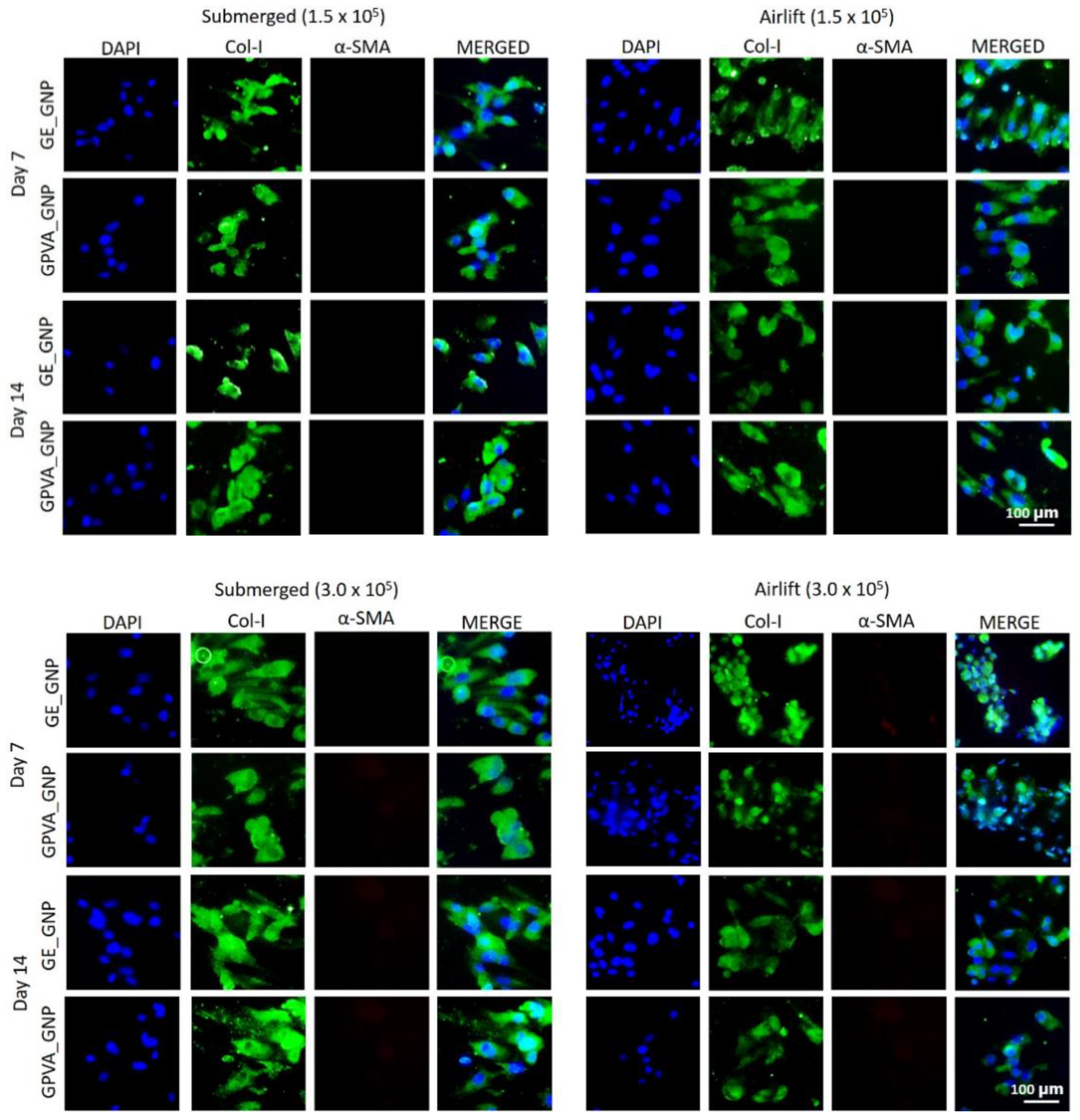
2.6. Immunocytochemistry (Collagen Type I and Alpha Smooth Muscle Actin)
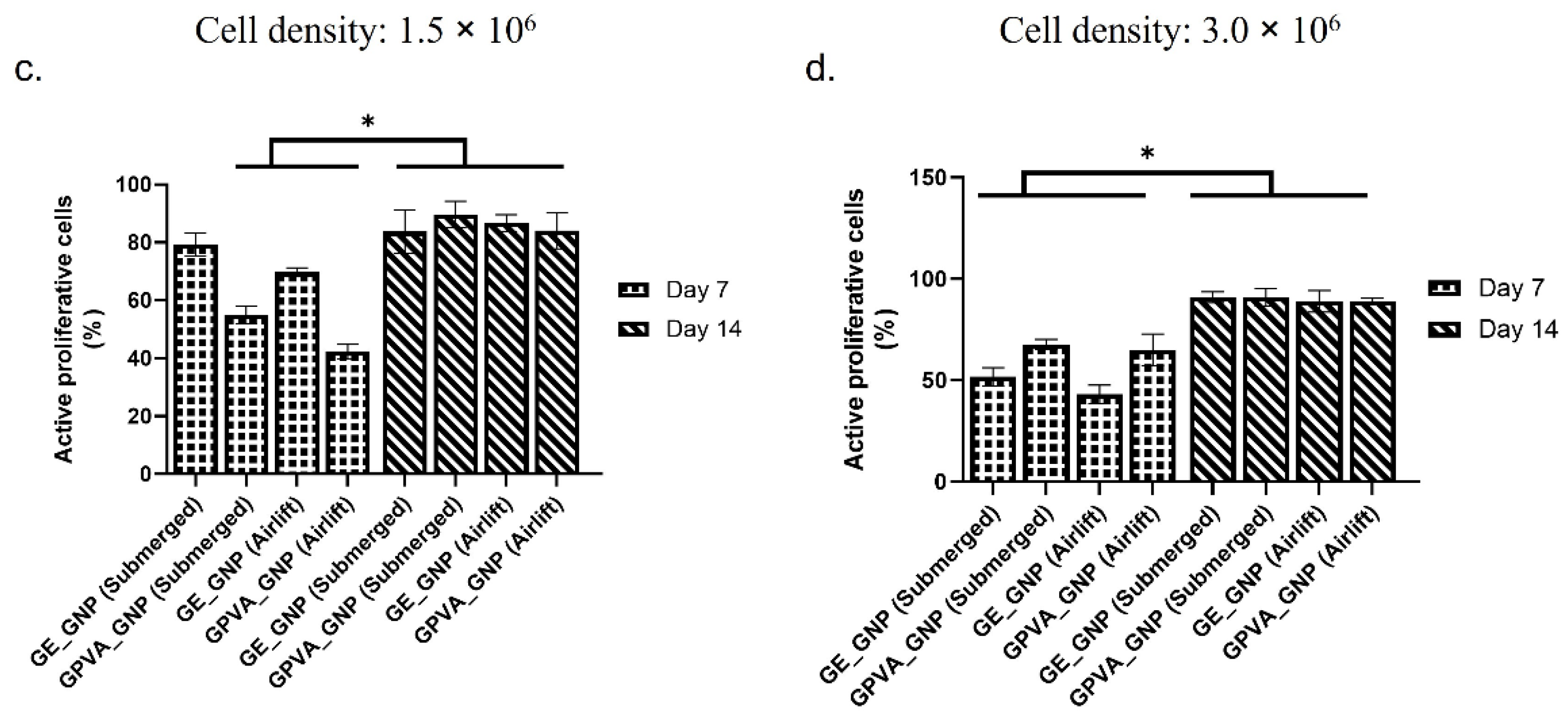
2.7. Cell Proliferation Activity (Ki-67)
3. Discussion
4. Materials and Methods
4.1. Reconstruction of 3D In-Vitro Skin Model (Single Dermal Layer) Using Bioinks
4.2. Printer Compatibility Testing
4.3. Gross Appearance Evaluation of 3D-Bioprinted Hydrogel
4.4. Microporous Structure of Hydrogels through Scanning-Electron Microscopy
4.5. Chemical Characterisation
4.6. Primary Skin Cell Isolations and Culture
4.7. Cytotoxicity Testing of 3D In Vitro Model
4.8. Cell Morphology Encapsulated in the Hydrogels
4.9. Cell Migration Activity
4.10. Immunocytochemistry (Protein Expression)
4.11. Statistical Analysis
5. Conclusions and Future Perspective
Author Contributions
Funding

Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Idrees, A.; Schmitz, I.; Zoso, A.; Gruhn, D.; Pacharra, S.; Shah, S.; Ciardelli, G.; Viebahn, R.; Chiono, V.; Salber, J. Fundamental in vitro 3D human skin equivalent tool development for assessing biological safety and biocompatibility–towards alternative for animal experiments. 4Open 2021, 4, 1. [Google Scholar] [CrossRef]
- Souci, L.; Denesvre, C. 3D skin models in domestic animals. Vet. Res. 2021, 52, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Medawar, P.B. The cultivation of adult mammalian skin epithelium in vitro. Q. J. Microsc. Sci. 1948, 89, 187–196. [Google Scholar] [CrossRef]
- Bojar, R.A. Studying the Human Skin Microbiome Using 3D In Vitro Skin Models. Appl. Vitr. Toxicol. 2015, 1, 165–171. [Google Scholar] [CrossRef]
- Netzlaff, F.; Lehr, C.M.; Wertz, P.W.; Schaefer, U.F. The human epidermis models EpiSkin®, SkinEthic® and EpiDerm®: An evaluation of morphology and their suitability for testing phototoxicity, irritancy, corrosivity, and substance transport. Eur. J. Pharm. Biopharm. 2005, 60, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Van den Broek, L.J.; Bergers, L.I.J.C.; Reijnders, C.M.A.; Gibbs, S. Progress and Future Prospectives in Skin-on-Chip Development with Emphasis on the use of Different Cell Types and Technical Challenges. Stem Cell Rev. Rep. 2017, 13, 418–429. [Google Scholar] [CrossRef]
- Pourchet, L.J.; Thepot, A.; Albouy, M.; Courtial, E.J.; Boher, A.; Blum, L.J.; Marquette, C.A. Human Skin 3D Bioprinting Using Scaffold-Free Approach. Adv. Healthc. Mater. 2017, 6, 1601101. [Google Scholar] [CrossRef] [PubMed]
- Varkey, M.; Visscher, D.O.; Van Zuijlen, P.P.M.; Atala, A.; Yoo, J.J. Skin bioprinting: The future of burn wound reconstruction? Burn. Trauma 2019, 7, 1–12. [Google Scholar] [CrossRef]
- Masri, S.; Fauzi, M.B. Current Insight of Printability Quality Improvement Strategies in Natural-Based Bioinks for Skin Regeneration and Wound Healing. Polymers 2021, 13, 1101. [Google Scholar] [CrossRef]
- Bishop, E.S.; Mostafa, S.; Pakvasa, M.; Luu, H.H.; Lee, M.J.; Wolf, J.M.; Ameer, G.A.; He, T.C.; Reid, R.R. 3-D bioprinting technologies in tissue engineering and regenerative medicine: Current and future trends. Genes Dis. 2017, 4, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Marques, C.F.; Diogo, G.S.; Pina, S.; Oliveira, J.M.; Silva, T.H.; Reis, R.L. Collagen-based bioinks for hard tissue engineering applications: A comprehensive review. J. Mater. Sci. Mater. Med. 2019, 30, 1–12. [Google Scholar] [CrossRef]
- Groll, J.; Burdick, J.A.; Cho, D.W.; Derby, B.; Gelinsky, M.; Heilshorn, S.C.; Jüngst, T.; Malda, J.; Mironov, V.A.; Nakayama, K.; et al. A definition of bioinks and their distinction from biomaterial inks. Biofabrication 2019, 11, 013001. [Google Scholar] [CrossRef] [PubMed]
- Nagarkar, R.; Patel, J. Polyvinyl Alcohol: A Comprehensive Study. Acta Sci. Pharm. Sci. 2019, 3, 34–44. [Google Scholar]
- He, P.; Zhao, J.; Zhang, J.; Li, B.; Gou, Z.; Gou, M.; Li, X. Bioprinting of skin constructs for wound healing. Burn. Trauma 2018, 6, 1–10. [Google Scholar] [CrossRef]
- Hoque, M.E.; Sharifi, S.; Hoque, M.E.; Nuge, T.; Yeow, T.K.; Nordin, N.; Prasad, R.G.S.V. Hybrid Nanofibre Mat rix for Regenerat ive Therapy Fabricated by Electrospinning: Effects of Process Parameters on the Fibre Efficacy. A review of key challenges of elect rospun scaffolds for t issue-engineering applicat ions gelatin based scaffolds for tissue engineering-a review. J. Nanomed. Biother. Discov. 2014, 4, 1. [Google Scholar]
- Nike, D.U.; Katas, H.; Mohd, N.F.; Hiraoka, Y.; Tabata, Y.; Idrus, R.B.H.; Fauzi, M.B. Characterisation of rapid in situ forming gelipin hydrogel for future use in irregular deep cutaneous wound healing. Polymers 2021, 13, 3152. [Google Scholar] [CrossRef] [PubMed]
- Bello, A.B.; Kim, D.; Kim, D.; Park, H.; Lee, S.H. Engineering and functionalization of gelatin biomaterials: From cell culture to medical applications. Tissue Eng.-Part B Rev. 2020, 26, 164–180. [Google Scholar] [CrossRef]
- Shanmugam, M.K.; Shen, H.; Tang, F.R.; Arfuso, F.; Rajesh, M.; Wang, L.; Kumar, A.P.; Bian, J.; Goh, B.C.; Bishayee, A.; et al. Potential role of genipin in cancer therapy. Pharmacol. Res. 2018, 133, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Khoeini, R.; Nosrati, H.; Akbarzadeh, A.; Eftekhari, A.; Kavetskyy, T.; Khalilov, R.; Ahmadian, E.; Nasibova, A.; Datta, P.; Roshangar, L.; et al. Natural and Synthetic Bioinks for 3D Bioprinting. Adv. NanoBiomed Res. 2021, 1, 2000097. [Google Scholar] [CrossRef]
- Salleh, A.; Mustafa, N.; Teow, Y.H.; Fatimah, M.N.; Khairudin, F.A. Dual-Layered Approach of Ovine Collagen-Gelatin/Cellulose Hybrid Biomatrix Containing Graphene Oxide-Silver Nanoparticles for Cutaneous Wound Healing: Fabrication, Physicochemical, Cytotoxicity and Antibacterial Characterisation. Biomedicines 2022, 10, 816. [Google Scholar] [CrossRef]
- Zulkiflee, I.; Fauzi, M.B. Gelatin-polyvinyl alcohol film for tissue engineering: A concise review. Biomedicines 2021, 9, 979. [Google Scholar] [CrossRef] [PubMed]
- Muppalaneni, S.; Omidian, H. Polyvinyl Alcohol in Medicine and Pharmacy: A Perspective. J. Dev. Drugs 2013, 2, 1–5. [Google Scholar] [CrossRef]
- Janik, H.; Marzec, M. A review: Fabrication of porous polyurethane scaffolds. Mater. Sci. Eng. C 2015, 48, 586–591. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.; Tanaka, M. Designing smart biomaterials for tissue engineering. Int. J. Mol. Sci. 2018, 19, 17. [Google Scholar] [CrossRef]
- Mather, J.A. Ethics and care: For animals, not just mammals. Animals 2019, 9, 1018. [Google Scholar] [CrossRef] [PubMed]
- Nayak, S.; Dey, S.; Kundu, S.C. Skin Equivalent Tissue-Engineered Construct: Co-Cultured Fibroblasts/ Keratinocytes on 3D Matrices of Sericin Hope Cocoons. PLoS ONE 2013, 8, e74779. [Google Scholar] [CrossRef]
- Yildirimer, L.; Hobson, D.; Lin, Z.Y.W.; Cui, W.; Zhao, X. Tissue-Engineered Human Skin Equivalents and Their Applications in Wound Healing. Tissue Eng. Artif. Organs Regen. Med. Smart Diagn. Pers. Med. 2016, 1–2, 215–241. [Google Scholar] [CrossRef]
- Tabatabaei, F.; Moharamzadeh, K.; Tayebi, L. Fibroblast encapsulation in gelatin methacryloyl (GelMA) versus collagen hydrogel as substrates for oral mucosa tissue engineering. J. Oral Biol. Craniofacial Res. 2020, 10, 573–577. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.S.; Xiang, Y.; Cui, Y.L.; Lin, K.M.; Zhang, X.F. Dietary blue pigments derived from genipin, attenuate inflammation by inhibiting LPS-induced iNOS and COX-2 expression via the NF-κB inactivation. PLoS ONE 2012, 7, e34122. [Google Scholar] [CrossRef]
- Kirchmajer, D.M.; Watson, C.A.; Ranson, M.; Panhuis, M.I.H. Gelapin, a degradable genipin cross-linked gelatin hydrogel. RSC Adv. 2013, 3, 1073–1081. [Google Scholar] [CrossRef]
- Fauzi, M.B.; Rashidbenam, Z.; Saim, A.B.; Idrus, R.B.H. Preliminary study of in vitro three-dimensional skin model using an ovine collagen type i sponge seeded with co-culture skin cells: Submerged versus air-liquid interface conditions. Polymers 2020, 12, 2784. [Google Scholar] [CrossRef] [PubMed]
- Rasoulianboroujeni, M.; Kiaie, N.; Tabatabaei, F.S.; Yadegari, A.; Fahimipour, F.; Khoshroo, K.; Tayebi, L. Dual Porosity Protein-based Scaffolds with Enhanced Cell Infiltration and Proliferation. Sci. Rep. 2018, 8, 14889. [Google Scholar] [CrossRef] [PubMed]
- Mu, C.; Zhang, K.; Lin, W.; Li, D. Ring-opening polymerization of genipin and its long-range crosslinking effect on collagen hydrogel. J. Biomed. Mater. Res.-Part A 2013, 101, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Zidaric, T.; Milojevic, M.; Gradisnik, L.; Kleinschek, K.S.; Maver, U.; Maver, T. Polysaccharide-Based Bioink Formulation for 3D Bioprinting of an In Vitro Model of the Human Dermis. Nanomaterials 2020, 10, 733. [Google Scholar] [CrossRef]
- Nguyen, T.-H.; Ventura, R.; Min, Y.-K.; Lee, B.-T. Genipin Cross-Linked Polyvinyl Alcohol-Gelatin Hydrogel for Bone Regeneration. J. Biomed. Sci. Eng. 2016, 9, 419–429. [Google Scholar] [CrossRef]
- Vila, A.; Torras, N.; Castaño, A.G.; García-Díaz, M.; Comelles, J.; Pérez-Berezo, T.; Corregidor, C.; Castaño, Ó.; Engel, E.; Fernández-Majada, V.; et al. pte ce d M pt. ACS Appl. Mater. Interfaces 2018, 10, 16. [Google Scholar]
- Yang, Y.; Xu, R.; Wang, C.; Guo, Y.; Sun, W.; Ouyang, L. Recombinant Human Collagen-Based Bioinks for the 3D Bioprinting of Full-thickness Human Skin Equivalent. Int. J. Bioprinting 2022, 8, 145–160. [Google Scholar] [CrossRef]
- Cao, Y.; Lee, B.H.; Irvine, S.A.; Wong, Y.S.; Peled, H.B.; Venkatraman, S. Inclusion of cross-linked elastin in gelatin/PEG hydrogels favourably influences fibroblast phenotype. Polymers 2020, 12, 670. [Google Scholar] [CrossRef]
- Dogan, A.A.; Dufva, M. Customized 3D-printed stackable cell culture inserts tailored with bioactive membranes. Sci. Rep. 2022, 12, 3694. [Google Scholar] [CrossRef] [PubMed]
- Smolar, J.; De Nardo, D.; Reichmann, E.; Gobet, R.; Eberli, D.; Horst, M. Detrusor bioengineering using a cell-enriched compressed collagen hydrogel. J. Biomed. Mater. Res.-Part B Appl. Biomater. 2020, 108, 3045–3055. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, N.; Geng, L.; Yue, Y.; Zhang, Q.; Wang, J. Fabrication of Sulfated Heterosaccharide/Poly(Vinyl Alcohol) Hydrogel Nanocomposite for Application as Wound Healing Dressing. Molecules 2022, 27, 1801. [Google Scholar] [CrossRef] [PubMed]
- Tracy, L.E.; Minasian, R.A.; Caterson, E.J. Extracellular Matrix and Dermal Fibroblast Function in the Healing Wound. Adv. Wound Care 2016, 5, 119–136. [Google Scholar] [CrossRef] [PubMed]
- Ibañez, R.I.R.; Do Amaral, R.J.F.C.; Reis, R.L.; Marques, A.P.; Murphy, C.M.; O’brien, F.J. 3D-Printed Gelatin Methacrylate Scaffolds With Controlled Architecture and Stiffness Modulate the Fibroblast Phenotype Towards Dermal Regeneration. Polymers 2021, 13, 2510. [Google Scholar] [CrossRef] [PubMed]
- Roosens, A.; Handoyo, Y.P.; Dubruel, P.; Declercq, H. Impact of modified gelatin on valvular microtissues. J. Tissue Eng. Regen. Med. 2019, 13, 771–784. [Google Scholar] [CrossRef]
- Lu, K.; Li, K.; Zhang, M.; Fang, Z.; Wu, P.; Feng, L.; Deng, K.; Yu, C.; Deng, Y.; Xiao, Y.; et al. Adipose-derived stem cells (ADSCs) and platelet-rich plasma (PRP) loaded gelatin/silk fibroin hydrogels for improving healing in a murine pressure ulcer model. Chem. Eng. J. 2021, 424, 130429. [Google Scholar] [CrossRef]
- Genç, H.; Hazur, J.; Karakaya, E.; Dietel, B.; Bider, F.; Groll, J.; Alexiou, C.; Boccaccini, A.R.; Detsch, R.; Cicha, I. Differential responses to bioink-induced oxidative stress in endothelial cells and fibroblasts. Int. J. Mol. Sci. 2021, 22, 2358. [Google Scholar] [CrossRef]




| Hydrogels | Amorphous (%) | Crystallinity (%) |
|---|---|---|
| GE_NC | 60.4% | 39.6% |
| GE_GNP | 43.6% | 56.4% |
| GPVA_NC | 56.9% | 44.1% |
| GPVA_GNP | 42.5% | 57.5% |
| Hydrogels | C (%) | O (%) | N (%) |
|---|---|---|---|
| GE_NC | 48.58 ± 1.02 | 34.46 ± 0.92 | 16.98 ± 1.38 |
| GE_GNP | 49.52 ± 1.2 | 30.74 ± 1.0 | 19.78 ± 1.6 |
| GPVA_NC | 51.72 ± 1.1 | 39.2 ± 0.18 | 9.04 ± 1.48 |
| GPVA_GNP | 54.12 ± 1.44 | 35.54 ± 1.18 | 10.36 ± 1.16 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Masri, S.; Fauzi, F.A.M.; Hasnizam, S.B.; Azhari, A.S.; Lim, J.E.A.; Hao, L.Q.; Maarof, M.; Motta, A.; Fauzi, M.B. Engineered-Skin of Single Dermal Layer Containing Printed Hybrid Gelatin-Polyvinyl Alcohol Bioink via 3D-Bioprinting: In Vitro Assessment under Submerged vs. Air-Lifting Models. Pharmaceuticals 2022, 15, 1328. https://doi.org/10.3390/ph15111328
Masri S, Fauzi FAM, Hasnizam SB, Azhari AS, Lim JEA, Hao LQ, Maarof M, Motta A, Fauzi MB. Engineered-Skin of Single Dermal Layer Containing Printed Hybrid Gelatin-Polyvinyl Alcohol Bioink via 3D-Bioprinting: In Vitro Assessment under Submerged vs. Air-Lifting Models. Pharmaceuticals. 2022; 15(11):1328. https://doi.org/10.3390/ph15111328
Chicago/Turabian StyleMasri, Syafira, Faraheda Amilia Mohd Fauzi, Sarah Batrisyia Hasnizam, Aizzaty Sulha Azhari, Juliana Edora Amin Lim, Looi Qi Hao, Manira Maarof, Antonella Motta, and Mh Busra Fauzi. 2022. "Engineered-Skin of Single Dermal Layer Containing Printed Hybrid Gelatin-Polyvinyl Alcohol Bioink via 3D-Bioprinting: In Vitro Assessment under Submerged vs. Air-Lifting Models" Pharmaceuticals 15, no. 11: 1328. https://doi.org/10.3390/ph15111328
APA StyleMasri, S., Fauzi, F. A. M., Hasnizam, S. B., Azhari, A. S., Lim, J. E. A., Hao, L. Q., Maarof, M., Motta, A., & Fauzi, M. B. (2022). Engineered-Skin of Single Dermal Layer Containing Printed Hybrid Gelatin-Polyvinyl Alcohol Bioink via 3D-Bioprinting: In Vitro Assessment under Submerged vs. Air-Lifting Models. Pharmaceuticals, 15(11), 1328. https://doi.org/10.3390/ph15111328










