The Therapeutic Potential of Plant Polysaccharides in Metabolic Diseases
Abstract
1. Introduction
2. Plant Polysaccharides
2.1. Type
2.2. Composition
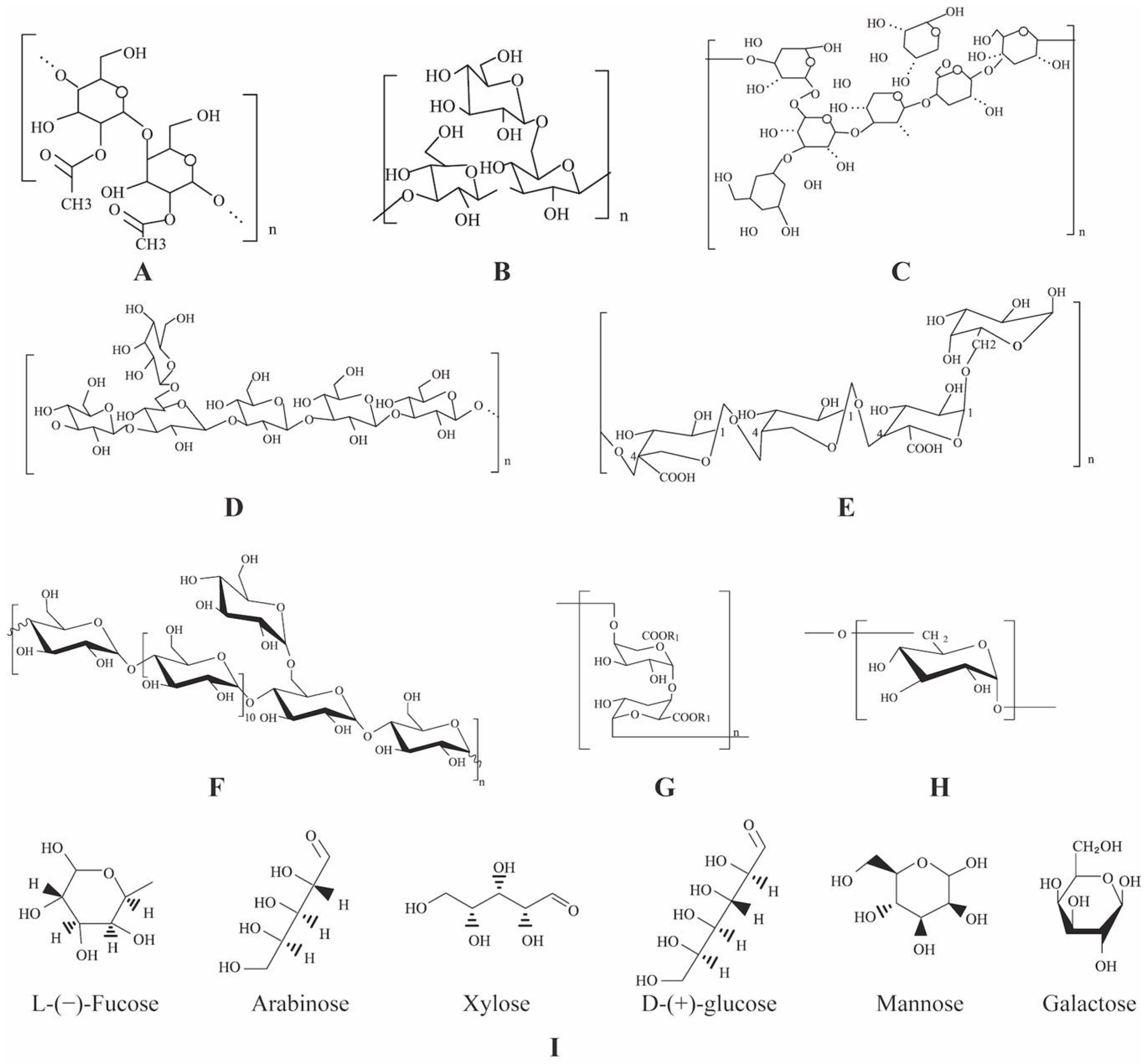
2.3. Structure
2.4. Functional Activity
| PPS | Plant Parts | Monosaccharides | MW (kDa) | Extract Methods | Functional Activities | Reference |
|---|---|---|---|---|---|---|
| APs | Stems and leaf | Glucose, mannose, galactose, arabinose, xylose | 200–523 | HWE, ETE | Regulating immunity, lowering blood glucose, inhibiting tumor progression, reducing inflammation, improving oral disease, regulating CVDs, promoting bone growth | [17,39,40,41,42,43,44] |
| ASPs | Rhizome | Glucose, mannose, galactose, rhamnose, arabinose, xylose | 5.1–2300 | HWE | Regulating immunity, inhibiting tumor progression, reducing radiation, improving hematopoiesis, lowering blood sugar and blood lipids, protecting the liver, inhibiting oxidative damage and protecting nerves, reducing joint inflammation | [35,36,37,45,46,47,48,49] |
| Pumpkin (Cucurbita moschata) polysaccharides (PPs) | Fruit | Galactose, glucose, arabinose, xylose, glucuronic acid | - | ALE, UAE, HWE | Inhibiting cancer progression, reducing oxidation, lowering blood sugar, reducing bacteria, reducing toxicity, reducing blood pressure, reducing blood lipids, lowering cholesterol levels, assisting the healing process in wounds | [19,50,51,52,53,54,55] |
| Lycium barbarum polysaccharides (LCPs) | Rhizome | Rhamnose, fucose, arabinose, galactose | 10–2300 | UAE, EAM, MAM, SFM | Enhancing the intestinal microbiota, boosting beneficial bacteria levels, modulating innate immune response, reducing oxidation, delaying aging, increasing metabolism lowing intraocular pressure, regulating immunity, inhibiting tumor progression, improving neurological diseases, lowering blood sugar | [56,57,58,59,60] |
| GPs | Rhizome | Arabinose, galactose, rhamnose, galacturonic acid, glucuronic acid | 3.2–1900 | ETE | Relieving depression, reducing blood glucose, regulating immunity, inhibiting cancer progression, reducing oxidation, reducing radiation | [38,61,62] |
| Schisandra chinensis polysaccharides (SCPs) | Fruit | Rhamnose, fucose, arabinose, xylose, mannose, glucose, galactose | - | ETE, HWE | Lowering blood sugar, relieving fatigue, relieving a cough, reducing inflammation, improving neurological diseases, reducing hyperprolactinemia, promoting regeneration, reversing liver injury, inhibiting cancer progression, protecting the intestines | [10,63,64,65,66,67,68,69] |
| Dioscorea opposita polysaccharides (DOPs) | Rhizome | Glucose, mannose, xylose, galactose, arabinose, fucose | - | HWE, ETE | Reducing blood sugar, inhibiting cancer progression, reducing oxidation, promoting endometrial epithelial proliferation, regulating immunity, protecting the heart | [31,70,71,72] |
| PCPs | Rhizome | Glucose, fucose, arabinose, xylose, mannose, galactose | 41–500 | HWE, MAE, EE, UE | Reducing liver injury, inhibiting cancer progression, reducing inflammatory factors and blood lipid levels, relieving depression, regulating immunity | [27,73,74,75,76,77,78] |
| Tea (Camellia sinensis) polysaccharides (TPs) | Rhizome | Glucose, rhamnose, arabinose, mannose, ribose, xylose, galactose, fucose, galacturonic acid | 1000–5000 | HWE, MAE, EE, UAE | Inhibiting cancer, reducing blood sugar, reducing oxidation, reducing inflammatory factors and blood lipid levels, relieving fatigue | [79,80] |
3. Metabolic Diseases
3.1. Common Risk Factors
3.2. Cardiovascular Disease
3.3. Type 2 Diabetes Mellitus
3.4. Nervous System Disease
3.5. Nonalcoholic Steatohepatitis
3.6. Other Metabolic Diseases
4. The Modulation of Plant Polysaccharides in Metabolic Disease
4.1. Aloe vera Polysaccharides
4.2. Angelica Sinensis Polysaccharides
4.3. Pumpkin Polysaccharides
4.4. Lycium Barbarum Polysaccharides
4.5. Ginseng Polysaccharides
4.6. Schisandra Chinensis Polysaccharides
4.7. Dioscorea Opposita Polysaccharides
4.8. Poria Cocos Polysaccharides
4.9. Tea (Camellia sinensis) Polysaccharides
| PPS | Dosage | Model | Effect | Mechanism | Diseases |
|---|---|---|---|---|---|
| APs | 60 mg/kg | MCAO male Wister rats (in vivo) | Regulating immunity, resisting tumor, protecting liver, and nourishing stomach | Inhibiting aoptosis | Cerebral ischemia [129] |
| 5, 10 and 20 mg/ml 100 mg/g | Palmitate-induced HIT-T15 cells (in vitro) db/db mice (in vivo) | Regulating ER stress, inhibiting neuronal apoptosis, reducing blood sugar | Inhibiting PERK and IRE1 pathways, inhibiting ROS generation | T2DM [7] | |
| ASPs | 50 mg/kg | Hippocampus was injected with Aß25 - 35 rats (in vivo) | Inhibiting inflammation and apoptosis | Activating the BDNF/TrkB/CREB pathway | AD [8] |
| 400 and 600 mg/kg | STZ-induced diabetic BALB/c mice (in vivo) | Inhibiting TNF-α, IL-1β, and TNF-α expression, inhibiting SOD and CAT activity, decreasing MDA content, inhibiting caspase-3 and Bax/Bcl-2 expression | Activating the BDNF/TrkB/CREB signaling pathway | T2DM [35] | |
| PPs | 100, 250 and 500 mg/kg | STZ-induced rats (in vivo) | Reducing FBG, IR and blood lipid TC, TG and LDL levels, improving blood glucose | T2DM [133] | |
| 95% (w/w) HF diet plus 5% (w/w) PP | Male Sprague Dawley rats (in vivo) | Reducing TG, TC, and plasma LDL-C, increasing the levels of fecal fat, cholesterol, and plasma HDL-C | Increasing the binding capacity of fat and cholesterol | Obesity [134] | |
| LBPs | 100, 250, and 500 mg/kg | STZ induced diabetic rat (in vivo) | Reducing the concentration of albuminuria, blood urea nitrogen, IL-2, IL-6, TNF-α, IFN-α, serum levels of MCP-1 and ICAM-1, increasing SOD and GSH Px activity | Inhibiting the NF-κB pathway | T2DM [137] |
| 0.2% LBPs water | HFD mice (in vivo) | Reducing TG, TC and LDL-C levels, increasing HDL-C and SCFA | Improving IR and fatty acid oxidation, activating the adenosine monophosphate activated protein kinase CoA carboxylase pathway | Obesity [138] | |
| GPs | 50 and 200 mg/kg | C57BL/6 anxiety mice (in vivo) | Increasing the walking distance and staying time in the central area of the mice, decreasing the average speed of mice | Reducing the expression of tyrosine hydroxylase (TH) in the midbrain and dopamine D1 receptor (DRD1) | Anxiety [140] |
| 0.2, 0.5 and 1 g/kg | High-sugar diet and STZ -induced rats (in vivo) | Reducing FBG, restoring disturbed intestinal flora, enhancing β- d-glucosidase, enhancing the hypoglycemic effect of ginsenoside Rdb1 | Changing the biotransformation pathway of ginsenoside Rb1, improving the biotransformation rate of ginsenoside Rb1 to CK | T2DM [141] | |
| SCPs | 200 mg/kg | CFS rats (in vivo) | Increasing food intake and body weight, improving the memory deficit | Promoting the recovery of tricarboxylic acid cycle metabolism pathway and alanine, aspartic acid and glutamate metabolism pathway | CFS [142] |
| 25, 50 or 100 mg/kg | STZ -induced rats (in vivo) | Reducing FBG, increasing fasting insulin level, improving glucose tolerance, and inhibiting the expression of proinflammatory cytokines | Downregulating NF- κ B and P-JNK signaling pathways, upregulating the IRS-1/PI3K/AKT signaling pathway | T2DM [143] | |
| 100 mg kg | High-fat diet-induced male Wistar rats (in vivo) | Reducing AST, ALT, TG, TC, and LDL-C, increasing HDL-C | Regulating UGP2, UGDH, ACC and FAS expression | NASH [144] | |
| OPs | alloxan diabetic rats (in vivo) | Reducing blood glucose, increasing insulin secretion, and improving the function of pancreatic β-cells | Reducing lipid peroxide and eliminating free radicals | Diabetes [146] | |
| 50, 100 and 150 mg/kg | Dexamethasone-induced IR glucose/lipid metabolism diabetic mice)(in vivo) | Reducing blood sugar | T2DM [147] | ||
| PCPs | 100, 200, and 400 mg/kg | ApoE−/− mice (in vivo) | Reduced serum TNF-α, IL-6, NO, LDL-C, TG and TC levels, decreasing MDA, and increasing SOD | Inhibiting the TLR4/NF-κB pathway | AS [9] |
| 3 g/day | High-fat diet-induced NAFLD mice (in vivo) | Increasing the lipid utilization, decreasing the lipid synthesis and absorption | Regulating fatty acid metabolism, bile acid metabolism, and tricarboxylic acid cycle | NAFLD [148] | |
| TPs | 200, 400 and 800 mg/kg | STZ-induced T2DM rats (in vivo) | Reducing intestinal flora | Regulating primary and secondary bile acid biosynthesis, downregulating the OD-like receptor signaling pathway | T2DM [149] |
| 3, 10, and 30 mg/kg | Formalin test and several behavioral animal models (in vivo) | Resisting anxiety, pain, anxiety | Anxiety [150] |
5. Conclusions and Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Saklayen, M.G. The Global Epidemic of the Metabolic Syndrome. Curr. Hypertens. Rep. 2018, 20, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Emanuela, F.; Grazia, M.; Marco, D.R.; Paola, L.M.; Giorgio, F.; Marco, B. Inflammation as a Link between Obesity and Metabolic Syndrome. J. Nutr. Metab. 2012, 2012, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Fahed, G.; Aoun, L.; Bou Zerdan, M.; Allam, S.; Bou Zerdan, M.; Bouferraa, Y.; Assi, H.I. Metabolic Syndrome: Updates on Pathophysiology and Management in 2021. Int. J. Mol. Sci. 2022, 23, 786. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Kuang, H.; Su, Y.; Sun, Y.; Feng, J.; Guo, R.; Chan, K. Naturally derived anti-inflammatory compounds from Chinese medicinal plants. J. Ethnopharmacol. 2013, 146, 9–39. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.-H.; Jin, M.-L.; Morris, G.A.; Zha, X.-Q.; Chen, H.-Q.; Yi, Y.; Li, J.-E.; Wang, Z.-J.; Gao, J.; Nie, S.-P.; et al. Advances on Bioactive Polysaccharides from Medicinal Plants. Crit. Rev. Food Sci. Nutr. 2016, 56, S60–S84. [Google Scholar] [CrossRef]
- Yin, M.; Zhang, Y.; Li, H. Advances in Research on Immunoregulation of Macrophages by Plant Polysaccharides. Front. Immunol. 2019, 10, 145. [Google Scholar] [CrossRef]
- Kim, K.; Chung, M.H.; Park, S.; Cha, J.; Baek, J.H.; Lee, S.-Y.; Choi, S.-Y. ER stress attenuation by Aloe-derived polysaccharides in the protection of pancreatic β-cells from free fatty acid-induced lipotoxicity. Biochem. Biophys. Res. Commun. 2018, 500, 797–803. [Google Scholar] [CrossRef] [PubMed]
- Du, Q.; Zhu, X.; Si, J. Angelica polysaccharide ameliorates memory impairment in Alzheimer’s disease rat through activating BDNF/TrkB/CREB pathway. Exp. Biol. Med. 2020, 245, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Yu, J.; Zhao, J.; Xiao, X.; Li, W.; Zang, L.; Yu, J.; Liu, H.; Niu, X. Poria cocos polysaccharides reduces high-fat diet-induced arteriosclerosis in ApoE −/− mice by inhibiting inflammation. Phytotherapy Res. 2021, 35, 2220–2229. [Google Scholar] [CrossRef] [PubMed]
- Su, L.; Mao, C.; Wang, X.; Li, L.; Tong, H.; Mao, J.; Ji, D.; Lu, T.; Hao, M.; Huang, Z.; et al. The Anti-colitis Effect of Schisandra chinensis Polysaccharide Is Associated With the Regulation of the Composition and Metabolism of Gut Microbiota. Front. Cell. Infect. Microbiol. 2020, 10, 519479. [Google Scholar] [CrossRef]
- Luo, L.; Zheng, S.; Huang, Y.; Qin, T.; Xing, J.; Niu, Y.; Bo, R.; Liu, Z.; Huang, Y.; Hu, Y.; et al. Preparation and characterization of Chinese yam polysaccharide PLGA nanoparticles and their immunological activity. Int. J. Pharm. 2016, 511, 140–150. [Google Scholar] [CrossRef] [PubMed]
- Bo, R.; Zheng, S.; Xing, J.; Luo, L.; Niu, Y.; Huang, Y.; Liu, Z.; Hu, Y.; Liu, J.; Wu, Y.; et al. The immunological activity of Lycium barbarum polysaccharides liposome in vitro and adjuvanticity against PCV2 in vivo. Int. J. Biol. Macromol. 2016, 85, 294–301. [Google Scholar] [CrossRef] [PubMed]
- Minjares-Fuentes, J.R.; Femenia, A.; Comas-Serra, F.; Rodríguez-González, V.M. Compositional and Structural Features of the Main Bioactive Polysaccharides Present in the Aloe vera Plant. J. AOAC Int. 2018, 101, 1711–1719. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Delbianco, M.; Seeberger, P.H. Automated Assembly of Starch and Glycogen Polysaccharides. J. Am. Chem. Soc. 2021, 143, 9758–9768. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.; Zhang, W.; Feng, Y.; Yu, X. Cloning and Characterization of a Sucrose Synthase-Encoding Gene from Muskmelon. Mol. Biol. Rep. 2010, 37, 695–702. [Google Scholar] [CrossRef]
- Qu, J.; Huang, P.; Zhang, L.; Qiu, Y.; Qi, H.; Leng, A.; Shang, D. Hepatoprotective effect of plant polysaccharides from natural resources: A review of the mechanisms and structure-activity relationship. Int. J. Biol. Macromol. 2020, 161, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Godoy, D.J.D.; Chokboribal, J.; Pauwels, R.; Banlunara, W.; Sangvanich, P.; Jaroenporn, S.; Thunyakitpisal, P. Acemannan increased bone surface, bone volume, and bone density in a calvarial defect model in skeletally-mature rats. J. Dent. Sci. 2018, 13, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhou, T.; Wang, H.; Cui, Z.; Cheng, F.; Wang, K.-P. Structural characterization and in vitro antitumor activity of an acidic polysaccharide from Angelica sinensis (Oliv.) Diels. Carbohydr. Polym. 2016, 147, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Wei, Y.; Liang, L.; Huang, L.; Yu, G.; Li, Q. A novel low-molecular-mass pumpkin polysaccharide: Structural characterization, antioxidant activity, and hypoglycemic potential. Carbohydr. Polym. 2021, 251, 117090. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Li, J.; Liu, Y.; Wu, D.; Cai, P.; Pan, Y. Structural characterization and immunomodulatory activity of a water soluble polysaccharide isolated from Botrychium ternatum. Carbohydr. Polym. 2017, 171, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Yang, M.; He, Y.; Zhai, C.; Li, C. A review on the production, structure, bioactivities and applications of Tremella polysaccharides. Int. J. Immunopathol. Pharmacol. 2021, 35, 20587384211000541. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Willför, S.; Xu, C. A review of bioactive plant polysaccharides: Biological activities, functionalization, and biomedical applications. Bioact. Carbohydrates Diet. Fibre 2015, 5, 31–61. [Google Scholar] [CrossRef]
- Rees, D.A.; Welsh, E.J. Secondary and Tertiary Structure of Polysaccharides in Solutions and Gels. Angew. Chem. Int. Ed. 1977, 16, 214–224. [Google Scholar] [CrossRef]
- Liu, C.; Cui, Y.; Pi, F.; Cheng, Y.; Guo, Y.; Qian, H. Extraction, Purification, Structural Characteristics, Biological Activities and Pharmacological Applications of Acemannan, a Polysaccharide from Aloe vera: A Review. Molecules 2019, 24, 1554. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.-Z.; He, X.; Yu, Z.; Wu, H.; Yang, T.-H. A Nano Drug Delivery System Based on Angelica sinensis Polysaccharide for Combination of Chemotherapy and Immunotherapy. Molecules 2020, 25, 3096. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Sun, Y.; Huang, G. Preparation and antioxidant activities of important traditional plant polysaccharides. Int. J. Biol. Macromol. 2018, 111, 780–786. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; He, Y.; Zeng, P.; Liu, Y.; Zhang, M.; Hao, C.; Wang, H.; Lv, Z.; Zhang, L. Molecular basis for Poria cocos mushroom polysaccharide used as an antitumour drug in China. J. Cell. Mol. Med. 2018, 23, 4–20. [Google Scholar] [CrossRef]
- Yoo, D.-G.; Kim, M.-C.; Park, M.-K.; Park, K.-M.; Quan, F.-S.; Song, J.-M.; Wee, J.J.; Wang, B.-Z.; Cho, Y.-K.; Compans, R.W.; et al. Protective Effect of Ginseng Polysaccharides on Influenza Viral Infection. PLoS ONE 2012, 7, e33678. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Zhao, J.; Wei, Y.; Yu, G.; Li, Q. Characterization of a neutral polysaccharide from pumpkin (Cucurbita moschata Duch) with potential immunomodulatory activity. Int. J. Biol. Macromol. 2021, 188, 729–739. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Zhou, X.; Huang, G. Preparation, structure, and properties of tea polysaccharide. Chem. Biol. Drug Des. 2022, 99, 75–82. [Google Scholar] [CrossRef]
- Huang, R.; Shen, M.; Yu, Y.; Liu, X.; Xie, J. Physicochemical characterization and immunomodulatory activity of sulfated Chinese yam polysaccharide. Int. J. Biol. Macromol. 2020, 165, 635–644. [Google Scholar] [CrossRef]
- Cheng, J.; Zhou, Z.W.; Sheng, H.P.; He, L.J.; Fan, X.W.; He, Z.X.; Sun, T.; Zhang, X.; Zhao, R.J.; Gu, L.; et al. An evidence-based update on the pharmacological activities and possible molecular targets of Lycium barbarum polysaccharides. Drug Des. Dev. Ther. 2014, 9, 33–78. [Google Scholar] [CrossRef]
- Xie, Y.; Zhang, B.; Zhang, Y. Protective effects of Acanthopanax polysaccharides on cerebral ischemia–reperfusion injury and its mechanisms. Int. J. Biol. Macromol. 2015, 72, 946–950. [Google Scholar] [CrossRef]
- Quezada, M.P.; Salinas, C.; Gotteland, M.; Cardemil, L. Acemannan and Fructans from Aloe vera (Aloe barbadensis Miller) Plants as Novel Prebiotics. J. Agric. Food Chem. 2017, 65, 10029–10039. [Google Scholar] [CrossRef]
- Liu, C.; Li, J.; Meng, F.Y.; Liang, S.X.; Deng, R.; Li, C.K.; Pong, N.; Lau, C.P.; Cheng, S.W.; Ye, J.Y.; et al. Polysaccharides from the root of Angelica sinensis promotes hematopoiesis and thrombopoiesis through the PI3K/AKT pathway. BMC Complement. Altern. Med. 2010, 10, 79. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Cao, P.; Shui, W.; Yang, Q.; Tang, Z.; Zhang, Y. Angelica sinensis polysaccharide regulates glucose and lipid metabolism disorder in prediabetic and streptozotocin-induced diabetic mice through the elevation of glycogen levels and reduction of inflammatory factors. Food Funct. 2015, 6, 902–909. [Google Scholar] [CrossRef]
- Cao, P.; Sun, J.; Sullivan, M.A.; Huang, X.; Wang, H.; Zhang, Y.; Wang, N.; Wang, K. Angelica sinensis polysaccharide protects against acetaminophen-induced acute liver injury and cell death by suppressing oxidative stress and hepatic apoptosis in vivo and in vitro. Int. J. Biol. Macromol. 2018, 111, 1133–1139. [Google Scholar] [CrossRef]
- Wang, J.; Flaisher-Grinberg, S.; Li, S.; Liu, H.; Sun, L.; Zhou, Y.; Einat, H. Antidepressant-like effects of the active acidic polysaccharide portion of ginseng in mice. J. Ethnopharmacol. 2010, 132, 65–69. [Google Scholar] [CrossRef]
- Yagi, A.; Hegazy, S.; Kabbash, A.; Wahab, E.A.-E. Possible hypoglycemic effect of Aloe vera L. high molecular weight fractions on type 2 diabetic patients. Saudi Pharm. J. 2009, 17, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Hęś, M.; Dziedzic, K.; Górecka, D.; Jędrusek-Golińska, A.; Gujska, E. Aloe vera (L.) Webb.: Natural Sources of Antioxidants — A Review. Mater. Veg. 2019, 74, 255–265. [Google Scholar] [CrossRef]
- Gentilini, R.; Bozzini, S.; Munarin, F.; Petrini, P.; Visai, L.; Tanzi, M.C. Pectins from Aloe Vera: Extraction and production of gels for regenerative medicine. J. Appl. Polym. Sci. 2014, 131. [Google Scholar] [CrossRef]
- Thunyakitpisal, P.; Ruangpornvisuti, V.; Kengkwasing, P.; Chokboribal, J.; Sangvanich, P. Acemannan increases NF-κB/DNA binding and IL-6/-8 expression by selectively binding Toll-like receptor-5 in human gingival fibroblasts. Carbohydr. Polym. 2017, 161, 149–157. [Google Scholar] [CrossRef]
- Harlev, E.; Nevo, E.; Lansky, E.P.; Ofir, R.; Bishayee, A. Anticancer Potential of Aloes: Antioxidant, Antiproliferative, and Immunostimulatory Attributes. Planta Med. 2012, 78, 843–852. [Google Scholar] [CrossRef]
- Bhalang, K.; Thunyakitpisal, P.; Rungsirisatean, N. Acemannan, a Polysaccharide Extracted from Aloe vera, Is Effective in the Treatment of Oral Aphthous Ulceration. J. Altern. Complement. Med. 2013, 19, 429–434. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.; Zhao, K.; Huang, Q.; Xu, C.; Shang, P. Isolation, structure and bioactivities of the polysaccharides from Angelica sinensis (Oliv.) Diels: A review. Carbohydr. Polym. 2012, 89, 713–722. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.; Zhao, K.; Huang, Q.; Shang, P. Structural features and biological activities of the polysaccharides from Astragalus membranaceus. Int. J. Biol. Macromol. 2014, 64, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-G.; Hsieh, W.-T.; Chen, S.-U.; Chiang, B.-H. Hematopoietic and myeloprotective activities of an acidic Angelica sinensis polysaccharide on human CD34+ stem cells. J. Ethnopharmacol. 2012, 139, 739–745. [Google Scholar] [CrossRef] [PubMed]
- Lei, T.; Li, H.; Fang, Z.; Lin, J.; Wang, S.; Xiao, L.; Yang, F.; Liu, X.; Zhang, J.; Liao, W.; et al. Polysaccharides from Angelica sinensis alleviate neuronal cell injury caused by oxidative stress. Neural Regen. Res. 2014, 9, 260–267. [Google Scholar] [CrossRef]
- Tian, S.; Hao, C.; Xu, G.; Yang, J.; Sun, R. Optimization conditions for extracting polysaccharide from Angelica sinensis and its antioxidant activities. J. Food Drug Anal. 2017, 25, 766–775. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhang, F.; Liu, X.; Ange, K.S.; Zhang, A.; Li, Q.; Linhardt, R.J. Isolation of a lectin binding rhamnogalacturonan-I containing pectic polysaccharide from pumpkin. Carbohydr. Polym. 2017, 163, 330–336. [Google Scholar] [CrossRef]
- Wu, H.; Zhu, J.; Diao, W.; Wang, C. Ultrasound-assisted enzymatic extraction and antioxidant activity of polysaccharides from pumpkin (Cucurbita moschata). Carbohydr. Polym. 2014, 113, 314–324. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Huang, G. Extraction, characterization and antioxidant activities of pumpkin polysaccharide. Int. J. Biol. Macromol. 2018, 118, 770–774. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Zhao, J.; Wei, Y.; Huang, L.; Li, F.; Zhang, Y.; Li, Q. Physicochemical Properties and Antioxidant Activity of Pumpkin Polysaccharide (Cucurbita moschata Duchesne ex Poiret) Modified by Subcritical Water. Foods 2021, 10, 197. [Google Scholar] [CrossRef] [PubMed]
- Qian, Z.-G. Cellulase-assisted extraction of polysaccharides from Cucurbita moschata and their antibacterial activity. Carbohydr. Polym. 2014, 101, 432–434. [Google Scholar] [CrossRef] [PubMed]
- Iwo, M.I.; Insanu, M.; Dass, C.A.S. Development of Immunonutrient from Pumpkin (Cucurbita Moschata Duchense Ex. Lamk.) Seed. Procedia Chem. 2014, 13, 105–111. [Google Scholar] [CrossRef]
- Ji, X.; Peng, Q.; Yuan, Y.; Liu, F.; Wang, M. Extraction and physicochemical properties of polysaccharides from Ziziphus Jujuba cv. Muzao by ultrasound-assisted aqueous two-phase extraction. Int. J. Biol. Macromol. 2018, 108, 541–549. [Google Scholar] [CrossRef]
- Skenderidis, P.; Petrotos, K.; Giavasis, I.; Hadjichristodoulou, C.; Tsakalof, A. Optimization of ultrasound assisted extraction of of goji berry (Lycium barbarum) fruits and evaluation of extracts’ bioactivity. J. Food Process Eng. 2016, 40, e12522. [Google Scholar] [CrossRef]
- Tian, X.; Liang, T.; Liu, Y.; Ding, G.; Zhang, F.; Ma, Z. Extraction, Structural Characterization, and Biological Functions of Lycium Barbarum Polysaccharides: A Review. Biomolecules 2019, 9, 389. [Google Scholar] [CrossRef]
- Reverchon, E.; De Marco, I. Supercritical fluid extraction and fractionation of natural matter. J. Supercrit. Fluids 2006, 38, 146–166. [Google Scholar] [CrossRef]
- Zhu, W.; Zhou, S.; Liu, J.; McLean, R.J.; Chu, W. Prebiotic, immuno-stimulating and gut microbiota-modulating effects of Lycium barbarum polysaccharide. Biomed. Pharmacother. 2019, 121, 109591. [Google Scholar] [CrossRef]
- Zeng, P.; Li, J.; Chen, Y.; Zhang, L. The structures and biological functions of polysaccharides from traditional Chinese herbs. Prog. Mol. Biol. Transl. Sci. 2019, 163, 423–444. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Zhang, N.; Feng, Q.; Li, H.; Wang, D.; Ma, L.; Liu, S.; Chen, C.; Wu, W.; Jiao, L. The core structure characterization and of ginseng neutral polysaccharide with the immune-enhancing activity. Int. J. Biol. Macromol. 2018, 123, 713–722. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Wang, J.; Zhang, X.; Yan, T.; Wu, B.; Bi, K.; Jia, Y. Polysaccharide from Schisandra chinensis acts via LRP-1 to reverse microglia activation through suppression of the NF-κB and MAPK signaling. J. Ethnopharmacol. 2020, 256, 112798. [Google Scholar] [CrossRef] [PubMed]
- Zhong, S.; Liu, X.-D.; Nie, Y.-C.; Gan, Z.-Y.; Yang, L.-Q.; Huang, C.-Q.; Lai, K.-F.; Zhong, N.-S. Antitussive activity of the Schisandra chinensis fruit polysaccharide (SCFP-1) in guinea pigs models. J. Ethnopharmacol. 2016, 194, 378–385. [Google Scholar] [CrossRef]
- Chi, A.; Zhang, Y.; Kang, Y.; Shen, Z. Metabolic mechanism of a polysaccharide from Schisandra chinensis to relieve chronic fatigue syndrome. Int. J. Biol. Macromol. 2016, 93, 322–332. [Google Scholar] [CrossRef]
- Jin, D.; Zhao, T.; Feng, W.-W.; Mao, G.-H.; Zou, Y.; Wang, W.; Li, Q.; Chen, Y.; Wang, X.-T.; Yang, L.-Q.; et al. Schisandra polysaccharide increased glucose consumption by up-regulating the expression of GLUT-4. Int. J. Biol. Macromol. 2016, 87, 555–562. [Google Scholar] [CrossRef]
- Hong, S.-H.; Li, M.; Jeung, E.-B.; Lee, G.-S.; Hong, E.-J.; Choi, Y.-W.; An, B.-S. Therapeutic effects of Schisandra chinensis on the hyperprolactinemia in rat. Int. J. Oncol. 2017, 50, 1448–1454. [Google Scholar] [CrossRef]
- Li, X.; Fan, X.; Zeng, X.; Wang, Y.; Zhou, Y.; Chen, Y.; Huang, M.; Bi, H. Schisandra sphenanthera extract facilitates liver regeneration after partial hepatectomy in mice. Drug Metab. Dispos. 2016, 44, 647–652. [Google Scholar] [CrossRef]
- Che, J.; Yang, S.; Qiao, Z.-J.; Li, H.; Sun, J.; Zhuang, W.; Chen, J.; Wang, C. Schisandra chinensis acidic polysaccharide partialy reverses acetaminophen-induced liver injury in mice. J. Pharmacol. Sci. 2019, 140, 248–254. [Google Scholar] [CrossRef]
- Ju, Y.; Xue, Y.; Huang, J.; Zhai, Q.; Wang, X.-H. Antioxidant Chinese yam polysaccharides and its pro-proliferative effect on endometrial epithelial cells. Int. J. Biol. Macromol. 2014, 66, 81–85. [Google Scholar] [CrossRef]
- Zhang, N.; Liang, T.; Jin, Q.; Shen, C.; Zhang, Y.; Jing, P. Chinese yam (Dioscorea opposita Thunb.) alleviates antibiotic-associated diarrhea, modifies intestinal microbiota, and increases the level of short-chain fatty acids in mice. Food Res. Int. 2019, 122, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Zeng, M.; Zhang, L.; Zhang, B.; Li, B.; Kan, Y.; Yang, H.; Feng, W.; Zheng, X. Chinese yam extract and adenosine attenuated LPS-induced cardiac dysfunction by inhibiting RAS and apoptosis via the ER-mediated activation of SHC/Ras/Raf1 pathway. Phytomedicine 2019, 61, 152857. [Google Scholar] [CrossRef]
- Afshari, K.; Samavati, V.; Shahidi, S.-A. Ultrasonic-assisted extraction and in-vitro antioxidant activity of polysaccharide from Hibiscus leaf. Int. J. Biol. Macromol. 2015, 74, 558–567. [Google Scholar] [CrossRef]
- Thirugnanasambandham, K.; Sivakumar, V.; Maran, J.P. Microwave-assisted extraction of polysaccharides from mulberry leaves. Int. J. Biol. Macromol. 2015, 72, 1–5. [Google Scholar] [CrossRef]
- Yan, Y.; Li, X.; Wan, M.; Chen, J.; Li, S.; Cao, M.; Zhang, D. Effect of extraction methods on property and bioactivity of water-soluble polysaccharides from Amomum villosum. Carbohydr. Polym. 2015, 117, 632–635. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, L.; Li, P.; Zhao, J.; Duan, J. Antidepressant and immunosuppressive activities of two polysaccharides from Poria cocos (Schw.) Wolf. Int. J. Biol. Macromol. 2018, 120, 1696–1704. [Google Scholar] [CrossRef]
- Wu, K.; Fan, J.; Huang, X.; Wu, X.; Guo, C. Hepatoprotective effects exerted by Poria Cocos polysaccharides against acetaminophen-induced liver injury in mice. Int. J. Biol. Macromol. 2018, 114, 137–142. [Google Scholar] [CrossRef]
- Tian, H.; Liu, Z.; Pu, Y.; Bao, Y. Immunomodulatory effects exerted by Poria Cocos polysaccharides via TLR4/TRAF6/NF-κB signaling in vitro and in vivo. Biomed. Pharmacother. 2019, 112, 108709. [Google Scholar] [CrossRef]
- Yao, J.; Liu, H.; Ma, C.; Pu, L.; Yang, W.; Lei, Z. A Review on the Extraction, Bioactivity, and Application of Tea Polysaccharides. Molecules 2022, 27, 4679. [Google Scholar] [CrossRef]
- Wang, Q.; Yang, X.; Zhu, C.; Liu, G.; Sun, Y.; Qian, L. Advances in the Utilization of Tea Polysaccharides: Preparation, Physicochemical Properties, and Health Benefits. Polymers 2022, 14, 2775. [Google Scholar] [CrossRef]
- Landrier, J.-F.; Derghal, A.; Mounien, L. MicroRNAs in Obesity and Related Metabolic Disorders. Cells 2019, 8, 859. [Google Scholar] [CrossRef] [PubMed]
- Dommermuth, R.; Ewing, K. Metabolic Syndrome: Systems thinking in heat disease. Prim. Care Clin. Off. Pract. 2018, 45, 109–129. [Google Scholar] [CrossRef] [PubMed]
- Kassi, E.; Pervanidou, P.; Kaltsas, G.; Chrousos, G. Metabolic syndrome: Definitions and controversies. BMC Med. 2011, 9, 48. [Google Scholar] [CrossRef]
- Matsuzawa, Y.; Funahashi, T.; Nakamura, T. The Concept of Metabolic Syndrome: Contribution of Visceral Fat Accumulation and Its Molecular Mechanism. J. Atheroscler. Thromb. 2011, 18, 629–639. [Google Scholar] [CrossRef] [PubMed]
- Sherling, D.H.; Perumareddi, P.; Hennekens, C.H. Metabolic Syndrome: Clinical and Policy Implications of the New Silent Killer. J. Cardiovasc. Pharmacol. Ther. 2017, 22, 365–367. [Google Scholar] [CrossRef] [PubMed]
- Stępień, M.; Stępień, A.; Wlazeł, R.N.; Paradowski, M.; Banach, M.; Rysz, J. Obesity indices and inflammatory markers in obese non-diabetic normo- and hypertensive patients: A comparative pilot study. Lipids Health Dis. 2014, 13, 29. [Google Scholar] [CrossRef] [PubMed]
- Clebak, K.T.; Morrison, A.; Croad, J.R. Gout: Rapid Evidence Review. Am. Fam. Physician 2020, 102, 533–538. [Google Scholar]
- Zhang, S.; Liu, Q.; Wang, J.; Harnish, D.C. Suppression of interleukin-6-induced C-reactive protein expression by FXR agonists. Biochem. Biophys. Res. Commun. 2009, 379, 476–479. [Google Scholar] [CrossRef]
- Rocha, V.Z.; Libby, P. Obesity, inflammation, and atherosclerosis. Nat. Rev. Cardiol. 2009, 6, 399–409. [Google Scholar] [CrossRef]
- Dai, Y.; Mercanti, F.; Dai, D.; Wang, X.; Ding, Z.; Pothineni, N.V.; Mehta, J.L. LOX-1, a bridge between GLP-1R and mitochondrial ROS generation in human vascular smooth muscle cells. Biochem. Biophys. Res. Commun. 2013, 437, 62–66. [Google Scholar] [CrossRef]
- Tooke, J.E.; Hannemann, M.M. Adverse endothelial function and the insulin resistance syndrome. J. Intern. Med. 2000, 247, 425–431. [Google Scholar] [CrossRef]
- Kivimaki, M.; Steptoe, A. Effects of stress on the development and progression of cardiovascular disease. Nat. Rev. Cardiol. 2017, 15, 215–229. [Google Scholar] [CrossRef] [PubMed]
- Galassi, A.; Reynolds, K.; He, J. Metabolic Syndrome and Risk of Cardiovascular Disease: A Meta-Analysis. Am. J. Med. 2006, 119, 812–819. [Google Scholar] [CrossRef]
- Moore, K.; Shah, R. Introduction to the Obesity, Metabolic Syndrome, and CVD Compendium. Circ. Res. 2020, 126, 1475–1476. [Google Scholar] [CrossRef] [PubMed]
- Hill, M.A.; Yang, Y.; Zhang, L.; Sun, Z.; Jia, G.; Parrish, A.R.; Sowers, J.R. Insulin resistance, cardiovascular stiffening and cardiovascular disease. Metabolism 2021, 119, 154766. [Google Scholar] [CrossRef]
- Petersen, M.C.; Shulman, G.I. Mechanisms of Insulin Action and Insulin Resistance. Physiol. Rev. 2018, 98, 2133–2223. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, J.; Cheng, Y.; Zhu, M.; Xiao, Z.; Ruan, G.; Wei, Y. Gut microbiota: A new target for T2DM prevention and treatment. Front. Endocrinol. 2022, 13, 958218. [Google Scholar] [CrossRef] [PubMed]
- Palano, F.; Paneni, F.; Sciarretta, S.; Tocci, G.; Volpe, M. Attuali concetti sullo sviluppo dell’insufficienza cardiaca nell’ipertensione. Recenti Prog. Med. 2011, 102, 461–467. [Google Scholar] [CrossRef]
- Litwin, M.; Kułaga, Z. Obesity, metabolic syndrome, and primary hypertension. Pediatr. Nephrol. 2021, 36, 825–837. [Google Scholar] [CrossRef] [PubMed]
- Tanase, D.M.; Gosav, E.M.; Costea, C.F.; Ciocoiu, M.; Lacatusu, C.M.; Maranduca, M.A.; Ouatu, A.; Floria, M. The Intricate Relationship between Type 2 Diabetes Mellitus (T2DM), Insulin Resistance (IR), and Nonalcoholic Fatty Liver Disease (NAFLD). J. Diabetes Res. 2020, 2020, 1–16. [Google Scholar] [CrossRef]
- Suzuki, A.; Angulo, P.; Lymp, J.; Sauver, J.S.; Muto, A.; Okada, T.; Lindor, K. Chronological development of elevated aminotransferases in a nonalcoholic population. Hepatology 2004, 41, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Milaneschi, Y.; Simmons, W.K.; Van Rossum, E.F.C.; Penninx, B.W. Depression and obesity: Evidence of shared biological mechanisms. Mol. Psychiatry 2019, 24, 18–33. [Google Scholar] [CrossRef] [PubMed]
- Kuriakose, D.; Xiao, Z. Pathophysiology and Treatment of Stroke: Present Status and Future Perspectives. Int. J. Mol. Sci. 2020, 21, 7609. [Google Scholar] [CrossRef]
- Liou, C.-W.; Tan, T.-Y.; Lin, T.-K.; Wang, P.-W.; Yip, H.-K. Metabolic syndrome and three of its components as risk factors for recurrent ischaemic stroke presenting as large-vessel infarction. Eur. J. Neurol. 2008, 15, 802–809. [Google Scholar] [CrossRef]
- Kernan, W.N.; Inzucchi, S.E.; Sawan, C.; Macko, R.F.; Furie, K.L. Obesity: A stubbornly obvious target for stroke prevention. Stroke 2013, 44, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Wang, K.; Hu, J. Effect of Probiotics on Depression: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients 2016, 8, 483. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.K.; Curhan, G. Independent Impact of Gout on Mortality and Risk for Coronary Heart Disease. Circulation 2007, 116, 894–900. [Google Scholar] [CrossRef] [PubMed]
- Evrensel, A.; Ceylan, A.M.E. The Gut-Brain Axis: The Missing Link in Depression. Clin. Psychopharmacol. Neurosci. 2015, 13, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Gold, P.W. The organization of the stress system and its dysregulation in depressive illness. Mol. Psychiatry 2015, 20, 32–47. [Google Scholar] [CrossRef]
- Abdelmalek, M.F. Nonalcoholic fatty liver disease: Another leap forward. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 85–86. [Google Scholar] [CrossRef]
- Csige, I.; Ujvárosy, D.; Szabó, Z.; Lőrincz, I.; Paragh, G.; Harangi, M.; Somodi, S. The Impact of Obesity on the Cardiovascular System. J. Diabetes Res. 2018, 2018, 1–12. [Google Scholar] [CrossRef]
- Brunt, E.M.; Kleiner, D.E.; Wilson, L.A.; Belt, P.; Neuschwander-Tetri, B.A. For the NASH Clinical Research Network (CRN) Nonalcoholic fatty liver disease (NAFLD) activity score and the histopathologic diagnosis in NAFLD: Distinct clinicopathologic meanings. Hepatology 2011, 53, 810–820. [Google Scholar] [CrossRef] [PubMed]
- Farrell, G.C.; Haczeyni, F.; Chitturi, S. Pathogenesis of NASH: How Metabolic Complications of Overnutrition Favour Lipotoxicity and Pro-Inflammatory Fatty Liver Disease. Obes. Fat. Liver Liver Cancer 2018, 1061, 19–44. [Google Scholar] [CrossRef]
- Caligiuri, A.; Gentilini, A.; Marra, F. Molecular Pathogenesis of NASH. Int. J. Mol. Sci. 2016, 17, 1575. [Google Scholar] [CrossRef] [PubMed]
- Stepanova, M.; Rafiq, N.; Makhlouf, H.; Agrawal, R.; Kaur, I.; Younoszai, Z.; McCullough, A.; Goodman, Z.; Younossi, Z.M. Predictors of All-Cause Mortality and Liver-Related Mortality in Patients with Non-Alcoholic Fatty Liver Disease (NAFLD). Am. J. Dig. Dis. 2013, 58, 3017–3023. [Google Scholar] [CrossRef]
- Esler, W.P.; Bence, K.K. Metabolic Targets in Nonalcoholic Fatty Liver Disease. Cell. Mol. Gastroenterol. Hepatol. 2019, 8, 247–267. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016, 64, 73–84. [Google Scholar] [CrossRef]
- Sheka, A.C.; Adeyi, O.; Thompson, J.; Hameed, B.; Crawford, P.A.; Ikramuddin, S. Nonalcoholic Steatohepatitis: A Review. JAMA 2020, 323, 1175–1183. [Google Scholar] [CrossRef]
- Bellastella, G.; Scappaticcio, L.; Esposito, K.; Giugliano, D.; Maiorino, M.I. Metabolic syndrome and cancer: “The common soil hypothesis”. Diabetes Res. Clin. Pract. 2018, 143, 389–397. [Google Scholar] [CrossRef]
- Miller, P.D. Management of severe osteoporosis. Expert Opin. Pharmacother. 2015, 17, 473–488. [Google Scholar] [CrossRef]
- Gathirua-Mwangi, W.G.; Monahan, P.O.; Murage, M.J.; Zhang, J. Metabolic syndrome and total cancer mortality in the Third National Health and Nutrition Examination Survey. Cancer Causes Control 2017, 28, 127–136. [Google Scholar] [CrossRef]
- Esposito, K.; Chiodini, P.; Colao, A.; Lenzi, A.; Giugliano, D. Metabolic Syndrome and Risk of Cancer: A systematic review and meta-analysis. Diabetes Care 2012, 35, 2402–2411. [Google Scholar] [CrossRef]
- Esposito, K.; Ciardiello, F.; Giugliano, D. Unhealthy diets: A common soil for the association of metabolic syndrome and cancer. Endocrine 2014, 46, 39–42. [Google Scholar] [CrossRef] [PubMed]
- Giugliano, D.; Ceriello, A.; Esposito, K. Are there specific treatments for the metabolic syndrome? Am. J. Clin. Nutr. 2008, 87, 8–11. [Google Scholar] [CrossRef]
- Bhole, V.; de Vera, M.; Rahman, M.M.; Krishnan, E.; Choi, H. Epidemiology of gout in women: Fifty-two-year followup of a prospective cohort. Arthritis Care Res. 2010, 62, 1069–1076. [Google Scholar] [CrossRef]
- Yong, E.; Logan, S. Menopausal osteoporosis: Screening, prevention and treatment. Singap. Med. J. 2021, 62, 159–166. [Google Scholar] [CrossRef]
- Armas, L.A.; Recker, R.R. Pathophysiology of Osteoporosis. Endocrinol. Metab. Clin. N. Am. 2012, 41, 475–486. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.-C.; Kim, S.Y.; Kim, Y.T.; Kim, E.-A.; Lee, S.-H.; Ko, S.-C.; Wijesinghe, W.; Samarakoon, K.W.; Kim, Y.-S.; Cho, J.H.; et al. In vitro and in vivo antioxidant activities of polysaccharide purified from aloe vera (Aloe barbadensis) gel. Carbohydr. Polym. 2014, 99, 365–371. [Google Scholar] [CrossRef]
- Lu, Z.-Q.; Deng, Y.-J.; Lu, J.-X. Effect of aloe polysaccharide on caspase-3 expression following cerebral ischemia and reperfusion injury in rats. Mol. Med. Rep. 2012, 6, 371–374. [Google Scholar] [CrossRef]
- Qian, W.; Cai, X.; Qian, Q.; Wang, D.; Zhang, L. Angelica Sinensis Polysaccharide Suppresses Epithelial-Mesenchymal Transition and Pulmonary Fibrosis via a DANCR/AUF-1/FOXO3 Regulatory Axis. Aging Dis. 2020, 11, 17–30. [Google Scholar] [CrossRef]
- Umavathi, S.; Keerthika, M.; Gopinath, K.; Kavitha, C.; Uddin, R.; Alagumanian, S.; Balalakshmi, C. Optimization of aqueous-assisted extraction of polysaccharides from pumpkin (Cucurbita moschata Duch) and their biological activities. Saudi J. Biol. Sci. 2021, 28, 6692–6700. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.-Q.; Ma, Z.-L.; Zhang, D.-X.; Wu, P.; Guo, Y.-H.; Yang, F.; Li, D.-Y. Sequential Extraction, Characterization, and Analysis of Pumpkin Polysaccharides for Their Hypoglycemic Activities and Effects on Gut Microbiota in Mice. Front. Nutr. 2021, 8, 769181. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Liang, L.; Yu, G.; Li, Q. Pumpkin polysaccharide modifies the gut microbiota during alleviation of type 2 diabetes in rats. Int. J. Biol. Macromol. 2018, 115, 711–717. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.-H.; Qian, L.; Yin, D.-L.; Zhou, Y. Hypolipidemic effect of the polysaccharides extracted from pumpkin by cellulase-assisted method on mice. Int. J. Biol. Macromol. 2014, 64, 137–138. [Google Scholar] [CrossRef]
- Skenderidis, P.; Lampakis, D.; Giavasis, I.; Leontopoulos, S.; Petrotos, K.; Hadjichristodoulou, C.; Tsakalof, A. Chemical Properties, Fatty-Acid Composition, and Antioxidant Activity of Goji Berry (Lycium barbarum L. and Lycium chinense Mill.) Fruits. Antioxidants 2019, 8, 60. [Google Scholar] [CrossRef]
- Potterat, O. Goji (Lycium barbarum and L. chinense): Phytochemistry, Pharmacology and Safety in the Perspective of Traditional Uses and Recent Popularity. Planta Medica 2010, 76, 7–19. [Google Scholar] [CrossRef]
- Du, M.; Hu, X.; Kou, L.; Zhang, B.; Zhang, C. Lycium barbarum Polysaccharide Mediated the Antidiabetic and Antinephritic Effects in Diet-Streptozotocin-Induced Diabetic Sprague Dawley Rats via Regulation of NF-κB. BioMed Res. Int. 2016, 2016, 1–9. [Google Scholar] [CrossRef]
- Yang, M.; Yin, Y.; Wang, F.; Zhang, H.; Ma, X.; Yin, Y.; Tan, B.; Chen, J. Supplementation With Lycium barbarum Polysaccharides Reduce Obesity in High-Fat Diet-Fed Mice by Modulation of Gut Microbiota. Front. Microbiol. 2021, 12, 719967. [Google Scholar] [CrossRef]
- Zhao, X.-Y.; Zhang, F.; Pan, W.; Yang, Y.-F.; Jiang, X.-Y. Clinical potentials of ginseng polysaccharide for treating gestational diabetes mellitus. World J. Clin. Cases 2021, 9, 4959–4979. [Google Scholar] [CrossRef]
- Wang, J.; Li, Y.; Luo, P.; Chen, Y.; Xi, Q.; Wu, H.; Zhao, W.; Shu, G.; Wang, S.; Gao, P.; et al. Oral supplementation with ginseng polysaccharide promotes food intake in mice. Brain Behav. 2019, 9, e01340. [Google Scholar] [CrossRef]
- Li, J.; Li, R.; Li, N.; Zheng, F.; Dai, Y.; Ge, Y.; Yue, H.; Yu, S. Mechanism of antidiabetic and synergistic effects of ginseng polysaccharide and ginsenoside Rb1 on diabetic rat model. J. Pharm. Biomed. Anal. 2018, 158, 451–460. [Google Scholar] [CrossRef]
- Lin, H.; Zhang, X.; Liu, J.; Yuan, L.; Liu, J.; Wang, C.; Sun, J.; Chen, J.; Jing, S.; Li, H. Schisantherin A improves learning and memory abilities partly through regulating the Nrf2/Keap1/ARE signaling pathway in chronic fatigue mice. Exp. Ther. Med. 2021, 21, 1–9. [Google Scholar] [CrossRef]
- Qiao, Z.; Du, X.; Zhuang, W.; Yang, S.; Li, H.; Sun, J.; Chen, J.; Wang, C. Schisandra Chinensis Acidic Polysaccharide Improves the Insulin Resistance in Type 2 Diabetic Rats by Inhibiting Inflammation. J. Med. Food 2020, 23, 358–366. [Google Scholar] [CrossRef]
- Feng, Y.; Li, H.; Chen, C.; Lin, H.; Xu, G.; Li, H.; Wang, C.; Chen, J.; Sun, J. Study on the Hepatoprotection of Schisandra chinensis Caulis Polysaccharides in Nonalcoholic Fatty Liver Disease in Rats Based on Metabolomics. Front. Pharmacol. 2021, 12, 727636. [Google Scholar] [CrossRef]
- Huang, R.; Xie, J.; Yu, Y.; Shen, M. Recent progress in the research of yam mucilage polysaccharides: Isolation, structure and bioactivities. Int. J. Biol. Macromol. 2020, 155, 1262–1269. [Google Scholar] [CrossRef]
- Fan, Y.; He, Q.; Luo, A.; Wang, M.; Luo, A. Characterization and Antihyperglycemic Activity of a Polysaccharide from Dioscorea opposita Thunb Roots. Int. J. Mol. Sci. 2015, 16, 6391–6401. [Google Scholar] [CrossRef]
- Li, Q.; Li, W.; Gao, Q.; Zou, Y. Hypoglycemic Effect of Chinese Yam (Dioscorea opposita rhizoma ) Polysaccharide in Different Structure and Molecular Weight. J. Food Sci. 2017, 82, 2487–2494. [Google Scholar] [CrossRef]
- Wang, J.; Zheng, D.; Huang, F.; Zhao, A.; Kuang, J.; Ren, Z.; Chen, T.; Lei, J.; Lin, J.; Wang, X.; et al. Theabrownin and Poria cocos Polysaccharide Improve Lipid Metabolism via Modulation of Bile Acid and Fatty Acid Metabolism. Front. Pharmacol. 2022, 13, 875549. [Google Scholar] [CrossRef]
- Wu, Z.; Zeng, W.; Zhang, X.; Yang, J. Characterization of Acidic Tea Polysaccharides from Yellow Leaves of Wuyi Rock Tea and Their Hypoglycemic Activity via Intestinal Flora Regulation in Rats. Foods 2022, 11, 617. [Google Scholar] [CrossRef]
- Chaves, P.F.P.; Hocayen, P.D.A.S.; Dallazen, J.L.; Werner, M.F.D.P.; Iacomini, M.; Andreatini, R.; Cordeiro, L.M. Chamomile tea: Source of a glucuronoxylan with antinociceptive, sedative and anxiolytic-like effects. Int. J. Biol. Macromol. 2020, 164, 1675–1682. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.-F.; Chen, X.; Tang, Y.; Wu, J.-M.; Qin, D.-L.; Yu, L.; Yu, C.-L.; Zhou, X.-G.; Wu, A.-G. The Therapeutic Potential of Plant Polysaccharides in Metabolic Diseases. Pharmaceuticals 2022, 15, 1329. https://doi.org/10.3390/ph15111329
Wang X-F, Chen X, Tang Y, Wu J-M, Qin D-L, Yu L, Yu C-L, Zhou X-G, Wu A-G. The Therapeutic Potential of Plant Polysaccharides in Metabolic Diseases. Pharmaceuticals. 2022; 15(11):1329. https://doi.org/10.3390/ph15111329
Chicago/Turabian StyleWang, Xiao-Fang, Xue Chen, Yong Tang, Jian-Ming Wu, Da-Lian Qin, Lu Yu, Chong-Lin Yu, Xiao-Gang Zhou, and An-Guo Wu. 2022. "The Therapeutic Potential of Plant Polysaccharides in Metabolic Diseases" Pharmaceuticals 15, no. 11: 1329. https://doi.org/10.3390/ph15111329
APA StyleWang, X.-F., Chen, X., Tang, Y., Wu, J.-M., Qin, D.-L., Yu, L., Yu, C.-L., Zhou, X.-G., & Wu, A.-G. (2022). The Therapeutic Potential of Plant Polysaccharides in Metabolic Diseases. Pharmaceuticals, 15(11), 1329. https://doi.org/10.3390/ph15111329







