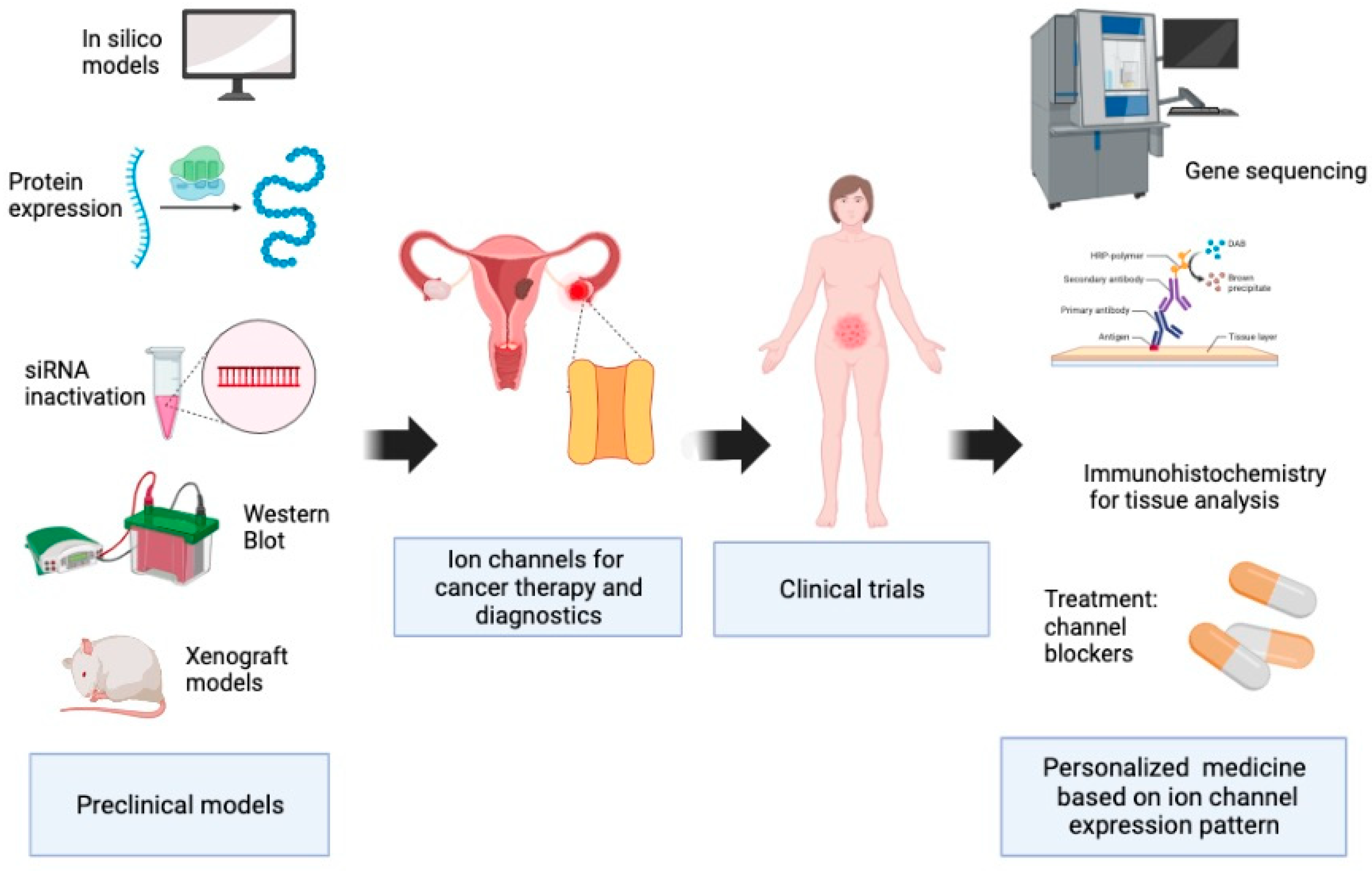
Ion Channels and Personalized Medicine in Gynecological Cancers
Abstract
1. Introduction
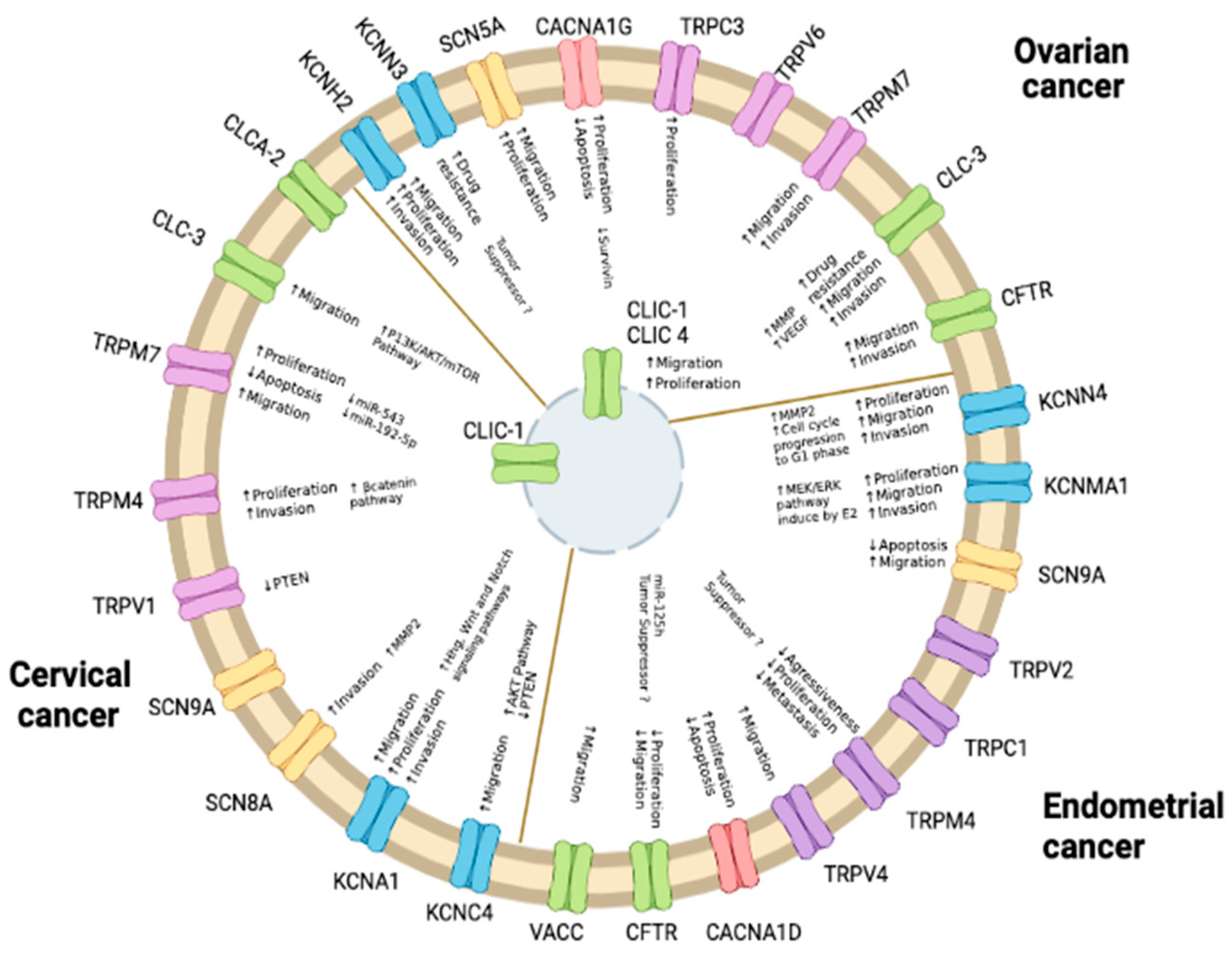
2. Potassium Channels
2.1. Endometrial Cancer
2.2. Ovarian Cancer
2.3. Cervical Cancer
| Channel | In Vitro | Animal Models | Clinical Observations | Reference |
|---|---|---|---|---|
| Endometrial Cancer | ||||
| KV11.1 | High expression of KCNE and HERG genes in the AN3-CA, KLE, and Ishikawa cell lines. | Higher frequency of RNA and protein expression in primary human tumors compared to non-cancerous tissues. | [24,25] | |
| KCa1.1 | Silencing with siRNA reduced cell proliferation, cell migration, and p-MEK1/2 and p-ERK1/2 expression in Ishikawa cells. Overexpression promotes cell proliferation and migration, and blockage with IBTX reduces cell proliferation in HEC-1-B cells. | Silencing in xenografts transplanted in nude mice produced smaller tumors compared to control mice. | Higher protein staining in type I endometrial adenocarcinoma tissue compared to normal and atypical endometrial tissues. | [14,15] |
| KCa3.1 | Downregulation by siRNA in HEC-1-A and KLE cells inhibits proliferation. Silencing by shRNA or blockage with TRAM-34 reduces cell cycle progression, and TRAM-34 diminishes migration and MMP2 expression in HEC-1-A and Ishikawa cells. | TRAM-34 and clotrimazole reduced tumor formation of HEC-1-A cells in nude mice. | Higher expression of mRNA and protein levels in endometrial cancer tissues compared to normal tissues. | [9,10] |
| Cervical cancer | ||||
| KV1.1 | Knockdown suppresses cell proliferation, migration, invasion, and protein levels of Hhg and Wnt1 in HeLa cells. | Knockdown in HeLa cells generated smaller xenograft tumors and prolonged survival in nude mice. | Higher protein expression in CCa tissues correlates with poor prognosis. | [22] |
| KV3.4 | Inactivation of the AKT pathway and inhibition of cell migration by blockage with BDS-II in Hela cells. | [21] | ||
| KV10.1 | Higher expression in HeLa, SiHa, and primary cultures of cervical cancer. Imipramine and astemizole decrease channel expression and increase apoptosis in E6/E7-transfected keratinocytes. Decreased proliferation and increased apoptosis of HeLa, SiHa, CaSki, INBL, and C-33A cells with astemizole treatment. Decreased mRNA and proliferation with calcitriol treatment in the SiHa cell line. | Inhibition of tumor growth in xenograft mice with tetrandrine treatment. Increased mRNA and protein expression in CCa tissues of transgenic mice K14E7 treated with estrogens. | Higher expression in high-grade cervical lesions compared to low-grade lesions and normal tissues. | [26,27,28,29,30] |
| Kir6.2 | Overexpression of mRNA in HeLa cells, and blockage with glibenclamide reduces cell viability. | Higher expression in invasive tumors compared to low or non-invasive tumors. | [31] | |
| KCa1.1 | Estradiol treatment increased protein and mRNA expression in K14E7 transgenic mice with CCa. | Higher intensity of immunostaining in biopsies of carcinoma in situ. | [12] | |
| KCa3.1 | Downregulation by siRNA increased apoptosis in HeLa cells. Increased uptake of dye H33258 dependent on KCa3.1 is observed in cervical carcinoma cell lines (CXT) compared to nonmalignant cervical epithelial cell strains (HCX). Clotrimazole reduces mRNA expression and changes HeLa cell morphology. | mRNA and protein overexpression in cervical cancer tissues. | [23,32,33] | |
| Ovarian cancer | ||||
| K2p2.1 K2p10.1 | Curcumin increases late apoptosis and decrease proliferation in SK-OV-3 and OVCAR-3 cells. | Expression is increased in cancer samples compared to normal ovarian samples. | [34] | |
| K2p9.1 | Reduction in proliferation and increase in late apoptosis in SK-OV-3 and OVCAR-3 cells with methanandamide treatment. | Significant correlation of immunostaining with tumor stage in patient biopsies. | [35] | |
| KV10.1 KV11.1 | 4-aminopyridine and tetraethylammonium inhibited proliferation in SK-OV-3 cells. Imipramine increases apoptosis levels and decreases proliferation in SK-OV-3 cells. Ergtoxin inhibits the proliferation of SK-OV-3 cells. | Higher expression in OC tissues compared to noncancerous tissue. | [36,37] | |
| KV10.1 | siRNA targeting sensitizes SK-OV-3 and TYK cells to cisplatin-induced apoptosis. | High expression compared to normal tissues. | [38] | |
| KV11.1 | Berberine reduces mRNA and protein levels in SK-OV-3 cells. | Berberine decreases tumor growth in xenografts compared to the control group. | High protein expression in tumor tissues compared to non-tumor tissues. | [17] |
| KCa1.1 | Correlation of miR-31 levels and resistance to cisplatin in A2780 cells. | Loss of expression is associated with cisplatin resistance. | [39] | |
| KCa2.3 | Low mRNA and protein expression in samples of ovarian serous cystadenocarcinomas compared to normal ovarian tissues and correlated with shorter disease-free and overal survival. | [19] | ||
3. Sodium Channels
3.1. Endometrial Cancer
3.2. Ovarian Cancer
3.3. Cervical Cancer
| Channel | In Vitro | Animal Models | Clinical Observations | Reference |
|---|---|---|---|---|
| Endometrial Cancer | ||||
| NaV1.7 | Blockade decreases invasion and promotes apoptosis, where activation increases invasion in primary cultures. | mRNA overexpression associated with tumor size, metastasis, and poor prognosis. | [42] | |
| Cervical cancer | ||||
| NaV1.2 | High mRNA expression in primary cultures transfected with the E7 oncogene. | High mRNA expression in cancerous biopsies compared to normal biopsies. | [52] | |
| NaV1.6 | mRNA overexpression in primary cultures increases invasive cell capacity, mediated by MMP2 activity. | More extensive protein pattern expression in tissue biopsies of cervical cancer compared to non-cancerous biopsies. | [49,50] | |
| NaV1.7 | mRNA overexpression in primary cultures. | More extensive protein pattern expression in tissue biopsies of cervical cancer compared to non-cancerous biopsies. | [49] | |
| Ovarian cancer | ||||
| NaV1.5 | Blockade with TTX decreases cell migration and invasion in Caov-3 and SK-OV-3 cells. EPA inactivates the channel and reduced migration and proliferation in SK-OV-3 cells. | Express in ovarian cancer with lymph node metastasis but not in normal ovary. | [43,53] | |
| NaV1.6 | RNAseq data analysis shows lower expression in 48 ovarian cell lines. | Higher overall survival in patients with lower expression. | [47] | |
| SCNN1A | Overexpressed in SK-OV-3, HO-8910, OVCAR-3, and CoC1 cell lines. | Overexpressed in sample patients obtained by database analysis. | [45] | |
4. Calcium and TRP Channels
4.1. Endometrial Cancer
4.2. Cervical Cancer
4.3. Ovarian Cancer
| Channel | In Vitro | Animal Models | Clinical Observations | Reference |
|---|---|---|---|---|
| Endometrial Cancer | ||||
| CaV1.3 | Increased expression with 17 β-estradiol treatment in Ishikawa cells. Blockage suppresses cell proliferation and promotes apoptosis and autophagy in HEC-1A. | High expression in atypical hyperplasia and endometrial carcinoma tissues, but low in benign endometrial tissues. | [78,79] | |
| TRPV2 | Overexpressed in metastatic biopsies and correlated with high-risk tumors. | [80] | ||
| TRPV1 | CBD triggers apoptosis in Ishikawa cells induced by channel activation. | [81] | ||
| TRPV4 | High levels of expression in Ishikawa cells are linked to migration. | Reduced metastatic peritoneal nodules in the shTRPV4 mice group. | Bioinformatic analysis found higher expression in of EC tissues. | [61] |
| TRPM4 | Silencing in AN3CA promoted proliferation, cell cycle, and migration in AN3CA. | Correlates with low-risk tumors in biopsies of EC and suggested as a protective gene (higher OS found by in silico analysis of RNA sequencing). | [58,62,63] | |
| CACNA2D1 | Overexpression in HEC-108, KLE, Ishikawa, and HEC-06 cells. Silencing with siRNA inhibited migration and proliferation of Ishikawa and HEC-108 cells. | Amlodipine treatment inhibits tumor growth in Balb/c mice. | In silico prognosis model of RNA sequencing found lower OS in samples overexpressing CACNA2D1, SLC8A1, and CCL2. | [60] |
| CACNA2D3 | Overexpression inhibits cell proliferation and migration and increases apoptosis in Ishikawa cells. | Overexpression reduces tumor growth in BALB/c nude mice. | Expression is downregulated in EC tissues. | [64] |
| Cervical cancer | ||||
| TRPV1 | Increased cell viability and colony formation. | Higher expression in CCa tissues. | [67] | |
| TRPV6 | Reduced expression is associated with poor prognosis in early-stage cervical squamous cell carcinoma. | [74] | ||
| TRPM4 | Overexpression in gene sequence analysis in CCa specimens. | [68] | ||
| TRPM7 | Silencing increases apoptosis and reduces migration in C-33A and SiHa cells. | Higher expression in CCa tissues. | [71] | |
| Ovarian cancer | ||||
| CaV1.2, CaV1.3, and CaV1.4 | Calcium channel blockers increase apoptosis in tumor stem cells. | Combination of manidipine and paclitaxel inhibits tumor growth in ovarian-CSC xenograft mouse models. | High expression of CACNA1D, CACNA1F, and CACNA1H is associated with low survival rates. | [77] |
| CaV1.2 | In silico: lower expression in tissues from patients with ovarian cancer compared to normal tissues. | [82] | ||
| CaV3.1 and CaV3.2 | Blockage with mibefradil and NNC 55-0396 reduces cell proliferation in HO8910 and A2780 cell lines. mRNA expression in A2780, A2780Cis, and IGROV-1 | NNC 55-0396 slows down the formation of tumors in nude mice. Mibefradil sensitizes tumors to carboplatin in a mouse model. | Increased expression in tissues of patients with ovarian cancer. | [75,76] |
| VGCC and KCa1.1 | Inhibition with trimebutine decreases stem cell properties in A2780-SP. | Trimebutine treatment reduces tumor growth in A2780-SP xenograft mice. | [83] | |
| T -Type and L-type calcium channels | Combination treatment of poziotinib and manidipine induces apoptosis in ovarian cancer stem cells in A2780-SP cells. | [84] | ||
| TRPC3 | Decreased expression by SKF96365 reduces SK-OV-3 proliferation. | Downregulation suppressed tumor development in nude mice. | High expression in ovarian epithelial tumors. | [85] |
| TRPV6 | SOR-C13 reduces tumor growth in SK-OV-3 xenografts. | High expression of mRNA and protein in ovarian cancer over normal tissue. SOR-C13 provides an antiproliferative mechanism through the inhibition of the channel activity. | [86,87] | |
| TRPM7 | Silencing inhibits migration and invasion in SK-OV-3 and OVCAR-3 cells. | Silencing prolongs the survival of mice bearing ovarian tumors. | Upregulated expression is associated with the EMT process. | [88] |
5. Chloride Channels
5.1. Endometrial Cancer
5.2. Cervical Cancer
5.3. Ovarian Cancer
| Channel | In Vitro | Animal Models | Clinical Observations | Reference |
|---|---|---|---|---|
| Endometrial Cancer | ||||
| VACC | Reduction of invasive endometrial cancer cells in the presence of a blocker in Ishikawa cells. | [92] | ||
| CFTR | Increased expression in Ishikawa cells. | mRNA upregulated expression in cancerous tissue. | [91] | |
| Cervical cancer | ||||
| CLIC1 | Increased mRNA levels expression in HeLa, SiHa, C-33A, and CaSki cells compared to normal human cervical epithelial cells. | Increased mRNA expression in mouse xenograft models. | Increased mRNA expression in cancerous tissue compared to normal samples. | [94] |
| CLC3 | Associated with migration and invasion of SiHa cells through the PI3K/Akt/mTOR pathway. | Increased mRNA and protein expression in cervical squamous carcinoma compared to para-carcinoma and normal tissue. | [103] | |
| CLCA2 | Decreased expression in cervical squamous cell carcinoma tissues compared to normal tissue. | [95] | ||
| VRAC | Channel blockage reduces the proliferation of SiHa cells. | [104] | ||
| Ovarian cancer | ||||
| CLIC1 | Gene silencing downregulates proliferation in A2780 cells. | Present in serum of xenograft mice model. Pulsed dendritic cells MtHsp70-CLIC1 improve the antitumor immune response in NOG mice. | High concentration in the serum of patients with ovarian cancer. High expression in ovarian cancer with peritoneal metastasis and contributes to tumorigenesis. | [96,97,98,105] |
| CLC3 | Overexpression in paclitaxel-resistant A2780 cells Channel antisense inhibits cell proliferation in SK-OV-3 cells. | [101,102] | ||
| CLIC4 | Present in the serum of OC patients. High expression in cancer tissue by immunohistochemistry. | [96,99] | ||
| CFTR | Gene knockdown inhibits cell motility and invasion in SK-OV-3 and A2780 cells. | Silencing inhibits xenograft tumor formation in nude mice. | Higher expression in ovarian cancer samples than in benign tumors and normal ovaries. | [106] |
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jentsch, T.J.; Hübner, C.A.; Fuhrmann, J.C. Ion channels: Function unravelled by dysfunction. Nat. Cell Biol. 2004, 6, 1039–1047. [Google Scholar] [CrossRef]
- Ramírez, A.; García-Quiroz, J.; Aguilar-Eslava, L.; Sánchez-Pérez, Y.; Camacho, J. Novel Therapeutic Approaches of Ion Channels and Transporters in Cancer. Rev. Physiol. Biochem. Pharmacol. 2022, 183, 45–101. [Google Scholar] [CrossRef]
- Prevarskaya, N.; Skryma, R.; Shuba, Y. Ion channels and the hallmarks of cancer. Trends Mol. Med. 2010, 16, 107–121. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef]
- Gutman, G.A.; Chandy, K.G.; Adelman, J.P.; Aiyar, J.; Bayliss, D.A.; Clapham, D.E.; Covarriubias, M.; Desir, G.V.; Furuichi, K.; Ganetzky, B.; et al. International Union of Pharmacology. XLI. Compendium of voltage-gated ion channels: Potassium channels. Pharmacol. Rev. 2003, 55, 583–586. [Google Scholar] [CrossRef]
- Pardo, L.A.; Stühmer, W. The roles of K+ channels in cancer. Nat. Rev. Cancer 2014, 14, 39–48. [Google Scholar] [CrossRef]
- Zuniga, L.; Cayo, A.; Gonzalez, W.; Vilos, C.; Zuniga, R. Potassium Channels as a Target for Cancer Therapy: Current Perspectives. OncoTargets Ther. 2022, 15, 783–797. [Google Scholar] [CrossRef]
- Costa, B.P.; Nunes, F.B.; Noal, F.C.; Branchini, G. Ion Channels in Endometrial Cancer. Cancers 2022, 14, 4733. [Google Scholar] [CrossRef]
- Wang, Z.H.; Shen, B.; Yao, H.L.; Jia, Y.C.; Ren, J.; Feng, Y.J.; Wang, Y.Z. Blockage of intermediate-conductance-Ca(2+) -activated K(+) channels inhibits progression of human endometrial cancer. Oncogene 2007, 26, 5107–5114. [Google Scholar] [CrossRef]
- Zhang, Y.; Feng, Y.; Chen, L.; Zhu, J. Effects of Intermediate-Conductance Ca(2+)-Activated K(+) Channels on Human Endometrial Carcinoma Cells. Cell Biochem. Biophys. 2015, 72, 515–525. [Google Scholar] [CrossRef]
- Erdem Kis, E.; Tiftik, R.N.; Al Hennawi, K.; Un, I. The role of potassium channels in the proliferation and migration of endometrial adenocarcinoma HEC1-A cells. Mol. Biol. Rep. 2022, 49, 7447–7454. [Google Scholar] [CrossRef]
- Ramírez, A.; Vera, E.; Gamboa-Domínguez, A.; Lambert, P.; Gariglio, P.; Camacho, J. Calcium-activated potassium channels as potential early markers of human cervical cancer. Oncol. Lett. 2018, 15, 7249–7254. [Google Scholar] [CrossRef]
- Oeggerli, M.; Tian, Y.; Ruiz, C.; Wijker, B.; Sauter, G.; Obermann, E.; Güth, U.; Zlobec, I.; Sausbier, M.; Kunzelmann, K.; et al. Role of KCNMA1 in breast cancer. PLoS ONE 2012, 7, e41664. [Google Scholar] [CrossRef]
- Wang, F.; Chen, Q.; Huang, G.; Guo, X.; Li, N.; Li, Y.; Li, B. BKCa participates in E2 inducing endometrial adenocarcinoma by activating MEK/ERK pathway. BMC Cancer 2018, 18, 1128. [Google Scholar] [CrossRef]
- Li, N.; Liu, L.; Li, G.; Xia, M.; Du, C.; Zheng, Z. The role of BKCa in endometrial cancer HEC-1-B cell proliferation and migration. Gene 2018, 655, 42–47. [Google Scholar] [CrossRef]
- He, S.; Moutaoufik, M.T.; Islam, S.; Persad, A.; Wu, A.; Aly, K.A.; Fonge, H.; Babu, M.; Cayabyab, F.S. HERG channel and cancer: A mechanistic review of carcinogenic processes and therapeutic potential. Biochim. Biophys. Acta Rev. Cancer 2020, 1873, 188355. [Google Scholar] [CrossRef]
- Zhi, D.; Zhou, K.; Yu, D.; Fan, X.; Zhang, J.; Li, X.; Dong, M. hERG1 is involved in the pathophysiological process and inhibited by berberine in SKOV3 cells. Oncol. Lett. 2019, 17, 5653–5661. [Google Scholar] [CrossRef]
- Cicek, M.S.; Koestler, D.C.; Fridley, B.L.; Kalli, K.R.; Armasu, S.M.; Larson, M.C.; Wang, C.; Winham, S.J.; Vierkant, R.A.; Rider, D.N.; et al. Epigenome-wide ovarian cancer analysis identifies a methylation profile differentiating clear-cell histology with epigenetic silencing of the HERG K+ channel. Hum. Mol. Genet. 2013, 22, 3038–3047. [Google Scholar] [CrossRef]
- Liu, X.; Wei, L.; Zhao, B.; Cai, X.; Dong, C.; Yin, F. Low expression of KCNN3 may affect drug resistance in ovarian cancer. Mol. Med. Rep. 2018, 18, 1377–1386. [Google Scholar] [CrossRef]
- Wang, X.; Chen, Y.; Li, J.; Guo, S.; Lin, X.; Zhang, H.; Zhan, Y.; An, H. Tetrandrine, a novel inhibitor of ether-à-go-go-1 (Eag1), targeted to cervical cancer development. J. Cell Physiol. 2019, 234, 7161–7173. [Google Scholar] [CrossRef]
- Sim, H.J.; Song, M.S.; Lee, S.Y. Kv3 channels contribute to cancer cell migration via vimentin regulation. Biochem. Biophys. Res. Commun. 2021, 551, 140–147. [Google Scholar] [CrossRef]
- Liu, L.; Chen, Y.; Zhang, Q.; Li, C. Silencing of KCNA1 suppresses the cervical cancer development via mitochondria damage. Channels 2019, 13, 321–330. [Google Scholar] [CrossRef]
- Bukhari, M.; Deng, H.; Sipes, D.; Ruane-Foster, M.; Purdy, K.; Woodworth, C.D.; Sur, S.; Samways, D.S.K. KCa3.1-dependent uptake of the cytotoxic DNA-binding dye Hoechst 33258 into cancerous but not healthy cervical cells. J. Biol. Chem. 2021, 296, 100084. [Google Scholar] [CrossRef]
- Cherubini, A.; Taddei, G.L.; Crociani, O.; Paglierani, M.; Buccoliero, A.M.; Fontana, L.; Noci, I.; Borri, P.; Borrani, E.; Giachi, M.; et al. HERG potassium channels are more frequently expressed in human endometrial cancer as compared to non-cancerous endometrium. Br. J. Cancer 2000, 83, 1722–1729. [Google Scholar] [CrossRef]
- Suzuki, T.; Takimoto, K. Selective expression of HERG and Kv2 channels influences proliferation of uterine cancer cells. Int. J. Oncol. 2004, 25, 153–159. [Google Scholar] [CrossRef]
- Díaz, L.; Ceja-Ochoa, I.; Restrepo-Angulo, I.; Larrea, F.; Avila-Chávez, E.; García-Becerra, R.; Borja-Cacho, E.; Barrera, D.; Ahumada, E.; Gariglio, P.; et al. Estrogens and human papilloma virus oncogenes regulate human ether-à-go-go-1 potassium channel expression. Cancer Res. 2009, 69, 3300–3307. [Google Scholar] [CrossRef]
- Ortiz, C.S.; Montante-Montes, D.; Saqui-Salces, M.; Hinojosa, L.M.; Gamboa-Dominguez, A.; Hernández-Gallegos, E.; Martínez-Benítez, B.; Del Rosario Solís-Pancoatl, M.; Garcia-Villa, E.; Ramírez, A.; et al. Eag1 potassium channels as markers of cervical dysplasia. Oncol. Rep. 2011, 26, 1377–1383. [Google Scholar] [CrossRef]
- de Guadalupe Chávez-López, M.; Hernández-Gallegos, E.; Vázquez-Sánchez, A.Y.; Gariglio, P.; Camacho, J. Antiproliferative and proapoptotic effects of astemizole on cervical cancer cells. Int. J. Gynecol. Cancer 2014, 24, 824–828. [Google Scholar] [CrossRef]
- Avila, E.; García-Becerra, R.; Rodríguez-Rasgado, J.A.; Díaz, L.; Ordaz-Rosado, D.; Zügel, U.; Steinmeyer, A.; Barrera, D.; Halhali, A.; Larrea, F.; et al. Calcitriol down-regulates human ether a go-go 1 potassium channel expression in cervical cancer cells. Anticancer Res. 2010, 30, 2667–2672. [Google Scholar]
- Farias, L.M.; Ocaña, D.B.; Díaz, L.; Larrea, F.; Avila-Chávez, E.; Cadena, A.; Hinojosa, L.M.; Lara, G.; Villanueva, L.A.; Vargas, C.; et al. Ether a go-go potassium channels as human cervical cancer markers. Cancer Res. 2004, 64, 6996–7001. [Google Scholar] [CrossRef]
- Vazquez-Sanchez, A.Y.; Hinojosa, L.M.; Parraguirre-Martinez, S.; Gonzalez, A.; Morales, F.; Montalvo, G.; Vera, E.; Hernandez-Gallegos, E.; Camacho, J. Expression of K(ATP) channels in human cervical cancer: Potential tools for diagnosis and therapy. Oncol. Lett. 2018, 15, 6302–6308. [Google Scholar] [CrossRef]
- Liu, L.; Zhan, P.; Nie, D.; Fan, L.; Lin, H.; Gao, L.; Mao, X. Intermediate-Conductance-Ca2-Activated K Channel IKCa1 Is Upregulated and Promotes Cell Proliferation in Cervical Cancer. Med. Sci. Monit. Basic Res. 2017, 23, 45–57. [Google Scholar] [CrossRef]
- Zhan, P.; Liu, L.; Nie, D.; Liu, Y.; Mao, X. Effect of inhibition of intermediate-conductance-Ca(2+)-activated K(+) channels on HeLa cell proliferation. J. Cancer Res. Ther. 2018, 14, S41–S45. [Google Scholar] [CrossRef]
- Innamaa, A.; Jackson, L.; Asher, V.; van Schalkwyk, G.; Warren, A.; Keightley, A.; Hay, D.; Bali, A.; Sowter, H.; Khan, R. Expression and effects of modulation of the K2P potassium channels TREK-1 (KCNK2) and TREK-2 (KCNK10) in the normal human ovary and epithelial ovarian cancer. Clin. Transl. Oncol. 2013, 15, 910–918. [Google Scholar] [CrossRef]
- Innamaa, A.; Jackson, L.; Asher, V.; Van Shalkwyk, G.; Warren, A.; Hay, D.; Bali, A.; Sowter, H.; Khan, R. Expression and prognostic significance of the oncogenic K2P potassium channel KCNK9 (TASK-3) in ovarian carcinoma. Anticancer Res. 2013, 33, 1401–1408. [Google Scholar]
- Asher, V.; Khan, R.; Warren, A.; Shaw, R.; Schalkwyk, G.V.; Bali, A.; Sowter, H.M. The Eag potassium channel as a new prognostic marker in ovarian cancer. Diagn. Pathol. 2010, 5, 78. [Google Scholar] [CrossRef]
- Asher, V.; Warren, A.; Shaw, R.; Sowter, H.; Bali, A.; Khan, R. The role of Eag and HERG channels in cell proliferation and apoptotic cell death in SK-OV-3 ovarian cancer cell line. Cancer Cell Int. 2011, 11, 6. [Google Scholar] [CrossRef]
- Hui, C.; Lan, Z.; Yue-li, L.; Li-lin, H.; Li-lin, H. Knockdown of Eag1 Expression by RNA Interference Increases Chemosensitivity to Cisplatin in Ovarian Cancer Cells. Reprod. Sci. 2015, 22, 1618–1626. [Google Scholar] [CrossRef]
- Samuel, P.; Pink, R.C.; Caley, D.P.; Currie, J.M.; Brooks, S.A.; Carter, D.R. Over-expression of miR-31 or loss of KCNMA1 leads to increased cisplatin resistance in ovarian cancer cells. Tumour Biol. 2016, 37, 2565–2573. [Google Scholar] [CrossRef]
- de Lera Ruiz, M.; Kraus, R.L. Voltage-Gated Sodium Channels: Structure, Function, Pharmacology, and Clinical Indications. J. Med. Chem. 2015, 58, 7093–7118. [Google Scholar] [CrossRef]
- Black, J.A.; Waxman, S.G. Noncanonical roles of voltage-gated sodium channels. Neuron 2013, 80, 280–291. [Google Scholar] [CrossRef]
- Liu, J.; Tan, H.; Yang, W.; Yao, S.; Hong, L. The voltage-gated sodium channel Na(v)1.7 associated with endometrial cancer. J. Cancer 2019, 10, 4954–4960. [Google Scholar] [CrossRef]
- Gao, R.; Shen, Y.; Cai, J.; Lei, M.; Wang, Z. Expression of voltage-gated sodium channel alpha subunit in human ovarian cancer. Oncol. Rep. 2010, 23, 1293–1299. [Google Scholar] [CrossRef]
- Liu, J.; Liu, D.; Liu, J.J.; Zhao, C.; Yao, S.; Hong, L. [Corrigendum] Blocking the Nav1.5 channel using eicosapentaenoic acid reduces migration and proliferation of ovarian cancer cells. Int. J. Oncol. 2020, 57, 1234. [Google Scholar] [CrossRef]
- Wu, L.; Ling, Z.H.; Wang, H.; Wang, X.Y.; Gui, J. Upregulation of SCNN1A Promotes Cell Proliferation, Migration, and Predicts Poor Prognosis in Ovarian Cancer Through Regulating Epithelial-Mesenchymal Transformation. Cancer Biother. Radiopharm. 2019, 34, 642–649. [Google Scholar] [CrossRef]
- Patel, F.; Brackenbury, W.J. Dual roles of voltage-gated sodium channels in development and cancer. Int. J. Dev. Biol. 2015, 59, 357–366. [Google Scholar] [CrossRef]
- Brummelhuis, I.S.; Fiascone, S.J.; Hasselblatt, K.T.; Frendl, G.; Elias, K.M. Voltage-Gated Sodium Channels as Potential Biomarkers and Therapeutic Targets for Epithelial Ovarian Cancer. Cancers 2021, 13, 5437. [Google Scholar] [CrossRef]
- Nelson, M.; Millican-Slater, R.; Forrest, L.C.; Brackenbury, W.J. The sodium channel beta1 subunit mediates outgrowth of neurite-like processes on breast cancer cells and promotes tumour growth and metastasis. Int. J. Cancer 2014, 135, 2338–2351. [Google Scholar] [CrossRef]
- Hernandez-Plata, E.; Ortiz, C.S.; Marquina-Castillo, B.; Medina-Martinez, I.; Alfaro, A.; Berumen, J.; Rivera, M.; Gomora, J.C. Overexpression of NaV 1.6 channels is associated with the invasion capacity of human cervical cancer. Int. J. Cancer 2012, 130, 2013–2023. [Google Scholar] [CrossRef]
- Lopez-Charcas, O.; Espinosa, A.M.; Alfaro, A.; Herrera-Carrillo, Z.; Ramirez-Cordero, B.E.; Cortes-Reynosa, P.; Perez Salazar, E.; Berumen, J.; Gomora, J.C. The invasiveness of human cervical cancer associated to the function of Na(V)1.6 channels is mediated by MMP-2 activity. Sci. Rep. 2018, 8, 12995. [Google Scholar] [CrossRef]
- Song, C.; Lee, Y.; Kim, S. Bioinformatic Analysis for the Prognostic Implication of Genes Encoding Epithelial Sodium Channel in Cervical Cancer. Int. J. Gen. Med. 2022, 15, 1777–1787. [Google Scholar] [CrossRef]
- Diaz, D.; Delgadillo, D.M.; Hernández-Gallegos, E.; Ramírez-Domínguez, M.E.; Hinojosa, L.M.; Ortiz, C.S.; Berumen, J.; Camacho, J.; Gomora, J.C. Functional expression of voltage-gated sodium channels in primary cultures of human cervical cancer. J. Cell Physiol. 2007, 210, 469–478. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, D.; Liu, J.J.; Zhao, C.; Yao, S.; Hong, L. Blocking the Nav1.5 channel using eicosapentaenoic acid reduces migration and proliferation of ovarian cancer cells. Int. J. Oncol. 2018, 53, 855–865. [Google Scholar] [CrossRef] [PubMed]
- Monteith, G.R.; Davis, F.M.; Roberts-Thomson, S.J. Calcium channels and pumps in cancer: Changes and consequences. J. Biol. Chem. 2012, 287, 31666–31673. [Google Scholar] [CrossRef]
- Catterall, W.A.; Perez-Reyes, E.; Snutch, T.P.; Striessnig, J. International Union of Pharmacology. XLVIII. Nomenclature and structure-function relationships of voltage-gated calcium channels. Pharmacol. Rev. 2005, 57, 411–425. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.J.; Sweet, T.B.; Clapham, D.E. International Union of Basic and Clinical Pharmacology. LXXVI. Current progress in the mammalian TRP ion channel family. Pharmacol. Rev. 2010, 62, 381–404. [Google Scholar] [CrossRef] [PubMed]
- Shapovalov, G.; Ritaine, A.; Skryma, R.; Prevarskaya, N. Role of TRP ion channels in cancer and tumorigenesis. Semin. Immunopathol. 2016, 38, 357–369. [Google Scholar] [CrossRef]
- Van den Eynde, C.; De Clercq, K.; Van Bree, R.; Luyten, K.; Annibali, D.; Amant, F.; Han, S.; Van Nieuwenhuysen, E.; Baert, T.; Peeraer, K.; et al. TRP channel expression correlates with the epithelial-mesenchymal transition and high-risk endometrial carcinoma. Cell. Mol. Life Sci. 2021, 79, 26. [Google Scholar] [CrossRef]
- Dolphin, A.C. Voltage-gated calcium channel α (2)δ subunits: An assessment of proposed novel roles. F1000Research 2018, 7, 1830. [Google Scholar] [CrossRef]
- Huang, T.; Feng, X.; Wang, J.; Zhou, J.; Wang, J. Calcium-Related Genes Predicting Outcomes and Serving as Therapeutic Targets in Endometrial Cancer. Cells 2022, 11, 3156. [Google Scholar] [CrossRef]
- Li, X.; Cheng, Y.; Wang, Z.; Zhou, J.; Jia, Y.; He, X.; Zhao, L.; Dong, Y.; Fan, Y.; Yang, X.; et al. Calcium and TRPV4 promote metastasis by regulating cytoskeleton through the RhoA/ROCK1 pathway in endometrial cancer. Cell Death Dis. 2020, 11, 1009. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Lin, J.; He, H. Identification of Potential Crucial Genes Associated With the Pathogenesis and Prognosis of Endometrial Cancer. Front. Genet. 2019, 10, 373. [Google Scholar] [CrossRef] [PubMed]
- Li, X.C.; Cheng, Y.; Yang, X.; Zhou, J.Y.; Dong, Y.Y.; Shen, B.Q.; Wang, J.Q.; Zhao, L.J.; Wang, Z.Q.; Li, X.P.; et al. Decreased expression of TRPM4 is associated with unfavorable prognosis and aggressive progression of endometrial carcinoma. Am. J. Transl. Res. 2020, 12, 3926–3939. [Google Scholar]
- Kong, X.; Li, M.; Shao, K.; Yang, Y.; Wang, Q.; Cai, M. Progesterone induces cell apoptosis via the CACNA2D3/Ca2+/p38 MAPK pathway in endometrial cancer. Oncol. Rep. 2020, 43, 121–132. [Google Scholar] [CrossRef]
- Huang, T.; Zhou, J.; Zhang, L.; Yang, X.; Cheng, Y.; Yin, S.; Wang, J.; Shen, B.; Feng, X.; Li, X.; et al. Azelnidipine nanoparticles break calcium homeostasis and induce severe ER stress combined with medroxyprogesterone acetate for endometrial cancer therapy. Nano Today 2022, 47, 101682. [Google Scholar] [CrossRef]
- Lei, J.; Deng, F.; Ding, H.; Fu, M.; Xu, T.; Ji, B.; Feng, L.; Li, M.; Qiu, J.; Gao, Q. Recent Developments on the Roles of Calcium Signals and Potential Therapy Targets in Cervical Cancer. Cells 2022, 11, 3003. [Google Scholar] [CrossRef] [PubMed]
- Han, G.H.; Chay, D.B.; Nam, S.; Cho, H.; Chung, J.Y.; Kim, J.H. The Combination of Transient Receptor Potential Vanilloid Type 1 (TRPV1) and Phosphatase and Tension Homolog (PTEN) is an Effective Prognostic Biomarker in Cervical Cancer. Int. J. Gynecol. Pathol. 2021, 40, 214–223. [Google Scholar] [CrossRef]
- Narayan, G.; Bourdon, V.; Chaganti, S.; Arias-Pulido, H.; Nandula, S.V.; Rao, P.H.; Gissmann, L.; Dürst, M.; Schneider, A.; Pothuri, B.; et al. Gene dosage alterations revealed by cDNA microarray analysis in cervical cancer: Identification of candidate amplified and overexpressed genes. Genes Chromosomes Cancer 2007, 46, 373–384. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, X. Targeting the Wnt/β-catenin signaling pathway in cancer. J. Hematol. Oncol. 2020, 13, 165. [Google Scholar] [CrossRef]
- Zhou, W.; Guo, S.; Xiong, Z.; Liu, M. Oncogenic role and therapeutic target of transient receptor potential melastatin 7 channel in malignancy. Expert Opin. Ther. Targets 2014, 18, 1177–1196. [Google Scholar] [CrossRef]
- Qi, H.; Lu, L.; Wang, L. Long Noncoding RNA ST7-AS1 Upregulates TRPM7 Expression by Sponging microRNA-543 to Promote Cervical Cancer Progression. Onco Targets Ther. 2020, 13, 7257–7269. [Google Scholar] [CrossRef]
- Dong, R.F.; Zhuang, Y.J.; Wang, Y.; Zhang, Z.Y.; Xu, X.Z.; Mao, Y.R.; Yu, J.J. Tumor suppressor miR-192-5p targets TRPM7 and inhibits proliferation and invasion in cervical cancer. Kaohsiung J. Med. Sci. 2021, 37, 699–708. [Google Scholar] [CrossRef]
- Liu, X.; Gan, L.; Zhang, J. miR-543 inhibites cervical cancer growth and metastasis by targeting TRPM7. Chem. Biol. Interact 2019, 302, 83–92. [Google Scholar] [CrossRef]
- Sun, F.; Xiao, L.; Jang, X.X.; Xiong, Y.; Li, Q.; Yue, X.J.; Wei, Y.J.; Wei, Y.X.; Ma, Y.L.; Yu, Y.H. TRPV6 is a prognostic marker in early-stage cervical squamous cell carcinoma. Tumour Biol. 2016, 37, 15743–15751. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhang, S.L.; Wang, N.; Zhang, B.B.; Li, M. Blockade of T-type Ca(2+) channels inhibits human ovarian cancer cell proliferation. Cancer Investig. 2011, 29, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Dziegielewska, B.; Casarez, E.V.; Yang, W.Z.; Gray, L.S.; Dziegielewski, J.; Slack-Davis, J.K. T-Type Ca2+ Channel Inhibition Sensitizes Ovarian Cancer to Carboplatin. Mol. Cancer Ther. 2016, 15, 460–470. [Google Scholar] [CrossRef]
- Lee, H.; Kim, J.W.; Kim, D.K.; Choi, D.K.; Lee, S.; Yu, J.H.; Kwon, O.B.; Lee, J.; Lee, D.S.; Kim, J.H.; et al. Calcium Channels as Novel Therapeutic Targets for Ovarian Cancer Stem Cells. Int. J. Mol. Sci. 2020, 21, 2327. [Google Scholar] [CrossRef] [PubMed]
- Hao, J.; Bao, X.; Jin, B.; Wang, X.; Mao, Z.; Li, X.; Wei, L.; Shen, D.; Wang, J.L. Ca2+ channel subunit α 1D promotes proliferation and migration of endometrial cancer cells mediated by 17β-estradiol via the G protein-coupled estrogen receptor. Faseb J. 2015, 29, 2883–2893. [Google Scholar] [CrossRef]
- Bao, X.X.; Xie, B.S.; Li, Q.; Li, X.P.; Wei, L.H.; Wang, J.L. Nifedipine induced autophagy through Beclin1 and mTOR pathway in endometrial carcinoma cells. Chin. Med. J. 2012, 125, 3120–3126. [Google Scholar]
- Marinelli, O.; Morelli, M.B.; Annibali, D.; Aguzzi, C.; Zeppa, L.; Tuyaerts, S.; Amantini, C.; Amant, F.; Ferretti, B.; Maggi, F.; et al. The Effects of Cannabidiol and Prognostic Role of TRPV2 in Human Endometrial Cancer. Int. J. Mol. Sci. 2020, 21, 5409. [Google Scholar] [CrossRef]
- Fonseca, B.M.; Correia-da-Silva, G.; Teixeira, N.A. Cannabinoid-induced cell death in endometrial cancer cells: Involvement of TRPV1 receptors in apoptosis. J. Physiol. Biochem. 2018, 74, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Chang, X.; Dong, Y. CACNA1C is a prognostic predictor for patients with ovarian cancer. J. Ovarian Res. 2021, 14, 88. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Kwon, O.B.; Lee, J.E.; Jeon, Y.H.; Lee, D.S.; Min, S.H.; Kim, J.W. Repositioning Trimebutine Maleate as a Cancer Treatment Targeting Ovarian Cancer Stem Cells. Cells 2021, 10, 918. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Kim, J.W.; Lee, D.S.; Min, S.H. Combined Poziotinib with Manidipine Treatment Suppresses Ovarian Cancer Stem-Cell Proliferation and Stemness. Int. J. Mol. Sci. 2020, 21, 7379. [Google Scholar] [CrossRef]
- Yang, S.L.; Cao, Q.; Zhou, K.C.; Feng, Y.J.; Wang, Y.Z. Transient receptor potential channel C3 contributes to the progression of human ovarian cancer. Oncogene 2009, 28, 1320–1328. [Google Scholar] [CrossRef]
- Xue, H.; Wang, Y.; MacCormack, T.J.; Lutes, T.; Rice, C.; Davey, M.; Dugourd, D.; Ilenchuk, T.T.; Stewart, J.M. Inhibition of Transient Receptor Potential Vanilloid 6 channel, elevated in human ovarian cancers, reduces tumour growth in a xenograft model. J. Cancer 2018, 9, 3196–3207. [Google Scholar] [CrossRef]
- Fu, S.; Hirte, H.; Welch, S.; Ilenchuk, T.T.; Lutes, T.; Rice, C.; Fields, N.; Nemet, A.; Dugourd, D.; Piha-Paul, S.; et al. Erratum to: First-in-human phase I study of SOR-C13, a TRPV6 calcium channel inhibitor, in patients with advanced solid tumors. Investig. New Drugs 2017, 35, 397. [Google Scholar] [CrossRef]
- Liu, L.; Wu, N.; Wang, Y.; Zhang, X.; Xia, B.; Tang, J.; Cai, J.; Zhao, Z.; Liao, Q.; Wang, J. TRPM7 promotes the epithelial-mesenchymal transition in ovarian cancer through the calcium-related PI3K/AKT oncogenic signaling. J. Exp. Clin. Cancer Res. 2019, 38, 106. [Google Scholar] [CrossRef]
- Alexander, S.P.H.; Mathie, A.; Peters, J.A. Guide to Receptors and Channels (GRAC), 4th edn. Br. J. Pharmacol. 2009, 158, S130–S134. [Google Scholar] [CrossRef]
- Kim, H.J.; Lee, P.C.; Hong, J.H. Chloride Channels and Transporters: Roles beyond Classical Cellular Homeostatic pH or Ion Balance in Cancers. Cancers 2022, 14, 856. [Google Scholar] [CrossRef]
- Xia, X.; Wang, J.; Liu, Y.; Yue, M. Lower Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Promotes the Proliferation and Migration of Endometrial Carcinoma. Med. Sci. Monit. 2017, 23, 966–974. [Google Scholar] [CrossRef]
- Li, M.; Wu, D.B.; Wang, J. Effects of volume-activated chloride channels on the invasion and migration of human endometrial cancer cells. Eur. J. Gynaecol. Oncol. 2013, 34, 60–64. [Google Scholar] [PubMed]
- Jóźwiak, P.; Ciesielski, P.; Forma, E.; Kozal, K.; Wójcik-Krowiranda, K.; Cwonda, Ł.; Bieńkiewicz, A.; Bryś, M.; Krześlak, A. Expression of voltage-dependent anion channels in endometrial cancer and its potential prognostic significance. Tumour Biol. 2020, 42, 1010428320951057. [Google Scholar] [CrossRef]
- Wang, W.; Li, X.; Xu, Y.; Guo, W.; Yu, H.; Zhang, L.; Wang, Y.; Chen, X. Acetylation-stabilized chloride intracellular channel 1 exerts a tumor-promoting effect on cervical cancer cells by activating NF-κB. Cell Oncol 2021, 44, 557–568. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Cao, J.L.; Yang, F.N.; Li, X.F.; Tao, L.M.; Wang, F. Decreased expression of CLCA2 and the correlating with immune infiltrates in patients with cervical squamous cell carcinoma: A bioinformatics analysis. Taiwan J. Obstet. Gynecol. 2021, 60, 480–486. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.Y.; Beer, L.A.; Tanyi, J.L.; Zhang, R.; Liu, Q.; Speicher, D.W. Protein isoform-specific validation defines multiple chloride intracellular channel and tropomyosin isoforms as serological biomarkers of ovarian cancer. J. Proteom. 2013, 89, 165–178. [Google Scholar] [CrossRef]
- Qu, H.; Chen, Y.; Cao, G.; Liu, C.; Xu, J.; Deng, H.; Zhang, Z. Identification and validation of differentially expressed proteins in epithelial ovarian cancers using quantitative proteomics. Oncotarget 2016, 7, 83187–83199. [Google Scholar] [CrossRef]
- Ye, Y.; Yin, M.; Huang, B.; Wang, Y.; Li, X.; Lou, G. CLIC1 a novel biomarker of intraperitoneal metastasis in serous epithelial ovarian cancer. Tumour Biol. 2015, 36, 4175–4179. [Google Scholar] [CrossRef]
- Singha, B.; Harper, S.L.; Goldman, A.R.; Bitler, B.G.; Aird, K.M.; Borowsky, M.E.; Cadungog, M.G.; Liu, Q.; Zhang, R.; Jean, S.; et al. CLIC1 and CLIC4 complement CA125 as a diagnostic biomarker panel for all subtypes of epithelial ovarian cancer. Sci. Rep. 2018, 8, 14725. [Google Scholar] [CrossRef]
- Yao, Q.; Qu, X.; Yang, Q.; Wei, M.; Kong, B. CLIC4 mediates TGF-beta1-induced fibroblast-to-myofibroblast transdifferentiation in ovarian cancer. Oncol. Rep. 2009, 22, 541–548. [Google Scholar] [CrossRef]
- Feng, J.; Peng, Z.; Gao, L.; Yang, X.; Sun, Z.; Hou, X.; Li, E.; Zhu, L.; Yang, H. ClC-3 promotes paclitaxel resistance via modulating tubulins polymerization in ovarian cancer cells. Biomed. Pharmacother. 2021, 138, 111407. [Google Scholar] [CrossRef]
- Li, M.; Wu, D.B.; Wang, J.; Chen, L. CLC-3 Cl- channel-mediated invasion and migration of human ovarian cancer cells. Eur. J. Gynaecol. Oncol. 2016, 37, 689–695. [Google Scholar]
- Guan, Y.T.; Xie, Y.; Zhou, H.; Shi, H.Y.; Zhu, Y.Y.; Zhang, X.L.; Luan, Y.; Shen, X.M.; Chen, Y.P.; Xu, L.J.; et al. Overexpression of chloride channel-3 (ClC-3) is associated with human cervical carcinoma development and prognosis. Cancer Cell Int. 2019, 19, 8. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.R.; Droogmans, G.; Eggermont, J.; Voets, T.; Ellory, J.C.; Nilius, B. Differential expression of volume-regulated anion channels during cell cycle progression of human cervical cancer cells. J. Physiol. 2000, 529, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Qu, H.; Cao, G.; Liu, C.; Deng, H.; Zhang, Z. MtHsp70-CLIC1-pulsed dendritic cells enhance the immune response against ovarian cancer. Biochem. Biophys. Res. Commun. 2017, 494, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Yong, M.; Li, J.; Dong, X.; Yu, T.; Fu, X.; Hu, L. High level of CFTR expression is associated with tumor aggression and knockdown of CFTR suppresses proliferation of ovarian cancer in vitro and in vivo. Oncol. Rep. 2015, 33, 2227–2234. [Google Scholar] [CrossRef]
- Sheth, M.; Esfandiari, L. Bioelectric Dysregulation in Cancer Initiation, Promotion, and Progression. Front. Oncol. 2022, 12, 846917. [Google Scholar] [CrossRef]
- Pardo, L.A. Voltage-Gated Potassium Channels Beyond the Action Potential. Bioelectricity 2022, 4, 117–125. [Google Scholar] [CrossRef]
- Singh, A.K.; Awasthi, R.; Malviya, R. Bioelectronic medicines: Therapeutic potential and advancements in next-generation cancer therapy. Biochim. Biophys. Acta Rev. Cancer 2022, 1877, 188808. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramírez, A.; Ogonaga-Borja, I.; Acosta, B.; Chiliquinga, A.J.; de la Garza, J.; Gariglio, P.; Ocádiz-Delgado, R.; Bañuelos, C.; Camacho, J. Ion Channels and Personalized Medicine in Gynecological Cancers. Pharmaceuticals 2023, 16, 800. https://doi.org/10.3390/ph16060800
Ramírez A, Ogonaga-Borja I, Acosta B, Chiliquinga AJ, de la Garza J, Gariglio P, Ocádiz-Delgado R, Bañuelos C, Camacho J. Ion Channels and Personalized Medicine in Gynecological Cancers. Pharmaceuticals. 2023; 16(6):800. https://doi.org/10.3390/ph16060800
Chicago/Turabian StyleRamírez, Ana, Ingrid Ogonaga-Borja, Brenda Acosta, Andrea Jazmín Chiliquinga, Jaime de la Garza, Patricio Gariglio, Rodolfo Ocádiz-Delgado, Cecilia Bañuelos, and Javier Camacho. 2023. "Ion Channels and Personalized Medicine in Gynecological Cancers" Pharmaceuticals 16, no. 6: 800. https://doi.org/10.3390/ph16060800
APA StyleRamírez, A., Ogonaga-Borja, I., Acosta, B., Chiliquinga, A. J., de la Garza, J., Gariglio, P., Ocádiz-Delgado, R., Bañuelos, C., & Camacho, J. (2023). Ion Channels and Personalized Medicine in Gynecological Cancers. Pharmaceuticals, 16(6), 800. https://doi.org/10.3390/ph16060800








