Photodynamic Therapy: A Prospective Therapeutic Approach for Viral Infections and Induced Neoplasia
Abstract
1. Introduction
1.1. Historical and Background of Photodynamic Therapy
1.2. Emerging Challenges
2. Therapeutic Improvements
2.1. Emergence of “Smart” Light-Sensitive Molecules
2.2. Light-Related Strategies
2.3. Few Supplying Oxygen Strategies
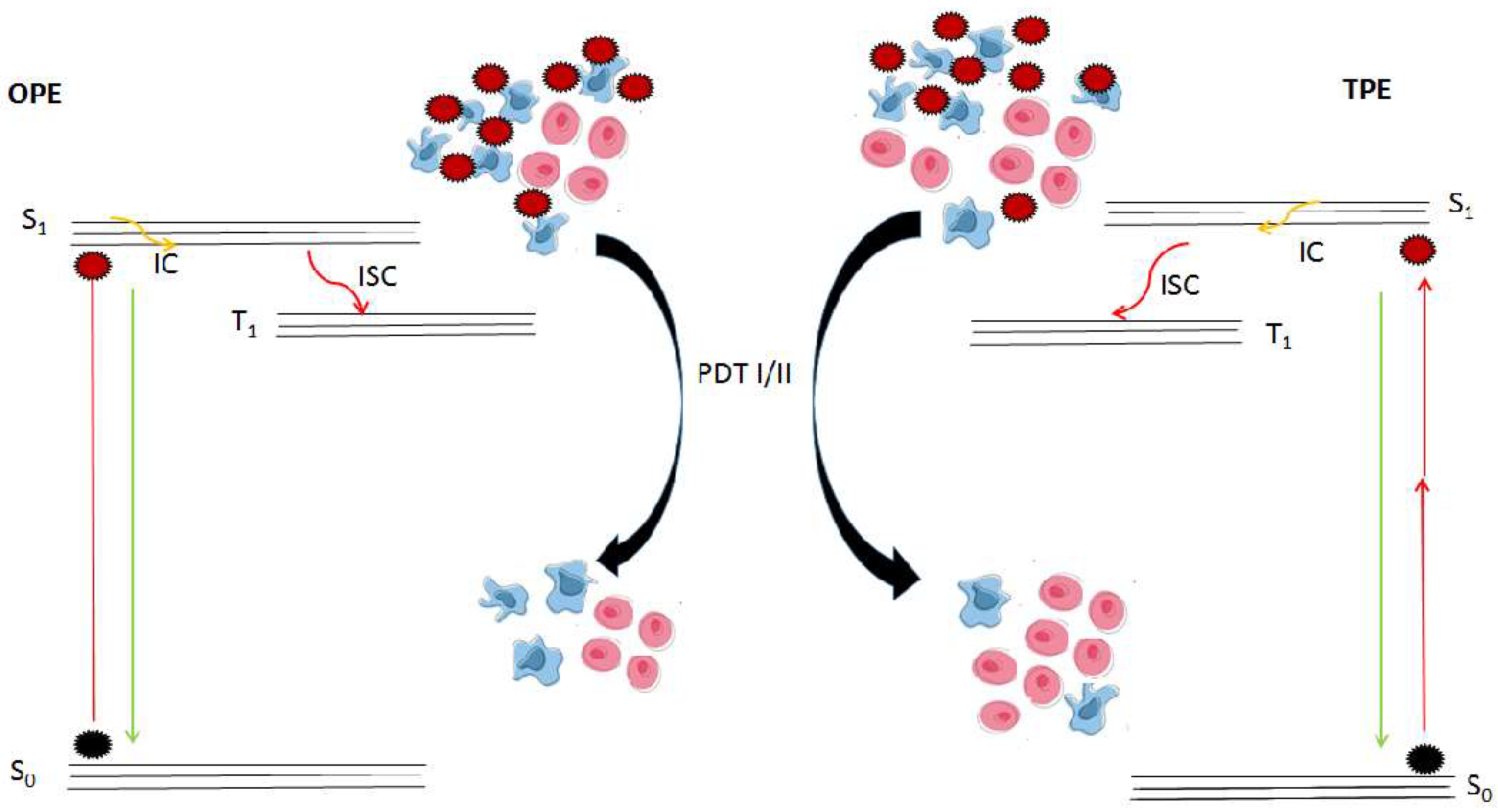
2.4. Mechanisms of Photodynamic Actions
3. Viral Infections and Photodynamic-Induced Immunity
3.1. Induced Immunity
3.2. Oncogenic Viruses
4. Oncogenic Viruses—Signaling Pathway Interactions
5. Future Perspective and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ABC | ATP-binding cassette | Kbp | Kilo base pair(s) |
| AIDS | Acquired immune deficiency syndrome | KSHV | Kaposi’s sarcoma herpes virus |
| AIE | Aggregation induced emission | LANA | Latent associated nuclear antigen |
| AMR | Antimicrobial resistance | MAPK | Mitogen-activated protein kinase |
| ATM | Ataxia-telangiectasia mutated | MCV | Merkel cell polymavirus |
| ATP | Adenosine triphosphate | MDR | Multiple drug resistance |
| ATR | Ataxia-telangiectasia Rad3 | MOF | Metal organic framework |
| ART | Antiretroviral treatment | MnO2 | Manganese oxide |
| CD | Carbon dots | mTOR | Mechanistic target of rapamycin |
| CHK | Checkpoint kinase | NF-kB | Nuclear factor kB |
| DAMPs | Damage associated molecular patterns | NIR | Near infrared |
| DNA | Deoxyribonucleic acid | NPs | Nanoparticles |
| EBV | Epstein–Barr Virus | 1O2 | Singlet oxygen |
| EPR | Enhanced permeability retention | OPE | One photon emission |
| FG | Fluorescence-guided | PDD | Photodynamic diagnosis |
| GBM | Glioblastoma | PDI | Photodynamic inactivation |
| HBV | Hepatitis B virus | PDT | Photodynamic therapy |
| HCV | Hepatitis C virus | PI3K | Phosphatidylinositol-3-kinase |
| HHV | Human herpes virus | PSs | Photosensitizers |
| HIV | Human immune virus | RNA | Ribonucleic acid |
| Hp | Hematoporphyrin | ROS | Reactive oxygen species |
| HpD | Hematoporphyrin derivatives | SARS | Severe acute respiratory syndrome |
| HTLV | Human T-lymphotropic virus | S0/S1 | Ground state/excited state |
| H2O2 | Hydrogen peroxide | T-ALL | T-cell acute lymphoblastic leukemia |
| IC | Internal conversion | TPE | Two photon emission |
| ICD | Immunogenic cell death | TME | Tumor microenvironment |
| ISC | Intersystem crossing | T1 | Excited triplet state |
References
- Krueger, B.P.; Fleming, G.R.; Longworth, J. Photochemical reaction. Ency. Brit. 2018. Available online: https://www.britannica.com/science/photochemical-reaction (accessed on 2 August 2022).
- Dougherty, T.J. Photodynamic Therapy. JNCI 1998, 90, 889–905. [Google Scholar] [CrossRef] [PubMed]
- Barreca, M.; Ingarra, A.M.; Raimondi, M.V.; Spanò, V.; de Franco, M.; Menilli, L.; Gandin, V.; Miolo, G.; Barraja, P.; Montalbano, A. Insight on pyrimido[5,4-g]indolizine and pyrimido[4,5-c]pyrrolo[1,2-a]azepine systems as promising photosensitizers on malignant cells. Eur. J. Med. Chem. 2022, 237, 114399. [Google Scholar] [CrossRef]
- Josefsen, L.B.; Boyle, R.W. Photodynamic Therapy and the Development of Metal-Based Photosensitisers. Met. Based Drugs 2008, 276109. [Google Scholar] [CrossRef] [PubMed]
- Wilson, B.T.; Mang, T. Photodynamic therapy for cutaneous malignancies. Clin. Dermatol. 1995, 13, 91–96. [Google Scholar] [CrossRef]
- Ackroyd, R.; Kelty, C.; Brown, N.; Reed, M. The History of Photodetection and Photodynamic Therapy. Photochem. Photobiol. 2001, 74, 656–669. [Google Scholar] [CrossRef]
- Daniel, M.D.; Hill, J.S. A history of photodynamic therapy. Aust. N. Z. J. Surg. 1991, 61, 340–348. [Google Scholar] [CrossRef]
- Lee, C.N.; Hsu, R.; Chen, H.; Wong, T.W. Daylight photodynamic therapy: An update. Molecules 2020, 25, 5195. [Google Scholar] [CrossRef]
- Li, W.P.; Yen, C.J.; Wu, B.S. Wong TW. Recent Advances in Photodynamic Therapy for Deep-Seated Tumors with the Aid of Nanomedicine. Biomedicine 2021, 9, 69. [Google Scholar]
- Fadeel, D.A.A.; Fadel, M.; Tawfik, A.; Omar, Y. Transfersomal eosin topical delivery assisted by fractional CO2 laser for photodynamic treatment of palmar hyperhidrosis: Case study. Drug Deliv. Transl. Res. 2022. [Google Scholar] [CrossRef]
- Szeimies, R.M.; Dräger, J.; Abels, C.; Landthaler, M. History of photodynamic therapy in dermatology. In Photodynamic Therapy and Fluorescence Diagnosis in Dermatology; Calzavara-Pinton, E.-G., Szeimies, R.-M., Ortel, B., Eds.; Elsevier: Amsterdam, The Netherlands, 2001; Volume 2, pp. 3–15. [Google Scholar]
- Moan, J.; Berg, K. The photodegradation of porphyrins in cells can be used to estimate the lifetime of singlet oxygen. Photochem. Photobiol. 1991, 53, 549–553. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Halls, S.; Dickey, D.; Tulip, J.; Moore, R.B. Fractionated versus Standard Continuous Light Delivery in Interstitial Photodynamic Therapy of dunning Prostate Carcinomas. Clin. Cancer Res. 2007, 13, 7496–7505. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Vaupel, P.; Thews, O.; Hoeckel, M. Treatment Resistance of Solid Tumors. Med. Oncol. 2001, 18, 243–260. [Google Scholar] [CrossRef] [PubMed]
- Henderson, B.W.; Waldow, S.M.; Mang, T.S.; Potter, W.R.; Malone, P.B.; Dougherty, T.J. Tumor Destruction and Kinetics of Tumor Cell Death in Two Experimental Mouse Tumors Following Photodynamic Therapy. Cancer Res. 1985, 45, 572–576. [Google Scholar]
- Dolmans, D.E.; Kadambi, A.; Hill, J.S.; Waters, C.A.; Robinson, B.C.; Walker, J.P.; Fukumura, D.; Jain, R.K. Vascular Accumulation of a Novel Photosensitizer, MV6401, Causes Selective Thrombosis in Tumor Vessels after Photodynamic Therapy. Cancer Res. 2002, 62, 2151–2156. [Google Scholar]
- Busch, T.M.; Wileyto, E.P.; Emanuele, M.J.; del Piero, F.; Marconato, L.; Glatstein, E.; Koch, C.J. Photodynamic Therapy Creates Fluence Rate-dependent Gradients in the Intratumoral Spatial Distribution of Oxygen. Cancer Res. 2002, 62, 7273–7279. [Google Scholar]
- Ossola, R.; Jönsson, O.M.; Moor, K.; McNeill, K. Singlet Oxygen Quantum Yields in Environmental Waters. Chem. Rev. 2001, 121, 4100–4146. [Google Scholar] [CrossRef]
- Li, Y.; Wang, J.; Zhang, X.; Guo, W.; Li, F.; Yu, M.; Kong, X.; Wu, W.; Hong, Z. Highly Water-Soluble and Tumor-Targeted Photosensitizers for Photodynamic Therapy. Org. Biomol. Chem. 2015, 13, 7681–7694. [Google Scholar] [CrossRef]
- Dubuc, C.; Langlois, R.; Bénard, F.; Cauchon, N.; Klarskov, K.; Tone, P.; van Lier, J.E. Targeting Gastrin-Releasing Peptide Receptors of Prostate Cancer Cells for Photodynamic Therapy with a Phthalocyanine-Bombesin Conjugate. Bioorg. Med. Chem. Lett. 2008, 18, 2424–2427. [Google Scholar] [CrossRef]
- Sekhosana, K.E.; Nyokong, T. Synthesis of Ytterbium Bisphthalocyanines: Photophysicochemical Properties and Nonlinear Absorption Behavior. Opt. Mater. 2014, 37, 139–146. [Google Scholar] [CrossRef]
- Rossetti, F.C.; Lopes, L.B.; Carollo, A.R.H.; Thomazini, J.A.; Tedesco, A.C.; Bentley, M.V.L.B. A Delivery System to Avoid Self-Aggregation and to Improve In Vitro and In Vivo Skin Delivery of a Phthalocyanine Derivative Used in the Photodynamic Therapy. J. Control Release 2011, 155, 400–408. [Google Scholar] [CrossRef] [PubMed]
- Kuruppuarachchi, M.; Savoie, H.; Lowry, A.; Alonso, C.; Boyle, R.W. Polyacrylamide Nanoparticles as a Delivery System in Photodynamic Therapy. Mol. Pharm. 2011, 8, 920–931. [Google Scholar] [CrossRef]
- Vasiliou, V.; Vasiliou, K.; Nebert, D.W. Human ATP-binding cassette (ABC) transporter family. Hum. Genom. 2009, 3, 281–290. [Google Scholar] [CrossRef]
- Mansoori, B.; Mohammadi, A.; Davudian, S.; Shirjang, S.; Baradaran, B. The different mechanisms of cancer drug resistance: A brief review. Adv. Pharm. Bull. 2017, 7, 339–348. [Google Scholar] [CrossRef]
- Aniogo, E.C.; George, B.P.A.; Abrahamse, H. The role of photodynamic therapy on multidrug resistant breast cancer. Cancer Cell Int. 2019, 19, 91. [Google Scholar] [CrossRef] [PubMed]
- Lucena, S.; Salazar, N.; Gracia-Cazaña, T.; Zamarrón, A.; González, S.; Juarranz, Á.; Gilaberte, Y. Combined Treatments with Photodynamic Therapy for Non-Melanoma Skin Cancer. Int. J. Mol. Sci. 2015, 16, 25912–25933. [Google Scholar] [CrossRef] [PubMed]
- Cree, I.A.; Charlton, P. Molecular chess? Hallmarks of anticancer drug resistance. BMC Cancer 2017, 17, 10. [Google Scholar] [CrossRef] [PubMed]
- Ambudkar, S.V.; Kimchi-Sarfaty, C.; Sauna, Z.E.; Gottesman, M.M. P-glycoprotein: From genomics to mechanism. Oncogene 2003, 22, 7468–7485. [Google Scholar] [CrossRef]
- CDC. Antibiotic Resistance Threats in the United States, 2019; U.S. Department of Health and Human Services, CDC: Atlanta, GA, USA, 2019.
- Rossolini, G.M.; Mantengoli, E. Antimicrobial resistance in Europe and its potential impact on empirical therapy. Clin. Microbiol. Infect. 2008, 14, 2–8. [Google Scholar] [CrossRef]
- Muehler, D.; Rupp, C.M.; Keceli, S.; Brochhausen, C.; Siegmund, H.; Maisch, T.; Hiller, K.A.; Buchalla, W.; Cieplik, F. Insights into Mechanisms of Antimicrobial Photodynamic Action Toward Biofilms Using Phenalen-1-One Derivatives as Photosensitizers. Front. Microbiol. 2020, 11, 589364. [Google Scholar] [CrossRef]
- Sibata, C.H.; Colussi, V.C.; Oleinick, N.L.; Kinsella, T.J. Photodynamic Therapy: A New Concept in Medical Treatment. Braz. J. Med. Biol. 2000, 33, 869–880. [Google Scholar] [CrossRef] [PubMed]
- Mfouo-Tynga, I.S.; Dias, L.D.; Inanda, N.M.; Kurachi, C. Features of Third Generation Photosensitizers Used in Anticancer Photodynamic Therapy: Review. Photodiagnosis Photodyn. Ther. 2021, 34, 102091. [Google Scholar] [CrossRef] [PubMed]
- Gomez, S.; Tsung, A.; Hu, Z. Current Targets and Bioconjugation Strategies in Photodynamic Diagnosis and Therapy of Cancer. Molecules 2020, 25, 4964. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, S.R.G.; Fernandes, R.; Sarmento, B.; Pereira, P.M.R.; Tome, J.P.C. Photoimmunoconjugates: Novel Synthetic strategies to Targeted and Treat Cancer by Photodynamic Therapy. Org. Biomol. Chem. 2019, 17, 2579–2593. [Google Scholar] [CrossRef] [PubMed]
- Sandland, J.; Boyle, R.W. Photosensitizer Antibody-Drug Conjugates: Past, Present and Future. Bioconjug. Chem. 2019, 30, 975–993. [Google Scholar] [CrossRef] [PubMed]
- Soares, S.; Sousa, J.; Pais, A.; Vitorino, C. Nanomedicine: Principles, Properties, and Regulatory Issues. Front. Chem. 2018, 6, 360. [Google Scholar] [CrossRef]
- Debele, T.A.; Yeh, C.F.; Su, W.P. Cancer Immunotherapy and Application of Nanoparticles in Cancers Immunotherapy as the Delivery of Immunotherapeutic Agents and as the Immunomodulators. Cancers 2020, 12, 3773. [Google Scholar] [CrossRef]
- Algorri, J.F.; Ochoa, M.; Roldan-Varona, P.; Rodriguez-Cobo, L.; Lopez-Higuera, J.M. Photodynamic Therapy: A Compendium of Latest Reviews. Cancers 2021, 13, 4447. [Google Scholar] [CrossRef]
- Mesquita, M.Q.; Dias, C.J.; Gamelas, S.; Fardilha, M.; Neves, M.G.P.M.S.; Faustino, M.A.F. An Insight on the Role of Photosensitizer Nanocarriers for Photodynamic Therapy. An. Acad. Bras. Cienc. 2018, 90, 1101–1130. [Google Scholar] [CrossRef]
- Lu, K.Y.; Li, R.; Hsu, C.H.; Lin, C.W.; Chou, S.C.; Tsai, M.L.; Mi, F.L. Development of a new type of multifunctional fucoidan-based nanoparticles for anticancer drug delivery. Carbohydr. Polym. 2017, 165, 410–420. [Google Scholar] [CrossRef]
- Zhen, Z.; Tang, W.; Chuang, Y.J.; Todd, T.; Zhang, W.; Lin, X.; Niu, G.; Liu, G.; Wang, L.; Pan, Z.; et al. Tumor vasculature targeted photodynamic therapy for enhanced delivery of nanoparticles. ACS Nano 2014, 8, 6004–6013. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Watanabe, R.; Choyke, P.L. Improving conventional enhanced permeability and retention (EPR) effects; What is the appropriate target? Theranostics 2013, 4, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Zamboni, W.C. Concept and clinical evaluation of carrier-mediated anticancer agents. Oncologist 2008, 13, 248–260. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Lee, Y.K.; Park, I.K.; Hwang, S.R. Current Limitations and Recent Progress in Nanomedicine for Clinically Available Photodynamic Therapy. Biomedicines 2021, 9, 85. [Google Scholar] [CrossRef]
- Yoon, I.; Li, J.Z.; Shim, Y.K. Advance in photosensitizers and light delivery for photodynamic therapy. Clin. Endosc. 2013, 46, 7. [Google Scholar] [CrossRef]
- Jared, R.J.; Lauren, E.A.; Neil, I.B.; Daniel, M.S. Transcranial red and near infrared light transmission in a cadaveric model. PLoS ONE 2012, 7, e47460. [Google Scholar]
- Benning, R.K.P.; Piston, D.W. Two-Photons Excitation Microscopy for the Study of Living Cells and Tissues. Curr. Protoc. Cell Biol. 2014, 65, 2–9. [Google Scholar]
- Zhu, C.; Kwok, R.T.K.; Lam, J.W.Y.; Tang, B.Z. Aggregation-induced emission: A trailblazing journey to the field of biomedicine. ACS Appl. Bio Mater. 2018, 1, 1768–1786. [Google Scholar] [CrossRef]
- Sun, X.; Zebibula, A.; Dong, X.; Zhang, G.; Zhang, D.; Qian, J.; He, S. Aggregation-induced emission nanoparticles encapsulated with pegylated nano graphene oxide and their applications in two-photon fluorescence bioimaging and photodynamic therapy in vitro and in vivo. ACS Appl. Mater. Interfaces 2018, 10, 25037–25046. [Google Scholar] [CrossRef]
- Zhuang, W.; Yang, L.; Ma, B.; Kong, Q.; Li, G.; Wang, Y.; Tang, B.Z. Multifunctional two-photon aie luminogens for highly mitochondria-specific bioimaging and efficient photodynamic therapy. ACS Appl. Mater. Interfaces 2019, 11, 20715–20724. [Google Scholar] [CrossRef]
- Wang, S.; Wu, W.; Manghnani, P.; Xu, S.; Wang, Y.; Goh, C.C.; Ng, L.G.; Liu, B. Polymerization-enhanced two-photon photosensitization for precise photodynamic therapy. ACS Nano 2019, 13, 3095–3105. [Google Scholar] [CrossRef] [PubMed]
- Ho-Wu, R.; Yau, S.H.; Goodson, T. III Efficient singlet oxygen generation in metal nanoclusters for two-photon photodynamic therapy applications. J. Phys. Chem. B 2017, 121, 10073–10080. [Google Scholar] [CrossRef] [PubMed]
- McLean, A.; Wang, R.; Huo, Y.; Cooke, A.; Hopkins, T.; Potter, N.; Li, Q.; Isaac, J.; Haidar, J.; Jin, R.; et al. Synthesis and optical properties of two-photon-absorbing au25 (captopril) 18-embedded polyacrylamide nanoparticles for cancer therapy. ACS Appl. Nano. Mater. 2020, 3, 1420–1430. [Google Scholar] [CrossRef]
- Fitzgerald, F. Photodynamic Therapy (PDT), Principles, Mechanisms and Applications; Nova Science Publishers, Inc.: New York, NY, USA, 2017. [Google Scholar]
- Overchuk, M.; Zheng, G. Overcoming obstacles in the tumor microenvironment: Recent advancements in nanoparticle delivery for cancer theranostics. Biomaterials 2018, 156, 217–237. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Huang, C.; Liu, L.; Hu, R.; Qu, J. Enhancing Type I Photochemistry in Photodynamic Therapy Under Near Infrared Light by Using Antennae–Fullerene Complexes. Cytom. Part A 2018, 93, 997–1003. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.Y.; Li, X.M.; Yao, C.; Wang, W.X.; Zhao, M.Y.; El -Toni, A.M.; Zhang, F. Orthogonal near-infrared upconversion co-regulated site-specific O2 delivery and photodynamic therapy for hypoxia tumor by using red blood cell microcarriers. Biomaterials 2017, 125, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Cui, Y.C.; Bu, H.X.; Chen, J.M.; Li, Y.; Tang, G.P.; Wang, L.Q. A photosensitizer loaded hemoglobin-polymer conjugate as a nanocarrier for enhanced photodynamic therapy. J. Mater. Chem. B 2018, 6, 1825–1833. [Google Scholar] [CrossRef]
- Xu, Z.; Pan, C.; Yuan, W. Light-enhanced hypoxia-responsive and azobenzene cleavage-triggered size-shrinkable micelles for synergistic photodynamic therapy and chemotherapy. Biomater. Sci. 2020, 8, 3348–3358. [Google Scholar] [CrossRef]
- Klimenko, V.V.; Knyazev, N.A.; Moiseenko, F.V.; Rusanov, A.A.; Bogdanov, A.A.; Dubina, M.V. Pulse mode of laser photodynamic treatment induced cell apoptosis. Photodiagnosis Photodyn. Ther. 2016, 13, 101–107. [Google Scholar] [CrossRef]
- Ma, S.; Zhou, J.; Zhang, Y.; Yang, B.; He, Y.; Tian, C.; Xu, X.; Gu, Z. An oxygen self-sufficient fluorinated nanoplatform for relieved tumor hypoxia and enhanced photodynamic therapy of cancers. ACS Appl. Mater. Interfaces 2019, 11, 7731–7742. [Google Scholar] [CrossRef]
- Hu, H.; Yan, X.; Wang, H.; Tanaka, J.; Wang, M.; You, W.; Li, Z. Perfluorocarbon-based o 2 nanocarrier for efficient photodynamic therapy. J. Mater. Chem. B 2019, 7, 1116–1123. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.; Zhong, L.; Wang, M.; Li, H.; Qu, Y.; Liu, Q.; Han, R.; Yuan, L.; Shi, K.; Peng, J.; et al. Perfluorocarbon-loaded and redox-activatable photosensitizing agent with oxygen supply for enhancement of fluorescence/photoacoustic imaging guided tumor photodynamic therapy. ACS Appl. Bio-Mater. 2019, 29, 1806199. [Google Scholar] [CrossRef]
- Liu, P.; Xie, X.; Shi, X.; Peng, Y.; Ding, J.; Zhou, W. Oxygen-self-supplying and hif-1α-inhibiting core–shell nanosystem for hypoxia-resistant photodynamic therapy. ACS Appl. Mater. Interfaces 2019, 11, 48261–48270. [Google Scholar] [CrossRef] [PubMed]
- Hai, L.; Zhang, A.; Wu, X.; Cheng, H.; He, D.; Wang, T.; He, X.; Wang, K. Liposome-stabilized black phosphorus for photothermal drug delivery and oxygen self-enriched photodynamic therapy. ACS Appl. Nano Mater. 2019, 3, 563–575. [Google Scholar] [CrossRef]
- Phua, S.Z.F.; Yang, G.; Lim, W.Q.; Verma, A.; Chen, H.; Thanabalu, T.; Zhao, Y. Catalase-integrated hyaluronic acid as nanocarriers for enhanced photodynamic therapy in solid tumor. ACS Nano 2019, 13, 4742–4751. [Google Scholar] [CrossRef]
- Gao, Z.; Li, Y.; Zhang, Y.; Cheng, K.; An, P.; Chen, F.; Chen, J.; You, C.; Zhu, Q.; Sun, B. Biomimetic platinum nanozyme immobilized on 2d metal–organic frameworks for mitochondrion-targeting and oxygen self-supply photodynamic therapy. ACS Appl. Mater. Interfaces 2019, 12, 1963–1972. [Google Scholar] [CrossRef]
- Liang, R.; Liu, L.; He, H.; Chen, Z.; Han, Z.; Luo, Z.; Wu, Z.; Zheng, M.; Ma, Y.; Cai, L. Oxygen-boosted immunogenic photodynamic therapy with gold nanocages@ manganese dioxide to inhibit tumor growth and metastases. Biomaterials 2018, 177, 149–160. [Google Scholar] [CrossRef]
- Feng, Y.; Ding, D.; Sun, W.; Qiu, Y.; Luo, L.; Shi, T.; Meng, S.; Chen, X.; Chen, H. Magnetic manganese oxide sweetgum-ball nanospheres with large mesopores regulate tumor microenvironments for enhanced tumor nanotheranostics. ACS Appl. Mater. Interfaces 2019, 11, 37461–37470. [Google Scholar] [CrossRef]
- Jia, Q.; Ge, J.; Liu, W.; Zheng, X.; Chen, S.; Wen, Y.; Zhang, H.; Wang, P. A magnetofluorescent carbon dot assembly as an acidic H2O2-driven oxygenerator to regulate tumor hypoxia for simultaneous bimodal imaging and enhanced photodynamic therapy. Adv. Mater. 2018, 30, 1706090. [Google Scholar] [CrossRef]
- Chaplin, D.D. Overview of the immune response. J Allergy Clin. Immunol. 2010, 125, S3–S23. [Google Scholar] [CrossRef]
- Reginato, E.; Wolf, P.; Hamblin, M.R. Immune response after photodynamic therapy increases anti-cancer and anti-bacterial effects. World J. Immunol. 2014, 4, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Reginato, E.; Lindenmann, J.; Langner, C.; Schweintzger, N.; Bambach, I.; Smolle-Juttner, F.; Wolf, P. Photodynamic therapy downregulates the function of regulatory T cells in patients with esophageal squamous cell carcinoma. Photochem. Photobiol. Sci. 2014, 13, 1281–1289. [Google Scholar] [CrossRef] [PubMed]
- Wachowska, M.; Muchowicz, A.; Demkow, U. Immunological aspects of antitumor photodynamic therapy outcome. Central-Eur. J. Immunol. 2015, 40, 481–485. [Google Scholar] [CrossRef] [PubMed]
- Domingo, E. (Ed.) Introduction to virus origins and their role in biological evolution. In Virus as Populations; Elsevier: Amsterdam, The Netherlands, 2015; pp. 1–34. [Google Scholar]
- Banerjee, N.; Mukhopadhyay, S. Viral glycoproteins: Biological role and application in diagnosis. Virusdisease 2016, 27, 1–11. [Google Scholar] [CrossRef]
- Sadraeian, M.; Junior, F.F.P.; Miranda, M.; Galinskas, J.; Fernandes, R.S.; da Cruz, E.F.; Fu, L.; Zhang, L.; Diaz, R.S.; Cabral-Miranda, G.; et al. Study of Viral Photoinactivation by UV-C Light and Photosensitizer Using a Pseudotyped Model. Pharmaceutics 2022, 14, 683. [Google Scholar] [CrossRef]
- Moore, P.S.; Chang, Y. Why do viruses cause cancer? Highlights of the first century of human tumour virology. Nat. Rev. Cancer 2010, 10, 878–889. [Google Scholar] [CrossRef]
- Boshart, M.; Gissmann, L.; Ikenberg, H.; Kleinheinz, A.; Scheurlen, W.; zur Hausen, H. A new type of papillomavirus DNA, its presence in genital cancer biopsies and in cell lines derived from cervical cancer. EMBO J. 1984, 3, 1151–1157. [Google Scholar] [CrossRef]
- Durst, M.; Gissmann, L.; Ikenberg, H.; zur Hausen, H. A papillomavirus DNA from a cervical carcinoma and its prevalence in cancer biopsy samples from different geographic regions. Proc. Natl. Acad. Sci. USA 1983, 80, 3812–3815. [Google Scholar] [CrossRef]
- Divya, C.S.; Pillai, M.R. Antitumor Action of Curcumin in Human Papillomavirus Associated Cells Involves Downregulation of Viral Oncogenes, Prevention of NFΚB and AP-1 Translocation, and Modulation of Apoptosis. Mol. Carcinog. 2006, 45, 320–332. [Google Scholar] [CrossRef]
- Vélez-Bohórquez, A.; Bohórquez-Lozano, M.; Echeverry-de-Polanco, M. The virus in the Human oncogenesis. Infectio 2018, 22, 213–222. [Google Scholar] [CrossRef]
- Bouza, E.; Jiménez, M.M.; Alemany, L.; Arribas, J.; Bañares, R.; Barragán, M.B.; Eiros Bouza, J.M.; Felip, E.; Fernández-Capetillo, O.; Gracia, D.; et al. Overview of virus and cancer relationships. Position paper. Rev. Esp. Quimioter. 2021, 34, 525–555. [Google Scholar] [CrossRef] [PubMed]
- Passos, A.M.; Granato, C.F.H. Cancer causing viruses and the role of laboratory medicine: Literature review and perspectives. J. Bras. Patol. Med. Lab. 2013, 49, 109–114. [Google Scholar] [CrossRef][Green Version]
- Epstein, M.A.; Achong, B.G.; Barr, Y.M. Virus particles in cultured lymphoblasts from Burkitt’s lymphoma. Lancet 1964, 1, 702–703. [Google Scholar] [CrossRef]
- Chang, Y.; Cesarman, E.; Pessin, M.S.; Lee, F.; Culpepper, J.; Knowles, D.M.; Moore, P.S. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi’s sarcoma. Science 1994, 266, 1865–1869. [Google Scholar] [CrossRef]
- Dane, D.S.; Cameron, C.H.; Briggs, M. Virus-like particles in serum of patients with Australia-antigen-associated hepatitis. Lancet 1970, 1, 695–698. [Google Scholar] [CrossRef]
- Soyama, T.; Sakuragi, A.; Oishi, D.; Kimura, Y.; Aoki, H.; Nomoto, A.; Yano, S.; Nishie, H.; Kataoka, H.; Aoyama, M. Photodynamic therapy exploiting the anti-tumor activity of mannose-conjugated chlorin e6 reduced M2-like tumor-associated macrophages. Transl. Oncol. 2021, 14, 101005. [Google Scholar] [CrossRef]
- Kim, H.J.; Yoo, H.S.; Kim, J.C.; Park, C.S.; Choi, M.S.; Kim, M.; Choi, H.; Min, J.S.; Kim, Y.S.; Yoon, S.W.; et al. Antiviral effect of Curcuma longa Linn extract against hepatitis B virus replication. J. Ethnopharmacol. 2009, 124, 189–196. [Google Scholar] [CrossRef]
- Steinmann, E.; Gravemann, U.; Friesland, M.; Doerrbecker, J.; Müller, T.H.; Pietschmann, T.; Seltsam, A. Two pathogen reduction technologies – methylene blue plus light and shortwave ultraviolet light—Effectively inactivate hepatitis C virus in blood products. Transfusion 2013, 53, 1010–1018. [Google Scholar] [CrossRef]
- Blumberg, B.S.; Alter, H.J.; Visnich, S.A. “new” antigen in leukemia sera. JAMA 1965, 191, 541–546. [Google Scholar] [CrossRef]
- Kim, K.; Kim, K.H.; Kim, H.Y.; Cho, H.K.; Sakamoto, N.; Cheong, J.H. Curcumin inhibits hepatits C virus replication via suppressing the Akt-SREBP-1 pathway. FEBS Lett. 2010, 584, 707–712. [Google Scholar] [CrossRef]
- Zhang, B.; Zheng, L.; Huang, Y.; Mo, Q.; Wang, X.; Qian, K. Detection of Nucleic Acid Lesions During Photochemical Inactivation of RNA Viruses by Treatment with Methylene Blue and Light Using Real-time PCR. Photochem. Photobiol. 2011, 87, 365–369. [Google Scholar] [CrossRef] [PubMed]
- Hoofnagle, J.H. Course and outcome of hepatitis C. Hepatology 2002, 36, S21–S29. [Google Scholar] [PubMed]
- Yoshida, M.; Miyoshi, I.; Hinuma, Y. Isolation and characterization of retrovirus from cell lines of human adult T-cell leukemia and its implication in the disease. Proc. Natl. Acad. Sci. USA 1982, 79, 2031–2035. [Google Scholar] [CrossRef]
- Seiki, M.; Hattori, S.; Hirayama, Y.; Yoshida, M. Human adult T-cell leukemia virus: Complete nucleotide sequence of the provirus genome integrated in leukemia cell DNA. Proc. Natl. Acad. Sci. USA 1983, 80, 3618–3622. [Google Scholar] [CrossRef] [PubMed]
- Poiesz, B.J.; Ruscetti, F.W.; Gazdar, A.F.; Bunn, P.A.; Minna, J.D.; Gallo, R.C. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc. Natl. Acad. Sci. USA 1980, 77, 7415–7419. [Google Scholar] [CrossRef] [PubMed]
- Oka, T.; Matsuoka, K.I.; Utsunomiya, A. Sensitive Photodynamic Detection of Adult T-cell Leukemia/Lymphoma and Specific Leukemic Cell Death Induced by Photodynamic Therapy: Current Status in Hematopoietic Malignancies. Cancers 2020, 12, 335. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, X.; Cheng, W.; Wang, Y.; Yi, K.; Wang, Z.; Zhang, Y.; Shao, L.; Zhao, T. Hypericin-photodynamic therapy inhibits the growth of adult T-cell leukemia cells through induction of apoptosis and suppression of viral transcription. Retrovirology 2019, 16, 5. [Google Scholar] [CrossRef]
- Akula, S.M.; Pramod, N.P.; Wang, F.Z.; Chandran, B. Integrin alpha3beta1 (CD 49c/29) is a cellular receptor for Kaposi’s sarcoma-associated herpesvirus (KSHV/HHV-8) entry into the target cells. Cell 2002, 108, 407–419. [Google Scholar] [CrossRef]
- Feng, H.; Shuda, M.; Chang, Y.; Moore, P.S. Clonal integration of a polyomavirus in human Merkel cell carcinoma. Science 2008, 319, 1096–1100. [Google Scholar] [CrossRef]
- Ikeda, I.; Yamashita, Y.; Ono, T.; Ogawa, H. Selective phototoxic destruction of rat Merkel cells abolishes responses of slowly adapting type I mechanoreceptor units. J Physiol. 1994, 479, 247–256. [Google Scholar] [CrossRef]
- Sixbey, J.W.; Nedrud, J.G.; Raab-Traub, N.; Hanes, R.A.; Pagano, J.S. Epstein-Barr virus replication in oropharyngeal epithelial cells. N. Engl. J. Med. 1984, 310, 1225–1230. [Google Scholar] [CrossRef] [PubMed]
- Robinson, W.S.; Clayton, D.A.; Greenman, R.L. DNA of a human hepatitis B virus candidate. J. Virol. 1974, 14, 384–391. [Google Scholar] [CrossRef] [PubMed]
- Kelly, G.L.; Rickinson, A.B. Burkitt lymphoma: Revisiting the pathogenesis of a virus-associated malignancy. Am. Soc. Hematol. Educ. Program 2007, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Porta, C.; Paglino, C.; Mosca, A. Targeting PI3K/Akt/mTOR signaling in cancer. Front. Oncol. 2014, 4, 64. [Google Scholar] [CrossRef]
- Dowling, R.J.; Topisirovic, I.; Alain, T.; Bidinosti, M.; Fonseca, B.D.; Petroulakis, E.; Wang, X.; Larsson, O.; Selvaraj, A.; Liu, Y.; et al. mTORC1-mediated cell proliferation, but not cell growth, controlled by the 4E-BPs. Science 2010, 328, 1172–1176. [Google Scholar] [CrossRef]
- Bossler, F.; Hoppe-Seyler, K.; Hoppe-Seyler, F. PI3K/AKT/mTOR Signaling Regulates the Virus/Host Cell Crosstalk in HPV-Positive Cervical Cancer Cells. Int. J. Mol. Sci. 2019, 20, 2188. [Google Scholar] [CrossRef]
- Serrano, M.; Lin, A.W.; McCurrach, M.E.; Beach, D.; Lowe, S.W. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell 1997, 88, 593–602. [Google Scholar] [CrossRef]
- Kim, E.K.; Choi, E.J. Compromised MAPK signaling in human diseases: An update. Arch. Toxicol. 2015, 89, 867–882. [Google Scholar] [CrossRef]
- Bourotto, M.; Chiou, V.L.; Lee, J.M.; Elise, C.; Kohn, M. The MAPK pathway across different malignancies: A new perspective. Cancer 2014, 120, 3446–3456. [Google Scholar] [CrossRef]
- Lobry, C.; Oh, P.; Aifantis, I. Oncogenic and tumor suppressor functions of Notch in cancer: It’s NOTCH what you think. J. Exp. Med. 2011, 208, 1931–1935. [Google Scholar] [CrossRef]
- Nusse, R.; Clevers, H. Wnt/β-catenin signaling, disease, and emerging therapeutic modalities. Cell 2017, 169, 985–999. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Li, X.; Liu, L.; Wang, M. β-Catenin: Oncogenic role and therapeutic target in cervical cancer. Biol. Res. 2020, 53, 33. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Lenardo, M.J.; Baltimore, D. 30 Years of NF-κB: A blossoming of relevance to human pathobiology. Cell 2017, 168, 37–57. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.C.; Cesarman, E. NF-κB as a target for oncogenic viruses. Curr. Top. Microbiol. Immunol. 2011, 349, 197–244. [Google Scholar]
| PSs | Tropisms | Cancer | Primary Infection | Viral Integration | Virus | Family | Virus Type | Transmission | Refs. |
|---|---|---|---|---|---|---|---|---|---|
| 5-ALA mTHPc Curcumin | Keratinocytes, stratified epithelial cells | Cervical carcinoma, pharyngeal carcinoma, anal carcinoma, penile carcinoma | Warts, condyloma, acumatum, oral and laryngeal papillomatosis | Yes | HPV-16 HPV-18 | Papillomavirus Viridae | Enveloped DNA | Sexual contact, Mucosal contact | [80,81,82,83,84,85,86] |
| PBNP Zn-Bc_Am | B-lymphocytes, epithelial cells | Burkitt’s lymphoma, nasopharyngeal carcinoma | Asymptomatic, mononucleosis | Yes | ERV/ HH-4 | Herpesvirus Viridae | Enveloped DNA | Saliva | [84,85,86,87] |
| Photosens Chlorin e6 | Macrophages, keratinocytes, endothelial cells, B cells, etc. | Kaposi’s sarcoma, effusion lymphoma, multicentric Castleman’s disease | Asymptomatic | Yes | KSHV/ HHV-8 | Herpesvirus Viridae | Enveloped DNA | Sexual contact | [84,85,86,88,89,90] |
| Curcumin, Methylene Blue | Hepatocytes | Hepatocellular carcinoma | Acute hepatitis and chronic (10%) | Yes | HBV | Hepadnavirus Viridae | Enveloped DNA | Sexual contact, parental | [84,85,86,89,91,92,93] |
| Curcumin, Methylene Blue | Hepatocytes, B-lymphocytes, dendritic cells | Hepatocellular carcinoma | Acute hepatitis and chronic (85%) | No | HCV | Flavivirus Viridae | Enveloped RNA | Sexual contact, parental | [84,85,86,94,95,96] |
| 5-ALA Hypericin | T-lymphocytes | Adult T-cell leukemia | Asymptomatic | Yes | HTLV-1 | Retrovirus Viridae | Enveloped RNA | Fluids with cells | [84,85,86,97,98,99,100,101] |
| Quinacrine | Merkel’s cells | Merkel cell carcinoma | Asymptomatic | Yes | MCV | Polymavirus Viridae | Naked DNA | Not clear (respiratory droplets) | [84,85,86,102,103,104] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mfouo-Tynga, I.S.; Mouinga-Ondeme, A.G. Photodynamic Therapy: A Prospective Therapeutic Approach for Viral Infections and Induced Neoplasia. Pharmaceuticals 2022, 15, 1273. https://doi.org/10.3390/ph15101273
Mfouo-Tynga IS, Mouinga-Ondeme AG. Photodynamic Therapy: A Prospective Therapeutic Approach for Viral Infections and Induced Neoplasia. Pharmaceuticals. 2022; 15(10):1273. https://doi.org/10.3390/ph15101273
Chicago/Turabian StyleMfouo-Tynga, Ivan S., and Augustin G. Mouinga-Ondeme. 2022. "Photodynamic Therapy: A Prospective Therapeutic Approach for Viral Infections and Induced Neoplasia" Pharmaceuticals 15, no. 10: 1273. https://doi.org/10.3390/ph15101273
APA StyleMfouo-Tynga, I. S., & Mouinga-Ondeme, A. G. (2022). Photodynamic Therapy: A Prospective Therapeutic Approach for Viral Infections and Induced Neoplasia. Pharmaceuticals, 15(10), 1273. https://doi.org/10.3390/ph15101273







