Abstract
Atopic dermatitis (AD) is a chronic inflammatory skin disease characterized by pruritus, dry skin and redness on the face and inside elbows or knees. Most patients with AD are children and youths, but it can also develop in adults. In the therapeutic aspect, treatment with corticosteroids for AD has several side effects, such as weight loss, atrophy and acne. In the current study, we examined the anti-inflammatory effect of Moringa concanensis leaves on HaCaT keratinocytes and 2,4-dinitrochlorobenzene (DNCB)-induced atopic dermatitis-like symptoms in BALB/c mice. We observed that M. concanensis treatment exhibited significant inhibition in the production of inflammatory mediators and proinflammatory cytokines, such as IL-1β, in LPS-induced HaCaT keratinocytes by downregulating the NLRP3 inflammasome activation. Moreover, M. concanensis inhibited the activation of JNK, AP-1 and p65, which resulted in the deformation of NLRP3 in LPS-stimulated HaCaT cells. In mice with DNCB-induced AD-like skin lesions, the administration of M. concanensis ameliorated the clinical symptoms, such as the dermatitis score, thickness of lesional ear skin and TEWL. Furthermore, M. concanensis could attenuate the activation of the immune system, such as reducing the spleen index, concentration of the IgE levels and expression of the NLRP3 inflammasome in ear tissues. Therefore, our results suggest that M. concanensis exerts anti-atopic dermatitis effects by inhibiting the NLRP3 inflammasome-mediated IL-1β.
1. Introduction
From various pathophysiological perspectives, atopic dermatitis (AD) is a chronic relapsing inflammatory skin disease associated with pruritus and redness, typically on the face and the inside of elbows and knees [1]. Therefore, AD is closely connected to patients’ quality of life and comorbidities [2]. AD can occur at any age and its prevalence is increasing. According to one report, the number of AD patients diagnosed in one year ranged from 13.5% to 41.9%, depending on the country [3]. The main symptoms of AD are characterized by a multidimensional patient burden, including persistent itching, dryness of the skin and depression [4,5]. Although the pathogenesis of AD is still unclear, some studies have suggested that most patients with AD are affected due to sensitization to environmental allergens, genetic backgrounds and increased serum immunoglobulin E (IgE) [6,7]. Based on clinical experiments, topical corticosteroids are commonly used in patients with AD [8]. However, the current treatments for AD, especially topical steroids, have various adverse effects, such as atrophy, acne and red burning skin [9]. Therefore, it is necessary to develop both effective and safer therapies for atopic dermatitis without side effects.
The skin barrier is an important defense system against potential allergens, microorganisms and pollutants [10]. When the barrier of the epidermis is damaged, there is increased epicutaneous absorption of environmental allergens and the activation of the immune response, which could stimulate the expression of inflammatory cytokines such as IL-4, IL-33 and IL-1β [11]. Several studies have reported that the nucleotide-binding oligomerization domain, leucine-rich repeat and pyrin domain-containing 3 (NLRP3) inflammasome, which includes NLRP3, ASC and caspase-1, can regulate the activation of IL-1β [12,13]. NLRP3 inflammasome-dependent IL-1β activation by cleaved caspase-1 is the main inflammatory cytokine in the progression of inflammation [14]. It was thought that the inflammasome could only be expressed in immune cells, but recent studies suggest that human keratinocytes result in the expression of IL-1β by activating the NLRP3 inflammasome [15].
Moringa concanensis Nimmo (Morigaceae) is an medicinally important plant, which is called Kattumurungai or Peyimurungai in Tamil [16]. M. concanensis inhabits India and Asian and Arab countries. It has been reported that M. concanensis leaves can be used to treat different medical conditions, including dysmenorrhea, hypertension, constipation and skin tumors [17]. Moreover, it has been reported that M. concanensis contains flavonoid compounds, such as quercetin [18]. Although the beneficial properties of Moringa oleifera in atopic dermatitis have been widely reported [19,20], the effects of M. concanensis in atopic dermatitis are still unknown. In this study, we investigated the anti-inflammatory and the therapeutic effects of M. concanensis leaves in LPS-stimulated HaCaT keratinocytes and a 2,4-dinitrochlorobenzene (DNCB)-induced atopic dermatitis murine model.
2. Results
2.1. Analysis of Quadrupole Time-of-Flight (Q-TOF) Mass Spectrometry of M. concanensis
To identify the major phytochemicals in the ethanol extracts of M. concanensis leaves, we conducted a QTOF-MS/MS analysis. The results of the QTOF-MS/MS analysis revealed the presence of 313 phytochemicals in the leaves of M. concanensis (Figure 1 and Table 1). The major phytochemicals included quinic acid (C7H12O6), coumaroylquinic acid (C16H18O8), coumaric acid (C9H8O3) and quercetin (C15H10O7). It has been indicated that quinic acid derivatives, coumaric acid and quercetin suppress immune responses [21,22,23]. In particular, some studies revealed that quercetin downregulated the production of IL-1β by inhibiting the NLRP3 inflammasome [24,25].
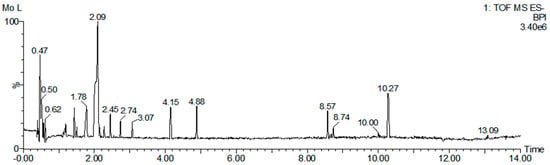
Figure 1.
UPLC−QTOF−MS/MS analysis of M. concanensis L. Base peak intensity (BPI) chromatogram.

Table 1.
Phytochemical profiling of M. concanensis Leaves.
2.2. M. concanensis Inhibits the LPS-Stimulated Inflammatory Mediators in HaCaT Cells
Recently, new insight into the pathogenesis of AD focused on abnormalities in the epidermal layer [26]. Furthermore, several studies have suggested that the downregulation of immune activation in epidermal keratinocytes plays a key role in ameliorating inflammatory skin diseases, such as AD [27,28]. Therefore, we investigated the effect of M. concanensis on LPS-stimulated inflammatory responses in keratinocytes. Firstly, an MTT assay was performed to determine the cytotoxic concentration of M. concanensis in HaCaT keratinocytes. The treatment of M. concanensis extract did not affect the viability of HaCaT cells at the concentration of 10–300 μg/mL (Figure 2A). HaCaT cells were pretreated with M. concanensis for 1 h and then treated with LPS (1 μg/mL) for 24 h. M. concanensis extract markedly reduced the production of NO and PGE2 at 100 and 300 μg/mL concentrations (Figure 2B,E). Moreover, M. concanensis inhibited the mRNA as well as protein expressions of iNOS and COX-2, which is related to the synthesis of NO and PGE2 production (Figure 2C,D,F,G). Our results suggest that M. concanensis can regulate the production of inflammatory mediators, such as NO and PGE2, via the inhibition of iNOS and COX-2 expressions.
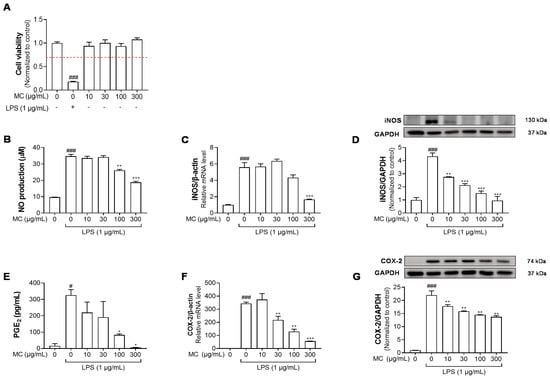
Figure 2.
Effects of M. concanensis on the inflammatory response of LPS−stimulated HaCaT cells. HaCaT cells were treated with M. concanensis (MC) for 24 h, and cell viability was determined by MTT assay (A). Cells were pretreated with MC for 1 h before LPS stimulation (1 μg/mL) for 24 h. The production of NO and PGE2 was determined by Griess reagent and ELISA kits, respectively (B,E). The level of mRNA expressions of iNOS and COX-2 was determined by RT-qPCR (C,F). The levels of iNOS and COX-2 proteins were measured by Western blotting analysis, and the quantifications were normalized to the control (D,G). The data presented are mean of three independent determinations and indicate the mean ± S.E.M. # p < 0.05, ### p < 0.001 compared to the vehicle-treated controls; * p < 0.05, ** p < 0.01 and *** p < 0.001 compared to the LPS-treated group.
2.3. M. concanensis Downregulated the Expression of Inflammatory Cytokines in LPS-Stimulated HaCaT Cells
The production of inflammatory cytokines, such as TNF-α, IL-1β and IL-6, due to the inflammatory reactions could control immune activation [29]. Therefore, we examined whether M. concanensis had an inhibitory effect on inflammatory cytokines, such as TNF-α, IL-1β and IL-6, in LPS-stimulated HaCaT cells. The cells were pretreated with M. concanensis for 1 h before LPS stimulation (1 μg/mL) for 24 h. The LPS-stimulated cells and mediums were collected to investigate the expression of inflammatory cytokines using RT-qPCR and ELISA kits. Figure 3A–C show that M. concanensis treatment significantly reduced the mRNA expression levels of TNF-α, IL-1β and IL-6 compared to those in the LPS-treated controls in HaCaT cells. Moreover, the secretion of TNF-α, IL-1β and IL-6 protein was markedly reduced by M. concanensis (Figure 3D–F).
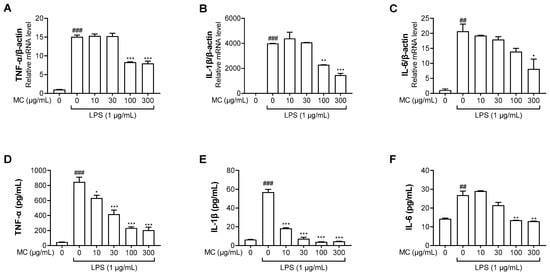
Figure 3.
Effects of M. concanensis on LPS−stimulated proinflammatory cytokine expression in HaCaT keratinocytes. Cells were pretreated with M. concanensis (MC) for 1 h before LPS stimulation (1 μg/mL) for 24 h. The level of mRNA expressions of TNF-α, IL-1β and IL-6 was determined by RT-qPCR (A–C). The level of TNF-α, IL-1β and IL-6 proteins was measured by ELISA kits (D–F). The data presented are the mean of three independent determinations and indicate the mean ± S.E.M. ## p < 0.01, ### p < 0.001 compared to the vehicle-treated controls; * p < 0.05, ** p < 0.01 and *** p < 0.001 compared to the LPS-treated group.
2.4. M. concanensis Reduced the Expression of IL-1β by Inhibiting the NLRP3 Inflammasome in HaCaT Cells
The NLRP3 inflammasome is a multiprotein complex that consists of NLRP3, ASC and caspase-1. The NLRP3 inflammasome initiates immune responses during exposure to a variety of stimuli, mainly pathogen and danger-related molecular patterns [30]. The activation of the inflammasome results in the secretion of cytokine IL-1β, which is correlated with chronic inflammatory diseases [31]. As shown in Figure 3B,E, we found that M. concanensis effectively reduced the expression of IL-1β in HaCaT cells stimulated with LPS. Therefore, we next examined the effects of M. concanensis on the NLRP3 inflammasome activation in LPS/ATP-stimulated HaCaT cells. The experimental data demonstrated that M. concanensis dose-dependently downregulated the expression of NLRP3 (Figure 4B). Furthermore, M. concanensis significantly attenuated the activation of ASC and cleaved caspase-1 at 300 μg/mL (Figure 4C,D). These data indicate that M. concanensis reduced the secretion of IL-1β by regulating the formation of the NLRP3 inflammasome.
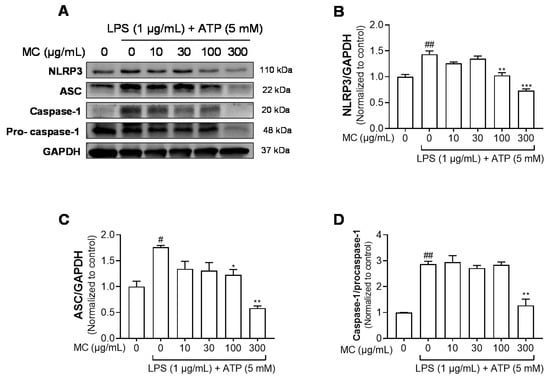
Figure 4.
Effects of M. concanensis on the NLRP3 inflammasome activation in HaCaT keratinocytes. Cells were pretreated with M. concanensis (MC) for 1 h before LPS stimulation (1 μg/mL) for 24 h and ATP (5 mM) for 1 h. The protein expressions of NLRP3, ASC and Caspase-1 in HaCaT cells were determined by immunoblot analysis (A–D). The data presented are the mean of three independent determinations and indicate the mean ± S.E.M. # p < 0.05 and ## p < 0.01 compared to the vehicle-treated controls; * p < 0.05, ** p < 0.01 and *** p < 0.001 compared to the LPS-treated group.
2.5. M. concanensis Inhibited the Phosphorylation of NF-κB, MAPK and AP-1 in HaCaT Cells Stimulated with LPS
ROS and NF-κB contribute to the mechanism underlying NLRP3 inflammasome activation [32]. Mounting evidence indicates that activated transcription factors, such as NF-κB, induce the priming of the NLRP3 inflammasome [33,34]. Hence, we studied whether M. concanensis suppressed the phosphorylation of NF-κB and its upstream MAPK in LPS-stimulated HaCaT cells. As shown in Figure 5A,B, LPS treatment significantly upregulated the phosphorylation of NF-κB. However, M. concanensis treatment significantly downregulated the phosphorylation of p65. Furthermore, LPS treatment increased the phosphorylation of JNK, p38 and ERK which are subunits of MAPK, but M. concanensis only exhibited a significant reduction in the phosphorylation of JNK (Figure 5A,C). In addition, AP-1, which is regulated by the activated MAPK family, such as JNK, can mediate the transcription of inflammatory mediators [35]. Our results show that M. concanensis also inhibited the phosphorylation of the AP-1 subunit c-fos (Figure 5A,D). Therefore, these results demonstrate that M. concanensis had anti-inflammatory properties and inhibited the priming of the NLRP3 inflammasome via the inhibition of phosphorylated p65, JNK and c-fos signaling.
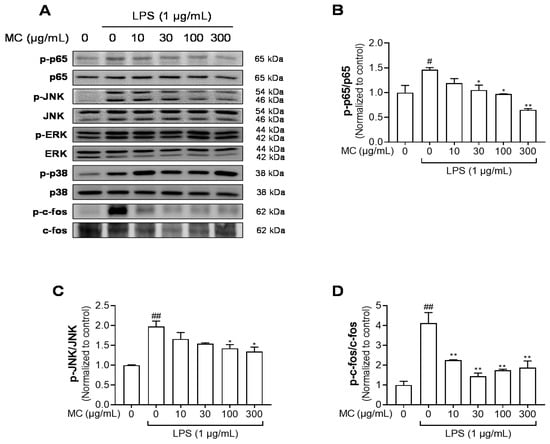
Figure 5.
Effects of M. concanensis on MAPK/AP-1/NF-κB signaling in HaCaT keratinocytes. Cells were pretreated with M. concanensis (MC) for 1 h prior to LPS stimulation (1 μg/mL) for 1 h. The expression of phospho-p65, JNK, p38, ERK, c-fos, p65, JNK, p38, ERK and c-fos was measured by a Western blot analysis (A). The phosphorylation level was normalized to the control (B–D). The data presented are the mean of three independent determinations and indicate the mean ± S.E.M. # p < 0.05 and ## p < 0.01 compared to the vehicle-treated controls; * p < 0.05 and ** p < 0.01 compared to the LPS-treated group.
2.6. M. concanensis Improved the Clinical Symptoms in Mice with AD-like Skin Lesions Induced by DNCB
Given the anti-inflammatory properties of M. concanensis in keratinocytes, we further investigated whether M. concanensis has anti-atopic dermatitis effects in mice with AD-like skin lesions induced by DNCB. BALB/c mice were shaved using a clipper for dorsal skin. After shaving, the mice were sensitized with 1% DNCB twice every 7 days. To evaluate the effects of M. concanensis on AD, the mice were orally administered with M. concanensis (100 and 200 mg/kg) for 14 days. In addition, 0.6% DNCB was topically applied to accelerate atopic dermatitis once every 2 days. There was no difference in the body weight of the AD mice in the M. concanensis-treated group, but a significant reduction in the body weight of dexamethasone (1 mg/kg)-administered group when compared with the DNCB-treated group (Figure 6B). A significant increase in the SCORAD index was observed in the DNCB-treated group compared with the normal group. However, we observed that the dermatitis scores were dose-dependently reduced by the administration of M. concanensis when compared with the DNCB-treated group (Figure 6A,C). Moreover, the mice treated with M. concanensis had reduced ear thickness and TEWL compared to the DNCB-treated group (Figure 6D,E). These results demonstrate that the M. concanensis oral administration can attenuate the clinical symptoms of AD without side effects, such as body weight loss, in a DNCB-induced AD mice model.
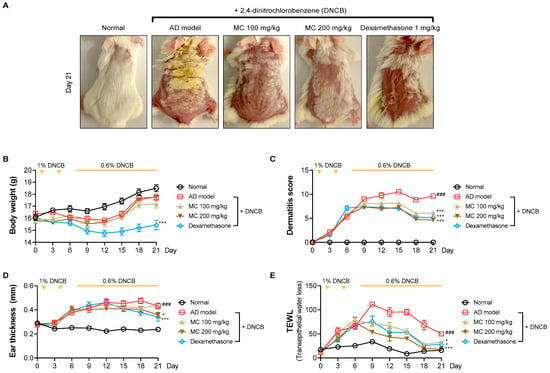
Figure 6.
Effects of M. concanensis on atopic dermatitis in BALB/c mice induced by DNCB. The dorsal skin images were obtained at identical magnification, and representative images obtained on day 21 are shown (A). The clinical symptoms, including body weight (B), dermatitis score (C), ear thickness (D) and TEWL (E), were measured every 3 days (n = 8). The data presented are the mean ± S.E.M. ### p < 0.001 versus Normal. * p < 0.05 and *** p < 0.001 compared to the DNCB-treated group. AD model; only DNCB-treated group, MC 100 mg/kg + DNCB; DNCB-induced mice administered with 100 mg/kg of M. concanensis, MC 200 mg/kg + DNCB; DNCB-induced mice administered with 200 mg/kg of M. concanensis.
2.7. M. concanensis Ameliorated the Immunological and Histological Changes in DNCB-Challenged BALB/c Mice
To determine whether the administration of M. concanensis affects immunological activation, the weights of the cernical lymph nodes and spleen and the plasma IgE concentration were calculated after sacrifice. The indices of the lymph nodes and spleen were significantly increased in the DNCB-only group when compared with the normal group. Although the lymph node index did not significantly decrease, the spleen index was significantly reduced by the oral administration of M. concanensis when compared with the DNCB-administered group (Figure 7A,B). In addition, it is well known that the upregulated IgE levels have been detected in patients with AD [36]. As shown in Figure 7C, M. concanensis dose-dependently inhibited the level of the plasma IgE compared to that in the DNCB-only group. These data indicate that the reduced spleen index and IgE levels could be therapeutic strategies in AD therapies.
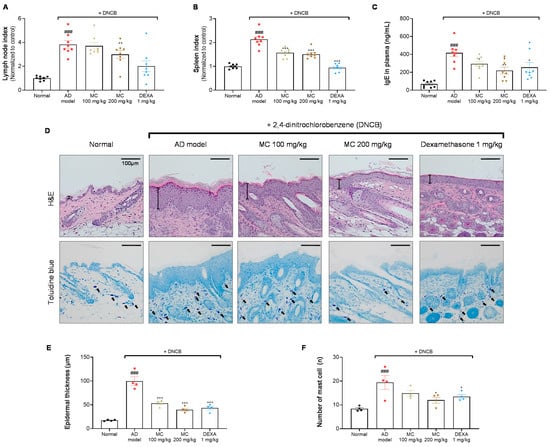
Figure 7.
Effects of M. concanensis on histological and immunological changes in DNCB-induced AD. On day 21, the mice were sacrificed to investigate the immunological and histological differences. The lymph node (A) and spleen (B) indices were normalized to those in the normal group [Index = (organ weight/body weight) × 100] (n = 8). The plasma IgE concentration (C) was measured by an ELISA kit (n = 8). Images of hematoxylin and eosin (H&E) and toluidine blue (TB) staining of lesional dorsal skin (D) were obtained at 200× magnification, and characteristic images are shown; scale bar: 100 μm. Epidermal thickness (E) and the number of mast cells (F) were counted (n = 4), and the data presented are mean ± S.E.M. ### p < 0.001 versus Normal. * p < 0.05, ** p < 0.01 and *** p < 0.001 versus the only DNCB-treated group. AD model; only DNCB-treated group, MC 100 mg/kg + DNCB; DNCB-induced mice administered with 100 mg/kg of M. concanensis, MC 200 mg/kg + DNCB; DNCB-induced mice administered with 200 mg/kg of M. concanensis, DEXA 1 mg/kg + DNCB; DNCB-induced mice administered with 1 mg/kg of dexamethasone.
To investigate the histological changes, epidermal hyperplasia and mast cell infiltration in lesional dorsal skin were investigated by H&E and toluidine blue staining, respectively (Figure 7D). A significant increase in the epidermal thickness in the DNCB-administered group was observed when compared with the normal group (Figure 7D,E). Furthermore, mast cell infiltration was considerably increased in the DNCB-challenged group (Figure 7D,F). However, the mice treated with M. concanensis showed a dose-dependent suppression of hyperplasia and mast cell infiltration in lesional dorsal skin tissues (Figure 7D,E,F). These data suggest that M. concanensis may regulate of the immune system activation and histological changes in DNCB-induced lesional dorsal skin.
2.8. M. concanensis Inhibited the Activation of the NLRP3 Inflammasome in DNCB-Treated BALB/c Mice
It has been shown that the upregulation of NLRP3 inflammasome is associated with the pathogenesis of chronic dermatitis in the skin of mice [37]. To determine whether the symptoms of AD were attenuated via the inhibition of the NLRP3 inflammasome, we investigated whether the application of M. concanensis inhibited the NLRP3 inflammasome in DNCB-induced lesional ear tissues. After the sacrifice, the lesional ear tissues were collected and analyzed for the expression of NLRP3, ASC and IL-1β. As shown in Figure 8, the NLRP3, ASC and IL-1β expressions were significantly increased in the DNCB-treated group. However, similar to Figure 4, we found that the mice treated with M. concanensis had significantly reduced NLRP3, ASC and IL-1β mRNA expression compared to mice in the DNCB-treated group (Figure 8A–C). Therefore, the results of the present study suggest that M. concanensis relieved AD-like symptoms by downregulating the expression of NLRP3, IL-1β and ASC in DNCB-induced lesional ear tissues.

Figure 8.
Effects of M. concanensis on the NLRP3 inflammasome in DNCB-induced AD lesional ear tissues. The mRNA levels of NLRP3 (A), ASC (B) and IL-1β (C) in DNCB-induced lesional ear tissue were measured by RT-qPCR (n = 4). The data presented are the mean of three independent determinations and indicate the mean ± S.E.M. ## p < 0.01 and ### p < 0.001 versus Normal. * p < 0.05, ** p < 0.01 and *** p < 0.001 versus the only DNCB-treated group. AD model; only DNCB-treated group, MC 100 mg/kg + DNCB; DNCB-induced mice administered with 100 mg/kg of M. concanensis, MC 200 mg/kg + DNCB; DNCB-induced mice administered with 200 mg/kg of M. concanensis, DEXA 1 mg/kg + DNCB; DNCB-induced mice administered with 1 mg/kg of dexamethasone.
3. Discussion
Atopic dermatitis is known as a typical chronic inflammatory disease, and its prevalence in patients with AD has consistently increased over the last decade [38]. This inflammatory skin disease is normally demonstrated during the first year of birth, however, it can occur in adults [39]. The pathogenesis of AD involves complex factors, including environmental provocation, genetic predisposition and immunological abnormalities [40]. Based on clinical research, AD cannot be completely cured [41]. Therefore, the main management of AD involves improving the clinical symptoms and achieving long-term disease control following treatment guidelines. The drugs used in the treatment of AD, such as glucocorticosteroids, antihistamines and calcineurin inhibitors, can improve itching, edema and skin inflammation [42]. However, several studies have shown that the prolonged use of these medications could cause various adverse effects, including skin atrophy, heart failure and high blood pressure [43,44]. In this study, we first found that M. concanensis alleviated AD-like lesions in BALB/c mice induced by DNCB. Furthermore, we revealed that M. concanensis blocked NLRP3 formation by inhibiting the JNK-NF-κB and AP-1 pathways. These observations indicate that M. concanensis could be a novel candidate for preventing and treating AD.
Keratinocytes play a potential role in skin immune responses that cause immune cells to produce proinflammatory cytokines [45]. The present study exhibited the anti-inflammatory properties of M. concanensis and its underlying mechanisms in LPS-stimulated HaCaT keratinocytes. After Toll-like receptor 4 (TLR4) recognizes LPS, the TLR4 signaling cascade regulates inflammatory mediators via the phosphorylation of transcription factors, mainly NF-κB [46,47]. A recent study indicated that the NF-κB activation could positively regulate the NLRP3 inflammasome, which aggravated immune-related skin diseases, such as AD [48]. In LPS-induced HaCaT keratinocytes, it was observed that M. concanensis reduced iNOS and COX-2 expressions, which synthesize NO and PGE2, inhibiting the secretion of inflammatory mediators. Furthermore, we found that M. concanensis inhibited the level of mRNA and protein expressions of TNF-α, IL-1β and IL-6 by inhibiting the phosphorylation of JNK/AP-1/NF-κB. Moreover, we confirmed that M. concanensis reduced the expression of IL-1β by inhibiting the formation of the NLRP3 inflammasome. The genus Moringa is known as a medicinal plant that has been traditionally used for diseases such as colds and diabetes [49]. Many Moringa species have been reported to inhibit the inflammatory response [50,51]. A previous study demonstrated that the hydroethanolic extract of Moringa oleifera flowers has anti-inflammatory potentials by preventing the phosphorylation of NF-κB in RAW 264.7 macrophages stimulated with LPS [52]. Moreover, the study showed that the ethanolic extract of M. concanensis relieved pain and had anti-inflammation effects by reducing the synthesis of prostaglandin [53]. Thus, these previous studies support our results that M. concanensis had inhibitory effects on LPS-induced inflammatory responses in HaCaT cells. In addition, in DNCB-challenged BALB/c mice, we found that the mice treated with M. concanensis had significantly improved skin lesions, ear thickness and TEWL. Note that, as shown in Figure 6E, the levels of TEWL were reduced from Day 15. It is thought that the TEWL levels were decreased by hair regrowth. Moreover, our results show that the mice treated with M. concanensis had a reduced spleen index, IgE levels in plasma, epidermal thickness, mast cell infiltration and NLRP3 inflammasome expression in lesional ear tissue. Therefore, our data indicate the anti-atopic properties of M. concanensis in DNCB-challenged BALB/c mice.
Accumulating evidence suggests that inflammasomes are associated with the inflammatory response in AD [54,55]. In particular, NLRP3 inflammasome-mediated IL-1β plays a critical role in the pathological process of inflammation-mediated skin diseases [56]. Thus, inhibition of NLRP3 inflammasome-dependent IL-1β could regulate the pathogenesis of AD. The present study revealed that M. concanensis inhibited the priming signal of the NLRP3 inflammasome by restraining NF-κB phosphorylation. This evidence supports the notion that M. concanensis inhibits the priming and activating signals of the NLRP3 inflammasome. Moreover, several studies reported that extracts of M. concanensis had antioxidative properties [57,58]. In this study, various chemical compounds, including quercetin and quinic acid, were detected using a UPLC-QTOF analysis of M. concanensis (Table 1). Some studies reported that quercetin and quinic acid derivatives had anti-inflammatory properties in an animal model of colitis and microglia, respectively [59,60,61]. Notably, previous studies reported that quercetin elicited an inhibitory effect of NLRP3 inflammasome activation in macrophages and endothelial cells [62,63]. These previous reports implicate that quercetin and quinic acid in M. concanensis may contribute to the anti-inflammatory effects of M. concanensis. Therefore, we considered that quercetin in M. concanensis may alleviate the AD symptoms by reducing the activation of NLRP3 inflammasome-mediated IL-1β. Although inhibitors of the NLRP3 inflammasome have anti-inflammatory properties in mice, evidence of similar effects in human skin diseases is still lacking. Therefore, clinical studies targeting the NLRP3 inflammasome for inflammatory skin diseases are needed.
4. Materials and Methods
4.1. Animals
BALB/c mice (female, 6 weeks old) were procured from Orient Bio (Seongnam, Korea). All animal experiments were performed with the approval of the Institutional Animal Care and Use Committee of the Laboratory Animal Research Center, Kangwon National University, Korea (KW-211208-5). Each cage contained four mice, which were housed under controlled conditions of 21–25 °C and a 12 h light and dark cycle. The animals were given free access to food and water throughout the experimental period.
4.2. Preparation of an Ethanolic Extract of M. concanensis
The leaves of M. concanensis were collected Coimbatore District, Tamil Nadu, India and the plant sample was authenticated (Letter No. BSI/SRC/5/23/2018/Tech-437) Botanical Survey of India, Southern Regional Centre, Coimbatore, Tamil Nadu, India. After washing with distilled water, the leaves were dried under light-shielding conditions. Then, 100 g of dried M. concanensis leaves (MC) were mixed with 1 L of 70% ethanol for extraction, twice for 2 h by using an ultrasonic bath. After the extraction, the filtrate was evaporated using a rotary vacuum evaporator. Then, the semisolid residue was lyophilized to produce the extract with 20%.
4.3. Identification of Phytochemicals in M. concanensis by UPLC-QTOF-MS/MS
The phytochemicals in M. concanensis L. were estimated by using ultra-performance liquid chromatography supplied with quadrupole time-of-flight mass spectrometry (UPLC/QTOF-MS/MS) (WATERS XEVO GS-XS QTOF analyzer). Ten milligrams of M. concanensis were dissolved in 10 mL of 70% ethanol, and then, 2 μL of M. concanensis were injected into a Waters ACQUITY UPLC BEH C18 column (50 × 2.1 mm, 1.7 μm). The flow rate of the column was altered at 0.3 mL/min. The mobile phase contained 0.1% formic acid in water (solvent A), and 0.1% formic acid in acetonitrile (solvent B). The column conditions and the characterization of chemical components were followed according to the method described by Oh et al. [64]. The chemical components in the leaves of M. concanensis were identified from the library of traditional Chinese medicine (TCM) using UNIFI 1.8 (Waters, Milford, MA, USA) software and an in-house library.
4.4. Materials
Dulbecco’s modified Eagle’s medium (DMEM), Dulbecco’s phosphate-buffered saline (DPBS), DEPC water and penicillin-streptomycin (P/S) were procured from Welgene (Gyeongsan, Korea). Fetal bovine serum (FBS) was provided by Atlas Biologicals (Fort Collins, CO, USA). Griess reagent, lipopolysaccharides from Escherichia coli O26:B6 (LPS), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT), dimethyl sulfoxide (DMSO), sodium nitrite, skim milk powder, 1-chloro-2,4-dinitrobenzene (DNCB) and dexamethasone were obtained from Sigma Chemical Co. (St. Louis, MO, USA). RNAiso Plus was purchased from Takara Bio Inc. (Kusatsu, Japan). Chloroform, 2-propyl alcohol, olive oil and acetone were purchased from Daejung (Seongnam, Korea). iNOS, COX-2, IL-6, IL-1β and β-actin oligonucleotide coupled primers were synthesized by Integrated DNA Technologies (Coralville, IA, USA). An enzyme-linked immunosorbent assay (ELISA) kit for prostaglandin E2 (PGE2) was obtained from R&D Systems (Minneapolis, MN, USA), and an ELISA kit for interleukin-6 (IL-6) was obtained from Abcam (Cambridge, UK). An ELISA kit for interleukin-1β (IL-1β) was obtained from Invitrogen (Carlsbad, CA, USA). TransScript® All-in-One First-Strand cDNA Synthesis SuperMix for qPCR (One-Step gDNA Removal) was purchased from TransGen Biotech Co. (Beijing, China). PowerSYBR® Green PCR Master Mix from Applied Biosystems was purchased from Thermo Fisher Scientific (Rockford, IL, USA). P38, c-Jun N-terminal kinase (JNK), extracellular signal-regulated kinase (ERK), P65, phosphorylated P38 (p-P38), phosphorylated JNK (p-JNK), phosphorylated ERK (p-ERK) and phosphorylated P65 (p-P65) antibodies were procured from Cell Signaling Technology (Danvers, MA, USA). All other reagents and materials were of the highest quality available.
4.5. Cell Culture
The human epidermal keratinocyte cells (HaCaT) were provided by Professor Ok-Hwan Lee from the Food Chemistry Laboratory at Kangwon National University. HaCaT cells (2 × 105) were cultured in DMEM supplemented with 10% FBS in a 5% CO2 incubator at 37 °C. LPS was used to stimulate the HaCaT cells at the concentration of 1 μg/mL for 1 h or 24 h.
4.6. Cell Viability
The viability of HaCaT cells was determined using an MTT assay. For this purpose, the cells were pretreated with M. concanensis for 24 h and incubated with MTT solution at 5 mg/mL for 4 h to form formazan crystals. After the incubation, the supernatant in each well was replaced with 100 μL of DMSO and isopropyl alcohol (1:1). The absorbance was measured at 540 nm on SpectraMax microplate reader (Molecular Devices, Sunnyvale, CA, USA).
4.7. Nitric Oxide Production
HaCaT cells were pretreated with different concentrations of M. concanensis (10, 30, 100 and 300 μg/mL) for 1 h and then treated with LPS (1 μg/mL) for 24 h. The production of nitric oxide (NO) was assessed by measuring the nitrite accumulation in the culture medium. The level of nitrite in the medium was measured by Griess reagent. Briefly, 100 μL of supernatant and Griess reagent were mixed and incubated for 10 min. The absorbance was measured at 540 nm on SpectraMax microplate reader (Molecular Devices, Sunnyvale, CA, USA). The level of nitrite in the culture supernatant of LPS-induced HaCaT cells was calculated using a sodium nitrite standard curve.
4.8. RNA Extraction and Real Time Quantitative Polymerase Chain Reaction (RT-qPCR)
The mRNA expression of iNOS, COX-2, TNF-α, IL-1β, IL-6, NLRP3 and ASC was measured using RT-qPCR. The total RNA was extracted using RNAiso PLUS (Takara, Otsu, Japan). Complementary DNA (cDNA) was synthesized from 1 μg of total RNA using All-in-One FirstStrand cDNA Synthesis SuperMix previously described by Ko et al. [65]. The synthesized cDNAs were used as a template for RT-qPCR using a QuantStudio 3 (Applied Biosystems, Foster City, CA, USA) system with POWER SYBR Green PCR master mix and gene-specific primers (Table 2). A dissociation curve analysis of iNOS, COX-2, IL-1β, IL-6, NLRP3, ASC and β-actin demonstrated a single peak. The expression levels of the target genes were quantified by duplicating measurements and normalized with the 2−ΔΔCT method relative to the control β-actin. The PCR analyses were performed under the following conditions: 40 cycles of 95 °C for 15 s; 57 °C for 20 s, and 72 °C for 40 s.

Table 2.
The list of primer sequences used in the RT-qPCR analyses.
4.9. PGE2, TNF-α, IL-1β, IL-6 and IgE Assays
The expression of PGE2, TNF-α, IL-1β and IL-6 in the culture supernatant was measured using ELISA kits (R&D Systems, Minneapolis, MN, USA). The cells were pretreated with M. concanensis at various concentrations (10, 30, 100 and 300 μg/mL) for 1 h and stimulated with LPS (1 μg/mL) for 24 h. The expression of IgE in plasma was also measured using an ELISA kit (R&D Systems, Minneapolis, MN, USA) according to the manufacturer’s protocol.
4.10. Western Blot Analysis
The cell cultures were washed with Dulbecco’s phosphate-buffered saline (DPBS) on ice and proteins were isolated from the cells using lysis buffer (Jubiotech, Daejeon, Korea) with a protease phosphatase inhibitor cocktail (Thermo Fisher Scientific, Rockford, IL, USA). The total cellular proteins were quantified using a Bradford assay, and protein (20 μg/well protein) was loaded onto 10% SDS-PAGE gels and then transferred to PVDF membranes [66]. The membranes were blocked with 5% skimmed milk for 2 h and then incubated with primary antibodies against iNOS (Cell Signaling Technology, 1:1000), COX-2 (Cell Signaling Technology, 1:1000), NLRP3 (Cell Signaling Technology, 1:500), ASC (Cell Signaling Technology, 1:500), Caspase-1 (Cell Signaling Technology, 1:500), p-p65 (Cell Signaling Technology, 1:1000), p-JNK (Cell Signaling Technology, 1:1000), p-ERK (Cell Signaling Technology, 1:1000), p-p38 (Cell Signaling Technology, 1:1000), p-c-fos (Cell Signaling Technology, 1:1000), p65 (Cell Signaling Technology, 1:1000), JNK (Cell Signaling Technology, 1:1000), ERK (Cell Signaling Technology, 1:1000), p38 (Cell Signaling Technology, 1:1000), c-fos (Cell Signaling Technology, 1:500), or GAPDH (Cell Signaling Technology, 1:500) at 4 °C overnight. After washing, the membranes were incubated for 2 h with a secondary antibody. The protein bands were detected using enhanced chemiluminescence (ECL) (General Electric, Boston, MA, USA). Subsequently, the proteins were visualized using a LAS-500 mini-imager and quantified with ImageJ software (version 1.51j8).
4.11. 2,4-Dinitrochlorobenzene (DNCB)-Induced Atopic Dermatitis Mice
BALB/c mice were topically sensitized with 200 μL of 1% DNCB diluted in a mixture of acetone and olive oil (3:1), on shaved dorsal skin and ears twice a week. The mice were divided into 5 groups (n = 8/group) as follows: an untreated group (Normal), an only DNCB-sensitized group (DNCB), a group receiving oral administration of 100 mg/kg M. concanensis (MC 100), a group receiving oral administration of 200 mg/kg M. concanensis (MC 200) and a group receiving the administration of 1 mg/kg dexamethasone (DEXA). Seven days later, the mice were stimulated with 0.6% DNCB on the dorsal skin (200 μL) and the right ear (20 μL for every 2 days). Mice with DNCB-induced AD-like skin lesions were orally treated with M. concanensis (100 and 200 mg/kg) and dexamethasone (1 mg/kg) every day (Figure 9).
Figure 9.
Diagram of the experimental procedures.
4.12. Measurement of Clinical Symptoms and Histological Changes
The severity of the skin lesions in DNCB-induced AD was estimated according to the SCORAD index, which is scored from 0 (none) to 3 (severe) based on erythema, pruritus/dry skin, edema and excoriation [67]. The thickness of the ear was measured using a Digimatic micrometer (Mitutoyo, Kawasaki, Japan). GPSKIN Barrier Pro (GPpower, Hanam, Korea) was used to measure transepithelial water loss (TEWL) in the dorsal skin using the GPSKIN Research program [68]. Changes in the clinical symptoms, such as body weight, ear thickness and TEWL, in the AD mice were measured every three days. To evaluate the histological examination, the dorsal skin tissues were punched using a 5 mm biopsy punch, fixed in 10% formalin solution and embedded in paraffin [69]. Each section slice of paraffin-embedded skin tissue was stained with hematoxylin and eosin (H&E) and toluidine blue (TB). The histological changes were examined by light microscopy (Olympus, Tokyo, Japan). The epidermal thickness was observed using H&E staining at 100× magnification. The infiltration of mast cells was analyzed with TB staining and the slices were examined in four randomly selected sections.
4.13. Statistical Analysis
The statistical analyses were performed using GraphPad Prism Version 8.0 (GraphPad, La Jolla, CA, USA). All data are expressed as the mean ± S.E.M. The data were analyzed by a one-way analysis of variance (ANOVA), followed by a Student–Newman–Keuls test for multiple comparisons. p < 0.05 was considered a significant statistical value.
5. Conclusions
In conclusion, our results show that M. concanensis inhibited the formation of the NLRP3 inflammasome through JNK/AP-1/NF-κB signaling in HaCaT keratinocytes. Furthermore, we suggest that M. concanensis attenuated DNCB-induced AD-like symptoms in BALB/c mice by inhibiting IL-1β mediated by the NLRP3 inflammasome. Therefore, M. concanensis has therapeutic properties in chronic inflammatory skin diseases, mainly AD.
Author Contributions
Writing—original draft preparation, K.-M.K.; writing—review and editing, S.J.P. and K.S.; conceptualization, K.-M.K. and S.J.P.; formal analysis, S.-Y.K., T.J.M., H.J.B., S.-H.C., Y.-Y.C., J.-Y.A., H.-J.K. and Y.E.C.; investigation, K.-M.K.; data curation, S.-Y.K., T.J.M., H.J.B., S.-H.C., Y.-Y.C., J.-Y.A., H.-J.K. and Y.E.C.; methodology, K.S.; supervision, S.J.P.; project administration, S.J.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Korean Ministry of Environment (2018002270002), the National Research Foundation of Korea (NRF) grant funded by the Ministry of Science, ICT and Future Planning (NRF-2020R1C1C1004911) and the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2021R1A6A1A03044242).
Institutional Review Board Statement
This study was approved by the Institutional Animal Care and Use Committee (IACUC) of the Laboratory Animal Research Center at Kangwon National University (KW-211208-5).
Informed Consent Statement
Not applicable.
Data Availability Statement
All data are included in the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Foley, C.; Tundia, N.; Simpson, E.; Teixeira, H.D.; Litcher-Kelly, L.; Bodhani, A. Development and content validity of new patient-reported outcome questionnaires to assess the signs and symptoms and impact of atopic dermatitis: The Atopic Dermatitis Symptom Scale (ADerm-SS) and the Atopic Dermatitis Impact Scale (ADerm-IS). Curr. Med. Res. Opin. 2019, 35, 1139–1148. [Google Scholar] [CrossRef]
- Zyriax, B.C.; Augustin, M.; Abeck, F.; Mohr, N.; Kirsten, N.; Langenbruch, A. Adherence to Guideline-Oriented Preventive Measures in Patients with Atopic Dermatitis in Germany. Dermatology 2021, 238, 307–312. [Google Scholar] [CrossRef]
- Silverberg, J.I.; Barbarot, S.; Gadkari, A.; Simpson, E.L.; Weidinger, S.; Mina-Osorio, P.; Rossi, A.B.; Brignoli, L.; Saba, G.; Guillemin, I.; et al. Atopic dermatitis in the pediatric population: A cross-sectional, international epidemiologic study. Ann. Allergy Asthma Immunol. 2021, 126, 417–428.e2. [Google Scholar] [CrossRef]
- Wei, W.; Anderson, P.; Gadkari, A.; Blackburn, S.; Moon, R.; Piercy, J.; Shinde, S.; Gomez, J.; Ghorayeb, E. Extent and consequences of inadequate disease control among adults with a history of moderate to severe atopic dermatitis. J. Dermatol. 2018, 45, 150–157. [Google Scholar] [CrossRef]
- Eichenfield, L.F.; Tom, W.L.; Chamlin, S.L.; Feldman, S.R.; Hanifin, J.M.; Simpson, E.L.; Berger, T.G.; Bergman, J.N.; Cohen, D.E.; Cooper, K.D.; et al. Guidelines of care for the management of atopic dermatitis: Section 1. Diagnosis and assessment of atopic dermatitis. J. Am. Acad. Dermatol. 2014, 70, 338–351. [Google Scholar] [CrossRef]
- Wollenberg, A.; Kraft, S.; Oppel, T.; Bieber, T. Atopic dermatitis: Pathogenetic mechanisms. Clin. Exp. Dermatol. 2000, 25, 530–534. [Google Scholar] [CrossRef] [PubMed]
- Eichenfield, L.F.; Hanifin, J.M.; Beck, L.A.; Lemanske, R.F., Jr.; Sampson, H.A.; Weiss, S.T.; Leung, D.Y. Atopic dermatitis and asthma: Parallels in the evolution of treatment. Pediatrics 2003, 111, 608–616. [Google Scholar] [CrossRef]
- Hussain, Z.; Sahudin, S.; Thu, H.E.; Shuid, A.N.; Bukhari, S.N.; Kumolosasi, E. Recent Advances in Pharmacotherapeutic Paradigm of Mild to Recalcitrant Atopic Dermatitis. Crit. Rev. Ther. Drug Carr. Syst. 2016, 33, 213–263. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Shi, T.; Hou, J.; Li, M. Ferulic acid alleviates atopic dermatitis-like symptoms in mice via its potent anti-inflammatory effect. Immunopharmacol. Immunotoxicol. 2020, 42, 156–164. [Google Scholar] [CrossRef]
- Rawlings, A.V.; Harding, C.R. Moisturization and skin barrier function. Dermatol. Ther. 2004, 17 (Suppl. 1), 43–48. [Google Scholar] [CrossRef]
- Zhu, T.H.; Zhu, T.R.; Tran, K.A.; Sivamani, R.K.; Shi, V.Y. Epithelial barrier dysfunctions in atopic dermatitis: A skin-gut-lung model linking microbiome alteration and immune dysregulation. Br. J. Dermatol. 2018, 179, 570–581. [Google Scholar] [CrossRef]
- Latz, E.; Xiao, T.S.; Stutz, A. Activation and regulation of the inflammasomes. Nat. Rev. Immunol. 2013, 13, 397–411. [Google Scholar] [CrossRef] [PubMed]
- Dunn, J.H.; Ellis, L.Z.; Fujita, M. Inflammasomes as molecular mediators of inflammation and cancer: Potential role in melanoma. Cancer Lett. 2012, 314, 24–33. [Google Scholar] [CrossRef]
- Zurier, R.B.; Rossetti, R.G.; Burstein, S.H.; Bidinger, B. Suppression of human monocyte interleukin-1beta production by ajulemic acid, a nonpsychoactive cannabinoid. Biochem. Pharmacol. 2003, 65, 649–655. [Google Scholar] [CrossRef]
- Dai, X.; Sayama, K.; Tohyama, M.; Shirakata, Y.; Hanakawa, Y.; Tokumaru, S.; Yang, L.; Hirakawa, S.; Hashimoto, K. Mite allergen is a danger signal for the skin via activation of inflammasome in keratinocytes. J. Allergy Clin. Immunol. 2011, 127, 806–814.e4. [Google Scholar] [CrossRef] [PubMed]
- Anbazhakan, S.; Dhandapani, R.; Anandhakumar, P.; Balu, S. Traditional Medicinal Knowledge on Moringa concanensis Nimmo of Perambalur District, Tamilnadu. Anc. Sci. Life 2007, 26, 42–45. [Google Scholar]
- Balakrishnan, B.B.; Krishnasamy, K.; Choi, K.C. Moringa concanensis Nimmo ameliorates hyperglycemia in 3T3-L1 adipocytes by upregulating PPAR-gamma, C/EBP-alpha via Akt signaling pathway and STZ-induced diabetic rats. Biomed. Pharmacother. 2018, 103, 719–728. [Google Scholar] [CrossRef]
- Maitreya, C.S.a.B. Seasonal analysis of phytochemicals in Moringa concanensis Nimmo ex Dalz. and Gibson from south saurasthara zone, Junagadh-Gujarat, India. Int. Assoc. Biol. Comput. Dig. 2021, 6, 116–125. [Google Scholar]
- Hur, S.S.; Choi, S.W.; Lee, D.R.; Park, J.H.; Chung, T.H. Advanced Effect of Moringa oleifera Bioconversion by Rhizopus oligosporus on the Treatment of Atopic Dermatitis: Preliminary Study. Evid. Based Complement. Altern. Med. 2018, 2018, 7827565. [Google Scholar] [CrossRef]
- Choi, E.J.; Debnath, T.; Tang, Y.; Ryu, Y.B.; Moon, S.H.; Kim, E.K. Topical application of Moringa oleifera leaf extract ameliorates experimentally induced atopic dermatitis by the regulation of Th1/Th2/Th17 balance. Biomed. Pharmacother. 2016, 84, 870–877. [Google Scholar] [CrossRef]
- Zeng, K.; Thompson, K.E.; Yates, C.R.; Miller, D.D. Synthesis and biological evaluation of quinic acid derivatives as anti-inflammatory agents. Bioorg. Med. Chem. Lett. 2009, 19, 5458–5460. [Google Scholar] [CrossRef]
- Pragasam, S.J.; Venkatesan, V.; Rasool, M. Immunomodulatory and anti-inflammatory effect of p-coumaric acid, a common dietary polyphenol on experimental inflammation in rats. Inflammation 2013, 36, 169–176. [Google Scholar] [CrossRef]
- Karuppagounder, V.; Arumugam, S.; Thandavarayan, R.A.; Sreedhar, R.; Giridharan, V.V.; Watanabe, K. Molecular targets of quercetin with anti-inflammatory properties in atopic dermatitis. Drug Discov. Today 2016, 21, 632–639. [Google Scholar] [CrossRef]
- Chanjitwiriya, K.; Roytrakul, S.; Kunthalert, D. Quercetin negatively regulates IL-1beta production in Pseudomonas aeruginosa-infected human macrophages through the inhibition of MAPK/NLRP3 inflammasome pathways. PLoS ONE 2020, 15, e0237752. [Google Scholar] [CrossRef]
- Xue, Y.; Du, M.; Zhu, M.J. Quercetin suppresses NLRP3 inflammasome activation in epithelial cells triggered by Escherichia coli O157:H7. Free Radic. Biol. Med. 2017, 108, 760–769. [Google Scholar] [CrossRef]
- Kim, J.; Kim, B.E.; Leung, D.Y.M. Pathophysiology of atopic dermatitis: Clinical implications. Allergy Asthma Proc. 2019, 40, 84–92. [Google Scholar] [CrossRef]
- Ahn, S.; Siddiqi, M.H.; Aceituno, V.C.; Simu, S.Y.; Zhang, J.; Jimenez Perez, Z.E.; Kim, Y.J.; Yang, D.C. Ginsenoside Rg5:Rk1 attenuates TNF-α/IFN-γ-induced production of thymus- and activation-regulated chemokine (TARC/CCL17) and LPS-induced NO production via downregulation of NF-κB/p38 MAPK/STAT1 signaling in human keratinocytes and macrophages. In Vitro Cell. Dev. Biol. Anim. 2016, 52, 287–295. [Google Scholar] [CrossRef]
- Lim, J.S.; Kim, J.Y.; Lee, S.; Choi, J.K.; Kim, E.N.; Choi, Y.A.; Jang, Y.H.; Jeong, G.S.; Kim, S.H. Bakuchicin attenuates atopic skin inflammation. Biomed. Pharmacother. 2020, 129, 110466. [Google Scholar] [CrossRef]
- Zhou, B.R.; Zhang, J.A.; Zhang, Q.; Permatasari, F.; Xu, Y.; Wu, D.; Yin, Z.Q.; Luo, D. Palmitic acid induces production of proinflammatory cytokines interleukin-6, interleukin-1beta, and tumor necrosis factor-alpha via a NF-κB-dependent mechanism in HaCaT keratinocytes. Mediat. Inflamm. 2013, 2013, 530429. [Google Scholar] [CrossRef]
- Zito, G.; Buscetta, M.; Cimino, M.; Dino, P.; Bucchieri, F.; Cipollina, C. Cellular Models and Assays to Study NLRP3 Inflammasome Biology. Int. J. Mol. Sci. 2020, 21, 4294. [Google Scholar] [CrossRef]
- Strowig, T.; Henao-Mejia, J.; Elinav, E.; Flavell, R. Inflammasomes in health and disease. Nature 2012, 481, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Kelley, N.; Jeltema, D.; Duan, Y.; He, Y. The NLRP3 Inflammasome: An Overview of Mechanisms of Activation and Regulation. Int. J. Mol. Sci. 2019, 20, 3328. [Google Scholar] [CrossRef] [PubMed]
- Bai, B.; Yang, Y.; Wang, Q.; Li, M.; Tian, C.; Liu, Y.; Aung, L.H.H.; Li, P.F.; Yu, T.; Chu, X.M. NLRP3 inflammasome in endothelial dysfunction. Cell Death Dis. 2020, 11, 776. [Google Scholar] [CrossRef] [PubMed]
- Budai, M.M.; Varga, A.; Milesz, S.; Tozser, J.; Benko, S. Aloe vera downregulates LPS-induced inflammatory cytokine production and expression of NLRP3 inflammasome in human macrophages. Mol. Immunol. 2013, 56, 471–479. [Google Scholar] [CrossRef]
- Subedi, L.; Lee, J.H.; Yumnam, S.; Ji, E.; Kim, S.Y. Anti-Inflammatory Effect of Sulforaphane on LPS-Activated Microglia Potentially through JNK/AP-1/NF-κB Inhibition and Nrf2/HO-1 Activation. Cells 2019, 8, 194. [Google Scholar] [CrossRef]
- Zitnik, S.E.; Ruschendorf, F.; Muller, S.; Sengler, C.; Lee, Y.A.; Griffioen, R.W.; Meglio, P.; Wahn, U.; Witt, H.; Nickel, R. IL13 variants are associated with total serum IgE and early sensitization to food allergens in children with atopic dermatitis. Pediatr. Allergy Immunol. 2009, 20, 551–555. [Google Scholar] [CrossRef]
- Zheng, J.; Yao, L.; Zhou, Y.; Gu, X.; Wang, C.; Bao, K.; Sun, Y.; Hong, M. A novel function of NLRP3 independent of inflammasome as a key transcription factor of IL-33 in epithelial cells of atopic dermatitis. Cell Death Dis. 2021, 12, 871. [Google Scholar] [CrossRef]
- Garmhausen, D.; Hagemann, T.; Bieber, T.; Dimitriou, I.; Fimmers, R.; Diepgen, T.; Novak, N. Characterization of different courses of atopic dermatitis in adolescent and adult patients. Allergy 2013, 68, 498–506. [Google Scholar] [CrossRef]
- Illi, S.; von Mutius, E.; Lau, S.; Nickel, R.; Gruber, C.; Niggemann, B.; Wahn, U.; The Multicenter Allergy Study Group. The natural course of atopic dermatitis from birth to age 7 years and the association with asthma. J. Allergy Clin. Immunol. 2004, 113, 925–931. [Google Scholar] [CrossRef]
- Skabytska, Y.; Kaesler, S.; Volz, T.; Biedermann, T. How the innate immune system trains immunity: Lessons from studying atopic dermatitis and cutaneous bacteria. J. Dtsch. Dermatol. Ges. 2016, 14, 153–156. [Google Scholar] [CrossRef][Green Version]
- Weidinger, S.; Novak, N. Atopic dermatitis. Lancet 2016, 387, 1109–1122. [Google Scholar] [CrossRef]
- Hajar, T.; Leshem, Y.A.; Hanifin, J.M.; Nedorost, S.T.; Lio, P.A.; Paller, A.S.; Block, J.; Simpson, E.L. A systematic review of topical corticosteroid withdrawal (“steroid addiction”) in patients with atopic dermatitis and other dermatoses. J. Am. Acad. Dermatol. 2015, 72, 541–549.e2. [Google Scholar] [CrossRef] [PubMed]
- Gutfreund, K.; Bienias, W.; Szewczyk, A.; Kaszuba, A. Topical calcineurin inhibitors in dermatology. Part I: Properties, method and effectiveness of drug use. Postep. Dermatol. Alergol. 2013, 30, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Eichenfield, L.F.; Tom, W.L.; Berger, T.G.; Krol, A.; Paller, A.S.; Schwarzenberger, K.; Bergman, J.N.; Chamlin, S.L.; Cohen, D.E.; Cooper, K.D.; et al. Guidelines of care for the management of atopic dermatitis: Section 2. Management and treatment of atopic dermatitis with topical therapies. J. Am. Acad. Dermatol. 2014, 71, 116–132. [Google Scholar] [CrossRef] [PubMed]
- Nestle, F.O.; Kaplan, D.H.; Barker, J. Psoriasis. N. Engl. J. Med. 2009, 361, 496–509. [Google Scholar] [CrossRef]
- Begon, E.; Michel, L.; Flageul, B.; Beaudoin, I.; Jean-Louis, F.; Bachelez, H.; Dubertret, L.; Musette, P. Expression, subcellular localization and cytokinic modulation of Toll-like receptors (TLRs) in normal human keratinocytes: TLR2 up-regulation in psoriatic skin. Eur. J. Dermatol. 2007, 17, 497–506. [Google Scholar] [CrossRef]
- Lee, S.H.; Kwon, N.S.; Baek, K.J.; Yun, H.Y.; Kim, D.S. LGI3 is secreted and binds to ADAM22 via TRIF-dependent NF-κB pathway in response to LPS in human keratinocytes. Cytokine 2020, 126, 154872. [Google Scholar] [CrossRef]
- Tang, L.; Zhou, F. Inflammasomes in Common Immune-Related Skin Diseases. Front. Immunol. 2020, 11, 882. [Google Scholar] [CrossRef]
- Abd Rani, N.Z.; Husain, K.; Kumolosasi, E. Moringa Genus: A Review of Phytochemistry and Pharmacology. Front. Pharmacol. 2018, 9, 108. [Google Scholar] [CrossRef]
- Arulselvan, P.; Tan, W.S.; Gothai, S.; Muniandy, K.; Fakurazi, S.; Esa, N.M.; Alarfaj, A.A.; Kumar, S.S. Anti-Inflammatory Potential of Ethyl Acetate Fraction of Moringa oleifera in Downregulating the NF-κB Signaling Pathway in Lipopolysaccharide-Stimulated Macrophages. Molecules 2016, 21, 1452. [Google Scholar] [CrossRef]
- Tamrat, Y.; Nedi, T.; Assefa, S.; Teklehaymanot, T.; Shibeshi, W. Anti-inflammatory and analgesic activities of solvent fractions of the leaves of Moringa stenopetala Bak. (Moringaceae) in mice models. BMC Complement. Altern. Med. 2017, 17, 473. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.S.; Arulselvan, P.; Karthivashan, G.; Fakurazi, S. Moringa oleifera Flower Extract Suppresses the Activation of Inflammatory Mediators in Lipopolysaccharide-Stimulated RAW 264.7 Macrophages via NF-κB Pathway. Mediat. Inflamm. 2015, 2015, 720171. [Google Scholar] [CrossRef] [PubMed]
- Chitra, M.J.a.M. Evaluation of anti-inflammatory, analgesic and antipyretic activity of Moringa concanensis Nimmo. J. Chem. Pharm. Res. 2011, 3, 802–806. [Google Scholar]
- Douglas, T.; Champagne, C.; Morizot, A.; Lapointe, J.M.; Saleh, M. The Inflammatory Caspases-1 and -11 Mediate the Pathogenesis of Dermatitis in Sharpin-Deficient Mice. J. Immunol. 2015, 195, 2365–2373. [Google Scholar] [CrossRef] [PubMed]
- Hiramoto, K.; Yamate, Y.; Yokoyama, S. Ultraviolet B eye irradiation aggravates atopic dermatitis via adrenocorticotropic hormone and NLRP3 inflammasome in NC/Nga mice. Photodermatol. Photoimmunol. Photomed. 2018, 34, 200–210. [Google Scholar] [CrossRef]
- Xiao, Y.; Xu, W.; Su, W. NLRP3 inflammasome: A likely target for the treatment of allergic diseases. Clin. Exp. Allergy 2018, 48, 1080–1091. [Google Scholar] [CrossRef] [PubMed]
- Dey, A.; Bhattacharyya, S.; Pal, T.K. Antioxidant Activities of Moringa concanensis Flowers (fresh and dried) Grown in West Bengal. Int. J. Res. Chem. Environ. 2014, 4, 64–70. [Google Scholar]
- Balakrishnan, B.B.; Krishnasamy, K. Evaluation of Free Radical Screening and Antioxidant Potential of Moringa concanensis Nimmo-a Medicinal Plant Used in Indian Traditional Medication System. Int. J. Pharm. Pharm. Sci. 2018, 10, 91–97. [Google Scholar] [CrossRef]
- Comalada, M.; Camuesco, D.; Sierra, S.; Ballester, I.; Xaus, J.; Gálvez, J.; Zarzuelo, A. In vivo quercitrin anti-inflammatory effect involves release of quercetin, which inhibits inflammation through down-regulation of the NF-κB pathway. Eur. J. Immunol. 2005, 35, 584–592. [Google Scholar] [CrossRef]
- Lesjak, M.; Beara, I.; Simin, N.; Pintać, D.; Majkić, T.; Bekvalac, K.; Orčić, D.; Mimica-Dukić, N. Antioxidant and anti-inflammatory activities of quercetin and its derivatives. J. Funct. Foods 2018, 40, 68–75. [Google Scholar] [CrossRef]
- Lee, S.Y.; Moon, E.; Kim, S.Y.; Lee, K.R. Quinic acid derivatives from Pimpinella brachycarpa exert anti-neuroinflammatory activity in lipopolysaccharide-induced microglia. Bioorg. Med. Chem. Lett. 2013, 23, 2140–2144. [Google Scholar] [CrossRef] [PubMed]
- Domiciano, T.P.; Wakita, D.; Jones, H.D.; Crother, T.R.; Verri, W.A., Jr.; Arditi, M.; Shimada, K. Quercetin Inhibits Inflammasome Activation by Interfering with ASC Oligomerization and Prevents Interleukin-1 Mediated Mouse Vasculitis. Sci. Rep. 2017, 7, 41539. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Xu, X.; Li, Y.; Kou, J.; Huang, F.; Liu, B.; Liu, K. Quercetin, luteolin and epigallocatechin gallate alleviate TXNIP and NLRP3-mediated inflammation and apoptosis with regulation of AMPK in endothelial cells. Eur. J. Pharmacol. 2014, 745, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Oh, M.; Park, S.; Kim, H.; Choi, G.J.; Kim, S.H. Application of UPLC-QTOF-MS Based Untargeted Metabolomics in Identification of Metabolites Induced in Pathogen-Infected Rice. Plants 2021, 10, 213. [Google Scholar] [CrossRef]
- Ko, W.K.; Lee, S.H.; Kim, S.J.; Jo, M.J.; Kumar, H.; Han, I.B.; Sohn, S. Anti-inflammatory effects of ursodeoxycholic acid by lipopolysaccharide-stimulated inflammatory responses in RAW 264.7 macrophages. PLoS ONE 2017, 12, e0180673. [Google Scholar] [CrossRef]
- Yang, Y.I.; Woo, J.H.; Seo, Y.J.; Lee, K.T.; Lim, Y.; Choi, J.H. Protective Effect of Brown Alga Phlorotannins against Hyper-inflammatory Responses in Lipopolysaccharide-Induced Sepsis Models. J. Agric. Food Chem. 2016, 64, 570–578. [Google Scholar] [CrossRef]
- Oranje, A.P.; Glazenburg, E.J.; Wolkerstorfer, A.; de Waard-van der Spek, F.B. Practical issues on interpretation of scoring atopic dermatitis: The SCORAD index, objective SCORAD and the three-item severity score. Br. J. Dermatol. 2007, 157, 645–648. [Google Scholar] [CrossRef]
- Logger, J.G.M.; Driessen, R.J.B.; de Jong, E.; van Erp, P.E.J. Value of GPSkin for the measurement of skin barrier impairment and for monitoring of rosacea treatment in daily practice. Skin Res. Technol. 2021, 27, 15–23. [Google Scholar] [CrossRef]
- Yang, H.; Jung, E.M.; Ahn, C.; Lee, G.S.; Lee, S.Y.; Kim, S.H.; Choi, I.G.; Park, M.J.; Lee, S.S.; Choi, D.H.; et al. Elemol from Chamaecyparis obtusa ameliorates 2,4-dinitrochlorobenzene-induced atopic dermatitis. Int. J. Mol. Med. 2015, 36, 463–472. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).