Synthesis, Biological Evaluation and Machine Learning Prediction Model for Fluorinated Cinchona Alkaloid-Based Derivatives as Cholinesterase Inhibitors
Abstract
1. Introduction
2. Results and Discussion
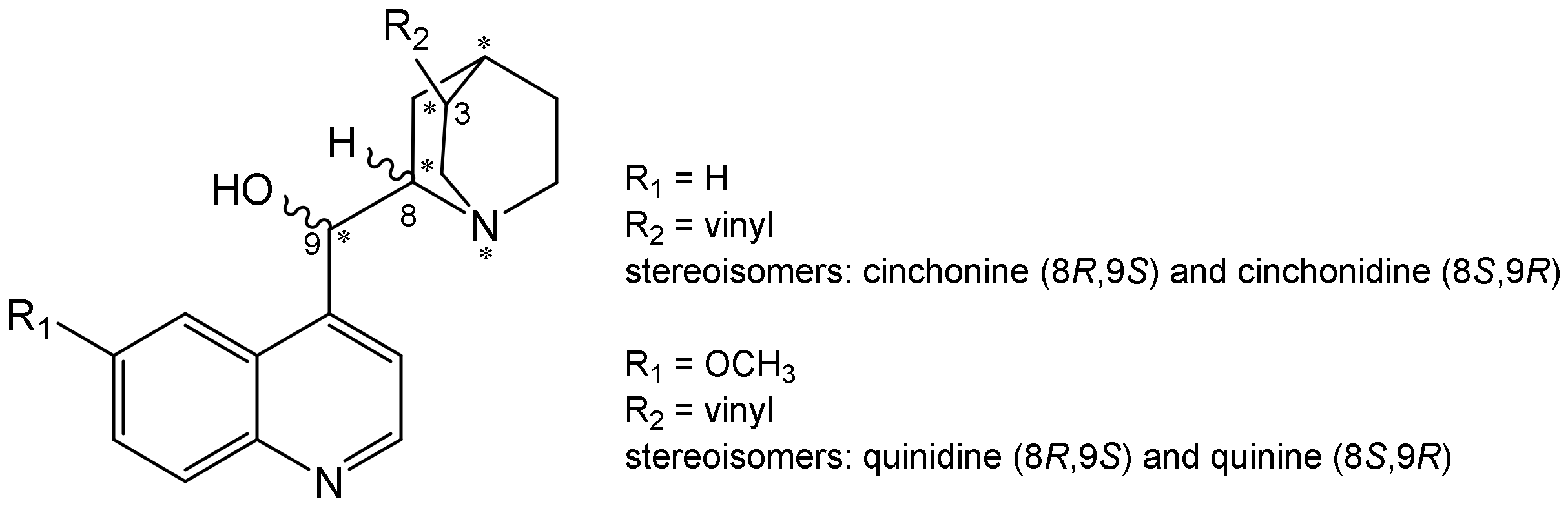
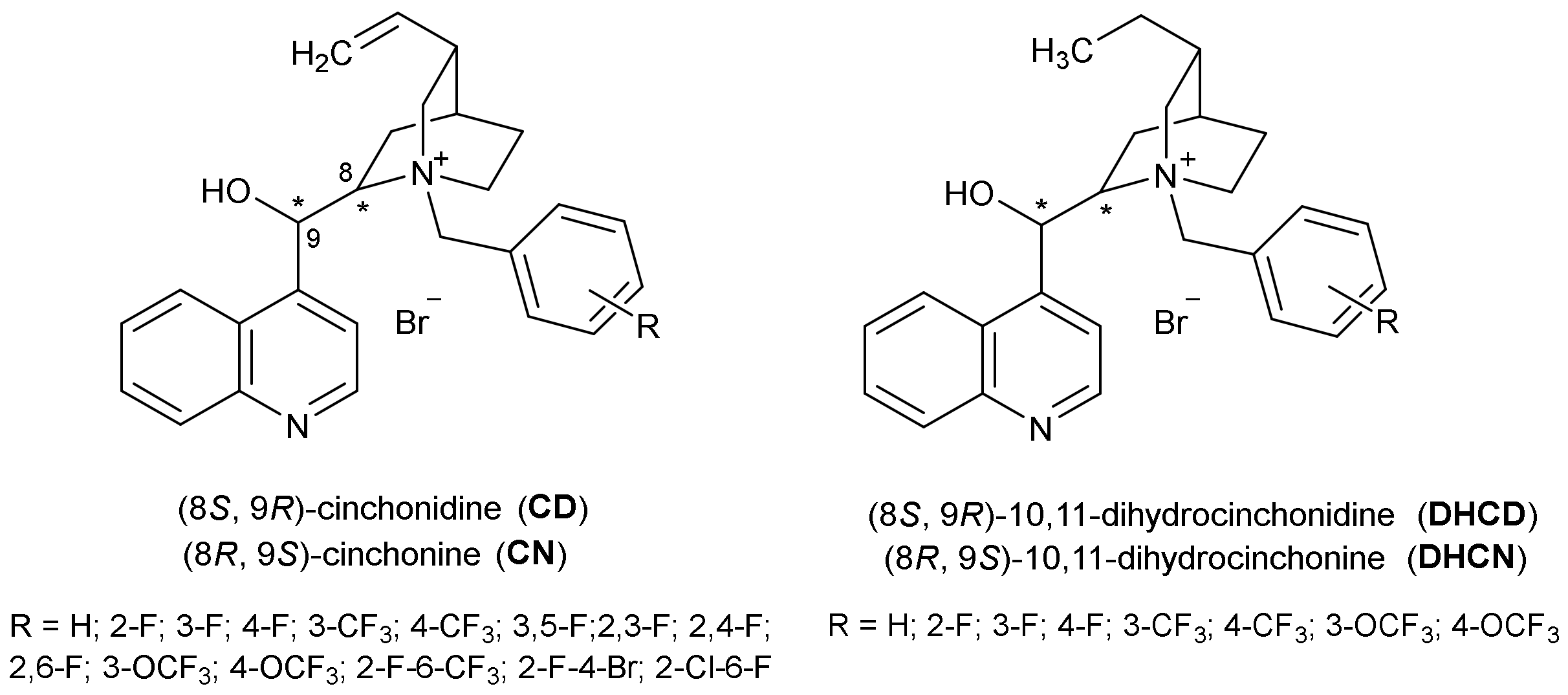
2.1. Synthesis
2.2. Inhibition of Cholinesterases
2.2.1. Inhibition by Cinchonine and Cinchonidine Derivatives
2.2.2. Inhibition by 10, 11-Dihydrocinchonine and 10, 11-Dihydrocinchonidine and Their Derivatives
2.2.3. Selectivity of Inhibition
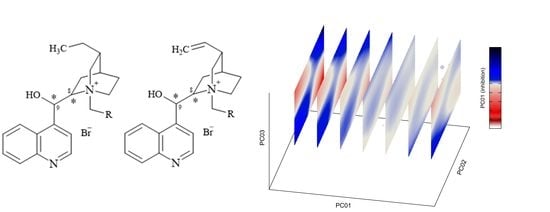
2.2.4. Stereoselectivity of Inhibition by Pseudo-Enantiomers
2.3. In Silico Modelling
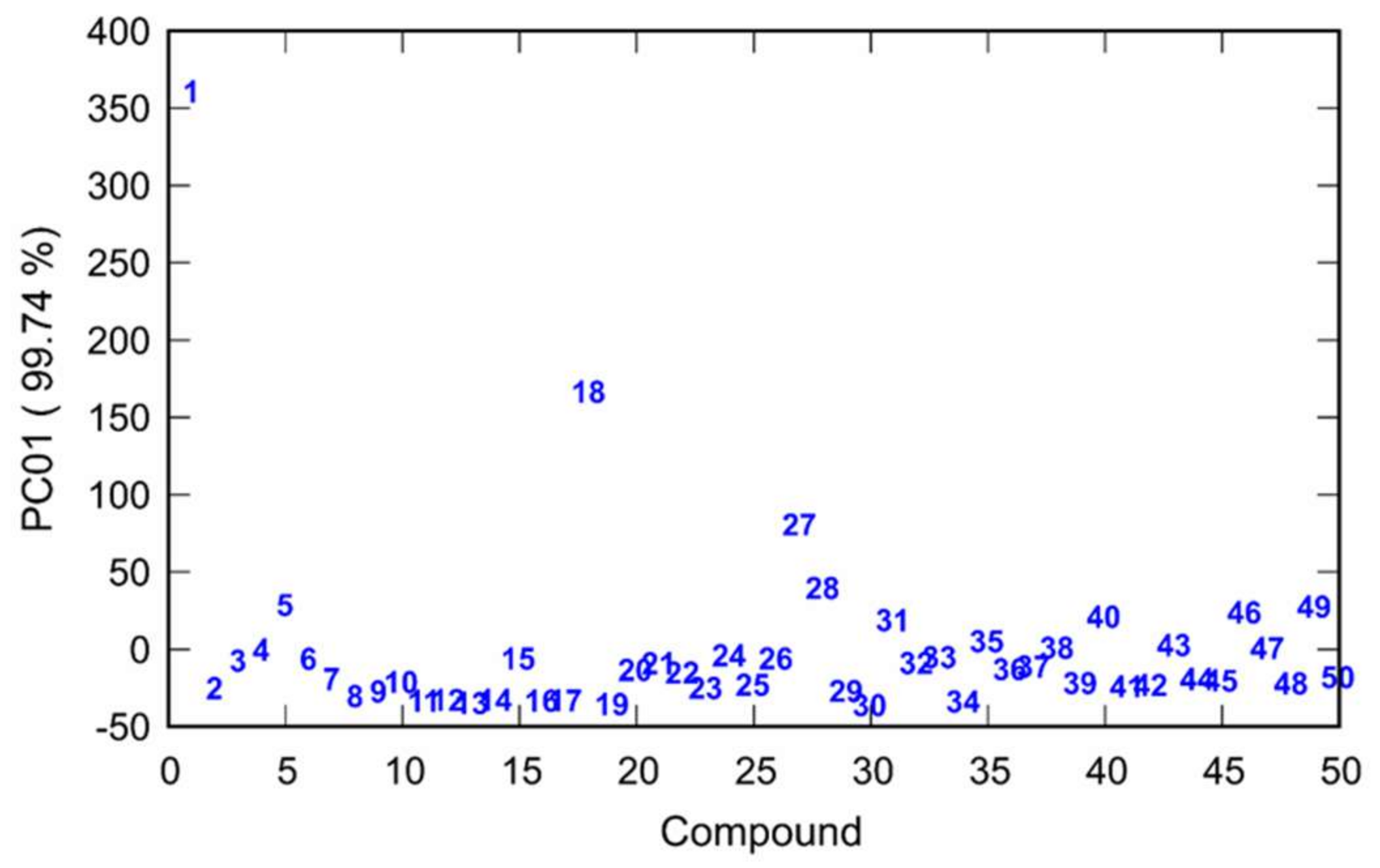
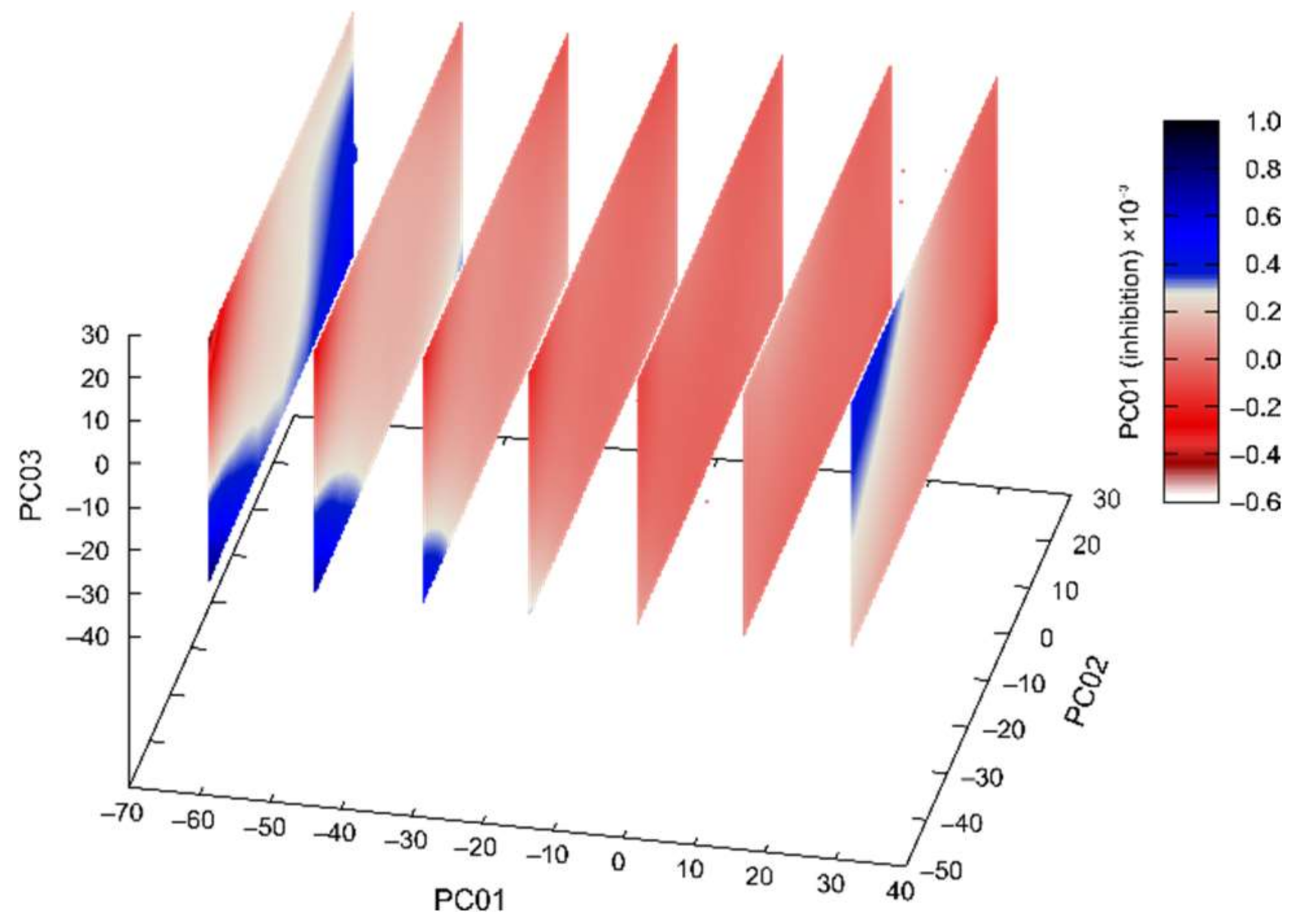
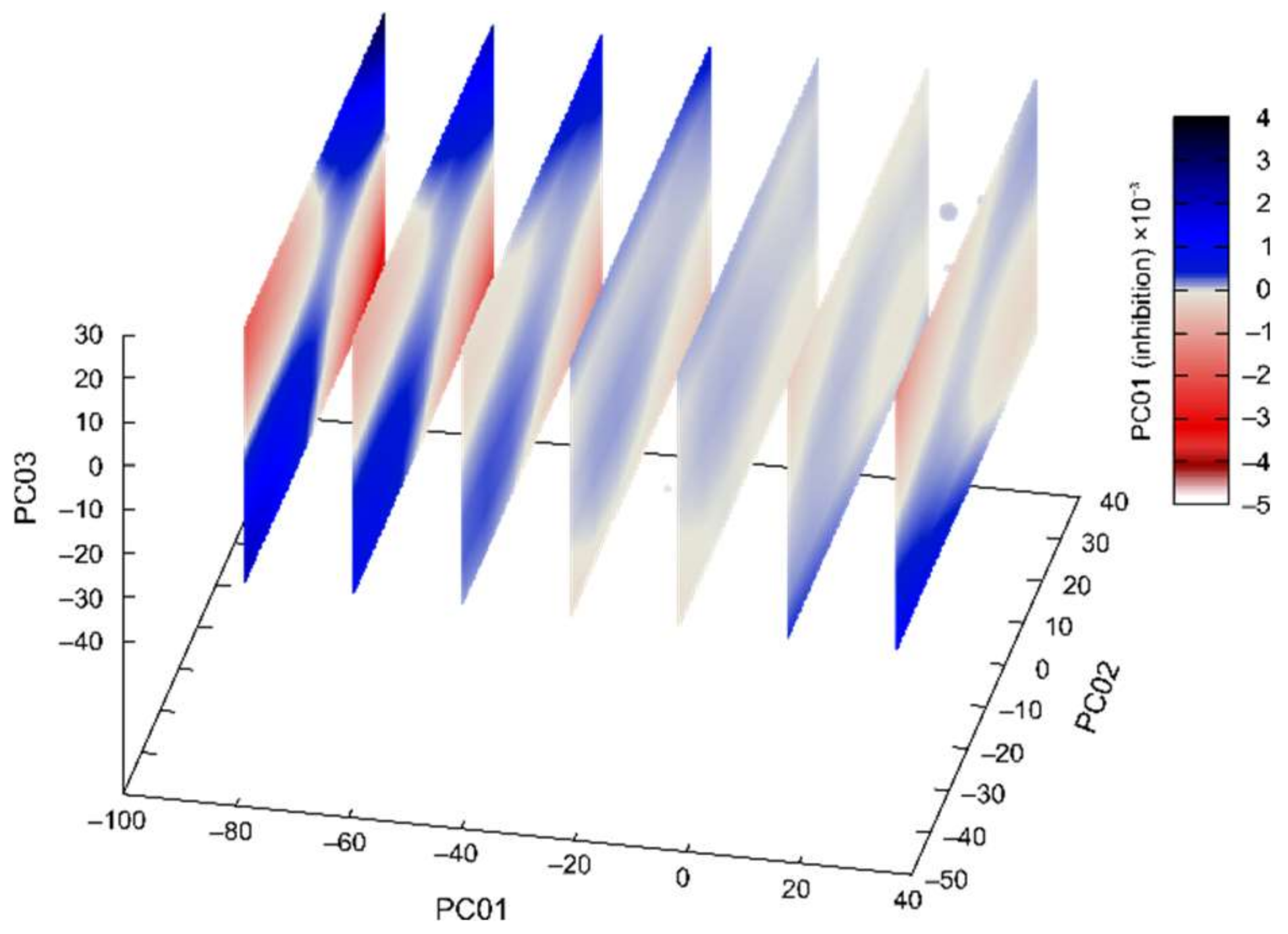
2.4. PCA and Activity/PES Model Established by Machine Learning
3. Materials and Methods
3.1. Chemicals
3.2. Synthesis
3.3. Kinetic Measurements
3.3.1. Enzymes
3.3.2. Enzymes Activity Measurement
3.3.3. Enzyme-Inhibitor Dissociation Constants
3.4. In Silico Prediction of Drug-Likeness
3.5. In Silico Prediction of Blood-Brain Barrier Penetration
3.6. Principal Component Analysis
3.7. Sampling of the Potential Energy Surfaces
3.8. Machine Learning Multivariate Linear Regression
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Giacobini, E.; Pepeu, G. The Brain Cholinergic System in Health and Disease; Informa Healthcare: London, UK, 2006. [Google Scholar] [CrossRef]
- Peterson, B. Alzheimer’s disease facts and figures. Alzheimers Dement. 2019, 15, 321–387. [Google Scholar] [CrossRef]
- Alzheimer’s Association. 2021 Alzheimer’s Disease Facts and Figures. Alzheimers Dement. 2021, 17, 327–406. [Google Scholar] [CrossRef]
- Contestabile, A. The history of the cholinergic hypothesis. Behav. Brain Res. 2011, 221, 334–340. [Google Scholar] [CrossRef]
- Gupta, S.; Mohan, C.G. Dual Binding Site and Selective Acetylcholinesterase Inhibitors Derived from Integrated Pharmacophore Models and Sequential Virtual Screening. Biomed. Res. Int. 2014, 2014, 291214. [Google Scholar] [CrossRef]
- Nalivaeva, N.N.; Turner, A.J. AChE and the amyloid precursor protein (APP)—Cross-talk in Alzheimer’s disease. Chem. Biol. Interact. 2016, 259, 301–306. [Google Scholar] [CrossRef]
- Giacobini, E.; Cuello, A.C.; Fisher, A. Reimagining cholinergic therapy for Alzheimer’s disease Get access Arrow. Brain 2022, 145, 2250–2275. [Google Scholar] [CrossRef]
- Greig, N.H.; Utsuki, T.; Ingram, D.K.; Wang, Y.; Pepeu, G.; Scali, C.; Yu, Q.-S.; Mamczarz, J.; Holloway, H.W.; Giordano, T.; et al. Selective butyrylcholinesterase inhibition elevates brain acetylcholine, augments learning and lowers Alzheimer-amyloid peptide in rodent. Proc. Natl. Acad. Sci. USA 2005, 102, 17213–17218. [Google Scholar] [CrossRef]
- Greig, N.H.; Lahiri, D.K.; Sambamurti, K. Butyrylcholinesterase: An important new target in Alzheimer’s disease therapy. Int. Psychogeriatr. 2002, 14, 77–91. [Google Scholar] [CrossRef]
- Kacprzak, K. Chemistry and Biology of Cinchona Alkaloids. In Natural Products; Ramawat, K.G., Mérillon, J.-M., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 605–641. [Google Scholar]
- Achan, J.; Talisuna, A.O.; Erhart, A.; Yeka, A.; Tibenderana, J.K.; Baliraine, F.N.; Rosenthal, P.J.; D’Alessandro, U. Quinine, an old anti-malarial drug in a modern world: Role in the treatment of malaria. Malar. J. 2011, 10, 144–156. [Google Scholar] [CrossRef]
- Laraia, L.; Ohsawa, K.; Konstantinidis, G.; Robke, L.; Wu, Y.-W.; Kumar, K.; Waldmann, H. Discovery of Novel Cinchona-Alkaloid-Inspired Oxazatwistane Autophagy Inhibitors. Angew. Chem. Int. Ed. 2017, 56, 2145–2150. [Google Scholar] [CrossRef]
- Wang, X.; Zeng, Y.; Sheng, L.; Larson, P.; Liu, X.; Zou, X.; Wang, S.; Guo, K.; Ma, C.; Zhang, G.; et al. A Cinchona Alkaloid Antibiotic That Appears To Target ATP Synthase in Streptococcus pneumoniae. J. Med. Chem. 2019, 62, 2305–2332. [Google Scholar] [CrossRef] [PubMed]
- Jo, Y.J.; Lee, H.I.; Kim, N.; Hwang, D.; Lee, J.; Lee, G.R.; Hong, S.E.; Lee, H.; Kwon, M.; Kim, N.Y.; et al. Cinchonine inhibits osteoclast differentiation by regulating TAK1 and AKT, and promotes osteogenesis. J. Cell. Physiol. 2021, 236, 1854–1865. [Google Scholar] [CrossRef] [PubMed]
- Che, Z.P.; Yang, J.M.; Zhang, S.; Sun, D.; Tian, Y.E.; Liu, S.M.; Lin, X.M.; Jiang, J.; Chen, G.Q. Synthesis of novel 9R/S-acyloxy derivatives of cinchonidine and cinchonine as insecticidal agents. J. Asian Nat. Prod. Res. 2021, 23, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Bosak, A.; Ramić, A.; Šmidlehner, T.; Hrenar, T.; Primožič, I.; Kovarik, Z. Design and evaluation of selective butyrylcholinesterase inhibitors based on Cinchona alkaloid scaffold. PLoS ONE 2018, 13, e0205193. [Google Scholar] [CrossRef] [PubMed]
- Meanwell, N.A. Fluorine and Fluorinated Motifs in the Design and Application of Bioisosteres for Drug Design. J. Med. Chem. 2018, 61, 5822–5880. [Google Scholar] [CrossRef] [PubMed]
- Richardson, P. Applications of fluorine to the construction of bioisosteric elements for the purposes of novel drug discovery. Expert Opin. Drug Discov. 2021, 16, 1261–1286. [Google Scholar] [CrossRef]
- Belyk, K.M.; Xiang, B.; Bulger, P.G.; Leonard, W.R.; Balsells, J.; Yin, J.; Chen, C.-Y. Enantioselective Synthesis of (1R,2S)-1-Amino-2-vinylcyclopropanecarboxylic Acid Ethyl Ester (Vinyl-ACCA-OEt) by Asymmetric Phase-Transfer Catalyzed Cyclopropanation of (E)-N-Phenylmethyleneglycine Ethyl Ester. Org. Process Res. Dev. 2010, 14, 692–700. [Google Scholar] [CrossRef]
- Nicolaou, K.C.; Liu, G.; Beabout, K.; McCurry, M.D.; Shamoo, Y. Asymmetric Alkylation of Anthrones, Enantioselective Total Synthesis of (-)- and (+)-Viridicatumtoxins B and Analogues Thereof: Absolute Configuration and Potent Antibacterial Agents. J. Am. Chem. Soc. 2017, 139, 3736–3746. [Google Scholar] [CrossRef]
- Jew, S.; Yoo, M.; Jeong, B.; Park, I.Y.; Park, H. An unusual electronic effect of an aromatic-F in phase-transfer catalysts derived from cinchona-alkaloid. Org. Lett. 2002, 4, 4245–4248. [Google Scholar] [CrossRef]
- Lian, M.; Li, Z.; Du, J.; Meng, Q.; Gao, Z. Asymmetric Direct α-Hydroxylation of β-Oxo Esters by Phase-Transfer Catalysis Using Chiral Quaternary Ammonium Salts. Eur. J. Org. Chem. 2010, 34, 6525–6530. [Google Scholar] [CrossRef]
- Aires-de-Sousa, J.; Prabhakar, S.; Lobo, A.M.; Rosa, A.M.; Gomes, M.J.S.; Corvo, M.C.; Williams, D.J.; White, A.J.P. Asymmetric synthesis of N-aryl aziridines. Tetrahedron Asymmetry 2002, 12, 3349–3365. [Google Scholar] [CrossRef]
- Shi, Q.; Lee, Y.; Kim, M.; Park, M.; Lee, K.; Song, H.; Cheng, M.; Jeong, B.; Park, H.; Jew, S. Highly efficient polymer supported phase-transfer catalysts containing hydrogen bond inducing functional groups. Tetrahedron Lett. 2008, 49, 1380–1383. [Google Scholar] [CrossRef]
- Denmark, S.E.; Cullen, L.R. Development of a Phase-Transfer-Catalyzed, [2,3]-Wittig Rearrangement. J. Org. Chem. 2015, 80, 11818–11848. [Google Scholar] [CrossRef] [PubMed]
- Vidal-Albalat, A.; Świderek, K.; Izquierdo, J.; Rodríguez, S.; Moliner, V.; González, F.V. Catalytic enantioselective epoxidation of nitroalkenes. Chem. Commun. 2016, 52, 10060–10063. [Google Scholar] [CrossRef]
- Simeon-Rudolf, V.; Šinko, G.; Štuglin, A.; Reiner, E. Inhibition of human blood acetylcholinesterase and butyrylcholinesterase by ethopropazine. Croat. Chem. Acta 2001, 74, 173–182. [Google Scholar]
- Saxena, A.; Fedorko, J.M.; Vinayaka, C.R.; Medhekar, R.; Radić, Z.; Taylor, P.; Lockridge, O.; Doctor, B.P. Aromatic amino-acid residues at the active and peripheral anionic sites control the binding of E2020 (Aricept) to cholinesterases. Eur. J. Biochem. 2003, 270, 4447–4458. [Google Scholar] [CrossRef]
- Reiner, E.; Radić, Z. Mechanism of action of cholinesterase inhibitors. In Cholinesterase and Cholinesterase Inhibitors, 1st ed.; Giacobini, E., Ed.; Martin Dunitz Ltd.: London, UK, 2000; pp. 103–120. [Google Scholar]
- Matošević, A.; Radman Kastelic, A.; Mikelić, A.; Zandona, A.; Katalinić, M.; Primožič, I.; Bosak, A.; Hrenar, T. Quinuclidine-Based Carbamates as Potential CNS Active Compounds. Pharmaceutics 2021, 13, 420. [Google Scholar] [CrossRef]
- Wu, H.; Hintermann, L. Transfer Hydrogenations of Alkenes with Formate on Pd/C: Synthesis of Dihydrocinchona Alkaloids. Synthesis 2013, 45, 888–892. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres, V.; Featherstone, R.M. New and rapid colorimetric determination of acetylcholin-esterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef]
- Veber, D.F.; Johnson, S.R.; Cheng, H.Y.; Smith, B.R.; Ward, K.W.; Kopple, K.D. Molecular properties that influence the oral bioavailability of drug candidates. J. Med. Chem. 2002, 45, 2615–2623. [Google Scholar] [CrossRef] [PubMed]
- Pajouhesh, H.; Lenz, G.R. Medicinal chemical properties of successful central nervous system drugs. NeuroRx 2005, 2, 541–553. [Google Scholar] [CrossRef] [PubMed]
- Chemicalize, Calculation Module 2, (2018) developed by Chemaxon Ltd. Available online: https://chemicalize.com/ (accessed on 30 September 2022).
- Egan, W.J.; Lauri, G. Prediction of intestinal permeability. Adv. Drug. Deliv. Rev. 2002, 54, 273–289. [Google Scholar] [CrossRef]
- Jolliffe, I.T. Principal Component Analysis; Springer: Berlin/Heidelberg, Germany, 1986. [Google Scholar]
- Smilde, A.; Bro, R.; Geladi, P. Multi-Way Analysis: Applications in the Chemical Sciences; John Wiley & Sons Ltd.: Chichester, UK, 2004. [Google Scholar]
- Beltrami, E. Sulle funzioni bilineari. G. Mat. Uso Stud. Univ. 1873, 11, 98–106. [Google Scholar]
- Pearson, K. LIII. On lines and planes of closest fit to systems of points in space. Lond. Edinb. Dublin Philos. Mag. J. Sci. 1901, 2, 559–572. [Google Scholar] [CrossRef]
- Hotelling, H. Analysis of a complex of statistical variables into principal components. J. Educ. Psychol. 1933, 24, 417–441. [Google Scholar] [CrossRef]
- Novak, P.; Kišić, A.; Hrenar, T.; Jednačak, T.; Miljanić, S.; Verbanec, G. In-line reaction monitoring of entacapone synthesis by Raman spectroscopy and multivariate analysis. J. Pharm. Biomed. Anal. 2011, 54, 660–666. [Google Scholar] [CrossRef]
- Jović, O.; Smolić, T.; Primožič, I.; Hrenar, T. Spectroscopic and Chemometric Analysis of Binary and Ternary Edible Oil Mixtures: Qualitative and Quantitative Study. Anal. Chem. 2016, 88, 4516–4524. [Google Scholar] [CrossRef]
- Hrenar, T. moonee, Program for Manipulation and Analysis of Multi- and Univariate Data; Revision 0.6827; University of Zagreb Faculty of Science: Zagreb, Croatia, 2021. [Google Scholar]
- Geladi, P.; Kowalski, B.R. Partial least-squares regression: A tutorial. Anal. Chim. Acta 1986, 185, 1–17. [Google Scholar] [CrossRef]
- Leach, A. Molecular Modelling: Principles and Applications, 2nd ed.; Pearson Education Limited: Dorchester, UK, 2001. [Google Scholar]
- Stewart, J.J. Optimization of parameters for semiempirical methods VI: More modifications to the NDDO approximations and re-optimization of parameters. J. Mol. Model. 2013, 19, 1–32. [Google Scholar] [CrossRef]
- Stewart, J.J. Stewart Computational Chemistry; MOPAC2016: Colorado Springs, CO, USA, 2016. [Google Scholar]
- Primožič, I.; Tomica, H.; Baumann, K.; Krišto, L.; Križić, I.; Tomić, S. Mechanochemical and Conformational Study of N-heterocyclic Carbonyl-Oxime Transformations. Croat. Chem. Acta 2014, 87, 153–160. [Google Scholar] [CrossRef]
- Kovačević, G.; Hrenar, T.; Došlić, N. Hydrogen bonding in malonaldehyde: A density functional and reparametrized semiempirical approach. Chem. Phys. 2003, 293, 41–52. [Google Scholar] [CrossRef]
| Compound | Ki/µM | SI * | Compound | Ki/µM | SI * | ||
|---|---|---|---|---|---|---|---|
| BChE | AChE | BChE | AChE | ||||
| CD Bzl | 0.075 ± 0.007 | 15 ± 2 | 200 | CN Bzl | 2.9 ± 0.3 | 121 ± 12 | 42 |
| CD 2F | 0.82 ± 0.03 | 33 ± 1 | 40 | CN 2F | 2.4 ± 0.1 | 80 ± 2 | 33 |
| CD 3F | 0.075 ± 0.005 | 40 ± 2 | 533 | CN 3F | 6.1 ± 0.3 | 13 ± 0.4 | 2.1 |
| CD 4F | 1.5 ± 0.1 | 69 ± 3 | 46 | CN 4F | 2.6 ± 0.1 | 3.9 ± 0.2 | 1.5 |
| CD 3CF3 | 2.4 ± 0.1 | 34 ± 1 | 14 | CN 3CF3 | 4.4 ± 0.2 | 59 ± 2 | 13 |
| CD 4CF3 | 2.0 ± 0.1 | 21 ± 1 | 11 | CN 4CF3 | 6.0 ± 0.3 | 31 ± 1 | 5.2 |
| CD 3,5F | 0.081 ± 0.01 | 10 ± 1 | 123 | CN 3,5F | 6.3 ± 0.2 | 34 ± 3 | 5.4 |
| CD 3,4F | 1.3 ± 0.1 | 13 ± 1 | 10 | CN 3,4F | 6.1 ± 0.2 | 14 ± 0.2 | 2.3 |
| CD 2,3F | 0.75 ±0.03 | 19 ± 1 | 25 | CN 2,3F | 9.6 ± 0.4 | 46 ± 3 | 4.8 |
| CD 2,4F | 6.1 ± 0.5 | 6.4 ± 0.3 | 1.1 | CN 2,4F | 6.0 ± 0.2 | 27 ± 1 | 4.5 |
| CD 2,6F | 9.9 ±0.4 | 7.7 ± 0.5 | 0.77 | CN 2,6F | 5.2 ± 0.2 | 30 ± 2 | 5.8 |
| CD 3OCF3 | 7.4 ±0.4 | 8.2 ± 1.1 | 1.1 | CN 3OCF3 | 4.7 ± 0.2 | 41 ± 2 | 8.7 |
| CD 4OCF3 | 7.6 ± 0.5 | 7.3 ± 0.5 | 0.96 | CN 4OCF3 | 7.8 ± 0.3 | 19 ± 2 | 2.4 |
| CD 2F-6CF3 | 5.7 ± 0.6 | 35 ± 4 | 6.1 | CN 2F-6CF3 | 7.7 ± 0.4 | 61 ± 2 | 7.9 |
| CD 2F-4Br | 0.68 ± 0.05 | 7.2 ± 0.4 | 10 | CN 2F-4Br | 5.5 ± 0.3 | 16 ± 1 | 2.9 |
| CD 2Cl-6F | 5.0 ± 0.3 | 9.9 ± 0.8 | 1.9 | CN 2Cl-6F | 1.2 ± 0.0 | 17 ± 1 | 14 |
| Compound | Ki/µM | SI * | Compound | Ki/µM | SI * | ||
|---|---|---|---|---|---|---|---|
| BChE | AChE | BChE | AChE | ||||
| DHCD | 19 ± 2 | 206 ± 6 | 11 | DHCN | 1.2 ± 0.1 | 43 ± 2 | 43 |
| DHCD Bzl | 0.4 ± 0.02 | 4.8 ± 0.4 | 12 | DHCN Bzl | 0.9 ± 0.04 | 21 ± 1 | 23 |
| DHCD 3F | 0.3 ± 0.02 | 27 ± 2 | 84 | DHCN 3F | 1.2 ± 0.1 | 20 ± 1 | 20 |
| DHCD 4F | 4.3 ± 0.2 | 31 ± 1 | 8 | DHCN 4F | 1.6 ± 0.1 | 64 ± 2 | 40 |
| DHCD 3CF3 | 1.4 ± 0.1 | 25 ± 1 | 18 | DHCN 3CF3 | 1.2 ± 0.05 | 41 ± 2 | 34 |
| DHCD 4CF3 | 3.2 ± 0.2 | 15 ± 1 | 5 | DHCN 4CF3 | 1.6 ± 0.1 | 18 ± 1 | 9 |
| DHCD 3OCF3 | 6.8 ± 0.3 | 36 ± 1 | 5 | DHCN 3OCF3 | 1.3 ± 0.5 | 68 ± 1 | 52 |
| DHCD 4OCF3 | 5.9 ± 0.2 | 17 ± 1 | 3 | DHCN 4OCF3 | 2.2 ± 0.1 | 22 ± 1 | 10 |
| 2F | 3F | 4F | 3CF3 | 4CF3 | 3,5F | 3,4F | 2,3F | 2,4F | 2,6F | 3OCF3 | 4OCF3 | 2F-6CF3 | 2F-4Br | 6F-2Cl | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ki(CN/CD) | BChE | 2.9 | 81 | 1.7 | 1.8 | 3.0 | 78 | 4.7 | 13 | 1.0 | 0.53 | 0.64 | 1.0 | 1.4 | 8.1 | 0.24 |
| AChE | 2.4 | 0.33 | 0.056 | 1.7 | 1.5 | 3.4 | 1.1 | 2.4 | 4.2 | 3.9 | 5 | 2.6 | 1.7 | 2.2 | 1.7 | |
| - | Bzl | 3F | 4F | 3CF3 | 4CF3 | 3OCF3 | 4OCF3 | ||
|---|---|---|---|---|---|---|---|---|---|
| Ki(DHCN/DHCD) | BChE | 0.063 | 2.2 | 4.0 | 0.37 | 0.86 | 0.50 | 0.19 | 0.37 |
| AChE | 0.21 | 4.4 | 0.74 | 2.1 | 1.6 | 1.2 | 1.9 | 1.3 | |
| Compounds | MW/100 | clogP | HBD | HBA | RB | PSA/10 |
|---|---|---|---|---|---|---|
| CD 2F, 3F, 4F | 4.83425 | 0.376 | 1 | 2 | 5 | 3.312 |
| CD 3CF3, 4CF3 | 5.33433 | 1.111 | 1 | 2 | 6 | 3.312 |
| CD 3,5F, 3,4F, 2,3F, 2,4F, 2,6F | 5.01416 | 0.519 | 1 | 2 | 5 | 3.312 |
| CD 3OCF3, 4OCF3 | 5.49432 | 1.664 | 1 | 3 | 7 | 4.235 |
| CD 2F-6CF3 | 5.51424 | 1.625 | 1 | 2 | 6 | 3.312 |
| CD 2F-4Br | 5.62321 | 1.145 | 1 | 2 | 5 | 3.312 |
| CD 6F-2Cl | 5.1787 | 0.98 | 1 | 2 | 5 | 3.312 |
| DHCD | 3.76319 | 2.975 | 1 | 3 | 3 | 3.636 |
| DHCD Bzl | 4.6616 | 0.537 | 1 | 2 | 5 | 3.312 |
| DHCD 3F, 4F | 4.85441 | 0.68 | 1 | 2 | 5 | 3.312 |
| DHCD 3CF3, 4CF3 | 5.341493 | 1.415 | 1 | 2 | 6 | 3.312 |
| DHCD 3OCF3, 4OCF3 | 5.550144 | 1.968 | 1 | 3 | 7 | 4.235 |
| Recommended values | 5 | 5 | 3 | 7 | 8 | 7 |
| Penetration Levels * | 0 | 1 |
|---|---|---|
| Compounds | CD 3OCF3, CD 4OCF3, CN 3OCF3, DHCN 3OCF3, DHCN 4OCF3, DHCD 3OCF3, DHCD 4OCF3 | CD 2F, CD 3F, CD 4F, CD 3CF3, CD 4CF3, CN 2F, CN 3F, CN 4F, CN 3CF3, CD 3,5F, CD 3,4F, CN 4CF3, CD 2,3F, CD 2,4F, CD 2,6F, CN 3,5F, CN 3,4F, CN 2,3F, CN 2,4F, CN 2,6F, CD 2F-6CF3, CD 2F-4Br, CD 2Cl-6F, CN 2F-6CF3, CN 2F-4Br, CN 2Cl-6F, DHCD, DHCD 3F, DHCD 4F, DHCD 4CF3, DHCD 3CF3, DHCD Bzl, DHCN, DHCN Bzl, DHCN 4CF3, DHCN 3F, DHCN 3CF3, Tacrine |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramić, A.; Matošević, A.; Debanić, B.; Mikelić, A.; Primožič, I.; Bosak, A.; Hrenar, T. Synthesis, Biological Evaluation and Machine Learning Prediction Model for Fluorinated Cinchona Alkaloid-Based Derivatives as Cholinesterase Inhibitors. Pharmaceuticals 2022, 15, 1214. https://doi.org/10.3390/ph15101214
Ramić A, Matošević A, Debanić B, Mikelić A, Primožič I, Bosak A, Hrenar T. Synthesis, Biological Evaluation and Machine Learning Prediction Model for Fluorinated Cinchona Alkaloid-Based Derivatives as Cholinesterase Inhibitors. Pharmaceuticals. 2022; 15(10):1214. https://doi.org/10.3390/ph15101214
Chicago/Turabian StyleRamić, Alma, Ana Matošević, Barbara Debanić, Ana Mikelić, Ines Primožič, Anita Bosak, and Tomica Hrenar. 2022. "Synthesis, Biological Evaluation and Machine Learning Prediction Model for Fluorinated Cinchona Alkaloid-Based Derivatives as Cholinesterase Inhibitors" Pharmaceuticals 15, no. 10: 1214. https://doi.org/10.3390/ph15101214
APA StyleRamić, A., Matošević, A., Debanić, B., Mikelić, A., Primožič, I., Bosak, A., & Hrenar, T. (2022). Synthesis, Biological Evaluation and Machine Learning Prediction Model for Fluorinated Cinchona Alkaloid-Based Derivatives as Cholinesterase Inhibitors. Pharmaceuticals, 15(10), 1214. https://doi.org/10.3390/ph15101214