Formulation of Solid Lipid Nanoparticles Loaded with Nociceptin/Orphanin FQ (N/OFQ) and Characterization in a Murine Model of Airway Hyperresponsiveness
Abstract
1. Introduction
2. Results
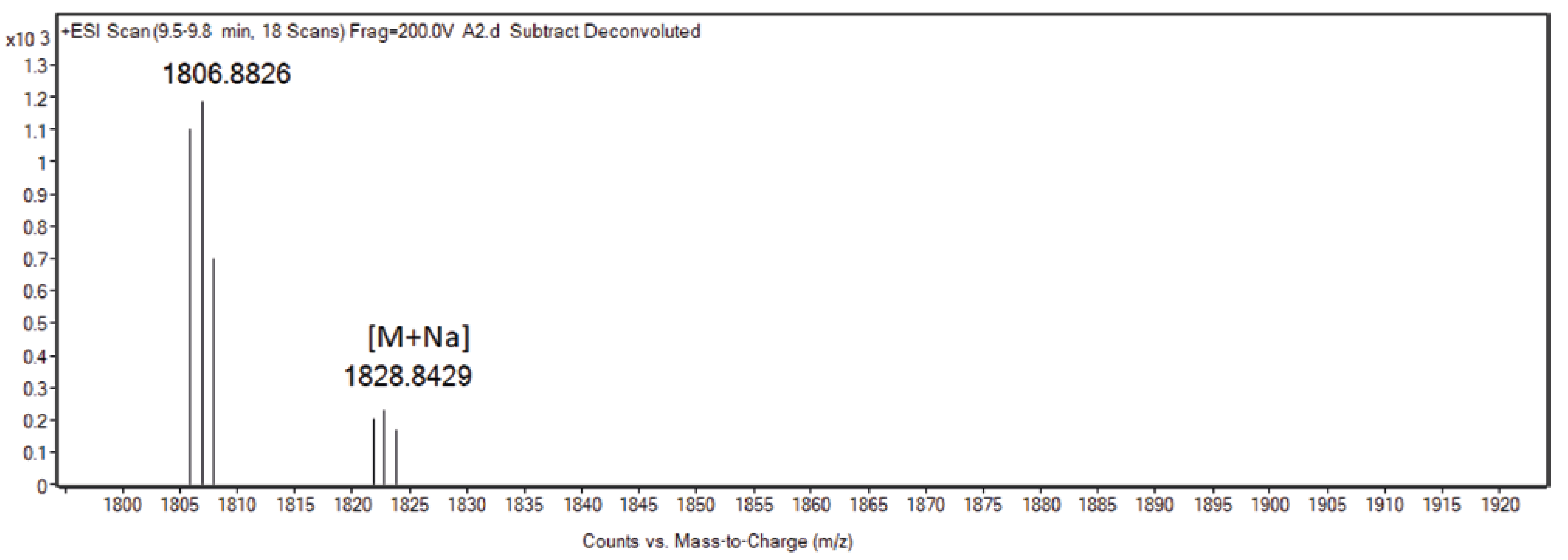
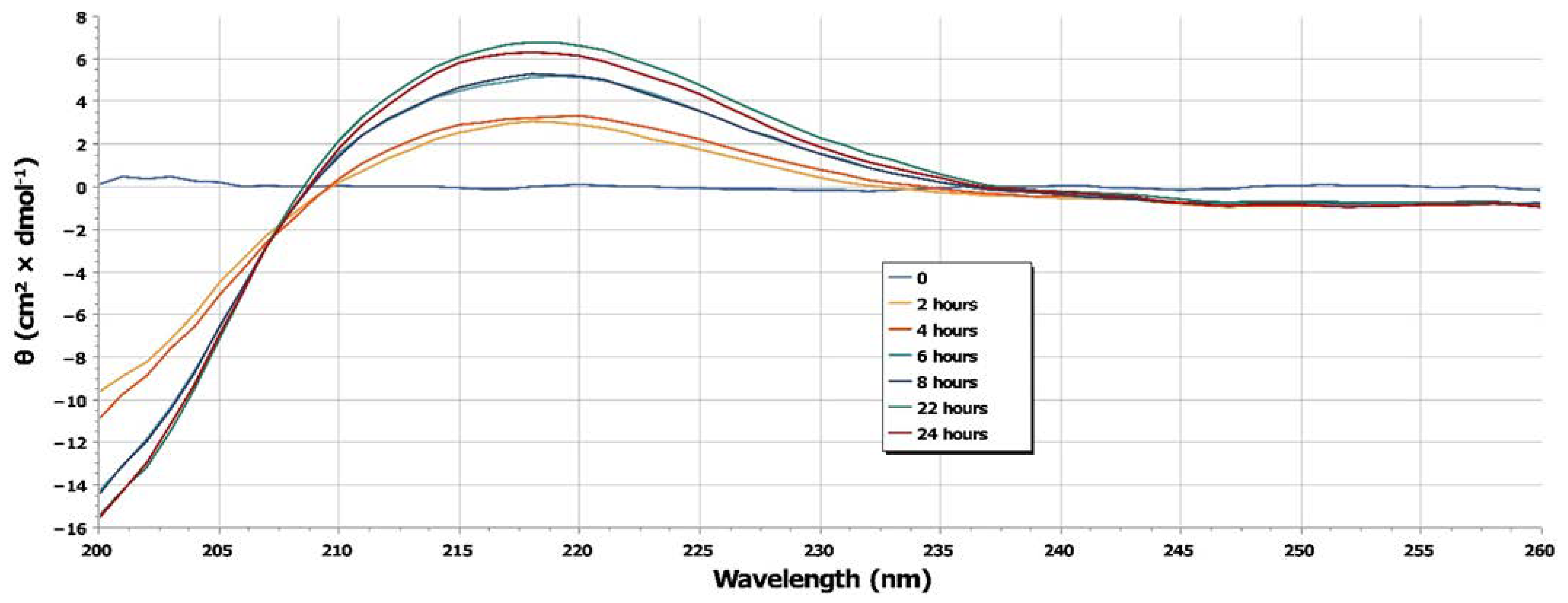
2.1. SLN Formulation and Characterization
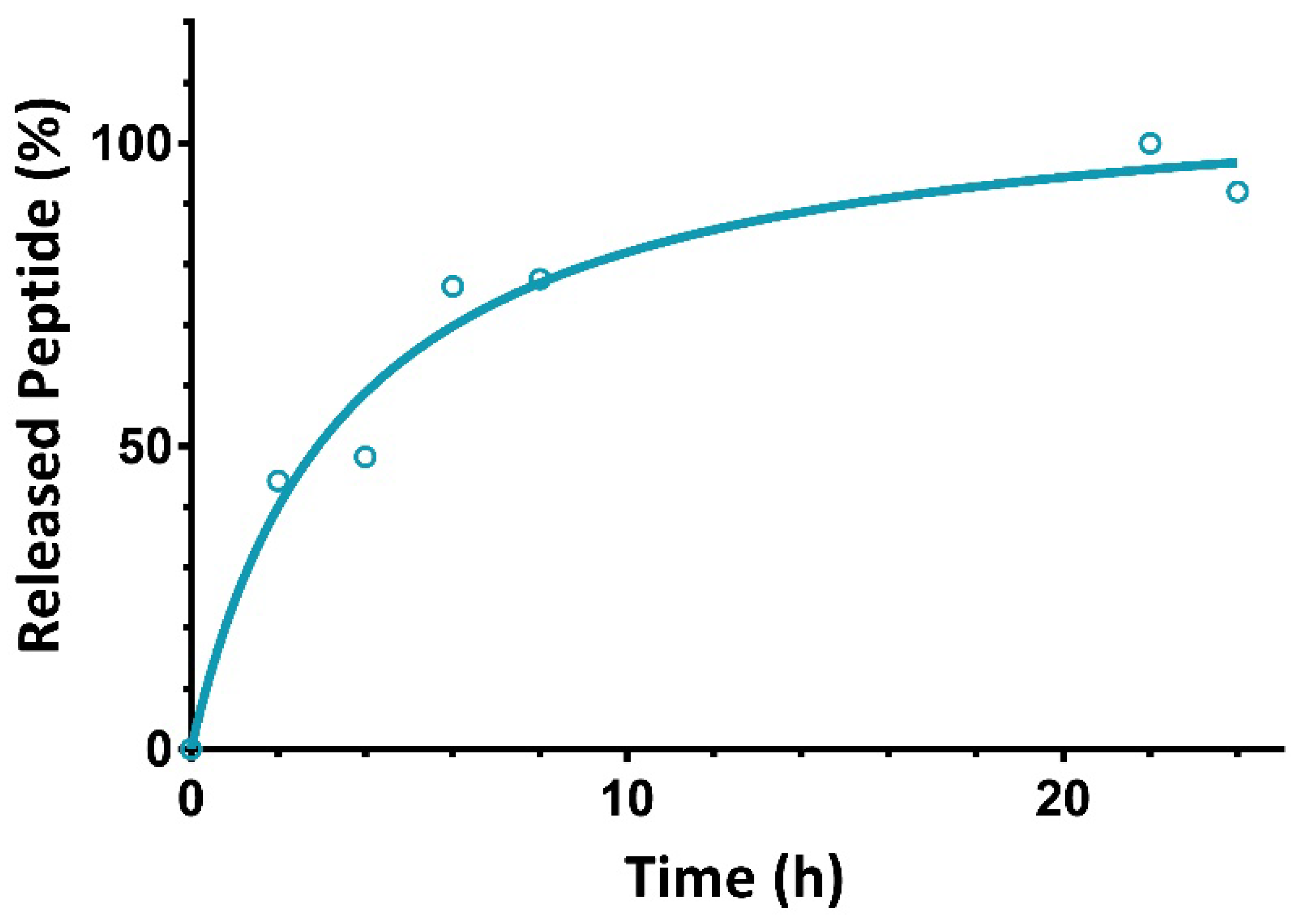
2.2. In Vitro Release of N/OFQ from SLN
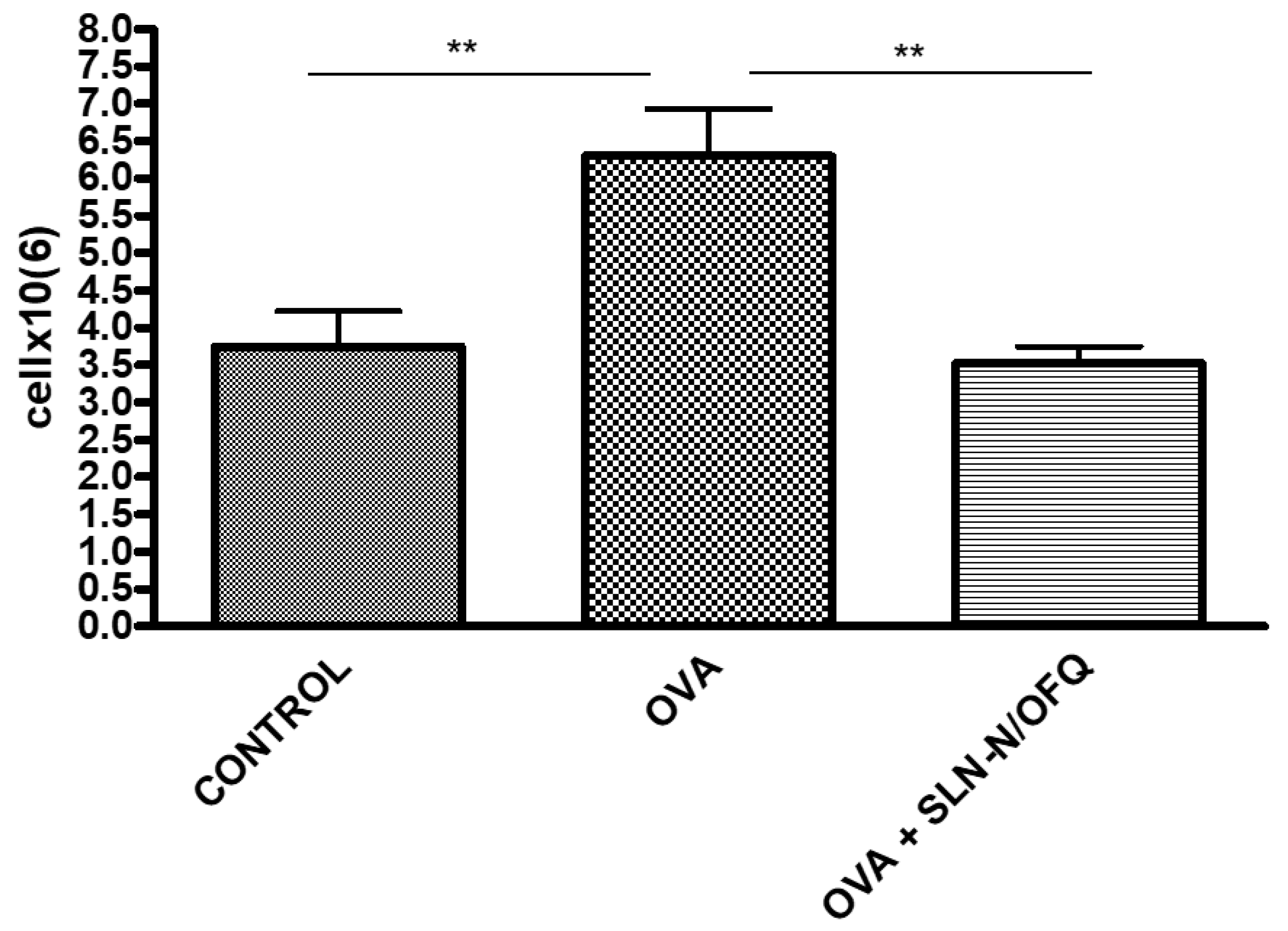
2.3. Functional and Cell Count Evaluations
3. Discussion
4. Materials and Methods
4.1. SLN Formulation
4.2. SLN Characterization
4.3. Scanning Electron Microscopy
4.4. Determination of Drug Loading
4.5. In Vitro Release Study
4.6. In Vitro Release Profile of N/OFQ from SLN by LC-MS
4.7. N/OFQ
4.8. Animal Study
4.9. Sensitization and Treatment
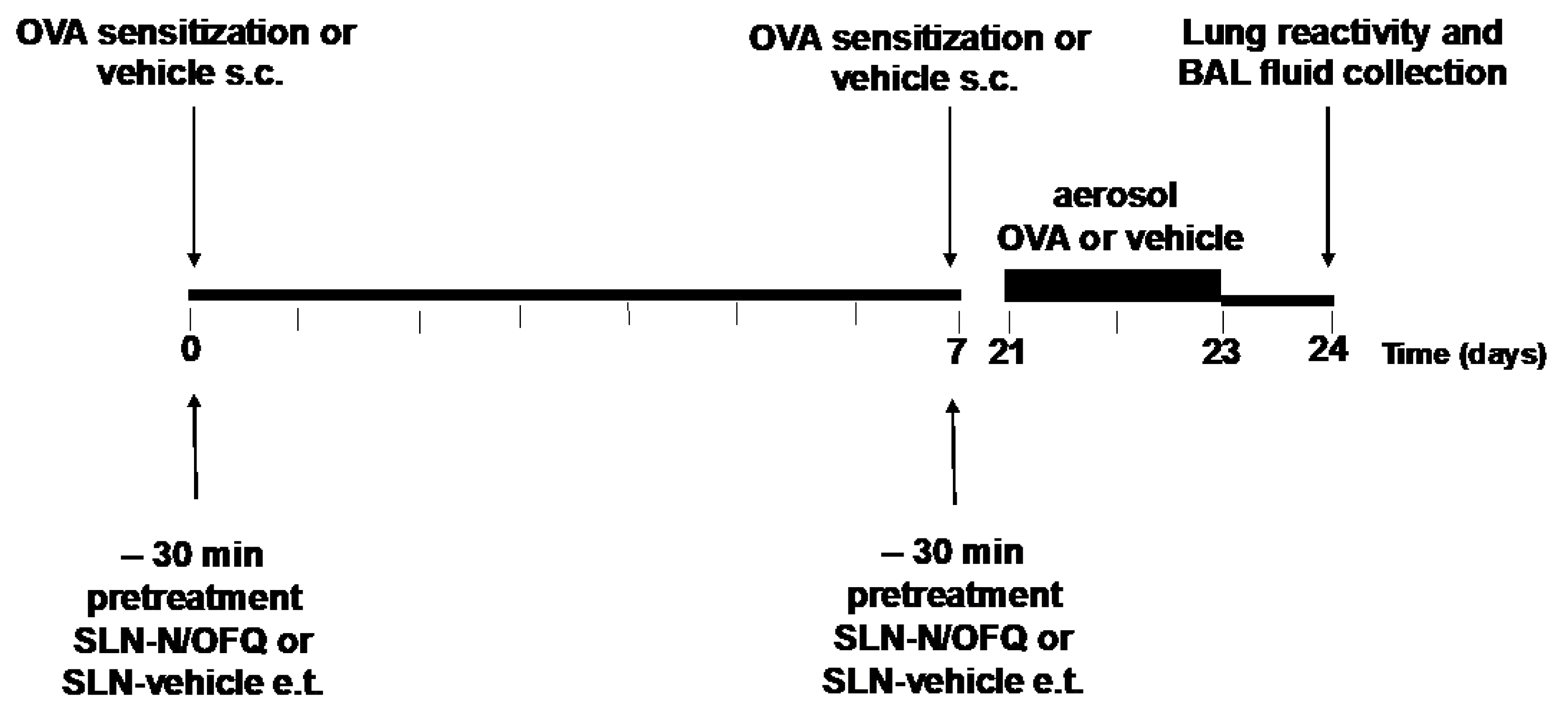
4.10. Experimental Design
4.11. Measurement of Airway Hyperresponsiveness (AHR)
4.12. Preparation and Analysis of Bronchoalveolar Lavage Fluids (BALF)
4.13. Total and Differential Cell Count
4.14. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Global Initiative for Asthma: Global Strategy for Asthma Management and Prevention. NHLBI/WHO Workshop Report. 2021. Available online: https://ginasthma.org/gina-reports/ (accessed on 28 June 2022).
- Busse, W.W.; Lemanske, R.F., Jr. Asthma. N. Engl. J. Med. 2001, 344, 350–362. [Google Scholar] [CrossRef] [PubMed]
- Errahmani, M.B.; Chebra, K.F.; Messaoudi, Z. Multidimensional analysis of genetic background and environmental factors: Cases of atopy in asthmatic children. Arch. Pediatr. 2020, 27, 315–321. [Google Scholar] [CrossRef]
- Koefoed, H.J.L.; Zwitserloot, A.M.; Vonk, J.M.; Koppelman, G.H. Asthma, bronchial hyperresponsiveness, allergy and lung function development until early adulthood: A systematic literature review. Pediatr. Allergy Immunol. 2021, 32, 1238–1254. [Google Scholar] [CrossRef] [PubMed]
- Mogil, J.S.; Pasternak, G.W. The molecular and behavioral pharmacology of the orphanin FQ/Nociceptin peptide and receptor family. Pharmacol. Rev. 2001, 53, 381–415. [Google Scholar] [PubMed]
- Wtorek, K.; Janecka, A. Potential of Nociceptin/Orphanin FQ Peptide Analogs for Drug Development. Chem. Biodivers. 2021, 18, e2000871. [Google Scholar] [CrossRef]
- Lambert, D.G. The nociceptin/orphanin FQ receptor: A target with broad therapeutic potential. Nat. Rev. Drug Discov. 2008, 7, 694–710. [Google Scholar] [CrossRef]
- Schröder, W.; Lambert, D.G.; Ko, M.C.; Koch, T. Functional plasticity of the N/OFQ-NOP receptor system determines analgesic properties of NOP receptor agonists. Br. J. Pharmacol. 2014, 171, 3777–3800. [Google Scholar] [CrossRef]
- Peiser, C.; Undem, B.J.; Fischer, A. Nociceptin effects in the airways. Peptides 2000, 21, 995–998. [Google Scholar] [CrossRef]
- D’Agostino, B.; Orlotti, D.; Calò, G.; Sullo, N.; Russo, M.; Guerrini, R.; De Nardo, M.; Mazzeo, F.; Candeletti, S.; Rossi, F. Nociceptin modulates bronchoconstriction induced by sensory nerve activation in mouse lung. Am. J. Respir. Cell Mol. Biol. 2010, 42, 250–254. [Google Scholar] [CrossRef]
- D’Agostino, B.; Marrocco, G.; De Nardo, M.; Calò, G.; Guerrini, R.; Gallelli, L.; Advenier, C.; Rossi, F. Activation of the nociceptin/orphanin FQ receptor reduces bronchoconstriction and microvascular leakage in a rabbit model of gastroesophageal reflux. Br. J. Pharmacol. 2005, 144, 813–820. [Google Scholar] [CrossRef]
- Rouget, C.; Cui, Y.Y.; D’Agostino, B.; Faisy, C.; Naline, E.; Bardou, M.; Advenier, C. Nociceptin inhibits airway microvascular leakage induced by HCl intra-oesophageal instillation. Br. J. Pharmacol. 2004, 141, 1077–1083. [Google Scholar] [CrossRef] [PubMed]
- Rizzi, A.; Malfacini, D.; Cerlesi, M.C.; Ruzza, C.; Marzola, E.; Bird, M.F.; Rowbotham, D.J.; Salvadori, S.; Guerrini, R.; Lambert, D.G.; et al. In vitro and in vivo pharmacological characterization of nociceptin/orphanin FQ tetrabranched derivatives. Br. J. Pharmacol. 2014, 171, 4138–4153. [Google Scholar] [CrossRef] [PubMed]
- Guerrini, R.; Marzola, E.; Trapella, C.; Pela’, M.; Molinari, S.; Cerlesi, M.C.; Malfacini, D.; Rizzi, A.; Salvadori, S.; Calo’, G. A novel and facile synthesis of tetra branched derivatives of nociceptin/orphanin FQ. Bioorganic Med. Chem. 2014, 22, 3703–3712. [Google Scholar] [CrossRef] [PubMed]
- Cerlesi, M.C.; Ding, H.; Bird, M.F.; Kiguchi, N.; Ferrari, F.; Malfacini, D.; Rizzi, A.; Ruzza, C.; Lambert, D.G.; Ko, M.C.; et al. Pharmacological studies on the NOP and opioid receptor agonist PWT2-[Dmt1]N/OFQ(1-13). Eur. J. Pharmacol. 2017, 794, 115–126. [Google Scholar] [CrossRef][Green Version]
- Spence, B.M.; Longest, W.; Wei, X.; Dhapare, S.; Hindle, M. Development of a High-Flow Nasal Cannula and Pharmaceutical Aerosol Combination Device. J. Aerosol Med. Pulm. Drug Deliv. 2019, 32, 224–241. [Google Scholar] [CrossRef]
- Dutta, R.; Spence, B.; Wei, X.; Dhapare, S.; Hindle, M.; Longest, P.W. CFD Guided Optimization of Nose-to-Lung Aerosol Delivery in Adults: Effects of Inhalation Waveforms and Synchronized Aerosol Delivery. Pharm. Res. 2020, 37, 199. [Google Scholar] [CrossRef]
- Longest, P.W.; Walenga, R.L.; Son, Y.J.; Hindle, M. High-efficiency generation and delivery of aerosols through nasal cannula during noninvasive ventilation. J. Aerosol Med. Pulm. Drug Deliv. 2013, 26, 266–279. [Google Scholar] [CrossRef]
- Arduin, M.; Spagnolo, B.; Calò, G.; Guerrini, R.; Carrà, G.; Fischetti, C.; Trapella, C.; Marzola, E.; McDonald, J.; Lambert, D.G.; et al. Synthesis and biological activity of nociceptin/orphanin FQ analogues substituted in position 7 or 11 with Calpha, alpha-dialkylated amino acids. Bioorganic Med. Chem. 2007, 15, 4434–4443. [Google Scholar] [CrossRef]
- Pudlarz, A.; Szemraj, J. Nanoparticles as Carriers of Proteins, Peptides and Other Therapeutic Molecules. Open Life Sci. 2018, 13, 285–298. [Google Scholar] [CrossRef]
- Tan, M.L.; Choong, P.F.; Dass, C.R. Recent developments in liposomes, microparticles and nanoparticles for protein and peptide drug delivery. Peptides 2010, 31, 184–193. [Google Scholar] [CrossRef]
- Puglia, C.; Offerta, A.; Carbone, C.; Bonina, F.; Pignatello, R.; Puglisi, G. Lipid nanocarriers (LNC) and their applications in ocular drug delivery. Curr. Med. Chem. 2015, 22, 1589–1602. [Google Scholar] [CrossRef] [PubMed]
- Kawashima, Y.; Niwa, T.; Handa, T.; Takeuchi, H.; Iwamoto, T.; Itoh, Y. Preparation of prolonged-release spherical micro-matrix of ibuprofen with acrylic polymer by the emulsion-solvent diffusion method for improving bioavailability. Chem. Pharm. Bull. 1989, 37, 425–429. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.C.; Kumari, A.; Yadav, R. Development of peptide and protein nanotherapeutics by nanoencapsulation and nanobioconjugation. Peptides 2011, 32, 173–187. [Google Scholar] [CrossRef] [PubMed]
- Moutinho, C.G.; Matos, C.M.; Teixeira, J.A.; Balcão, V.M.J. Nanocarrier possibilities for functional targeting of bioactive peptides and proteins: State-of-the-art. Drug Target. 2012, 20, 114–141. [Google Scholar] [CrossRef]
- Agrahari, V.; Agrahari, V.; Mitra, A.K. Nanocarrier fabrication and macromolecule drug delivery: Challenges and opportunities. Ther. Deliv. 2016, 7, 257–278. [Google Scholar] [CrossRef]
- Puglia, C.; Santonocito, D.; Ostacolo, C.; Maria Sommella, E.; Campiglia, P.; Carbone, C.; Drago, F.; Pignatello, R.; Bucol, C. Ocular Formulation Based on Palmitoylethanolamide-Loaded Nanostructured Lipid Carriers: Technological and Pharmacological Profile. Nanomaterials 2020, 10, 287. [Google Scholar] [CrossRef]
- Ferreira, K.C.B.; Valle, A.B.C.D.S.; Paes, C.Q.; Tavares, G.D.; Pittella, F. Nanostructured Lipid Carriers for the Formulation of Topical Anti-Inflammatory Nanomedicines Based on Natural Substances. Pharmaceutics 2021, 13, 1454. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Rodriguez, S.; Ford, W.R.; Broadley, K.J.; Kidd, E.J. Establishing the phenotype in novel acute and chronic murine models of allergic asthma. Int. Immunopharmacol. 2008, 8, 756–763. [Google Scholar] [CrossRef]
- Shin, Y.S.; Takeda, K.; Gelfand, E.W. Understanding asthma using animal models. Allergy Asthma Immunol. Res. 2009, 1, 10–18. [Google Scholar] [CrossRef]
- Klaudel, L.; Legowska, A.; Brzozowski, K.; Silberring, J.; Wójcik, J. Solution conformational study of nociception an(d its 1-13 and 1-11 fragments using circular dichroism and two-dimensional NMR in conjunction with theoretical conformational analysis. J. Pept. Sci. 2004, 10, 678–690. [Google Scholar] [CrossRef]
- Liparulo, A.; Esposito, R.; Santonocito, D.; Muñoz-Ramírez, A.; Spaziano, G.; Bruno, F.; Xiao, J.; Puglia, C.; Filosa, R.; Berrino, L.; et al. Formulation and Characterization of Solid Lipid Nanoparticles Loading RF22-c, a Potent and Selective 5-LO Inhibitor, in a Monocrotaline-Induced Model of Pulmonary Hypertension. Front. Pharmacol. 2020, 11, 83. [Google Scholar] [CrossRef] [PubMed]
- Patlolla, R.R.; Chougule, M.; Patel, A.R.; Jackson, T.; Tata, P.N.; Singh, M. Formulation, characterization and pulmonary deposition of nebulized celecoxib encapsulated nanostructured lipid carriers. J. Control. Release 2010, 144, 233–241. [Google Scholar] [CrossRef]
- Ahmed, M.M.; Fatima, F.; Anwer, M.K.; Aldawsari, M.F.; Alsaidan, Y.S.M.; Alfaiz, S.A.; Haque, A.; Az, A.; Alhazzani, K. Development and characterization of Brigatinib loaded solid lipid nanoparticles: In-vitro cytotoxicity against human carcinoma A549 lung cell lines. Chem. Phys. Lipids 2020, 233, 105003. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Wu, M.; Ye, W.; Huang, Z.; Ma, X.; Wang, W.; Wang, W.; Huang, Y.; Pan, X.; Wu, C. Inhalable solid lipid nanoparticles for intracellular tuberculosis infection therapy: Macrophage-targeting and pH-sensitive properties. Drug Deliv. Transl. Res. 2021, 11, 1218–1235. [Google Scholar] [CrossRef] [PubMed]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriguez-Torres, M.D.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.F.; Liu, W.H.; Song, W.S.; Chiang, K.L.; Ma, H.I.; Kao, C.L.; Chen, M.T. Nanomedicine-based neuroprotective strategies in patient specific-iPSC and personalized medicine. Int. J. Mol. Sci. 2014, 15, 3904–3925. [Google Scholar] [CrossRef] [PubMed]
- Jain, K.K. An Overview of Drug Delivery Systems. Methods Mol. Biol. 2020, 2059, 1–54. [Google Scholar] [CrossRef]
- Mukherjee, S.; Ray, S.; Thakur, R.S. Solid lipid nanoparticles: A modern formulation approach in drug delivery system. Indian J. Pharm. Sci. 2009, 71, 349–358. [Google Scholar] [CrossRef]
- Rostami, E.; Kashanian, S.; Azandaryani, A.H.; Faramarzi, H.; Dolatabadi, J.E.; Omidfar, K. Drug targeting using solid lipid nanoparticles. Chem. Phys. Lipids. 2014, 181, 56–61. [Google Scholar] [CrossRef]
- Lv, C.; Li, H.; Cui, H.; Bi, Q.; Wang, M. Solid lipid nanoparticle delivery of rhynchophylline enhanced the efficiency of allergic asthma treatment via the upregulation of suppressor of cytokine signaling 1 by repressing the p38 signaling pathway. Bioengineered 2021, 12, 8635–8649. [Google Scholar] [CrossRef]
- Wang, W.; Zhu, R.; Xie, Q.; Li, A.; Xiao, Y.; Li, K.; Liu, H.; Cui, D.; Chen, Y.; Wang, S. Enhanced bioavailability and efficiency of curcumin for the treatment of asthma by its formulation in solid lipid nanoparticles. Int. J. Nanomed. 2012, 7, 3667–3677. [Google Scholar] [CrossRef]
- Prasanna, P.; Rathee, S.; Upadhyay, A.; Sulakshana, S. Nanotherapeutics in the treatment of acute respiratory distress syndrome. Life Sci. 2021, 276, 119428. [Google Scholar] [CrossRef]
- Castellani, S.; Trapani, A.; Spagnoletta, A.; di Toma, L.; Magrone, T.; Di Gioia, S.; Mandracchia, D.; Trapani, G.; Jirillo, E.; Conese, M. Nanoparticle delivery of grape seed-derived proanthocyanidins to airway epithelial cells dampens oxidative stress and inflammation. J. Transl. Med. 2018, 16, 140. [Google Scholar] [CrossRef]
- Uhlig, S.; Wollin, L. An improved setup for the isolated perfused rat lung. J. Pharmacol. Toxicol. Methods 1994, 31, 85–94. [Google Scholar] [CrossRef]
- Spaziano, G.; Cappetta, D.; Urbanek, K.; Piegari, E.; Esposito, G.; Matteis, M.; Sgambato, M.; Tartaglione, G.; Russo, R.; De Palma, R.; et al. New Role of Adult Lung c-kit+ Cells in a Mouse Model of Airway Hyperresponsiveness. Mediat. Inflamm. 2016, 2016, 3917471. [Google Scholar] [CrossRef]
- Singh, S.R.; Sullo, N.; Matteis, M.; Spaziano, G.; McDonald, J.; Saunders, R.; Woodman, L.; Urbanek, K.; De Angelis, A.; De Palma, R.; et al. Nociceptin/orphanin FQ (N/OFQ) modulates immunopathology and airway hyperresponsiveness representing a novel target for the treatment of asthma. Br. J. Pharmacol. 2016, 173, 1286–1301. [Google Scholar] [CrossRef]
- McHugh, M.L. Multiple comparison analysis testing in ANOVA. Biochem. Med. 2011, 21, 203–209. [Google Scholar] [CrossRef]
- Armstrong, R.A. When to use the Bonferroni correction. Ophthalmic Physiol. Opt. 2014, 34, 502–508. [Google Scholar] [CrossRef]



| Formulation | Z-Ave (nm ± SD) | PDI (-) ± SD | ZP (mV ± SD) |
|---|---|---|---|
| Unloaded SLN | 248.6 ± 0.10 | 0.289 ± 0.16 | −27.6 ± 0.32 |
| SLN-N/OFQ | 239.6 ± 0.12 | 0.479 ± 0.18 | −29.5 ± 0.30 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mirra, D.; Spaziano, G.; Esposito, R.; Santonocito, D.; Filosa, R.; Roviezzo, F.; Malgieri, G.; D’Abrosca, G.; Iovino, P.; Gallelli, L.; et al. Formulation of Solid Lipid Nanoparticles Loaded with Nociceptin/Orphanin FQ (N/OFQ) and Characterization in a Murine Model of Airway Hyperresponsiveness. Pharmaceuticals 2022, 15, 1210. https://doi.org/10.3390/ph15101210
Mirra D, Spaziano G, Esposito R, Santonocito D, Filosa R, Roviezzo F, Malgieri G, D’Abrosca G, Iovino P, Gallelli L, et al. Formulation of Solid Lipid Nanoparticles Loaded with Nociceptin/Orphanin FQ (N/OFQ) and Characterization in a Murine Model of Airway Hyperresponsiveness. Pharmaceuticals. 2022; 15(10):1210. https://doi.org/10.3390/ph15101210
Chicago/Turabian StyleMirra, Davida, Giuseppe Spaziano, Renata Esposito, Debora Santonocito, Rosanna Filosa, Fiorentina Roviezzo, Gaetano Malgieri, Gianluca D’Abrosca, Pasquale Iovino, Luca Gallelli, and et al. 2022. "Formulation of Solid Lipid Nanoparticles Loaded with Nociceptin/Orphanin FQ (N/OFQ) and Characterization in a Murine Model of Airway Hyperresponsiveness" Pharmaceuticals 15, no. 10: 1210. https://doi.org/10.3390/ph15101210
APA StyleMirra, D., Spaziano, G., Esposito, R., Santonocito, D., Filosa, R., Roviezzo, F., Malgieri, G., D’Abrosca, G., Iovino, P., Gallelli, L., Fattorusso, R., Puglia, C., & D’Agostino, B. (2022). Formulation of Solid Lipid Nanoparticles Loaded with Nociceptin/Orphanin FQ (N/OFQ) and Characterization in a Murine Model of Airway Hyperresponsiveness. Pharmaceuticals, 15(10), 1210. https://doi.org/10.3390/ph15101210








