The Antimicrobial Effects of Saudi Sumra Honey against Drug Resistant Pathogens: Phytochemical Analysis, Antibiofilm, Anti-Quorum Sensing, and Antioxidant Activities
Abstract
1. Introduction
2. Results
2.1. Antibacterial Potential of Sumra Honey
2.2. Antifungal Activity of Sumra Honey
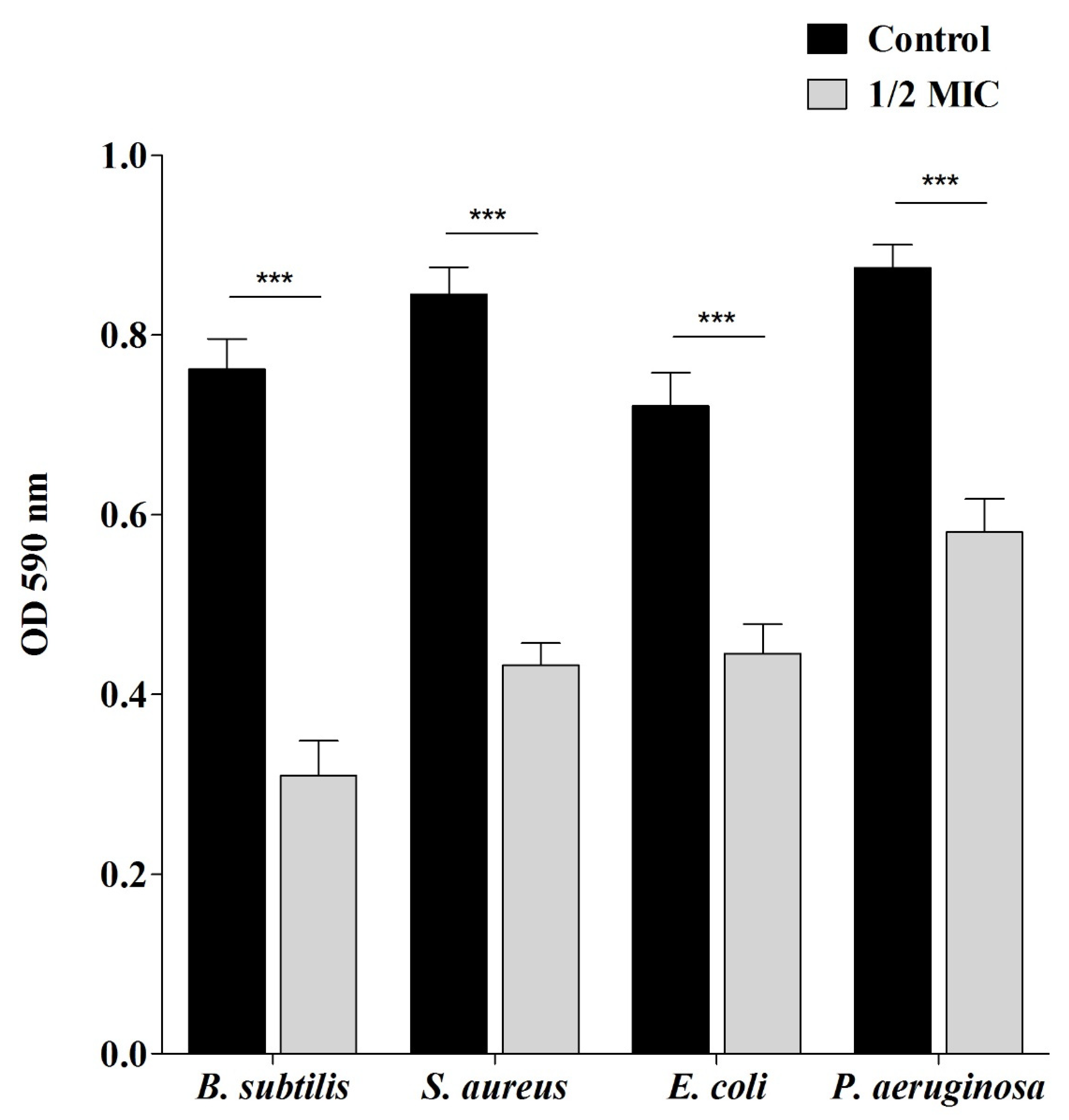
2.3. Antibiofilm Properties of Sumra Honey
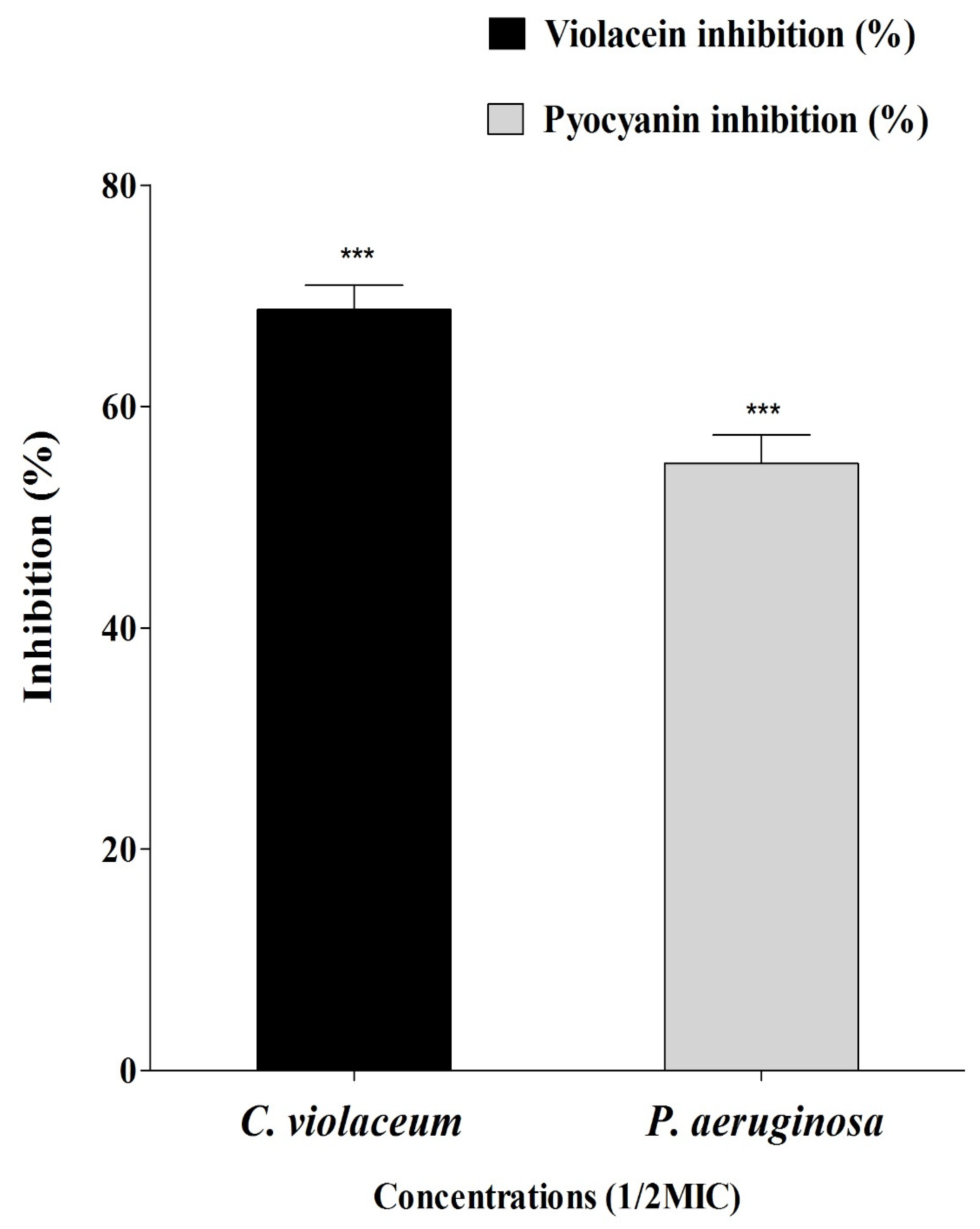
2.4. Anti-Quorum Sensing Properties of Sumra Honey
2.5. Antioxidant Properties of Sumra Honey
2.6. Identification of Bioactive Constituents from Sumra Honey by Gas Chromatography-Mass Spectrometry (GC-MS)
3. Discussion
4. Materials and Methods
4.1. Screening of Antibacterial and Antifungal Activity of Honey Sample
4.1.1. Honey Sample, Bacterial, and Fungal Strains
4.1.2. Determination of Minimum Inhibitory Concentration (MIC) against Bacterial Isolates
4.1.3. Determination of Minimum Inhibitory Concentration (MIC) against Fungal Isolates
4.1.4. Determination of Minimum Bactericidal Concentration (MBC)
4.2. Antibiofilm Assay of Sumra Honey
4.3. Inhibition of Quorum Sensing by Sumra Honey
4.4. Violacein Inhibition Assay in C. violaceum
4.5. Pyocyanin Inhibition Assay in P. aeruginosa
4.6. Antioxidant Assays of Sumra Honey
4.6.1. Scavenging Activity of DPPH Free Radicals
(Acontrol)
4.6.2. Scavenging Activity of ABTS Free Radicals
(Acontrol)
4.7. β-Carotene Bleaching Assay
(Bcontrol)
4.8. Gas Chromatography-Mass Spectrophotometry (GC–MS) Analysis
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- O’Neill, J. Antimicrobial Resistance: Tackling a Crisis for the Health and Wealth of Nations; The Review on Antimicrobial Resistance: London, UK, 2014. [Google Scholar]
- Obey, J.K.; Ngeiywa, M.M.; Lehesvaara, M.; Kauhanen, J.; von Wright, A.; Tikkanen-Kaukanen, C. Antimicrobial activity of commercial organic honeys against clinical isolates of human pathogenic bacteria. Org. Agric. 2022, 12, 267–277. [Google Scholar] [CrossRef]
- Garcia, M.; Lipskiy, N.; Tyson, J.; Watkins, R.; Esser, E.S.; Kinley, T. Centers for Disease Control and Prevention 2019 novel coronavirus disease (COVID-19) information management: Addressing national health-care and public health needs for standardized data definitions and codified vocabulary for data exchange. J. Am. Med. Inf. Assoc. 2020, 27, 1476–1487. [Google Scholar] [CrossRef] [PubMed]
- European Centre for Disease Prevention and Control; World Health Organization; Regional Office for Europe. Antimicrobial Resistance Surveillance in Europe 2022—2020 Data; World Health Organization: Geneva, Switzerland; Regional Office for Europe: Copenhagen, Denmark, 2022.
- Bazaid, A.S.; Saeed, A.; Alrashidi, A.; Alrashidi, A.; Alshaghdali, K.; Hammam, S.A.; Alreshidi, T.; Alshammary, M.; Alarfaj, A.; Thallab, R.; et al. Antimicrobial Surveillance for Bacterial Uropathogens in Ha’il, Saudi Arabia: A Five-Year Multicenter Retrospective Study. Infect. Drug. Resist. 2021, 14, 1455–1465. [Google Scholar] [CrossRef] [PubMed]
- Bazaid, A.S.; Aldarhami, A.; Gattan, H.; Barnawi, H.; Qanash, H.; Alsaif, G.; Alharbi, B.; Alrashidi, A.; Eldrehmy, E.H. Antibiogram of Urinary Tract Infections and Sepsis among Infants in Neonatal Intensive Care Unit. Children 2022, 9, 629. [Google Scholar] [CrossRef]
- Bazaid, A.S.; Barnawi, H.; Qanash, H.; Alsaif, G.; Aldarhami, A.; Gattan, H.; Alharbi, B.; Alrashidi, A.; Al-Soud, W.A.; Moussa, S.; et al. Bacterial Coinfection and Antibiotic Resistance Profiles among Hospitalised COVID-19 Patients. Microorganisms 2022, 10, 495. [Google Scholar] [CrossRef]
- Bush, K.; Bradford, P.A. β-Lactams and β-Lactamase Inhibitors: An Overview. Cold Spring Harb. Perspect. Med. 2016, 6, a025247. [Google Scholar] [CrossRef]
- Romero-Calle, D.; Guimarães Benevides, R.; Góes-Neto, A.; Billington, C. Bacteriophages as Alternatives to Antibiotics in Clinical Care. Antibiotics 2019, 8, 138. [Google Scholar] [CrossRef]
- Harman, R.M.; Yang, S.; He, M.K.; Van de Walle, G.R. Antimicrobial peptides secreted by equine mesenchymal stromal cells inhibit the growth of bacteria commonly found in skin wounds. Stem. Cell Res. Ther. 2017, 8, 157. [Google Scholar] [CrossRef]
- Bazaid, A.S.; Aldarhami, A.; Gattan, H.; Aljuhani, B. Saudi Honey: A Promising Therapeutic Agent for Treating Wound Infections. Cureus 2021, 13, e18882. [Google Scholar] [CrossRef]
- Qanash, H.; Yahya, R.; Bakri, M.M.; Bazaid, A.S.; Qanash, S.; Shater, A.F.; Abdelghany, T.M. Anticancer, antioxidant, antiviral and antimicrobial activities of Kei Apple (Dovyalis caffra) fruit. Sci. Rep. 2022, 12, 5914. [Google Scholar] [CrossRef]
- Abdelghany, T.M.; Yahya, R.; Bakri, M.M.; Ganash, M.; Amin, B.H.; Qanash, H. Effect of Thevetia peruviana seeds extract for microbial pathogens and cancer control. Int. J. Pharmacol. 2021, 17, 643–655. [Google Scholar] [CrossRef]
- Saeed, F.; Afzaal, M.; Tufail, T.; Ahmad, A. Use of Natural Antimicrobial Agents: A Safe Preservation Approach; Active Antimicrobial Food Packaging (IntechOpen): London, UK, 2019. [Google Scholar]
- Ye, G.; Wu, H.; Huang, J.; Wang, W.; Ge, K.; Li, G.; Zhong, J.; Huang, Q. LAMP2: A major update of the database linking antimicrobial peptides. Database 2020, 2020. [Google Scholar] [CrossRef]
- Dixon, B. Bacteria can’t resist honey. Lancet Infect. Dis. 2003, 3, 116. [Google Scholar] [CrossRef]
- Al-Ghamdi, A.A.; Ansari, M.J. Biological and therapeutic roles of Saudi Arabian honey: A comparative review. J. King Saud. Univ.-Sci. 2021, 33, 101329. [Google Scholar] [CrossRef]
- Al-Rajhi, A.M.H.; Qanash, H.; Almuhayawi, M.S.; Al Jaouni, S.K.; Bakri, M.M.; Ganash, M.; Salama, H.M.; Selim, S.; Abdelghany, T.M. Molecular Interaction Studies and Phytochemical Characterization of Mentha pulegium L. Constituents with Multiple Biological Utilities as Antioxidant, Antimicrobial, Anticancer and Anti-Hemolytic Agents. Molecules 2022, 27, 4824. [Google Scholar] [CrossRef]
- Samarghandian, S.; Farkhondeh, T.; Samini, F. Honey and Health: A Review of Recent Clinical Research. Pharmacogn. Res. 2017, 9, 121–127. [Google Scholar] [CrossRef]
- Mandal, M.D.; Mandal, S. Honey: Its medicinal property and antibacterial activity. Asian Pac. J. Trop. Biomed. 2011, 1, 154–160. [Google Scholar] [CrossRef]
- Maddocks, S.E.; Jenkins, R.E. Honey: A sweet solution to the growing problem of antimicrobial resistance? Future Microbiol. 2013, 8, 1419–1429. [Google Scholar] [CrossRef]
- Cooper, R.A.; Jenkins, L.; Henriques, A.F.; Duggan, R.S.; Burton, N.F. Absence of bacterial resistance to medical-grade manuka honey. Eur. J. Clin. Microbiol. Infect. Dis. 2010, 29, 1237–1241. [Google Scholar] [CrossRef]
- Visavadia, B.G.; Honeysett, J.; Danford, M.H. Manuka honey dressing: An effective treatment for chronic wound infections. Br. J. Oral Maxillofac. Surg. 2008, 46, 55–56. [Google Scholar] [CrossRef]
- Lusby, P.E.; Coombes, A.L.; Wilkinson, J.M. Bactericidal activity of different honeys against pathogenic bacteria. Arch. Med. Res. 2005, 36, 464–467. [Google Scholar] [CrossRef] [PubMed]
- Hegazi, A.G.; Guthami, F.M.; Gethami, A.F.; Allah, F.M.; Saleh, A.A.; Fouad, E.A. Potential antibacterial activity of some Saudi Arabia honey. Vet. World 2017, 10, 233–237. [Google Scholar] [CrossRef]
- Al-Nahari, A.A.; Almasaudi, S.B.; Abd El-Ghany El, S.M.; Barbour, E.; Al Jaouni, S.K.; Harakeh, S. Antimicrobial activities of Saudi honey against Pseudomonas aeruginosa. Saudi. J. Biol. Sci. 2015, 22, 521–525. [Google Scholar] [CrossRef]
- Halawani, E.; Shohayeb, M. Survey of the antibacterial activity of Saudi and some international honeys. J. Microbiol. Antimicrob. 2011, 3, 94–101. [Google Scholar]
- Molan, P.C.; Cooper, R.A. Honey and sugar as a dressing for wounds and ulcers. Trop. Doct. 2000, 30, 249–250. [Google Scholar] [CrossRef] [PubMed]
- Alnaimat, S.; Wainwright, M.; Al’Abri, K. Antibacterial potential of honey from different origins: A comparison with Manuka honey. J. Microbiol. Biotechnol. Food Sci. 2012, 1, 1328–1338. [Google Scholar]
- Mohd Ramli, E.S.; Sukalingam, K.; Kamaruzzaman, M.A.; Soelaiman, I.N.; Pang, K.L.; Chin, K.Y. Direct and Indirect Effect of Honey as a Functional Food Against Metabolic Syndrome and Its Skeletal Complications. Diabetes Metab. Syndr. Obes. 2021, 14, 241–256. [Google Scholar] [CrossRef]
- Anand, S.; Deighton, M.; Livanos, G.; Morrison, P.D.; Pang, E.C.K.; Mantri, N. Antimicrobial Activity of Agastache Honey and Characterization of Its Bioactive Compounds in Comparison With Important Commercial Honeys. Front. Microbiol. 2019, 10, 263. [Google Scholar] [CrossRef]
- Bogdanov, S. Non-Peroxide Antibacterial Activity of Honey; Springer: Boston, MA, USA, 1997; pp. 39–47. [Google Scholar]
- Libonatti, C.; Varela, S.; Basualdo, M. Antibacterial activity of honey: A review of honey around the world. J. Microbiol. Antimicrob. 2014, 6, 51–56. [Google Scholar] [CrossRef]
- Weston, R.J. The contribution of catalase and other natural products to the antibacterial activity of honey: A review. Food Chem. 2000, 71, 235–239. [Google Scholar] [CrossRef]
- Althagafi, I.; El-Metwaly, N.; Farghaly, T.A. New Series of Thiazole Derivatives: Synthesis, Structural Elucidation, Antimicrobial Activity, Molecular Modeling and MOE Docking. Molecules 2019, 24, 1741. [Google Scholar] [CrossRef] [PubMed]
- Estrada, H.; Gamboa Mdel, M.; Arias, M.L.; Chaves, C. Evaluation of the antimicrobial action of honey against Staphylococcus aureus, Staphylococcus epidermidis, Pseudomonas aeruginosa, Escherichia coli, Salmonella enteritidis, Listeria monocytogenes and Aspergillus niger. Evaluation of its microbiological charge. Arch. Lat. Nutr. 2005, 55, 167–171. [Google Scholar]
- Ilyasov, R.; Gaifullina, L.; Saltykova, E.; Poskryakov, A.; Nikolenko, A. Review of the Expression of Antimicrobial Peptide Defensin in Honey Bees Apis mellifera L. J. Apic. Sci. 2012, 56, 115–124. [Google Scholar] [CrossRef]
- Kwakman, P.H.; Zaat, S.A. Antibacterial components of honey. IUBMB Life 2012, 64, 48–55. [Google Scholar] [CrossRef]
- Klaudiny, J.; Albert, S.; Bachanová, K.; Kopernický, J.; Simúth, J. Two structurally different defensin genes, one of them encoding a novel defensin isoform, are expressed in honeybee Apis mellifera. Insect. Biochem. Mol. Biol. 2005, 35, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Yaouba, S.; Koch, A.; Guantai, E.M.; Derese, S.; Irungu, B.; Heydenreich, M.; Yenesew, A. Alkenyl cyclohexanone derivatives from Lannea rivae and Lannea schweinfurthii. Phytochem. Lett. 2018, 23, 141–148. [Google Scholar] [CrossRef]
- Alsamarrai, A.S.H.; Abdulghani, S.S. Microwave-Assisted Synthesis, Structural Characterization and Assessment of the Antibacterial Activity of Some New Aminopyridine, Pyrrolidine, Piperidine and Morpholine Acetamides. Molecules 2021, 26, 533. [Google Scholar] [CrossRef]
- Alsayari, A.; Asiri, Y.I.; Muhsinah, A.B.; Hassan, M.Z. Synthesis and antimicrobial activity of aryldiazenyl/arylhydrazono pyrazoles. J. Chem. Res. 2021, 45, 1093–1099. [Google Scholar] [CrossRef]
- Spassov, A.; Golovinsky, E.; Spassovska, N.; Maneva, L. Effect of Thio- and Hydrazino-derivatives of Uracil, 6-Azauracil and 6-Azathymine on the Growth of Some Microorganisms in Vitro. Z. Für. Nat. B 1972, 27, 818–821. [Google Scholar] [CrossRef][Green Version]
- Kalt, F.R.; Cock, I.E. Gas chromatography-mass spectroscopy analysis of bioactive petalostigma extracts: Toxicity, antibacterial and antiviral activities. Pharm. Mag. 2014, 10, S37–S49. [Google Scholar] [CrossRef]
- Hattori, N.; Nakajima, M.O.; O’Hara, K.; Sawai, T. Novel antibiotic susceptibility tests by the ATP-bioluminescence method using filamentous cell treatment. Antimicrob. Agents. Chemother. 1998, 42, 1406–1411. [Google Scholar] [CrossRef] [PubMed]
- Kabuki, T.; Uenishi, H.; Seto, Y.; Yoshioka, T.; Nakajima, H. A unique lantibiotic, thermophilin 1277, containing a disulfide bridge and two thioether bridges. J. Appl. Microbiol. 2009, 106, 853–862. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Carr, P.; Gardiner, M.G.; Banwell, M.G.; Elbanna, A.H.; Khalil, Z.G.; Capon, R.J. Levoglucosenone and Its Pseudoenantiomer iso-Levoglucosenone as Scaffolds for Drug Discovery and Development. ACS Omega 2020, 5, 13926–13939. [Google Scholar] [CrossRef] [PubMed]
- Rashid, T.; Sijam, K.; Kadir, J.; Halimi, M.; Awla, H.; Zulperi, D.; Mohd Hata, E. Screening for active compounds in Rhus coriaria L. crude extract that inhibit the growth of Pseudomonas syringae and Ralstonia solanacearum. Indian J. Agric. Res. 2016, 50, 1–7. [Google Scholar] [CrossRef][Green Version]
- Alandejani, T.; Marsan, J.; Ferris, W.; Slinger, R.; Chan, F. Effectiveness of honey on Staphylococcus aureus and Pseudomonas aeruginosa biofilms. Otolaryngol. Head. Neck Surg. 2009, 141, 114–118. [Google Scholar] [CrossRef]
- Jervis-Bardy, J.; Foreman, A.; Bray, S.; Tan, L.; Wormald, P.J. Methylglyoxal-infused honey mimics the anti-Staphylococcus aureus biofilm activity of manuka honey: Potential implication in chronic rhinosinusitis. Laryngoscope 2011, 121, 1104–1107. [Google Scholar] [CrossRef]
- Anyanwu, C. Investigation of in vitro antifungal activity of honey. J. Med. Plants Res. 2012, 6, 3512–3516. [Google Scholar] [CrossRef]
- de Groot, T.; Janssen, T.; Faro, D.; Cremers, N.A.J.; Chowdhary, A.; Meis, J.F. Antifungal Activity of a Medical-Grade Honey Formulation against Candida auris. J. Fungi 2021, 7, 50. [Google Scholar] [CrossRef]
- Moussa, A.; Noureddine, D.; Saad, A.; Abdelmelek, M.; Abdelkader, B. Antifungal activity of four honeys of different types from Algeria against pathogenic yeast: Candida albicans and Rhodotorula sp. Asian Pac. J. Trop. Biomed. 2012, 2, 554–557. [Google Scholar] [CrossRef]
- Khosravi, A.R.; Shokri, H.; Katiraee, F.; Ziglari, T.; Forsi, M. Fungicidal potential of different Iranian honeys against some pathogenic Candida species. J. Apic. Res. 2008, 47, 256–260. [Google Scholar] [CrossRef]
- Estevinho, M.L.; Afonso, S.E.; Feás, X. Antifungal effect of lavender honey against Candida albicans, Candida krusei and Cryptococcus neoformans. J. Food Sci. Technol. 2011, 48, 640–643. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.B.; Bassler, B.L. Quorum sensing in bacteria. Annu. Rev. Microbiol. 2001, 55, 165–199. [Google Scholar] [CrossRef]
- Hawver, L.A.; Jung, S.A.; Ng, W.L. Specificity and complexity in bacterial quorum-sensing systems. FEMS Microbiol. Rev. 2016, 40, 738–752. [Google Scholar] [CrossRef]
- Liu, L.; Wu, R.; Zhang, J.; Shang, N.; Li, P. D-Ribose Interferes with Quorum Sensing to Inhibit Biofilm Formation of Lactobacillus paraplantarum L-ZS9. Front. Microbiol. 2017, 8, 1860. [Google Scholar] [CrossRef]
- Jiang, Q.; Chen, J.; Yang, C.; Yin, Y.; Yao, K. Quorum Sensing: A Prospective Therapeutic Target for Bacterial Diseases. Biomed. Res. Int. 2019, 2019, 2015978. [Google Scholar] [CrossRef] [PubMed]
- Alzahrani, H.A.; Boukraa, L.; Bellik, Y.; Abdellah, F.; Bakhotmah, B.A.; Kolayli, S.; Sahin, H. Evaluation of the antioxidant activity of three varieties of honey from different botanical and geographical origins. Glob. J. Health Sci. 2012, 4, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Badolato, M.; Carullo, G.; Cione, E.; Aiello, F.; Caroleo, M.C. From the hive: Honey, a novel weapon against cancer. Eur. J. Med. Chem. 2017, 142, 290–299. [Google Scholar] [CrossRef] [PubMed]
- Sant’Ana, L.D.; Sousa, J.P.; Salgueiro, F.B.; Lorenzon, M.C.; Castro, R.N. Characterization of monofloral honeys with multivariate analysis of their chemical profile and antioxidant activity. J. Food Sci. 2012, 77, C135–C140. [Google Scholar] [CrossRef]
- Can, Z.; Yildiz, O.; Sahin, H.; Akyuz Turumtay, E.; Silici, S.; Kolayli, S. An investigation of Turkish honeys: Their physico-chemical properties, antioxidant capacities and phenolic profiles. Food Chem. 2015, 180, 133–141. [Google Scholar] [CrossRef]
- Stagos, D.; Soulitsiotis, N.; Tsadila, C.; Papaeconomou, S.; Arvanitis, C.; Ntontos, A.; Karkanta, F.; Adamou-Androulaki, S.; Petrotos, K.; Spandidos, D.A.; et al. Antibacterial and antioxidant activity of different types of honey derived from Mount Olympus in Greece. Int. J. Mol. Med. 2018, 42, 726–734. [Google Scholar] [CrossRef]
- Chan, C.W.; Deadman, B.J.; Manley-Harris, M.; Wilkins, A.L.; Alber, D.G.; Harry, E. Analysis of the flavonoid component of bioactive New Zealand mānuka (Leptospermum scoparium) honey and the isolation, characterisation and synthesis of an unusual pyrrole. Food Chem. 2013, 141, 1772–1781. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Suarez, J.M.; Gasparrini, M.; Forbes-Hernández, T.Y.; Mazzoni, L.; Giampieri, F. The Composition and Biological Activity of Honey: A Focus on Manuka Honey. Foods 2014, 3, 420–432. [Google Scholar] [CrossRef]
- Stephens, J.M.; Schlothauer, R.C.; Morris, B.D.; Yang, D.; Fearnley, L.; Greenwood, D.R.; Loomes, K.M. Phenolic compounds and methylglyoxal in some New Zealand manuka and kanuka honeys. Food Chem. 2010, 120, 78–86. [Google Scholar] [CrossRef]
- Bazaid, A.S.; Forbes, S.; Humphreys, G.J.; Ledder, R.G.; O’Cualain, R.; McBain, A.J. Fatty Acid Supplementation Reverses the Small Colony Variant Phenotype in Triclosan-Adapted Staphylococcus aureus: Genetic, Proteomic and Phenotypic Analyses. Sci. Rep. 2018, 8, 3876. [Google Scholar] [CrossRef] [PubMed]
- Lemos, A.S.O.; Campos, L.M.; Melo, L.; Guedes, M.; Oliveira, L.G.; Silva, T.P.; Melo, R.C.N.; Rocha, V.N.; Aguiar, J.A.K.; Apolônio, A.C.M.; et al. Antibacterial and Antibiofilm Activities of Psychorubrin, a Pyranonaphthoquinone Isolated From Mitracarpus frigidus (Rubiaceae). Front. Microbiol. 2018, 9, 724. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.; Siddiqui, A.J.; Hamadou, W.S.; Surti, M.; Awadelkareem, A.M.; Ashraf, S.A.; Alreshidi, M.; Snoussi, M.; Rizvi, S.M.D.; Bardakci, F.; et al. Inhibition of Bacterial Adhesion and Antibiofilm Activities of a Glycolipid Biosurfactant from Lactobacillus rhamnosus with Its Physicochemical and Functional Properties. Antibiotics 2021, 10, 1546. [Google Scholar] [CrossRef]
- Fidaleo, M.; Lavecchia, R.; Zuorro, A. Antibacterial and anti-quorum sensing activities of selected Italian honeys against antibiotic-resistant pathogens. Online J. Biol. Sci. 2015, 15, 236–243. [Google Scholar] [CrossRef]
- Blosser, R.S.; Gray, K.M. Extraction of violacein from Chromobacterium violaceum provides a new quantitative bioassay for N-acyl homoserine lactone autoinducers. J. Microbiol. Methods 2000, 40, 47–55. [Google Scholar] [CrossRef]
- Isla, M.I.; Craig, A.; Ordoñez, R.; Zampini, C.; Sayago, J.; Bedascarrasbure, E.; Alvarez, A.; Salomón, V.; Maldonado, L. Physico chemical and bioactive properties of honeys from Northwestern Argentina. LWT-Food Sci. Technol. 2011, 44, 1922–1930. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Ferreira, I.C.F.R.; Aires, E.; Barreira, J.C.M.; Estevinho, L.M. Antioxidant activity of Portuguese honey samples: Different contributions of the entire honey and phenolic extract. Food Chem. 2009, 114, 1438–1443. [Google Scholar] [CrossRef]
| Bacterial Isolates | Gram-Stain | MIC (mg/mL) | MBC (mg/mL) | MBC/MIC Ratio |
|---|---|---|---|---|
| Clinical Isolates | ||||
| Staphylococcus aureus | Positive | 300 | 350 | 1.16 |
| Methicillin-resistant Staphylococcus aureus- Uni 1 | Positive | 300 | >450 | >1.5 |
| Methicillin-resistant Staphylococcus aureus- Uni 2 | Positive | 250 | >450 | >1.8 |
| Pseudomonas aeruginosa | Negative | 250 | 300 | 1.2 |
| Escherichia coli | Negative | 350 | >450 | >1.28 |
| Acinetobacter baumannii | Negative | 250 | 450 | 1.8 |
| Reference isolates | ||||
| Bacillus subtilis MTCC 121 | Positive | 80 | 100 | 1.25 |
| Staphylococcus aureus MTCC 96 | Positive | 90 | 150 | 1.66 |
| Escherichia coli MTCC 9537 | Negative | 100 | 200 | 2.0 |
| Pseudomonas aeruginosa MTCC 741 | Negative | 120 | 250 | 2.08 |
| Fungal Strains | MIC (mg/mL) |
|---|---|
| Clinical isolates | |
| Candida auris | 600 |
| Cryptococcus neoformans | ≥1000 |
| Reference strains | |
| Candida krusei CCUG 74256 | >1000 |
| Candida albicans CCUG 74255 | 700 |
| DPPH IC50 (mg/mL) | ABTS IC50 (mg/mL) | β-Carotene IC50 (mg/mL) | |
|---|---|---|---|
| Sumra Honey | 7.7 | 5.4 | >20 |
| Ascorbic acid | 0.023 | 0.021 | 0.017 |
| Identified Compound | Class | Area (%) | Retention Time [min] | Molecular Weight [g/mol] |
|---|---|---|---|---|
| 5-Methyl-2-ethylamino-2-thiazoline | Amino acids | 10.59 | 7.223 | 144.24 |
| 4-Hydroxy-1-[4-(hydroxymethyl)-3,6- dioxabicyclo[3.1.0]hexan-2-yl]-5-methylpyrimidin-2-one | Organic compound | 3.93 | 8.515 | 152.15 |
| 2-chloro-Propanoic acid | Organic Acids | 3.31 | 1.555 | 108.52 |
| Cyclohexanone | Ketone | 2.84 | 11.63 | 98.14 |
| 4-[3-(4-Fluorobenzyloxy)propyl]-1H-imidazole | Fatty acids | 2.11 | 14.124 | 234.27 |
| Dimethyl (R)-(+)-malate, O-ethoxycarbonyl- | Organic acids | 2.05 | 8.775 | 162.14 |
| Bicyclo[2.2.1]heptane-1-carboxylic 7,7-dimethyl- | Acid esters | 1.36 | 11.81 | 557.6 |
| 2,4-Dihydroxy-2,5-dimethyl-3(2H)-furan-3-one | Ketone | 1.34 | 5.623 | 144.12 |
| 1-Methylcyclopropanemethanol | Amino acids | 1.18 | 3.199 | 86.13 |
| Acetamide | Organic Acids | 1.18 | 3.394 | 399.4 |
| 3,5-Methano-2H-cyclopenta[b]furan-2-one | Organic compound | 1.15 | 12.049 | 340.16 |
| 3H-Pyrazol-3-one, 2,4-dihydro-5-methyl- | Organic compound | 0.87 | 5.049 | 98.1 |
| Azathymine | Ketone | 0.78 | 6.545 | 127.1 |
| Oxabicyclo[6.1.0]non-6-en-2-one | Organic compound | 0.59 | 7.58 | 138.16 |
| 3,4-Furandimethanol | Organic alcohol | 0.54 | 13.075 | 128.13 |
| 2-Furanmethanol | Organic alcohol | 0.45 | 5.32 | 155.19 |
| Levoglucosenone | Ketone | 0.41 | 6.853 | 126.11 |
| Trispiro[4.2.4.2.4.2.]heneicosane | Organic compound | 0.41 | 12.903 | 288.5 |
| Cirsiumaldehyde | Aldehyde | 0.38 | 16.925 | 132.16 |
| 2,5-Furandione | Organic acids | 0.36 | 10.381 | 98.06 |
| Ethanethiol | Alcohol | 0.34 | 2.958 | 62.14 |
| 2-Pyridinemethanol | Alcohol | 0.3 | 9.217 | 109.13 |
| 2-Amino-2-methyl-1,3-propanediol | Organic compound | 0.27 | 2.431 | 105.14 |
| Cirsiumaldehyde | Aldehyde | 0.26 | 14.317 | 132.16 |
| Hexadecanoic acid | Fatty acid | 0.16 | 14.847 | 258.41 |
| 1,4-Benzodioxan-5-carboxylic | Organic compound | 0.09 | 10.492 | 234.13 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bazaid, A.S.; Aldarhami, A.; Patel, M.; Adnan, M.; Hamdi, A.; Snoussi, M.; Qanash, H.; Imam, M.; Monjed, M.K.; Khateb, A.M. The Antimicrobial Effects of Saudi Sumra Honey against Drug Resistant Pathogens: Phytochemical Analysis, Antibiofilm, Anti-Quorum Sensing, and Antioxidant Activities. Pharmaceuticals 2022, 15, 1212. https://doi.org/10.3390/ph15101212
Bazaid AS, Aldarhami A, Patel M, Adnan M, Hamdi A, Snoussi M, Qanash H, Imam M, Monjed MK, Khateb AM. The Antimicrobial Effects of Saudi Sumra Honey against Drug Resistant Pathogens: Phytochemical Analysis, Antibiofilm, Anti-Quorum Sensing, and Antioxidant Activities. Pharmaceuticals. 2022; 15(10):1212. https://doi.org/10.3390/ph15101212
Chicago/Turabian StyleBazaid, Abdulrahman S., Abdu Aldarhami, Mitesh Patel, Mohd Adnan, Assia Hamdi, Mejdi Snoussi, Husam Qanash, Mohammed Imam, Mohammad Khalil Monjed, and Aiah Mustafa Khateb. 2022. "The Antimicrobial Effects of Saudi Sumra Honey against Drug Resistant Pathogens: Phytochemical Analysis, Antibiofilm, Anti-Quorum Sensing, and Antioxidant Activities" Pharmaceuticals 15, no. 10: 1212. https://doi.org/10.3390/ph15101212
APA StyleBazaid, A. S., Aldarhami, A., Patel, M., Adnan, M., Hamdi, A., Snoussi, M., Qanash, H., Imam, M., Monjed, M. K., & Khateb, A. M. (2022). The Antimicrobial Effects of Saudi Sumra Honey against Drug Resistant Pathogens: Phytochemical Analysis, Antibiofilm, Anti-Quorum Sensing, and Antioxidant Activities. Pharmaceuticals, 15(10), 1212. https://doi.org/10.3390/ph15101212










