Some Common Medicinal Plants with Antidiabetic Activity, Known and Available in Europe (A Mini-Review)
Abstract
1. Introduction
2. Medicinal Plants with Antidiabetic Activity
- Alteration of glucose metabolism: inhibition of renal reabsorption of glucose [29], inhibition of β-galactosidase [30], inhibition of β-glucosidase [30,31], inhibition of α-amylase [30,31], glycogenesis stimulation [32], hepaticglycolysis stimulation [32], starch conversion to glucose inhibited [30,31];
- Hypolipidemic effect: lipid peroxidation decrease [33];
- Pancreatic effect: effect of regeneration/repairing of β-cells [34], protective effect on β-cells [35], effect of increasing number and/or size of cells in Langerhans islets [34], insulin resistance reduction [36], insulin secretion stimulation [36,37], inhibition of degradative processes of insulin [36];
- Antioxidative effect: protection against the effects of oxidative stress responsible for β-cell dysfunction [38] by scavenging free radicals, reducing H2O2 formation, inhibition of ROS production, modulation of enzymes (cyclooxygenase, microsomal monooxygenase, NADH oxidase, xanthine oxidase, lipoxygenase, succinoxidase) [39], regulation of antioxidant:oxidant balance in cells [33], induction of enzymes (glutathione peroxidase, catalase, superoxide dismutase) [33], improvement of antioxidant capacity in plasma [33];
- Diabetes complication treatment: inhibition of pro-inflammatory pathway of NF-κβ, resulting in vascular complications [40];
- Insulin-like effect.
2.1. White Mulberry (Morus alba L.)
- Influence on the appetite [90];
2.2. Fenugreek (Trigonella foenum-graecum L.)
2.3. Ceylon cinnamon (Cinnamomum zeylanicum J.Presl)
- Cinnamon fiber delays the emptying of the stomach;
- Eugenol from cinnamon acts as an inhibitor of α-glucosidase in the intestines;
- In the myocyte, there is an improvement of insulin receptor phosphorylation, synthesis, and translocation of GLUT-4 to glucose uptake and, therefore, an increase of glycogen;
- Cinnamaldehyde provides sympathetic actions; increased noradrenaline may increase the heart rate and thermogenic influence on brown adipose tissue;
- The proposed mechanism of body fat loss across cinnamon intake occurs from the UCP1 activation in the mitochondria of brown adipose tissue and greater PPAR-α expression in white adipose tissue and, consequently, increases β-oxidation by means of enzymatic action of acyl-CoA oxidase;
- Expected improvement of glycaemic, lipid, and antioxidant parameters.
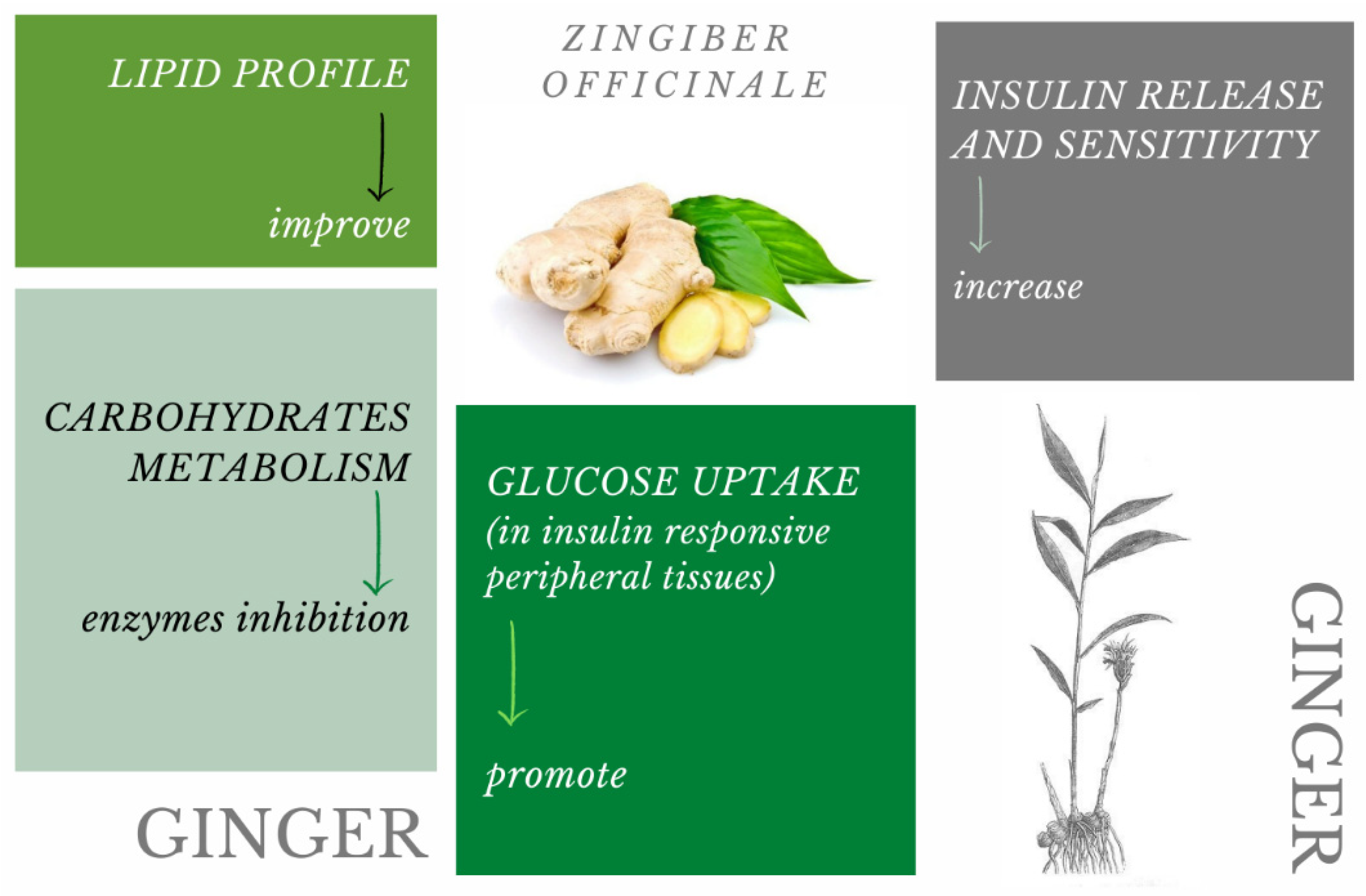
2.4. Ginger (Zingiber officinale Rosc.)
2.5. Common Bean (Phasolus vulgaris L.)
2.6. Ginseng (Panax ginseng C.A.Meyer)
3. Conclusions and Future Perspectives
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| EFSA | European Food Safety Authority |
| BMI | body mass index |
| HbA1c | glycated hemoglobin |
| FBG | fasting blood glucose |
| TC | total cholesterol |
| TG | triglycerides |
| H2O2 | hydrogen peroxide |
| SI | serum insulin |
| FSI | fasting serum insulin |
| ROS | reactive oxygen species |
| NF-κβ | nuclear factor-κβ |
| NADH | nicotinamide adenine dinucleotide + hydrogen |
| HOMA-IR | homeostasis model assessment insulin resistance |
| GLUT-4 | glucose transporter type 4 |
| GLUT-1 | glucose transporter type 1 |
| PPG60min | postprandial glucose level (60 min) |
| PPG30min | postprandial glucose level (30 min) |
| PPG120min | postprandial glucose level (120 min) |
| OGTT | oral glucose tolerance test |
| NOAEL | not observed adverse effect level |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| ABTS | 2,2’-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) |
| FRAP | ferric reducing antioxidant power |
| LDL | low-density lipoprotein |
| UCP-1 | uncoupling protein 1 |
| PPAR-α | peroxisome proliferator-activated receptor alpha |
| CRP | C-reactive protein |
| FDA | Food and Drug Administration |
References
- Alem Geberemeskel, G.; Godefa Debebe, Y.; Abraha Nguse, N. Clinical study antidiabetic effect of fenugreek seed powder solution (Trigonella foenum-graecum L.) on hyperlipidemia in diabetic patients. J. Diabetes Res. 2019, 2019, 8507453. [Google Scholar] [CrossRef]
- Statista Diabetics Percentage Worldwide. 2019. Available online: https://www.statista.com/statistics/271464/percentage-of-diabetics-worldwide/ (accessed on 2 November 2020).
- Shaw, J.E.; Sicree, R.A.; Zimmet, P.Z. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res. Clin. Pract. 2010, 87, 4–14. [Google Scholar] [CrossRef]
- Roglic, G.; Unwin, N. Mortality attributable to diabetes: Estimates for the year 2010. Diabetes Res. Clin. Pract. 2010, 87, 15–19. [Google Scholar] [CrossRef]
- Wu, Z.; Tang, Y.; Cheng, Q. Diabetes increases the mortality of patients with COVID-19: A meta-analysis. Acta Diabetol. 2021, 58, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Szczepaniak, O.; Ligaj, M.; Kobus-Cisowska, J.; Tichoniuk, M.; Dziedziński, M.; Przeor, M.; Szulc, P. The genoprotective role of naringin. Biomolecules 2020, 10, 700. [Google Scholar] [CrossRef] [PubMed]
- Dziedzinski, M.; Kobus-Cisowska, J.; Szymanowska, D.; Stuper-Szablewska, K.; Baranowska, M. Identification of polyphenols from coniferous shoots as natural antioxidants and antimicrobial compounds. Molecules 2020, 25, 3527. [Google Scholar] [CrossRef] [PubMed]
- Telichowska, A.; Kobus-Cisowska, J.; Szulc, P. Phytopharmacological possibilities of bird cherry Prunus padus L. and Prunus serotina L. species and their bioactive phytochemicals. Nutrients 2020, 12, 1966. [Google Scholar] [CrossRef]
- Kobus-Cisowska, J.; Szymanowska-Powałowska, D.; Szczepaniak, O.; Kmiecik, D.; Przeor, M.; Gramza-Michałowska, A.; Cielecka-Piontek, J.; Smuga-Kogut, M.; Szulc, P. Composition and in vitro effects of cultivars of Humulus lupulus L. Hops on cholinesterase activity and microbial growth. Nutrients 2019, 11, 1377. [Google Scholar] [CrossRef] [PubMed]
- Szczepaniak, O.M.; Kobus-Cisowska, J.; Kusek, W.; Przeor, M. Functional properties of Cornelian cherry (Cornus mas L.): A comprehensive review. Eur. Food Res. Technol. 2019, 245, 2071–2087. [Google Scholar] [CrossRef]
- Przeor, M.; Flaczyk, E.; Beszterda, M.; Szymandera-Buszka, K.E.; Piechocka, J.; Kmiecik, D.; Szczepaniak, O.; Kobus-Cisowska, J.; Jarzębski, M.; Tylewicz, U. Air-drying temperature changes the content of the phenolic acids and flavonols in white mulberry (Morus alba L.) leaves. Cienc. Rural 2019, 49. [Google Scholar] [CrossRef]
- Tylewicz, U.; Oliveira, G.; Alminger, M.; Nohynek, L.; Dalla Rosa, M.; Romani, S. Antioxidant and antimicrobial properties of organic fruits subjected to PEF-assisted osmotic dehydration. Innov. Food Sci. Emerg. Technol. 2020, 62, 102341. [Google Scholar] [CrossRef]
- Cavalcanti, V.P.; Aazza, S.; Bertolucci, S.K.V.; Pereira, M.M.A.; Cavalcanti, P.P.; Buttrós, V.H.T.; de Oliveira e Silva, A.M.; Pasqual, M.; Dória, J. Plant, pathogen and biocontrol agent interaction effects on bioactive compounds and antioxidant activity in garlic. Physiol. Mol. Plant Pathol. 2020, 112, 101550. [Google Scholar] [CrossRef]
- Tajner-Czopek, A.; Gertchen, M.; Rytel, E.; Kita, A.; Kucharska, A.Z.; Sokół-Łętowska, A. Study of antioxidant activity of some medicinal plants having high content of caffeic acid derivatives. Antioxidants 2020, 9, 412. [Google Scholar] [CrossRef] [PubMed]
- Waśkiewicz, A.; Beszterda, M.; Bocianowski, J.; Goliński, P. Natural occurrence of fumonisins and ochratoxin A in some herbs and spices commercialized in Poland analyzed by UPLC-MS/MS method. Food Microbiol. 2013, 36, 426–431. [Google Scholar] [CrossRef] [PubMed]
- Sowa, P.; Marcinčáková, D.; Miłek, M.; Sidor, E.; Legáth, J.; Dżugan, M. Analysis of cytotoxicity of selected asteraceae plant extracts in real time, their antioxidant properties and polyphenolic profile. Molecules 2020, 25, 5517. [Google Scholar] [CrossRef]
- Kozlowska, M.; Zbikowska, A.; Marciniak-Lukasiak, K.; Kowalska, M. Herbal extracts incorporated into shortbread cookies: Impact on color and fat quality of the cookies. Biomolecules 2019, 9, 858. [Google Scholar] [CrossRef]
- Przeor, M.; Flaczyk, E.; Kmiecik, D.; Kobus-Cisowska, J.; Bueschke, M.; Kulczyński, B. Polish consumers’ awareness and knowledge about functional food. Univ. Technol. Stetin. Agric. Aliment. Pisc. Zootech 2018, 341, 59–68. [Google Scholar] [CrossRef]
- Musabayane, C.T.; Bwititi, P.T.; Ojewole, J.A.O. Effects of oral administration of some herbal extracts on food consumption and blood glucose levels in normal and streptozotocin-treated diabetic rats. Methods Find. Exp. Clin. Pharmacol. 2006, 28, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Baset, M.; Ali, T.; Elshamy, H.; El Sadek, A.; Sami, D.; Badawy, M.; Abou-Zekry, S.; Heiba, H.; Saadeldin, M.; Abdellatif, A. Anti-diabetic effects of fenugreek (Trigonella foenum-graecum): A comparison between oral and intraperitoneal—An animal study. Int. J. Funct. Nutr. 2020, 1, 2. [Google Scholar] [CrossRef]
- Salehi, B.; Ata, A.; Anil Kumar, N.V.; Sharopov, F.; Ramírez-Alarcón, K.; Ruiz-Ortega, A.; Abdulmajid Ayatollahi, S.; Tsouh Fokou, P.V.; Kobarfard, F.; Amiruddin Zakaria, Z.; et al. Antidiabetic potential of medicinal plants and their active components. Biomolecules 2019, 9, 551. [Google Scholar] [CrossRef]
- Singab, A.N.; Youssef, F.S.; Ashour, M.L. Medicinal plants with potential antidiabetic activity and their assessment. Med. Aromat. Plants 2014, 3, 151. [Google Scholar] [CrossRef]
- Arumugam, G.; Manjula, P.; Paari, N. A review: Anti diabetic medicinal plants used for diabetes mellitus. J. Acute Dis. 2013, 2, 196–200. [Google Scholar] [CrossRef]
- Chhetri, D.R.; Parajuli, P.; Subba, G.C. Antidiabetic plants used by Sikkim and Darjeeling Himalayan tribes, India. J. Ethnopharmacol. 2005, 99, 199–202. [Google Scholar] [CrossRef] [PubMed]
- Przeor, M.; Flaczyk, E.; Kmiecik, D.; Buchowski, M.S.; Staniek, H.; Tomczak-Graczyk, A.; Kobus-Cisowska, J.; Gramza-Michałowska, A.; Foksowicz-Flaczyk, J. Functional properties and antioxidant activity of Morus alba L. leaves var. Zolwinska Wielkolistna (WML-P)—the effect of controlled conditioning process. Antioxidants 2020, 9, 668. [Google Scholar] [CrossRef]
- Chinsembu, K.C. Diabetes mellitus and nature’s pharmacy of putative antidiabetic plants. J. Herb. Med. 2019, 15, 100230. [Google Scholar] [CrossRef]
- Durazzo, A.; D’Addezio, L.; Camilli, E.; Piccinelli, R.; Turrini, A.; Marletta, L.; Marconi, S.; Lucarini, M.; Lisciani, S.; Gabrielli, P.; et al. From plant compounds to botanicals and back: A current snapshot. Molecules 2018, 23, 1844. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.H.; Ngoh, G.C.; Yusoff, R. A brief review on anti diabetic plants: Global distribution, active ingredients, extraction techniques and acting mechanisms. Pharmacogn. Rev. 2012, 6, 22–28. [Google Scholar] [CrossRef]
- Eddouks, M.; Maghrani, M.; Lemhadri, A.; Ouahidi, M.L.; Jouad, H. Ethnopharmacological survey of medicinal plants used for the treatment of diabetes mellitus, hypertension and cardiac diseases in the south-east region of Morocco (Tafilalet). J. Ethnopharmacol. 2002, 82, 97–103. [Google Scholar] [CrossRef]
- Gholap, S.; Kar, A. Hypoglycaemic effects of some plant extracts are possibly mediated through inhibition in corticosteroid concentration. Pharmazie 2004, 59, 876–878. [Google Scholar]
- Heidari, R.; Zareae, S.; Heidarizadeh, M. Extraction, purification, and inhibitory effect of alpha-amylase inhibitor from wheat (Triticum aestivum Var. Zarrin). Pakistan J. Nutr. 2005, 4, 101–105. [Google Scholar] [CrossRef]
- Miura, T.; Itoh, C.; Iwamoto, N.; Kato, M.; Kawai, M.; Park, S.R.; Suzuki, I. Hypoglycemic activity of the fruit of the Momordica charantia in type 2 diabetic mice. J. Nutr. Sci. Vitaminol. 2001, 47, 340–344. [Google Scholar] [CrossRef] [PubMed]
- Crespy, V.; Williamson, G. A review of the health effects of green tea catechins in in vivo animal models. J. Nutr. 2004, 134. [Google Scholar] [CrossRef] [PubMed]
- Bnouham, M.; Ziyyat, A.; Mekhfi, H.; Tahri, A.; Legssyer, A. Medicinal plants with potential antidiabetic activity-A review of ten years of herbal medicine research (1990–2000). Int J Diabetes Metab. 2006, 14, 1–25. [Google Scholar] [CrossRef]
- Kim, M.J.; Ryu, G.R.; Chung, J.S.; Sim, S.S.; Rhie, D.J.; Yoon, S.H.; Hahn, S.J.; Kim, M.S.; Jo, Y.H. Protective effects of epicatechin against the toxic effects of streptozotocin on rat pancreatic islets: In vivo and in vitro. Pancreas 2003, 26, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, P.K.; Maiti, K.; Mukherjee, K.; Houghton, P.J. Leads from Indian medicinal plants with hypoglycemic potentials. J. Ethnopharmacol. 2006, 106, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Esmaeili, M.A.; Yazdanparast, R. Hypoglycaemic effect of Teucrium polium: Studies with rat pancreatic islets. J. Ethnopharmacol. 2004, 95, 27–30. [Google Scholar] [CrossRef]
- Kaneto, H.; Matsuoka, T.; Nakatani, Y.; Kawamori, D.; Matsuhisa, M.; Yamasaki, Y. Oxidative stress and the JNK pathway in diabetes. Curr. Diabetes Rev. 2005, 1, 65–72. [Google Scholar] [CrossRef]
- Shin, A.H.; Oh, C.J.; Park, J.-P. Glycation-induced inactivation of antioxidant enzymes and modulation of cellular redox status in lens cells. Arch. Pharmacal Res. 2006, 29, 577–581. [Google Scholar] [CrossRef]
- Suryavanshi, S.V.; Kulkarni, Y.A. NF-κβ: A potential target in the management of vascular complications of diabetes. Front. Pharmacol. 2017, 8, 798. [Google Scholar] [CrossRef]
- Herrera, T.; Navarro del Hierro, J.; Fornari, T.; Reglero, G.; Martin, D. Inhibitory effect of quinoa and fenugreek extracts on pancreatic lipase and α-amylase under in vitro traditional conditions or intestinal simulated conditions. Food Chem. 2019, 270, 509–517. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Ni, X.; Kai, G.; Chen, X. A review on structure-activity relationship of dietary polyphenols inhibiting α-amylase. Crit. Rev. Food Sci. Nutr. 2013, 53, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.; Houghton, P.J.; Soumyanath, A. α-Amylase inhibitory activity of some Malaysian plants used to treat diabetes; with particular reference to Phyllanthus amarus. J. Ethnopharmacol. 2006, 107, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Ozkan, G.; Kamiloglu, S.; Ozdal, T.; Boyacioglu, D.; Capanoglu, E. Potential use of Turkish medicinal plants in the treatment of various diseases. Molecules 2016, 21, 257. [Google Scholar] [CrossRef]
- Kumari, R.; Srivastava, S.; Srivastava, R.P. Nutritional evaluation of fresh leaves of mulberry genotypes. Agric. Sci. Dig. 2009, 29, 198–201. [Google Scholar] [CrossRef]
- Seneta, W.; Dolatowski, J. Dendrologia; Wydawnictwo Naukowe PWN: Warsaw, Poland, 2007. [Google Scholar]
- Przeor, M.; Flaczyk, E. Antioxidant properties of Paratha type flat bread enriched with white mulberry leaf extract. Indian J. Tradit. Knowl. 2016, 15, 237–244. [Google Scholar]
- Kobus-Cisowska, J.; Gramza-Michalowska, A.; Kmiecik, D.; Flaczyk, E.; Korczak, J. Mulberry fruit as an antioxidant component in muesli. Agric. Sci. 2013, 4, 130–135. [Google Scholar] [CrossRef]
- Kobus-Cisowska, J.; Dziedziński, M.; Szymanowska, D.; Szczepaniak, O.; Byczkiewicz, S.; Telichowska, A.; Szulc, P. The effects of Morus alba L. fortification on the quality, functional properties and sensory attributes of bread stored under refrigerated conditions. Sustainability 2020, 12, 6691. [Google Scholar] [CrossRef]
- Przeor, M.; Flaczyk, E. Morwa biała—Nieocenione znaczenie zdrowotne. Przem. Spożywczy 2016, 5, 33–35. [Google Scholar] [CrossRef]
- Grześkowiak, J.; Łochyńska, M. Związki biologicznie aktywne morwy białej (Morus alba L.) i ich działanie lecznicze. Postępy Fitoter. 2017, 18, 31–35. [Google Scholar]
- Butt, M.S.; Nazir, A.; Sultan, M.T.; Schroën, K. Morus alba L. nature’s functional tonic. Trends Food Sci. Technol. 2008, 19, 505–512. [Google Scholar] [CrossRef]
- Kwon, H.J.; Chung, J.Y.; Kim, J.Y.; Kwon, O. Comparison of 1-deoxynojirimycin and aqueous mulberry leaf extract with emphasis on postprandial hypoglycemic effects: In vivo and in vitro studies. J. Agric. Food Chem. 2011, 59, 3014–3019. [Google Scholar] [CrossRef]
- Yin, Z.; Zhang, W.; Feng, F.; Zhang, Y.; Kang, W. α-Glucosidase inhibitors isolated from medicinal plants. Food Sci. Hum. Wellness 2014, 3, 136–174. [Google Scholar] [CrossRef]
- Liu, Q.; Li, X.; Li, C.; Zheng, Y.; Peng, G. 1-Deoxynojirimycin alleviates insulin resistance via activation of insulin signaling PI3K/AKT pathway in skeletal muscle of db/db mice. Molecules 2015, 20, 21700–21714. [Google Scholar] [CrossRef]
- Pandey, V.K.; Mathur, A.; Khan, M.F.; Kakkar, P. Activation of PERK-eIF2α-ATF4 pathway contributes to diabetic hepatotoxicity: Attenuation of ER stress by Morin. Cell. Signal. 2019, 59, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Shane-McWhorter, L. Biological complementary therapies: A focus on botanical products in diabetes. Diabetes Spectr. 2001, 14, 199–208. [Google Scholar] [CrossRef]
- Broca, C.; Gross, R.; Petit, P.; Sauvaire, Y.; Manteghetti, M.; Tournier, M.; Masiello, P.; Gomis, R.; Ribes, G. 4-hydroxyisoleucine: Experimental evidence of its insulinotropic and antidiabetic properties. Am. J. Physiol.-Endocrinol. Metab. 1999, 277, E617–E623. [Google Scholar] [CrossRef] [PubMed]
- Sanlier, N.; Gencer, F. Role of spices in the treatment of diabetes mellitus: A minireview. Trends Food Sci. Technol. 2020, 99, 441–449. [Google Scholar] [CrossRef]
- Yoshinari, O.; Igarashi, K. Anti-diabetic effect of trigonelline and nicotinic acid, on KK-Ay Mice. Curr. Med. Chem. 2010, 17, 2196–2202. [Google Scholar] [CrossRef] [PubMed]
- Jarvill-Taylor, K.J.; Anderson, R.A.; Graves, D.J. A hydroxychalcone derived from cinnamon functions as a mimetic for insulin in 3T3-L1 adipocytes. J. Am. Coll. Nutr. 2001, 20, 327–336. [Google Scholar] [CrossRef]
- Subash Babu, P.; Prabuseenivasan, S.; Ignacimuthu, S. Cinnamaldehyde—A potential antidiabetic agent. Phytomedicine 2007, 14, 15–22. [Google Scholar] [CrossRef]
- Santos, H.O.; da Silva, G.A.R. To what extent does cinnamon administration improve the glycemic and lipid profiles? Clin. Nutr. ESPEN 2018, 27, 1–9. [Google Scholar] [CrossRef]
- Singh, P.; Jayaramaiah, R.H.; Agawane, S.B.; Vannuruswamy, G.; Korwar, A.M.; Anand, A.; Dhaygude, V.S.; Shaikh, M.L.; Joshi, R.S.; Boppana, R.; et al. Potential dual role of eugenol in inhibiting advanced glycation end products in diabetes: Proteomic and mechanistic insights. Sci. Rep. 2016, 6, 18798. [Google Scholar] [CrossRef] [PubMed]
- Ali, B.H.; Blunden, G.; Tanira, M.O.; Nemmar, A. Some phytochemical, pharmacological and toxicological properties of ginger (Zingiber officinale Roscoe): A review of recent research. Food Chem. Toxicol. 2008, 46, 409–420. [Google Scholar] [CrossRef]
- Ezzat, S.M.; Ezzat, M.I.; Okba, M.M.; Menze, E.T.; Abdel-Naim, A.B. The hidden mechanism beyond ginger (Zingiber officinale Rosc.) potent in vivo and in vitro anti-inflammatory activity. J. Ethnopharmacol. 2018, 214, 113–123. [Google Scholar] [CrossRef]
- Chavan, J.J.; Kshirsagar, P.R.; Pai, S.R.; Pawar, N.V. Micropropagation, metabolite profiling, antioxidant activities and chromatographic determination of bioactive molecules across in vitro conditions and subsequent field cultivation stages of ‘Shampoo Ginger’ (Zingiber zerumbet L. Roscoe ex Sm). Biocatal. Agric. Biotechnol. 2018, 16, 79–89. [Google Scholar] [CrossRef]
- Tran, N.; Pham, B.; Le, L. Bioactive compounds in anti-diabetic plants: From herbal medicine to modern drug discovery. Biology 2020, 9, 252. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-C.; Lin, C.-Y.; Huang, S.-F.; Lin, H.-C.; Chang, W.-L.; Chang, T.-C. Effect and mechanism of ginsenosides CK and Rg1 on stimulation of glucose uptake in 3T3-L1 adipocytes. J. Agric. Food Chem. 2010, 58, 6039–6047. [Google Scholar] [CrossRef]
- Kim, K.-S.; Yang, H.J.; Lee, I.-S.; Kim, K.-H.; Park, J.; Jeong, H.-S.; Kim, Y.; Ahn, K.S.; Na, Y.-C.; Jang, H.-J. The aglycone of ginsenoside Rg3 enables glucagon-like peptide-1 secretion in enteroendocrine cells and alleviates hyperglycemia in type 2 diabetic mice OPEN. Sci. Rep. 2015, 5, 18325. [Google Scholar] [CrossRef]
- Tian, W.; Chen, L.; Zhang, L.; Wang, B.; Li, X.; Fan, K.; Ai, C.; Xia, X.; Li, S.; Li, Y. Effects of ginsenoside Rg1 on glucose metabolism and liver injury in streptozotocin-induced type 2 diabetic rats. Genet. Mol. Res. 2017, 16, 16019463. [Google Scholar] [CrossRef] [PubMed]
- Merck | Poland. Available online: https://www.sigmaaldrich.com/PL/pl (accessed on 13 December 2021).
- Yuan, Q.; Xie, Y.; Wang, W.; Yan, Y.; Ye, H.; Jabbar, S.; Zeng, X. Extraction optimization, characterization and antioxidant activity in vitro of polysaccharides from mulberry (Morus alba L.) leaves. Carbohydr. Polym. 2015, 128, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Flaczyk, E.; Kobus-Cisowska, J.; Przeor, M.; Korczak, J.; Remiszewski, M.; Korbas, E.; Buchowski, M. Chemical characterization and antioxidative properties of Polish variety of Morus alba L. leaf aqueous extracts from the laboratory and pilot-scale processes. Agric. Sci. 2013, 4, 141–147. [Google Scholar] [CrossRef]
- Zhang, D.-Y.; Wan, Y.; Hao, J.-Y.; Hu, R.-Z.; Chen, C.; Yao, X.-H.; Zhao, W.-G.; Liu, Z.-Y.; Li, L. Evaluation of the alkaloid, polyphenols, and antioxidant contents of various mulberry cultivars from different planting areas in eastern China. Ind. Crop. 2018, 122, 298–307. [Google Scholar] [CrossRef]
- Cheng, K.C.; Wang, C.J.; Chang, Y.C.; Hung, T.W.; Lai, C.J.; Kuo, C.W.; Huang, H.P. Mulberry fruits extracts induce apoptosis and autophagy of liver cancer cell and prevent hepatocarcinogenesis in vivo. J. Food Drug Anal. 2020, 28, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Riche, D.M.; Riche, K.D.; East, H.E.; Barrett, E.K.; May, W.L. Impact of mulberry leaf extract on type 2 diabetes (Mul-DM): A randomized, placebo-controlled pilot study. Complement. Ther. Med. 2017, 32, 105–108. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Zhang, B.; Chen, G.; Fu, X. The novel contributors of anti-diabetic potential in mulberry polyphenols revealed by UHPLC-HR-ESI-TOF-MS/MS. Food Res. Int. 2017, 100, 873–884. [Google Scholar] [CrossRef] [PubMed]
- Ranjan, B.; Kumar, R.; Verma, N.; Mittal, S.; Pakrasi, P.L.; Venkatesh Kumar, R. Evaluation of the antidiabetic properties of S-1708 mulberry variety. Pharmacogn. Mag. 2017, 13, S280–S288. [Google Scholar] [CrossRef]
- Gryn-Rynko, A.; Bazylak, G.; Olszewska-Slonina, D. New potential phytotherapeutics obtained from white mulberry (Morus alba L.) leaves. Biomed. Pharmacother. 2016, 84, 628–636. [Google Scholar] [CrossRef]
- Harauma, A.; Murayama, T.; Ikeyama, K.; Sano, H.; Arai, H.; Takano, R.; Kita, T.; Hara, S.; Kamei, K.; Yokode, M. Mulberry leaf powder prevents atherosclerosis in apolipoprotein E-deficient mice. Biochem. Biophys. Res. Commun. 2007, 358, 751–756. [Google Scholar] [CrossRef]
- Rodrigues, E.L.; Marcelino, G.; Silva, G.T.; Figueiredo, P.S.; Garcez, W.S.; Corsino, J.; Guimarães, R.D.C.A.; Freitas, K.D.C. Nutraceutical and medicinal potential of the Morus species in metabolic dysfunctions. Int. J. Mol. Sci. 2019, 20, 301. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.Y.; Sung, W.C.; Shyu, Y.S. Effect of mulberry lees addition on dough mixing characteristics and the quality of mulberry toast. J. Mar. Sci. Technol. 2008, 16, 103–108. [Google Scholar] [CrossRef]
- Wen, P.; Hu, T.-G.; Linhardt, R.J.; Liao, S.-T.; Wu, H.; Zou, Y.-X. Mulberry: A review of bioactive compounds and advanced processing technology. Trends Food Sci. Technol. 2019, 83, 138–158. [Google Scholar] [CrossRef]
- Akhtar, N.; Hisham, J.; Shoaib Khan, H.M.; Ali Khan, B.; Mahmood, T.; Saeed, T. Whitening and antierythemic effect of a cream containing Morus alba extract. Hygeia J. Drugs Med. 2012, 4, 97–103. [Google Scholar]
- Adisakwattana, S.; Intrawangso, J.; Hemrid, A.; Chanathong, B.; Mäkynen, K. Extracts of edible plants inhibit pancreatic lipase, cholesterol esterase and cholesterol micellization, and bind bile acids. Food Technol. Biotechnol. 2012, 50, 11–16. [Google Scholar]
- Adisakwattana, S.; Ruengsamran, T.; Kampa, P.; Sompong, W. In vitro inhibitory effects of plant-based foods and their combinations on intestinal α-glucosidase and pancreatic α-amylase. BMC Complement. Altern. Med. 2012, 12, 110. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.H.; Yang, S.J.; Kim, Y.; Lee, M.; Lim, Y. Combined treatment of mulberry leaf and fruit extract ameliorates obesity-related inflammation and oxidative stress in high fat diet-induced obese mice. J. Med. Food 2013, 16, 673–680. [Google Scholar] [CrossRef] [PubMed]
- Mahboubi, M. Morus alba (mulberry), a natural potent compound in management of obesity. Pharmacol. Res. 2019, 146, 104341. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.H.; Liu, L.K.; Chuang, C.M.; Chyau, C.C.; Huang, C.N.; Wang, C.J. Mulberry water extracts possess an anti-obesity effect and ability to inhibit hepatic lipogenesis and promote lipolysis. J. Agric. Food Chem. 2011, 59, 2663–2671. [Google Scholar] [CrossRef] [PubMed]
- Tond, S.B.; Fallah, S.; Salemi, Z.; Seifi, M. Influence of mulberry leaf extract on serum adiponectin, visfatin and lipid profile levels in type 2 diabetic rats. Brazilian Arch. Biol. Technol. 2016, 59, 16160297. [Google Scholar] [CrossRef]
- Ann, J.Y.; Eo, H.; Lim, Y. Mulberry leaves (Morus alba L.) ameliorate obesity-induced hepatic lipogenesis, fibrosis, and oxidative stress in high-fat diet-fed mice. Genes Nutr. 2015, 10, 46. [Google Scholar] [CrossRef]
- Liu, L.K.; Lee, H.J.; Shih, Y.W.; Chyau, C.C.; Wang, C.J. Mulberry anthocyanin extracts inhibit LDL oxidation and macrophage-derived foam cell formation induced by oxidative LDL. J. Food Sci. 2008, 73, H113–H121. [Google Scholar] [CrossRef]
- Katsube, T.; Yamasaki, M.; Shiwaku, K.; Ishijima, T.; Matsumoto, I.; Abe, K.; Yamasaki, Y. Effect of flavonol glycoside in mulberry (Morus alba L.) leaf on glucose metabolism and oxidative stress in liver in diet-induced obese mice. J. Sci. Food Agric. 2010, 90, 2386–2392. [Google Scholar] [CrossRef]
- Metwally, F.M.; Ahmed, H.H.; Rashad, H.; Zaazaa, A.M. Insights into the role of Morus alba in reversing obesity-associated hepatic steatosis and related metabolic disorder in rats. Asian J. Pharm. Clin. Res. 2016, 9, 231–238. [Google Scholar] [CrossRef][Green Version]
- Ren, C.; Zhang, Y.; Cui, W.; Lu, G.; Wang, Y.; Gao, H.; Huang, L.; Mu, Z. A polysaccharide extract of mulberry leaf ameliorates hepatic glucose metabolism and insulin signaling in rats with type 2 diabetes induced by high fat-diet and streptozotocin. Int. J. Biol. Macromol. 2015, 72, 951–959. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.; Sun, W.; Fan, Y.; Guo, X.; Xu, G.; Xu, T.; Hou, Y.; Zhao, B.; Feng, X.; Liu, T. Effect of mulberry leaf (Folium Mori) on insulin resistance via IRS-1/PI3K/Glut-4 signalling pathway in type 2 diabetes mellitus rats. Pharm. Biol. 2016, 54, 2685–2691. [Google Scholar] [CrossRef]
- Sudha, P.; Zinjarde, S.S.; Bhargava, S.Y.; Kumar, A.R. Potent a-amylase inhibitory activity of Indian Ayurvedic medicinal plants. BMC Complement. Altern. Med. 2011, 11, 5. [Google Scholar] [CrossRef]
- Kimura, T.; Nakagawa, K.; Kubota, H.; Kojima, Y.; Goto, Y.; Yamgishi, K.; Oita, S.; Oikawa, S.; Miyazawa, T. Food-grade mulberry powder enriched with 1-deoxynojirimycin suppresses the elevation of postprandial blood glucose in humans. J. Agric. Food Chem. 2007, 55, 5869–5874. [Google Scholar] [CrossRef] [PubMed]
- Hansawasdi, C.; Kawabata, J. α-Glucosidase inhibitory effect of mulberry (Morus alba) leaves on Caco-2. Fitoterapia 2006, 77, 568–573. [Google Scholar] [CrossRef]
- Jiao, Y.; Wang, X.; Jiang, X.; Kong, F.; Wang, S.; Yan, C. Antidiabetic effects of Morus alba fruit polysaccharides on high-fat diet- and streptozotocin-induced type 2 diabetes in rats. J. Ethnopharmacol. 2017, 199, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.G.; Wen, P.; Liu, J.; Long, X.S.; Liao, S.T.; Wu, H.; Zou, Y.X. Combination of mulberry leaf and oat bran possessed greater hypoglycemic effect on diabetic mice than mulberry leaf or oat bran alone. J. Funct. Foods 2019, 61, 103503. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, X.; Liang, C.; Hu, J.; Yu, Z. Safety evaluation of mulberry leaf extract: Acute, subacute toxicity and genotoxicity studies. Regul. Toxicol. Pharmacol. 2018, 95, 220–226. [Google Scholar] [CrossRef]
- EU Novel Food Catalogue (v.1.1). Available online: https://ec.europa.eu/food/safety/novel_food/catalogue/search/public/index.cfm (accessed on 4 November 2020).
- Wani, S.A.; Kumar, P. Fenugreek: A review on its nutraceutical properties and utilization in various food products. J. Saudi Soc. Agric. Sci. 2018, 17, 97–106. [Google Scholar] [CrossRef]
- Ahmad, A.; Alghamdi, S.S.; Mahmood, K.; Afzal, M. Fenugreek a multipurpose crop: Potentialities and improvements. Saudi J. Biol. Sci. 2016, 23, 300–310. [Google Scholar] [CrossRef] [PubMed]
- El Nasri, N.A.; El Tinay, A.H. Functional properties of fenugreek (Trigonella foenum graecum) protein concentrate. Food Chem. 2007, 103, 582–589. [Google Scholar] [CrossRef]
- Acharya, S.; Srichamroen, A.; Basu, S.; Ooraikul, B.; Basu, T. Improvement in the nutraceutical properties of fenugreek (Trigonella foenum-graecum L.). Songklanakarin J. Sci. Technol. 2006, 28, 1–9. [Google Scholar]
- He, Y.; Ding, C.; Wang, X.; Wang, H.; Suo, Y. Using response surface methodology to optimize countercurrent chromatographic separation of polyphenol compounds from fenugreek (Trigonella foenum-graecum L.) seeds. J. Liq. Chromatogr. Relat. Technol. 2015, 38, 29–35. [Google Scholar] [CrossRef]
- Gaur, V.; Bodhankar, S.L.; Mohan, V.; Thakurdesai, P.A. Neurobehavioral assessment of hydroalcoholic extract of Trigonella foenum-graecum seeds in rodent models of Parkinson’s disease. Pharm. Biol. 2013, 51, 550–557. [Google Scholar] [CrossRef]
- Król-Kogus, B.; Krauze-Baranowska, M. Kozieradka pospolita (Trigonella foenum graecum L.)—tradycja stosowania na tle wyników badań naukowych. Postępy Fitoter. 2011, 3, 185–190. [Google Scholar]
- Kumar, P.; Bhandari, U.; Jamadagni, S. Fenugreek seed extract inhibit fat accumulation and ameliorates dyslipidemia in high fat diet-induced obese rats. Biomed. Res. Int. 2014, 2014, 606021. [Google Scholar] [CrossRef]
- Kaviarasan, S.; Naik, G.H.; Gangabhagirathi, R.; Anuradha, C.V.; Priyadarsini, K.I. In vitro studies on antiradical and antioxidant activities of fenugreek (Trigonella foenum graecum) seeds. Food Chem. 2007, 103, 31–37. [Google Scholar] [CrossRef]
- Kania, M.; Derebecka, N. Medicinal plants in type 2 diabetes mellitus. Postępy Fitoter. 2010, 2, 76–84. [Google Scholar]
- Rao, M.U.; Sreenivasulu, M.; Chengaiah, B.; Reddy, K.J.; Chetty, C.M. Herbal medicines for diabetes mellitus: A review. Int. J. PharmTech Res. 2010, 2, 1883–1892. [Google Scholar]
- Bawadi, H.A.; Maghaydah, S.N.; Tayyem, R.F.; Tayyem, R.F. The postprandial hypoglycemic activity of fenugreek seed and seeds’ extract in type 2 diabetics: A pilot study. Pharmacogn. Mag. 2009, 4, 134–138. [Google Scholar]
- Arivalagan, M.; Gangopadhyay, K.K.; Kumar, G. Determination of steroidal saponins and fixed oil content in fenugreek (Trigonella foenum-graecum) genotypes. Indian J. Pharm. Sci. 2013, 75, 110–113. [Google Scholar] [CrossRef]
- Eidi, A.; Eidi, M.; Sokhteh, M. Effect of fenugreek (Trigonella foenum-graecum L) seeds on serum parameters in normal and streptozotocin-induced diabetic rats. Nutr. Res. 2007, 27, 728–733. [Google Scholar] [CrossRef]
- Verma, N.; Usman, K.; Patel, N.; Jain, A.; Dhakre, S.; Swaroop, A.; Bagchi, M.; Kumar, P.; Preuss, H.G.; Bagchi, D. A multicenter clinical study to determine the efficacy of a novel fenugreek seed (Trigonella foenum-graecum) extract (FenfuroTM) in patients with type 2 diabetes. Food Nutr. Res. 2016, 60, 32382. [Google Scholar] [CrossRef]
- Singh, A.; Rai, J.; Mahajan, D.S. Comparative evaluation of glipizide and fenugreek (Trigonella foenum-graecum) seeds as monotherapy and combination therapy on glycaemic control and lipid profile in patients with type 2 diabetes mellitus. Int. J. Basic Clin. Pharmacol. 2016, 3, 942–950. [Google Scholar] [CrossRef]
- Fæste, C.K.; Namork, E.; Lindvik, H. Allergenicity and antigenicity of fenugreek (Trigonella foenum-graecum) proteins in foods. J. Allergy Clin. Immunol. 2009, 123, 187–194. [Google Scholar] [CrossRef]
- Ouzir, M.; El Bairi, K.; Amzazi, S. Toxicological properties of fenugreek (Trigonella foenum graecum). Food Chem. Toxicol. 2016, 96, 145–154. [Google Scholar] [CrossRef]
- Momtaz, S.; Hassani, S.; Khan, F.; Ziaee, M.; Abdollahi, M. Cinnamon, a promising prospect towards Alzheimer’s disease. Pharmacol. Res. 2018, 130, 241–258. [Google Scholar] [CrossRef] [PubMed]
- Tapsell, L.C.; Hemphill, I.; Cobiac, L.; Patch, C.S.; Sullivan, D.R.; Fenech, M.; Roodenrys, S.; Keogh, J.B.; Clifton, P.M.; Williams, P.G.; et al. Health benefits of herbs and spices: The past, the present, the future. Med. J. Aust. 2006, 185, 1–24. [Google Scholar] [CrossRef]
- Vasconcelos, N.G.; Croda, J.; Simionatto, S. Antibacterial mechanisms of cinnamon and its constituents: A review. Microb. Pathog. 2018, 120, 198–203. [Google Scholar] [CrossRef]
- Chanotiya, C.S.; Yadav, A. Enantioenriched (3s)-(+)-linalool in the leaf oil of cinnamomum tamala nees et eberm. From Kumaon. J. Essent. Oil Res. 2010, 22, 593–596. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Shi, Y.; Pan, X.; Lu, Y.; Cao, P. Antibacterial effects of cinnamon (Cinnamomum zeylanicum) bark essential oil on Porphyromonas gingivalis. Microb. Pathog. 2018, 116, 26–32. [Google Scholar] [CrossRef]
- Lu, M.; Yuan, B.; Zeng, M.; Chen, J. Antioxidant capacity and major phenolic compounds of spices commonly consumed in China. Food Res. Int. 2011, 44, 530–536. [Google Scholar] [CrossRef]
- Sharafeldin, K.; Rizvi, M.R. Effect of traditional plant medicines (Cinnamomum zeylanicum and Syzygium cumini) on oxidative stress and insulin resistance in streptozotocin-induced diabetic rats. J. Basic Appl. Zool. 2015, 72, 126–134. [Google Scholar] [CrossRef]
- Mirfeizi, M.; Mehdizadeh Tourzani, Z.; Mirfeizi, S.Z.; Asghari Jafarabadi, M.; Rezvani, H.R.; Afzali, M. Controlling type 2 diabetes mellitus with herbal medicines: A triple-blind randomized clinical trial of efficacy and safety. J. Diabetes 2016, 8, 647–656. [Google Scholar] [CrossRef] [PubMed]
- Vafa, M.; Mohammadi, F.; Shidfar, F.; Sormaghi, M.S.; Heidari, I.; Golestan, B.; Amiri, F. Effects of cinnamon consumption on glycemic status, lipid profile and body composition in type 2 diabetic patients. Int. J. Prev. Med. 2012, 3, 531–536. [Google Scholar]
- Unlu, M.; Ergene, E.; Unlu, G.V.; Zeytinoglu, H.S.; Vural, N. Composition, antimicrobial activity and in vitro cytotoxicity of essential oil from Cinnamomum zeylanicum Blume (Lauraceae). Food Chem. Toxicol. 2010, 48, 3274–3280. [Google Scholar] [CrossRef] [PubMed]
- Qadir, M.M.F.; Bhatti, A.; Ashraf, M.U.; Sandhu, M.A.; Anjum, S.; John, P. Immunomodulatory and therapeutic role of Cinnamomum verum extracts in collagen-induced arthritic BALB/c mice. Inflammopharmacology 2018, 26, 157–170. [Google Scholar] [CrossRef] [PubMed]
- Kamath, J.V.; Rana, A.C.; Roy Chowdhury, A. Pro-healing effect of Cinnamomum zeylanicum bark. Phyther. Res. 2003, 17, 970–972. [Google Scholar] [CrossRef]
- Jamali, N.; Jalali, M.; Saffari-Chaleshtori, J.; Samare-Najaf, M.; Samareh, A. Effect of cinnamon supplementation on blood pressure and anthropometric parameters in patients with type 2 diabetes: A systematic review and meta-analysis of clinical trials. Diabetes Metab. Syndr. Clin. Res. Rev. 2020, 14, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Zachariah, T.J. Ginger. In Chemistry of Spices; Parthasarathy, V.A., Chempakam, B., Zachariah, T.J., Eds.; CABI: London, UK, 2008; pp. 70–97. ISBN 1845934059. [Google Scholar]
- An, K.; Zhao, D.; Wang, Z.; Wu, J.; Xu, Y.; Xiao, G. Comparison of different drying methods on Chinese ginger (Zingiber officinale Roscoe): Changes in volatiles, chemical profile, antioxidant properties, and microstructure. Food Chem. 2016, 197, 1292–1300. [Google Scholar] [CrossRef]
- Malhotra, S.; Singh, A.P. Medicinal properties of Ginger (Zingiber officinale Rosc.). Nat. Prod. Radiance 2003, 2, 296–301. [Google Scholar]
- Shanmugam, K.R.; Mallikarjuna, K.; Nishanth, K.; Kuo, C.H.; Reddy, K.S. Protective effect of dietary ginger on antioxidant enzymes and oxidative damage in experimental diabetic rat tissues. Food Chem. 2011, 124, 1436–1442. [Google Scholar] [CrossRef]
- Young, H.Y.; Luo, Y.L.; Cheng, H.Y.; Hsieh, W.C.; Liao, J.C.; Peng, W.H. Analgesic and anti-inflammatory activities of [6]-gingerol. J. Ethnopharmacol. 2005, 96, 207–210. [Google Scholar] [CrossRef] [PubMed]
- Elshater, A.-E.A.; Salman, M.M.A.; Moussa, M.M.A. Effect of Ginger Extract Consumption on levels of blood Glucose, Lipid Profile and Kidney Functions in Alloxan Induced-Diabetic Rats. Egypt. Acad. J. Biol. Sci 2009, 2, 153–162. [Google Scholar] [CrossRef][Green Version]
- Sheikhhossein, F.; Borazjani, M.; Jafari, A.; Askari, M.; Vataniyan, E.; Gholami, F.; Amini, M.R. Effects of ginger supplementation on biomarkers of oxidative stress: A systematic review and meta-analysis of randomized controlled trials. Clin. Nutr. ESPEN 2021, 45, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, U.; Sharma, J.N.; Zafar, R. The protective action of ethanolic ginger (Zingiber officinale) extract in cholesterol fed rabbits. J. Ethnopharmacol. 1998, 61, 167–171. [Google Scholar] [CrossRef]
- Funk, J.L.; Frye, J.B.; Oyarzo, J.N.; Chen, J.; Zhang, H.; Timmermann, B.N. Anti-inflammatory effects of the essential oils of ginger (Zingiber officinale Roscoe) in experimental rheumatoid arthritis. PharmaNutrition 2016, 4, 123–131. [Google Scholar] [CrossRef]
- Jafarzadeh, A.; Nemati, M. Therapeutic potentials of ginger for treatment of Multiple sclerosis: A review with emphasis on its immunomodulatory, anti-inflammatory and anti-oxidative properties. J. Neuroimmunol. 2018, 324, 54–75. [Google Scholar] [CrossRef]
- Haksar, A.; Sharma, A.; Chawla, R.; Kumar, R.; Arora, R.; Singh, S.; Prasad, J.; Gupta, M.; Tripathi, R.P.; Arora, M.P.; et al. Zingiber officinale exhibits behavioral radioprotection against radiation-induced CTA in a gender-specific manner. Pharmacol. Biochem. Behav. 2006, 84, 179–188. [Google Scholar] [CrossRef]
- Ryan, J.L.; Morrow, G.R. Ginger. Oncol. Nurse Ed. 2010, 24, 46. [Google Scholar] [PubMed]
- Akhani, S.P.; Vishwakarma, S.L.; Goyal, R.K. Anti-diabetic activity of Zingiber officinale in streptozotocin-induced type I diabetic rats. J. Pharm. Pharmacol. 2004, 56, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Abdulrazaq, N.B.; Cho, M.; Win, N.N.; Zaman, R.; Rahman, M.T. Beneficial effects of ginger (Zingiber officinale) on carbohydrate metabolism in streptozotocin-induced diabetic rats. Br. J. Nutr. 2012, 108, 1194–1201. [Google Scholar] [CrossRef] [PubMed]
- Ramudu, S.K.; Korivi, M.; Kesireddy, N.; Lee, L.-C.; Cheng, I.-S.; Kuo, C.-H.; Kesireddy, S.R. Nephro-protective effects of a ginger extract on cytosolic and mitochondrial enzymes against streptozotocin (STZ)-induced diabetic complications in rats. Chin. J. Physiol. 2011, 54, 79–86. [Google Scholar] [CrossRef]
- Akash, M.S.H.; Rehman, K.; Tariq, M.; Chen, S. Zingiber officinale and type 2 diabetes mellitus: Evidence from experimental studies. Crit. Rev. Eukaryot. Gene Expr. 2015, 25, 91–112. [Google Scholar] [CrossRef]
- Rani, M.P.; Krishna, M.S.; Padmakumari, K.P.; Raghu, K.G.; Sundaresan, A. Zingiber officinale extract exhibits antidiabetic potential via modulating glucose uptake, protein glycation and inhibiting adipocyte differentiation: An in vitro study. J. Sci. Food Agric. 2012, 92, 1948–1955. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Tran, V.H.; Duke, C.C.; Roufogalis, B.D. Gingerols of Zingiber officinale enhance glucose uptake by increasing cell surface GLUT4 in cultured L6 myotubes. Planta Med. 2012, 78, 1549–1555. [Google Scholar] [CrossRef]
- El Gayar, M.H.; Aboromia, M.M.M.; Ibrahim, N.A.; Abdel Hafiz, M.H. Effects of ginger powder supplementation on glycemic status and lipid profile in newly diagnosed obese patients with type 2 diabetes mellitus. Obes. Med. 2019, 14, 100094. [Google Scholar] [CrossRef]
- Khandouzi, N.; Shidfar, F.; Rajab, A.; Rahideh, T.; Hosseini, P.; Taheri, M.M. The effects of Ginger on fasting blood sugar, hemoglobin A1c, apolipoprotein B, apolipoprotein A-I and malondialdehyde in type 2 diabetic patients. Iran. J. Pharm. Res. 2015, 14, 131–140. [Google Scholar]
- Shidfar, F.; Rajab, A.; Rahideh, T.; Khandouzi, N.; Hosseini, S.; Shidfar, S. The effect of ginger (Zingiber officinale) on glycemic markers in patients with type 2 diabetes. J. Complement. Integr. Med. 2015, 12, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Mahluji, S.; Attari, V.E.; Mobasseri, M.; Payahoo, L.; Ostadrahimi, A.; Golzari, S.E. Effects of ginger (Zingiber officinale) on plasma glucose level, HbA1c and insulin sensitivity in type 2 diabetic patients. Int. J. Food Sci. Nutr. 2013, 64, 682–686. [Google Scholar] [CrossRef]
- Li, Y.; Tran, V.H.; Duke, C.C.; Roufogalis, B.D. Preventive and protective properties of Zingiber officinale (Ginger) in diabetes mellitus, diabetic complications, and associated lipid and other metabolic disorders: A brief review. Evid.-Based Complement. Altern. Med. 2012, 2012, 516870. [Google Scholar] [CrossRef]
- Popowski, D.; Pawłowska, K.A.; Deipenbrock, M.; Hensel, A.; Kruk, A.; Melzig, M.F.; Piwowarski, J.P.; Granica, S. Antiadhesive activity of hydroethanolic extract from bean pods of Phaseolus vulgaris (common bean) against uropathogenic E. coli and permeability of its constituents through Caco-2 cells monolayer. J. Ethnopharmacol. 2021, 274, 114053. [Google Scholar] [CrossRef]
- Madrera, R.R.; Negrillo, A.C.; Valles, B.S.; Fernández, J.J.F. Phenolic content and antioxidant activity in seeds of common bean (Phaseolus vulgaris L.). Foods 2021, 10, 864. [Google Scholar] [CrossRef]
- Bljajić, K.; Brajković, A.; Čačić, A.; Vujić, L.; Jablan, J.; Carvalho, I.S.D.; Končić, M.Z. Chemical composition, antioxidant, and α-glucosidase-inhibiting activity of aqueous and hydroethanolic extracts of traditional antidiabetics from Croatian ethnomedicine. Horticulturae 2021, 7, 15. [Google Scholar] [CrossRef]
- Damián-Medina, K.; Salinas-Moreno, Y.; Milenkovic, D.; Figueroa-Yáñez, L.; Marino-Marmolejo, E.; Higuera-Ciapara, I.; Vallejo-Cardona, A.; Lugo-Cervantes, E. In silico analysis of antidiabetic potential of phenolic compounds from blue corn (Zea mays L.) and black bean (Phaseolus vulgaris L.). Heliyon 2020, 6, e03632. [Google Scholar] [CrossRef]
- Lomas-Soria, C.; Pérez-Ramírez, I.F.; Caballero-Pérez, J.; Guevara-Gonzalez, R.G.; Guevara-Olvera, L.; Loarca-Piña, G.; Guzman-Maldonado, H.S.; Reynoso-Camacho, R. Cooked common beans (Phaseolus vulgaris L.) modulate renal genes in streptozotocin-induced diabetic rats. J. Nutr. Biochem. 2015, 26, 761–768. [Google Scholar] [CrossRef]
- Halenova, T.; Raksha, N.; Kravchenko, O.; Vovk, T.; Yurchenko, A.; Vareniuk, I.; Savchuk, O.; Ostapchenko, L. Hypoglycemic activity of Phaseolus vulgaris (L.) aqueous extract in type 1 diabetic rats. Curr. Issues Pharm. Med. Sci. 2019, 32, 210–218. [Google Scholar] [CrossRef]
- Almuaigel, M.F.; Seif, M.A.; Albuali, H.W.; Alharbi, O.; Alhawash, A. Hypoglycemic and hypolipidemic effects of aqueous extract of Phaseolus vulgaris pods in streptozotocin-diabetic rats. Biomed. Pharmacother. 2017, 94, 742–746. [Google Scholar] [CrossRef]
- Ocho-Anin Atchibri, A.L.; Brou, K.D.; Kouakou, T.H.; Kouadio, Y.J.; Gnakri, D. Screening for antidiabetic activity and phytochemical constituents of common bean (Phaseolus vulgaris L.) seeds. J. Med. Plants Res. 2010, 4, 1757–1761. [Google Scholar] [CrossRef]
- Shi, Z.; Zhang, X.; Zhu, Y.; Yao, Y.; Ren, G. Natural extracts from white common bean (Phaseolus vulgaris L.) inhibit 3T3-L1 adipocytes differentiation. Appl. Sci. 2020, 11, 167. [Google Scholar] [CrossRef]
- Karłowicz-Bodalska, K. Żeń-szeń—wszechlek Dalekiego Wschodu. Postęoy Fitoter. 2004, 4, 183–188. [Google Scholar]
- Wolski, T.; Ludwiczuk, A.; Baj, T.; Głowniak, K.; Świątek, Ł. Rodzaj Panax—systematyka, skład chemiczny, działanie i zastosowanie oraz analiza fitochemiczna nadziemnych i podziemnych organów żeń-szenia amerykańskiego—Panax quinquefolium L. Cz. I. Postępy Fitoter. 2008, 2, 96–114. [Google Scholar]
- Park, H.J.; Kim, D.H.; Park, S.J.; Kim, J.M.; Ryu, J.H. Ginseng in traditional herbal prescriptions. J. Ginseng Res. 2012, 36, 225–241. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, S.C.; Lee, H.Y.; Cho, D.Y.; Jung, J.G.; Kang, D.; Kang, S.S.; Cho, K.M. Changes in nutritional compositions of processed mountain-cultivated ginseng sprouts (Panax ginseng) and screening for their antioxidant and anti-inflammatory properties. J. Funct. Foods 2021, 86, 104668. [Google Scholar] [CrossRef]
- Guo, N.; Yang, Y.; Yang, X.; Guan, Y.; Yang, J.; Quan, J.; Yan, H.; Hou, W.; Zhang, G. Growth age of mountain cultivated ginseng affects its chemical composition. Ind. Crops Prod. 2021, 167, 113531. [Google Scholar] [CrossRef]
- Qi, H.; Zhang, Z.; Liu, J.; Chen, Z.; Huang, Q.; Li, J.; Chen, J.; Wang, M.; Zhao, D.; Wang, Z.; et al. Comparisons of isolation methods, structural features, and bioactivities of the polysaccharides from three common Panax species: A review of recent progress. Molecules 2021, 26, 4997. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Xu, F.R.; Wang, Y.Z. Traditional uses, chemical diversity and biological activities of Panax L. (Araliaceae): A review. J. Ethnopharmacol. 2020, 263, 112792. [Google Scholar] [CrossRef]
- Szczuka, D.; Nowak, A.; Zakłos-Szyda, M.; Kochan, E.; Szymańska, G.; Motyl, I.; Blasiak, J. American ginseng (Panax quinquefolium L.) as a source of bioactive phytochemicals with pro-health properties. Nutrients 2019, 11, 1041. [Google Scholar] [CrossRef]
- Fan, S.; Zhang, Z.; Su, H.; Xu, P.; Qi, H.; Zhao, D.; Li, X. Panax ginseng clinical trials: Current status and future perspectives. Biomed. Pharmacother. 2020, 132, 110832. [Google Scholar] [CrossRef]
- Dzimitrowicz, A.; Cyganowski, P.; Pohl, P.; Milkowska, W.; Jermakowicz-Bartkowiak, D.; Jamroz, P. Plant extracts activated by cold atmospheric pressure plasmas as suitable tools for synthesis of gold nanostructures with catalytic uses. Nanomaterials 2020, 10, 1088. [Google Scholar] [CrossRef] [PubMed]
- Panossian, A.; Abdelfatah, S.; Efferth, T. Network pharmacology of ginseng (Part II): The differential effects of red ginseng and ginsenoside Rg5 in cancer and heart diseases as determined by transcriptomics. Pharmaceuticals 2021, 14, 1010. [Google Scholar] [CrossRef]
- Chou, C.-K.; Huang, Y.-S.; Lin, P.-Y.; Imai, K.; Chen, S.-M.; Lee, J.-A. The ginsenoside Rg1 rescues mitochondrial disorders in aristolochic acid-induced nephropathic mice. Life 2021, 11, 1018. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Park, K.H.; Kim, D.H.; Chae, H.J.; Sung, G.H.; Kim, Y.O. In vitro assessments of bone microcomputed tomography in an aged male rat model supplemented with Panax ginseng. Saudi J. Biol. Sci. 2018, 25, 1135–1139. [Google Scholar] [CrossRef]
- Hwang, J.H.; Park, S.H.; Choi, E.K.; Jung, S.J.; Pyo, M.K.; Chae, S.W. A randomized, double-blind, placebo-controlled pilot study to assess the effects of protopanaxadiol saponin–enriched ginseng extract and pectinase-processed ginseng extract on the prevention of acute respiratory illness in healthy people. J. Ginseng Res. 2020, 44, 697–703. [Google Scholar] [CrossRef]
- Lee, S.T.; Chu, K.; Sim, J.Y.; Heo, J.H.; Kim, M. Panax ginseng enhances cognitive performance in Alzheimer disease. Alzheimer Dis. Assoc. Disord. 2008, 22, 222–226. [Google Scholar] [CrossRef]
- Cui, Y.; Shu, X.-O.; Gao, Y.-T.; Cai, H.; Tao, M.-H.; Zheng, W. Association of ginseng use with survival and quality of life among breast cancer patients. Am. J. Epidemiol. 2006, 163, 645–653. [Google Scholar] [CrossRef]
- Jung, S.J.; Hwang, J.H.; Park, S.H.; Choi, E.K.; Ha, K.C.; Baek, H.I.; Shin, D.G.; Seo, J.H.; Chae, S.W. A 12-week, randomized, double-blind study to evaluate the efficacy and safety of liver function after using fermented ginseng powder (GBCK25). Food Nutr. Res. 2020, 64. [Google Scholar] [CrossRef]
- Kochan, E.; Nowak, A.; Zakłos-Szyda, M.; Szczuka, D.; Szymańska, G.; Motyl, I. Panax quinquefolium L. ginsenosides from hairy root cultures and their clones exert cytotoxic, genotoxic and pro-apoptotic activity towards human colon adenocarcinoma cell line Caco-2. Molecules 2020, 25, 2262. [Google Scholar] [CrossRef]
- Tardy, A.L.; De Fer, B.B.; Cañigueral, S.; Kennedy, D.; Scholey, A.; Hitier, S.; Aran, A.; Pouteau, E. Reduced self-perception of fatigue after intake of Panax ginseng root extract (G115®) formulated with vitamins and minerals—an open-label study. Int. J. Environ. Res. Public Health 2021, 18, 6257. [Google Scholar] [CrossRef]
- Yoon, J.W.; Kang, S.M.; Vassy, J.L.; Shin, H.; Lee, Y.H.; Ahn, H.Y.; Choi, S.H.; Park, K.S.; Jang, H.C.; Lim, S. Efficacy and safety of ginsam, a vinegar extract from Panax ginseng, in type 2 diabetic patients: Results of a double-blind, placebo-controlled study. J. Diabetes Investig. 2012, 3, 309–317. [Google Scholar] [CrossRef]
- Park, S.H.; Oh, M.R.; Choi, E.K.; Kim, M.G.; Ha, K.C.; Lee, S.K.; Kim, Y.G.; Park, B.H.; Kim, D.S.; Soo-Wan, C. An 8-wk, randomized, double-blind, placebo-controlled clinical trial for the antidiabetic effects of hydrolyzed ginseng extract. J. Ginseng Res. 2014, 38, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Oh, M.-R.; Park, S.-H.; Kim, S.-Y.; Back, H.-I.; Kim, M.-G.; Jeon, J.-Y.; Ha, K.-C.; Na, W.-T.; Cha, Y.-S.; Park, B.-H.; et al. Postprandial glucose-lowering effects of fermented red ginseng in subjects with impaired fasting glucose or type 2 diabetes: A randomized, double-blind, placebo-controlled clinical trial. BMC Complement. Altern. Med. 2014, 14, 237. [Google Scholar] [CrossRef]
- Hye Cho, Y.; Cheol Ahn, S.; Yeoup Lee, S.; Wook Jeong, D.; Jung Choi, E.; Jin Kim, Y.; Gyu Lee, J.; Lee, Y.-H.; Shin OMD, B.-C. Effect of Korean red ginseng on insulin sensitivity in non-diabetic healthy overweight and obese adults. Asia Pac. J. Clin. Nutr. 2013, 22, 365–371. [Google Scholar] [CrossRef]
- Kwon, D.H.; Bose, S.; Song, M.Y.; Lee, M.J.; Lim, C.Y.; Kwon, B.S.; Kim, H.J. Efficacy of Korean red ginseng by single nucleotide polymorphism in obese women: Randomized, double-blind, placebo-controlled trial. J. Ginseng Res. 2012, 36, 176. [Google Scholar] [CrossRef]
- Zhu, J.; Chen, H.; Song, Z.; Wang, X.; Sun, Z. Effects of ginger (Zingiber officinale Roscoe) on type 2 diabetes mellitus and components of the metabolic syndrome: A systematic review and meta-analysis of randomized controlled trials. Evid.-Based Complement. Altern. Med. 2018, 2018, 5692962. [Google Scholar] [CrossRef]
- Jeon, W.J.; Oh, J.S.; Park, M.S.; Ji, G.E. Anti-hyperglycemic effect of fermented ginseng in type 2 diabetes mellitus mouse model. Phyther. Res. 2013, 27, 166–172. [Google Scholar] [CrossRef]
- Kim, S.T.; Kim, H.B.; Lee, K.H.; Choi, Y.R.; Kim, H.J.; Shin, I.S.; Gyoung, Y.S.; Joo, S.S. Steam-dried ginseng berry fermented with Lactobacillus plantarum controls the increase of blood glucose and body weight in type 2 obese diabetic db/db mice. J. Agric. Food Chem. 2012, 60, 5438–5445. [Google Scholar] [CrossRef]
- Raposo, A.; Saraiva, A.; Ramos, F.; Carrascosa, C.; Raheem, D.; Bárbara, R.; Silva, H. The role of food supplementation in microcirculation—A comprehensive review. Biology 2021, 10, 616. [Google Scholar] [CrossRef]
| How Can the Medicinal Plant Be Introduced into the Diet? | ||
|---|---|---|
| First way | ||
| Eat on your own | Basic form: | whole leaves |
| whole seeds | ||
| whole shoots | ||
| whole fruits | ||
| Modified form: | ground | |
| crushed | ||
| dried | ||
| cut | ||
| Changed state of matter: | brew | |
| tea | ||
| extract | ||
| Second way | ||
| Change the matrix | Functional food products | |
| Dietary supplements | ||
| Medicinal Plant | Biologically Active Compound Probably Responsible for the Antidiabetic Activity | Main Antidiabetic Mechanism of Action on Organism | Source | |
|---|---|---|---|---|
| White mulberry Morus alba L. | 1.5-dideoxy-1.5-imino- D-sorbitol (DNJ) | 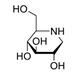 | inhibition of α-amylase; inhibition of α-glucosidase; hypolipidemic; antioxidant | [53,54,55] |
| morin | 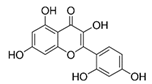 | [21,56] | ||
| Fenugreek Trigonella foenum-graecum L. | galactomannans | - | decrease blood glucose concentration | [57] |
| 4-hydroxyisoleucine | 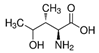 | [58] | ||
| saponins | - | [59] | ||
| trigonelline + nicotinic acid | 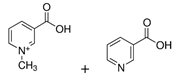 | [60] | ||
| Ceylon cinnamon Cinnamomum zeylanicum J.Presl | methylhydroxychalcone polymer | - | elevation in plasma insulin; hypoglycaemic; hypocholesterolemic; stimulate glucose uptake by adipocytes; | [61] |
| cinnamaldehyde | 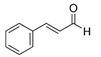 | [62,63] | ||
| eugenol | 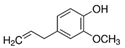 | [64] | ||
| Ginger Zingiber officinale Rosc. | shogaol | 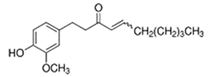 | increase insulin level; decrease fasting glucose level | [65,66] |
| gingerol | 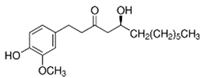 | [66,67] | ||
| Common bean Phaseolus vulgaris L. | phaseolamin | - | hypoglycaemic; inhibit α-amylase activity; antioxidant; hypolipidemic | [68] |
| Ginseng Panax ginseng C.A.Meyer | ginsenoside Rg1 | 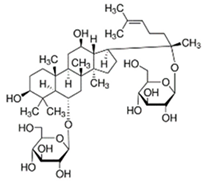 | lowering blood glucose level; slows down glucose absorption; obesity reduction; increase in the expression of GLUT-1 and GLUT-4 | [69] |
| ginsenoside C-K |  | [69] | ||
| ginsenoside Rg3 | 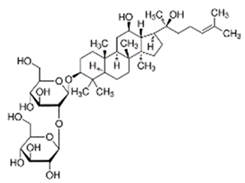 | [70] | ||
| ginsenoside Rb1 | 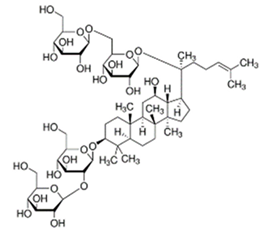 | [71] | ||
| Medicinal Plant | Morphological Element Used in Folk Medicine | Registered as Novel Food |
|---|---|---|
| Morus alba L. White mulberry | fruits | NO |
| young leaves | N/A * but authorized in food supplement use | |
| stems | N/A * but authorized in food supplement use | |
| rhizome (root bark) | N/A * but authorized in food supplement use | |
| root | N/A | |
| twigs | N/A | |
| Trigonella foenum-graecum L. Fenugreek | seeds | NO |
| leaves | N/A | |
| Cinnamomum zeylanicum J.Presl Ceylon cinnamon | bark of the branches | NO |
| leaves | NO * only applies to food supplements | |
| oil of the leaves | N/A | |
| flowers | N/A | |
| Phaseolus vulgaris L. Common bean | seeds | NO |
| pods | NO | |
| Zingiber officinale Rosc. Ginger | rhizome | NO |
| Panax ginseng C.A.Meyer Ginseng | root | NO |
| berries | NO | |
| leaves | NO | |
| oil | NO |
| Medicinal Plants | Effective Dose, Intake Duration, and Form of Plant Material | Effects of Consumption in In Vivo Models (Level of Change) | Source |
|---|---|---|---|
| Morus alba L. White mulberry | 20 mg/100 g b.w./d|5 w|leaf extract | R: FBG reduction (5%) | [19] |
| 0.8 g and 1.2 g|single dose|leaf powder enriched with DNJ (1.5%) | H: PPG60min, PPG90min inhibition, insulin secretion inhibition | [99] | |
| 100 mL (1 g of leaves)|tea | R: inhibition of α- glucosidase activity | [100] | |
| 400 mg/kg b.w.|7 w|fruits (polysaccharides) | R: FBG reduction (31.9–47.5%), FSI reduction (3.41–4.19 mIU/L), OGTT reduction (18.12–19.30) | [101] | |
| 6 g/kg b.w.|28 d|leaves with oat bran (1:1) | M: FBG reduction, PPG60min reduction, aspartate transaminase inhibition, alanine transaminase inhibition | [102] | |
| 20 mg and 40 mg and 80 mg/kg|4 w|DNJ extracted from leaves | M: BG reduction, b.w. reduction, SI reduction, HOMA-IR index reduction | [55] | |
| 30 mg/kg b.w.|4 w|morin from leaves | R: downregulation of PERK-eIF2α-ATF4 pathway, BG reduction (69.42%) | [56] | |
| 600 mg/kg b.w./d|6 w| ethanolic leaf extract or leaf powder | R: FBG reduction, TC reduction, TG reduction, LDL reduction; leaf powder more effective than leaf extract | [91] | |
| 2 g/kg b.w./d|4 w|leaf extract | R: FBG reduction, OGTT reduction, HOMA-IR reduction, TC reduction, TG reduction, LDL reduction, insulin resistance improved | [97] | |
| Trigonella foenum-graecum L. Fenugreek | 50 mg/d|30 d|seed powder solution | H: TC reduction (13.6%), TG reduction (23,53%), LDL reduction (23,4%), HDL improved (21.7%) | [1] |
| 50 mg/kg b.w./d|6 d|4-hydroxyisoleucine | R: BG reduction (from 163.5 mg/dL to 143.6 mg/dL), FSI reduction (from 1.96 ng/mL to 1.52 ng/mL), glucose tolerance improved | [58] | |
| 0.25 g and 0.5 g/kg b.w./d|14 d|ethanolic seeds extract | R: serum glucose reduction (similar to glibenclamide effect), TG reduction, TC reduction, b.w. reduction (5.5% and 9.5%) | [118] | |
| 100 mg/kg b.w./d|4 w|fenugreek extract | R: BG reduction, level of liver enzymes (aspartate aminotransferase and alanine aminotransferase) reduction, TG reduction | [20] | |
| 500 mg/d|30 d or 60 d or 90 d|seed extract enriched with 40% furostanolic saponins | H: FBG reduction (6.69%, 10.31%, 21.98%); PPG60min (13.7%, 20.6%, 30.4%); HbA1c reduction | [119] | |
| 1000 mg/d|12 w|seed extract | H: FBG reduction (24.62%), HbA1c reduction (9.38%); TC reduction (5.66%), TG reduction (17.23%), LDL reduction (4.15%) | [120] | |
| Cinnamomum zeylanicum J.Presl Ceylon cinnamon | 5 mg and 10 mg and 20 mg/kg b.w./d|45 d |cinnamaldehyde | R: BG reduction (60.8, 139.3 and 219.0 mg/dL) | [62] |
| 200 mg/kg b.w./w|4 w|ethanolic extract | R: BG reduction (from 257 mg/dL to 122.9 mg/dL), HbA1c reduction (2.51%) | [129] | |
| 1 g/d|90 d| cinnamon supplement | H: PPG90min reduced to 224 mg/dL (with cinnamon) vs. reduced to 270 mg/dL (without cinnamon) | [130] | |
| 3000 mg/d|8 w|cinnamon powder | H: SI reduction (by 12.87 mIU/L), FBG reduction (by 0.45 mg/dL) | [131] | |
| Zingiber officinale Rosc. Ginger | 25 mg and 50 mg and 100 mg and 200 mg/kg b.w./d|single dose|aqueous extract | R: edemas reduction, NOx reduction similar to indomethacin | [66] |
| 4 mL/kg b.w./d|6 w|ginger juice | R: flattening BG curve, flattening the insulinemia curve | [148] | |
| 100–500 mg/kg b.w./d|30 d|aqueous ginger extract | R: activity of glycolytic enzymes improved | [149] | |
| 200 mg/kg b.w./d|30 d|ethanolic ginger extract | R: reversed hyperglycaemia, activity of extra-mitochondrial and intra-mitochondrial enzymes improved | [150] | |
| 1800 mg/d|8w|dried ginger | H: BMI reduction (0.54 kg/m2), HbA1c (1.11%), FBG (51.15 mg/dL), FSI (7.88 mIU/L), TC (31.10 mg/dL), LDL (17.70 mg/dL) | [154] | |
| 2 g/d|12 w|ginger powder | H: FBG reduction (19,41 mg/dL); HbA1c reduction (0.77%); apolipoprotein B reduction (12.45 mg/dL) | [155] | |
| 3 g/d|12 w|ginger powder | H: serum glucose reduction (19.41 mg/dL), HbA1c (0.77%), SI reduction (1.46 µIU/mL), insulin resistance reduction (16.38); high-sensitive CRP reduction (2.78 mg/dL) | [156] | |
| 2 g/d|8 w|ginger powder | H: SI reduction (13µU/mL), LDL reduction (13.7%), TG reduction (11.7%), HOMA-IR reduction (8.1%) | [157] | |
| Phaseolus vulgaris L. Common bean | 200 mg and 400 mg/kg b.w./d|28 d|aqueous ginger extract | R: GLUT-4 in skeletal muscles increase | [164] |
| 100 mg/kg b.w./d|2 w or 4 w|cooked common bean | R: FBG reduction (25% or 35%) | [163] | |
| 50 mg and 100 mg and 200 mg and 250 mg/kg b.w./d|40 d|aqueous bean extract | R: FBG reduction (25% or 50%), TC reduction, TG reduction | [165] | |
| 300 mg/kg b.w./d|single dose|bean | R: dose of glibenclamide reduction to improve glycaemia | [166] | |
| Panax ginseng C.A.Meyer Ginseng | 1500–3000 mg/d|8 w|vinegar ginseng extract | H: HbA1c reduction (0.29–0.56%), FBG reduction (6.76–21.4 mg/dL) compared to placebo | [187] |
| 2 capsules/d|8 w|30% of hydrolyzed ginseng extract | H: FBG reduction, PPG60min | [188] | |
| 2.7 g/d|4 w|fermented ginseng | H: PPG120min reduction (17.2%), glucose curve flattened (27.4%), no effect on FBG | [189] | |
| 6 g/d|12 w|ginseng | H: no effect on SI level, no effect on insulin sensitivity | [190] | |
| 6 g/d|8 w|ginseng | H: obesity level reduced | [191] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Przeor, M. Some Common Medicinal Plants with Antidiabetic Activity, Known and Available in Europe (A Mini-Review). Pharmaceuticals 2022, 15, 65. https://doi.org/10.3390/ph15010065
Przeor M. Some Common Medicinal Plants with Antidiabetic Activity, Known and Available in Europe (A Mini-Review). Pharmaceuticals. 2022; 15(1):65. https://doi.org/10.3390/ph15010065
Chicago/Turabian StylePrzeor, Monika. 2022. "Some Common Medicinal Plants with Antidiabetic Activity, Known and Available in Europe (A Mini-Review)" Pharmaceuticals 15, no. 1: 65. https://doi.org/10.3390/ph15010065
APA StylePrzeor, M. (2022). Some Common Medicinal Plants with Antidiabetic Activity, Known and Available in Europe (A Mini-Review). Pharmaceuticals, 15(1), 65. https://doi.org/10.3390/ph15010065






