Synthesis of Novel (S)-3-(1-Aminoethyl)-8-pyrimidinyl-2-phenylisoquinolin-1(2H)-ones by Suzuki–Miyaura Coupling and Their Cell Toxicity Activities
Abstract
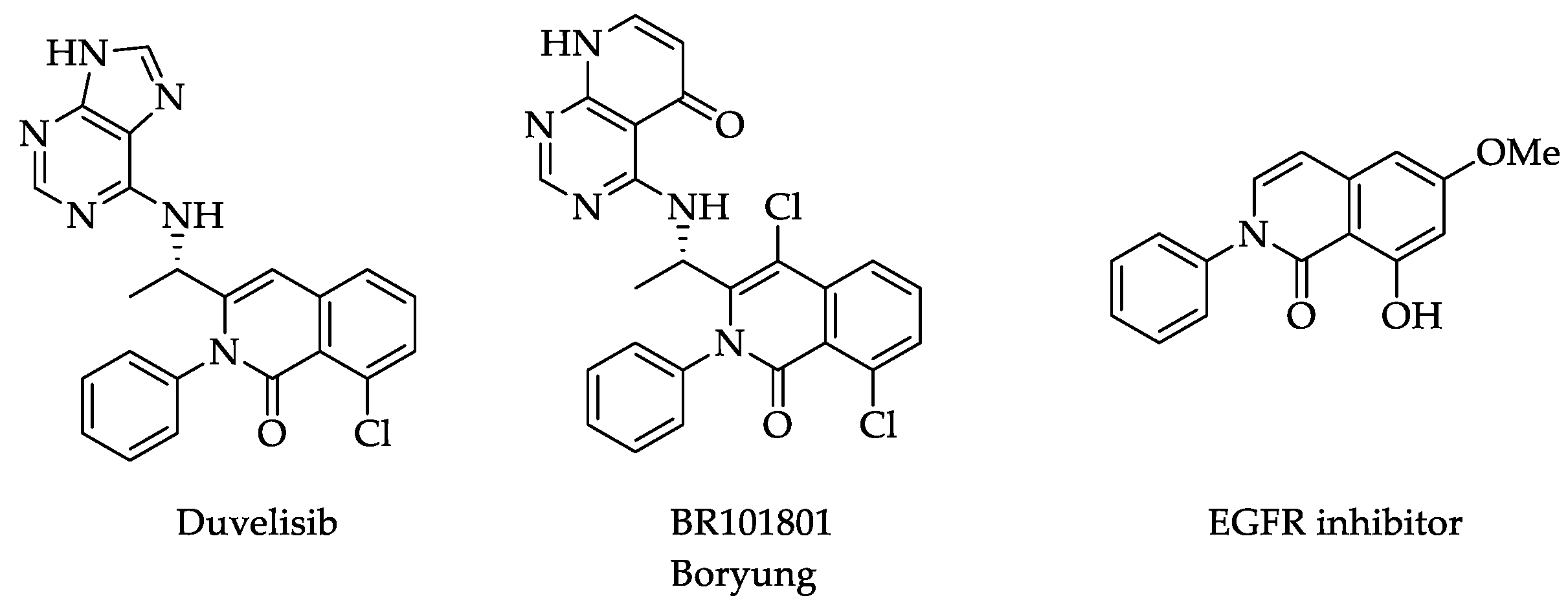
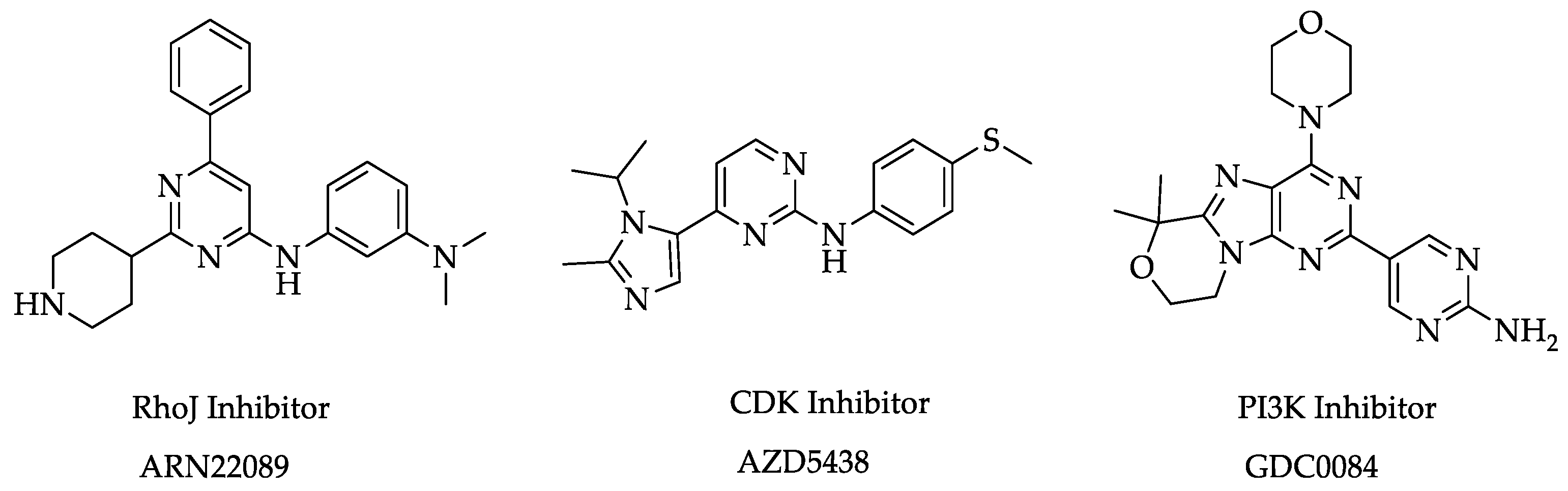
:1. Introduction
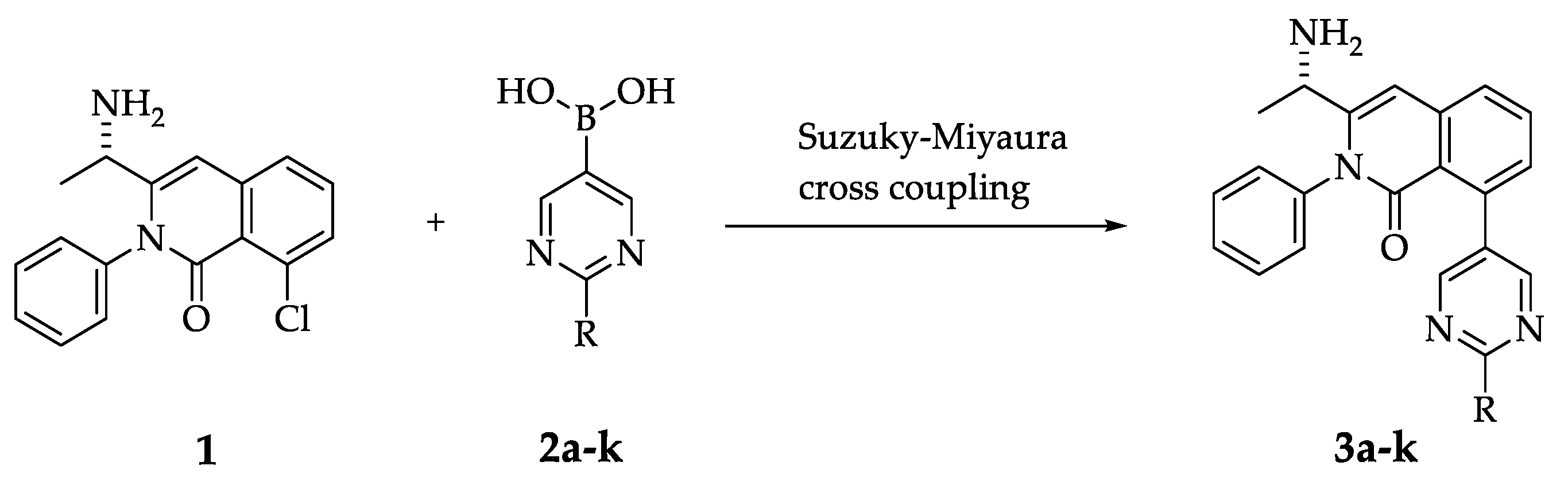
2. Results and Discussion
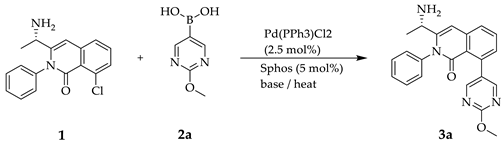
2.1. Synthesis of Compounds
2.2. Anti-Cancer Activity
3. Materials and Methods
3.1. General
3.2. General Procedure for the Suzuki-Miyaura Coupling Reactions (3a~3k)
3.3. Scale up Procedure for the Synthesis of 3-[(1S)-1-Aminoethyl]-8-(2-Methoxypyrimidin-5-yl)-2-Phenyl-Isoquinolin-1-one (3a)
3.4. Cell Culture and Viability Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Glushkov, V.A.; Shklyaev, Y.V. Synthesis of 1(2H)-isoquinolones. Chem. Heterocycl. Compd. 2001, 37, 663. [Google Scholar] [CrossRef]
- Matsui, T.; Sugiura, T.; Nakui, H.; Iguch, S.; Shigeoka, S.; Tukedu, H.; Odagaki, T.; Ushio, Y.; Ohmoto, K.; Iwamani, M.; et al. Novel 5-HT3A ntagonists. Isoquinolinones and 3-Aryl-2-pyridones. J. Med. Chem. 1992, 35, 3307. [Google Scholar] [CrossRef] [PubMed]
- Chao, Q.; Deng, L.; Shih, H.; Leoni, L.M.; Genini, D.; Carson, D.A.; Cottam, H.B. Substituted Isoquinolines and Quinazolines as Potential Antiinflammatory Agents. Synthesis and Biologocal Evaluation of Inhibitors of Tumor Necrosis Factor α. J. Med. Chem. 1999, 42, 3860–3873. [Google Scholar] [CrossRef] [PubMed]
- Evans, C.A.; Liu, T.; Lescarbeau, A.; Nair, S.J.; Grenier, L.; Pradeilles, J.A.; Glenadel, Q.; Tibbitts, T.; Rowley, A.M.; DiNitto, J.P.; et al. Discovery of a Selective Phosphoinositide-3-Kinase (PI3K)-γ Inhibitor (IPI-549) as an Immuno-Oncology Clinical Candidate. ACS Med. Chem. Lett. 2016, 7, 862–867. [Google Scholar] [CrossRef] [Green Version]
- Kang, B.R.; Shan, A.L.; Li, Y.P.; Xu, J.; Lu, S.M.; Zhang, S.Q. Discovery of 2-aryl-8-hydroxy (or methoxy)-isoquinolin-1 (2H)-ones as Novel EGFR Inhibitor by Scaffold Hopping. Bioorg. Med. Chem. 2013, 21, 6956–6964. [Google Scholar] [CrossRef]
- Rodrigues, D.A.; Sagrillo, F.S.; Fraga, C.A.M. Duvelisib: A 2018 Novel FDA-Approved Small Molecule Inhibiting Phosphoinositide 3-Kinases. Pharmaceuticals 2019, 12, 69. [Google Scholar] [CrossRef] [Green Version]
- Son, M.K.; Jeon, B.; Wang, J.S.; Kim, B.K.; Lee, B.R.; Choi, Y.S.; Kim, N.H.; Myung, J.; Kim, D.H. BR101801: A first-in-class dual inhibitor of PI3Kδ and DNA-PK in non-Hodgkin’s lymphoma. Cancer Res. 2018, 78, 871. [Google Scholar] [CrossRef]
- Flesher, J.L.; Jahid, S.; Ortega, J.A.; Sala, G.L.; Brindani, N.; Arencibia, J.M.; Manigrasso, J.; Hachey, S.; Chen, C.; Hughes, C.; et al. Structure-based design of CDC42/RHOJ effector inhibitors for the treatment of cancer. Cancer Res. 2020, 80, 5324. [Google Scholar] [CrossRef]
- Byth, K.F.; Thomas, A.; Hughes, G.; Forder, C.; McGregor, A.; Geh, C.; Oakes, S.; Green, C.; Walker, M.; Newcombe, N.; et al. AZD5438, a Potent Oral Inhibitor of Cyclin-Dependent Kinases 1, 2, And 9, Leads to Pharmacodynamic Changes and Potent Antitumor Effects in Human Tumor Xenografts. Mol. Cancer Ther. 2009, 8, 1856–1866. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heffron, T.P.; Ndubaku, C.O.; Salphati, L.; Alicke, B.; Cheong, J.; Drobnick, J.; Edgar, K.; Gould, S.E.; Lee, L.B.; Lesnick, J.D.; et al. Discovery of Clinical Development Candidate GDC-0084, a Brain Penetrant Inhibitor of PI3K and mTOR. ACS Med. Chem. Lett. 2016, 7, 351–356. [Google Scholar] [CrossRef] [Green Version]
- Roy, D.; Uozumi, Y. Recent Advances in Palladium-Catalyzed Cross-Coupling Reactions at ppm to ppb Molar Catalyst Loadings. Adv. Synth. Catal. 2018, 360, 620. [Google Scholar] [CrossRef]
- Littke, A.F.; Dai, C.; Fu, G.C. Versatile Catalysts for the Suzuki Cross-Coupling of Arylboronic Acids with Aryl and Vinyl Halides and Triflates under Mild Conditions. J. Am. Chem. Soc. 2000, 122, 4020–4028. [Google Scholar] [CrossRef]
- Ashworth, I.W.; Campbell, A.D.; Cherryman, J.H.; Clark, J.; Crampton, A.; Eden-Rump, E.G.; Evans, M.; Jones, M.F.; McKeever-Abbas, S.; Meadows, R.E.; et al. Process development of a Suzuki reaction used in the manufacture of lanabecestat. Org. Process Res. Dev. 2018, 22, 1801–1808. [Google Scholar] [CrossRef]
- Irfan, M.; Fuchs, M.; Glasnov, T.N.; Kappe, C.O. Microwave-Assisted Cross-Coupling and Hydrogenation Chemistry by Using Heterogeneous Transition-Metal Catalysts: An Evaluation of the Role of Selective Catalyst Heating. Chem. Eur. J. 2009, 15, 11608–11618. [Google Scholar] [CrossRef]
- Lee, G.H.; Lim, H.J.; Cho, H.Y.; Park, W.K.; Kim, S.H.; Choi, J.H. Heteroaryl Derivative or Pharmaceutically Acceptable Salt Thereof, Preparation Method Therefor, and Pharmaceutical Composition for Preventing or Treating Diseases Associated with Pi3 Kinases, Containing Same as Active Ingredient. U.S. Patent WO2016204429, 22 December 2016. [Google Scholar]
- Knapp, D.M.; Gillis, E.P.; Burke, M.D. A General Solution for Unstable Boronic Acids: Slow-Release Cross-Coupling from Air-Stable MIDA Boronates. J. Am. Chem. Soc. 2009, 131, 6961–6963. [Google Scholar] [CrossRef]
- Bender, A.M.; Chopko, T.C.; Bridges, T.M.; Lindsley, C.W. Preparation of Unsymmetrical 1,2,4,5-Tetrazines via a Mild Suzuki Cross-Coupling Reaction. Org. Lett. 2017, 19, 5693–5696. [Google Scholar] [CrossRef]
- Takahashi, R.; Kubota, K.; Ito, H. Air- and Moisture-Stable Xantphos-Ligated Palladium Dialkyl Complex as a Precatalyst for Cross-Coupling Reactions. Chem. Commun. 2020, 56, 407–410. [Google Scholar] [CrossRef]
- Martin, R.; Buchwald, S.L. Palladium-Catalyzed Suzuki-Miyaura Cross-Coupling Reactions Employing Dialkylbiaryl Phosphine Ligands. Acc. Chem. Res. 2008, 41, 1461–1473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolfe, J.P.; Singer, R.A.; Yang, B.H.; Buchwald., S.L. Highly Active Palladium Catalysts for Suzuki Coupling Reactions. J. Am. Chem. Soc. 1999, 121, 9550–9561. [Google Scholar] [CrossRef]
- Billingsley, K.; Buchwald, S.L. Highly Efficient Monophosphine-Based Catalyst for the Palladium-Catalyzed Suzuki-Miyaura Reaction of Heteroaryl Halides and Heteroaryl Boronic Acids and Esters. J. Am. Chem. Soc. 2007, 129, 3358–3366. [Google Scholar] [CrossRef] [PubMed]
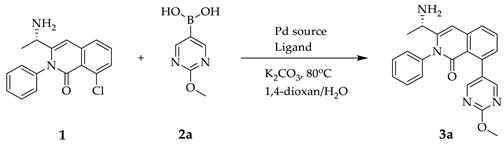 | ||||
|---|---|---|---|---|
| Entry | Catalyst (Mol%) | Ligand (Mol%) | 3a(%) b | 1(%) b |
| 1 | Pd(PPh3)4 (5) | - | 18.5 | 74.5 |
| 2 | Pd(PPh3)Cl2 (5) | - | 28.7 | 63.5 |
| 3 | Pd(OAc)2 (5) | PPh3 (10) | 19.4 | 73.3 |
| 4 | PdCl2 (5) | PPh3 (10) | 21.4 | 72.4 |
| 5 | Pd(PPh3)2Cl2 (5) | PPh3 (10) | 39.2 | 53.5 |
| 6 | Pd(PPh3)2Cl2 (2.5) | PPh3 (5) | 28.2 | 64.5 |
| 7 | Pd(PPh3)2Cl2 (2.5) | P(O-tol)3 (5) | 31.2 | 62.9 |
| 8 | Pd(PPh3)2Cl2 (2.5) | P(Cy)3 (5) | 66.2 | 27.4 |
| 9 | Pd(PPh3)2Cl2 (2.5) | Dppf (5) | 35.6 | 58.4 |
| 10 | Pd(PPh3)2Cl2 (2.5) | Aphos (5) | 76.9 | 17.8 |
| 11 | Pd(PPh3)2Cl2 (2.5) | Xantphos (5) | 65.6 | 29.2 |
| 12 | Pd(PPh3)2Cl2 (2.5) | Xphos (5) | 86.3 | 9.0 |
| 13 | Pd(PPh3)2Cl2 (2.5) | Sphos (5) | 96.5 a | 0 |
| 14 | Pd(PPh3)2Cl2 (2.5) | Ruphos (5) | 96.4 a | 0 |
| 15 | Pd(PPh3)2Cl2 (2.5) | Davephos (5) | 96.1 a | 0 |
 | |||||
|---|---|---|---|---|---|
| Entry | Base | Solvent | T(℃) | 3a(%) c | 1(%) c |
| 1 | - | 1,4-dioxane/H2O a | 80 | 0 | 96.0 |
| 2 | KtOBu | 1,4-dioxane/H2O a | 80 | 31.4 | 0 |
| 3 | Na2CO3 | 1,4-dioxane/H2O a | 80 | 96.0 | 0 |
| 4 | KOH | 1,4-dioxane/H2O a | 80 | 35.4 | 0 |
| 5 | KOAc | 1,4-dioxane/H2O a | 80 | 95.1 | 0.1 |
| 6 | Cs2CO3 | 1,4-dioxane/H2O a | 80 | 96.9 | 0.1 |
| 7 | K2CO3 | 1,4-dioxane/H2O a | 80 | 96.9 | 0 |
| 8 | K2CO3 | THF | 64 b | 25.1 | 70.3 |
| 9 | K2CO3 | 1,4-dioxane | 104 b | 9.1 | 67.5 |
| 10 | K2CO3 | EtOH | 80 b | 87.4 | 0 |
| 11 | K2CO3 | H2O | 101 b | 49.7 | 45.3 |
| 12 | K2CO3 | THF/H2O a | 65 b | 99.0 | 0 |
| 13 | K2CO3 | EtOH/H2O a | 78 b | 97.6 | 0 |
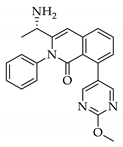 | 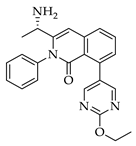 | 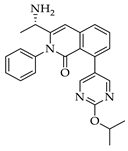 | 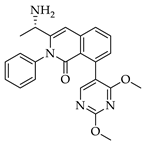 |
| 3a 98% a | 3b 97% a | 3c 95% b | 3d 45% b |
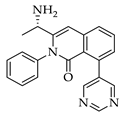 | 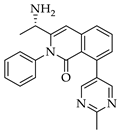 | 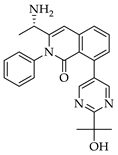 | 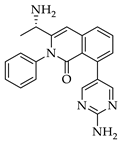 |
| 3e 91% b | 3f 95% b | 3g 94% c | 3h 61% b |
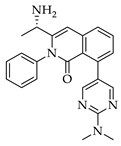 | 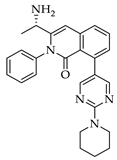 | 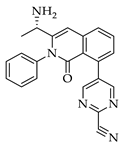 | |
| 3i 87% a | 3j 92% a | 3k 40% d |
| Entry | Compound | MDA-MB231 IC50 (μM) a | HeLa IC50 (μM) a | HepG2 IC50 (μM) a |
|---|---|---|---|---|
| 1 | 1 | ND b | ND b | ND b |
| 2 | 3a | ND b | 5.07 ± 0.13 | 2.20 ± 0.26 |
| 3 | 3b | 2.28 ± 0.10 | 1.55 ± 0.15 | 2.01 ± 0.05 |
| 4 | 3c | 2.83 ± 0.10 | 1.86 ± 0.12 | 1.79 ± 0.13 |
| 5 | 3d | ND b | 2.07 ± 0.46 | 4.85 ± 0.21 |
| 6 | 3e | ND b | 2.12 ± 0.17 | 5.12 ± 0.23 |
| 7 | 3f | ND b | 5.38 ± 0.22 | 5.22 ± 0.23 |
| 8 | 3g | 5.25 ± 0.09 | 2.43 ± 0.25 | 5.27 ± 0.39 |
| 9 | 3h | 2.72 ± 0.24 | 1.94 ± 0.11 | 2.93 ± 0.10 |
| 10 | 3i | 2.29 ± 0.11 | 1.42 ± 0.19 | 3.08 ± 0.20 |
| 11 | 3j | 1.62 ± 0.12 | 1.11 ± 0.70 | 3.87 ± 1.58 |
| 12 | 3k | 1.18 ± 0.08 | 1.99 ± 0.28 | 1.57 ± 0.17 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, O.K.; Sun, Y.H.; Lee, H.; Lee, J.K.; Lee, T.H.; Kim, H. Synthesis of Novel (S)-3-(1-Aminoethyl)-8-pyrimidinyl-2-phenylisoquinolin-1(2H)-ones by Suzuki–Miyaura Coupling and Their Cell Toxicity Activities. Pharmaceuticals 2022, 15, 64. https://doi.org/10.3390/ph15010064
Choi OK, Sun YH, Lee H, Lee JK, Lee TH, Kim H. Synthesis of Novel (S)-3-(1-Aminoethyl)-8-pyrimidinyl-2-phenylisoquinolin-1(2H)-ones by Suzuki–Miyaura Coupling and Their Cell Toxicity Activities. Pharmaceuticals. 2022; 15(1):64. https://doi.org/10.3390/ph15010064
Chicago/Turabian StyleChoi, Ok Kyoung, Yong Ho Sun, Hyemi Lee, Joon Kwang Lee, Tae Hoon Lee, and Hakwon Kim. 2022. "Synthesis of Novel (S)-3-(1-Aminoethyl)-8-pyrimidinyl-2-phenylisoquinolin-1(2H)-ones by Suzuki–Miyaura Coupling and Their Cell Toxicity Activities" Pharmaceuticals 15, no. 1: 64. https://doi.org/10.3390/ph15010064
APA StyleChoi, O. K., Sun, Y. H., Lee, H., Lee, J. K., Lee, T. H., & Kim, H. (2022). Synthesis of Novel (S)-3-(1-Aminoethyl)-8-pyrimidinyl-2-phenylisoquinolin-1(2H)-ones by Suzuki–Miyaura Coupling and Their Cell Toxicity Activities. Pharmaceuticals, 15(1), 64. https://doi.org/10.3390/ph15010064






