Synthesis and Evaluation of 3-Halobenzo[b]thiophenes as Potential Antibacterial and Antifungal Agents
Abstract
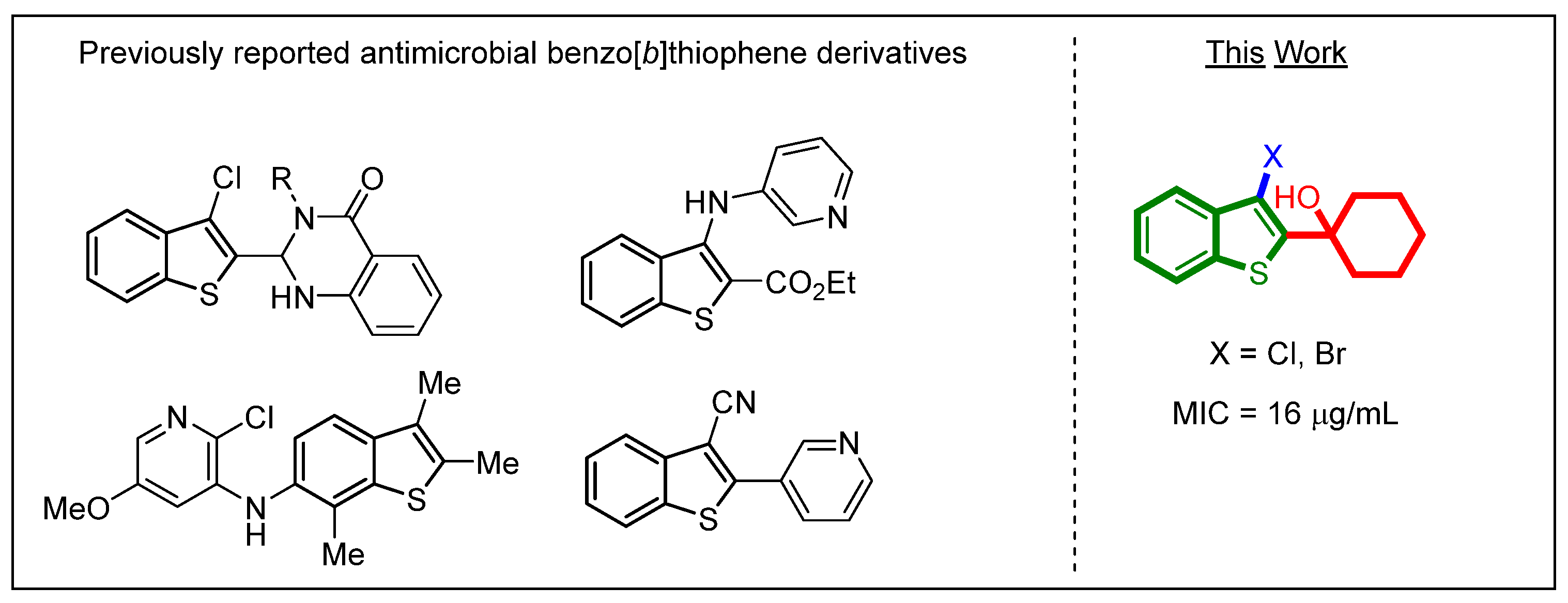
:1. Introduction
2. Results and Discussion
2.1. Synthesis of Benzo[b]thiophene Derivatives
2.2. Minimum Inhibitory Concentration Determination
2.3. Time-Kill Kinetics
2.4. In Silico ADME Properties
3. Materials and Methods
3.1. General Procedure for the Electrophilic Cyclization Reaction
3.1.1. 3-chloro-2-cyclohexylbenzo[b]thiophene (28)
3.1.2. 3-bromo-2-cyclohexylbenzo[b]thiophene (29)
3.1.3. 2-(3-chlorobenzo[b]thiophen-2-yl)propan-2-ol (30)
3.1.4. 2-(3-bromobenzo[b]thiophen-2-yl)propan-2-ol (31)
3.1.5. 1-(3-chlorobenzo[b]thiophen-2-yl)cyclopentan-1-ol (32)
3.2. Chemicals and Microbial Strains
3.3. Determination of Minimum Inhibitory Concentration Values
3.4. Time-Kill Assay
3.5. Predicted In Silico ADME Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guo, Y.; Song, G.; Sun, M.; Wang, J.; Wang, Y. Prevalence and Therapies of Antibiotic-Resistance in Staphylococcus aureus. Front. Cell. Infect. Microbiol. 2020, 10, 107. [Google Scholar] [CrossRef] [Green Version]
- Joshi, S.; Shallal, A.; Zervos, M. Vancomycin-Resistant Enterococci: Epidemiology, Infection Prevention, and Control. Infect. Dis. Clin. N. Am. 2021, 35, 953–968. [Google Scholar] [CrossRef] [PubMed]
- Holmes, A.H.; Moore, L.S.; Sundsfjord, A.; Steinbakk, M.; Regmi, S.; Karkey, A.; Guerin, P.J.; Piddock, L.J. Understanding the mechanisms and drivers of antimicrobial resistance. Lancet 2016, 387, 176–187. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). Antibiotic Resistance Threats in the United States, 2019; U.S. Department of Health and Human Services, CDC: Atlanta, GA, USA, 2019.
- Levy, S.B.; Marshall, B. Antibacterial resistance worldwide: Causes, challenges and responses. Nat. Med. 2004, 10, S122–S129. [Google Scholar] [CrossRef]
- Durand, G.A.; Raoult, D.; Dubourg, G. Antibiotic discovery: History, methods and perspectives. Int. J. Antimicrob. Agents 2019, 53, 371–382. [Google Scholar] [CrossRef]
- Naganagowda, G.; Padmashali, B. Utility of 3-Chlorobenzothiophene-2-Carbonylisothiocyanate for the Synthesis of Some Novel Biheterocycles of Expected Biological Activity. Phosphorus Sulfur Silicon Relat. Elem. 2010, 185, 1369–1380. [Google Scholar] [CrossRef]
- Pinto, E.; Queiroz, M.-J.R.P.; Vale-Silva, L.A.; Oliveira, J.F.; Begouin, A.; Begouin, J.-M.; Kirsch, G. Antifungal activity of synthetic di(hetero)arylamines based on the benzo[b]thiophene moiety. Bioorganic Med. Chem. 2008, 16, 8172–8177. [Google Scholar] [CrossRef]
- Ryu, C.-K.; Lee, S.-K.; Han, J.-Y.; Jung, O.-J.; Lee, J.Y.; Jeong, S.H. Synthesis and antifungal activity of 5-arylamino-4,7-dioxobenzo[b]thiophenes. Bioorganic Med. Chem. Lett. 2005, 15, 2617–2620. [Google Scholar] [CrossRef]
- Martorana, A.; Gentile, C.; Perricone, U.; Piccionello, A.P.; Bartolotta, R.; Terenzi, A.; Pace, A.; Mingoia, F.; Almerico, A.M.; Lauria, A. Synthesis, antiproliferative activity, and in silico insights of new 3-benzoylamino-benzo[b]thiophene derivatives. Eur. J. Med. Chem. 2015, 90, 537–546. [Google Scholar] [CrossRef]
- Jianqi, L.; Kai, G.; Na, L. Benzothiophene Alkanol Piperazine Derivatives and Their Use as Antidepressant. U.S. Patent No. 8,680,097, 25 March 2014. [Google Scholar]
- Wardakhan, W.W.; Abdel-Salam, O.M.; Elmegeed, G.A. Screening for antidepressant, sedative and analgesic activities of novel fused thiophene derivatives. Acta Pharm. 2008, 58, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Rask-Madsen, J.; Bukhave, K.; Laursen, L.S.; Lauritsen, K. 5-Lipoxygenase inhibitors for the treatment of inflammatory bowel disease. Agents Actions 1992, 36, C37–C46. [Google Scholar] [CrossRef]
- Fakhr, I.M.I.; Radwan, M.A.A.; El-Batran, S.; Abd El-Salam, O.M.E.; El-Shenawy, S.M. Synthesis and pharmacological evaluation of 2-substituted benzo[b]thiophenes as anti-inflammatory and analgesic agents. Eur. J. Med. Chem. 2009, 44, 1718–1725. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, I.C.; Queiroz, M.J.; Vilas-Boas, M.; Estevinho, L.M.; Begouin, A.; Kirsch, G. Evaluation of the antioxidant properties of diarylamines in the benzo[b]thiophene series by free radical scavenging activity and reducing power. Bioorg. Med. Chem. Lett. 2006, 16, 1384–1387. [Google Scholar] [CrossRef]
- Liu, F.; Hu, J.; Chang, L.; Jiang, Y. In vitro anti-mycobacterial activity of novel benzo(c)thiophene-1,3-dione: A novel scaffold against Mycobacterium tuberculosis. Microb. Pathog. 2020, 148, 104466. [Google Scholar] [CrossRef]
- Chandrasekera, N.S.; Bailey, M.A.; Files, M.; Alling, T.; Florio, S.K.; Ollinger, J.; Odingo, J.O.; Parish, T. Synthesis and anti-tubercular activity of 3-substituted benzo[b]thiophene-1,1-dioxides. PeerJ 2014, 2, e612. [Google Scholar] [CrossRef] [Green Version]
- Keri, R.S.; Chand, K.; Budagumpi, S.; Balappa Somappa, S.; Patil, S.A.; Nagaraja, B.M. An overview of benzo[b]thiophene-based medicinal chemistry. Eur. J. Med. Chem. 2017, 138, 1002–1033. [Google Scholar] [CrossRef]
- Santillan, A., Jr.; McClure, K.J.; Allison, B.D.; Lord, B.; Boggs, J.D.; Morton, K.L.; Everson, A.M.; Nepomuceno, D.; Letavic, M.A.; Lee-Dutra, A.; et al. Indole- and benzothiophene-based histamine H3 antagonists. Bioorg. Med. Chem. Lett. 2010, 20, 6226–6230. [Google Scholar] [CrossRef]
- Johnson, D.S.; Ahn, K.; Kesten, S.; Lazerwith, S.E.; Song, Y.; Morris, M.; Fay, L.; Gregory, T.; Stiff, C.; Dunbar, J.B.; et al. Benzothiophene piperazine and piperidine urea inhibitors of fatty acid amide hydrolase (FAAH). Bioorganic Med. Chem. Lett. 2009, 19, 2865–2869. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davis, R.L.; Kahraman, M.; Prins, T.J.; Beaver, Y.; Cook, T.G.; Cramp, J.; Cayanan, C.S.; Gardiner, E.M.M.; McLaughlin, M.A.; Clark, A.F.; et al. Benzothiophene containing Rho kinase inhibitors: Efficacy in an animal model of glaucoma. Bioorganic Med. Chem. Lett. 2010, 20, 3361–3366. [Google Scholar] [CrossRef]
- Duc, X.D. Recent Progress in the Synthesis of Benzo[b]thiophene. Curr. Org. Chem. 2020, 24, 2256–2271. [Google Scholar] [CrossRef]
- Matsuzawa, T.; Hosoya, T.; Yoshida, S. One-step synthesis of benzo[b]thiophenes by aryne reaction with alkynyl sulfides. Chem. Sci. 2020, 11, 9691–9696. [Google Scholar] [CrossRef]
- Hari, D.P.; Hering, T.; König, B. Visible Light Photocatalytic Synthesis of Benzothiophenes. Org. Lett. 2012, 14, 5334–5337. [Google Scholar] [CrossRef] [PubMed]
- Yan, K.; Yang, D.; Zhang, M.; Wei, W.; Liu, Y.; Tian, L.; Wang, H. Facile Access to Benzothiophenes through Metal-Free Iodine–Catalyzed Intermolecular Cyclization of Thiophenols and Alkynes. Synlett 2015, 26, 1890–1894. [Google Scholar] [CrossRef]
- Ulyankin, E.B.; Kostyuchenko, A.S.; Chernenko, S.A.; Bystrushkin, M.O.; Samsonenko, A.L.; Shatsauskas, A.L.; Fisyuk, A.S. A Simple and Efficient Synthesis of Fused Benzo[b]thiophene Derivatives. Synthesis 2021, 53, 2422–2434. [Google Scholar]
- Sajal, S.; Barnali, D. Synthesis and evaluation of some novel thiophenes as potential antibacterial and mycolytic agents. Der Pharma Chem. 2011, 3, 103–111. [Google Scholar]
- Chawla, R.; Arora, A.; Parameswaran, M.K.; Sharma, P.C.; Michael, S.; Ravi, T.K. Synthesis of novel 1, 3, 4-oxadiazole derivatives as potential antimicrobial agents. Synthesis 2010, 181, 23. [Google Scholar]
- Ferreira, I.C.; Calhelha, R.C.; Estevinho, L.M.; Queiroz, M.-J.R. Screening of antimicrobial activity of diarylamines in the 2, 3, 5-trimethylbenzo [b] thiophene series: A structure–activity evaluation study. Bioorganic Med. Chem. Lett. 2004, 14, 5831–5833. [Google Scholar] [CrossRef] [Green Version]
- Nagesh, H.; Padmashali, B.; Sandeep, C.; Yuvaraj, T.; Siddesh, M.; Mallikarjuna, S. Synthesis and antimicrobial activity of benzothiophene substituted coumarins, pyrimidines and pyrazole as new scaffold. Int. J. Pharm. Sci. Rev. Res. 2014, 28, 6–10. [Google Scholar]
- Kumara, T.; Mahadevan, K.; Harishkumar, H.; Padmashali, B.; Naganagowda, G. Synthesis of Benzo[b]thiophene Substituted Carbamates, Ureas, Semicarbazides, and Pyrazoles and Their Antimicrobial and Analgesic Activity. Phosphorus Sulfur Silicon Relat. Elem. 2009, 184, 1866–1879. [Google Scholar] [CrossRef]
- Naganagowda, G.; Petsom, A. Synthesis and antimicrobial activity of some new 2-(3-chloro-1-benzothiophen-2-yl)-3-(substituted-phenyl)-4-(3H)-quinazolinones derivatives. J. Sulfur Chem. 2011, 32, 223–233. [Google Scholar] [CrossRef]
- Gouda, M.A.; Berghot, M.A.; Abd El-Ghani, G.E.; Khalil, A.M. Synthesis and antimicrobial activities of some new thiazole and pyrazole derivatives based on 4,5,6,7-tetrahydrobenzothiophene moiety. Eur. J. Med. Chem. 2010, 45, 1338–1345. [Google Scholar] [CrossRef] [PubMed]
- Queiroz, M.-J.R.P.; Ferreira, I.C.F.R.; Gaetano, Y.D.; Kirsch, G.; Calhelha, R.C.; Estevinho, L.M. Synthesis and antimicrobial activity studies of ortho-chlorodiarylamines and heteroaromatic tetracyclic systems in the benzo[b]thiophene series. Bioorganic Med. Chem. 2006, 14, 6827–6831. [Google Scholar] [CrossRef] [PubMed]
- Fournier dit Chabert, J.; Marquez, B.; Neville, L.; Joucla, L.; Broussous, S.; Bouhours, P.; David, E.; Pellet-Rostaing, S.; Marquet, B.; Moreau, N.; et al. Synthesis and evaluation of new arylbenzo[b]thiophene and diarylthiophene derivatives as inhibitors of the NorA multidrug transporter of Staphylococcus aureus. Bioorganic Med. Chem. 2007, 15, 4482–4497. [Google Scholar] [CrossRef] [PubMed]
- Naganagowda, G.; Thamyongkit, P.; Klai-U-dom, R.; Ariyakriangkrai, W.; Luechai, A.; Petsom, A. Synthesis and biological activity of some more heterocyclic compounds containing benzothiophene moiety. J. Sulfur Chem. 2011, 32, 235–247. [Google Scholar] [CrossRef]
- Isloor, A.M.; Kalluraya, B.; Sridhar Pai, K. Synthesis, characterization and biological activities of some new benzo[b]thiophene derivatives. Eur. J. Med. Chem. 2010, 45, 825–830. [Google Scholar] [CrossRef] [PubMed]
- Hernandes, M.Z.; Cavalcanti, S.M.; Moreira, D.R.; de Azevedo Junior, W.F.; Leite, A.C. Halogen atoms in the modern medicinal chemistry: Hints for the drug design. Curr. Drug Targets 2010, 11, 303–314. [Google Scholar] [CrossRef]
- Voth, A.R.; Khuu, P.; Oishi, K.; Ho, P.S. Halogen bonds as orthogonal molecular interactions to hydrogen bonds. Nat. Chem. 2009, 1, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Yang, Z.; Liu, Y.; Lu, Y.; Chen, K.; Zhu, W. Halogen Bond: Its Role beyond Drug–Target Binding Affinity for Drug Discovery and Development. J. Chem. Inf. Modeling 2014, 54, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Gillis, E.P.; Eastman, K.J.; Hill, M.D.; Donnelly, D.J.; Meanwell, N.A. Applications of Fluorine in Medicinal Chemistry. J. Med. Chem. 2015, 58, 8315–8359. [Google Scholar] [CrossRef] [PubMed]
- Liger, F.; Bouhours, P.; Ganem-Elbaz, C.; Jolivalt, C.; Pellet-Rostaing, S.; Popowycz, F.; Paris, J.M.; Lemaire, M. C2 Arylated Benzo[b]thiophene Derivatives as Staphylococcus aureus NorA Efflux Pump Inhibitors. ChemMedChem 2016, 11, 320–330. [Google Scholar] [CrossRef] [PubMed]
- Bhaskar, B.V.; Babu, T.M.C.; Reddy, N.V.; Rajendra, W. Homology modeling, molecular dynamics, and virtual screening of NorA efflux pump inhibitors of Staphylococcus aureus. Drug Des. Dev. Ther. 2016, 10, 3237. [Google Scholar] [CrossRef] [Green Version]
- Fontaine, F.; Hequet, A.; Voisin-Chiret, A.-S.; Bouillon, A.; Lesnard, A.; Cresteil, T.; Jolivalt, C.; Rault, S. First identification of boronic species as novel potential inhibitors of the Staphylococcus aureus NorA efflux pump. J. Med. Chem. 2014, 57, 2536–2548. [Google Scholar] [CrossRef]
- Godoi, B.; Schumacher, R.F.; Zeni, G. Synthesis of Heterocycles via Electrophilic Cyclization of Alkynes Containing Heteroatom. Chem. Rev. 2011, 111, 2937–2980. [Google Scholar] [CrossRef] [PubMed]
- Yue, D.; Larock, R.C. Synthesis of 2,3-Disubstituted Benzo[b]thiophenes via Palladium-Catalyzed Coupling and Electrophilic Cyclization of Terminal Acetylenes. J. Org. Chem. 2002, 67, 1905–1909. [Google Scholar] [CrossRef] [PubMed]
- Larock, R.C.; Yue, D. Synthesis of benzo[b]thiophenes by electrophilic cyclization. Tetrahedron Lett. 2001, 42, 6011–6013. [Google Scholar] [CrossRef]
- Kim, S.; Dahal, N.; Kesharwani, T. Environmentally benign process for the synthesis of 2, 3-disubstituted benzo[b]thiophenes using electrophilic cyclization. Tetrahedron Lett. 2013, 54, 4373–4376. [Google Scholar] [CrossRef]
- Kesharwani, T.; Kornman, C.; Tonnaer, A.; Hayes, A.; Kim, S.; Dahal, N.; Romero, R.; Royappa, A. Sodium halides as the source of electrophilic halogens in green synthesis of 3-halo- and 3,n-dihalobenzo[b]thiophenes. Tetrahedron 2018, 74, 2973–2984. [Google Scholar] [CrossRef] [PubMed]
- Kesharwani, T.; Kornman, C.T.; Tonnaer, A.L.; Royappa, A.D. Green synthesis of benzo[b]thiophenes via iron (III) mediated 5-endo-dig iodocyclization of 2-alkynylthioanisoles. Tetrahedron Lett. 2016, 57, 411–414. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute (CLSI). Reference method for broth dilution antifungal susceptibility testing of yeasts. In CLSI Document M27, 4th ed.; CLSI: Wayne, PA, USA, 2017; p. 46. [Google Scholar]
- Clinical and Laboratory Standards Institute (CLSI). Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. In CLSI Standard M07, 11th ed.; CLSI: Wayne, PA, USA, 2018; p. 112. [Google Scholar]
- Miller, S.I. Antibiotic Resistance and Regulation of the Gram-Negative Bacterial Outer Membrane Barrier by Host Innate Immune Molecules. mBio 2016, 7, e01541-16. [Google Scholar] [CrossRef] [Green Version]
- Breijyeh, Z.; Jubeh, B.; Karaman, R. Resistance of Gram-Negative Bacteria to Current Antibacterial Agents and Approaches to Resolve It. Molecules 2020, 25, 1340. [Google Scholar] [CrossRef] [Green Version]
- Baecker, D.; Sesli, Ö.; Knabl, L.; Huber, S.; Orth-Höller, D.; Gust, R. Investigating the antibacterial activity of salen/salophene metal complexes: Induction of ferroptosis as part of the mode of action. Eur. J. Med. Chem. 2021, 209, 112907. [Google Scholar] [CrossRef]
- Baltoumas, F.A.; Hamodrakas, S.J.; Iconomidou, V.A. The gram-negative outer membrane modeler: Automated building of lipopolysaccharide-rich bacterial outer membranes in four force fields. J. Comput. Chem. 2019, 40, 1727–1734. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standards Institute (CLSI). Methods for determining bactericidal activity of antimicrobial agents: Approved guidelines. In CLSI Document M26-A; CLSI: Wayne, PA, USA, 1999; p. 32. [Google Scholar]
- Petersen, P.J.; Jones, C.H.; Bradford, P.A. In vitro antibacterial activities of tigecycline and comparative agents by time-kill kinetic studies in fresh Mueller-Hinton broth. Diagn. Microbiol. Infect. Dis. 2007, 59, 347–349. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef]
- Veber, D.F.; Johnson, S.R.; Cheng, H.Y.; Smith, B.R.; Ward, K.W.; Kopple, K.D. Molecular properties that influence the oral bioavailability of drug candidates. J. Med. Chem. 2002, 45, 2615–2623. [Google Scholar] [CrossRef] [PubMed]
- Ghose, A.K.; Viswanadhan, V.N.; Wendoloski, J.J. A knowledge-based approach in designing combinatorial or medicinal chemistry libraries for drug discovery. 1. A qualitative and quantitative characterization of known drug databases. J. Comb. Chem. 1999, 1, 55–68. [Google Scholar] [CrossRef]
- Egan, W.J.; Merz, K.M., Jr.; Baldwin, J.J. Prediction of drug absorption using multivariate statistics. J. Med. Chem. 2000, 43, 3867–3877. [Google Scholar] [CrossRef]
- Muegge, I.; Heald, S.L.; Brittelli, D. Simple selection criteria for drug-like chemical matter. J. Med. Chem. 2001, 44, 1841–1846. [Google Scholar] [CrossRef]
- Baell, J.B.; Holloway, G.A. New substructure filters for removal of pan assay interference compounds (PAINS) from screening libraries and for their exclusion in bioassays. J. Med. Chem. 2010, 53, 2719–2740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brenk, R.; Schipani, A.; James, D.; Krasowski, A.; Gilbert, I.H.; Frearson, J.; Wyatt, P.G. Lessons learnt from assembling screening libraries for drug discovery for neglected diseases. ChemMedChem 2008, 3, 435–444. [Google Scholar] [CrossRef]
- Montanari, F.; Ecker, G.F. Prediction of drug-ABC-transporter interaction—Recent advances and future challenges. Adv. Drug Deliv. Rev. 2015, 86, 17–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharom, F.J. ABC multidrug transporters: Structure, function and role in chemoresistance. Pharmacogenomics 2008, 9, 105–127. [Google Scholar] [CrossRef]
- Szakacs, G.; Varadi, A.; Ozvegy-Laczka, C.; Sarkadi, B. The role of ABC transporters in drug absorption, distribution, metabolism, excretion and toxicity (ADME-Tox). Drug Discov. Today 2008, 13, 379–393. [Google Scholar] [CrossRef] [PubMed]
- Denison, M.S.; Whitlock, J.P., Jr. Xenobiotic-inducible transcription of cytochrome P450 genes. J. Biol. Chem. 1995, 270, 18175–18178. [Google Scholar] [CrossRef] [Green Version]
- Zanger, U.M.; Schwab, M. Cytochrome P450 enzymes in drug metabolism: Regulation of gene expression, enzyme activities, and impact of genetic variation. Pharmacol. Ther. 2013, 138, 103–141. [Google Scholar] [CrossRef] [PubMed]



| Compound | Bacteria | Fungi | ||
|---|---|---|---|---|
| S. aureus | E. faecalis | B. cereus | C. albicans | |
| 10 | >512 | >512 | >512 | >512 |
| 11 | >512 | >512 | >512 | >512 |
| 12 | >512 | >512 | >512 | >512 |
| 13 | >512 | >512 | >512 | >512 |
| 14 | >512 | >512 | >512 | 512 |
| 15 | >512 | >512 | >512 | 512 |
| 16 | >512 | >512 | >512 | >512 |
| 17 | >512 | >512 | >512 | >512 |
| 18 | >512 | >512 | >512 | >512 |
| 19 | 256 | 256 | 128 | 128 |
| 20 | >512 | >512 | >512 | >512 |
| 21 | >512 | >512 | >512 | >512 |
| 22 | 512 | >512 | 512 | 512 |
| 23 | 512 | >512 | 512 | 256 |
| 24 | >512 | >512 | >512 | >512 |
| 25 | 16 | 16 | 16 | 16 |
| 26 | 16 | 16 | 16 | 32 |
| 27 | >512 | >512 | >512 | >512 |
| 28 | >512 | >512 | >512 | 512 |
| 29 | >512 | >512 | >512 | >512 |
| 30 | 64 | 64 | 16 | 64 |
| 31 | 64 | 128 | 32 | 64 |
| 32 | 128 | 128 | 64 | 128 |
| Ampicillin | 8 | 8 | 32 | - |
| Chloramphenicol | 8 | 4 | 2 | - |
| Kanamycin | 2 | 32 | 2 | - |
| Fluconazole | - | - | - | 0.5 |
| Compound | MW a | nHA b | nAHA c | nRotB d | nHBA e | nHBD f | MR g | TPSA h | MLOGP i | ESOL j |
|---|---|---|---|---|---|---|---|---|---|---|
| 25 | 266.8 | 17 | 9 | 1 | 1 | 1 | 74.77 | 48.47 | 3.82 | MS |
| 26 | 311.2 | 17 | 9 | 1 | 1 | 1 | 77.46 | 48.47 | 3.95 | MS |
| 30 | 226.7 | 14 | 9 | 1 | 1 | 1 | 62.46 | 48.47 | 3.03 | S |
| 31 | 271.2 | 14 | 9 | 1 | 1 | 1 | 65.15 | 48.47 | 3.17 | MS |
| 32 | 252.8 | 16 | 9 | 1 | 1 | 1 | 69.96 | 48.47 | 3.57 | MS |
| Compound | Lipinski | Ghose | Veber | Egan | Muegge | PAINS a | Brenk |
|---|---|---|---|---|---|---|---|
| 25 | Yes | Yes | Yes | Yes | Yes | 0 | 0 |
| 26 | Yes | Yes | Yes | Yes | Yes | 0 | 0 |
| 30 | Yes | Yes | Yes | Yes | Yes | 0 | 0 |
| 31 | Yes | Yes | Yes | Yes | Yes | 0 | 0 |
| 32 | Yes | Yes | Yes | Yes | Yes | 0 | 0 |
| Compound | GI a Absorption | BBB b Permeant | P-gp c Substrate | CYP1A2 Inhibitor | CYP2C19 Inhibitor | CYP2C9 Inhibitor | CYP2D6 Inhibitor | CYP3A4 Inhibitor | Log Kp (cm/s) |
|---|---|---|---|---|---|---|---|---|---|
| 25 | High | Yes | Yes | Yes | Yes | Yes | Yes | No | −4.86 |
| 26 | High | Yes | Yes | Yes | Yes | Yes | Yes | No | −5.09 |
| 30 | High | Yes | No | Yes | Yes | No | No | No | −5.33 |
| 31 | High | Yes | No | Yes | Yes | No | No | No | −5.56 |
| 32 | High | Yes | Yes | Yes | Yes | No | Yes | No | −5.16 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Masih, P.J.; Kesharwani, T.; Rodriguez, E.; Vertudez, M.A.; Motakhaveri, M.L.; Le, T.K.; Tran, M.K.T.; Cloyd, M.R.; Kornman, C.T.; Phillips, A.M. Synthesis and Evaluation of 3-Halobenzo[b]thiophenes as Potential Antibacterial and Antifungal Agents. Pharmaceuticals 2022, 15, 39. https://doi.org/10.3390/ph15010039
Masih PJ, Kesharwani T, Rodriguez E, Vertudez MA, Motakhaveri ML, Le TK, Tran MKT, Cloyd MR, Kornman CT, Phillips AM. Synthesis and Evaluation of 3-Halobenzo[b]thiophenes as Potential Antibacterial and Antifungal Agents. Pharmaceuticals. 2022; 15(1):39. https://doi.org/10.3390/ph15010039
Chicago/Turabian StyleMasih, Prerna J., Tanay Kesharwani, Elivet Rodriguez, Mia A. Vertudez, Mina L. Motakhaveri, Terelan K. Le, Minh Kieu T. Tran, Matthew R. Cloyd, Cory T. Kornman, and Aimee M. Phillips. 2022. "Synthesis and Evaluation of 3-Halobenzo[b]thiophenes as Potential Antibacterial and Antifungal Agents" Pharmaceuticals 15, no. 1: 39. https://doi.org/10.3390/ph15010039
APA StyleMasih, P. J., Kesharwani, T., Rodriguez, E., Vertudez, M. A., Motakhaveri, M. L., Le, T. K., Tran, M. K. T., Cloyd, M. R., Kornman, C. T., & Phillips, A. M. (2022). Synthesis and Evaluation of 3-Halobenzo[b]thiophenes as Potential Antibacterial and Antifungal Agents. Pharmaceuticals, 15(1), 39. https://doi.org/10.3390/ph15010039








