Distinctive Supramolecular Features of β-Cyclodextrin Inclusion Complexes with Antidepressants Protriptyline and Maprotiline: A Comprehensive Structural Investigation
Abstract
:1. Introduction
2. Results and Discussion
2.1. PRT and MPL Are Conformationally Distinct in the β-CD Cavity Confinement
2.2. Structural Adaptability of β-CD Macrocycles to the Inclusion of PRT and MPL
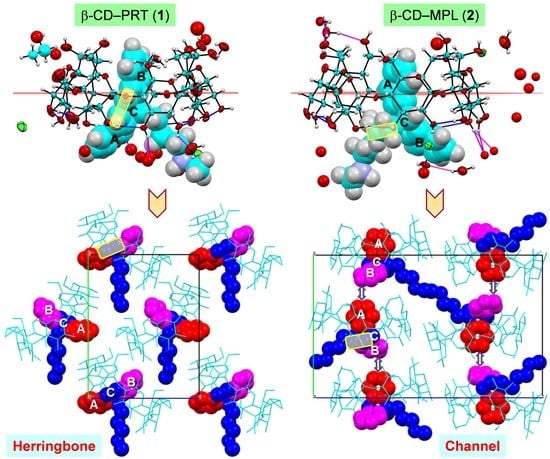
2.3. PRT Ethylene Group (1) Makes the Distinction in the Complex 3D Arrangements
2.4. Different Inclusion Stability of 1 and 2 in Solution and Gas Phase
3. Materials and Methods
3.1. Materials
3.2. X-ray Crystallography
3.2.1. Single-Crystal Preparation
3.2.2. X-ray Diffraction Experiment
3.2.3. Structure Solution and Refinement
3.3. DFT Calculations
3.3.1. Full-Geometry Optimization
3.3.2. Dispersion and BSSE Corrections
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization (WHO). Depression. 2019. Available online: https://www.who.int/en/news-room/fact-sheets/detail/depression (accessed on 11 June 2021).
- Our World in Data. Total Confirmed COVID-19 Cases. 8 June 2021. Available online: https://ourworldindata.org/grapher/covid-cases-income (accessed on 11 June 2021).
- Rogers, J.P.; Watson, C.J.; Badenoch, J.; Cross, B.; Butler, M.; Song, J.; Hafeez, D.; Morrin, H.; Rengasamy, E.R.; Thomas, L.; et al. Neurology and neuropsychiatry of COVID-19: A systematic review and meta-analysis of the early literature reveals frequent CNS manifestations and key emerging narratives. J. Neurol. Neurosurg. Psychiatry 2021, 92, 932–941. [Google Scholar] [CrossRef] [PubMed]
- Perlis, R.H.; Ognyanova, K.; Santillana, M.; Baum, M.A.; Lazer, D.; Druckman, J.; Della Volpe, J. Association of Acute Symptoms of COVID-19 and Symptoms of Depression in Adults. JAMA Netw. Open 2021, 4, e213223. [Google Scholar] [CrossRef] [PubMed]
- Nogrady, T.; Weaver, D.F. Medicinal Chemistry: A Molecular and Biochemical Approach; Oxford University Press: Oxford, UK, 2005. [Google Scholar]
- Cowen, P.J. 6.06—Psychopharmacology. In Comprehensive Clinical Psychology; Bellack, A.S., Hersen, M., Eds.; Pergamon: Oxford, UK, 1998; pp. 135–161. [Google Scholar] [CrossRef]
- Iodine.com. Compare Maprotiline vs. Vivactil. 2021. Available online: https://www.iodine.com/compare/maprotiline-vs-vivactil (accessed on 15 June 2021).
- Bansode, S.B.; Jana, A.K.; Batkulwar, K.; Warkad, S.D.; Joshi, R.S.; Sengupta, N.; Kulkarni, M.J. Molecular Investigations of Protriptyline as a Multi-Target Directed Ligand in Alzheimer’s Disease. PLoS ONE 2014, 9, e105196. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, V.; Mishra, A.; Singh, S.; Mishra, S.K.; Sahu, K.K.; Parul; Kulkarni, M.J.; Shukla, R.; Shukla, S. Protriptyline improves spatial memory and reduces oxidative damage by regulating NFκB-BDNF/CREB signaling axis in streptozotocin-induced rat model of Alzheimer’s disease. Brain Res. 2021, 1754, 147261. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Zhu, Y.; Liu, Q.; Luo, T.; Xu, W. Maprotiline Suppresses Cholesterol Biosynthesis and Hepatocellular Carcinoma Progression Through Direct Targeting of CRABP1. Front. Pharmacol. 2021, 12, 689767. [Google Scholar] [CrossRef] [PubMed]
- Astray, G.; Gonzalez-Barreiro, C.; Mejuto, J.; Rial-Otero, R.; Simal-Gándara, J. A review on the use of cyclodextrins in foods. Food Hydrocoll. 2009, 23, 1631–1640. [Google Scholar] [CrossRef]
- Bilensoy, E. (Ed.) Cyclodextrins in Pharmaceutics, Cosmetics, and Biomedicine: Current and Future Industrial Applications; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Szejtli, J. Cyclodextrin Technology (Vol. 1); Springer Science & Business Media: Berlin, Germany, 2013. [Google Scholar]
- Morin-Crini, N.; Fourmentin, S.; Fenyvesi, E.; Lichtfouse, E.; Torri, G.; Fourmentin, M.; Crini, G. 130 years of cyclodextrin discovery for health, food, agriculture, and the industry: A review. Environ. Chem. Lett. 2021, 19, 2581–2617. [Google Scholar] [CrossRef]
- Saenger, W. Cyclodextrin Inclusion Compounds in Research and Industry. Angew. Chem. Int. Ed. Engl. 1980, 19, 344–362. [Google Scholar] [CrossRef]
- Dodziuk, H. Cyclodextrins and Their Complexes: Chemistry, Analytical Methods, Applications; Wiley-VCH: Weinheim, Germany, 2006. [Google Scholar]
- Diniz, T.C.; Pinto, T.C.C.; Menezes, P.D.P.; Silva, J.C.; Teles, R.B.D.A.; Ximenes, R.C.C.; Guimarães, A.G.; Serafini, M.R.; Araújo, A.A.D.S.; Júnior, L.J.Q.; et al. Cyclodextrins improving the physicochemical and pharmacological properties of antidepressant drugs: A patent review. Expert Opin. Ther. Patents 2017, 28, 81–92. [Google Scholar] [CrossRef]
- Georgiou, M.E.; Koupparis, M.A.; Georgiou, C. Rapid automated spectrophotometric competitive complexation studies of drugs with cyclodextrins using the flow injection gradient technique: Tricyclic antidepressant drugs with α-cyclodextrin. Analyst 1999, 124, 391–396. [Google Scholar] [CrossRef]
- Valsami, G.; Koupparis, M.A.; Macheras, P.E. Complexation Studies of Cyclodextrins with Tricyclic Antidepressants Using Ion-Selective Electrodes. Pharm. Res. 1992, 9, 94–100. [Google Scholar] [CrossRef]
- Piperaki, S.; Parissi-Poulou, M.; Koupparis, M. A Separation Study of Tricyclic Antidepressant Drugs by HPLC with β-Cyclodextrin Bonded Stationary Phase. J. Liq. Chromatogr. Relat. Technol. 1993, 16, 3487–3508. [Google Scholar] [CrossRef]
- Hoshino, T.; Hirayama, F.; Uekama, K.; Yamasaki, M. Reduction of protriptyline-photosensitized hemolysis by β-cyclodextrin complexations. Int. J. Pharm. 1989, 50, 45–52. [Google Scholar] [CrossRef]
- Kundu, M.; Roy, M.N. Preparation, interaction and spectroscopic characterization of inclusion complex of a cyclic oligosaccharide with an antidepressant drug. J. Incl. Phenom. Macrocycl. Chem. 2017, 89, 177–187. [Google Scholar] [CrossRef]
- Aree, T. β-Cyclodextrin encapsulation of nortriptyline HCl and amitriptyline HCl: Molecular insights from single-crystal X-ray diffraction and DFT calculation. Int. J. Pharm. 2020, 575, 118899. [Google Scholar] [CrossRef] [PubMed]
- Junquera, E.; Romero, J.C.; Aicart, E. Behavior of Tricyclic Antidepressants in Aqueous Solution: Self-Aggregation and Association with β-Cyclodextrin. Langmuir 2001, 17, 1826–1832. [Google Scholar] [CrossRef]
- Viswalingam, M.; Prabu, S.; Sivakumar, K.; Rajamohan, R. Spectral characteristics of desipramine in β-cyclodextrin cavity through inclusion complex. J. Macromol. Sci. Part A 2016, 53, 781–790. [Google Scholar] [CrossRef]
- Jalali, F.; Ezzati, N. Spectrofluorimetric study and determination of desipramine in the presence of β-cyclodextrin. J. Anal. Chem. 2014, 69, 367–370. [Google Scholar] [CrossRef]
- Misiuk, W. Study on the Inclusion Complex Formation of Desipramine with Β-Cyclodextrin and Its Pharmaceutical Application. World J. Pharm. Pharm. Sci. 2015, 4, 18–33. Available online: https://www.wjpps.com/Wjpps_controller/abstract_id/3840 (accessed on 15 June 2021).
- Aree, T. β-Cyclodextrin Inclusion Complexation with Tricyclic Antidepressants Desipramine and Imipramine: A Structural Chemistry Perspective. J. Pharm. Sci. 2020, 109, 3086–3094. [Google Scholar] [CrossRef] [PubMed]
- Misiuk, W.; Zalewska, M. Study on the Inclusion Interactions of β-Cyclodextrin and Its Derivative with Clomipramine by Spectroscopy and Its Analytic Application. Anal. Lett. 2008, 41, 543–560. [Google Scholar] [CrossRef]
- Sankaranarayanan, R.; Siva, S.; Venkatesh, G.; Prabhu, A.A.M.; Rajendiran, N. Dual fluorescence of dothiepin, doxepin drugs—effect of solvents and β-cyclodextrin. J. Mol. Liq. 2011, 161, 107–114. [Google Scholar] [CrossRef]
- Spencer, B.J.; Zhang, W.; Purdy, W.C. Capillary electrophoretic separation of tricyclic antidepressants using charged carboxymethyl-β-cyclodextrin as a buffer additive. Electrophoresis 1997, 18, 736–744. [Google Scholar] [CrossRef] [PubMed]
- Kou, H.-S.; Chen, C.-C.; Huang, Y.-H.; Ko, W.-K.; Wu, H.-L.; Wu, S.-M. Method for simultaneous determination of eight cyclic antidepressants by cyclodextrin-modified capillary zone electrophoresis: Applications in pharmaceuticals. Anal. Chim. Acta 2004, 525, 23–30. [Google Scholar] [CrossRef]
- Uekama, K.; Irie, T.; Otagiri, M.; Hoshino, T.; Yamada, Y.; Ohtani, Y. Protective effects of cyclodextrins on the haemolysis induced with imipramine in vitro. Membrane 1983, 8, 315–321. [Google Scholar] [CrossRef]
- Castiglione, F.; Ganazzoli, F.; Malpezzi, L.; Mele, A.; Panzeri, W.; Raffaini, G. Inclusion complexes of β-cyclodextrin with tricyclic drugs: An X-ray diffraction, NMR and molecular dynamics study. Beilstein J. Org. Chem. 2017, 13, 714–719. [Google Scholar] [CrossRef] [Green Version]
- Aree, T. Supramolecular Complexes of β-Cyclodextrin with Clomipramine and Doxepin: Effect of the Ring Substituent and Component of Drugs on Their Inclusion Topologies and Structural Flexibilities. Pharmaceuticals 2020, 13, 278. [Google Scholar] [CrossRef] [PubMed]
- Cremer, D.; Pople, J.A. General definition of ring puckering coordinates. J. Am. Chem. Soc. 1975, 97, 1354–1358. [Google Scholar] [CrossRef]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge Structural Database. Acta Cryst. 2016, 72, 171–179. [Google Scholar] [CrossRef]
- Rose, P.W.; Prlic, A.; Bi, C.; Bluhm, W.F.; Christie, C.H.; Dutta, S.; Green, R.K.; Goodsell, D.S.; Westbrook, J.D.; Woo, J.; et al. The RCSB Protein Data Bank: Views of structural biology for basic and applied research and education. Nucleic Acids Res. 2015, 43, D345–D356. [Google Scholar] [CrossRef]
- Ventimiglia, G.; Magrone, D.; Allegrini, P.; Razzetti, G. Protriptyline Hydrochloride Crystalline Form. U.S. Patent Application No. 11/598,619, 17 May 2007. Available online: https://patents.google.com/patent/US20070112073A1/en (accessed on 2 April 2021).
- Brouant, P.; Reboul, J.P.; Siri, D.; Soyfer, J.C.; Barbe, J.; Pèpe, G. Un sel de Maprotiline (Ludiomil), Médicament Psychotrope, C20H24N+. C21H22NO2−. 0,5H2O. Acta Cryst. 1995, C51, 970–974. [Google Scholar] [CrossRef]
- Soltani, M.; Mash, B.L.; Henseler, J.; Badri, S.; Zeller, M.; Salter, E.A.; Wierzbicki, A.; Stenson, A.C.; Davis, J.H. Unorthodox crystalline drug salts via the reaction of amine-containing drugs with CO2. Chem. Commun. 2019, 55, 13546–13549. [Google Scholar] [CrossRef]
- Koshland, D.E. Protein shape and biological control. Sci. Am. 1973, 229, 52–67. Available online: https://www.jstor.org/stable/24923220 (accessed on 12 July 2021). [CrossRef] [PubMed]
- French, A.D.; Johnson, G.P. Linkage and pyranosyl ring twisting in cyclodextrins. Carbohydr. Res. 2007, 342, 1223–1237. [Google Scholar] [CrossRef] [PubMed]
- Aree, T.; Jongrungruangchok, S. Crystallographic evidence for β-cyclodextrin inclusion complexation facilitating the improvement of antioxidant activity of tea (+)-catechin and (−)-epicatechin. Carbohydr. Polym. 2016, 140, 362–373. [Google Scholar] [CrossRef] [PubMed]
- Lindner, K.; Saenger, W. Crystal and molecular structure of cyclohepta-amylose dodecahydrate. Carbohydr. Res. 1982, 99, 103–115. [Google Scholar] [CrossRef]
- Aree, T. β-Cyclodextrin Inclusion Complexes with Catechol-Containing Antioxidants Protocatechuic Aldehyde and Protocatechuic Acid—An Atomistic Perspective on Structural and Thermodynamic Stabilities. Molecules 2021, 26, 3574. [Google Scholar] [CrossRef]
- Ikuta, D.; Hirata, Y.; Wakamori, S.; Shimada, H.; Tomabechi, Y.; Kawasaki, Y.; Ikeuchi, K.; Hagimori, T.; Matsumoto, S.; Yamada, H. Conformationally supple glucose monomers enable synthesis of the smallest cyclodextrins. Science 2019, 364, 674–677. [Google Scholar] [CrossRef]
- Saenger, W. Nature and size of included guest molecule determines architecture of crystalline cyclodextrin host matrix. Isr. J. Chem. 1985, 25, 43–50. [Google Scholar] [CrossRef]
- Bruker. APEX2, SADABS and SHELXTL; Bruker AXS Inc.: Madison, WI, USA, 2014. [Google Scholar]
- Bruker. SAINT and XPREP; Bruker AXS Inc.: Madison, WI, USA, 2008. [Google Scholar]
- Emsley, P.; Lohkamp, B.; Scott, W.; Cowtan, K. Features and development of Coot. Acta Cryst. 2010, D66, 486–501. [Google Scholar] [CrossRef] [Green Version]
- Allen, F.H.; Bruno, I.J. Bond lengths in organic and metal-organic compounds revisited: X—H bond lengths from neutron diffraction data. Acta Cryst. 2010, B66, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Schnupf, U.; Momany, F.A. DFT energy optimization of a large carbohydrate: Cyclomaltohexa icosaose (CA-26). J. Phys. Chem. 2012, B116, 6618–6627. [Google Scholar] [CrossRef] [PubMed]
- Aree, T.; Arunchai, R.; Koonrugsa, N.; Intasiri, A. Fluorometric and theoretical studies on inclusion complexes of β-cyclodextrin and D-, L-phenylalanine. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2012, 96, 736–743. [Google Scholar] [CrossRef]
- Aree, T. Effect of the ring size and asymmetry of cyclodextrins on their inclusion ability: A theoretical study. J. Incl. Phenom. Macrocycl. Chem. 2013, 77, 439–445. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. GAUSSIAN09, Revision A.01; Gaussian Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Boys, S.F.; Bernardi, F.J.M.P. The calculation of small molecular interactions by the differences of separate total energies. Some procedures with reduced errors. Mol. Phys. 1970, 19, 553–566. [Google Scholar] [CrossRef]
- Lenze, E.J.; Mattar, C.; Zorumski, C.F.; Stevens, A.; Schweiger, J.; Nicol, G.E.; Miller, J.P.; Yang, L.; Yingling, M.; Avidan, M.S.; et al. Fluvoxamine vs placebo and clinical deterioration in outpatients with symptomatic COVID-19: A randomized clinical trial. JAMA 2020, 324, 2292–2300. [Google Scholar] [CrossRef]
- Schloer, S.; Brunotte, L.; Mecate-Zambrano, A.; Zheng, S.; Tang, J.; Ludwig, S.; Rescher, U. Drug synergy of combinatory treatment with remdesivir and the repurposed drugs fluoxetine and itraconazole effectively impairs SARS-CoV-2 infection in vitro. Br. J. Pharmacol. 2021, 178, 2339–2350. [Google Scholar] [CrossRef]
- Hoertel, N.; Sánchez-Rico, M.; Vernet, R.; Beeker, N.; Jannot, A.-S.; Neuraz, A.; Salamanca, E.; Paris, N.; Daniel, C.; Gramfort, A.; et al. Association between antidepressant use and reduced risk of intubation or death in hospitalized patients with COVID-19: Results from an observational study. Mol. Psychiatry 2021, 26, 1–14. [Google Scholar] [CrossRef]











| Host | Guest | Ratio | Inclusion Mode a | Ka, M−1 | Ref. | |
|---|---|---|---|---|---|---|
| Aromatic | Side-Chain | (Tech.) b | ||||
| α-CD | PRT | 1:1 | ✓ | 120 (FI) | [18] | |
| α-CD | PRT | 1:1 | ✓ | 0.11 × 103 (I) | [19] | |
| β-CD | PRT | 1:1 | ✓ | 18.04 × 103 (I) | [19] | |
| β-CD | PRT | 1:1 | ✓ A/B | 24.0 × 103 (H) | [20] | |
| β-CD | PRT | 1:1 | ✓ A/B | 14.2 × 103 (U) | [21] | |
| β-CD | PRT | 1:1 | ND c | ND (CD) | [21] | |
| β-CD | PRT | 1:1 | ✓ A/B | ND (F) | [21] | |
| β-CD | PRT | 1:1 | ✓ A/B | ND (N) | [21] | |
| β-CD | PRT | 1:1 | ✓ B | ND (X) | This work | |
| β-CD | PRT | 1:1 | ✓ B | ND (Tg) | This work | |
| DIMEB | PRT | 1:1 | ✓ A/B | 17.3 × 103 (U) | [21] | |
| DIMEB | PRT | 1:1 | ND | ND (CD) | [21] | |
| DIMEB | PRT | 1:1 | ✓ A/B | ND (F) | [21] | |
| DIMEB | PRT | 1:1 | ✓ A/B | ND (N) | [21] | |
| α-CD | MPL | 1:1 | ✓ | 240 (FI) | [18] | |
| α-CD | MPL | 1:1 | ✓ | 0.12 × 103 (I) | [19] | |
| β-CD | MPL | 1:1 | ✓ | 4.81 × 103 (I) | [19] | |
| β-CD | MPL | 1:1 | ✓ A/B | 4.9 × 103 (H) | [20] | |
| β-CD | MPL | 1:1 | ✓ A | ND (X) | This work | |
| β-CD | MPL | 1:1 | ✓ A | ND (Tg) | This work | |
| α-CD | NRT | 1:1 | ✓ | 70 (FI) | [18] | |
| α-CD | NRT | 1:1 | ✓ | 0.09 × 103 (I) | [19] | |
| β-CD | NRT | 1:1 | ✓ | 16.77 × 103 (I) | [19] | |
| β-CD | NRT | 1:1 | ✓ | ✓ | 235 (U), 211 (F) | [22] |
| β-CD | NRT | 1:1 | ✓ A/B | 16.1×103 (H) | [20] | |
| β-CD | NRT | 1:1 | ✓ A | ND (X) | [23] | |
| β-CD | NRT | 1:1 | ✓ | ND (Tgl) | [23] | |
| β-CD | DPM | 1:1 | ✓ | 2.04 × 103 (C) | [24] | |
| β-CD | DPM | 1:1 | ✓ | ✓ | 42.2 (U), 32.0 (F) | [25] |
| β-CD | DPM | 2:1 | ✓ A + B | ND (F) | [26] | |
| β-CD | DPM | 1:1 | ✓ | 8.92 × 103 (U) | [27] | |
| β-CD | DPM | 1:1 | ✓ A | ND (X) | [28] | |
| β-CD | DPM | 1:1 | ✓ A | ✓ | ND (Tg) | [28] |
| PRT (1) | MPL (2) | NRT (i) b | DPM (ii) c | |
|---|---|---|---|---|
| (1) Geometrical parameters | ||||
| (6-7/6-6)-Tricyclic core | ||||
| Butterfly angle ε (°) d | 131.4(4) | 118.6(3) | 116.7(3) | 120.0(3) |
| Annellation angle η (°) e | 26.1(1) | 2.5(3) | 24.5(3) | 28.9(3) |
| Twist angle τ (°) f | –178.3(4) | 1.7(3) | 4.7(4) | –8.1(5) |
| C15–C10–C11–C12 torsion angle (°) | 1.4(19) | – | 44.1(16) | –53.7(12) |
| A-ring-centroid–B-ring-centroid distance dAB (Å) | 4.978 | 4.483 | 4.814 | 4.757 |
| Side-chain at N5/C5 | ||||
| C13–N5/C5–C16–C17 torsion angle (°) | 59.1(10) | –167.4(7) | 174.4(5) | –64.3(7) |
| N5/C5–C16–C17–C18 torsion angle (°) | 179.3(8) | –172.1(10) | 140.0(6) | –171.9(5) |
| N5’–A-ring centroid distance dNA (Å) | 5.928(9) | 7.619(8) | 7.441(6) | 6.564(6) |
| N5’–B-ring centroid distance dNB (Å) | 7.543(8) | 6.111(8) | 6.055(6) | 6.136(6) |
| dNA/dNB ratio | 0.786 | 1.247 | 1.229 | 1.070 |
| (2) Inclusion structure | ||||
| Interplanar angle (°) | ||||
| A-ring vs. β-CD O4 plane | 41.8(3) | 85.4(2) | 88.4(2) | 83.7(2) |
| B-ring vs. β-CD O4 plane | 89.7(2) | 34.0(2) | 25.1(1) | 25.8(1) |
| Rings embedded in the β-CD cavity | B, C | A, C | A, C | A, C |
| Distance from TCA to β-CD (Å) | ||||
| A/B-ring centroid to O4 centroid (diagonal) g | 1.384 | 0.549 | 0.822 | 0.467 |
| A/B-ring centroid to O4 plane (vertical) | 1.384 | 0.551 | 0.822 | 0.464 |
| C-ring centroid to O4 centroid | –1.286 | –1.927 | –1.819 | –2.295 |
| B/A-ring centroid to O4 centroid | –3.741 | –4.127 | –4.111 | –4.475 |
| Interaction a | D–H | H···A | D···A | ∠(DHA) | Interaction a | D–H | H···A | D···A | ∠(DHA) |
|---|---|---|---|---|---|---|---|---|---|
| X-ray | DFT e | ||||||||
| β-CD–PRT HCl (1) | β-CD–PRT base | ||||||||
| N5’P–H1···O21 i,b | 0.89 | 2.41 | 3.087(10) | 133.1 | C52–H···Cg2 c | 1.10 | 3.75 | 4.80 | 162.2 |
| N5’P–H1···O31 i | 0.89 | 2.06 | 2.856(11) | 148.6 | C55–H···Cg2 | 1.10 | 4.61 | 3.56 | 161.6 |
| N5’P–H1···O21 i | 0.89 | 2.41 | 3.087(10) | 133.1 | C31–H···Cg1 c | 1.10 | 4.49 | 3.46 | 157.1 |
| C51–H···Cg2 c | 0.98 | 3.69 | 4.461 | 137.8 | |||||
| C55–H···Cg2 | 0.98 | 3.39 | 4.239 | 146.3 | |||||
| C32–H···Cg1 | 0.98 | 3.21 | 4.135 | 158.9 | |||||
| β-CD–MPL HCl (2) | β-CD–MPL base | ||||||||
| O61–H···N5’M ii,b | 0.82 | 2.44 | 3.159(12) | 146.4 | C51–H···Cg1 c | 1.10 | 3.93 | 4.98 | 160.7 |
| N5’M–H1···O52 iii | 0.89 | 2.50 | 3.141(8) | 129.2 | C31–H···Cg2 c | 1.10 | 3.37 | 4.30 | 143.5 |
| N5’M–H1···O62 iii | 0.89 | 2.10 | 2.930(11) | 155.7 | |||||
| N5’M–H2···O61 iii | 0.89 | 2.60 | 3.159(12) | 121.7 | |||||
| C51–H···Cg1 d | 0.98 | 3.577 | 4.515 | 161.1 | |||||
| C55–H···Cg1 | 0.98 | 3.843 | 4.782 | 161.4 | |||||
| C31–H···Cg2 | 0.98 | 3.018 | 3.903 | 150.9 | |||||
| O21–H···Cg2 | 0.82 | 4.394 | 4.499 | 92.1 | |||||
| O37–H···Cg2 | 0.82 | 3.930 | 4.520 | 132.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aree, T. Distinctive Supramolecular Features of β-Cyclodextrin Inclusion Complexes with Antidepressants Protriptyline and Maprotiline: A Comprehensive Structural Investigation. Pharmaceuticals 2021, 14, 812. https://doi.org/10.3390/ph14080812
Aree T. Distinctive Supramolecular Features of β-Cyclodextrin Inclusion Complexes with Antidepressants Protriptyline and Maprotiline: A Comprehensive Structural Investigation. Pharmaceuticals. 2021; 14(8):812. https://doi.org/10.3390/ph14080812
Chicago/Turabian StyleAree, Thammarat. 2021. "Distinctive Supramolecular Features of β-Cyclodextrin Inclusion Complexes with Antidepressants Protriptyline and Maprotiline: A Comprehensive Structural Investigation" Pharmaceuticals 14, no. 8: 812. https://doi.org/10.3390/ph14080812
APA StyleAree, T. (2021). Distinctive Supramolecular Features of β-Cyclodextrin Inclusion Complexes with Antidepressants Protriptyline and Maprotiline: A Comprehensive Structural Investigation. Pharmaceuticals, 14(8), 812. https://doi.org/10.3390/ph14080812