Extracellular Vesicles in Skin Wound Healing
Abstract
1. Introduction
- (i).
- the current knowledge about EV involvement in each stage of natural skin repair;
- (i).
- (ii). the current efforts applying EVs for skin regeneration, wound healing, and treatment of dermal diseases, including the cases of engineered EVs and those of non-human origin.
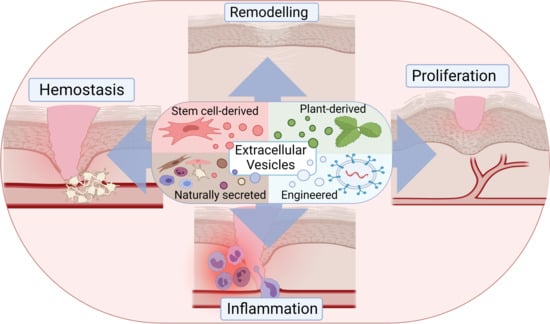
2. The Role of Extracellular Vesicles in Natural Wound Repair
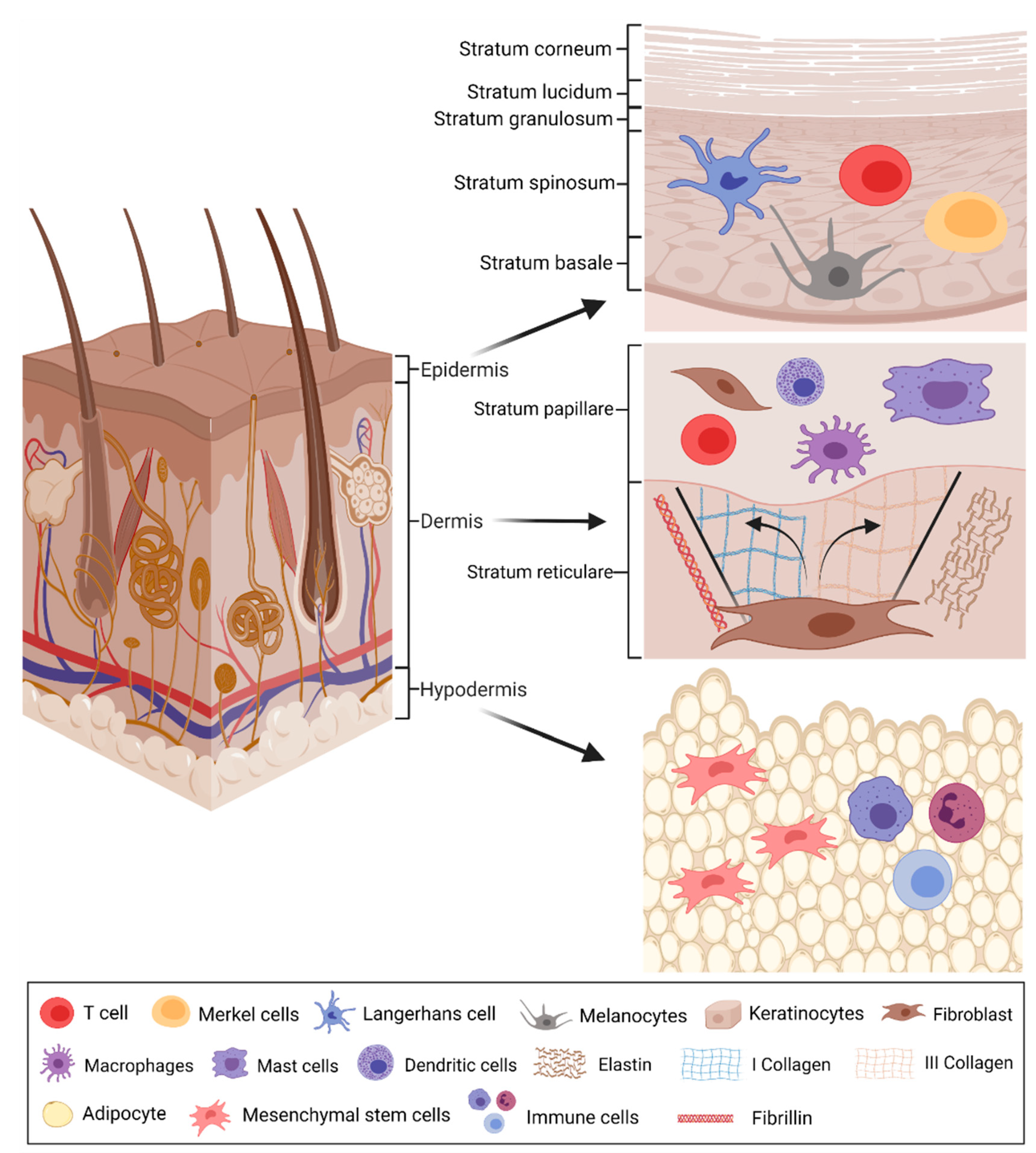
2.1. Physiology of Healthy and Wounded Skin
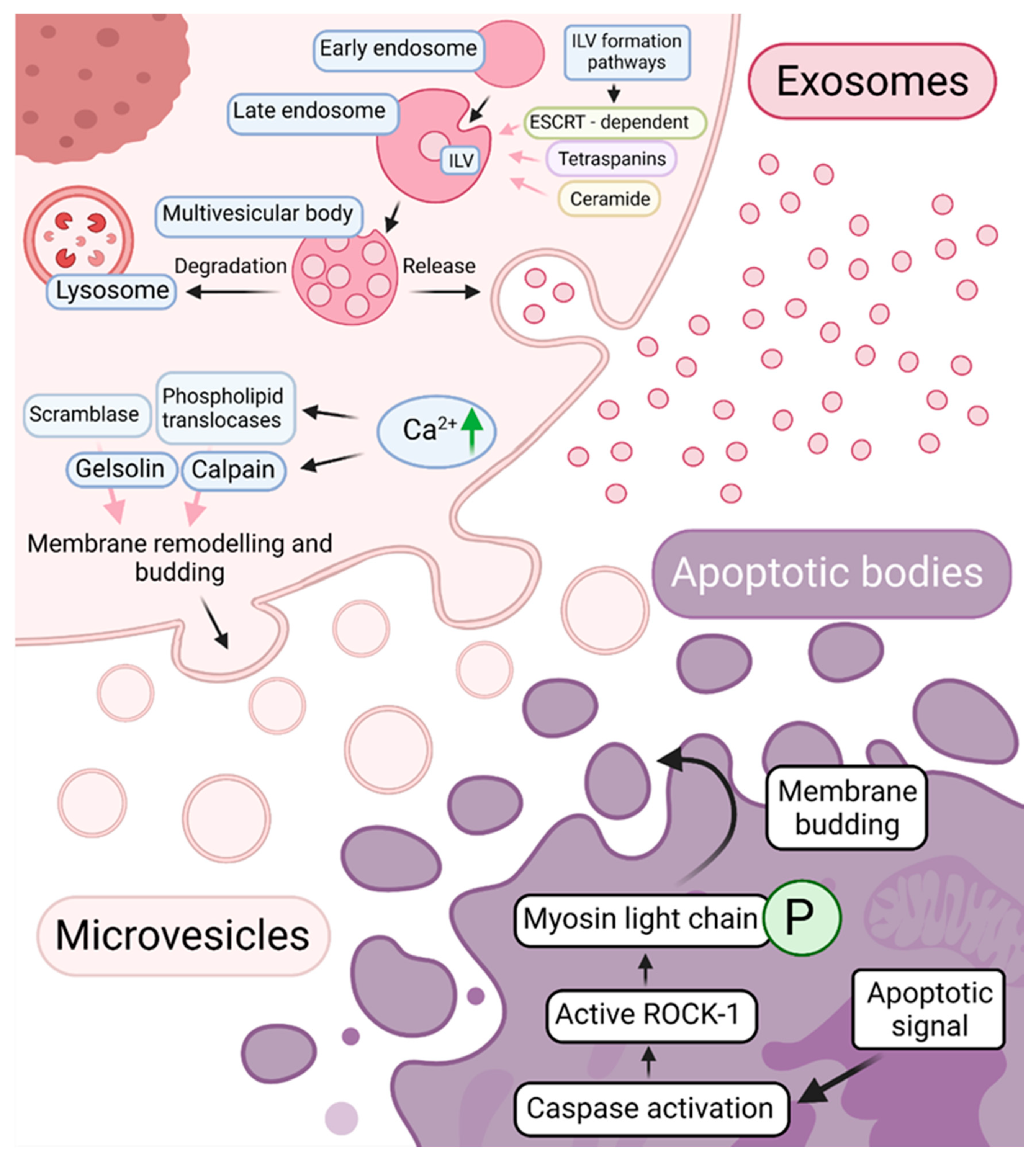
2.2. Extracellular Vesicles—Biogenesis, Composition, and Function
2.3. Physiological Role of Extracellular Vesicles in Wound Healing
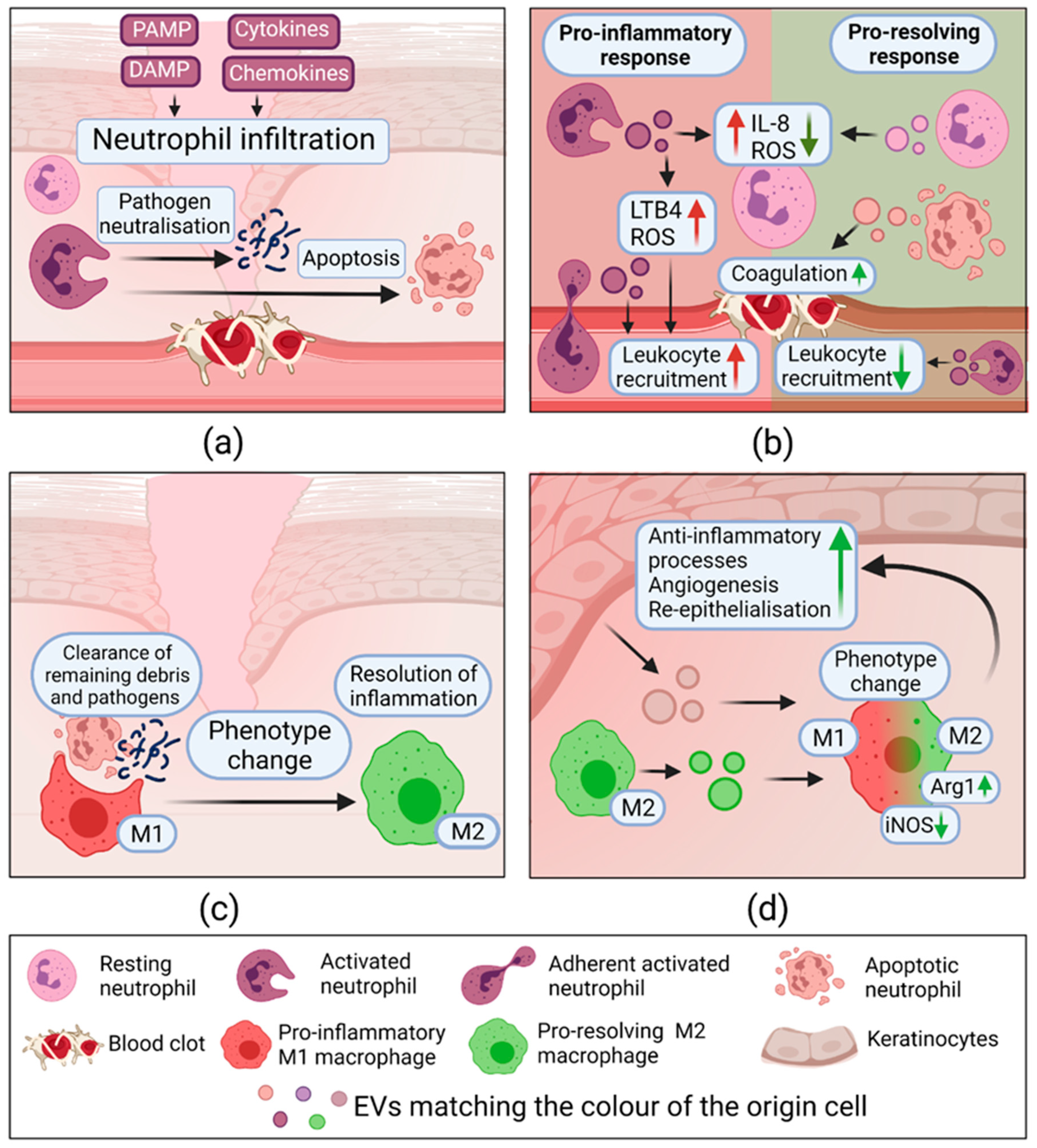
2.3.1. Extracellular Vesicles in Hemostasis
2.3.2. Extracellular Vesicles in Inflammation
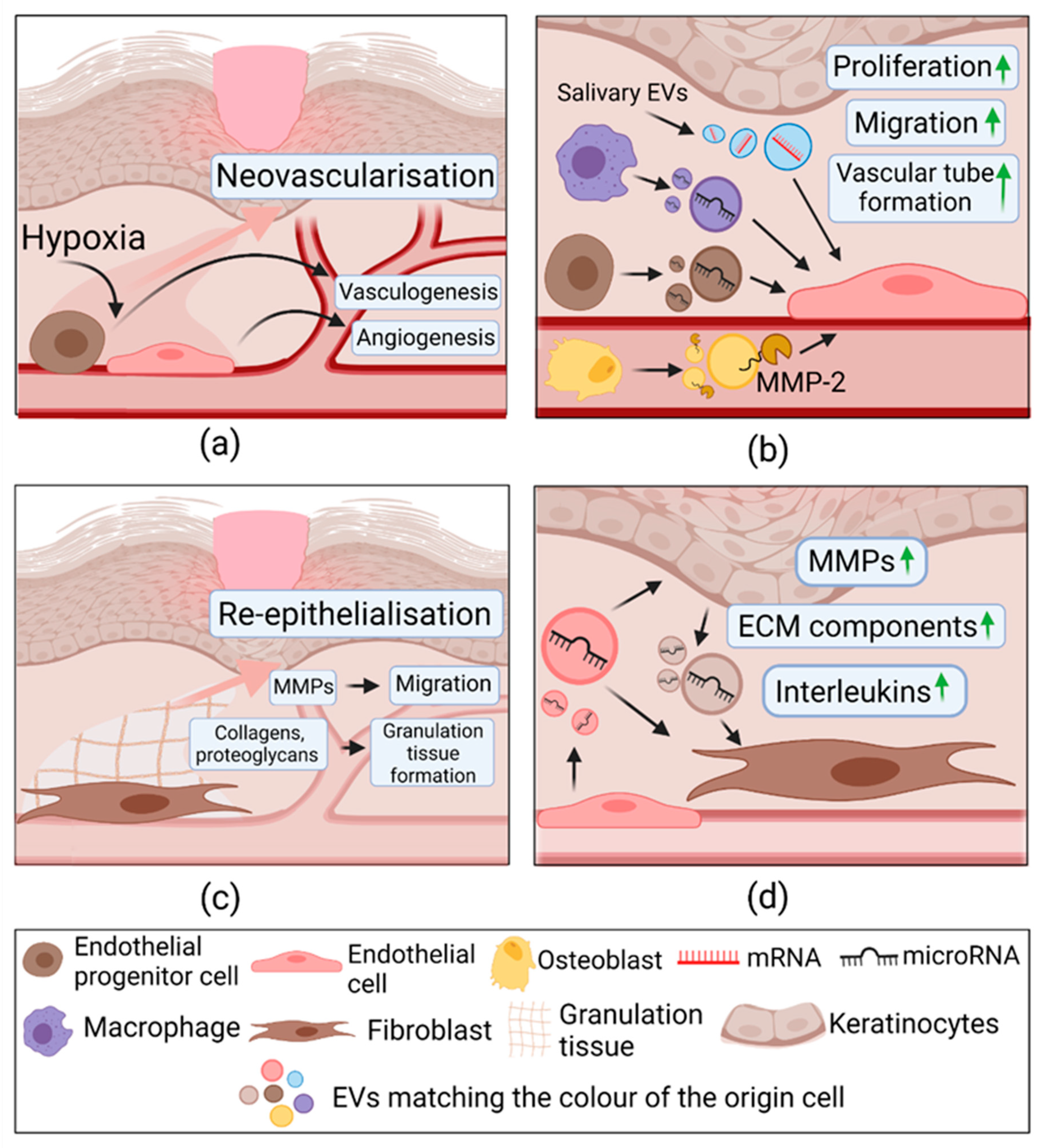
2.3.3. Extracellular Vesicles in Proliferation
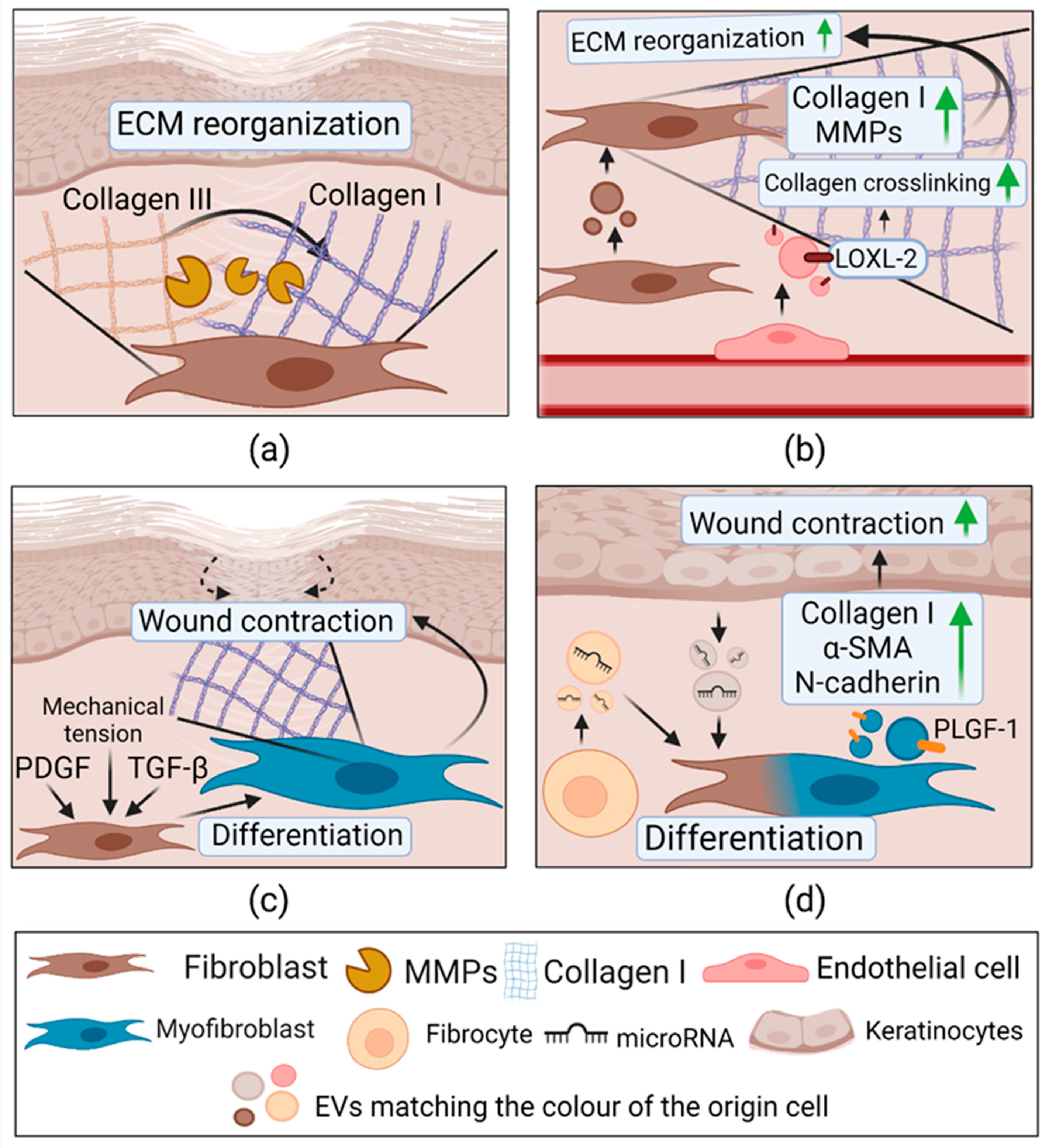
2.3.4. Extracellular Vesicles in Remodeling
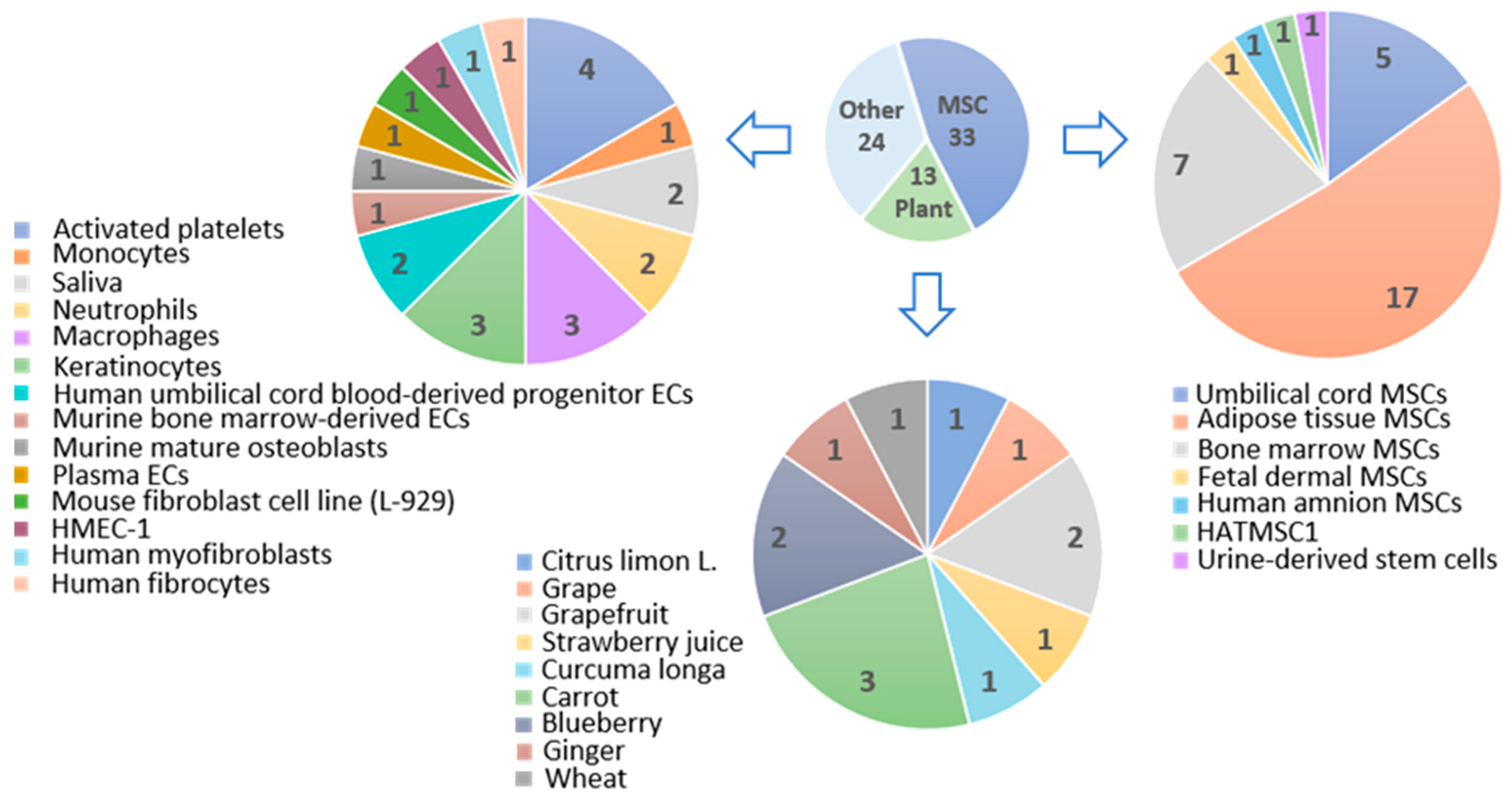
3. Stem Cell-Derived Extracellular Vesicles in Skin Wound Healing
3.1. Mesenchymal Stem Cell-Derived Extracellular Vesicles in Hemostasis
3.2. Mesenchymal Stem Cell-Derived Extracellular Vesicles in Inflammation
3.3. Extracellular Vesicles from Mesenchymal Stem Cells in Proliferation
3.4. Extracellular Vesicles from Mesenchymal Stem Cells in Remodelling
4. Plant-Derived Extracellular Vesicles
4.1. Plant-Derived Extracellular Vesicles in Hemostasis
4.2. Actual and Predictive Role of Plant-Derived Extracellular Vesicles in Inflammation
4.3. Plant-Derived Extracellular Vesicles in Proliferation
4.4. Plant-Derived Extracellular Vesicles in Remodelling
5. Therapeutical Application of Extracellular Vesicles for Skin Wound Healing
5.1. Extracellular Vesicle-Loaded Scaffolds
5.2. Application of Extracellular Vesicles for Treatment of Dermal Diseases
6. Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Phase | Parental Cells | Recipient Cells | Effects | Cargo/Signaling Pathway | Reference |
|---|---|---|---|---|---|
| Hemostasis | Thrombin activated platelets | Platelets | Formation of fibrin in vitro ↑, bleeding time and blood loss in vivo ↓ | Activated form of integrin αIIbβ3 | [103] |
| ADP activated platelets | Plasma | Formation of fibrin ↑; Provide pro-coagulant surface and bind PSGL-1 | PS, P-selectin | [104] | |
| Sepsis activated platelets | Plasma | Formation of thrombin ↑; Activate intrinsic and extrinsic coagulation pathways. | PS, Factor XII and TF. | [106] | |
| Monocytes | Collagen-activated platelets | Transfer of molecules to platelet membrane | TF, PSGL-1 | [109] | |
| Saliva | Plasma | Formation of fibrin ↑; Activate TF-dependent coagulation pathway. | CD24, TF | [110] | |
| CRP or TRAP-6 activated platelets | Platelets | Platelet activation ↑; Generate superoxide and activate platelets via GPVI receptor, increase P-selectin exposure. | NOX-1 | [111] | |
| Inflammation | Opsonized zymosan A activated neutrophils | Neutrophils, HUVECs | ↑ ROS, IL-8; E-selectin, VCAM-1 | Not determined | [112] |
| Resting state neutrophils | Neutrophils, HUVECs, plasma | ↓ ROS, IL-8, ↑ Coagulation | Not determined | [112] | |
| Apoptosing neutrophils | Plasma | ↑ Coagulation | Not determined | [112] | |
| Non-adherent fMLF activated neutrophils | EVs alone HUVECs | ↑ ROS, ↑LTB4 Migrated towards chemotactic gradient ↓ STAT1, NFKBIZ, CCL8, CXCL6 | NOX-2 LKHA4 5-LOX | [113] | |
| Adherent fMLF activated neutrophils | EVs alone HUVECs | ↑ LTB4; Migrated towards chemotactic gradient ↑IL1β, CCL3L1, STAT3 | Not determined | [113] | |
| Bone marrow derived-IL-4 activated—M2 macrophages | Bone marrow derived-IFN-γ activated—M1 macrophages In vivo healthy mouse model | Macrophage reprogramming M1 → M2 ↓iNOS, ↑Arg1; Reprogrammed M2 ↑ fibroblast migration, EC tube formation; In vivo: ↑ wound healing | CCL24, CCL22, MFG-E8 | [117] | |
| Mouse wound edge KCs | EVs alone; Mouse wound macrophages (M) In vivo healthy mouse model | Glycan ions with high mannose, ↑M uptake; M reprogramming ↓NOS2, CD74, TNFα; ↑ CL3; In vivo: ↓accumulation of M; ↓iNOS, ↑Arg1; ↑Skin barrier-function | Not determined | [7] | |
| Proliferation | Human umbilical cord blood derived endothelial progenitor cells | HMEC-1 In vivo diabetic rat model | ↑ Proliferation, migration, tube formation. ↑ ANG-1, E-selectin, FGF1, CXCL16, eNOS, VEGFA, VEGFR-2, IL8; ↓MMP-9. In vivo: ↑ Wound healing | Not determined | [118] |
| ↑ Proliferation, migration, tube formation. ↑ FGF-1, IL8, IL6, VEGFA, COX-2; c-Myc, Id1, pERK1/2 expression gene and protein expression; In vivo: ↑ Wound healing, angiogenesis | miR-21; Activated ERK1/2 | [119] | |||
| Murine bone marrow-derived ECs | In vivo healthy and diabetic mice | ↑ skin wound healing; ↑ VEGF, PECAM-1, Ki67. | miR-221-3p | [120] | |
| Macrophages (RAW 264.7) | Mouse endothelial cell line SVEC4-10EHR1; In vivo healthy mouse model | ↑ Proliferation, migration, tube formation In vivo: ↑ Angiogenesis | VEGF, Wnt3a, miR-130a, miR-126, miR-210. | [122] | |
| Diabetic HUVECs In vivo diabetic rat model | ↑Angiogenesis, cell migration, proliferation via ↓ IL-6 and TNF-α production; In vivo: ↓ IL-6, TNF-α; ↑ p-Akt, ↓MMP-9; ↑collagen deposition | Activated Akt/VEGF | [121] | ||
| Murine mature osteoblasts | Brain-derived endothelial cell line (bEnd.3) | ↑ Proliferation, migration, tube formation; ↑ p-VEGFR2, pERK1/2 expression, MMP-2 | MMP-2 Activated:VEGF/ERK1/2 | [123] | |
| Saliva | HUVECs In vivo healthy mice | ↑ Proliferation, migration, tube formation; ↓SMAD-6; ↑BMP2; In vivo: ↑ wound healing | UBE2O mRNA | [124] | |
| Plasma ECs | Diabetic skin fibroblasts; HaCaT; In vivo diabetic mouse model | ↑Proliferation, migration; ↓ Senescence markers; ↑ YAP dephosphorylation and nuclear translocation In vivo: ↑ wound healing, ↓fibroblast senescence | Activated PI3K/Akt/mTOR | [125] | |
| HaCaT; HEKa; NHEK | Human dermal fibroblasts | ↑ TGFBRII, CCN2, FGF2; laminin-111, collagen IV, IL-8, MMP-1, IL6 ↑ IL-6; MMP-1; MMP-3; THBS protein expression; ↑migration, fibroblast-mediated endothelial tube formation. ↓TIMP3; TIMP4; | Activated: ERK1/2, Smad, p38; JNK. | [127] | |
| HEKa | Human foreskin fibroblasts In vivo diabetic rat model | ↑migration, fibroblast-mediated endothelial tube formation. ↑ IL-6, IL-8 gene and protein expression; ↓PTEN, RECK; ↑ α-SMA and N-cadherin; ↑ pERK1/2 In vivo: ↑ wound healing. | miR-21; Activated: ERK1/2 | [128] | |
| Remodeling | Mouse fibroblast cell line (L-929) | Mouse fibroblast cell line (L-929); mouse endothelial cell line SVEC4-10EHR1; In vivo healthy mouse model | ↑Collagen I, MMP-1, MMP-3; ↑ Proliferation, migration, tube formation; Combination with fibrin glue ↑ wound healing, collagen deposition; ↑ VEGF | Not determined | [130] |
| HMEC-1 under hypoxic conditions | ECM | ↑LOX activity; ↑Collagen gel contraction | LOXL-2 | [131] | |
| Human myofibroblasts from normal skin wound | Human skin fibroblasts | ↑ Migration, collagen I; | PLGF-1, LTA, VEGF, IL-23 | [133] | |
| Human fibrocytes stimulated with PDGF, TGF-β, FGF-1 | Diabetic human ECs, KCs, dermal fibroblasts; In vivo diabetic mouse model | ↑ Proliferation, migration, tube formation; ↑Collagen I, Collagen III, α-SMA in fibroblasts In vivo: ↑ wound healing. | HSP-90α, total and activated STAT3, miR-124a, miR-125b, miR-126, miR-130a, miR-132, miR-21 | [134] |
| Phase | EVs Source | Effects | EVs Molecules Involved | Signaling Pathway | Reference |
|---|---|---|---|---|---|
| Hemostasis | Umbilical cord MSCs | Coagulation activation ↑Clot firmness and area ↓Clotting time ↓Clot formation ↓Lag period of spontaneous clotting | PS, CD9, Histones, Myosin-9, Talin-1, cytoplasmic 1 and 2 actin, annexin V | Not specified | [143] |
| Adipose tissue MSCs Bone marrow MSCs | ↑Peak of thrombin activity ↑Thrombin generation ↓Thrombin activation times Tendency to faster clot formation | PS, TF | Not specified | [144] | |
| Adipose tissue MSCs Bone marrow MSCs | Procoagulant activity | TF | Not specified | [145] | |
| Adipose tissue MSCs | Procoagulant activity | Not determined | Extrinsic and intrinsic | [147] | |
| Inflammation | Bone marrow MSCs | Macrophage polarization and reprogrammation M1→M2 ↑CD206 M2 marker ↑IL-10 and ↓TNFα In vivo: ↑Wound closure | miR-223 | pknox1 regulation | [148] |
| Adipose tissue MSCs | Macrophages polarization M1 → M2 ↑Arg1 and ↑CD206 M2 markers ↓TNFα, ↓IL-6, ↓IL-8 and ↑IL-10, ↑TGFβ1, ↑TSG6, ↑collagen III and I, ↑fibrinonectin In vitro: ↓Wound area | miR-34a-5p, miR-124-3p miR-146a-5p, miR-132 miR-21, miR-29a miR-223-3p, miR-203b-5p | Notch1 Mef2c Targeting TNFα, IL-24 | [149] | |
| Bone marrow MSCs | ↓IL-1β, ↓TNFα, ↓iNOS, ↑IL-10, ↑Arg1 ↑PTEN, inhibiting p-AKT, ↑M2/M1 ratio In vivo: ↑Angiogenesis and ↑collagen synthesis | Not determined | PTEN/AKT | [150] | |
| Adipose tissue MSCs | ↑Bcl-2 and ↑IL10 ↓C-caspase3 and ↓IL-6 ↑Cell viability, ↓apoptosis ↑ KCs migration | miRNA-19b | CCL1/TGF-β | [151] | |
| Adipose tissue MSCs | ↓ROS, ↓NOX1, ↓NOX4 ↓IL-1β, ↓TNFα, ↓IL-6 ↑SMP30, ↑VEGF, ↑p-VEGFR2 | Nrf2 | Nrf2 overexpression | [152] | |
| Umbilical cord MSCs | ↓NF-κB activation ↓IL-1β, ↓TNFα and ↓IL-10 | miR-181c | TLR4 | [153] | |
| Proliferation | Adipose tissue MSCs | ↑Fibroblast migration ↑Angiogenesis In vivo: ↓ischemic wounds | MALAT1 | Not specified | [155] |
| Human amnion MSCs | ↑Fibroblast migration, proliferation ↓E-Cadherin, ↓N-Cadherin, ↓LATS2, ↑αSMA In vivo: ↑Wound healing, new granulation tissue, ↓inflammatory cells amount | miR-135a | LATS2 | [156] | |
| Adipose tissue MSCs | ↑Fibroblast migration and proliferation, ↑N-cadherin, ↑cyclin-1, ↑PCNA, ↑collagen I and III In vivo: ↑Cutaneous wound healing and ↑collagen synthesis | Not determined | Not specified | [157] | |
| Adipose tissue MSCs | ↑Fibroblast migration and proliferation, ↑collagen I and III, ↑MMP1, ↑bFGF, ↑TGF-β1, ↑p-Akt/Akt, ↑collagen I and III, ↑MMP1, ↑bFGF, ↑TGF-β1. In vitro and in vivo: promote and optimize collagen deposition | Not determined | PI3K/Akt | [158] | |
| Fetal dermal MSCs | ↑Fibroblast migration, proliferation, viability and activity In vitro: ↑collagen I and III, ↑elastin, ↑fibronectin-1, ↑αSMA In vivo: ↑collagen deposition, ECM synthesis | Jagged 1 | Notch | [159] | |
| Adipose tissue MSCs | ↑Fibroblast migration, proliferation and invasion | lncRNA H19 | Wnt/β-catenin lncRNA H19/miR-19b/SOX9 axis | [160] | |
| Bone marrow MSCs | ↑Fibroblast migration and proliferation, ↑tube formation ↑c-myc, ↑cyclin A1, ↑cyclin D2, ↑HGF, ↑IGF1, ↑NGF, ↑SDF1 | STAT3 | Akt, ERK1/2 and STAT3 | [161] | |
| Adipose tissue MSCs | In vitro and in vivo: ↑angiogenesis In vitro: ↑Tube length and branches number, ↑Ang1, ↑Flk1, ↓Vash1 and ↓TSP1 | miR-125a | suppress DLL4 expression | [162] | |
| Immortalized adipose MSCs line HATMSC1 | ↑Proliferation and proangiogenic properties of ECs | EGF, bFGF, IL-8, VEGF, TIMP-1, TIMP-2, miR-210 miR-296, miR-126 miR-378, miR-221, miR-222, miR-92a | VEGFR2 | [163] | |
| Umbilical cord blood | ↑Number of new vessels, ↑tube length and branches amount, ↑wound closure, ↑collagen fibers, ↓scar widths, ↓PTEN ↓SPRY1 | miR-21-3p miR-214-5p miR-19b-5p | PI3K/Akt ERK 1/2 | [164] | |
| Urine-derived stem cells | In vitro: ↑Fibroblast proliferation and migration In vivo: ↑Pro-angiogenesic effects (number and density of new vessels), ↓scar widths | DMTB1 | Not specified | [165] | |
| Human umbilical cord MSCs | ↑Migration, proliferation, and tube formation In vivo: ↑wound closure, ↑angiogenesis | Angiopoietin-1 and 2 | Angiopoietin/ TIE | [166] | |
| Adipose tissue MSCs | ↑KCs migration, proliferation, ↑p-AKT, ↑HIF-1α In vivo: ↓Wound area | Not determined | AKT/HIF-1α | [167] | |
| Adipose tissue MSCs | ↑KCs proliferation and migration, ↑MMP-9, ↑TIMP2 | miRNA-21 | P13K/AKT | [168] | |
| Adipose tissue MSCs | ↑KCs proliferation and migration, ↓apoptosis ↑β-catenin In vivo: ↓wound area | Not determined | Wnt/β-catenin | [169] | |
| Umbilical cord MSCs | ↑Re-epithelization | Wnt4 | Wnt/β-catenin | [170] | |
| Adipose tissue MSCs Bone marrow MSCs | ↑Fibroblast, KCs, ECs migration, proliferation, cell viability ↑Tube formation ↑Wound closure | CD73 and various miRNAs | Both types EVs: EGFR receptor axis, PI3K/Akt, MAPK, Wnt Only AdMSCs-EVs: TGF-β and HIF-1α | [171] | |
| Remodeling | Bone marrow MSCs | ↓TGF-β1, ↓Smad2, ↓Smad3, ↓Smad4, ↑TGF-β3, ↑Smad7, ↑α-SMA, ↑VEGF ↑KCs and fibroblast proliferation In vivo: ↑wound healing, ↓wound area, ↑cutaneous appendages | Not determined | TGF-β/Smad | [172] |
| Adipose tissue MSCs | ECM remodeling ↑rate of collagen III/collagen I, ↑TGFβ3/TGFβ1, ↑MMP3 ↑Scarless wound healing | Not determined | ERK/MAPK | [173] | |
| Adipose tissue MSCs | In vivo: ↑Skin elasticity and barrier integrity, ↑re-epithelization, ↑wound closure, ↑angiogenesis, ↑collagen synthesis ↑PCNA, VEGF, filaggrin, loricrin, AQP3 ↓TNFα | Not determined | Not specified | [174] |
| Phase | EVs Source | Effects | EVs Molecules Involved | Signalling Pathway | Reference |
|---|---|---|---|---|---|
| Inflammation | Citrus limon L. | Anti-oxidative effect on mesenchymal stromal cells | Citrate Vitamin C Short miRNAs with unknown function | Not specified | [183] |
| Strawberry juice Fragaria x ananassa | ↓ROS production | Vitamin C Small RNAs (17–30 nt) miR166g | Not specified | [184] | |
| Blueberry | ↓Oxidative stress ↓ROS levels ↑Antioxidant genes expression ↑Bcl2 ↑HO-1 ↓Bax ↓FAS ↓ACC1 | Not determined | Nrf2 | [185] | |
| Blueberry | ↓TNFα induced ROS generation ↑cell viability | miR-156e, miR-162, and miR-319d | Involved in 340 canonical pathways, 121 KEGG pathways, and 121 GO Biological processes | [186] | |
| Grape | ↓DSS induced colitis | Not determined | Wnt/β-catenin pathway | [187] | |
| Curcuma Longa | ↓colitis ↑promote intestinal wound repair ↑HO-1 ↓IL-6 ↓IL-1β ↓TNF-α | Not determined | Nf-κB pathway | [188] | |
| Ginger | ↑HO-1 ↑IL-10 ↑IL-6 ↑nuclear factor like (erythroid-derived 2) | Not determined | Wnt/TCF4 | [189] | |
| Grapefruit | ↑nuclear factor like (erythroid-derived 2) | Not determined | Wnt/TCF4 | [189] | |
| Carrot | ↑nuclear factor like (erythroid-derived 2) | Not determined | Wnt/TCF4 | [189] | |
| Ginger | ↓lung inflammation ↓Nsp12 | aly-miR396a-5p and rlcv-miR-rL1-28-3p | NK-κB | [190] | |
| Proliferation | Grapefruit | ↑HaCaT cells’ viability ↑cell migration ↓intracellular ROS production ↑HUVECs tube formation capabilities | Not determined | Not specified | [192] |
| Wheat (Triticum aestivum) | ↑endothelial, epithelial and dermal fibroblast cells’ viability and migration ↑vascularization and angiogenesis | Not determined | Not specified | [177] | |
| Ginger | ↑intestinal wound healing ↓TNF-α, IL-6 and IL-1β ↑IL-10 and IL-22 | phosphatidic acid, digalactosyldiacylglycerol, monogalactosyldiacyglycerol, actin and proteolysis enzymes, aquaporin and chloride channels, ~125 miRNAs, 6-gingerol, 6-shogaol | Not specified | [193] |
References
- Frykberg, R.G.; Banks, J. Challenges in the Treatment of Chronic Wounds. Adv. Wound Care 2015, 4, 560–582. [Google Scholar] [CrossRef] [PubMed]
- Limandjaja, G.C.; Niessen, F.B.; Scheper, R.J.; Gibbs, S. Hypertrophic scars and keloids: Overview of the evidence and practical guide for differentiating between these abnormal scars. Exp. Dermatol. 2021, 30, 146–161. [Google Scholar] [CrossRef]
- Guest, J.F.; Vowden, K.; Vowden, P. The health economic burden that acute and chronic wounds impose on an average clinical commissioning group/ health board in the UK. J. Wound Care 2017, 26, 292–303. [Google Scholar] [CrossRef] [PubMed]
- Sen, C.K. Human Wounds and Its Burden: An Updated Compendium of Estimates. Adv. Wound Care 2019, 8, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Chen, Q.; Lin, L.; Sha, C.; Li, T.; Liu, Y.; Yin, X.; Xu, Y.; Chen, L.; Gao, W.; et al. Regulation of exosome production and cargo sorting. Int. J. Biol. Sci. 2020, 17, 163. [Google Scholar] [CrossRef]
- Chung, I.M.; Rajakumar, G.; Venkidasamy, B.; Subramanian, U.; Thiruvengadam, M. Exosomes: Current use and future applications. Clin. Chim. Acta 2020, 500, 226–232. [Google Scholar] [CrossRef]
- Clemmer, D.E.; Ghatak, S.; Zhou, X.; Brown, B.A.; Siegel, A.P.; El Masry, M.S.; Zeng, X.; Song, W.; Das, A.; Khandelwal, P.; et al. Exosome-mediated crosstalk between keratinocytes and macrophages in cutaneous wound healing. ACS Nano 2020, 14, 12732–12748. [Google Scholar] [CrossRef]
- Tran, P.H.L.; Xiang, D.; Tran, T.T.D.; Yin, W.; Zhang, Y.; Kong, L.; Chen, K.; Sun, M.; Li, Y.; Hou, Y.; et al. Exosomes and Nanoengineering: A Match Made for Precision Therapeutics. Adv. Mater. 2020, 32, 1904040. [Google Scholar] [CrossRef]
- Rahmati, S.; Shojaei, F.; Shojaeian, A.; Rezakhani, L.; Dehkordi, M.B. An overview of current knowledge in biological functions and potential theragnostic applications of exosomes. Chem. Phys. Lipids 2020, 226, 104836. [Google Scholar] [CrossRef]
- Meldolesi, J. Exosomes and Ectosomes in Intercellular Communication. Curr. Biol. 2018, 28, R435–R444. [Google Scholar] [CrossRef]
- Shin, K.O.; Ha, D.H.; Kim, J.O.; Crumrine, D.A.; Meyer, J.M.; Wakefield, J.S.; Lee, Y.; Kim, B.; Kim, S.; Kim, H.K.; et al. Exosomes from Human Adipose Tissue-Derived Mesenchymal Stem Cells Promote Epidermal Barrier Repair by Inducing de Novo Synthesis of Ceramides in Atopic Dermatitis. Cells 2020, 9, 680. [Google Scholar] [CrossRef]
- Wu, J.; Yang, Q.; Wu, S.; Yuan, R.; Zhao, X.; Li, Y.; Wu, W.; Zhu, N. Adipose-Derived Stem Cell Exosomes Promoted Hair Regeneration. Tissue Eng. Regen. Med. 2021, 18, 685–691. [Google Scholar] [CrossRef]
- Qiu, X.; Liu, J.; Zheng, C.; Su, Y.; Bao, L.; Zhu, B.; Liu, S.; Wang, L.; Wang, X.; Wang, Y.; et al. Exosomes released from educated mesenchymal stem cells accelerate cutaneous wound healing via promoting angiogenesis. Cell Prolif. 2020, 53, e12830. [Google Scholar] [CrossRef] [PubMed]
- Ha, D.H.; Kim, H.K.; Lee, J.; Kwon, H.H.; Park, G.H.; Yang, S.H.; Jung, J.Y.; Choi, H.; Lee, J.H.; Sung, S.; et al. Mesenchymal Stem/Stromal Cell-Derived Exosomes for Immunomodulatory Therapeutics and Skin Regeneration. Cells 2020, 9, 1157. [Google Scholar] [CrossRef] [PubMed]
- Gilaberte, Y.; Prieto-Torres, L.; Pastushenko, I.; Juarranz, Á. Anatomy and Function of the Skin. In Nanoscience in Dermatology; Elsevier: Amsterdam, The Netherlands, 2016; pp. 1–14. [Google Scholar] [CrossRef]
- Yousef, H.; Alhajj, M.; Sharma, S. Anatomy, Skin (Integument), Epidermis; StatPearls Publishing: Treasure Island, FL, USA, 2017. [Google Scholar]
- Medzhitov, R. Toll-like receptors and innate immunity. Nat. Rev. Immunol. 2001, 1, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Köllisch, G.; Kalali, B.N.; Voelcker, V.; Wallich, R.; Behrendt, H.; Ring, J.; Bauer, S.; Jakob, T.; Mempel, M.; Ollert, M. Various members of the Toll-like receptor family contribute to the innate immune response of human epidermal keratinocytes. Immunology 2005, 114, 531–541. [Google Scholar] [CrossRef]
- Kennedy-Crispin, M.; Billick, E.; Mitsui, H.; Gulati, N.; Fujita, H.; Gilleaudeau, P.; Sullivan-Whalen, M.; Johnson-Huang, L.M.; Suárez-Fariñas, M.; Krueger, J.G. Human keratinocytes’ response to injury upregulates CCL20 and other genes linking innate and adaptive immunity. J. Investig. Dermatol. 2012, 132, 105–113. [Google Scholar] [CrossRef]
- Woodley, D.T. Distinct Fibroblasts in the Papillary and Reticular Dermis: Implications for Wound Healing. Dermatol. Clin. 2017, 35, 95–100. [Google Scholar] [CrossRef]
- Brown, T.M.; Krishnamurthy, K. Histology, Dermis; In StatPerls [Internet]. StatPearls Publishing, Treasure Island (FL). 2021. [Google Scholar]
- Cole, M.A.; Quan, T.; Voorhees, J.J.; Fisher, G.J. Extracellular matrix regulation of fibroblast function: Redefining our perspective on skin aging. J. Cell Commun. Signal. 2018, 12, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Gaur, M.; Dobke, M.; Lunyak, V.V. Mesenchymal stem cells from adipose tissue in clinical applications for dermatological indications and skin aging. Int. J. Mol. Sci. 2017, 18, 208. [Google Scholar] [CrossRef] [PubMed]
- Mraz, M.; Haluzik, M. The role of adipose tissue immune cells in obesity and low-grade inflammation. J. Endocrinol. 2014, 222, R113–R127. [Google Scholar] [CrossRef]
- Chen, S.X.; Zhang, L.J.; Gallo, R.L. Dermal White Adipose Tissue: A Newly Recognized Layer of Skin Innate Defense. J. Investig. Dermatol. 2019, 139, 1002–1009. [Google Scholar] [CrossRef]
- Ezure, T.; Amano, S. Adiponectin and leptin up-regulate extracellular matrix production by dermal fibroblasts. Biofactors 2007, 31, 229–236. [Google Scholar] [CrossRef]
- Jackson, W.M.; Nesti, L.J.; Tuan, R.S. Concise review: Clinical translation of wound healing therapies based on mesenchymal stem cells. Stem Cells Transl. Med. 2012, 1, 44–50. [Google Scholar] [CrossRef]
- Naderi, N.; Combellack, E.J.; Griffin, M.; Sedaghati, T.; Javed, M.; Findlay, M.W.; Wallace, C.G.; Mosahebi, A.; Butler, P.E.; Seifalian, A.M.; et al. The regenerative role of adipose-derived stem cells (ADSC) in plastic and reconstructive surgery. Int. Wound J. 2017, 14, 112–124. [Google Scholar] [CrossRef]
- Jackson, W.M.; Nesti, L.J.; Tuan, R.S. Mesenchymal stem cell therapy for attenuation of scar formation during wound healing. Stem Cell Res. Ther. 2012, 3, 20. [Google Scholar] [CrossRef] [PubMed]
- Niu, P.; Smagul, A.; Wang, L.; Sadvakas, A.; Sha, Y.; Pérez, L.M.; Nussupbekova, A.; Amirbekov, A.; Akanov, A.A.; Gálvez, B.G.; et al. Transcriptional profiling of interleukin-2-primed human adipose derived mesenchymal stem cells revealed dramatic changes in stem cells response imposed by replicative senescence. Oncotarget 2015, 6, 17983. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.; Kosaric, N.; Bonham, C.A.; Gurtner, G.C. Wound healing: A cellular perspective. Physiol. Rev. 2019, 99, 665–706. [Google Scholar] [CrossRef] [PubMed]
- Joo, S.J. Mechanisms of platelet activation and integrin αIIβ3. Korean Circ. J. 2012, 42, 295–301. [Google Scholar] [CrossRef]
- Li, Z.; Delaney, M.K.; O’Brien, K.A.; Du, X. Signaling during platelet adhesion and activation. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 2341–2349. [Google Scholar] [CrossRef]
- Bowler, P.G.; Duerden, B.I.; Armstrong, D.G. Wound microbiology and associated approaches to wound management. Clin. Microbiol. Rev. 2001, 14, 244–269. [Google Scholar] [CrossRef] [PubMed]
- Kalan, L.; Grice, E.A. Fungi in the wound microbiome. Adv. Wound Care 2018, 7, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Gillitzer, R.; Goebeler, M. Chemokines in cutaneous wound healing. J. Leukoc. Biol. 2001, 69. [Google Scholar] [CrossRef]
- Sorg, H.; Tilkorn, D.J.; Hager, S.; Hauser, J.; Mirastschijski, U. Skin Wound Healing: An Update on the Current Knowledge and Concepts. Eur. Surg. Res. 2017, 58, 81–94. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.H.; Huang, B.S.; Horng, H.C.; Yeh, C.C.; Chen, Y.J. Wound healing. J. Chin. Med. Assoc. 2018, 81, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Balaji, S.; King, A.; Crombleholme, T.M.; Keswani, S.G. The Role of Endothelial Progenitor Cells in Postnatal Vasculogenesis: Implications for Therapeutic Neovascularization and Wound Healing. Adv. Wound Care 2013, 2, 283–295. [Google Scholar] [CrossRef]
- Guo, S.; DiPietro, L.A. Critical review in oral biology & medicine: Factors affecting wound healing. J. Dent. Res. 2010, 89, 219–229. [Google Scholar] [CrossRef]
- Dunnill, C.; Patton, T.; Brennan, J.; Barrett, J.; Dryden, M.; Cooke, J.; Leaper, D.; Georgopoulos, N.T. Reactive oxygen species (ROS) and wound healing: The functional role of ROS and emerging ROS-modulating technologies for augmentation of the healing process. Int. Wound J. 2015, 14, 89–96. [Google Scholar] [CrossRef]
- Edwards, R.; Harding, K.G. Bacteria and wound healing. Curr. Opin. Infect. Dis. 2004, 17, 91–96. [Google Scholar] [CrossRef]
- Gould, L.; Abadir, P.; Brem, H.; Carter, M.; Conner-Kerr, T.; Davidson, J.; Dipietro, L.; Falanga, V.; Fife, C.; Gardner, S.; et al. Chronic wound repair and healing in older adults: Current status and future research. Wound Repair Regen. 2015, 63, 427–438. [Google Scholar] [CrossRef]
- Hardman, M.J.; Ashcroft, G.S. Estrogen, not intrinsic aging, is the major regulator of delayed human wound healing in the elderly. Genome Biol. 2008, 9, R80. [Google Scholar] [CrossRef]
- Christian, L.M.; Graham, J.E.; Padgett, D.A.; Glaser, R.; Kiecolt-Glaser, J.K. Stress and wound healing. Neuroimmunomodulation 2007, 13, 337–346. [Google Scholar] [CrossRef]
- Patel, S.; Srivastava, S.; Singh, M.R.; Singh, D. Mechanistic insight into diabetic wounds: Pathogenesis, molecular targets and treatment strategies to pace wound healing. Biomed. Pharmacother. 2019, 112, 108615. [Google Scholar] [CrossRef]
- Pan, B.T.; Johnstone, R.M. Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: Selective externalization of the receptor. Cell 1983, 33, 967–978. [Google Scholar] [CrossRef]
- Kupsco, A.; Prada, D.; Valvi, D.; Hu, L.; Petersen, M.S.; Coull, B.; Grandjean, P.; Weihe, P.; Baccarelli, A.A. Human milk extracellular vesicle miRNA expression and associations with maternal characteristics in a population-based cohort from the Faroe Islands. Sci. Rep. 2021, 11, 5840. [Google Scholar] [CrossRef] [PubMed]
- Musante, L.; Bontha, S.V.; La Salvia, S.; Fernandez-Piñeros, A.; Lannigan, J.; Le, T.H.; Mas, V.; Erdbrügger, U. Rigorous characterization of urinary extracellular vesicles (uEVs) in the low centrifugation pellet—A neglected source for uEVs. Sci. Rep. 2020, 10, 3701. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Yuan, T.; Tschannen, M.; Sun, Z.; Jacob, H.; Du, M.; Liang, M.; Dittmar, R.L.; Liu, Y.; Liang, M.; et al. Characterization of human plasma-derived exosomal RNAs by deep sequencing. BMC Genom. 2013, 14, 319. [Google Scholar] [CrossRef]
- Srinivasan, S.; Vannberg, F.O.; Dixon, J.B. Lymphatic transport of exosomes as a rapid route of information dissemination to the lymph node. Sci. Rep. 2016, 6, 24436. [Google Scholar] [CrossRef]
- Chiabotto, G.; Gai, C.; Deregibus, M.C.; Camussi, G. Salivary Extracellular Vesicle-Associated exRNA as Cancer Biomarker. Cancers 2019, 11, 891. [Google Scholar] [CrossRef]
- Guha, D.; Lorenz, D.R.; Misra, V.; Chettimada, S.; Morgello, S.; Gabuzda, D. Proteomic analysis of cerebrospinal fluid extracellular vesicles reveals synaptic injury, inflammation, and stress response markers in HIV patients with cognitive impairment. J. Neuroinflamm. 2019, 16, 254. [Google Scholar] [CrossRef] [PubMed]
- Vojtech, L.; Zhang, M.; Davé, V.; Levy, C.; Hughes, S.M.; Wang, R.; Calienes, F.; Prlic, M.; Nance, E.; Hladik, F. Extracellular vesicles in human semen modulate antigen-presenting cell function and decrease downstream antiviral T cell responses. PLoS ONE 2019, 14, e0223901. [Google Scholar] [CrossRef] [PubMed]
- Frühbeis, C.; Fröhlich, D.; Krämer-Albers, E.M. Emerging roles of exosomes in neuron-glia communication. Front. Physiol. 2012, 3, 119. [Google Scholar] [CrossRef] [PubMed]
- Marcilla, A.; Trelis, M.; Cortés, A.; Sotillo, J.; Cantalapiedra, F.; Minguez, M.T.; Valero, M.L.; Sánchez del Pino, M.M.; Muñoz-Antoli, C.; Toledo, R.; et al. Extracellular Vesicles from Parasitic Helminths Contain Specific Excretory/Secretory Proteins and Are Internalized in Intestinal Host Cells. PLoS ONE 2012, 7, e45974. [Google Scholar] [CrossRef] [PubMed]
- Barteneva, N.S.; Maltsev, N.; Vorobjev, I.A. Microvesicles and intercellular communication in the context of parasitism. Front. Cell. Infect. Microbiol. 2013, 3, 49. [Google Scholar] [CrossRef]
- Doyle, L.; Wang, M. Overview of Extracellular Vesicles, Their Origin, Composition, Purpose, and Methods for Exosome Isolation and Analysis. Cells 2019, 8, 727. [Google Scholar] [CrossRef]
- Zaborowski, M.P.; Balaj, L.; Breakefield, X.O.; Lai, C.P. Extracellular Vesicles: Composition, Biological Relevance, and Methods of Study. Bioscience 2015, 65, 783–797. [Google Scholar] [CrossRef]
- Kerris, E.W.J.; Hoptay, C.; Calderon, T.; Freishtat, R.J. Platelets and platelet extracellular vesicles in hemostasis and sepsis. J. Investig. Med. 2020, 68, 813–820. [Google Scholar] [CrossRef]
- Elmore, S. Apoptosis: A Review of Programmed Cell Death. Toxicol. Pathol. 2007, 35, 495. [Google Scholar] [CrossRef]
- Leverrier, Y.; Ridley, A.J. Apoptosis: Caspases orchestrate the ROCK “n” bleb. Nat. Cell Biol. 2001, 3, E91–E92. [Google Scholar] [CrossRef]
- Wickman, G.R.; Julian, L.; Mardilovich, K.; Schumacher, S.; Munro, J.; Rath, N.; Zander, S.A.; Mleczak, A.; Sumpton, D.; Morrice, N.; et al. Blebs produced by actin–myosin contraction during apoptosis release damage-associated molecular pattern proteins before secondary necrosis occurs. Cell Death Differ. 2013, 20, 1293–1305. [Google Scholar] [CrossRef]
- Martínez, M.C.; Freyssinet, J.M. Deciphering the plasma membrane hallmarks of apoptotic cells: Phosphatidylserine transverse redistribution and calcium entry. BMC Cell Biol. 2001, 2, 20. [Google Scholar] [CrossRef]
- Mathieu, M.; Martin-Jaular, L.; Lavieu, G.; Théry, C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat. Cell Biol. 2019, 21, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Huotari, J.; Helenius, A. Endosome maturation. EMBO J. 2011, 30, 3481. [Google Scholar] [CrossRef] [PubMed]
- Baietti, M.F.; Zhang, Z.; Mortier, E.; Melchior, A.; Degeest, G.; Geeraerts, A.; Ivarsson, Y.; Depoortere, F.; Coomans, C.; Vermeiren, E.; et al. Syndecan–syntenin–ALIX regulates the biogenesis of exosomes. Nat. Cell Biol. 2012, 14, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Van Niel, G.; Charrin, S.; Simoes, S.; Romao, M.; Rochin, L.; Saftig, P.; Marks, M.S.; Rubinstein, E.; Raposo, G. The tetraspanin CD63 regulates ESCRT-independent and dependent endosomal sorting during melanogenesis. Dev. Cell 2011, 21, 708. [Google Scholar] [CrossRef] [PubMed]
- Ghossoub, R.; Chéry, M.; Audebert, S.; Leblanc, R.; Egea-Jimenez, A.L.; Lembo, F.; Mammar, S.; Le Dez, F.; Camoin, L.; Borg, J.-P.; et al. Tetraspanin-6 negatively regulates exosome production. Proc. Natl. Acad. Sci. USA 2020, 117, 5913–5922. [Google Scholar] [CrossRef]
- Trajkovic, K.; Hsu, C.; Chiantia, S.; Rajendran, L.; Wenzel, D.; Wieland, F.; Schwille, P.; Brügger, B.; Simons, M. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science 2008, 319, 1244–1247. [Google Scholar] [CrossRef]
- Verderio, C.; Gabrielli, M.; Giussani, P. Role of sphingolipids in the biogenesis and biological activity of extracellular vesicles. J. Lipid Res. 2018, 59, 1325–1340. [Google Scholar] [CrossRef] [PubMed]
- Grant, B.D.; Donaldson, J.G. Pathways and mechanisms of endocytic recycling. Nat. Rev. Mol. Cell Biol. 2009, 10, 597–608. [Google Scholar] [CrossRef]
- Stahl, P.D.; Raposo, G. Extracellular Vesicles: Exosomes and Microvesicles, Integrators of Homeostasis. Physiology 2019, 34, 169–177. [Google Scholar] [CrossRef]
- Nabhan, J.F.; Hu, R.; Oh, R.S.; Cohen, S.N.; Lu, Q. Formation and release of arrestin domain-containing protein 1-mediated microvesicles (ARMMs) at plasma membrane by recruitment of TSG101 protein. Proc. Natl. Acad. Sci. USA. 2012, 109, 4146–4151. [Google Scholar] [CrossRef]
- Piccin, A.; Murphy, W.G.; Smith, O.P. Circulating microparticles: Pathophysiology and clinical implications. Blood Rev. 2007, 21, 157–171. [Google Scholar] [CrossRef]
- Heijnen, H.F.G.; Schiel, A.E.; Fijnheer, R.; Geuze, H.J.; Sixma, J.J. Activated platelets release two types of membrane vesicles: Microvesicles by surface shedding and exosomes derived from exocytosis of multivesicular bodies and α-granules. Blood 1999, 94, 3791–3799. [Google Scholar] [CrossRef]
- Tauro, B.J.; Greening, D.W.; Mathias, R.A.; Ji, H.; Mathivanan, S.; Scott, A.M.; Simpson, R.J. Comparison of ultracentrifugation, density gradient separation, and immunoaffinity capture methods for isolating human colon cancer cell line LIM1863-derived exosomes. Methods 2012, 56, 293–304. [Google Scholar] [CrossRef]
- Morita, E.; Sandrin, V.; Chung, H.Y.; Morham, S.G.; Gygi, S.P.; Rodesch, C.K.; Sundquist, W.I. Human ESCRT and ALIX proteins interact with proteins of the midbody and function in cytokinesis. EMBO J. 2007, 26, 4215–4227. [Google Scholar] [CrossRef]
- Géminard, C.; De Gassart, A.; Blanc, L.; Vidal, M. Degradation of AP2 during reticulocyte maturation enhances binding of hsc70 and Alix to a common site on TFR for sorting into exosomes. Traffic 2004, 5, 181–193. [Google Scholar] [CrossRef]
- Buschow, S.I.; Van Balkom, B.W.; Aalberts, M.; Heck, A.J.; Wauben, M.; Stoorvogel, W. MHC class II-associated proteins in B-cell exosomes and potential functional implications for exosome biogenesis. Immunol. Cell Biol. 2010, 88, 851–856. [Google Scholar] [CrossRef]
- Dervin, F.; Wynne, K.; Maguire, P.B. Human Platelet Exosome Proteomics Leads to the Identification of WNT Positive Exosomes Which Impact Canonical WNT Signalling in Target Cells. Blood 2014, 124, 2758. [Google Scholar] [CrossRef]
- Palmisano, G.; Jensen, S.S.; Le Bihan, M.C.; Lainé, J.; McGuire, J.N.; Pociot, F.; Larsen, M.R. Characterization of membrane-shed microvesicles from cytokine-stimulated β-cells using proteomics strategies. Mol. Cell. Proteom. 2012, 11, 230–243. [Google Scholar] [CrossRef]
- Théry, C.; Boussac, M.; Véron, P.; Ricciardi-Castagnoli, P.; Raposo, G.; Garin, J.; Amigorena, S. Proteomic Analysis of Dendritic Cell-Derived Exosomes: A Secreted Subcellular Compartment Distinct from Apoptotic Vesicles. J. Immunol. 2001, 166, 7309–7318. [Google Scholar] [CrossRef]
- Escrevente, C.; Keller, S.; Altevogt, P.; Costa, J. Interaction and uptake of exosomes by ovarian cancer cells. BMC Cancer 2011, 11, 108. [Google Scholar] [CrossRef]
- Christianson, H.C.; Svensson, K.J.; Van Kuppevelt, T.H.; Li, J.P.; Belting, M. Cancer cell exosomes depend on cell-surface heparan sulfate proteoglycans for their internalization and functional activity. Proc. Natl. Acad. Sci. USA 2013, 110, 17380–17385. [Google Scholar] [CrossRef] [PubMed]
- Sinha, A.; Ignatchenko, V.; Ignatchenko, A.; Mejia-Guerrero, S.; Kislinger, T. In-depth proteomic analyses of ovarian cancer cell line exosomes reveals differential enrichment of functional categories compared to the NCI 60 proteome. Biochem. Biophys. Res. Commun. 2014, 445, 694–701. [Google Scholar] [CrossRef] [PubMed]
- Sano, S.; Izumi, Y.; Yamaguchi, T.; Yamazaki, T.; Tanaka, M.; Shiota, M.; Osada-Oka, M.; Nakamura, Y.; Wei, M.; Wanibuchi, H.; et al. Lipid synthesis is promoted by hypoxic adipocyte-derived exosomes in 3T3-L1 cells. Biochem. Biophys. Res. Commun. 2014, 445, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Subra, C.; Grand, D.; Laulagnier, K.; Stella, A.; Lambeau, G.; Paillasse, M.; De Medina, P.; Monsarrat, B.; Perret, B.; Silvente-Poirot, S.; et al. Exosomes account for vesicle-mediated transcellular transport of activatable phospholipases and prostaglandins. J. Lipid Res. 2010, 51, 2105–2120. [Google Scholar] [CrossRef] [PubMed]
- Guescini, M.; Genedani, S.; Stocchi, V.; Agnati, L.F. Astrocytes and Glioblastoma cells release exosomes carrying mtDNA. J. Neural Transm. 2010, 117, 1. [Google Scholar] [CrossRef]
- Balaj, L.; Lessard, R.; Dai, L.; Cho, Y.J.; Pomeroy, S.L.; Breakefield, X.O.; Skog, J. Tumour microvesicles contain retrotransposon elements and amplified oncogene sequences. Nat. Commun. 2011, 2, 180. [Google Scholar] [CrossRef]
- Kahlert, C.; Melo, S.A.; Protopopov, A.; Tang, J.; Seth, S.; Koch, O.; Zhang, J.; Weitz, J.; Chin, L.; Futreal, A.; et al. Identification of doublestranded genomic dna spanning all chromosomes with mutated KRAS and P53 DNA in the serum exosomes of patients with pancreatic cancer. J. Biol. Chem. 2014, 289, 3869–3875. [Google Scholar] [CrossRef] [PubMed]
- Thakur, B.K.; Zhang, H.; Becker, A.; Matei, I.; Huang, Y.; Costa-Silva, B.; Zheng, Y.; Hoshino, A.; Brazier, H.; Xiang, J.; et al. Double-stranded DNA in exosomes: A novel biomarker in cancer detection. Cell Res. 2014, 24, 766–769. [Google Scholar] [CrossRef]
- Eirin, A.; Riester, S.M.; Zhu, X.Y.; Tang, H.; Evans, J.M.; O’Brien, D.; van Wijnen, A.J.; Lerman, L.O. MicroRNA and mRNA cargo of extracellular vesicles from porcine adipose tissue-derived mesenchymal stem cells. Gene 2014, 551, 55–64. [Google Scholar] [CrossRef]
- Mittelbrunn, M.; Gutiérrez-Vázquez, C.; Villarroya-Beltri, C.; González, S.; Sánchez-Cabo, F.; González, M.Á.; Bernad, A.; Sánchez-Madrid, F. Unidirectional transfer of microRNA-loaded exosomes from T cells to antigen-presenting cells. Nat. Commun. 2011, 2, 12859. [Google Scholar] [CrossRef]
- Skog, J.; Würdinger, T.; van Rijn, S.; Meijer, D.H.; Gainche, L.; Curry, W.T.; Carter, B.S.; Krichevsky, A.M.; Breakefield, X.O. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat. Cell Biol. 2008, 10, 1470–1476. [Google Scholar] [CrossRef]
- Valadi, H.; Ekström, K.; Bossios, A.; Sjöstrand, M.; Lee, J.J.; Lötvall, J.O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007, 9, 654–659. [Google Scholar] [CrossRef]
- Redzic, J.S.; Balaj, L.; van der Vos, K.E.; Breakefield, X.O. Extracellular RNA mediates and marks cancer progression. Semin. Cancer Biol. 2014, 28, 14–23. [Google Scholar] [CrossRef]
- Bolukbasi, M.F.; Mizrak, A.; Ozdener, G.B.; Madlener, S.; Ströbel, T.; Erkan, E.P.; Fan, J.-B.; Breakefield, X.O.; Saydam, O. miR-1289 and “Zipcode”-like Sequence Enrich mRNAs in Microvesicles. Mol. Ther. Nucleic Acids 2012, 1, e10. [Google Scholar] [CrossRef]
- Villarroya-Beltri, C.; Gutiérrez-Vázquez, C.; Sánchez-Cabo, F.; Pérez-Hernández, D.; Vázquez, J.; Martin-Cofreces, N.; Martinez-Herrera, D.J.; Pascual-Montano, A.; Mittelbrunn, M.; Sánchez-Madrid, F. Sumoylated hnRNPA2B1 controls the sorting of miRNAs into exosomes through binding to specific motifs. Nat. Commun. 2013, 4, 2980. [Google Scholar] [CrossRef]
- Koppers-Lalic, D.; Hackenberg, M.; Bijnsdorp, I.V.; van Eijndhoven, M.A.J.; Sadek, P.; Sie, D.; Zini, N.; Middeldorp, J.M.; Ylstra, B.; de Menezes, R.X.; et al. Nontemplated nucleotide additions distinguish the small RNA composition in cells from exosomes. Cell Rep. 2014, 8, 1649–1658. [Google Scholar] [CrossRef]
- Arraud, N.; Linares, R.; Tan, S.; Gounou, C.; Pasquet, J.M.; Mornet, S.; Brisson, A.R. Extracellular vesicles from blood plasma: Determination of their morphology, size, phenotype and concentration. J. Thromb. Haemost. 2014, 12, 614–627. [Google Scholar] [CrossRef]
- Lopez, E.; Srivastava, A.K.; Burchfield, J.; Wang, Y.W.; Cardenas, J.C.; Togarrati, P.P.; Miyazawa, B.; Gonzalez, E.; Holcomb, J.B.; Pati, S.; et al. Platelet-derived- Extracellular Vesicles Promote Hemostasis and Prevent the Development of Hemorrhagic Shock. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef]
- Lee, J.H.; Jung, H.; Song, J.; Choi, E.S.; You, G.; Mok, H. Activated Platelet-Derived Vesicles for Efficient Hemostatic Activity. Macromol. Biosci. 2020, 20, 1900338. [Google Scholar] [CrossRef]
- Gasecka, A.; Nieuwland, R.; van der Pol, E.; Hajji, N.; Cwiek, A.; Pluta, K.; Konwerski, M.; Filipiak, K.J. P2y12 antagonist ticagrelor inhibits the release of procoagulant extracellular vesicles from activated platelets. Cardiol. J. 2019, 26, 782–789. [Google Scholar] [CrossRef]
- Owens, A.P.; MacKman, N. Microparticles in hemostasis and thrombosis. Circ. Res. 2011, 108, 1284–1297. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, S.; Luo, L.; Norström, E.; Braun, O.; Mörgelin, M.; Thorlacius, H. Platelet-derived microparticles regulates thrombin generation via phophatidylserine in abdominal sepsis. J. Cell. Physiol. 2018, 233, 1051–1060. [Google Scholar] [CrossRef]
- Van Es, N.; Bleker, S.; Sturk, A.; Nieuwland, R. Clinical Significance of Tissue Factor-Exposing Microparticles in Arterial and Venous Thrombosis; In Proceedings of the Seminars in Thrombosis and Hemostasis; Thieme Medical Publishers, Inc.: New York, NY, USA, 2015; Volume 41, pp. 718–727. [Google Scholar]
- Puhm, F.; Boilard, E.; MacHlus, K.R. Platelet Extracellular Vesicles: Beyond the Blood. Arterioscler. Thromb. Vasc. Biol. 2020, 41, 87–96. [Google Scholar] [CrossRef]
- Del Conde, I.; Shrimpton, C.N.; Thiagarajan, P.; López, J.A. Tissue-factor-bearing microvesicles arise from lipid rafts and fuse with activated platelets to initiate coagulation. Blood 2005, 106, 1604–1611. [Google Scholar] [CrossRef]
- Yu, Y.; Gool, E.; Berckmans, R.J.; Coumans, F.A.W.; Barendrecht, A.D.; Maas, C.; van der Wel, N.N.; Altevogt, P.; Sturk, A.; Nieuwland, R. Extracellular vesicles from human saliva promote hemostasis by delivering coagulant tissue factor to activated platelets. J. Thromb. Haemost. 2018, 16, 1153–1163. [Google Scholar] [CrossRef] [PubMed]
- Gaspar, R.S.; Ferreira, P.M.; Mitchell, J.L.; Pula, G.; Gibbins, J.M. Platelet-derived extracellular vesicles express NADPH oxidase-1 (Nox-1), generate superoxide and modulate platelet function. Free Radic. Biol. Med. 2021, 165, 395–400. [Google Scholar] [CrossRef]
- Kolonics, F.; Kajdácsi, E.; Farkas, V.J.; Veres, D.S.; Khamari, D.; Kittel, Á.; Merchant, M.L.; McLeish, K.R.; Lőrincz, Á.M.; Ligeti, E. Neutrophils produce proinflammatory or anti-inflammatory extracellular vesicles depending on the environmental conditions. J. Leukoc. Biol. 2021, 109, 793–806. [Google Scholar] [CrossRef]
- Dalli, J.; Montero-Melendez, T.; Norling, L.V.; Yin, X.; Hinds, C.; Haskard, D.; Mayr, M.; Perretti, M. Heterogeneity in neutrophil microparticles reveals distinct proteome and functional properties. Mol. Cell. Proteom. 2013, 12, 2205–2219. [Google Scholar] [CrossRef]
- Das, A.; Sinha, M.; Datta, S.; Abas, M.; Chaffee, S.; Sen, C.K.; Roy, S. Monocyte and Macrophage Plasticity in Tissue Repair and Regeneration. Am. J. Pathol. 2015, 185, 2596–2606. [Google Scholar] [CrossRef]
- Kim, H.; Wang, S.Y.; Kwak, G.; Yang, Y.; Kwon, I.C.; Kim, S.H. Exosome-Guided Phenotypic Switch of M1 to M2 Macrophages for Cutaneous Wound Healing. Adv. Sci. 2019, 6, 1900513. [Google Scholar] [CrossRef]
- Carmeliet, P.; Jain, R.K. Molecular mechanisms and clinical applications of angiogenesis. Nature 2011, 473, 298–307. [Google Scholar] [CrossRef]
- Kim, J.Y.; Song, S.H.; Kim, K.L.; Ko, J.J.; Im, J.E.; Yie, S.W.; Ahn, Y.K.; Kim, D.K.; Suh, W. Human cord blood-derived endothelial progenitor cells and their conditioned media exhibit therapeutic equivalence for diabetic wound healing. Cell Transplant. 2010, 19, 1635–1644. [Google Scholar] [CrossRef]
- Li, X.; Jiang, C.; Zhao, J. Human endothelial progenitor cells-derived exosomes accelerate cutaneous wound healing in diabetic rats by promoting endothelial function. J. Diabetes Complicat. 2016, 30, 986–992. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, C.; Hu, B.; Niu, X.; Liu, X.; Zhang, G.; Zhang, C.; Li, Q.; Wang, Y. Exosomes derived from human endothelial progenitor cells accelerate cutaneous wound healing by promoting angiogenesis through Erk1/2 signaling. Int. J. Biol. Sci. 2016, 12, 1472–1487. [Google Scholar] [CrossRef]
- Xu, J.; Bai, S.; Cao, Y.; Liu, L.; Fang, Y.; Du, J.; Luo, L.; Chen, M.; Shen, B.; Zhang, Q. MiRNA-221-3p in endothelial progenitor cell-derived exosomes accelerates skin wound healing in diabetic mice. Diabetes Metab. Syndr. Obes. Targets Ther. 2020, 13, 1259–1270. [Google Scholar] [CrossRef]
- Li, M.; Wang, T.; Tian, H.; Wei, G.; Zhao, L.; Shi, Y. Macrophage-derived exosomes accelerate wound healing through their anti-inflammation effects in a diabetic rat model. Artif. Cells Nanomed. Biotechnol. 2019, 47, 3793–3803. [Google Scholar] [CrossRef]
- Gangadaran, P.; Rajendran, R.L.; Oh, J.M.; Hong, C.M.; Jeong, S.Y.; Lee, S.W.; Lee, J.; Ahn, B.C. Extracellular vesicles derived from macrophage promote angiogenesis In vitro and accelerate new vasculature formation In vivo. Exp. Cell Res. 2020, 394, 112146. [Google Scholar] [CrossRef]
- Tang, H.; He, Y.; Li, L.; Mao, W.; Chen, X.; Ni, H.; Dong, Y.; Lyu, F. Exosomal MMP2 derived from mature osteoblasts promotes angiogenesis of endothelial cells via VEGF/Erk1/2 signaling pathway. Exp. Cell Res. 2019, 383, 111541. [Google Scholar] [CrossRef]
- Mi, B.; Chen, L.; Xiong, Y.; Yan, C.; Xue, H.; Panayi, A.C.; Liu, J.; Hu, L.; Hu, Y.; Cao, F.; et al. Saliva exosomes-derived UBE2O mRNA promotes angiogenesis in cutaneous wounds by targeting SMAD6. J. Nanobiotechnol. 2020, 18, 68. [Google Scholar] [CrossRef]
- Wei, F.; Wang, A.; Wang, Q.; Han, W.; Rong, R.; Wang, L.; Liu, S.; Zhang, Y.; Dong, C.; Li, Y. Plasma endothelial cells-derived extracellular vesicles promote wound healing in diabetes through YAP and the PI3K/Akt/mTOR pathway. Aging 2020, 12, 12002–12018. [Google Scholar] [CrossRef]
- Lipson, K.E.; Wong, C.; Teng, Y.; Spong, S. CTGF is a central mediator of tissue remodeling and fibrosis and its inhibition can reverse the process of fibrosis. Fibrogenesis Tissue Repair 2012, 5, S24. [Google Scholar] [CrossRef]
- Huang, P.; Bi, J.; Owen, G.R.; Chen, W.; Rokka, A.; Koivisto, L.; Heino, J.; Häkkinen, L.; Larjava, H. Keratinocyte microvesicles regulate the expression of multiple genes in dermal fibroblasts. J. Investig. Dermatol. 2015, 135, 3051–3059. [Google Scholar] [CrossRef]
- Li, Q.; Zhao, H.; Chen, W.; Huang, P.; Bi, J. Human keratinocyte-derived microvesicle miRNA-21 promotes skin wound healing in diabetic rats through facilitating fibroblast function and angiogenesis. Int. J. Biochem. Cell Biol. 2019, 114, 105570. [Google Scholar] [CrossRef]
- Mathew-Steiner, S.S.; Roy, S.; Sen, C.K. Collagen in wound healing. Bioengineering 2021, 8, 63. [Google Scholar] [CrossRef]
- Oh, E.J.; Gangadaran, P.; Rajendran, R.L.; Kim, H.M.; Oh, J.M.; Choi, K.Y.; Chung, H.Y.; Ahn, B.C. Extracellular vesicles derived from fibroblasts promote wound healing by optimizing fibroblast and endothelial cellular functions. Stem Cells 2021, 39, 266–279. [Google Scholar] [CrossRef]
- De Jong, O.G.; van Balkom, B.W.M.; Gremmels, H.; Verhaar, M.C. Exosomes from hypoxic endothelial cells have increased collagen crosslinking activity through up-regulation of lysyl oxidase-like 2. J. Cell. Mol. Med. 2016, 20, 342–350. [Google Scholar] [CrossRef]
- Grotendorst, G.R.; Rahmanie, H.; Duncan, M.R. Combinatorial signaling pathways determine fibroblast proliferation and myofibroblast differentiation. FASEB J. 2004, 18, 469–479. [Google Scholar] [CrossRef]
- Arif, S.; Larochelle, S.; Moulin, V.J. PLGF-1 contained in normal wound myofibroblast-derived microvesicles stimulated collagen production by dermal fibroblasts. J. Cell Commun. Signal. 2020, 14, 427–438. [Google Scholar] [CrossRef]
- Geiger, A.; Walker, A.; Nissen, E. Human fibrocyte-derived exosomes accelerate wound healing in genetically diabetic mice. Biochem. Biophys. Res. Commun. 2015, 467, 303–309. [Google Scholar] [CrossRef]
- Suga, H.; Rennert, R.C.; Rodrigues, M.; Sorkin, M.; Glotzbach, J.P.; Januszyk, M.; Fujiwara, T.; Longaker, M.T.; Gurtner, G.C. Tracking the elusive fibrocyte: Identification and characterization of collagen-producing hematopoietic lineage cells during murine wound healing. Stem Cells 2014, 32, 1347–1360. [Google Scholar] [CrossRef]
- Bhatia, A.; O’Brien, K.; Chen, M.; Woodley, D.T.; Li, W. Keratinocyte-Secreted Heat Shock Protein-90alpha: Leading Wound Reepithelialization and Closure. Adv. Wound Care 2016, 5, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Dauer, D.J.; Ferraro, B.; Song, L.; Yu, B.; Mora, L.; Buettner, R.; Enkemann, S.; Jove, R.; Haura, E.B. Stat3 regulates genes common to both wound healing and cancer. Oncogene 2005, 24, 3397–3408. [Google Scholar] [CrossRef]
- Akbari, A.; Jabbari, N.; Sharifi, R.; Ahmadi, M.; Vahhabi, A.; Seyedzadeh, S.J.; Nawaz, M.; Szafert, S.; Mahmoodi, M.; Jabbari, E.; et al. Free and hydrogel encapsulated exosome-based therapies in regenerative medicine. Life Sci. 2020, 249, 117447. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Zhang, X.; Li, X. Exosomes Derived from Mesenchymal Stem Cells. Int. J. Mol. Sci 2014, 15, 4142–4157. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Viejo, M. Mesenchymal stem cells from different sources and their derived exosomes: A pre-clinical perspective. World J. Stem Cells 2020, 12, 100–109. [Google Scholar] [CrossRef]
- Rani, S.; Ryan, A.E.; Griffin, M.D.; Ritter, T. Mesenchymal stem cell-derived extracellular vesicles: Toward cell-free therapeutic applications. Mol. Ther. 2015, 23, 812–823. [Google Scholar] [CrossRef]
- Golchin, A.; Hosseinzadeh, S.; Ardeshirylajimi, A. The exosomes released from different cell types and their effects in wound healing. J. Cell. Biochem. 2018, 119, 5043–5052. [Google Scholar] [CrossRef]
- Silachev, D.N.; Goryunov, K.V.; Shpilyuk, M.A.; Beznoschenko, O.S.; Morozova, N.Y.; Kraevaya, E.E.; Popkov, V.A.; Pevzner, I.B.; Zorova, L.D.; Evtushenko, E.A.; et al. Effect of MSCs and MSC-Derived Extracellular Vesicles on Human Blood Coagulation. Cells 2019, 8, 258. [Google Scholar] [CrossRef]
- Chance, T.C.; Rathbone, C.R.; Kamucheka, R.M.; Peltier, G.C.; Cap, A.P.; Bynum, J.A. The effects of cell type and culture condition on the procoagulant activity of human mesenchymal stromal cell-derived extracellular vesicles. J. Trauma Acute Care Surg. 2019, 87, S74–S82. [Google Scholar] [CrossRef]
- Christy, B.A.; Herzig, M.C.; Montgomery, R.K.; Delavan, C.; Bynum, J.A.; Reddoch, K.M.; Cap, A.P. Pro-coagulant activity of human mesenchymal stem cells. J. Trauma Acute Care Surg. 2017, 83, S164–S169. [Google Scholar] [CrossRef] [PubMed]
- Margetic, S. Infl ammation and haemostasis. Biochem. Med. 2012, 22, 49–62. [Google Scholar] [CrossRef]
- Fiedler, T.; Rabe, M.; Mundkowski, R.G.; Oehmcke-Hecht, S.; Peters, K. Adipose-derived mesenchymal stem cells release microvesicles with procoagulant activity. Int. J. Biochem. Cell Biol. 2018, 100, 49–53. [Google Scholar] [CrossRef]
- He, X.; Dong, Z.; Cao, Y.; Wang, H.; Liu, S.; Liao, L.; Jin, Y.; Yuan, L.; Li, B.; Bolontrade, M.F. MSC-Derived Exosome Promotes M2 Polarization and Enhances Cutaneous Wound Healing. Stem Cells Int. 2019, 2019. [Google Scholar] [CrossRef]
- Heo, J.S.; Kim, S.; Yang, C.E.; Choi, Y.; Song, S.Y.; Kim, H.O. Human Adipose Mesenchymal Stem Cell-Derived Exosomes: A Key Player in Wound Healing. Tissue Eng. Regen. Med. 2021, 18, 537–548. [Google Scholar] [CrossRef]
- Liu, W.; Yu, M.; Xie, D.; Wang, L.; Ye, C.; Zhu, Q.; Liu, F.; Yang, L. Melatonin-stimulated MSC-derived exosomes improve diabetic wound healing through regulating macrophage M1 and M2 polarization by targeting the PTEN/AKT pathway. Stem Cell Res. Ther. 2020, 11, 259. [Google Scholar] [CrossRef]
- Cao, G.; Chen, B.; Zhang, X.; Chen, H. Human Adipose-Derived Mesenchymal Stem Cells-Derived Exosomal microRNA-19b Promotes the Healing of Skin Wounds Through Modulation of the CCL1/TGF-β Signaling Axis. Clin. Cosmet. Investig. Dermatol. 2020, 13, 957. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xie, X.; Lian, W.; Shi, R.; Han, S.; Zhang, H.; Lu, L.; Li, M. Exosomes from adipose-derived stem cells overexpressing Nrf2 accelerate cutaneous wound healing by promoting vascularization in a diabetic foot ulcer rat model. Exp. Mol. Med. 2018, 50, 29. [Google Scholar] [CrossRef]
- Li, X.; Liu, L.; Yang, J.; Yu, Y.; Chai, J.; Wang, L.; Ma, L.; Yin, H. Exosome Derived From Human Umbilical Cord Mesenchymal Stem Cell Mediates MiR-181c Attenuating Burn-induced Excessive Inflammation. EBioMedicine 2016, 8, 72–82. [Google Scholar] [CrossRef]
- Sonnemann, K.J.; Bement, W.M. Wound repair: Toward understanding and integration of single-cell and multicellular wound responses. Annu. Rev. Cell Dev. Biol. 2011, 27, 237–263. [Google Scholar] [CrossRef]
- Cooper, D.R.; Wang, C.; Patel, R.; Trujillo, A.; Patel, N.A.; Prather, J.; Gould, L.J.; Wu, M.H. Human Adipose-Derived Stem Cell Conditioned Media and Exosomes Containing MALAT1 Promote Human Dermal Fibroblast Migration and Ischemic Wound Healing. Adv. Wound Care 2018, 7, 299–308. [Google Scholar] [CrossRef]
- Gao, S.; Chen, T.; Hao, Y.; Zhang, F.; Tang, X.; Wang, D.; Wei, Z.; Qi, J. Exosomal miR-135a derived from human amnion mesenchymal stem cells promotes cutaneous wound healing in rats and fibroblast migration by directly inhibiting LATS2 expression. Stem Cell Res. Ther. 2020, 11, 56. [Google Scholar] [CrossRef]
- Hu, L.; Wang, J.; Zhou, X.; Xiong, Z.; Zhao, J.; Yu, R.; Huang, F.; Zhang, H.; Chen, L. Exosomes derived from human adipose mensenchymal stem cells accelerates cutaneous wound healing via optimizing the characteristics of fibroblasts. Sci. Rep. 2016, 6, 32993. [Google Scholar] [CrossRef]
- Zhang, W.; Bai, X.; Zhao, B.; Li, Y.; Zhang, Y.; Li, Z.; Wang, X.; Luo, L.; Han, F.; Zhang, J.; et al. Cell-free therapy based on adipose tissue stem cell-derived exosomes promotes wound healing via the PI3K/Akt signaling pathway. Exp. Cell Res. 2018, 370, 333–342. [Google Scholar] [CrossRef]
- Wang, X.; Jiao, Y.; Pan, Y.; Zhang, L.; Gong, H.; Qi, Y.; Wang, M.; Gong, H.; Shao, M.; Wang, X.; et al. Fetal Dermal Mesenchymal Stem Cell-Derived Exosomes Accelerate Cutaneous Wound Healing by Activating Notch Signaling. Stem Cells Int. 2019, 2019, 2402916. [Google Scholar] [CrossRef]
- Qian, L.; Pi, L.; Fang, B.R.; Meng, X.X. Adipose mesenchymal stem cell-derived exosomes accelerate skin wound healing via the lncRNA H19/miR-19b/SOX9 axis. Lab. Investig. 2021. [Google Scholar] [CrossRef]
- Shabbir, A.; Cox, A.; Rodriguez-Menocal, L.; Salgado, M.; Van Badiavas, E. Mesenchymal Stem Cell Exosomes Induce Proliferation and Migration of Normal and Chronic Wound Fibroblasts, and Enhance Angiogenesis in Vitro. Stem Cells Dev. 2015, 24, 1635–1647. [Google Scholar] [CrossRef]
- Liang, X.; Zhang, L.; Wang, S.; Han, Q.; Zhao, R.C. Exosomes secreted by mesenchymal stem cells promote endothelial cell angiogenesis by transferring miR-125a. J. Cell Sci. 2016, 129, 2182–2189. [Google Scholar] [CrossRef] [PubMed]
- Krawczenko, A.; Bielawska-Pohl, A.; Paprocka, M.; Kraskiewicz, H.; Szyposzynska, A.; Wojdat, E.; Klimczak, A. Microvesicles from Human Immortalized Cell Lines of Endothelial Progenitor Cells and Mesenchymal Stem/Stromal Cells of Adipose Tissue Origin as Carriers of Bioactive Factors Facilitating Angiogenesis. Stem Cells Int. 2020. [Google Scholar] [CrossRef]
- Hu, Y.; Rao, S.-S.; Wang, Z.-X.; Cao, J.; Tan, Y.-J.; Luo, J.; Li, H.-M.; Zhang, W.-S.; Chen, C.-Y.; Xie, H. Exosomes from human umbilical cord blood accelerate cutaneous wound healing through miR-21-3p-mediated promotion of angiogenesis and fibroblast function. Theranostics 2018, 8, 1. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Rao, S.-S.; Ren, L.; Hu, X.-K.; Tan, Y.-J.; Hu, Y.; Luo, J.; Liu, Y.-W.; Yin, H.; Huang, J.; et al. Exosomal DMBT1 from human urine-derived stem cells facilitates diabetic wound repair by promoting angiogenesis. Int. Publ. Theranostics 2018, 8, 1607–1623. [Google Scholar] [CrossRef]
- Liu, J.; Yan, Z.; Yang, F.; Huang, Y.; Yu, Y.; Zhou, L.; Sun, Z.; Cui, D.; Yan, Y. Exosomes Derived from Human Umbilical Cord Mesenchymal Stem Cells Accelerate Cutaneous Wound Healing by Enhancing Angiogenesis through Delivering Angiopoietin-2. Stem Cell Rev. Rep. 2015, 17, 305–317. [Google Scholar] [CrossRef]
- Zhang, Y.; Han, F.; Gu, L.; Ji, P.; Yang, X.; Liu, M.; Tao, K.; Hu, D. Adipose mesenchymal stem cell exosomes promote wound healing through accelerated keratinocyte migration and proliferation by activating the AKT/HIF-1α axis. J. Mol. Histol. 2020, 51, 375–383. [Google Scholar] [CrossRef]
- Yang, C.; Luo, L.; Bai, X.; Shen, K.; Liu, K.; Wang, J.; Hu, D. Highly-expressed micoRNA-21 in adipose derived stem cell exosomes can enhance the migration and proliferation of the HaCaT cells by increasing the MMP-9 expression through the PI3K/AKT pathway. Arch. Biochem. Biophys. 2020, 681, 108259. [Google Scholar] [CrossRef]
- Ma, T.; Fu, B.; Yang, X.; Xiao, Y.; Pan, M. Adipose mesenchymal stem cell-derived exosomes promote cell proliferation, migration, and inhibit cell apoptosis via Wnt/β-catenin signaling in cutaneous wound healing. J. Cell. Biochem. 2019, 120, 10847–10854. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, M.; Gong, A.; Zhang, X.U.; Wu, X.; Zhu, Y.; Shi, H.; Wu, L.; Zhu, W.; Qian, H.; et al. HucMSC-Exosome Mediated-Wnt4 Signaling Is Required for Cutaneous Wound Healing. Stem Cells 2015, 33, 2158–2168. [Google Scholar] [CrossRef]
- Pomatto, M.; Gai, C.; Negro, F.; Cedrino, M.; Grange, C.; Ceccotti, E.; Togliatto, G.; Collino, F.; Tapparo, M.; Figliolini, F.; et al. Differential Therapeutic Effect of Extracellular Vesicles Derived by Bone Marrow and Adipose Mesenchymal Stem Cells on Wound Healing of Diabetic Ulcers and Correlation to Their Cargoes. Int. J. Mol. Sci. Artic. 2021, 22, 3851. [Google Scholar] [CrossRef]
- Jiang, T.; Wang, Z.; Sun, J. Human bone marrow mesenchymal stem cell-derived exosomes stimulate cutaneous wound healing mediates through TGF-β/Smad signaling pathway. Stem Cell Res. Ther. 2020, 11, 198. [Google Scholar] [CrossRef]
- Wang, L.; Hu, L.; Zhou, X.; Xiong, Z.; Zhang, C.; Shehada, H.M.A.; Hu, B.; Song, J.; Chen, L. Exosomes secreted by human adipose mesenchymal stem cells promote scarless cutaneous repair by regulating extracellular matrix remodelling. Sci. Rep. 2017, 7, 7066. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhao, B.; Zhang, X.-L.; Lu, Y.; Lu, S.-T.; Cheng, J.; Fu, Y.; Lin, L.; Zhang, N.-Y.; Li, P.-X.; et al. Combined topical and systemic administration with human adipose-derived mesenchymal stem cells (hADSC) and hADSC-derived exosomes markedly promoted cutaneous wound healing and regeneration. Stem Cell Res. Ther. 2021, 12, 257. [Google Scholar] [CrossRef]
- Dad, H.A.; Gu, T.W.; Zhu, A.Q.; Huang, L.Q.; Peng, L.H. Plant Exosome-like Nanovesicles: Emerging Therapeutics and Drug Delivery Nanoplatforms. Mol. Ther. 2021, 29, 13–31. [Google Scholar] [CrossRef]
- Kocak, P.; Kala, E.Y.; Gunes, M.; Unsal, N.; Yilmaz, H.; Metin, B.; Sahin, F. Edible plant-derived exosomes and their therapeutic applicatons. J Biomed Imag Bioeng 2020, 4, 130–135. [Google Scholar]
- Şahin, F.; Koçak, P.; Yıldırım Güneş, M.; Özkan, İ.; Yıldırım, E.; Yağmur Kala, E. In Vitro Wound Healing Activity of Wheat-Derived Nanovesicles. Appl. Biochem. Biotechnol. 2010, 188, 381–394. [Google Scholar] [CrossRef] [PubMed]
- Kahroba, H.; Davatgaran-Taghipour, Y. Exosomal Nrf2: From anti-oxidant and anti-inflammation response to wound healing and tissue regeneration in aged-related diseases. Biochimie 2020, 171, 103–109. [Google Scholar] [CrossRef]
- Omar, G.; Abdallah, L.; Rahim, A.; Othman, R.; Barakat, A. Selected Wild Plants Ethanol Extracts Bioactivity on the Coagulation Cascade. J. Sci. Res. Rep. 2017, 13, 1–10. [Google Scholar] [CrossRef]
- Abu, M.; Nyeem, B.; Mannan, M.A. Rubia cordifolia-phytochemical and Pharmacological evaluation of indigenous medicinal plant: A review. Int. J. Physiol. 2018, 3, 766–771. [Google Scholar]
- Chamara, A.M.R.; Thiripuranathar, G. Assessment of Haemostatic Activity of Medicinal Plants Using In Vitro Methods: A Concise Review. J. Pharm. Biol. Sci. 2020, 15, 26–34. [Google Scholar] [CrossRef]
- Thakur, R.; Jain, N.; Pathak, R.; Sandhu, S.S. Review Article Practices in Wound Healing Studies of Plants. Evid. Based Complement. Altern. Med. 2011, 2011, 17. [Google Scholar] [CrossRef]
- Baldini, N.; Torreggiani, E.; Roncuzzi, L.; Perut, F.; Zini, N.; Avnet, S. Exosome-like Nanovesicles Isolated from Citrus limon L. Exert Antioxidative Effect. Curr. Pharm. Biotechnol. 2018, 19, 877–885. [Google Scholar] [CrossRef]
- Perut, F.; Roncuzzi, L.; Avnet, S.; Massa, A.; Zini, N.; Sabbadini, S.; Giampieri, F.; Mezzetti, B.; Baldini, N. Strawberry-derived exosome-like nanoparticles prevent oxidative stress in human mesenchymal stromal cells. Biomolecules 2021, 11, 87. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.J.; Bian, Y.P.; Wang, Q.H.; Yin, F.; Yin, L.; Zhang, Y.L.; Liu, J.H. Blueberry-derived exosomes-like nanoparticles ameliorate nonalcoholic fatty liver disease by attenuating mitochondrial oxidative stress. Acta Pharmacol. Sin. 2021. [Google Scholar] [CrossRef]
- De Robertis, M.; Sarra, A.; D’oria, V.; Mura, F.; Bordi, F.; Postorino, P.; Fratantonio, D. Blueberry-derived exosome-like nanoparticles counters the response to TNF-α-induced change on gene expression in ea.Hy926 cells. Biomolecules 2020, 10, 742. [Google Scholar] [CrossRef]
- Ju, S.; Mu, J.; Dokland, T.; Zhuang, X.; Wang, Q.; Jiang, H.; Xiang, X.; Deng, Z.-B.; Wang, B.; Zhang, L.; et al. Grape exosome-like nanoparticles induce intestinal stem cells and protect mice from DSS-induced colitis. Mol. Ther. 2013, 21, 1345–1357. [Google Scholar] [CrossRef]
- Zhang, M.; Merlin, D. Curcuma Longa-Derived Nanoparticles Reduce Colitis and Promote Intestinal Wound Repair by Inactivating the NF-ΚB Pathway. Gastroenterology 2017, 152, S567. [Google Scholar] [CrossRef]
- Mu, J.; Zhuang, X.; Wang, Q.; Jiang, H.; Deng, Z.-B.; Wang, B.; Zhang, L.; Kakar, S.; Jun, Y.; Miller, D.; et al. Interspecies communication between plant and mouse gut host cells through edible plant derived exosome-like nanoparticles. Mol. Nutr. Food Res. 2014, 58, 1561–1573. [Google Scholar] [CrossRef] [PubMed]
- Teng, Y.; Xu, F.; Zhang, X.; Mu, J.; Sayed, M.; Hu, X.; Lei, C.; Sriwastva, M.; Kumar, A.; Sundaram, K.; et al. Plant-derived exosomal microRNAs inhibit lung inflammation induced by exosomes SARS-CoV-2 Nsp12. Mol. Ther. 2021. [Google Scholar] [CrossRef] [PubMed]
- Cundell, J. Chapter Two—Diabetic Foot Ulcers: Assessment, Treatment, and Management. In Smart Bandage Technologies; Academic Press: New York, NY, USA, 2016; pp. 37–61. [Google Scholar] [CrossRef]
- Savci, Y.; Kirbas, O.K.; Bozkurt, B.T.; Abdik, E.A.; Tasli, P.N.; Sahin, F.; Abdik, H. Grapefruit-derived extracellular vesicles as a promising cell-free therapeutic tool for wound healing. Food Funct. 2021, 12, 5144–5156. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Viennois, E.; Prasad, M.; Zhang, Y.; Wang, L.; Zhang, Z.; Han, M.K.; Xiao, B.; Xu, C.; Srinivasan, S.; et al. Edible ginger-derived nanoparticles: A novel therapeutic approach for the prevention and treatment of inflammatory bowel disease and colitis-associated cancer. Biomaterials 2016, 101, 321–340. [Google Scholar] [CrossRef] [PubMed]
- Xue, M.; Jackson, C.J. Extracellular Matrix Reorganization During Wound Healing and Its Impact on Abnormal Scarring. Adv. Wound Care 2015, 4, 119–136. [Google Scholar] [CrossRef]
- Lingzhi, Z.; Meirong, L.; Xiaobing, F. Biological approaches for hypertrophic scars. Int. Wound J. 2020, 17, 405–418. [Google Scholar] [CrossRef]
- Goodarzi, P.; Larijani, B.; Alavi-Moghadam, S.; Tayanloo-Beik, A.; Mohamadi-Jahani, F.; Ranjbaran, N.; Payab, M.; Falahzadeh, K.; Mousavi, M.; Arjmand, B. Mesenchymal stem cells-derived exosomes for wound regeneration. Cell Biol. Transl. Med. 2018, 4, 119–131. [Google Scholar] [CrossRef]
- Cai, Y.; Liu, W.; Lian, L.; Xu, Y.; Bai, X.; Xu, S.; Zhang, J. Stroke treatment: Is exosome therapy superior to stem cell therapy? Biochimie 2020, 179, 190–204. [Google Scholar] [CrossRef]
- Casado-Díaz, A.; Quesada-Gómez, J.M.; Dorado, G. Extracellular Vesicles Derived From Mesenchymal Stem Cells (MSC) in Regenerative Medicine: Applications in Skin Wound Healing. Front. Bioeng. Biotechnol. 2020, 8, 146. [Google Scholar] [CrossRef]
- Negut, I.; Dorcioman, G.; Grumezescu, V. Scaffolds for wound healing applications. Polymers 2020, 12, 2010. [Google Scholar] [CrossRef]
- Kalantari, K.; Mostafavi, E.; Afifi, A.M.; Izadiyan, Z.; Jahangirian, H.; Rafiee-Moghaddam, R.; Webster, T.J. Wound dressings functionalized with silver nanoparticles: Promises and pitfalls. Nanoscale 2020, 12, 2268–2291. [Google Scholar] [CrossRef] [PubMed]
- Kusuma, G.D.; Hagemeyer, C.E.; Donnini, S.; Riau, A.K.; Mehta, J.S.; Ong, H.S.; Yam, G.H.F. Sustained Delivery System for Stem Cell-Derived Exosomes. Front. Pharmacol. 2019, 10, 1368. [Google Scholar] [CrossRef]
- Las Heras, K.; Igartua, M.; Santos-Vizcaino, E.; Hernandez, R.M. Chronic wounds: Current status, available strategies and emerging therapeutic solutions. J. Control. Release 2020, 328, 532–550. [Google Scholar] [CrossRef]
- Pan, Z.; Ye, H.; Wu, D. Recent advances on polymeric hydrogels as wound dressings. APL Bioeng. 2021, 5, 011504. [Google Scholar] [CrossRef]
- Nooshabadi, V.T.; Khanmohamadi, M.; Valipour, E.; Mahdipour, S.; Salati, A.; Malekshahi, Z.V.; Shafei, S.; Amini, E.; Farzamfar, S.; Ai, J. Impact of exosome-loaded chitosan hydrogel in wound repair and layered dermal reconstitution in mice animal model. J. Biomed. Mater. Res. Part A 2020, 108, 2138–2149. [Google Scholar] [CrossRef]
- Qian, Z.; Bai, Y.; Zhou, J.; Li, L.; Na, J.; Fan, Y.; Guo, X.; Liu, H. A moisturizing chitosan-silk fibroin dressing with silver nanoparticles-adsorbed exosomes for repairing infected wounds. J. Mater. Chem. B 2020, 8, 7197–7212. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Liang, C.; Wang, R.; Yao, X.; Guo, P.; Yuan, W.; Liu, Y.; Song, Y.; Li, Z.; Xie, X. The fabrication of a highly efficient self-healing hydrogel from natural biopolymers loaded with exosomes for the synergistic promotion of severe wound healing. Biomater. Sci. 2020, 8, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Yu, Z.; Li, Y.; Wang, Y.; Li, Q.; Han, D. GelMA combined with sustained release of HUVECs derived exosomes for promoting cutaneous wound healing and facilitating skin regeneration. J. Mol. Histol. 2020, 51, 251–263. [Google Scholar] [CrossRef]
- Shiekh, P.A.; Singh, A.; Kumar, A. Data supporting exosome laden oxygen releasing antioxidant and antibacterial cryogel wound dressing OxOBand alleviate diabetic and infectious wound healing. Data Br. 2020, 31, 105671. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.; Xiao, C.; Miao, Y.; Wang, J.; Chen, R.; Fan, Z.; Hu, Z. Human acellular amniotic membrane incorporating exosomes from adipose- derived mesenchymal stem cells promotes diabetic wound healing. Stem Cell Res. Ther. 2021, 12, 255. [Google Scholar] [CrossRef] [PubMed]
- Seung Cho, B.; Lee, J.; Won, Y.; Duncan, D.I.; Jin, R.C.; Lee, J.; Hoon Kwon, H.; Park, G.-H.; Hoseong Yang, S.; Cheol Park, B.; et al. cosmetics Skin Brightening Efficacy of Exosomes Derived from Human Adipose Tissue-Derived Stem/Stromal Cells: A Prospective, Split-Face, Randomized Placebo-Controlled Study. Cosmetics 2020, 7, 90. [Google Scholar] [CrossRef]
- Gentile, P.; Garcovich, S. biomedicines Adipose-Derived Mesenchymal Stem Cells (AD-MSCs) against Ultraviolet (UV) Radiation Effects and the Skin Photoaging. Biomedicines 2021, 9, 532. [Google Scholar] [CrossRef] [PubMed]
- Ajit, A.; Nair, M.D.; Venugopal, B. Exploring the Potential of Mesenchymal Stem Cell-Derived Exosomes for the Treatment of Alopecia. Regen. Eng. Transl. Med. 2021, 7, 119–128. [Google Scholar] [CrossRef]
- Saxena, N.; Mok, K.-W.; Rendl, M. An updated classification of hair follicle morphogenesis. Exp. Dermatol. 2019, 28, 332–344. [Google Scholar] [CrossRef]
- Millar, S.E. Molecular mechanisms regulating hair follicle development. J. Investig. Dermatol. 2002, 118, 216–225. [Google Scholar] [CrossRef]
- Kim, J.H. Nanoparticle Composition for Prevention of Hair Loss and Promotion of Hair Growth. U.S. Patent Application 12/520,951, 29 April 2010. [Google Scholar]
- Rathi, V.; Rathi, J.C.; Tamizharasi, S.; Kumar, A. Plants used for hair growth promotion: A review. Rev. Lit. Arts Am. 2008, 2, 185–187. [Google Scholar]
- Lv, Q.; Deng, J.; Chen, Y.; Wang, Y.; Liu, B.; Liu, J. Engineered Human Adipose Stem-Cell-Derived Exosomes Loaded with miR-21-5p to Promote Diabetic Cutaneous Wound Healing. Mol. Pharm. 2020, 17, 1723–1733. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Yu, M.; Yin, W.; Liang, B.; Li, A.; Li, J.; Li, X.; Zhao, S.; Liu, F. Development of a novel RNAi therapy: Engineered miR-31 exosomes promoted the healing of diabetic wounds. Bioact. Mater. 2021, 6, 2841–2853. [Google Scholar] [CrossRef]
- An, Y.; Lin, S.; Tan, X.; Zhu, S.; Nie, F.; Zhen, Y.; Gu, L.; Zhang, C.; Wang, B.; Wei, W.; et al. Exosomes from adipose-derived stem cells and application to skin wound healing. Cell Prolif. 2021, 54, e12993. [Google Scholar] [CrossRef]
- Woith, E.; Fuhrmann, G.; Melzig, M.F. Extracellular vesicles—connecting kingdoms. Int. J. Mol. Sci. 2019, 20, 5695. [Google Scholar] [CrossRef]
- Raimondo, S.; Giavaresi, G.; Lorico, A.; Alessandro, R. Extracellular vesicles as biological shuttles for targeted therapies. Int. J. Mol. Sci. 2019, 20, 1848. [Google Scholar] [CrossRef]
- Urzì, O.; Raimondo, S.; Alessandro, R. Extracellular vesicles from plants: Current knowledge and open questions. Int. J. Mol. Sci. 2021, 22, 5366. [Google Scholar] [CrossRef]
- Huang, C.C.; Kang, M.; Shirazi, S.; Lu, Y.; Cooper, L.F.; Gajendrareddy, P.; Ravindran, S. 3D Encapsulation and tethering of functionally engineered extracellular vesicles to hydrogels. Acta Biomater. 2021, 126, 199–210. [Google Scholar] [CrossRef]
- Bai, Q.; Han, K.; Dong, K.; Zheng, C.; Zhang, Y.; Long, Q.; Lu, T. Potential applications of nanomaterials and technology for diabetic wound healing. Int. J. Nanomed. 2020, 15, 9717. [Google Scholar] [CrossRef] [PubMed]
- Elkhoury, K.; Koçak, P.; Kang, A.; Arab-Tehrany, E.; Ward, J.E.; Shin, S.R. Engineering Smart Targeting Nanovesicles and Their Combination with Hydrogels for Controlled Drug Delivery. Pharmaceutics 2020, 12, 849. [Google Scholar] [CrossRef] [PubMed]
- Ashammakhi, N.; Darabi, M.A.; Kehr, N.S.; Erdem, A.; Hu, S.K.; Dokmeci, M.R.; Nasr, A.S.; Khademhosseini, A. Advances in Controlled Oxygen Generating Biomaterials for Tissue Engineering and Regenerative Therapy. Biomacromolecules 2020, 21, 56–72. [Google Scholar] [CrossRef] [PubMed]
- Harrison, B.S.; Eberli, D.; Lee, S.J.; Atala, A.; Yoo, J.J. Oxygen producing biomaterials for tissue regeneration. Biomaterials 2007, 28, 4628–4634. [Google Scholar] [CrossRef] [PubMed]


| Scaffold Materials | Scaffold Formation and EV Loading Method | EVs Source | Evs Characteristics | Therapeutic Effects | References | ||
|---|---|---|---|---|---|---|---|
| Size | Surface Marker | ||||||
| Mouse full-thickness excisional wound model | Chitosan-glycerol hydrogel | Electrostatic interaction between chitosan and glycerol groups; hydrogen-bonding interactions between the chitosan chains. EVs were mixed in to the scaffold mixture | Human endometrial stem cell (hEnSC) | 40–150 nm | CD63 | ↑ angiogenesis, epidermal layer and tissue granulation formation | [204] |
| Mouse infected full-thickness wound model | Chitosan—silk fibroin/sodium alginate (CTS-SF/SA) dressing with incorporated silver nanoparticle-EVs composites (AgNPs-EVs) | Lyophilized CTS-SF rehydrated, frozen and SA solution added on the surface of the CTS-SF dressing. AgNPs-EVs mixture was prepared by sonication and integrated into CTS-SF/SA by secondary freeze-drying | Human umbilical cord MSCs | 30–70 nm | No data | Broad-spectrum antimicrobial activity, ↑angiogenesis, collagen deposition and nerve repair, oxygen and nutrient transfer to the wound was maintained due to moisture retention feature of the dressing | [205] |
| Diabetic mouse full-thickness wound model | Methylcellulose-chitosan hydrogel | Hydrogel was prepared by one pot mixing of aldehyde modified methyl-cellulose, chitosan grafted poly(ethylene glycol) and EVs. Self-healing properties of the hydrogel determined by dynamic Schiff base linkages between aldehyde and amino groups | Placental MSCs | About 62.5 nm | CD9, CD63, CD81 | ↑ migration of fibroblasts and KCs and other cells, angiogenesis, re-epithelialization. Inhibition of apoptosis | [206] |
| Full-thickness cutaneous wound model | Gelatin methacryloyl (GelMA) hydrogel | GelMA was made by reaction between gelatin and methacrylic anhydride. The polymer was dialyzed and freeze-dried. EVs were incorporated by ultraviolet light-induced crosslinking | HUVECs | 50–140 nm | CD9, CD63, CD81, HSP70 | ↑ wound healing, angiogenesis, collagen deposition, re-epithelialization, migration and proliferation of KCs and fibroblasts | [207] |
| Diabetic rat wound model | Polyurethane-based oxygen releasing antioxidant scaffold (PUAO-CPO) | PUAO-CPO was made by synthesis of PUAO via addition of ascorbic acid to the backbone chain of polyurethane, and subsequent incorporation of calcium peroxide into PUAO cryogels. EVs were attached by incubation forming OxOBand wound dressing (PUAO-CPO-EXO) | AdMSCs | 100–300 nm | CD81 | ↑ vascularization, ↑ KCs and fibroblast migration, proliferation, ↑ collagen remodeling, ↓ oxidative stress | [208] |
| Diabetic mouse skin wound model | Human acellular amniotic membrane (hAAM) | Decellularization of amniotic tissue | AdMSCs | 47.7–150 nm | CD9, CD81 | ↑ wound healing ↑ vascularization, ↑ ECM production, collagen deposition | [209] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Narauskaitė, D.; Vydmantaitė, G.; Rusteikaitė, J.; Sampath, R.; Rudaitytė, A.; Stašytė, G.; Aparicio Calvente, M.I.; Jekabsone, A. Extracellular Vesicles in Skin Wound Healing. Pharmaceuticals 2021, 14, 811. https://doi.org/10.3390/ph14080811
Narauskaitė D, Vydmantaitė G, Rusteikaitė J, Sampath R, Rudaitytė A, Stašytė G, Aparicio Calvente MI, Jekabsone A. Extracellular Vesicles in Skin Wound Healing. Pharmaceuticals. 2021; 14(8):811. https://doi.org/10.3390/ph14080811
Chicago/Turabian StyleNarauskaitė, Deimantė, Gabrielė Vydmantaitė, Justina Rusteikaitė, Revathi Sampath, Akvilė Rudaitytė, Gabija Stašytė, María Isabel Aparicio Calvente, and Aistė Jekabsone. 2021. "Extracellular Vesicles in Skin Wound Healing" Pharmaceuticals 14, no. 8: 811. https://doi.org/10.3390/ph14080811
APA StyleNarauskaitė, D., Vydmantaitė, G., Rusteikaitė, J., Sampath, R., Rudaitytė, A., Stašytė, G., Aparicio Calvente, M. I., & Jekabsone, A. (2021). Extracellular Vesicles in Skin Wound Healing. Pharmaceuticals, 14(8), 811. https://doi.org/10.3390/ph14080811