Micro- and Nano-Based Transdermal Delivery Systems of Photosensitizing Drugs for the Treatment of Cutaneous Malignancies
Abstract
:1. Introduction
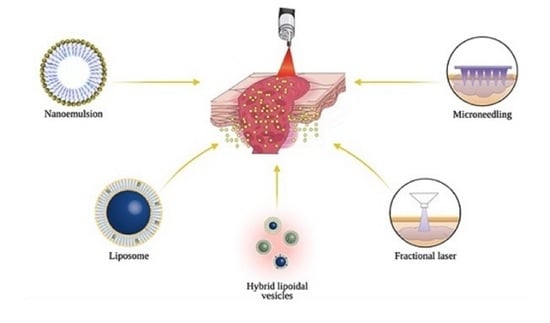
2. Transdermal Drug Delivery of PDT Agents
3. Chemical Approaches for Transdermal Delivery
3.1. Nanoemulsion (NE)
3.1.1. ALA-Nanoemulsion (BF-200 ALA)
3.1.2. Temoporfin (mTHPC)
3.1.3. Pc and Derivatives
3.2. Lipid-Based Vesicular Systems
3.2.1. ALA and Derivatives
3.2.2. Temoporfin (mTHPC)
3.2.3. Pc and Derivatives
3.2.4. Chlorophyll (CHL) and Derivatives
4. Physical Approaches for Transdermal Delivery
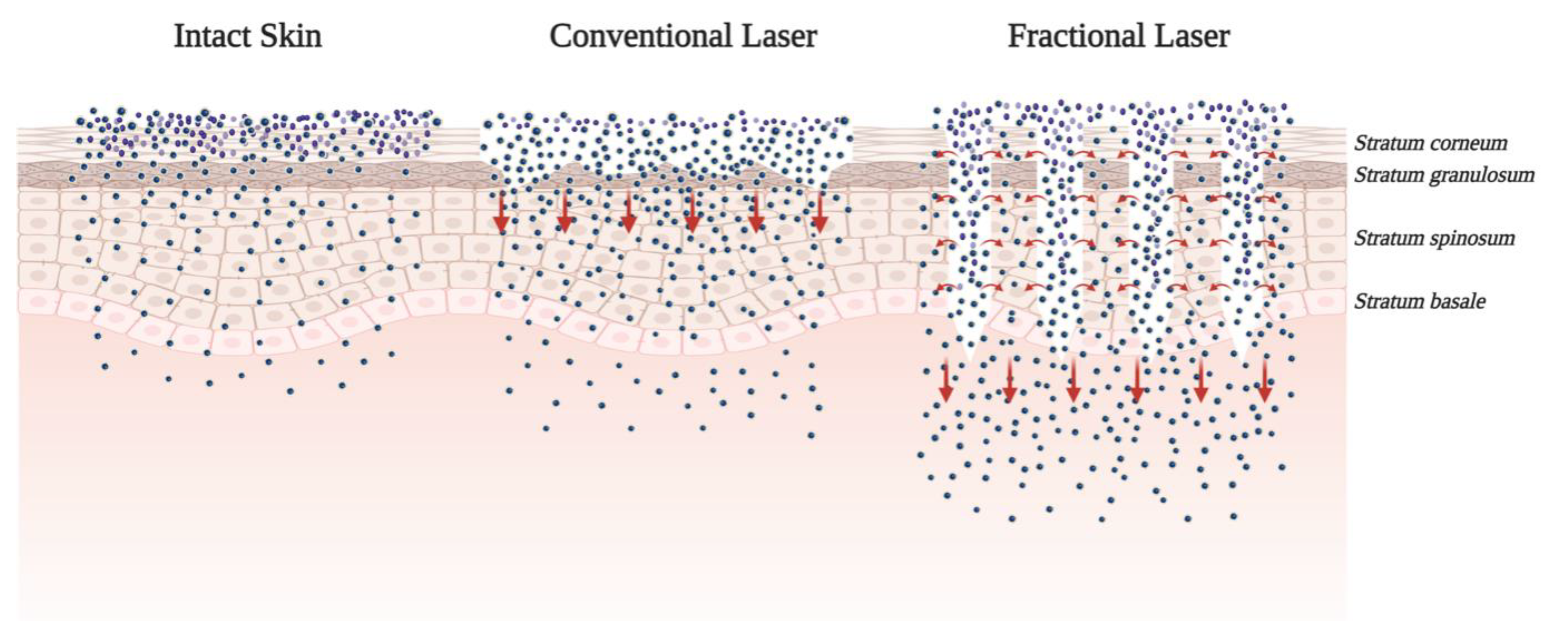
4.1. Ablative Fractional Technology
ALA and Its Derivatives
4.2. Microneedling
4.2.1. Bacteriochlorin
4.2.2. Pc and Derivatives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 5-aminolevulinic acid | ALA |
| ablative fractional laser | AFXL |
| actinic keratosis | AK |
| ALA-nanoemulsion | BF-200 ALA |
| aluminum (III) phthalocyanine tetrasulfonate | AlPcS4 |
| antimicrobial PDT | aPDT |
| basal cell carcinoma | BCC |
| benzoporphyrin derivative monoacid ring A | BPD-MA |
| chloroaluminum phthalocyanine | ClAlPc |
| chlorophyll | CHL |
| drug delivery systems | DDS |
| daylight PDT | dPDT |
| ferrous chlorophyllin | Fe-CHL |
| head-and-neck squamous carcinoma | HNSC |
| hematoporphyrin derivative | HpD |
| indoor daylight | IDL |
| laser-assisted drug delivery | LADD |
| meso-tetra (hydroxyphenyl)-chlorin | mTHPC |
| methyl aminolevulinate | MAL |
| nanoemulsion | NE |
| near-infrared | NIR |
| nitrosyl ruthenium complex [Ru (NH.NHq)(tpy)NO]3+ | RuNO |
| non-melanoma skin cancer | NMSC |
| photosensitizers | PSs |
| photodynamic therapy | PDT |
| phthalocyanine | Pc |
| protoporphyrin IX | PpIX |
| radiofrequency | RF |
| reactive oxygen species | ROS |
| stratum corneum | SC |
| transdermal delivery systems | tDDS |
| TMFI | Tixel®, Novoxel®, Israel |
| zinc phthalocyanine | ZnPc |
References
- Allison, R.R.; Moghissi, K. Photodynamic therapy (PDT): PDT mechanisms. Clin. Endosc. 2013, 46, 24. [Google Scholar] [CrossRef]
- Hamblin, M.R.; Abrahamse, H. Inorganic salts and antimicrobial photodynamic therapy: Mechanistic conundrums? Molecules 2018, 23, 3190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vallejo, M.; Moura, N.M.M.; Ferreira Faustino, M.A.; Almeida, A.; Gonçalves, I.; Serra, V.V.; Neves, M.G.P.M.S. An insight into the role of non-porphyrinoid photosensitizers for skin wound healing. Int. J. Mol. Sci. 2020, 22, 234. [Google Scholar] [CrossRef] [PubMed]
- Vallejo, M.C.S.; Moura, N.M.M.; Gomes, A.T.P.C.; Joaquinito, A.S.M.; Faustino, M.A.F.; Almeida, A.; Gonçalves, I.; Serra, V.V.; Neves, M.G.P.M.S. The role of porphyrinoid photosensitizers for skin wound healing. Int. J. Mol. Sci. 2021, 22, 4121. [Google Scholar] [CrossRef]
- Yang, D.; Lei, S.; Pan, K.; Chen, T.; Lin, J.; Ni, G.; Liu, J.; Zeng, X.; Chen, Q.; Dan, H. Application of photodynamic therapy in immune-related diseases. Photodiagn. Photodyn. Ther. 2021, 34, 102318. [Google Scholar] [CrossRef]
- Mik, E.G. Measuring mitochondrial oxygen tension: From basic principles to application in humans. Anesth. Analg. 2013, 117, 834–846. [Google Scholar] [CrossRef] [PubMed]
- Gunaydin, G.; Gedik, M.E.; Ayan, S. Photodynamic therapy—Current limitations and novel approaches. Front. Chem. 2021, 9, 400. [Google Scholar] [CrossRef]
- Berg, K.; Selbo, P.K.; Weyergang, A.; Dietze, A.; Prasmickaite, L.; Bonsted, A.; Engesaeter, B.O.; Angell-Petersen, E.; Warloe, T.; Frandsen, N.; et al. Porphyrin-related photosensitizers for cancer imaging and therapeutic applications. J. Microsc. 2005, 218, 133–147. [Google Scholar] [CrossRef]
- Dolmans, D.E.J.G.J.; Fukumura, D.; Jain, R.K. Photodynamic therapy for cancer. Nat. Rev. Cancer 2003, 3, 380–387. [Google Scholar] [CrossRef]
- Lipson, R.L.; Baldes, E.J.; Olsen, A.M. The use of a derivative of hematoporhyrin in tumor detection. J. Natl. Cancer Inst. 1961, 26, 1–11. [Google Scholar] [PubMed]
- Dougherty, T.J.; Grindey, G.B.; Fiel, R.; Weishaupt, K.R.; Boyle, D.G. Photoradiation therapy. II. Cure of animal tumors with hematoporphyrin and light23. J. Natl. Cancer Inst. 1975, 55, 115–121. [Google Scholar] [CrossRef]
- Dougherty, T.J.; Kaufman, J.E.; Goldfarb, A.; Weishaupt, K.R.; Boyle, D.; Mittleman, A. Photoradiation therapy for the treatment of malignant tumors. Cancer Res. 1978, 38, 2628–2635. [Google Scholar]
- van Straten, D.; Mashayekhi, V.; de Bruijn, H.S.; Oliveira, S.; Robinson, D.J. Oncologic photodynamic therapy: Basic principles, current clinical status and future directions. Cancers 2017, 9, 19. [Google Scholar] [CrossRef] [PubMed]
- Baskaran, R.; Lee, J.; Yang, S.-G. Clinical development of photodynamic agents and therapeutic applications. Biomater. Res. 2018, 22, 1–8. [Google Scholar] [CrossRef]
- Rkein, A.M.; Ozog, D.M. Photodynamic Therapy. Dermatol. Clin. 2014, 32, 415–425. [Google Scholar] [CrossRef]
- Kwiatkowski, S.; Knap, B.; Przystupski, D.; Saczko, J.; Kedzierska, E.; Knap-Czop, K.; Kotlinska, J.; Michel, O.; Kotowski, K.; Kulbacka, J. Photodynamic therapy-mechanisms, photosensitizers and combinations. Biomed. Pharmacother. 2018, 106, 1098–1107. [Google Scholar] [CrossRef] [PubMed]
- Chizenga, E.P.; Abrahamse, H. Nanotechnology in modern photodynamic therapy of cancer: A review of cellular resistance patterns affecting the therapeutic response. Pharmaceutics 2020, 12, 632. [Google Scholar] [CrossRef]
- Monge-Fuentes, V.; Muehlmann, L.A.; De Azevedo, R.B. Perspectives on the application of nanotechnology in photodynamic therapy for the treatment of melanoma. Nano Rev. 2014, 5, 24381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Alessandro, S.; Priefer, R. Non-porphyrin dyes used as photosensitizers in photodynamic therapy. J. Drug Deliv. Sci. Technol. 2020, 60, 101979. [Google Scholar] [CrossRef]
- Muehlmann, L.A.; Joanitti, G.A.; Silva, J.R.; Longo, J.P.F.; Azevedo, R.B. Liposomal photosensitizers: Potential platforms for anticancer photodynamic therapy. Braz. J. Med. Biol. Res. 2011, 44, 729–737. [Google Scholar] [CrossRef] [Green Version]
- Senapati, S.; Mahanta, A.K.; Kumar, S.; Maiti, P. Controlled drug delivery vehicles for cancer treatment and their performance. Signal Transduct. Target. Ther. 2018, 3, 1–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morrow, D.I.J.; Mccarron, P.A.; Woolfson, A.D.; Donnelly, R.F. Innovative strategies for enhancing topical and transdermal drug delivery. Open Drug Deliv. J. 2007, 1, 36–59. [Google Scholar] [CrossRef]
- Supe, S.; Takudage, P. Methods for evaluating penetration of drug into the skin: A review. Ski. Res. Technol. 2021, 27, 299–308. [Google Scholar] [CrossRef]
- Keservani, R.K.; Bandopadhyay, S.; Bandyopadhyay, N.; Sharma, A.K. Design and fabrication of transdermal/skin drug-delivery system. In Drug Delivery Systems; Elsevier: Amsterdam, The Netherlands, 2020; pp. 131–178. [Google Scholar] [CrossRef]
- Desai, P.; Patlolla, R.R.; Singh, M. Interaction of nanoparticles and cell-penetrating peptides with skin for transdermal drug delivery. Mol. Membr. Biol. 2010, 27, 247–259. [Google Scholar] [CrossRef] [Green Version]
- Cevc, G. Lipid vesicles and other colloids as drug carriers on the skin. Adv. Drug Deliv. Rev. 2004, 56, 675–711. [Google Scholar] [CrossRef]
- Chen, B.; Pogue, B.W.; Hasan, T. Liposomal delivery of photosensitising agents. Expert Opin. Drug Deliv. 2005, 2, 477–487. [Google Scholar] [CrossRef] [PubMed]
- Damoiseau, X.; Schuitmaker, H.J.; Lagerberg, J.W.M.; Hoebeke, M. Increase of the photosensitizing efficiency of the Bacteriochlorin a by liposome-incorporation. J. Photochem. Photobiol. B Biol. 2001, 60, 50–60. [Google Scholar] [CrossRef] [Green Version]
- Derycke, A.S.L.; de Witte, P.A.M. Liposomes for photodynamic therapy. Adv. Drug Deliv. Rev. 2004, 56, 17–30. [Google Scholar] [CrossRef]
- Wu, Y.; Li, Y.-H.; Gao, X.-H.; Chen, H.-D. The application of nanoemulsion in dermatology: An overview. J. Drug Target. 2013, 21, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Becher, P. Emulsions: Theory and Practice; Oxford University Press: Oxford, UK, 2001. [Google Scholar]
- De Rosa, F.S.; Bentley, M. Photodynamic therapy of skin cancers: Sensitizers, clinical studies and future directives. Pharm. Res. 2000, 17, 1447–1455. [Google Scholar] [CrossRef]
- Ma, L.; Bagdonas, S.; Moan, J. The photosensitizing effect of the photoproduct of protoporphyrin IX. J. Photochem. Photobiol. B Biol. 2001, 60, 108–113. [Google Scholar] [CrossRef]
- Casas, A.; Batlle, A. Aminolevulinic acid derivatives and liposome delivery as strategies for improving 5-aminolevulinic acid-mediated photodynamic therapy. Curr. Med. Chem. 2006, 13, 1157–1168. [Google Scholar] [CrossRef] [PubMed]
- Maisch, T.; Santarelli, F.; Schreml, S.; Babilas, P.; Szeimies, R.-M. Fluorescence induction of protoporphyrin IX by a new 5-aminolevulinic acid nanoemulsion used for photodynamic therapy in a full-thickness ex vivo skin model. Exp. Dermatol. 2009, 19, e302–e305. [Google Scholar] [CrossRef]
- Gholam, P.; Weberschock, T.; Denk, K.; Enk, A. Treatment with 5-Aminolaevulinic acid methylester is less painful than treatment with 5-Aminolaevulinic acid nanoemulsion in topical photodynamic therapy for actinic keratosis. Dermatology 2011, 222, 358–362. [Google Scholar] [CrossRef] [PubMed]
- Reinhold, U. A review of BF-200 ALA for the photodynamic treatment of mild-to-moderate actinic keratosis. Future Oncol. 2017, 13, 2413–2428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szeimies, R.-M.; Radny, P.; Sebastian, M.; Borrosch, F.; Dirschka, T.; Krähn-Senftleben, G.; Reich, K.; Pabst, G.; Voss, D.; Foguet, M.; et al. Photodynamic therapy with BF-200 ALA for the treatment of actinic keratosis: Results of a prospective, randomized, double-blind, placebo-controlled phase III study. Br. J. Dermatol. 2010, 163, 386–394. [Google Scholar] [CrossRef]
- Dirschka, T.; Radny, P.; Dominicus, R.; Mensing, H.; Brüning, H.; Jenne, L.; Karl, L.; Sebastian, M.; Oster-Schmidt, C.; Klövekorn, W.; et al. Photodynamic therapy with BF-200 ALA for the treatment of actinic keratosis: Results of a multicentre, randomized, observer-blind phase III study in comparison with a registered methyl-5-aminolaevulinate cream and placebo. Br. J. Dermatol. 2012, 166, 137–146. [Google Scholar] [CrossRef]
- Dirschka, T.; Ekanayake-Bohlig, S.; Dominicus, R.; Aschoff, R.; Herrera-Ceballos, E.; Botella-Estrada, R.; Hunfeld, A.; Kremser, M.; Schmitz, B.; Lübbert, H.; et al. A randomized, intraindividual, non-inferiority, Phase III study comparing daylight photodynamic therapy with BF-200 ALA gel and MAL cream for the treatment of actinic keratosis. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 288–297. [Google Scholar] [CrossRef]
- Collier, N.J.; Rhodes, L.E. Photodynamic therapy for basal cell carcinoma: The clinical context for future research priorities. Molecules 2020, 25, 5398. [Google Scholar] [CrossRef]
- Primo, F.L.; Bentley, M.V.L.B.; Tedesco, A.C. Photophysical studies and in vitro skin permeation/retention of Foscan/nanoemulsion (NE) applicable to photodynamic therapy skin cancer treatment. J. Nanosci. Nanotechnol. 2008, 8, 340–347. [Google Scholar] [CrossRef]
- Primo, F.L.; Michieleto, L.; Rodrigues, M.A.M.; Macaroff, P.P.; Morais, P.C.; Lacava, Z.G.M.; Bentley, M.V.L.B.; Tedesco, A.C. Magnetic nanoemulsions as drug delivery system for Foscan®: Skin permeation and retention in vitro assays for topical application in photodynamic therapy (PDT) of skin cancer. J. Magn. Magn. Mater. 2007, 311, 354–357. [Google Scholar] [CrossRef]
- Primo, F.L.; Rodrigues, M.M.A.; Simioni, A.R.; Bentley, M.V.L.B.; Morais, P.C.; Tedesco, A.C. In vitro studies of cutaneous retention of magnetic nanoemulsion loaded with zinc phthalocyanine for synergic use in skin cancer treatment. J. Magn. Magn. Mater. 2008, 320, e211–e214. [Google Scholar] [CrossRef]
- Ashtikar, M.; Nagarsekar, K.; Fahr, A. Transdermal delivery from liposomal formulations-Evolution of the technology over the last three decades. J. Control Release 2016, 242, 126–140. [Google Scholar] [CrossRef]
- Skupin-Mrugalska, P.; Piskorz, J.; Goslinski, T.; Mielcarek, J.; Konopka, K.; Duzgunes, N. Current status of liposomal porphyrinoid photosensitizers. Drug Discov. Today 2013, 18, 776–784. [Google Scholar] [CrossRef]
- De Leeuw, J.; De Vijlder, H.; Bjerring, P.; Neumann, H. Liposomes in dermatology today. J. Eur. Acad. Dermatol. Venereol. 2009, 23, 505–516. [Google Scholar] [CrossRef] [PubMed]
- Casas, A.; Fukuda, H.; Di Venosa, G. The influence of the vehicle on the synthesis of porphyrins after topical application of 5-aminolaevulinic acid. Implications in cutaneous photodynamic sensitization. Br. J. Dermatol. 2000, 143, 564–572. [Google Scholar] [CrossRef]
- Oh, E.K.; Jin, S.-E.; Kim, J.-K.; Park, J.-S.; Park, Y.; Kim, C.-K. Retained topical delivery of 5-aminolevulinic acid using cationic ultradeformable liposomes for photodynamic therapy. Eur. J. Pharm. Sci. 2011, 44, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Pierre, M.B.R.; Tedesco, A.C.; Marchetti, J.M.; Bentley, M.V.L. Stratum corneum lipids liposomes for the topical delivery of 5-aminolevulinic acid in photodynamic therapy of skin cancer: Preparation and in vitro permeation study. BMC Dermatol. 2001, 1, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Tsai, J.-C.; Chen, I.-H.; Wong, T.-W.; Lo, Y.-L. In vitro/in vivo correlations between transdermal delivery of 5-aminolaevulinic acid and cutaneous protoporphyrin IX accumulation and effect of formulation. Br. J. Dermatol. 2002, 146, 853–862. [Google Scholar] [CrossRef] [PubMed]
- Perotti, C.; Fukuda, H.; Divenosa, G.; Macrobert, A.J.; Batlle, A.; Casas, A. Porphyrin synthesis from ALA derivatives for photodynamic therapy. In vitro and in vivo studies. Br. J. Cancer 2004, 90, 1660–1665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Leeuw, J.; Van Der Beek, N.; Neugebauer, W.D.; Bjerring, P.; Neumann, H.A.M. Fluorescence detection and diagnosis of non-melanoma skin cancer at an early stage. Lasers Surg. Med. 2009, 41, 96–103. [Google Scholar] [CrossRef]
- Osiecka, B.; Jurczyszyn, K.; Symonowicz, K.; Bronowicz, A.; Ostasiewicz, P.; Czapińska, E.; Hotowy, K.; Krzystek-Korpacka, M.; Gębarowska, E.; Iżykowska, I.; et al. In vitro and in vivo matrix metalloproteinase expression after photodynamic therapy with a liposomal formulation of aminolevulinic acid and its methyl ester. Cell. Mol. Biol. Lett. 2010, 15, 630–650. [Google Scholar] [CrossRef]
- Yu, X.; Du, L.; Li, Y.; Fu, G.; Jin, Y. Improved anti-melanoma effect of a transdermal mitoxantrone ethosome gel. Biomed. Pharmacother. 2015, 73, 6–11. [Google Scholar] [CrossRef]
- Touitou, E.; Dayan, N.; Bergelson, L.; Godin, B.; Eliaz, M. Ethosomes—Novel vesicular carriers for enhanced delivery: Characterization and skin penetration properties. J. Control. Release 2000, 65, 403–418. [Google Scholar] [CrossRef]
- Niu, X.-Q.; Zhang, D.-P.; Bian, Q.; Feng, X.-F.; Li, H.; Rao, Y.-F.; Shen, Y.-M.; Geng, F.-N.; Yuan, A.-R.; Ying, X.-Y.; et al. Mechanism investigation of ethosomes transdermal permeation. Int. J. Pharm. X 2019, 1, 100027. [Google Scholar] [CrossRef]
- Fang, Y.-P.; Tsai, Y.-H.; Wu, P.-C.; Huang, Y.-B. Comparison of 5-aminolevulinic acid-encapsulated liposome versus ethosome for skin delivery for photodynamic therapy. Int. J. Pharm. 2008, 356, 144–152. [Google Scholar] [CrossRef]
- Fang, Y.-P.; Huang, Y.-B.; Wu, P.-C.; Tsai, Y.-H. Topical delivery of 5-aminolevulinic acid-encapsulated ethosomes in a hyperproliferative skin animal model using the CLSM technique to evaluate the penetration behavior. Eur. J. Pharm. Biopharm. 2009, 73, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Bragagni, M.; Scozzafava, A.; Mastrolorenzo, A.; Supuran, C.T.; Mura, P. Development and ex vivo evaluation of 5-aminolevulinic acid-loaded niosomal formulations for topical photodynamic therapy. Int. J. Pharm. 2015, 494, 258–263. [Google Scholar] [CrossRef]
- Bendsoe, N.; Persson, L.; Johansson, A.; Axelsson, J.; Svensson, J.; Grafe, S.; Trebst, T.; Andersson-Engels, S.; Svanberg, S.; Svanberg, K. Fluorescence monitoring of a topically applied liposomal temoporfin formulation and photodynamic therapy of nonpigmented skin malignancies. J. Environ. Pathol. Toxicol. Oncol. 2007, 26, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Johansson, A.; Svensson, J.; Bendsoe, N.; Svanberg, K.; Alexandratou, E.; Kyriazi, M.; Yova, D.; GräFe, S.; Trebst, T.; Andersson-Engels, S. Fluorescence and absorption assessment of a lipid mTHPC formulation following topical application in a non-melanotic skin tumor model. J. Biomed. Opt. 2007, 12, 034026. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buchholz, J.; Wergin, M.; Walt, H.; Gräfe, S.; Bley, C.R.; Kaser-Hotz, B. Photodynamic therapy of feline cutaneous squamous cell carcinoma using a newly developed liposomal photosensitizer: Preliminary results concerning drug safety and efficacy. J. Vet. Intern. Med. 2007, 21, 770–775. [Google Scholar] [CrossRef] [PubMed]
- Dragicevic-Curic, N.; Gräfe, S.; Albrecht, V.; Fahr, A. Topical application of temoporfin-loaded invasomes for photodynamic therapy of subcutaneously implanted tumours in mice: A pilot study. J. Photochem. Photobiol. B Biol. 2008, 91, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Dragicevic-Curic, N.; Gräfe, S.; Gitter, B.; Winter, S.; Fahr, A. Surface charged temoporfin-loaded flexible vesicles: In vitro skin penetration studies and stability. Int. J. Pharm. 2010, 384, 100–108. [Google Scholar] [CrossRef]
- Dragicevic-Curic, N.; Scheglmann, D.; Albrecht, V.; Fahr, A. Development of liposomes containing ethanol for skin delivery of temoporfin: Characterization and in vitro penetration studies. Colloids Surf. B Biointerfaces 2009, 74, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Kassab, K.; El Fadeel, D.A.; Fadel, M. Topical photodynamic therapy using transfersomal aluminum phthalocyanine tetrasulfonate: In vitro and in vivo study. Lasers Med. Sci. 2013, 28, 1353–1361. [Google Scholar] [CrossRef]
- Bolfarini, G.C.; Siqueira-Moura, M.P.; Demets, G.J.F.; Tedesco, A.C. Preparation, characterization, and in vitro phototoxic effect of zinc phthalocyanine cucurbit [7] uril complex encapsulated into liposomes. Dye. Pigment. 2014, 100, 162–167. [Google Scholar] [CrossRef]
- De Lima, R.G.; Tedesco, A.C.; Da Silva, R.S.; Lawrence, M.J. Ultradeformable liposome loaded with zinc phthalocyanine and [Ru(NH.NHq)(tpy)NO] 3+ for photodynamic therapy by topical application. Photodiagn. Photodyn. Ther. 2017, 19, 184–193. [Google Scholar] [CrossRef] [Green Version]
- Rady, M.; Gomaa, I.; Afifi, N.; Abdel-Kader, M. Dermal delivery of Fe-chlorophyllin via ultradeformable nanovesicles for photodynamic therapy in melanoma animal model. Int. J. Pharm. 2018, 548, 480–490. [Google Scholar] [CrossRef]
- Vilsinski, B.H.; Witt, M.A.; Barbosa, P.M.; Montanha, M.C.; Nunes, C.S.; Bellettini, I.C.; De Castro, L.V.; Sato, F.; Baesso, M.L.; Muniz, E.C.; et al. Formulation of chloroaluminum phthalocyanine incorporated into PS-b-PAA diblock copolymer nanomicelles. J. Mol. Liq. 2018, 271, 949–958. [Google Scholar] [CrossRef]
- Almeida, E.D.P.; Dipieri, L.V.; Rossetti, F.C.; Marchetti, J.M.; Bentley, M.V.L.B.; Nunes, R.D.S.; Sarmento, V.H.V.; Valerio, M.E.G.; Rodrigues Júnior, J.J.; Montalvão, M.M.; et al. Skin permeation, biocompatibility and antitumor effect of chloroaluminum phthalocyanine associated to oleic acid in lipid nanoparticles. Photodiagn. Photodyn. Ther. 2018, 24, 262–273. [Google Scholar] [CrossRef]
- Shi, Y.; Liu, S.; Liu, Y.; Sun, C.; Chang, M.; Zhao, X.; Hu, C.; Pang, M. Facile fabrication of nanoscale porphyrinic covalent organic polymers for combined photodynamic and photothermal cancer therapy. ACS Appl. Mater. Interfaces 2019, 11, 12321–12326. [Google Scholar] [CrossRef]
- Gomaa, I.; Sebak, A.; Afifi, N.; Abdel-Kader, M. Liposomal delivery of ferrous chlorophyllin: A novel third generation photosensitizer for in vitro PDT of melanoma. Photodiagn. Photodyn. Ther. 2017, 18, 162–170. [Google Scholar] [CrossRef]
- Nasr, S.; Rady, M.; Gomaa, I.; Syrovets, T.; Simmet, T.; Fayad, W.; Abdel-Kader, M. Ethosomes and lipid-coated chitosan nanocarriers for skin delivery of a chlorophyll derivative: A potential treatment of squamous cell carcinoma by photodynamic therapy. Int. J. Pharm. 2019, 568, 118528. [Google Scholar] [CrossRef]
- Ibrahim, O.; Wenande, E.; Hogan, S.; Arndt, K.A.; Haedersdal, M.; Dover, J.S. Challenges to laser-assisted drug delivery: Applying theory to clinical practice. Lasers Surg. Med. 2018, 50, 20–27. [Google Scholar] [CrossRef]
- Alegre-Sánchez, A.; Jiménez-Gómez, N.; Boixeda, P. Laser-assisted drug delivery. Actas Dermosifiliogr. 2018, 109, 858–867. [Google Scholar] [CrossRef]
- Lin, C.-H.; Aljuffali, I.A.; Fang, J.-Y. Lasers as an approach for promoting drug delivery via skin. Expert Opin. Drug Deliv. 2014, 11, 599–614. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.C.; Lee, W.R.; Fang, Y.P.; Hu, C.H.; Fang, J.Y. In vitro percutaneous absorption and in vivo protoporphyrin IX accumulation in skin and tumors after topical 5-aminolevulinic acid application with enhancement using an erbium:YAG laser. J. Pharm. Sci. 2006, 95, 929–938. [Google Scholar] [CrossRef] [PubMed]
- Haedersdal, M.; Sakamoto, F.H.; Farinelli, W.A.; Doukas, A.G.; Tam, J.; Anderson, R.R. Fractional CO2 laser-assisted drug delivery. Lasers Surg. Med. 2010, 42, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Haedersdal, M.; Katsnelson, J.; Sakamoto, F.H.; Farinelli, W.A.; Doukas, A.G.; Tam, J.; Anderson, R.R. Enhanced uptake and photoactivation of topical methyl aminolevulinate after fractional CO2 laser pretreatment. Lasers Surg. Med. 2011, 43, 804–813. [Google Scholar] [CrossRef]
- Haedersdal, M.; Sakamoto, F.H.; Farinelli, W.A.; Doukas, A.G.; Tam, J.; Anderson, R.R. Pretreatment with ablative fractional laser changes kinetics and biodistribution of topical 5-aminolevulinic acid (ALA) and methyl aminolevulinate (MAL). Lasers Surg. Med. 2014, 46, 462–469. [Google Scholar] [CrossRef] [PubMed]
- Paasch, U.; Said, T. Treating field cancerization by ablative fractional laser and indoor daylight: Assessment of efficacy and tolerability. J. Drugs Dermatol. 2020, 19, 425–427. [Google Scholar] [CrossRef] [PubMed]
- Park, J.M.; Jeong, K.-H.; Bae, M.I.; Lee, S.-J.; Kim, N.-I.; Shin, M.K. Fractional radiofrequency combined with sonophoresis to facilitate skin penetration of 5-aminolevulinic acid. Lasers Med. Sci. 2016, 31, 113–118. [Google Scholar] [CrossRef]
- Shavit, R.; Dierickx, C. A new method for percutaneous drug delivery by thermo-mechanical fractional injury. Lasers Surg. Med. 2020, 52, 61–69. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Zhang, Y.; Wang, P.; Wang, B.; Zhang, G.; Wang, X. Plum-blossom needling promoted PpIX fluorescence intensity from 5-aminolevulinic acid in porcine skin model and patients with actnic keratosis. Photodiagn. Photodyn. Ther. 2016, 15, 182–190. [Google Scholar] [CrossRef]
- Li, D.; Hu, D.; Xu, H.; Patra, H.K.; Liu, X.; Zhou, Z.; Tang, J.; Slater, N.; Shen, Y. Progress and perspective of microneedle system for anti-cancer drug delivery. Biomaterials 2021, 264, 120410. [Google Scholar] [CrossRef]
- Donnelly, R.F.; Morrow, D.I.J.; McCarron, P.A.; Woolfson, A.D.; Morrissey, A.; Juzenas, P.; Juzeniene, A.; Iani, V.; McCarthy, H.O.; Moan, J. Microneedle-mediated intradermal delivery of 5-aminolevulinic acid: Potential for enhanced topical photodynamic therapy. J. Control. Release 2008, 129, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, R.F.; Morrow, D.I.J.; Mccarron, P.A.; David Woolfson, A.; Morrissey, A.; Juzenas, P.; Juzeniene, A.; Iani, V.; Mccarthy, H.O.; Moan, J. Microneedle arrays permit enhanced intradermal delivery of a preformed photosensitizer. Photochem. Photobiol. 2009, 85, 195–204. [Google Scholar] [CrossRef]
- Donnelly, R.F.; Morrow, D.I.J.; Fay, F.; Scott, C.J.; Abdelghany, S.; Singh, R.R.T.; Garland, M.J.; David Woolfson, A. Microneedle-mediated intradermal nanoparticle delivery: Potential for enhanced local administration of hydrophobic pre-formed photosensitisers. Photodiagn. Photodyn. Ther. 2010, 7, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, R.F.; Morrow, D.I.J.; Mccrudden, M.T.C.; Alkilani, A.Z.; Vicente-Pérez, E.M.; O’Mahony, C.; González-Vázquez, P.; Mccarron, P.A.; Woolfson, A.D. Hydrogel-forming and dissolving microneedles for enhanced delivery of photosensitizers and precursors. Photochem. Photobiol. 2014, 90, 641–647. [Google Scholar] [CrossRef] [Green Version]
- Garland, M.J.; Cassidy, C.M.; Woolfson, D.; Donnelly, R.F. Designing photosensitizers for photodynamic therapy: Strategies, challenges and promising developments. Future Med. Chem. 2009, 1, 667–691. [Google Scholar] [CrossRef]
- Morrow, D.; Donnelly, R.; Juzenas, P.; Moan, J.; Morrissey, A.; Wilke, N.; McCarron, P. Microfabricated microneedles: A novel strategy for enhancing topical delivery of 5-aminolevulinic acid and preformed photosensitisers: 117. J. Pharm. Pharmacol. 2006, 58, A43–A44. [Google Scholar]
- Kearney, M.-C.; Brown, S.; McCrudden, M.T.C.; Brady, A.J.; Donnelly, R.F. Potential of microneedles in enhancing delivery of photosensitising agents for photodynamic therapy. Photodiagn. Photodyn. Ther. 2014, 11, 459–466. [Google Scholar] [CrossRef]
- Zhao, X.; Li, X.; Zhang, P.; Du, J.; Wang, Y. Tip-loaded fast-dissolving microneedle patches for photodynamic therapy of subcutaneous tumor. J. Control. Release 2018, 286, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.K.; Lee, C.H.; Gill, H.S. 5-Aminolevulinic acid coated microneedles for photodynamic therapy of skin tumors. J. Control. Release 2016, 239, 72–81. [Google Scholar] [CrossRef]
- Hamdan, I.M.N.; Tekko, I.A.; Matchett, K.B.; Arnaut, L.G.; Silva, C.S.; McCarthy, H.O.; Donnelly, R.F. Intradermal delivery of a near-infrared photosensitizer using dissolving microneedle arrays. J. Pharm. Sci. 2018, 107, 2439–2450. [Google Scholar] [CrossRef] [PubMed]
- Tham, H.P.; Xu, K.; Lim, W.Q.; Chen, H.; Zheng, M.; Thng, T.G.S.; Venkatraman, S.S.; Xu, C.; Zhao, Y. Microneedle-assisted topical delivery of photodynamically active mesoporous formulation for combination therapy of deep-Seated melanoma. ACS Nano 2018, 12, 11936–11948. [Google Scholar] [CrossRef]
- Alimardani, V.; Abolmaali, S.S.; Yousefi, G.; Rahiminezhad, Z.; Abedi, M.; Tamaddon, A.; Ahadian, S. Microneedle arrays combined with nanomedicine approaches for transdermal delivery of therapeutics. J. Clin. Med. 2021, 10, 181. [Google Scholar] [CrossRef]
- Chen, S.-X.; Ma, M.; Xue, F.; Shen, S.; Chen, Q.; Kuang, Y.; Liang, K.; Wang, X.; Chen, H. Construction of microneedle-assisted co-delivery platform and its combining photodynamic/immunotherapy. J. Control. Release 2020, 324, 218–227. [Google Scholar] [CrossRef] [PubMed]





| Types | Nano-/Micro-Emulsions | Conventional Liposomes | Niosomes | Transfersomes | Ethosomes | Invasomes |
|---|---|---|---|---|---|---|
| Structure |  |  |  |  |  |  |
| Timeline | 1961 | 1965 | 1970 | 1991 | 1997 | 2004 |
| Composition | Oil or main oleaginous ingredient Surfactant/emulsifier Water | Phospholipids and cholesterol | Non-ionic surfactants 5–10% Other additives Buffer QS | Lipid 5% w/v Edge activator 1% w/v Buffer QS | Phospholipid 5–10% w/w Ethanol 20-50% w/w Buffer QS | Soy-phosphatidylcholine 10% w/w and Lyso soy-phosphatidylcholine 0.7% Ethanol 10% w/w Terpenes 1% w/w Buffer QS |
| Characteristics | A liquid colloidal dispersion system which is kinetically stable, with a droplet size <100 nm | Microscopic spheres | Microscopic spheres | Ultra-deformable liposome | Elastic liposome | Ultraflexible and Elastic liposome |
| Flexibility | - | Rigid | Rigid | High deformability due to edge activator (surfactant) | High deformability and elasticity due to ethanol | High deformability and elasticity |
| Permeation Mechanism | Diffusion/Fusion/Lipolysis | Diffusion/Fusion/Lipolysis | Diffusion/Fusion/Lipolysis | Deformation | Lipid perturbation | Deformation and Lipid perturbation |
| Trademarks of topical photosensitizers | 5-ALA Ameluz® | Lipoxala® spray, mTHPC Foslip®, BPD-MA Visudyne®, ZnPc CGP55847® BPD-MA | - | mTHPC Fospeg® | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Portugal, I.; Jain, S.; Severino, P.; Priefer, R. Micro- and Nano-Based Transdermal Delivery Systems of Photosensitizing Drugs for the Treatment of Cutaneous Malignancies. Pharmaceuticals 2021, 14, 772. https://doi.org/10.3390/ph14080772
Portugal I, Jain S, Severino P, Priefer R. Micro- and Nano-Based Transdermal Delivery Systems of Photosensitizing Drugs for the Treatment of Cutaneous Malignancies. Pharmaceuticals. 2021; 14(8):772. https://doi.org/10.3390/ph14080772
Chicago/Turabian StylePortugal, Isabella, Sona Jain, Patrícia Severino, and Ronny Priefer. 2021. "Micro- and Nano-Based Transdermal Delivery Systems of Photosensitizing Drugs for the Treatment of Cutaneous Malignancies" Pharmaceuticals 14, no. 8: 772. https://doi.org/10.3390/ph14080772
APA StylePortugal, I., Jain, S., Severino, P., & Priefer, R. (2021). Micro- and Nano-Based Transdermal Delivery Systems of Photosensitizing Drugs for the Treatment of Cutaneous Malignancies. Pharmaceuticals, 14(8), 772. https://doi.org/10.3390/ph14080772