Antileukemic Activity and Molecular Docking Study of a Polyphenolic Extract from Coriander Seeds
Abstract
:1. Introduction
2. Results and Discussion
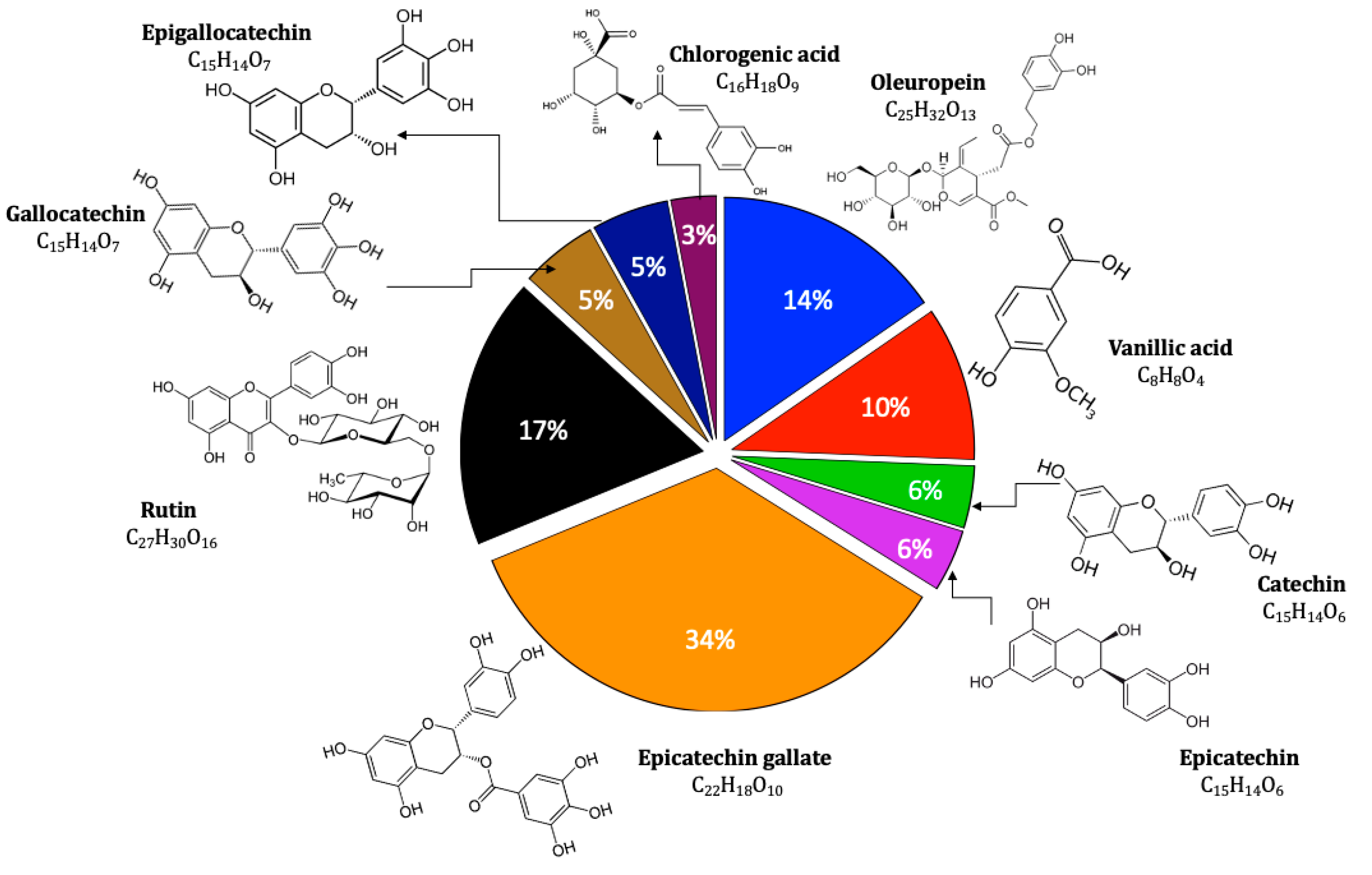
2.1. Extract Analysis
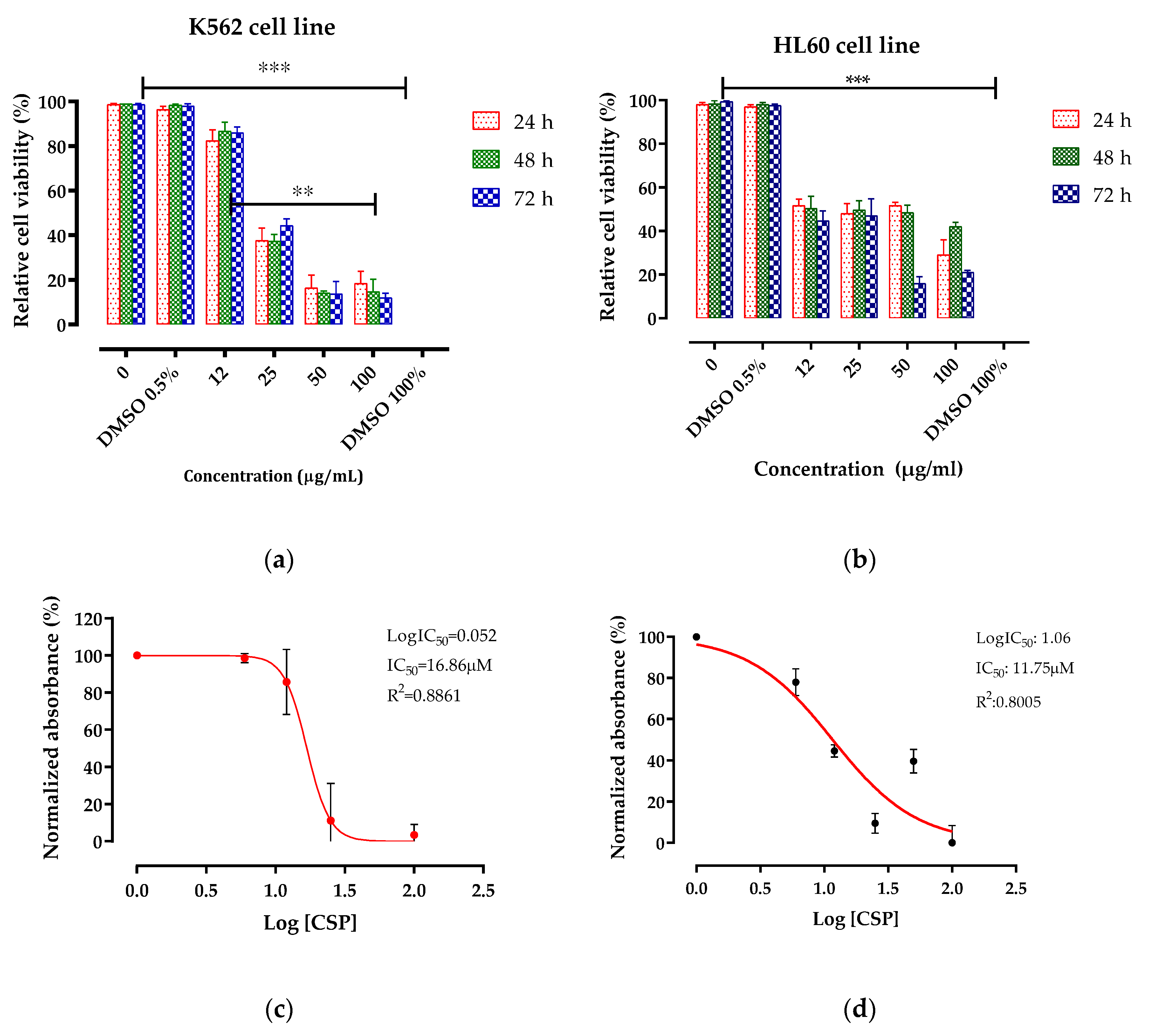
2.2. Antileukemic Activity
2.3. Acute Toxicity Study
2.3.1. Behavioral Studies
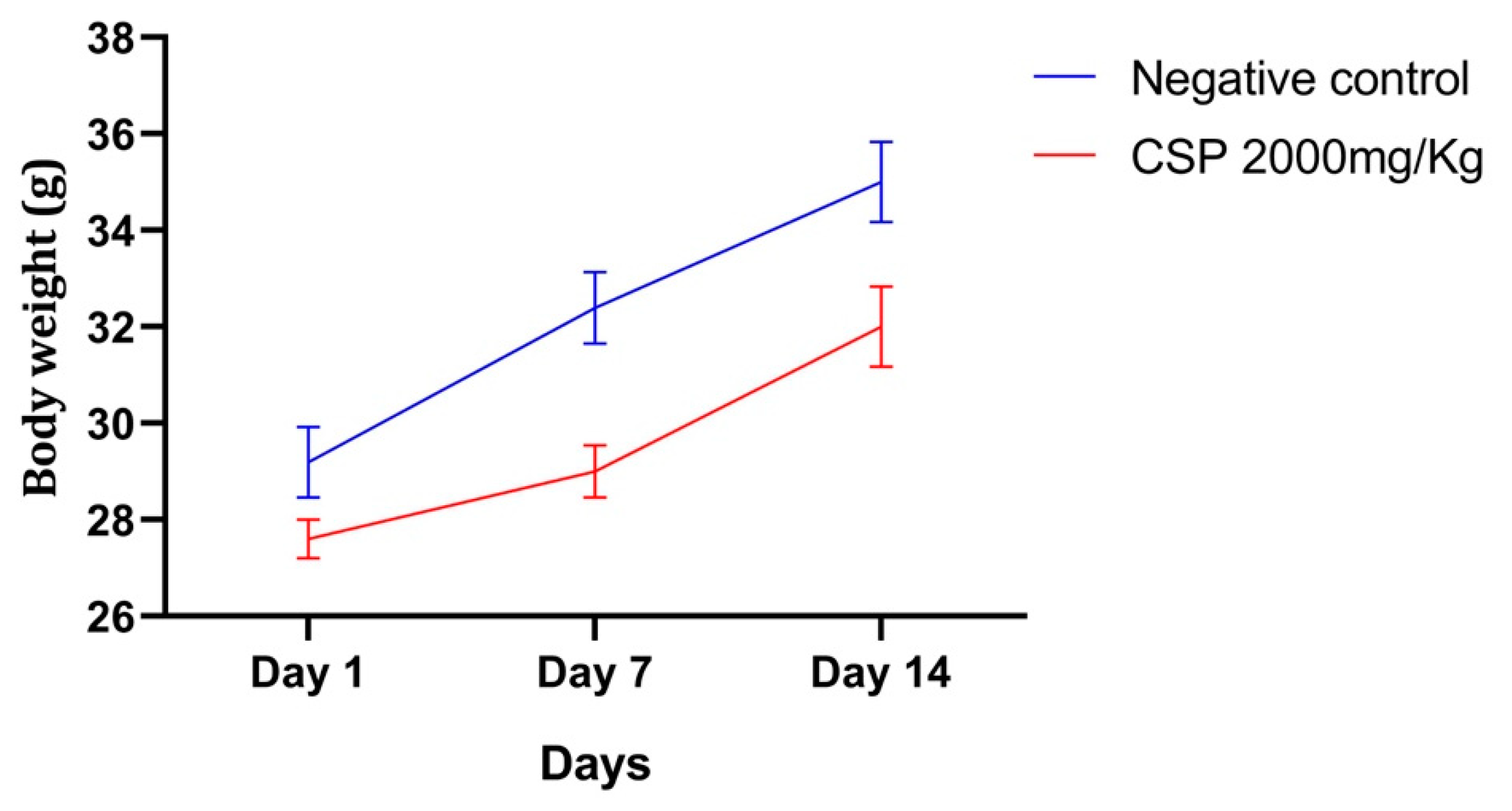
2.3.2. Relative Weight
2.3.3. Biochemical and Hematological Analyses
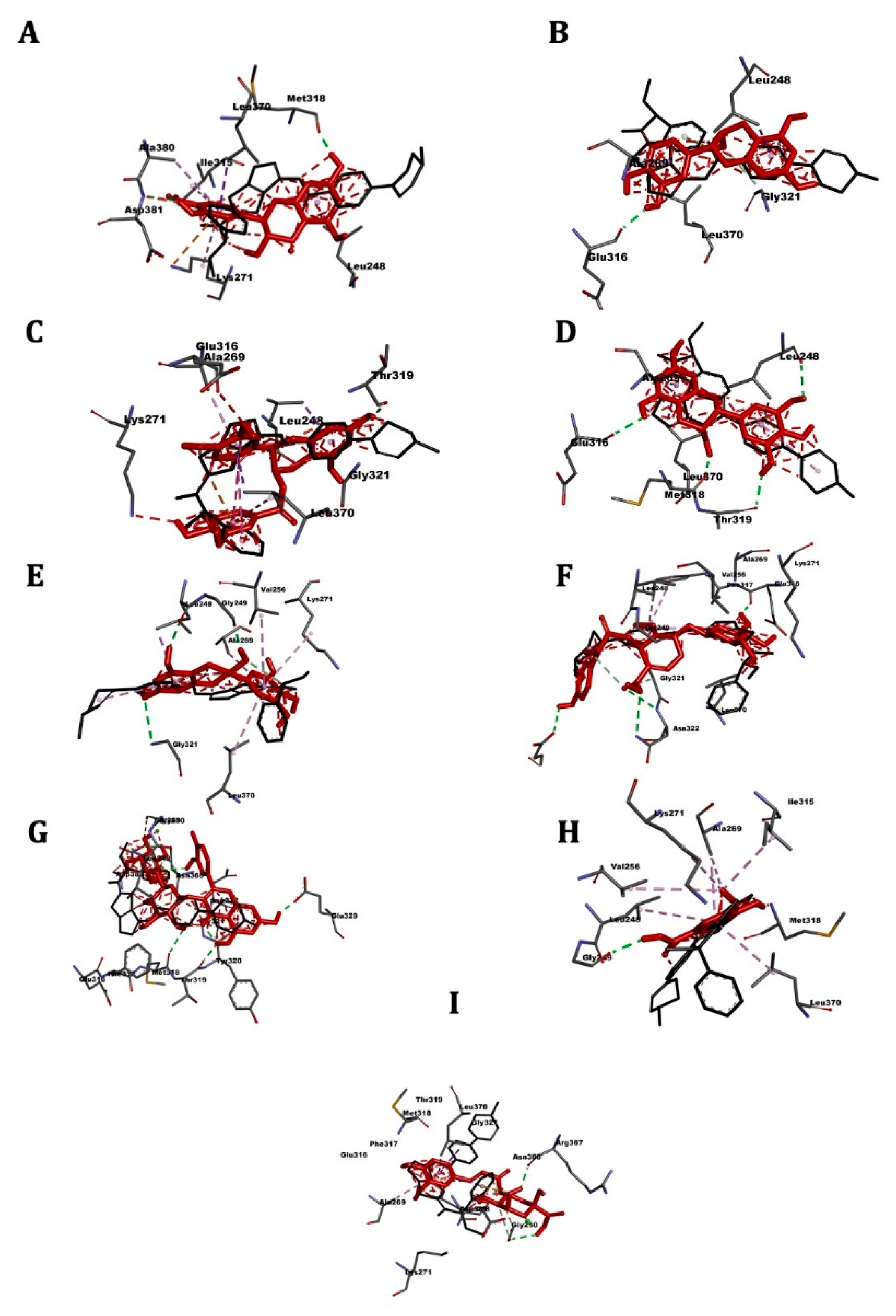
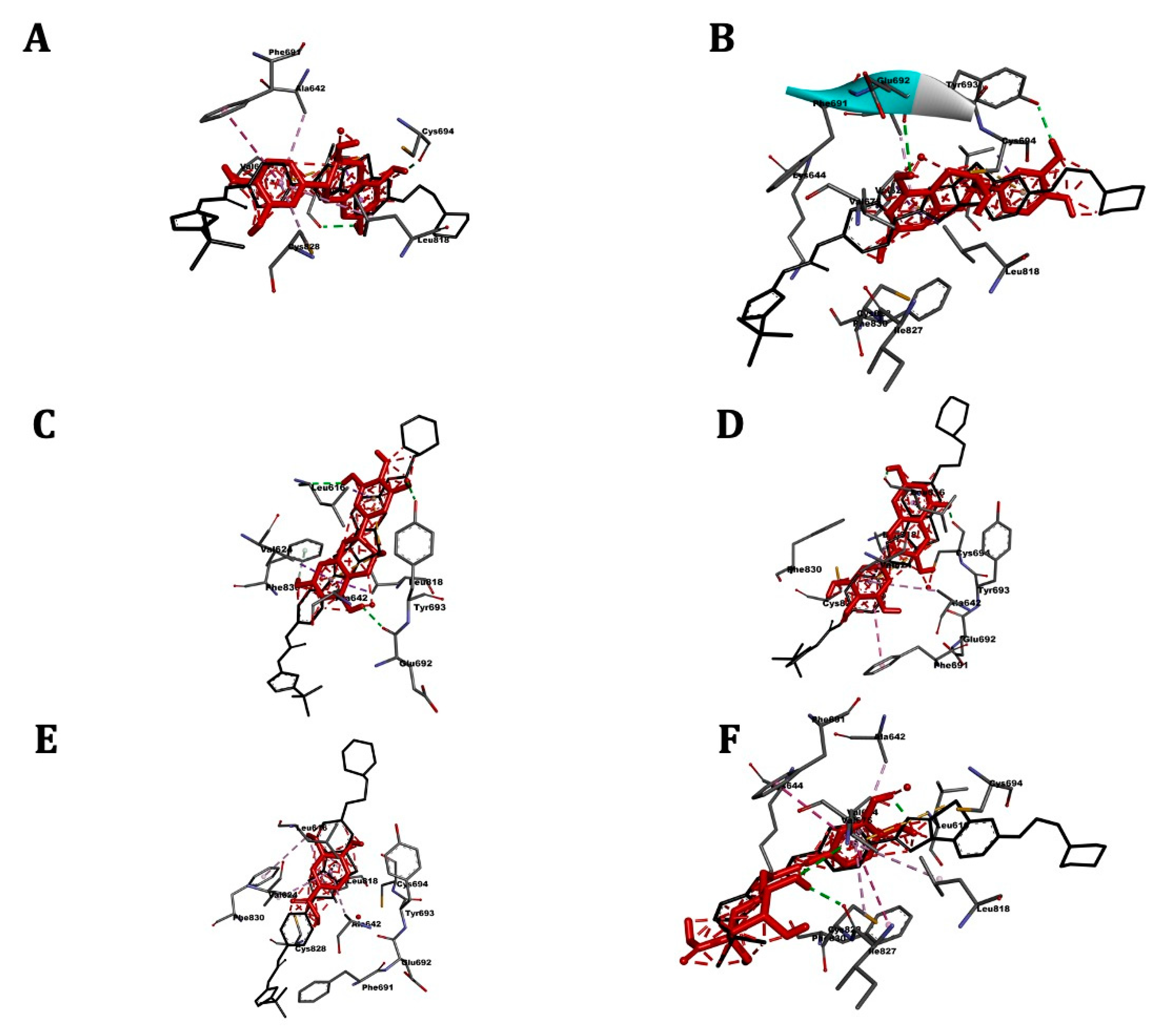
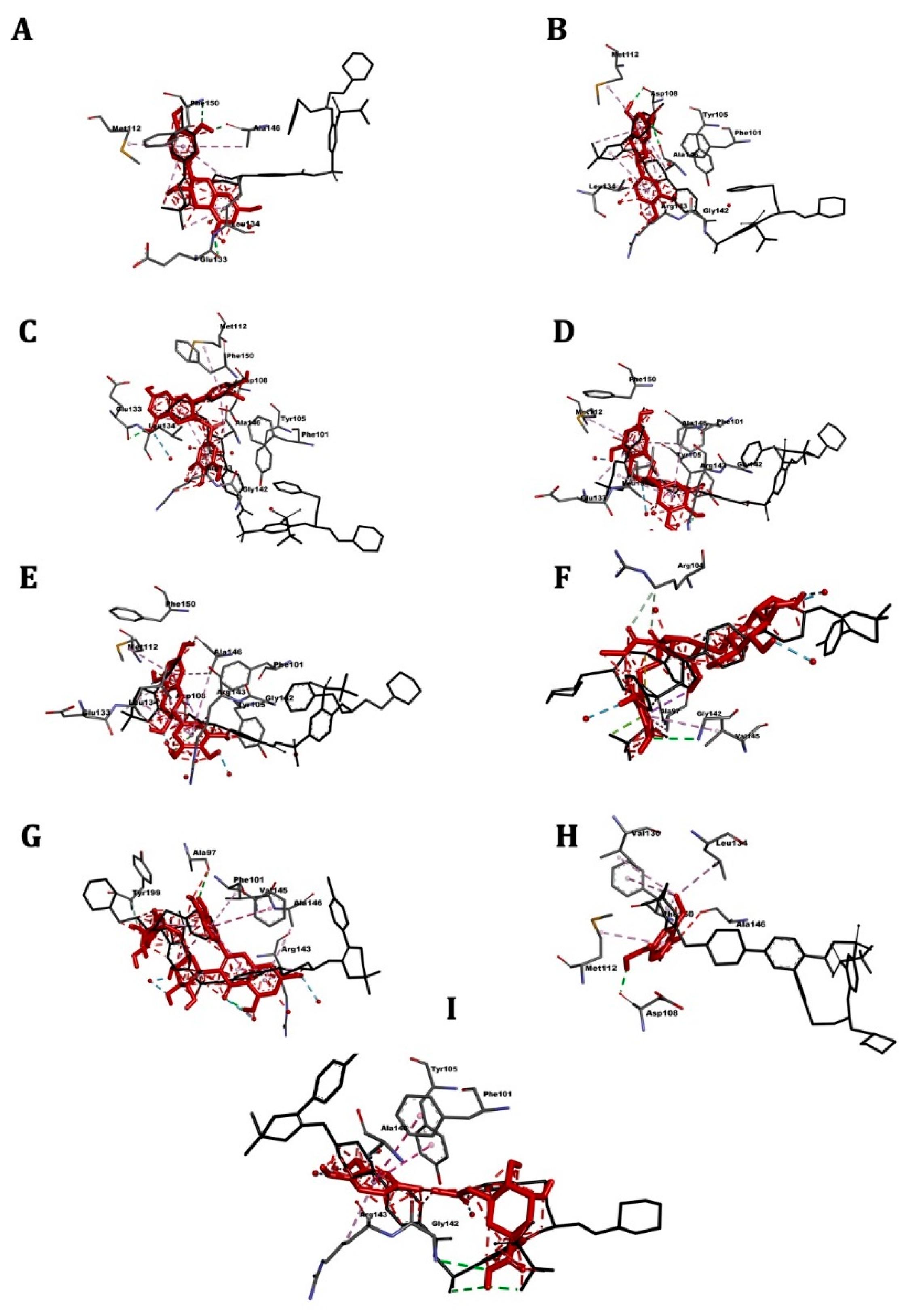
2.4. Molecular Docking
2.5. ABL Kinase
2.6. ABL1
2.7. FLT3
2.8. Bcl-2
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Plant Material
3.3. Preparation and Analysis of the Polyphenolic Extract
3.4. Cell Culture
3.5. Cytotoxicity Assay
3.6. Acute Toxicity Study
3.7. Molecular Docking
3.7.1. Ligand Preparation
3.7.2. Preparation of Receptors
3.7.3. Docking Simulations
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Blumenreich, M.S. The white blood cell and differential count. In Clinical Methods: The History, Physical, and Laboratory Examinations; Walker, H.K., Hall, W.D., Hurst, J.W., Eds.; Butterworths: Boston, MA, USA, 1990; ISBN 978-0-409-90077-4. [Google Scholar]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics, 2020. CA 2020, 70, 7–30. [Google Scholar] [CrossRef]
- Bhat, A.A.; Younes, S.N.; Raza, S.S.; Zarif, L.; Nisar, S.; Ahmed, I.; Mir, R.; Kumar, S.; Sharawat, S.K.; Hashem, S. Role of non-coding RNA networks in leukemia progression, metastasis and drug resistance. Mol. Cancer 2020, 19, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Matsukawa, T.; Aplan, P.D. Clinical and molecular consequences of fusion genes in myeloid malignancies. Stem Cells 2020, 38, 1366–1374. [Google Scholar] [CrossRef] [PubMed]
- Shankar, D.B.; Sakamoto, K.M. The role of cyclic-amp binding protein (CREB) in leukemia cell proliferation and acute leukemias. Leuk. Lymphoma 2004, 45, 265–270. [Google Scholar] [CrossRef]
- Ikeda, A.; Shankar, D.B.; Watanabe, M.; Tamanoi, F.; Moore, T.B.; Sakamoto, K.M. Molecular targets and the treatment of myeloid leukemia. Mol. Genet. Metab. 2006, 88, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Corey, S.J. New agents in the treatment of childhood leukemias and myelodysplastic syndromes. Curr. Oncol. Rep. 2005, 7, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Ling, Y.; Xie, Q.; Zhang, Z.; Zhang, H. Protein kinase inhibitors for acute leukemia. Biomark. Res. 2018, 6, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Saraswathy, M.; Gong, S. Different strategies to overcome multidrug resistance in cancer. Biotechnol. Adv. 2013, 31, 1397–1407. [Google Scholar] [CrossRef]
- Roy, A.; Jauhari, N.; Bharadvaja, N. Medicinal plants as a potential source of chemopreventive agents. In Anticancer Plants: Natural Products and Biotechnological Implements; Springer: Berlin/Heidelberg, Germany, 2018; pp. 109–139. [Google Scholar]
- Mechchate, H.; Es-safi, I.; Haddad, H.; Bekkari, H.; Grafov, A.; Bousta, D. Combination of catechin, epicatechin, and rutin:optimization of a novel complete antidiabetic formulation using a mixture design approach. J. Nutr. Biochem. 2020, 88, 108520. [Google Scholar] [CrossRef]
- Mundlia, J.; Ahuja, M.; Kumar, P. Enhanced biological activity of polyphenols on conjugation with gellan gum. Int. J. Polym. Mater. Polym. Biomater. 2020, 70, 712–729. [Google Scholar] [CrossRef]
- Szwajgier, D.; Paduch, R.; Kukuła-Koch, W.; Polak-Berecka, M.; Waśko, A. Study on biological activity of bread enriched with natural polyphenols in terms of growth inhibition of tumor intestine cells. J. Med. Food 2020, 23, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Nadeem, M.; Muhammad Anjum, F.; Issa Khan, M.; Tehseen, S.; El-Ghorab, A.; Iqbal Sultan, J. Nutritional and medicinal aspects of coriander (Coriandrum Sativum L.): A review. Br. Food J. 2013, 115, 743–755. [Google Scholar] [CrossRef]
- Jana, S.; Patra, K.; Sarkar, S.; Jana, J.; Mukherjee, G.; Bhattacharjee, S.; Mandal, D.P. Antitumorigenic potential of linalool is accompanied by modulation of oxidative stress: An in vivo study in sarcoma-180 solid tumor model. Nutr. Cancer 2014, 66, 835–848. [Google Scholar] [CrossRef]
- Villaseñor, I.M.; Bravo, N.F.C.; Ortega, K.J.L. Anti-skin tumor activity of nutraceuticals from strawberry, coriander, red coral lettuce and chinese chives. Philipp. Agric. Sci. 2009, 92, 338–343. [Google Scholar]
- Mechchate, H.; Es-safi, I.; Amaghnouje, A.; Boukhira, S.; Alotaibi, A.A.; Al-zharani, M.; Nasr, F.A.; Noman, O.M.; Conte, R.; Amal, E.H.E.Y.; et al. Antioxidant, anti-inflammatory and antidiabetic proprieties of LC-MS/MS identified polyphenols from coriander seeds. Molecules 2021, 26, 487. [Google Scholar] [CrossRef]
- Bernatoniene, J.; Kopustinskiene, D.M. The role of catechins in cellular responses to oxidative stress. Molecules 2018, 23, 965. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernando, W.; Rupasinghe, H.P.V.; Hoskin, D.W. Dietary phytochemicals with anti-oxidant and pro-oxidant activities: A double-edged sword in relation to adjuvant chemotherapy and radiotherapy? Cancer Lett. 2019, 452, 168–177. [Google Scholar] [CrossRef]
- Naghma, K.; Hasan, M. Tea and health: Studies in humans. Curr. Pharm. Des. 2013, 19, 6141–6147. [Google Scholar]
- Zhang, L.; Ho, C.-T.; Zhou, J.; Santos, J.S.; Armstrong, L.; Granato, D. Chemistry and biological activities of processed camellia sinensis teas: A comprehensive review. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1474–1495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, Y.; Zhi, F.; Chen, P.; Zhao, K.; Xiang, H.; Mao, Q.; Wang, X.; Zhang, X. Green tea and the risk of prostate cancer: A systematic review and meta-analysis. Medicine 2017, 96, e6426. [Google Scholar] [CrossRef]
- Najaf Najafi, M.; Salehi, M.; Ghazanfarpour, M.; Hoseini, Z.S.; Khadem-Rezaiyan, M. The association between green tea consumption and breast cancer risk: A systematic review and meta-analysis. Phytother. Res. 2018, 32, 1855–1864. [Google Scholar] [CrossRef]
- Naponelli, V.; Ramazzina, I.; Lenzi, C.; Bettuzzi, S.; Rizzi, F. Green tea catechins for prostate cancer prevention: Present achievements and future challenges. Antioxidants 2017, 6, 26. [Google Scholar] [CrossRef] [Green Version]
- Rashmi, H.B.; Negi, P.S. Phenolic acids from vegetables: A review on processing stability and health benefits. Food Res. Int. 2020, 136, 109298. [Google Scholar] [CrossRef]
- Anantharaju, P.G.; Gowda, P.C.; Vimalambike, M.G.; Madhunapantula, S.V. An overview on the role of dietary phenolics for the treatment of cancers. Nutr. J. 2016, 15, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, N.; Goel, N. Phenolic acids: Natural versatile molecules with promising therapeutic applications. Biotechnol. Rep. 2019, 24, e00370. [Google Scholar] [CrossRef] [PubMed]
- Santana-Gálvez, J.; Castrejón, J.V.; Serna-Saldívar, S.O.; Jacobo-Velázquez, D.A. Anticancer potential of dihydrocaffeic acid: A chlorogenic acid metabolite. CyTA J. Food 2020, 18, 245–248. [Google Scholar] [CrossRef] [Green Version]
- Matsunaga, K.; Katayama, M.; Sakata, K.; Kuno, T.; Yoshida, K.; Yamada, Y.; Hirose, Y.; Yoshimi, N.; Mori, H. Inhibitory effects of chlorogenic acid on azoxymethane-induced colon carcinogenesis in male F344 rats. Asian Pac. J. Cancer Prev. 2002, 3, 163–166. [Google Scholar]
- Gong, J.; Zhou, S.; Yang, S. Vanillic acid suppresses HIF-1α expression via inhibition of MTOR/P70S6K/4E-BP1 and Raf/MEK/ERK pathways in human colon cancer HCT116 cells. Int. J. Mol. Sci. 2019, 20, 465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nouri, Z.; Fakhri, S.; Nouri, K.; Wallace, C.E.; Farzaei, M.H.; Bishayee, A. Targeting multiple signaling pathways in cancer: The rutin therapeutic approach. Cancers 2020, 12, 2276. [Google Scholar] [CrossRef] [PubMed]
- Caparica, R.; Júlio, A.; Araújo, M.E.M.; Baby, A.R.; Fonte, P.; Costa, J.G.; de Almeida, T.S. Anticancer activity of rutin and its combination with ionic liquids on renal cells. Biomolecules 2020, 10, 233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, H.; Miao, Q.; Geng, M.; Liu, J.; Hu, Y.; Tian, L.; Pan, J.; Yang, Y. Anti-tumor effect of rutin on human neuroblastoma cell lines through inducing G2/M cell cycle arrest and promoting apoptosis. Sci. World J. 2013, 2013, 269165. [Google Scholar] [CrossRef] [Green Version]
- Grzesik, M.; Naparło, K.; Bartosz, G.; Sadowska-Bartosz, I. Antioxidant properties of catechins: Comparison with other antioxidants. Food Chem. 2018, 241, 480–492. [Google Scholar] [CrossRef] [PubMed]
- Shirakami, Y.; Shimizu, M. Possible mechanisms of green tea and its constituents against cancer. Molecules 2018, 23, 2284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deguchi, Y.; Kimura, S.; Ashihara, E.; Niwa, T.; Hodohara, K.; Fujiyama, Y.; Maekawa, T. Comparison of imatinib, dasatinib, nilotinib and INNO-406 in imatinib-resistant cell lines. Leuk. Res. 2008, 32, 980–983. [Google Scholar] [CrossRef]
- Gontarewicz, A.; Balabanov, S.; Keller, G.; Colombo, R.; Graziano, A.; Pesenti, E.; Benten, D.; Bokemeyer, C.; Fiedler, W.; Moll, J.; et al. Simultaneous targeting of aurora kinases and Bcr-Abl kinase by the small molecule inhibitor PHA-739358 is effective against imatinib-resistant BCR-ABL mutations including T315I. Blood 2008, 111, 4355–4364. [Google Scholar] [CrossRef] [Green Version]
- Kampa-Schittenhelm, K.M.; Heinrich, M.C.; Akmut, F.; Döhner, H.; Döhner, K.; Schittenhelm, M.M. Quizartinib (AC220) is a potent second generation class III tyrosine kinase inhibitor that displays a distinct inhibition profile against mutant-FLT3, -PDGFRA and -KIT isoforms. Mol. Cancer 2013, 12, 19. [Google Scholar] [CrossRef]
- Casson, L.; Howell, L.; Mathews, L.A.; Ferrer, M.; Southall, N.; Guha, R.; Keller, J.M.; Thomas, C.; Siskind, L.J.; Beverly, L.J. Inhibition of ceramide metabolism sensitizes human leukemia cells to inhibition of BCL2-like proteins. PLoS ONE. 2013, 8, e54525. [Google Scholar] [CrossRef]
- Pan, X.; Matsumoto, M.; Nishimoto, Y.; Ogihara, E.; Zhang, J.; Ukiya, M.; Tokuda, H.; Koike, K.; Akihisa, M.; Akihisa, T. Cytotoxic and nitric oxide production-inhibitory activities of limonoids and other compounds from the leaves and bark of melia azedarach. Chem. Biodivers. 2014, 11, 1121–1139. [Google Scholar] [CrossRef] [PubMed]
- Han, D.H.; Kim, J.H. Difference in growth suppression and apoptosis induction of EGCG and EGC on human promyelocytic leukemia HL-60 cells. Arch. Pharm. Res. 2009, 32, 543–547. [Google Scholar] [CrossRef]
- Araújo, K.C.F.; de MBCosta, E.M.; Pazini, F.; Valadares, M.C.; de Oliveira, V. Bioconversion of quercetin and rutin and the cytotoxicity activities of the transformed products. Food Chem. Toxicol. 2013, 51, 93–96. [Google Scholar] [CrossRef]
- Chiang, L.-C.; Chiang, W.; Chang, M.-Y.; Ng, L.-T.; Lin, C.-C. Antileukemic activity of selected natural products in taiwan. Am. J. Chin. Med. 2003, 31, 37–46. [Google Scholar] [CrossRef]
- Kharchoufa, L.; Bouhrim, M.; Bencheikh, N.; Addi, M.; Hano, C.; Mechchate, H.; Elachouri, M. Potential Toxicity of Medicinal Plants Inventoried in Northeastern Morocco: An Ethnobotanical Approach. Plants 2021, 10, 1108. [Google Scholar] [CrossRef]
- Bolkent, S.; Yanardag, R.; Ozsoy-Sacan, O.; Karabulut-Bulan, O. Effects of parsley (Petroselinum crispum) on the liver of diabetic rats: A morphological and biochemical study. Phytother. Res. 2004, 18, 996–999. [Google Scholar] [CrossRef] [PubMed]
- Lorusso, D.; Bria, E.; Costantini, A.; Di Maio, M.; Rosti, G.; Mancuso, A. Patients’ perception of chemotherapy side effects: Expectations, doctor–patient communication and impact on quality of life—An italian survey. Eur. J. Cancer Care 2017, 26, e12618. [Google Scholar] [CrossRef] [PubMed]
- Haq, M.I.; Nixon, J.; Stanley, A.J. Imatinib and liver toxicity. BMJ Case Rep. 2018, 11, e226740. [Google Scholar] [CrossRef] [PubMed]
- Efficace, F.; Baccarani, M.; Breccia, M.; Alimena, G.; Rosti, G.; Cottone, F.; Deliliers, G.L.; Baratè, C.; Rossi, A.R.; Fioritoni, G.; et al. Chronic fatigue is the most important factor limiting health-related quality of life of chronic myeloid leukemia patients treated with imatinib. Leukemia 2013, 27, 1511–1519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hensley, M.L.; Ford, J.M. Imatinib treatment: Specific issues related to safety, fertility, and pregnancy. Semin. Hematol. 2003, 40, 21–25. [Google Scholar] [CrossRef]
- Schirrmacher, V. From chemotherapy to biological therapy: A review of novel concepts to reduce the side effects of systemic cancer treatment. Int. J. Oncol. 2019, 54, 407–419. [Google Scholar]
- Iacobucci, I. Mechanism of resistance to tyrosine kinase inhibitors in philadelphia-positive acute lymphblastic leukaemia (all): From genetic alterations to impaired RNA editing. Mol. Sci. 2008, 20, 6141. [Google Scholar]
- Park, H.; Hong, S.; Kim, J.; Hong, S. Discovery of picomolar abl kinase inhibitors equipotent for wild type and T315I mutant via structure-based de novo design. J. Am. Chem. Soc. 2013, 135, 8227–8237. [Google Scholar] [CrossRef]
- Kazi, J.U.; Rönnstrand, L. The role of src family kinases in FLT3 signaling. Int. J. Biochem. Cell Biol. 2019, 107, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Gruszka, A.M.; Valli, D.; Alcalay, M. Wnt signalling in acute myeloid leukaemia. Cells 2019, 8, 1403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shokouhian, M.; Bagheri, M.; Poopak, B.; Chegeni, R.; Davari, N.; Saki, N. Altering chromatin methylation patterns and the transcriptional network involved in regulation of hematopoietic stem cell fate. J. Cell. Physiol. 2020, 235, 6404–6423. [Google Scholar] [CrossRef]
- Distelhorst, C.W.; Bootman, M.D. Creating a new cancer therapeutic agent by targeting the interaction between Bcl-2 and IP3 receptors. Cold Spring Harb. Perspect. Biol. 2019, 11, a035196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santucci, R.; Sinibaldi, F.; Cozza, P.; Polticelli, F.; Fiorucci, L. Cytochrome c: An extreme multifunctional protein with a key role in cell fate. Int. J. Biol. Macromol. 2019, 136, 1237–1246. [Google Scholar] [CrossRef]
- Yogarajah, M.; Stone, R.M. A concise review of BCL-2 inhibition in acute myeloid leukemia. Expert Rev. Hematol. 2018, 11, 145–154. [Google Scholar] [CrossRef] [PubMed]
- OECD. Test. No. 423: Acute Oral toxicity—Acute Toxic Class. Method. In OECD Guidelines for the Testing of Chemicals, Section 4; OECD Publishing: Paris, France, 2002. [Google Scholar] [CrossRef]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. Autodock4 and autodocktools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berman, H.M.; Battistuz, T.; Bhat, T.N.; Bluhm, W.F.; Bourne, P.E.; Burkhardt, K.; Feng, Z.; Gilliland, G.L.; Iype, L.; Jain, S.; et al. The protein data bank. Acta Crystallogr. Sect. D Biol. Crystallogr. 2002, 58, 899–907. [Google Scholar] [CrossRef] [PubMed]
- Discovery Studio Visualizer Version 21; Dassault Systèmes: San Diego, CA, USA, 2020.
- Trott, O.; Olson, A.J. Autodock vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2009, 31, 455–461. [Google Scholar] [CrossRef] [Green Version]


| Compounds | IC50 (µM) | ||
|---|---|---|---|
| K562 Cell Line | HL60 Cell Line | Vero Cell Line | |
| CSP | 16.86 | 11.75 | >100 |
| Nilotinib [36] | 0.0243 | >2 | - |
| Danusertib [37] | 0.15 | 3.06 | - |
| Quizartinib [38] | >10 | >10 | - |
| Navitoclax [39] | 0.4 | - | - |
| Catechin [40] | - | >100 | - |
| Epicatechin [40] | - | >100 | - |
| Epigallocatechin [41] | - | 107.7 | - |
| Epigallocatechin Gallate [41] | - | 60 | - |
| Rutin [41,42] | - | 14 | - |
| Vanillic acid [43] | 56 | - | - |
| Groups | Liver (g) * | Kidney (g) * | Spleen (g) * |
|---|---|---|---|
| Control | 8.65 ± 0.52 | 1.79 ± 0.18 | 0.74 ± 0.15 |
| CSP, 2000 mg/kg | 7.96 ± 0.05 | 1.78 ± 0.02 | 0.75 ± 0.05 |
| Parameter | Control * | CSP 2 g/kg * |
|---|---|---|
| Urea (g/L) | 0.28 ± 0.02 | 0.21 ± 0.03 |
| Creatinine (mg/L) | 3.40 ± 0.31 | 3.80 ± 0.25 |
| ALT (U/L) | 45.80 ± 2.11 | 58.20 ± 4.71 |
| AST (U/L) | 397.7 ± 30.37 | 354.7 ± 23.42 |
| Triglycerides (mg/dL) | 87.33 ± 16.25 | 85.66 ± 12.85 |
| Total Cholesterol (mg/dL) | 93.00 ± 7.93 | 91.33 ± 8.96 |
| HDL (mg/dL) | 45.33 ± 3.05 | 41.33 ± 4.72 |
| VLDL (mg/dL) | 17.33 ± 3.51 | 17.00 ± 2.64 |
| Total Proteins (gm/dL) | 6.16 ± 0.15 | 5.90 ± 0.17 |
| Albumin (gm/dL) | 3.33 ± 0.15 | 3.06 ± 0.30 |
| ALP (IU/L) | 92.33 ± 9.29 | 78.66 ± 4.50 |
| Total Bilirubin (mg/dL) | 1.00 ± 0.26 | 0.96 ± 0.05 |
| Direct Bilirubin (mg/dL) | 0.40 ± 0.10 | 0.43 ± 0.15 |
| Parameter. | Control * | CSP 2 g/kg * |
|---|---|---|
| Total Hb (g/dL) | 12.60 ± 1.15 | 12.96 ± 0.85 |
| Total RBC (106/µL) | 11.20 ± 0.35 | 10.83 ± 0.22 |
| Total WBC (103/µL) | 2.22 ± 0.85 | 3.00 ± 1.12 |
| Platelet Count (103/µL) | 512.33 ± 37.68 | 502.0 ± 45.51 |
| HCT (%) | 47.20 ± 3.27 | 43.10 ± 1.71 |
| Granulocytes (%) | 22.76 ± 2.01 | 20.06 ± 2.36 |
| Lymphocytes (%) | 55.26 ± 4.73 | 63.36 ± 6.29 |
| Monocytes (%) | 13.16 ± 1.76 | 12.62 ± 2.58 |
| Receptor | Reference | Affinity (kcal/mol) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CAT | EPI | EPG | EGC | GC | OLE | RU | CA | VA | ||
| ABL kinase | −9.9 a | −8.8 | −9.6 | −8.1 | −8.2 | −7.2 | −6.4 | −9.3 | −7.6 | −6.5 |
| ABL1 | −8.5 b | −8.2 | −7.7 | −9.2 | −7.9 | −7.9 | −6.9 | −8.6 | −6.8 | −5.4 |
| BCL2 | −11.5 c | −6.8 | −6.8 | −7.8 | −6.9 | −6.8 | −6.8 | −7.6 | −6.9 | −5.3 |
| FLT3 | −10.1 d | −9.1 | −8.3 | None | −8.3 | −8.9 | None | None | −8.3 | −5.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mechchate, H.; Costa de Oliveira, R.; Es-safi, I.; Vasconcelos Mourão, E.M.; Bouhrim, M.; Kyrylchuk, A.; Soares Pontes, G.; Bousta, D.; Grafov, A. Antileukemic Activity and Molecular Docking Study of a Polyphenolic Extract from Coriander Seeds. Pharmaceuticals 2021, 14, 770. https://doi.org/10.3390/ph14080770
Mechchate H, Costa de Oliveira R, Es-safi I, Vasconcelos Mourão EM, Bouhrim M, Kyrylchuk A, Soares Pontes G, Bousta D, Grafov A. Antileukemic Activity and Molecular Docking Study of a Polyphenolic Extract from Coriander Seeds. Pharmaceuticals. 2021; 14(8):770. https://doi.org/10.3390/ph14080770
Chicago/Turabian StyleMechchate, Hamza, Regiane Costa de Oliveira, Imane Es-safi, Emmily Myrella Vasconcelos Mourão, Mohamed Bouhrim, Andrii Kyrylchuk, Gemilson Soares Pontes, Dalila Bousta, and Andriy Grafov. 2021. "Antileukemic Activity and Molecular Docking Study of a Polyphenolic Extract from Coriander Seeds" Pharmaceuticals 14, no. 8: 770. https://doi.org/10.3390/ph14080770
APA StyleMechchate, H., Costa de Oliveira, R., Es-safi, I., Vasconcelos Mourão, E. M., Bouhrim, M., Kyrylchuk, A., Soares Pontes, G., Bousta, D., & Grafov, A. (2021). Antileukemic Activity and Molecular Docking Study of a Polyphenolic Extract from Coriander Seeds. Pharmaceuticals, 14(8), 770. https://doi.org/10.3390/ph14080770






