Zebrafish Model in Ophthalmology to Study Disease Mechanism and Drug Discovery
Abstract
1. Introduction
2. Anatomy and Development of Zebrafish Eye
2.1. Anatomy of Zebrafish Eye
2.1.1. Cornea
2.1.2. Iridocorneal Angle
2.1.3. Lens
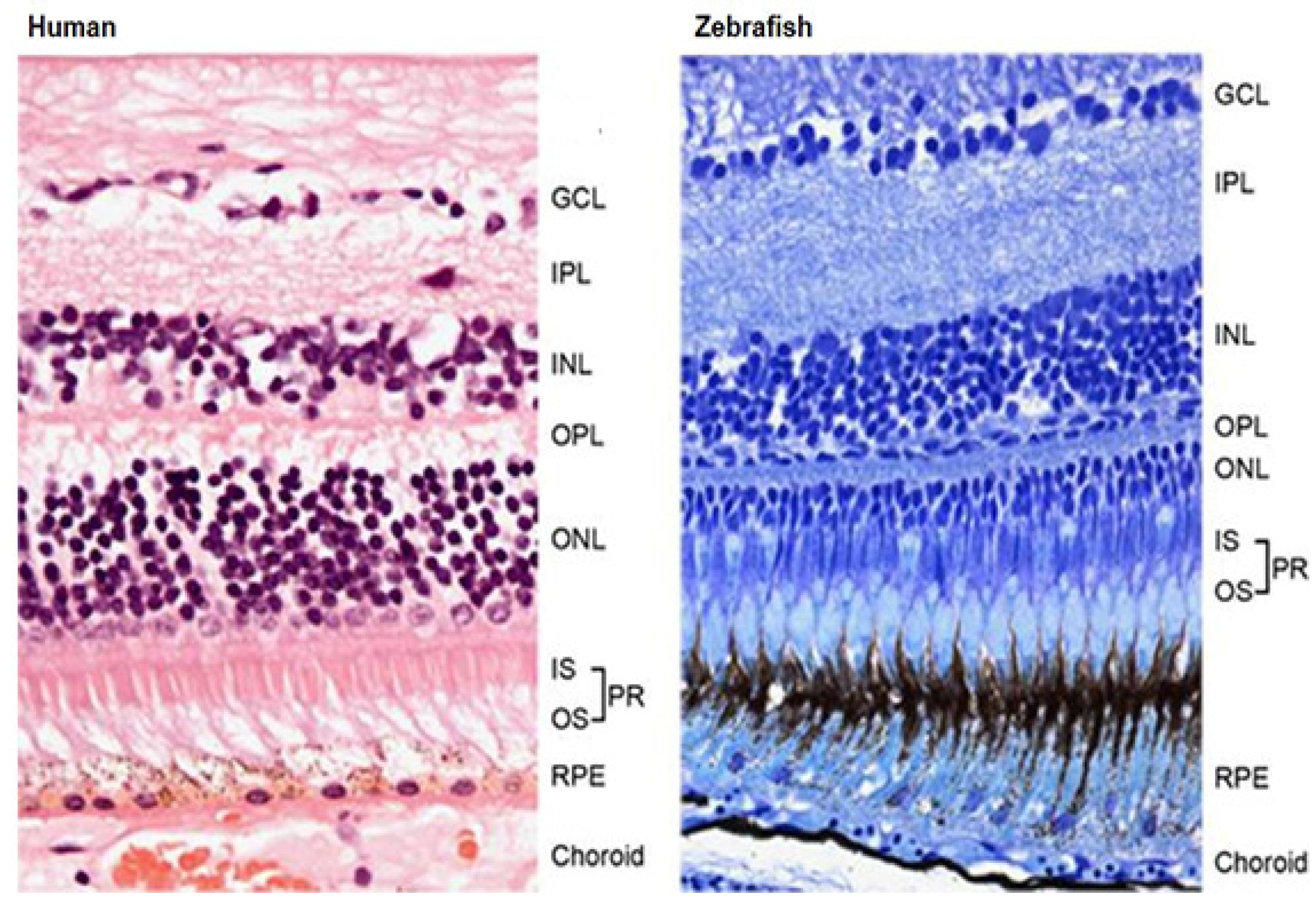
2.1.4. Visual System
2.1.5. Vasculature System
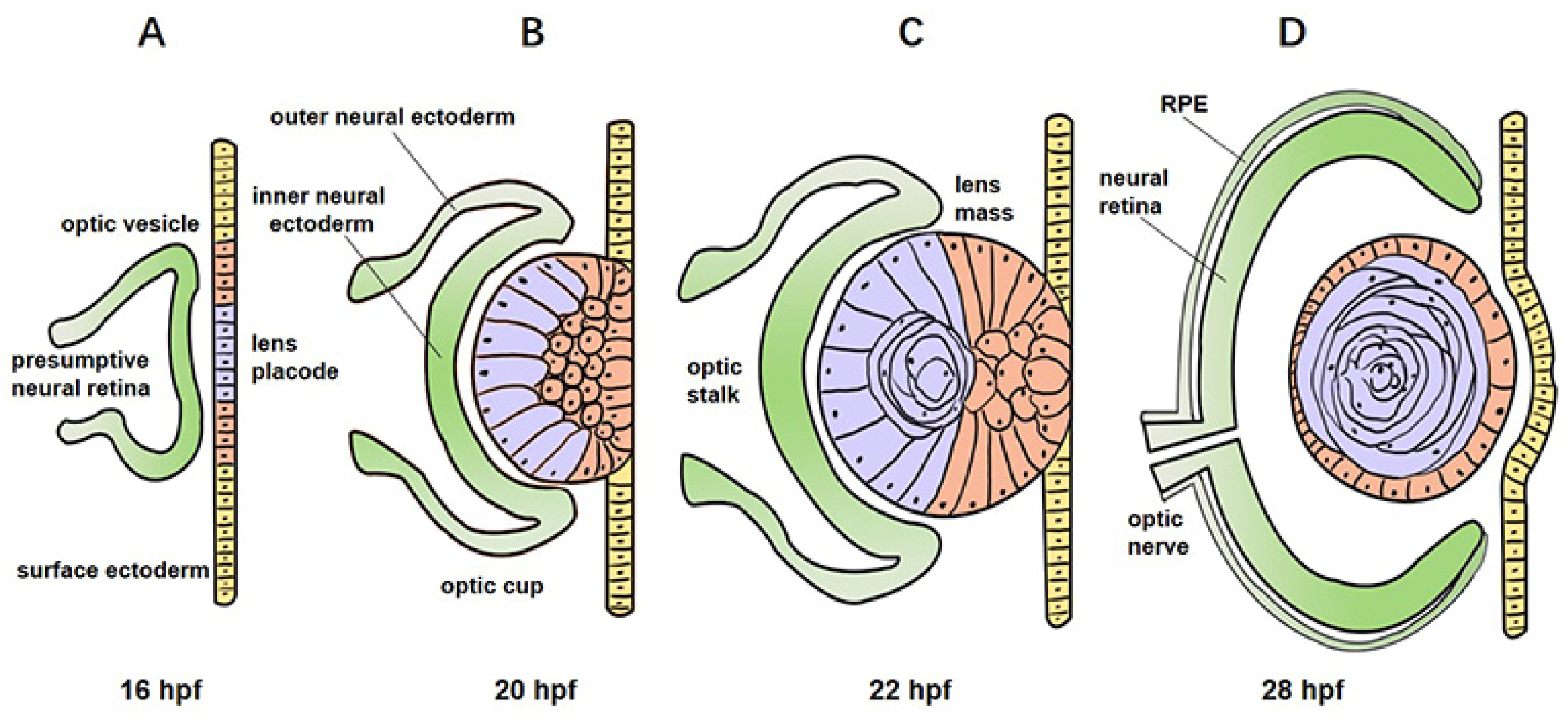
2.2. Development of Zebrafish Eye
3. Zebrafish as a Model for Studying Mechanisms of Eye Disorders
3.1. Corneal Dystrophy
3.2. Cataract
3.3. Glaucoma
3.4. Vascular Disease
3.4.1. Diabetic Retinopathy
3.4.2. Retinopathy of Prematurity
3.4.3. Age-Related Macular Degeneration
3.5. Photoreceptor Degeneration
3.5.1. Retinitis Pigmentosa
3.5.2. Leber Congenital Amaurosis
4. Zebrafish as a Model for the Drug Discovery of Eye Disorders
4.1. Anti-Angiogenic Compounds
4.2. Neuroprotective Drugs
4.3. Drug Oculotoxicity
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bourne, R.; Steinmetz, J.D.; Flaxman, S.; Briant, P.S.; Taylor, H.R.; Resnikoff, S.; Casson, R.J.; Abdoli, A.; Abu-Gharbieh, E.; Afshin, A.; et al. Trends in prevalence of blindness and distance and near vision impairment over 30 years: An analysis for the Global Burden of Disease Study. Lancet Glob. Health 2021, 9, e130–e143. [Google Scholar] [CrossRef]
- Steinmetz, J.D.; Bourne, R.R.A.; Briant, P.S.; Flaxman, S.R.; Taylor, H.R.B.; Jonas, J.B.; Abdoli, A.A.; Abrha, W.A.; Abualhasan, A.; Abu-Gharbieh, E.G.; et al. Causes of blindness and vision impairment in 2020 and trends over 30 years, and prevalence of avoidable blindness in relation to VISION 2020: The Right to Sight: An analysis for the Global Burden of Disease Study. Lancet Glob. Health 2021, 9, e144–e160. [Google Scholar] [CrossRef]
- Bibliowicz, J.; Tittle, R.K.; Gross, J.M. Toward a better understanding of human eye disease insights from the zebrafish, Danio rerio. Prog. Mol. Biol. Transl. Sci. 2011, 100, 287–330. [Google Scholar] [CrossRef]
- Ablain, J.; Zon, L.I. Of fish and men: Using zebrafish to fight human diseases. Trends Cell Biol. 2013, 23, 584–586. [Google Scholar] [CrossRef]
- MacRae, C.A.; Peterson, R.T. Zebrafish as tools for drug discovery. Nat. Rev. Drug Discov. 2015, 14, 721–731. [Google Scholar] [CrossRef] [PubMed]
- Richardson, R.; Tracey-White, D.; Webster, A.; Moosajee, M. The zebrafish eye—a paradigm for investigating human ocular genetics. Eye 2017, 31, 68–86. [Google Scholar] [CrossRef]
- Zhao, X.C.; Yee, R.W.; Norcom, E.; Burgess, H.; Avanesov, A.S.; Barrish, J.P.; Malicki, J. The Zebrafish Cornea: Structure and Development. Investig. Opthalmol. Vis. Sci. 2006, 47, 4341–4348. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Puzzolo, D.; Pisani, A.; Malta, C.; Santoro, G.; Meduri, A.; Abbate, F.; Montalbano, G.; Wylegala, E.; Rana, R.A.; Bucchieri, F.; et al. Structural, ultrastructural, and morphometric study of the zebrafish ocular surface: A model for human corneal diseases? Curr. Eye Res. 2018, 43, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Gestri, G.; Link, B.A.; Neuhauss, S.C. The visual system of zebrafish and its use to model human ocular diseases. Dev. Neurobiol. 2012, 72, 302–327. [Google Scholar] [CrossRef] [PubMed]
- Link, B.A.; Gray, M.P.; Smith, R.S.; John, S.W.M. Intraocular Pressure in Zebrafish: Comparison of Inbred Strains and Identification of a Reduced Melanin Mutant with Raised IOP. Investig. Opthalmol. Vis. Sci. 2004, 45, 4415–4422. [Google Scholar] [CrossRef]
- Dahm, R.; Schonthaler, H.B.; Soehn, A.S.; van Marle, J.; Vrensen, G.F. Development and adult morphology of the eye lens in the zebrafish. Exp. Eye Res. 2007, 85, 74–89. [Google Scholar] [CrossRef]
- Vihtelic, T.S. Teleost Lens Development and Degeneration. Int. Rev. Cell Mol. Biol. 2008, 269, 341–373. [Google Scholar] [CrossRef] [PubMed]
- Noel, N.C.L.; Macdonald, I.M.; Allison, W.T. Zebrafish Models of Photoreceptor Dysfunction and Degeneration. Biomolecules 2021, 11, 78. [Google Scholar] [CrossRef] [PubMed]
- Yoshimatsu, T.; Schröder, C.; Nevala, N.E.; Berens, P.; Baden, T. Fovea-like Photoreceptor Specializations Underlie Single UV Cone Driven Prey-Capture Behavior in Zebrafish. Neuron 2020, 107, 320–337.e6. [Google Scholar] [CrossRef]
- Portugues, R.; Engert, F. The neural basis of visual behaviors in the larval zebrafish. Curr. Opin. Neurobiol. 2009, 19, 644–647. [Google Scholar] [CrossRef] [PubMed]
- Bollmann, J.H. The Zebrafish Visual System: From Circuits to Behavior. Annu. Rev. Vis. Sci. 2019, 5, 269–293. [Google Scholar] [CrossRef]
- Hartsock, A.; Lee, C.; Arnold, V.; Gross, J.M. In vivo analysis of hyaloid vasculature morphogenesis in zebrafish: A role for the lens in maturation and maintenance of the hyaloid. Dev. Biol. 2014, 394, 327–339. [Google Scholar] [CrossRef] [PubMed]
- Rezzola, S.; Belleri, M.; Gariano, G.; Ribatti, D.; Costagliola, C.; Semeraro, F.; Presta, M. In vitro and ex vivo retina angiogenesis assays. Angiogenesis 2013, 17, 429–442. [Google Scholar] [CrossRef]
- Merrigan, S.L.; Kennedy, B.N. Vitamin D receptor agonists regulate ocular developmental angiogenesis and modulate expression of dre-miR-21 and VEGF. Br. J. Pharmacol. 2017, 174, 2636–2651. [Google Scholar] [CrossRef]
- Alvarez, Y.; Cederlund, M.L.; Cottell, D.C.; Bill, B.R.; Ekker, S.C.; Torres-Vazquez, J.; Weinstein, B.M.; Hyde, D.R.; Vihtelic, T.S.; Kennedy, B.N. Genetic determinants of hyaloid and retinal vasculature in zebrafish. BMC Dev. Biol. 2007, 7, 114. [Google Scholar] [CrossRef]
- Xie, J.; Farage, E.; Sugimoto, M.; Anand-Apte, B. A novel transgenic zebrafish model for blood-brain and blood-retinal barrier development. BMC Dev. Biol. 2010, 10, 76. [Google Scholar] [CrossRef]
- Lu, J.; Liu, R.; Miao, A.; Chen, X.; Xiao, W.; Wang, Y.; Cao, D.; Pan, J.; Li, L.; Luo, Y. The role of cldnh during the early retinal development in zebrafish. Exp. Eye Res. 2020, 200, 108207. [Google Scholar] [CrossRef]
- Lawson, N.; Weinstein, B.M. In Vivo Imaging of Embryonic Vascular Development Using Transgenic Zebrafish. Dev. Biol. 2002, 248, 307–318. [Google Scholar] [CrossRef]
- Choi, J.; Dong, L.; Ahn, J.; Dao, D.; Hammerschmidt, M.; Chen, J.-N. FoxH1 negatively modulates flk1 gene expression and vascular formation in zebrafish. Dev. Biol. 2007, 304, 735–744. [Google Scholar] [CrossRef] [PubMed]
- Olivares, A.M.; Althoff, K.; Chen, G.F.; Wu, S.; Morrisson, M.A.; DeAngelis, M.M.; Haider, N. Animal Models of Diabetic Retinopathy. Curr. Diabetes Rep. 2017, 17, 93. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Sánchez, B.; Clément, A.; Phillips, J.; Westerfield, M. Zebrafish models of human eye and inner ear diseases. Methods Cell Biol. 2017, 138, 415–467. [Google Scholar] [CrossRef] [PubMed]
- Easter, J.S.S.; Nicola, G.N. The Development of Vision in the Zebrafish (Danio rerio). Dev. Biol. 1996, 180, 646–663. [Google Scholar] [CrossRef] [PubMed]
- Stella, S.; Geathers, J.; Weber, S.; Grillo, M.; Barber, A.; Sundstrom, J.; Grillo, S. Neurodegeneration, Neuroprotection and Regeneration in the Zebrafish Retina. Cells 2021, 10, 633. [Google Scholar] [CrossRef]
- Schmitt, E.A.; Dowling, J.E. Early-eye morphogenesis in the zebrafish, Brachydanio rerio. J. Comp. Neurol. 1994, 344, 532–542. [Google Scholar] [CrossRef] [PubMed]
- Greiling, T.M.; Clark, J.I. Early lens development in the zebrafish: A three-dimensional time-lapse analysis. Dev. Dyn. 2009, 238, 2254–2265. [Google Scholar] [CrossRef]
- Greiling, T.M.S.; Aose, M.; Clark, J.I. Cell Fate and Differentiation of the Developing Ocular Lens. Investig. Opthalmol. Vis. Sci. 2010, 51, 1540–1546. [Google Scholar] [CrossRef]
- Mochizuki, T.; Luo, Y.-J.; Tsai, H.-F.; Hagiwara, A.; Masai, I. Cell division and cadherin-mediated adhesion regulate lens epithelial cell movement in zebrafish. Development 2017, 144, 708–719. [Google Scholar] [CrossRef]
- Gath, N.; Gross, J.M. Zebrafish mab21l2 mutants possess severe defects in optic cup morphogenesis, lens and cornea development. Dev. Dyn. 2019, 248, 514–529. [Google Scholar] [CrossRef] [PubMed]
- Lisch, W.; Weiss, J.S. Clinical and genetic update of corneal dystrophies. Exp. Eye Res. 2019, 186, 107715. [Google Scholar] [CrossRef] [PubMed]
- Boisset, G.; Polok, B.K.; Schorderet, D. Characterization of pip5k3 fleck corneal dystrophy-linked gene in zebrafish. Gene Expr. Patterns 2008, 8, 404–410. [Google Scholar] [CrossRef]
- Yeh, L.-K.; Liu, C.-Y.; Chien, C.-L.; Converse, R.L.; Kao, W.W.-Y.; Chen, M.-S.; Hu, F.-R.; Hsieh, F.-J.; Wang, I.-J. Molecular Analysis and Characterization of Zebrafish Keratocan (zKera) Gene. J. Biol. Chem. 2008, 283, 506–517. [Google Scholar] [CrossRef] [PubMed]
- Oliver, V.F.; Van Bysterveldt, K.A.; Cadzow, M.; Steger, B.; Romano, V.; Markie, D.; Hewitt, A.; Mackey, D.A.; Willoughby, C.; Sherwin, T.; et al. A COL17A1 Splice-Altering Mutation Is Prevalent in Inherited Recurrent Corneal Erosions. Ophthalmology 2016, 123, 709–722. [Google Scholar] [CrossRef] [PubMed]
- Semina, E.V.; Bosenko, D.V.; Zinkevich, N.C.; Soules, K.A.; Hyde, D.R.; Vihtelic, T.S.; Willer, G.B.; Gregg, R.; Link, B.A. Mutations in laminin alpha 1 result in complex, lens-independent ocular phenotypes in zebrafish. Dev. Biol. 2006, 299, 63–77. [Google Scholar] [CrossRef] [PubMed]
- Shiels, A.; Hejtmancik, J.F. Biology of Inherited Cataracts and Opportunities for Treatment. Annu. Rev. Vis. Sci. 2019, 5, 123–149. [Google Scholar] [CrossRef]
- Shiels, A.; Bennett, T.M.; Hejtmancik, J.F. Cat-Map: Putting cataract on the map. Mol. Vis. 2010, 16, 2007–2015. [Google Scholar]
- Mishra, S.; Wu, S.-Y.; Fuller, A.W.; Wang, Z.; Rose, K.L.; Schey, K.L.; Mchaourab, H.S. Loss of αB-crystallin function in zebrafish reveals critical roles in the development of the lens and stress resistance of the heart. J. Biol. Chem. 2018, 293, 740–753. [Google Scholar] [CrossRef] [PubMed]
- Goishi, K.; Shimizu, A.; Najarro, G.; Watanabe, S.; Rogers, R.; Zon, L.I.; Klagsbrun, M. AlphaA-crystallin expression prevents gamma-crystallin insolubility and cataract formation in the zebrafish cloche mutant lens. Development 2006, 133, 2585–2593. [Google Scholar] [CrossRef] [PubMed]
- Aryal, S.; Viet, J.; Weatherbee, B.; Siddam, A.D.; Hernandez, F.G.; Gautier-Courteille, C.; Paillard, L.; Lachke, S.A. The cataract-linked RNA-binding protein Celf1 post-transcriptionally controls the spatiotemporal expression of the key homeodomain transcription factors Pax6 and Prox1 in lens development. Hum. Genet. 2020, 139, 1541–1554. [Google Scholar] [CrossRef] [PubMed]
- Heisenberg, C.; Brand, M.; Jiang, Y.; Warga, R.; Beuchle, D.; van Eeden, F.; Furutani-Seiki, M.; Granato, M.; Haffter, P.; Hammerschmidt, M.; et al. Genes involved in forebrain development in the zebrafish, Danio rerio. Development 1996, 123, 191–203. [Google Scholar] [CrossRef]
- Shi, X.; Bosenko, D.; Zinkevich, N.; Foley, S.; Hyde, D.; Semina, E.; Vihtelic, T.S. Zebrafish pitx3 is necessary for normal lens and retinal development. Mech. Dev. 2005, 122, 513–527. [Google Scholar] [CrossRef]
- Shi, X.; Luo, Y.; Howley, S.; Dzialo, A.; Foley, S.; Hyde, D.R.; Vihtelic, T.S. Zebrafish foxe3: Roles in ocular lens morphogenesis through interaction with pitxMech. Development 2006, 123, 761–782. [Google Scholar] [CrossRef]
- Swindell, E.C.; Zilinski, C.A.; Hashimoto, R.; Shah, R.; Lane, M.E.; Jamrich, M. Regulation and function offoxe3 during early zebrafish development. Genes 2008, 46, 177–183. [Google Scholar] [CrossRef]
- Anand, D.; Agrawal, S.A.; Slavotinek, A.; Lachke, S.A. Mutation update of transcription factor genes FOXE3, HSF4, MAF, and PITX3 causing cataracts and other developmental ocular defects. Hum. Mutat. 2018, 39, 471–494. [Google Scholar] [CrossRef]
- Krall, M.; Htun, S.; Anand, D.; Hart, D.; Lachke, S.A.; Slavotinek, A.M. A zebrafish model of foxe3 deficiency demonstrates lens and eye defects with dysregulation of key genes involved in cataract formation in humans. Hum. Genet. 2018, 137, 315–328. [Google Scholar] [CrossRef]
- Gao, M.; Huang, Y.; Wang, L.; Huang, M.; Liu, F.; Liao, S.; Yu, S.; Lu, Z.; Han, S.; Hu, X.; et al. HSF4 regulates lens fiber cell differentiation by activating p53 and its downstream regulators. Cell Death Dis. 2017, 8, e3082. [Google Scholar] [CrossRef]
- Shiels, A.; Hejtmancik, J.F. Mutations and mechanisms in congenital and age-related cataracts. Exp. Eye Res. 2017, 156, 95–102. [Google Scholar] [CrossRef]
- Greiling, T.M.; Houck, S.A.; Clark, J.I. The zebrafish lens proteome during development and aging. Mol. Vis. 2009, 15, 2313–2325. [Google Scholar]
- Peschek, J.; Braun, N.; Rohrberg, J.; Back, K.C.; Kriehuber, T.; Kastenmüller, A.; Weinkauf, S.; Buchner, J. Regulated structural transitions unleash the chaperone activity of B-crystallin. Proc. Natl. Acad. Sci. USA 2013, 110, E3780–E3789. [Google Scholar] [CrossRef]
- Wu, S.-Y.; Zou, P.; Mishra, S.; Mchaourab, H.S. Transgenic zebrafish models reveal distinct molecular mechanisms for cataract-linked αA-crystallin mutants. PLoS ONE 2018, 13, e0207540. [Google Scholar] [CrossRef]
- Li, X.-Q.; Cai, H.-C.; Zhou, S.; Yang, J.-H.; Xi, Y.-B.; Gao, X.-B.; Zhao, W.-J.; Li, P.; Zhao, G.-Y.; Tong, Y.; et al. A novel mutation impairing the tertiary structure and stability of γC-crystallin (CRYGC) leads to cataract formation in humans and zebrafish lens. Hum. Mutat. 2012, 33, 391–401. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.-Y.; Zou, P.; Fuller, A.W.; Mishra, S.; Wang, Z.; Schey, K.L.; Mchaourab, H.S. Expression of Cataract-linked γ-Crystallin Variants in Zebrafish Reveals a Proteostasis Network That Senses Protein Stability. J. Biol. Chem. 2016, 291, 25387–25397. [Google Scholar] [CrossRef]
- Zhang, J.; Cui, W.-W.; Du, C.; Huang, Y.; Pi, X.; Guo, W.; Wang, J.; Huang, W.; Chen, D.; Li, J.; et al. Knockout of DNase1l1l abrogates lens denucleation process and causes cataract in zebrafish. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165724. [Google Scholar] [CrossRef] [PubMed]
- Harding, P.; Toms, M.; Schiff, E.; Owen, N.; Bell, S.; Lloyd, I.; Moosajee, M. EPHA2 Segregates with Microphthalmia and Congenital Cataracts in Two Unrelated Families. Int. J. Mol. Sci. 2021, 22, 2190. [Google Scholar] [CrossRef]
- Taler, K.; Weiss, O.; Rotem-Bamberger, S.; Rubinstein, A.M.; Seritrakul, P.; Gross, J.M.; Inbal, A. Lysyl hydroxylase 3 is required for normal lens capsule formation and maintenance of lens epithelium integrity and fate. Dev. Biol. 2020, 458, 177–188. [Google Scholar] [CrossRef]
- Shao, M.; Lu, T.; Zhang, C.; Zhang, Y.-Z.; Kong, S.-H.; Shi, D.-L. Rbm24 controls poly(A) tail length and translation efficiency of crystallin mRNAs in the lens via cytoplasmic polyadenylation. Proc. Natl. Acad. Sci. USA 2020, 117, 7245–7254. [Google Scholar] [CrossRef] [PubMed]
- Vorontsova, I.; Gehring, I.; Hall, J.E.; Schilling, T.F. Aqp0a Regulates Suture Stability in the Zebrafish Lens. Investig. Opthalmol. Vis. Sci. 2018, 59, 2869–2879. [Google Scholar] [CrossRef]
- Ping, X.; Liang, J.; Shi, K.; Bao, J.; Wu, J.; Yu, X.; Tang, X.; Zou, J.; Shentu, X. Rapamycin relieves the cataract caused by ablation of Gja8b through stimulating autophagy in zebrafish. Autophagy 2021, 2021, 1–15. [Google Scholar] [CrossRef]
- Ping, X.; Cheng, Y.; Bao, J.; Shi, K.; Zou, J.; Shentu, X. KPNA4 is involved in cataract formation via the nuclear import of p53. Gene 2021, 786, 145621. [Google Scholar] [CrossRef]
- Jones, J.L.; Corbett, M.A.; Yeaman, E.; Zhao, D.; Gecz, J.; Gasperini, R.J.; Charlesworth, J.C.; Mackey, D.A.; Elder, J.E.; Craig, J.E.; et al. A 127 kb truncating deletion of PGRMC1 is a novel cause of X-linked isolated paediatric cataract. Eur. J. Hum. Genet. 2021, 1–10. [Google Scholar] [CrossRef]
- Siddam, A.D.; Gautier-Courteille, C.; Perez-Campos, L.; Anand, D.; Kakrana, A.; Dang, C.A.; Legagneux, V.; Méreau, A.; Viet, J.; Gross, J.M.; et al. The RNA-binding protein Celf1 post-transcriptionally regulates p27Kip1 and Dnase2b to control fiber cell nuclear degradation in lens development. PLoS Genet. 2018, 14, e1007278. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Allingham, R.R. Major review: Molecular genetics of primary open-angle glaucoma. Exp. Eye Res. 2017, 160, 62–84. [Google Scholar] [CrossRef]
- Youngblood, H.; Hauser, M.A.; Liu, Y. Update on the genetics of primary open-angle glaucoma. Exp. Eye Res. 2019, 188, 107795. [Google Scholar] [CrossRef]
- Chen, C.-C.; Yeh, L.-K.; Liu, C.-Y.; Kao, W.W.-Y.; Samples, J.R.; Lin, S.-J.; Hu, F.-R.; Wang, I.-J. Morphological differences between the trabecular meshworks of zebrafish and mammals. Curr. Eye Res. 2008, 33, 59–72. [Google Scholar] [CrossRef] [PubMed]
- Gray, M.P.; Smith, R.S.; Soules, K.A.; John, S.W.M.; Link, B.A. The Aqueous Humor Outflow Pathway of Zebrafish. Investig. Opthalmol. Vis. Sci. 2009, 50, 1515–1521. [Google Scholar] [CrossRef]
- Iglesias, A.I.; Springelkamp, H.; Van Der Linde, H.; Severijnen, L.-A.; Amin, N.; Oostra, B.; Kockx, C.E.M.; Hout, M.C.G.N.V.D.; Van Ijcken, W.F.J.; Hofman, A.; et al. Exome sequencing and functional analyses suggest that SIX6 is a gene involved in an altered proliferation–differentiation balance early in life and optic nerve degeneration at old age. Hum. Mol. Genet. 2013, 23, 1320–1332. [Google Scholar] [CrossRef]
- Carnes, M.; Liu, Y.P.; Allingham, R.R.; Whigham, B.T.; Havens, S.; Garrett, M.E.; Qiao, C.; Katsanis, N.; Wiggs, J.L.; Pasquale, L.R.; et al. Discovery and Functional Annotation of SIX6 Variants in Primary Open-Angle Glaucoma. PLoS Genet. 2014, 10, e1004372. [Google Scholar] [CrossRef]
- Shah, M.H.; Tabanera, N.; Krishnadas, S.R.; Pillai, M.R.; Bovolenta, P.; Sundaresan, P. Identification and characterization of variants and a novel 4 bp deletion in the regulatory region of SIX6, a risk factor for primary open-angle glaucoma. Mol. Genet. Genom. Med. 2017, 5, 323–335. [Google Scholar] [CrossRef] [PubMed]
- Bailey, J.N.C.; ANZRAG Consortium; Loomis, S.J.; Kang, J.H.; Allingham, R.R.; Gharahkhani, P.; Khor, C.C.; Burdon, K.P.; Aschard, H.; Chasman, D.I.; et al. Genome-wide association analysis identifies TXNRD2, ATXN2 and FOXC1 as susceptibility loci for primary open-angle glaucoma. Nat. Genet. 2016, 48, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Berry, F.B.; Skarie, J.M.; Mirzayans, F.; Fortin, Y.; Hudson, T.J.; Raymond, V.; Link, B.A.; Walter, M.A. FOXC1 is required for cell viability and resistance to oxidative stress in the eye through the transcriptional regulation of FOXO1A. Hum. Mol. Genet. 2007, 17, 490–505. [Google Scholar] [CrossRef] [PubMed]
- Umali, J.; Hawkey-Noble, A.; French, C.R. Loss of foxc1 in zebrafish reduces optic nerve size and cell number in the retinal ganglion cell layer. Vis. Res. 2019, 156, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Veth, K.N.; Willer, J.R.; Collery, R.F.; Gray, M.P.; Willer, G.B.; Wagner, D.S.; Mullins, M.C.; Udvadia, A.J.; Smith, R.S.; John, S.W.M.; et al. Mutations in Zebrafish lrp2 Result in Adult-Onset Ocular Pathogenesis That Models Myopia and Other Risk Factors for Glaucoma. PLoS Genet. 2011, 7, e1001310. [Google Scholar] [CrossRef] [PubMed]
- Stujenske, J.M.; Dowling, J.E.; Emran, F. TheBugeyeMutant Zebrafish Exhibits Visual Deficits that Arise with the Onset of an Enlarged Eye Phenotype. Investig. Opthalmol. Vis. Sci. 2011, 52, 4200–4207. [Google Scholar] [CrossRef] [PubMed]
- Sheng, W.; Lu, Y.; Mei, F.; Wang, N.; Liu, Z.-Z.; Han, Y.-Y.; Wang, H.-T.; Zou, S.; Xu, H.; Zhang, X. Effect of Resveratrol on Sirtuins, OPA1, and Fis1 Expression in Adult Zebrafish Retina. Investig. Opthalmol. Vis. Sci. 2018, 59, 4542–4551. [Google Scholar] [CrossRef]
- Huang, W.; Hu, F.; Wang, M.; Gao, F.; Xu, P.; Xing, C.; Sun, X.; Zhang, S.; Wu, J. Comparative analysis of retinal ganglion cell damage in three glaucomatous rat models. Exp. Eye Res. 2018, 172, 112–122. [Google Scholar] [CrossRef]
- Aslan, M.; Cort, A.; Yucel, I. Oxidative and nitrative stress markers in glaucoma. Free. Radic. Biol. Med. 2008, 45, 367–376. [Google Scholar] [CrossRef]
- Giannaccini, M.; Usai, A.; Chiellini, F.; Guadagni, V.; Andreazzoli, M.; Ori, M.; Pasqualetti, M.; Dente, L.; Raffa, V. Neurotrophin-conjugated nanoparticles prevent retina damage induced by oxidative stress. Cell. Mol. Life Sci. 2018, 75, 1255–1267. [Google Scholar] [CrossRef]
- Sherpa, T.; Fimbel, S.M.; Mallory, D.E.; Maaswinkel, H.; Spritzer, S.D.; Sand, J.A.; Li, L.; Hyde, D.R.; Stenkamp, D.L. Ganglion cell regeneration following whole-retina destruction in zebrafish. Dev. Neurobiol. 2007, 68, 166–181. [Google Scholar] [CrossRef] [PubMed]
- Bonet-Fernández, J.-M.; Aroca-Aguilar, J.-D.; Corton, M.; Ramírez, A.-I.; Alexandre-Moreno, S.; García-Antón, M.-T.; Salazar, J.-J.; Ferre-Fernández, J.-J.; Atienzar-Aroca, R.; Villaverde, C.; et al. CPAMD8 loss-of-function underlies non-dominant congenital glaucoma with variable anterior segment dysgenesis and abnormal extracellular matrix. Hum Genet. 2020, 139, 1209–1231. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.L.; Eason, J.; Chawla, B.; Bohnsack, B.L. Cyp1b1 Regulates Ocular Fissure Closure through a Retinoic Acid–Independent Pathway. Investig. Opthalmol. Vis. Sci. 2017, 58, 1084–1097. [Google Scholar] [CrossRef]
- Skarie, J.M.; Link, B.A. FoxC1Is Essential for Vascular Basement Membrane Integrity and Hyaloid Vessel Morphogenesis. Investig. Opthalmol. Vis. Sci. 2009, 50, 5026–5034. [Google Scholar] [CrossRef]
- Ferre-Fernández, J.-J.; Aroca-Aguilar, J.-D.; Medina-Trillo, C.; Bonet-Fernández, J.-M.; Méndez-Hernández, C.-D.; Morales-Fernández, L.; Corton, M.; Cabañero-Valera, M.-J.; Gut, M.; Tonda, R.; et al. Whole-Exome Sequencing of Congenital Glaucoma Patients Reveals Hypermorphic Variants in GPATCH3, a New Gene Involved in Ocular and Craniofacial Development. Sci. Rep. 2017, 7, 46175. [Google Scholar] [CrossRef] [PubMed]
- Morales-Cámara, S.; Alexandre-Moreno, S.; Bonet-Fernández, J.-M.; Atienzar-Aroca, R.; Aroca-Aguilar, J.-D.; Ferre-Fernández, J.-J.; Méndez, C.-D.; Morales, L.; Fernández-Sánchez, L.; Cuenca, N.; et al. Role of GUCA1C in Primary Congenital Glaucoma and in the Retina: Functional Evaluation in Zebrafish. Genes 2020, 11, 550. [Google Scholar] [CrossRef] [PubMed]
- Luo, N.; West, C.C.; Murga-Zamalloa, C.A.; Sun, L.; Anderson, R.M.; Wells, C.D.; Weinreb, R.N.; Travers, J.; Khanna, H.; Sun, Y. OCRL localizes to the primary cilium: A new role for cilia in Lowe syndrome. Hum. Mol. Genet. 2012, 21, 3333–3344. [Google Scholar] [CrossRef]
- Hendee, K.E.; Sorokina, E.A.; Muheisen, S.S.; Reis, L.M.; Tyler, R.C.; Markovic, V.; Cuturilo, G.; Link, B.A.; Semina, E.V. PITX2 deficiency and associated human disease: Insights from the zebrafish model. Hum. Mol. Genet. 2018, 27, 1675–1695. [Google Scholar] [CrossRef] [PubMed]
- Lahola-Chomiak, A.A.; Footz, T.; Nguyen-Phuoc, K.; Neil, G.J.; Fan, B.; Allen, K.F.; Greenfield, D.S.; Parrish, R.K.; Linkroum, K.; Pasquale, L.R.; et al. Non-Synonymous variants in premelanosome protein (PMEL) cause ocular pigment dispersion and pigmentary glaucoma. Hum. Mol. Genet. 2018, 28, 1298–1311. [Google Scholar] [CrossRef]
- Protas, M.E.; Weh, E.; Footz, T.; Kasberger, J.; Baraban, S.C.; Levin, A.V.; Katz, L.J.; Ritch, R.; Walter, M.A.; Semina, E.V.; et al. Mutations of conserved non-coding elements of PITX2 in patients with ocular dysgenesis and developmental glaucoma. Hum. Mol. Genet. 2017, 26, 3630–3638. [Google Scholar] [CrossRef]
- Gallenberger, M.; Meinel, D.; Kroeber, M.; Wegner, M.; Milkereit, P.; Bösl, M.R.; Tamm, E.R. Lack of WDR36 leads to preimplantation embryonic lethality in mice and delays the formation of small subunit ribosomal RNA in human cells in vitro. Hum. Mol. Genet. 2010, 20, 422–435. [Google Scholar] [CrossRef]
- Heckler, K.; Kroll, J. Zebrafish as a Model for the Study of Microvascular Complications of Diabetes and Their Mechanisms. Int. J. Mol. Sci. 2017, 18, 2002. [Google Scholar] [CrossRef] [PubMed]
- Gleeson, M.; Connaughton, V.; Arneson, L.S. Induction of hyperglycaemia in zebrafish (Danio rerio) leads to morphological changes in the retina. Acta Diabetol. 2007, 44, 157–163. [Google Scholar] [CrossRef]
- Alvarez, Y.; Chen, K.; Reynolds, A.; Waghorne, N.; O’Connor, J.; Kennedy, B. Predominant cone photoreceptor dysfunction in a hyperglycaemic model of non-proliferative diabetic retinopathy. Dis. Model. Mech. 2010, 3, 236–245. [Google Scholar] [CrossRef]
- Tanvir, Z.; Nelson, R.F.; DeCicco-Skinner, K.; Connaughton, V.P. One month of hyperglycemia alters spectral responses of the zebrafish photopic electroretinogram. Dis. Model. Mech. 2018, 11. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.-H.; Kim, Y.S.; Lee, Y.-R.; Kim, J.S. High glucose-induced changes in hyaloid-retinal vessels during early ocular development of zebrafish: A short-term animal model of diabetic retinopathy. Br. J. Pharmacol. 2015, 173, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Ali, Z.; Zang, J.; Lagali, N.; Schmitner, N.; Salvenmoser, W.; Mukwaya, A.; Neuhauss, S.C.; Jensen, L.D.; Kimmel, R.A. Photoreceptor Degeneration Accompanies Vascular Changes in a Zebrafish Model of Diabetic Retinopathy. Investig. Opthalmol. Vis. Sci. 2020, 61, 43. [Google Scholar] [CrossRef]
- Salehpour, A.; Rezaei, M.; Khoradmehr, A.; Tahamtani, Y.; Tamadon, A. Which Hyperglycemic Model of Zebrafish (Danio rerio) Suites My Type 2 Diabetes Mellitus Research? A Scoring System for Available Methods. Front. Cell Dev. Biol. 2021, 9. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Yang, J. Development of a zebrafish screening model for diabetic retinopathy induced by hyperglycemia: Reproducibility verification in animal model. Biomed. Pharmacother. 2021, 135, 111201. [Google Scholar] [CrossRef] [PubMed]
- Sen, P.; Wu, W.-C.; Chandra, P.; Vinekar, A.; Manchegowda, P.T.; Bhende, P. Retinopathy of prematurity treatment: Asian perspectives. Eye 2020, 34, 632–642. [Google Scholar] [CrossRef]
- Wu, Y.-C.; Chang, C.-Y.; Kao, A.; Hsi, B.; Lee, S.-H.; Chen, Y.-H.; Wang, I.-J. Hypoxia-Induced Retinal Neovascularization in Zebrafish Embryos: A Potential Model of Retinopathy of Prematurity. PLoS ONE 2015, 10, e0126750. [Google Scholar] [CrossRef]
- Cao, Z.; Jensen, L.D.; Rouhi, P.; Hosaka, K.; Länne, T.; Steffensen, J.F.; Wahlberg, E.; Cao, Y. Hypoxia-induced retinopathy model in adult zebrafish. Nat. Protoc. 2010, 5, 1903–1910. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, P.; Liew, G.; Gopinath, B.; Wong, T.Y. Age-related macular degeneration. Lancet 2018, 392, 1147–1159. [Google Scholar] [CrossRef]
- van Rooijen, E.; Voest, E.E.; Logister, I.; Bussmann, J.; Korving, J.; van Eeden, F.J.; Giles, R.H.; Schulte-Merker, S. von Hippel-Lindau tumor suppressor mutants faithfully model pathological hypoxia-driven angiogenesis and vascular retinopathies in zebrafish. Dis. Model. Mech. 2010, 3, 343–353. [Google Scholar] [CrossRef]
- Biehlmaier, O.; Neuhauss, S.C.F.; Kohler, K. Double cone dystrophy and RPE degeneration in the retina of the zebrafish gnn mutant. Investig. Opthalmol. Vis. Sci. 2003, 44, 1287–1298. [Google Scholar] [CrossRef] [PubMed]
- Oura, Y.; Nakamura, M.; Takigawa, T.; Fukushima, Y.; Wakabayashi, T.; Tsujikawa, M.; Nishida, K. High-Temperature Requirement A 1 Causes Photoreceptor Cell Death in Zebrafish Disease Models. Am. J. Pathol. 2018, 188, 2729–2744. [Google Scholar] [CrossRef] [PubMed]
- Noel, N.C.L.; Nadolski, N.J.; Hocking, J.C.; Macdonald, I.M.; Allison, W.T. Progressive Photoreceptor Dysfunction and Age-Related Macular Degeneration-Like Features in rp1l1 Mutant Zebrafish. Cells 2020, 9, 2214. [Google Scholar] [CrossRef]
- Bujakowska, K.; Liu, Q.; Pierce, E.A. Photoreceptor Cilia and Retinal Ciliopathies. Cold Spring Harb. Perspect. Biol. 2017, 9, a028274. [Google Scholar] [CrossRef]
- Nakao, T.; Tsujikawa, M.; Notomi, S.; Ikeda, Y.; Nishida, K. The Role of Mislocalized Phototransduction in Photoreceptor Cell Death of Retinitis Pigmentosa. PLoS ONE 2012, 7, e32472. [Google Scholar] [CrossRef]
- Zelinka, C.P.; Sotolongo-Lopez, M.; Fadool, J.M. Targeted disruption of the endogenous zebrafish rhodopsin locus as models of rapid rod photoreceptor degeneration. Mol. Vis. 2018, 24, 587–602. [Google Scholar] [PubMed]
- Eroglu, A.U.; Mulligan, T.S.; Zhang, L.; White, D.T.; Sengupta, S.; Nie, C.; Lu, N.Y.; Qian, J.; Xu, L.; Pei, W.; et al. Multiplexed CRISPR/Cas9 Targeting of Genes Implicated in Retinal Regeneration and Degeneration. Front. Cell Dev. Biol. 2018, 6, 88. [Google Scholar] [CrossRef] [PubMed]
- Lyraki, R.; Megaw, R.; Hurd, T. Disease mechanisms of X-linked retinitis pigmentosa due to RP2 and RPGR mutations. Biochem. Soc. Trans. 2016, 44, 1235–1244. [Google Scholar] [CrossRef] [PubMed]
- Shu, X.; Zeng, Z.; Gautier, P.; Lennon, A.; Gakovic, M.; Cheetham, M.; Patton, E.E.; Wright, A.F. Knockdown of the Zebrafish Ortholog of the Retinitis Pigmentosa 2 (RP2) Gene Results in Retinal Degeneration. Investig. Opthalmol. Vis. Sci. 2011, 52, 2960–2966. [Google Scholar] [CrossRef]
- Liu, F.; Chen, J.; Yu, S.; Raghupathy, R.K.; Liu, X.; Qin, Y.; Li, C.; Huang, M.; Liao, S.; Wang, J.; et al. Knockout ofRP2decreases GRK1 and rod transducin subunits and leads to photoreceptor degeneration in zebrafish. Hum. Mol. Genet. 2015, 24, 4648–4659. [Google Scholar] [CrossRef][Green Version]
- Shu, X.; Zeng, Z.; Gautier, P.; Lennon, A.; Gakovic, M.; Patton, E.E.; Wright, A.F. Zebrafish Rpgr is required for normal retinal development and plays a role in dynein-based retrograde transport processes. Hum. Mol. Genet. 2009, 19, 657–670. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, C.; Shen, Y.; Chen, N.; Wang, L.; Liang, L.; Guo, T.; Yin, X.; Ma, Z.; Zhang, B.; et al. A mutation in ADIPOR1 causes nonsyndromic autosomal dominant retinitis pigmentosa. Hum. Genet. 2016, 135, 1375–1387. [Google Scholar] [CrossRef]
- Yu, S.; Li, C.; Biswas, L.; Hu, X.; Liu, F.; Reilly, J.; Liu, X.; Liu, Y.; Huang, Y.; Lu, Z.; et al. CERKL gene knockout disturbs photoreceptor outer segment phagocytosis and causes rod-cone dystrophy in zebrafish. Hum. Mol. Genet. 2017, 26, 2335–2345. [Google Scholar] [CrossRef]
- Yi, Z.; Ouyang, J.; Sun, W.; Li, S.; Xiao, X.; Zhang, Q. Comparative exome sequencing reveals novel candidate genes for retinitis pigmentosa. EBioMedicine 2020, 56, 102792. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Hu, X.; Liu, F.; Soares, D.; Liu, X.; Yu, S.; Gao, M.; Han, S.; Qin, Y.; Li, C.; et al. Ablation of EYS in zebrafish causes mislocalisation of outer segment proteins, F-actin disruption and cone-rod dystrophy. Sci. Rep. 2017, 7, srep46098. [Google Scholar] [CrossRef]
- Coomer, C.E.; Wilson, S.G.; Titialii-Torres, K.F.; Bills, J.D.; Krueger, L.A.; Petersen, R.A.; Turnbaugh, E.M.; Janesch, E.L.; Morris, A.C. Her9/Hes4 is required for retinal photoreceptor development, maintenance, and survival. Sci. Rep. 2020, 10, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Feng, D.; Chen, Z.; Yang, K.; Miao, S.; Xu, B.; Kang, Y.; Xie, H.; Zhao, C. The cytoplasmic tail of rhodopsin triggers rapid rod degeneration in kinesin-2 mutants. J. Biol. Chem. 2017, 292, 17375–17386. [Google Scholar] [CrossRef] [PubMed]
- Wasfy, M.M.; Matsui, J.I.; Miller, J.; Dowling, J.E.; Perkins, B.D. myosin 7aa−/−mutant zebrafish show mild photoreceptor degeneration and reduced electroretinographic responses. Exp. Eye Res. 2014, 122, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Hubshman, M.W.; Broekman, S.; Van Wijk, E.; Cremers, F.; Abu-Diab, A.; Khateb, S.; Tzur, S.; Lagovsky, I.; Smirin-Yosef, P.; Sharon, D.; et al. Whole-exome sequencing reveals POC5 as a novel gene associated with autosomal recessive retinitis pigmentosa. Hum. Mol. Genet. 2017, 27, 614–624. [Google Scholar] [CrossRef]
- Lu, Z.; Hu, X.; Reilly, J.; Jia, D.; Liu, F.; Yu, S.; Liu, X.; Xie, S.; Qu, Z.; Qin, Y.; et al. Deletion of the transmembrane protein Prom1b in zebrafish disrupts outer-segment morphogenesis and causes photoreceptor degeneration. J. Biol. Chem. 2019, 294, 13953–13963. [Google Scholar] [CrossRef]
- Li, J.; Liu, F.; Lv, Y.; Sun, K.; Zhao, Y.; Reilly, J.; Zhang, Y.; Tu, J.; Yu, S.; Liu, X.; et al. Prpf31 is essential for the survival and differentiation of retinal progenitor cells by modulating alternative splicing. Nucleic Acids Res. 2021, 49, 2027–2043. [Google Scholar] [CrossRef]
- Zhuang, Y.-Y.; Xiang, L.; Wen, X.-R.; Shen, R.-J.; Zhao, N.; Zheng, S.-S.; Han, R.-Y.; Qu, J.; Lu, F.; Jin, Z.-B. Slc7a14 Is Indispensable in Zebrafish Retinas. Front. Cell Dev. Biol. 2019, 7, 333. [Google Scholar] [CrossRef]
- Zhang, T.; Bai, J.; Zhang, X.; Zheng, X.; Lu, N.; Liang, Z.; Lin, L.; Chen, Y. SNRNP200 Mutations Cause Autosomal Dominant Retinitis Pigmentosa. Front. Med. 2021, 7. [Google Scholar] [CrossRef]
- Han, S.; Liu, X.; Xie, S.; Gao, M.; Liu, F.; Yu, S.; Sun, P.; Wang, C.; Archacki, S.; Lu, Z.; et al. Knockout of ush2a gene in zebrafish causes hearing impairment and late onset rod-cone dystrophy. Hum. Genet. 2018, 137, 779–794. [Google Scholar] [CrossRef] [PubMed]
- Kondkar, A.A.; Abu-Amero, K.K. Leber congenital amaurosis: Current genetic basis, scope for genetic testing and personalized medicine. Exp. Eye Res. 2019, 189, 107834. [Google Scholar] [CrossRef]
- Baye, L.M.; Patrinostro, X.; Swaminathan, S.; Beck, J.S.; Zhang, Y.; Stone, E.M.; Sheffield, V.C.; Slusarski, D.C. The N-terminal region of centrosomal protein 290 (CEP290) restores vision in a zebrafish model of human blindness. Hum. Mol. Genet. 2011, 20, 1467–1477. [Google Scholar] [CrossRef] [PubMed]
- Qu, Z.; Yimer, T.A.; Xie, S.; Wong, F.; Yu, S.; Liu, X.; Han, S.; Ma, J.; Lu, Z.; Hu, X.; et al. Knocking out lca5 in zebrafish causes cone-rod dystrophy due to impaired outer segment protein trafficking. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 2694–2705. [Google Scholar] [CrossRef] [PubMed]
- Kumaran, N.; Moore, A.T.; Weleber, R.G.; Michaelides, M. Leber congenital amaurosis/early-onset severe retinal dystrophy: Clinical features, molecular genetics and therapeutic interventions. Br. J. Ophthalmol. 2017, 101, 1147–1154. [Google Scholar] [CrossRef] [PubMed]
- Krock, B.L.; Perkins, B.D. The intraflagellar transport protein IFT57 is required for cilia maintenance and regulates IFT-particle–kinesin-II dissociation in vertebrate photoreceptors. J. Cell Sci. 2008, 121, 1907–1915. [Google Scholar] [CrossRef] [PubMed]
- Sukumaran, S.; Perkins, B.D. Early defects in photoreceptor outer segment morphogenesis in zebrafish ift57, ift88 and ift172 Intraflagellar Transport mutants. Vis. Res. 2009, 49, 479–489. [Google Scholar] [CrossRef]
- Lee, C.; Wallingford, J.B.; Gross, J.M. Cluap1 is essential for ciliogenesis and photoreceptor maintenance in the vertebrate eye. Invest. Ophthalmol. Vis. Sci. 2014, 55, 4585–4592. [Google Scholar] [CrossRef]
- Pooranachandran, N.; Malicki, J.J. Unexpected Roles for Ciliary Kinesins and Intraflagellar Transport Proteins. Genetics 2016, 203, 771–785. [Google Scholar] [CrossRef] [PubMed]
- Raghupathy, R.K.; Zhang, X.; Alhasani, R.H.; Zhou, X.; Mullin, M.; Reilly, J.; Li, W.-C.; Liu, M.; Shu, X. Abnormal photoreceptor outer segment development and early retinal degeneration inkif3amutant zebrafish. Cell Biochem. Funct. 2016, 34, 429–440. [Google Scholar] [CrossRef]
- Das, B.C.; McCormick, L.; Thapa, P.; Karki, R.; Evans, T. Use of zebrafish in chemical biology and drug discovery. Future Med. Chem. 2013, 5, 2103–2116. [Google Scholar] [CrossRef]
- Kitambi, S.S.; McCulloch, K.J.; Peterson, R.T.; Malicki, J.J. Small molecule screen for compounds that affect vascular development in the zebrafish retina. Mech. Dev. 2009, 126, 464–477. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, Y.; Astudillo, O.; Jensen, L.; Reynolds, A.L.; Waghorne, N.; Brazil, D.; Cao, Y.; O’Connor, J.J.; Kennedy, B.N. Selective Inhibition of Retinal Angiogenesis by Targeting PI3 Kinase. PLoS ONE 2009, 4, e7867. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhu, M.; Wu, W.; Qin, B.; Gu, J.; Tu, Y.; Chen, J.; Liu, D.; Shi, Y.; Liu, X.; et al. Brivanib, a multitargeted small-molecule tyrosine kinase inhibitor, suppresses laser-induced CNV in a mouse model of neovascular AMD. J. Cell. Physiol. 2020, 235, 1259–1273. [Google Scholar] [CrossRef]
- Arjamaa, O.; Nikinmaa, M. Oxygen-dependent diseases in the retina: Role of hypoxia-inducible factors. Exp. Eye Res. 2006, 83, 473–483. [Google Scholar] [CrossRef]
- Ohnesorge, N.; Sasore, T.; Hillary, D.; Alvarez, Y.; Carey, M.; Kennedy, B.N. Orthogonal Drug Pooling Enhances Phenotype-Based Discovery of Ocular Antiangiogenic Drugs in Zebrafish Larvae. Front. Pharmacol. 2019, 10, 508. [Google Scholar] [CrossRef]
- Rihel, J.; Prober, D.A.; Arvanites, A.; Lam, K.; Zimmerman, S.; Jang, S.; Haggarty, S.J.; Kokel, D.; Rubin, L.; Peterson, R.T.; et al. Zebrafish Behavioral Profiling Links Drugs to Biological Targets and Rest/Wake Regulation. Science 2010, 327, 348–351. [Google Scholar] [CrossRef] [PubMed]
- Kokel, D.; Bryan, J.; Laggner, C.; White, R.; Cheung, C.Y.J.; Mateus, R.; Healey, D.; Kim, S.; Werdich, A.A.; Haggarty, S.J.; et al. Rapid behavior-based identification of neuroactive small molecules in the zebrafish. Nat. Chem. Biol. 2010, 6, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Ganzen, L.; Venkatraman, P.; Pang, C.P.; Leung, Y.F.; Zhang, M. Utilizing Zebrafish Visual Behaviors in Drug Screening for Retinal Degeneration. Int. J. Mol. Sci. 2017, 18, 1185. [Google Scholar] [CrossRef] [PubMed]
- Ganzen, L.; Ko, M.J.; Zhang, M.; Xie, R.; Chen, Y.; Zhang, L.; James, R.; Mumm, J.; van Rijn, R.M.; Zhong, W.; et al. Drug screening with zebrafish visual behavior identifies carvedilol as a potential treatment for an autosomal dominant form of retinitis pigmentosa. Sci. Rep. 2021, 11, 1–14. [Google Scholar] [CrossRef]
- Zhang, L.; Xiang, L.; Liu, Y.; Venkatraman, P.; Chong, L.; Cho, J.; Bonilla, S.; Jin, Z.-B.; Pang, C.P.; Ko, K.M.; et al. A Naturally-Derived Compound Schisandrin B Enhanced Light Sensation in the pde6c Zebrafish Model of Retinal Degeneration. PLoS ONE 2016, 11, e0149663. [Google Scholar] [CrossRef][Green Version]
- Sancho-Pelluz, J.; Alavi, M.; Sahaboglu, A.; Kustermann, S.; Farinelli, P.; Azadi, S.; Van Veen, T.; Romero, F.J.; Paquet-Durand, F.; Ekström, P. Excessive HDAC activation is critical for neurodegeneration in the rd1 mouse. Cell Death Dis. 2010, 1, e24. [Google Scholar] [CrossRef] [PubMed]
- Kawase, R.; Nishimura, Y.; Ashikawa, Y.; Sasagawa, S.; Murakami, S.; Yuge, M.; Okabe, S.; Kawaguchi, K.; Yamamoto, H.; Moriyuki, K.; et al. EP300 Protects from Light-Induced Retinopathy in Zebrafish. Front. Pharmacol. 2016, 7. [Google Scholar] [CrossRef]
- Daly, C.; Shine, L.; Heffernan, T.; Deeti, S.; Reynolds, A.L.; O’Connor, J.J.; Dillon, E.T.; Duffy, D.J.; Kolch, W.; Cagney, G.; et al. A Brain-Derived Neurotrophic Factor Mimetic Is Sufficient to Restore Cone Photoreceptor Visual Function in an Inherited Blindness Model. Sci. Rep. 2017, 7, 11320. [Google Scholar] [CrossRef] [PubMed]
- Leyk, J.; Daly, C.; Janssen-Bienhold, U.; Kennedy, B.; Richter-Landsberg, C. HDAC6 inhibition by tubastatin A is protective against oxidative stress in a photoreceptor cell line and restores visual function in a zebrafish model of inherited blindness. Cell Death Dis. 2017, 8, e3028. [Google Scholar] [CrossRef] [PubMed]
- Sundaramurthi, H.; Roche, S.L.; Grice, G.L.; Moran, A.; Dillion, E.T.; Campiani, G.; Nathan, J.A.; Kennedy, B.N. Selective Histone Deacetylase 6 Inhibitors Restore Cone Photoreceptor Vision or Outer Segment Morphology in Zebrafish and Mouse Models of Retinal Blindness. Front. Cell Dev. Biol. 2020, 8. [Google Scholar] [CrossRef]
- Cassar, S.; Adatto, I.; Freeman, J.; Gamse, J.T.; Iturria, I.; Lawrence, C.; Muriana, A.; Peterson, R.T.; Van Cruchten, S.; Zon, L.I. Use of Zebrafish in Drug Discovery Toxicology. Chem. Res. Toxicol. 2020, 33, 95–118. [Google Scholar] [CrossRef]
- Deeti, S.; O’Farrell, S.; Kennedy, B.N. Early safety assessment of human oculotoxic drugs using the zebrafish visualmotor response. J. Pharmacol. Toxicol. Methods 2014, 69, 1–8. [Google Scholar] [CrossRef]
- Sadamoto, K.; Yamagiwa, Y.; Sakaki, H.; Kurata, M. Absence of histopathological changes in the retina of zebrafish treated with sodium iodate. J. Veter. Med. Sci. 2018, 80, 901–908. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.-J.; Huang, G.-Y.; Jiang, Y.-X.; Ma, D.-D.; Chen, H.-X.; Huang, M.-Z.; Hou, L.-P.; Xie, L.; Ying, G.-G. Medroxyprogesterone acetate affects eye growth and the transcription of associated genes in zebrafish. Ecotoxicol. Environ. Saf. 2020, 193, 110371. [Google Scholar] [CrossRef]
- Hoshijima, K.; Jurynec, M.J.; Shaw, D.K.; Jacobi, A.M.; Behlke, M.A.; Grunwald, D.J. Highly Efficient CRISPR-Cas9-Based Methods for Generating Deletion Mutations and F0 Embryos that Lack Gene Function in Zebrafish. Dev. Cell 2019, 51, 645–657.e4. [Google Scholar] [CrossRef] [PubMed]

| Function | Mutant Gene | Ocular Phenotype | Reference |
|---|---|---|---|
| encoding crystallins | CRYAA(αA-crystallin) | crystal-like opacity sporadically spreading across the lens, or frequent droplets covering a large fraction of the lens | [54] |
| CRYAB(αB-crystallin) | same as CRYAA | [41] | |
| CRYGC(γC-crystalline) | same as CRYAA | [55] | |
| CRYGD(γD-crystallin) | same as CRYAA | [56] | |
| encoding developmental factors | DNase1l1l | retaining nuclei in lens fiber cells | [57] |
| epha2 | smaller eye, lens opacification and coloboma | [58] | |
| mab21l2 | microphthalmia, colobomas, small and disorganized lenses, cornea dysgenesis | [33] | |
| plod3 | distorted and dislocated lenses from an early stage dislocated, lack of normal lens capsule | [59] | |
| rbm24 | coarse and irregular lens, small-size retina and lens | [60] | |
| encoding membrane proteins | aqp0a | nuclear opacity and widespread cortical fiber-to-fiber membrane stacking defects | [61] |
| gja8 | various sizes of lens opacity | [62] | |
| kpna4 | rugged and cloudy center part of the lens | [63] | |
| pgrmc1 | minor or mild nuclear central mass with fiber cell disorganization, and moderate or severe nuclear density with pitting | [64] | |
| encoding transcription factors | celf1 | lens defects and cataract | [65] |
| foxe3 | smaller eye and small, deformed or absent lenses | [49] | |
| hsf4 | cataract with overproliferation of the lens epithelial cells and excessive accumulation of fiber cells | [50] | |
| pitx3 | severe lens degeneration, lack of anterior chambers and outer segment structures | [45] |
| Method | Injury Paradigm | Ocular Phenotype | Model | Reference |
|---|---|---|---|---|
| Gene-Targeted | cpamd8 | Iridocorneal angle hypoplasia | POAG | [83] |
| cyp1b1 | Neural crest migration into the anterior segment | POAG | [84] | |
| foxc1 | RGC loss | POAG | [85] | |
| gpatch3 | Anterior chamber angle hypoplasia and a decreased number of iridophores | POAG | [86] | |
| guca1c | RGC apoptosis | POAG | [87] | |
| ocrl | Defective cilia formation in Kupffer vesicles | POAG | [88] | |
| pitx2 | Abnormal development of the cornea, iris, and iridocorneal angle | POAG | [89] | |
| pmel | Profound pigmentation defects and enlarged anterior segments | Pigmentary glaucoma | [90] | |
| six6 | Smaller eyes and reduced number of RGC | POAG | [70] | |
| Tg (Bugeye) | Decreased retinal cell densities and diminished outer retinal function | POAG | [91] | |
| wdr36 | Thinner retinal layers and smaller eyes | POAG | [92] | |
| Chemical- Induced | N-Methyl-D-aspartic acid (NMDA) | RGC loss | Glaucoma | [78] |
| Oxidative Stress-Induced | hydrogen peroxide | RGC injury | Glaucoma | [81] |
| Gene | Photoreceptor Features | Reference |
|---|---|---|
| adipor1 | Decrease in rod photoreceptors | [117] |
| cerkl | Photoreceptor functional defects at 7 dpf. Rod OS defects at 3 months, cone OS defects at 7 months. Notable thinning of the photoreceptor layer and cell death by 12 months | [118] |
| dact2 | Photoreceptor disc membrane disarrangement at 5 dpf | [119] |
| eys | Progressive photoreceptor loss; cone degeneration at 6 months, rod degeneration at 14 months | [120] |
| her9 | Decrease in rod photoreceptors at 5 dpf. Few double cones with short OSs at 12 dpf | [121] |
| kif3b | Delayed OS development. Rapid rod degeneration by 5 dpf | [122] |
| myo7aa | Decreased photoreceptor function at 5dpf. Reduced rods at 8 dpf | [123] |
| poc1 | Decrease length of photoreceptor OSs at 4 dpf | [124] |
| prom1 | Decrease in cone photoreceptors at 7 dpf. Longer rod Oss. Delayed development of OSs | [125] |
| prpf31 | Decreased in neuronal precursors and mature neurons at both 48 and 60 hpf | [126] |
| rho | Rod loss observed at 6 dpf. Degeneration continues into adulthood | [112] |
| rp1l1 | Rod dysfunction at 6 months. Subretinal drusenoid deposits at 11 months. Photoreceptor loss at 12 months | [108] |
| rp2 | Photoreceptor functional defects at 7 dpf. Short rod OSs at 2 months; cone OS defects at 4 months; significant rod OS loss and decreased cone OSs by 7 months | [115] |
| rpgrip1 | No rod OSs at 5 dpf. Cone dysfunction at 7 dpf. Severe rod degeneration by 3 months, followed by cone degeneration. Degeneration of most photoreceptors by 23 months | [116] |
| slc7a14 | Decreased photoreceptor function at 5 dpf. Reduced rod photoreceptors and peripheral RPE at 5 dpf | [127] |
| SNRNP200 | Photoreceptors loss at 3 dpf | [128] |
| ush2a | Decreased photoreceptor function at 5–7 dpf and increased photoreceptor apoptosis at 8 dpf. Notable rod OS degeneration at 12 months, cone OS degeneration at 20 months | [129] |
| Disease Model | Highlights | Drawbacks |
|---|---|---|
| Corneal dystrophy | Able to identify related specific gene mutations | Not suitable for modeling other corneal diseases |
| Cataract | Feasible to study disease mechanisms, especially those involved in crystallins | Unavailable to model ARC |
| Glaucoma | Available to test specific hypotheses associated with glaucoma | Unsuccessful at establishing POCG models |
| Zebrafish bugeye mutant with high IOP | Regenerative capability of retinal neurons, especially RGC cells | |
| Able to induce model of RGC loss | ||
| Vascular disease | Available to identify related genes and mechanisms | Regenerative capability of retinal neurons |
| Transgenic zebrafish lines expressing fluorescent reporter proteins in the vascular system | ||
| The pdx1 mutant zebrafish presenting hyperglycemia-induced retinal angiogenesis | Without ideal model for neovascular AMD | |
| Transgenic overexpression of human HTRA1 zebrafish eye with the features of early AMD | ||
| Feasible to help screen new anti-angiogenic drugs | ||
| Photoreceptor Degeneration | Available to have large array of functional and behavioral tests | Regenerative capability of retinal neurons |
| Able to identify new neuroprotective drugs using large-scale discovery | ||
| Feasible to identify related mutations by genetic screens |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, Y.; Luo, Y. Zebrafish Model in Ophthalmology to Study Disease Mechanism and Drug Discovery. Pharmaceuticals 2021, 14, 716. https://doi.org/10.3390/ph14080716
Hong Y, Luo Y. Zebrafish Model in Ophthalmology to Study Disease Mechanism and Drug Discovery. Pharmaceuticals. 2021; 14(8):716. https://doi.org/10.3390/ph14080716
Chicago/Turabian StyleHong, Yiwen, and Yan Luo. 2021. "Zebrafish Model in Ophthalmology to Study Disease Mechanism and Drug Discovery" Pharmaceuticals 14, no. 8: 716. https://doi.org/10.3390/ph14080716
APA StyleHong, Y., & Luo, Y. (2021). Zebrafish Model in Ophthalmology to Study Disease Mechanism and Drug Discovery. Pharmaceuticals, 14(8), 716. https://doi.org/10.3390/ph14080716





