Usage of Synthetic Peptides in Cosmetics for Sensitive Skin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Collection
2.2. Data Analysis
2.2.1. Peptides Usage Frequency
2.2.2. Top Peptides for Sensitive Skin
2.2.3. Scientific Evidence Supporting the Efficacy in Sensitive Skin Care
3. Results and Discussion
3.1. Top Ingredients for Sensitive Skin
3.2. Scientific Evidence Supporting the Efficacy in Sensitive Skin Care
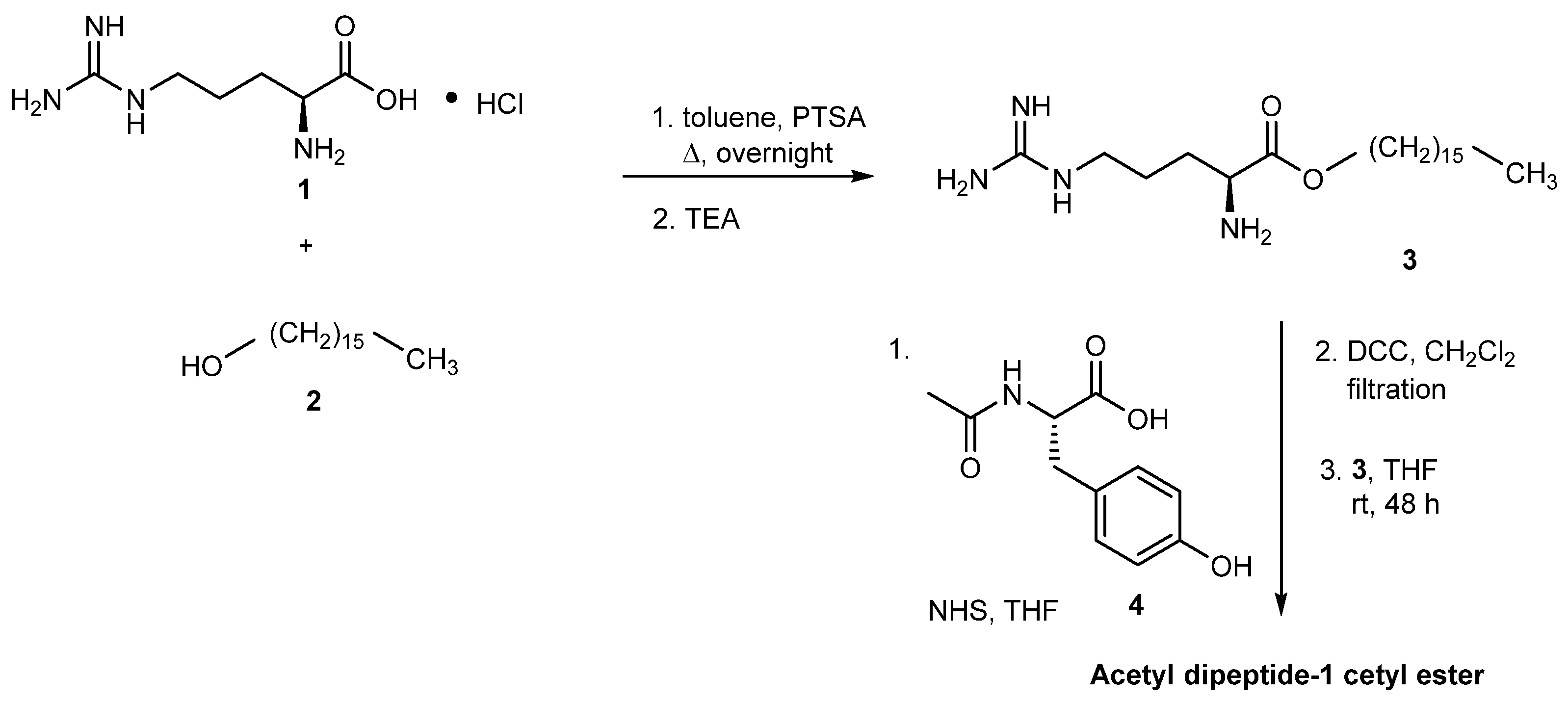
3.2.1. Acetyl Dipeptide-1 Cetyl Ester
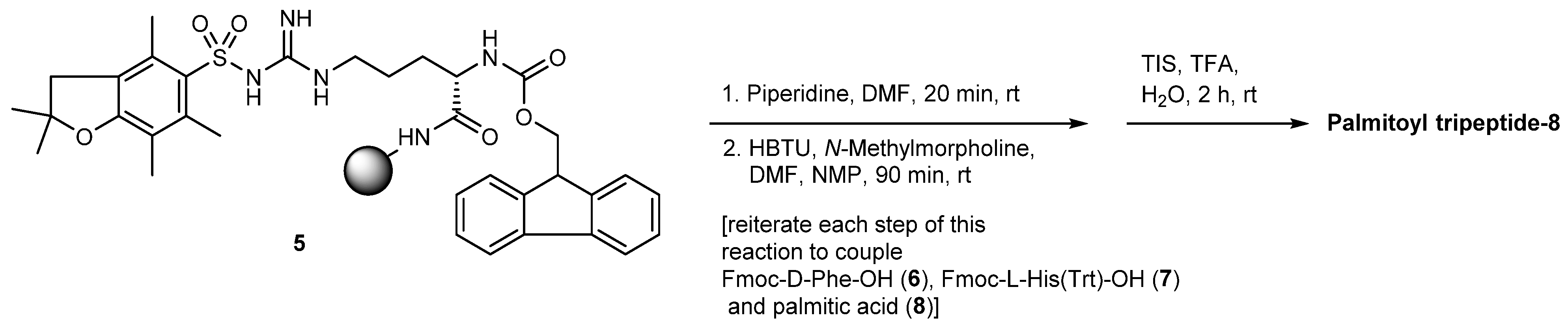
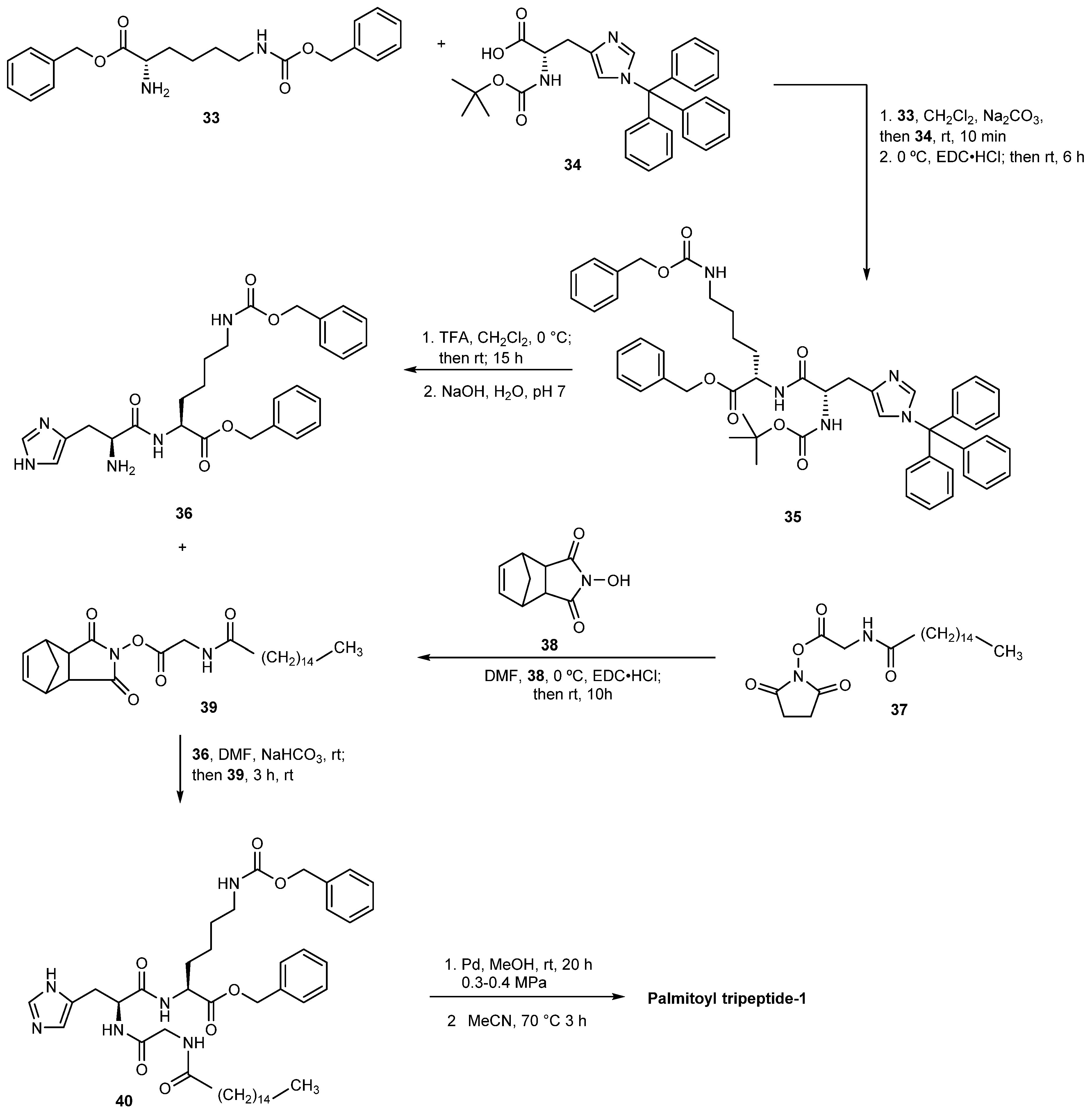
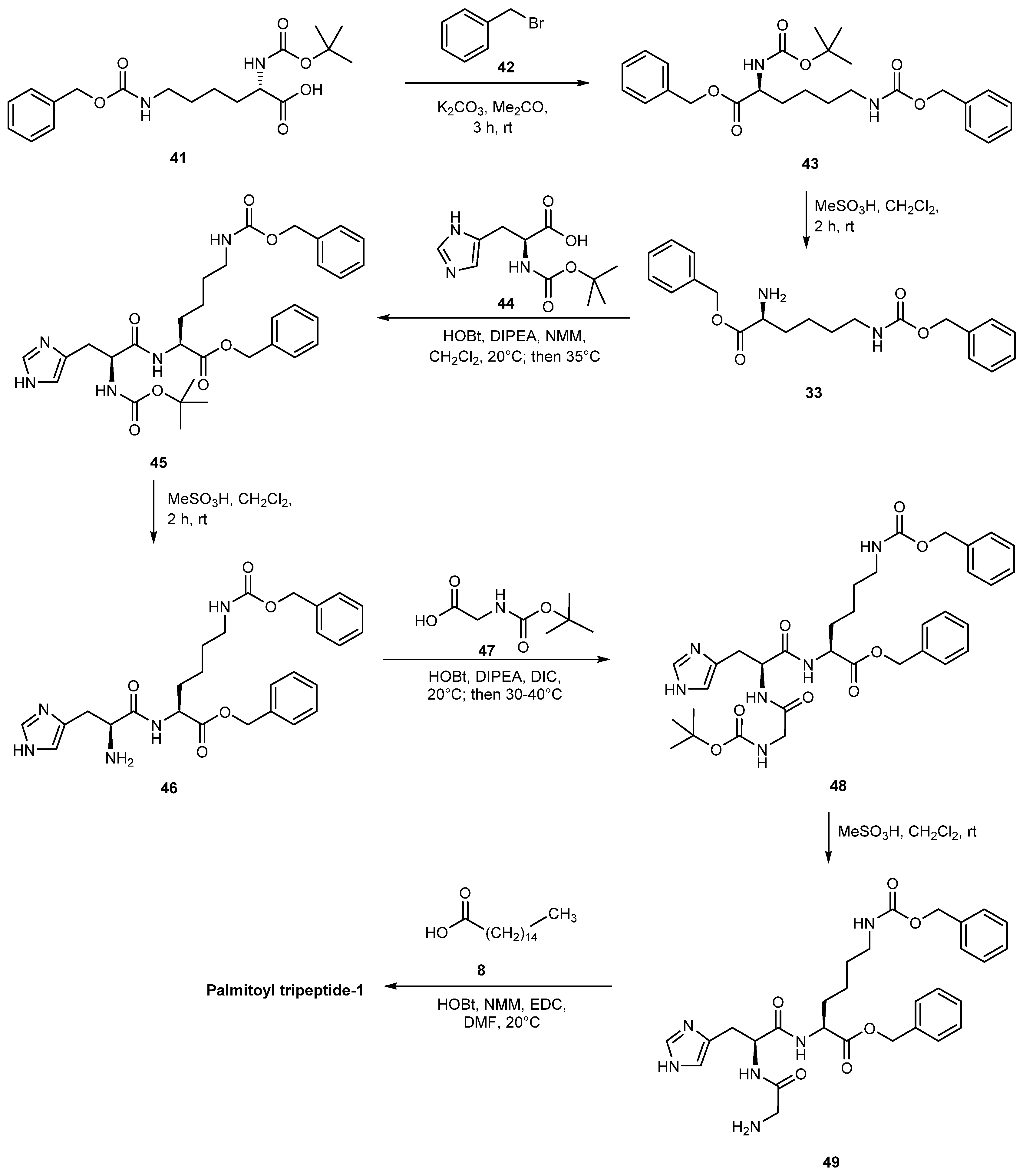
3.2.2. Palmitoyl Tripeptide-8
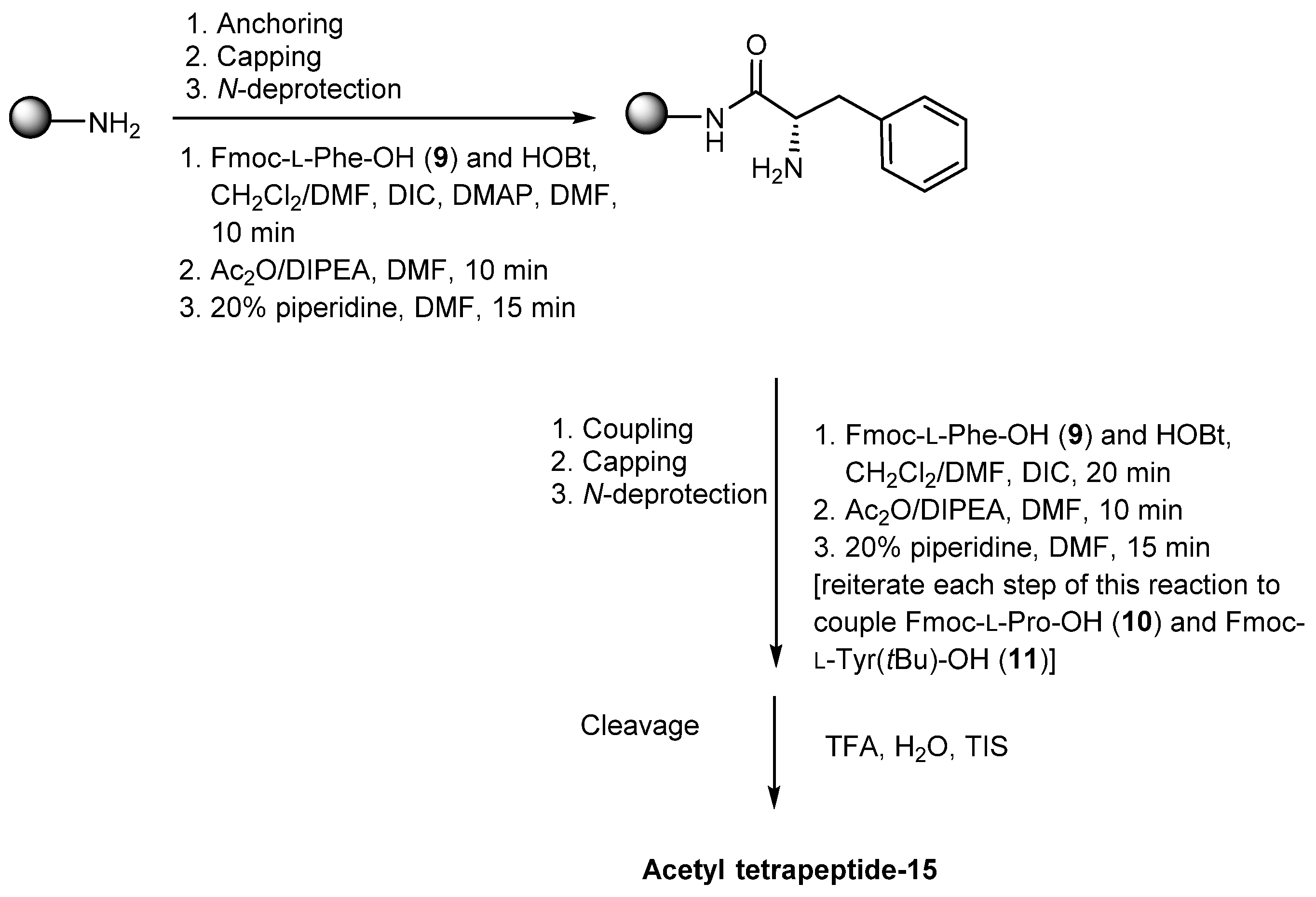
3.2.3. Acetyl Tetrapeptide-15
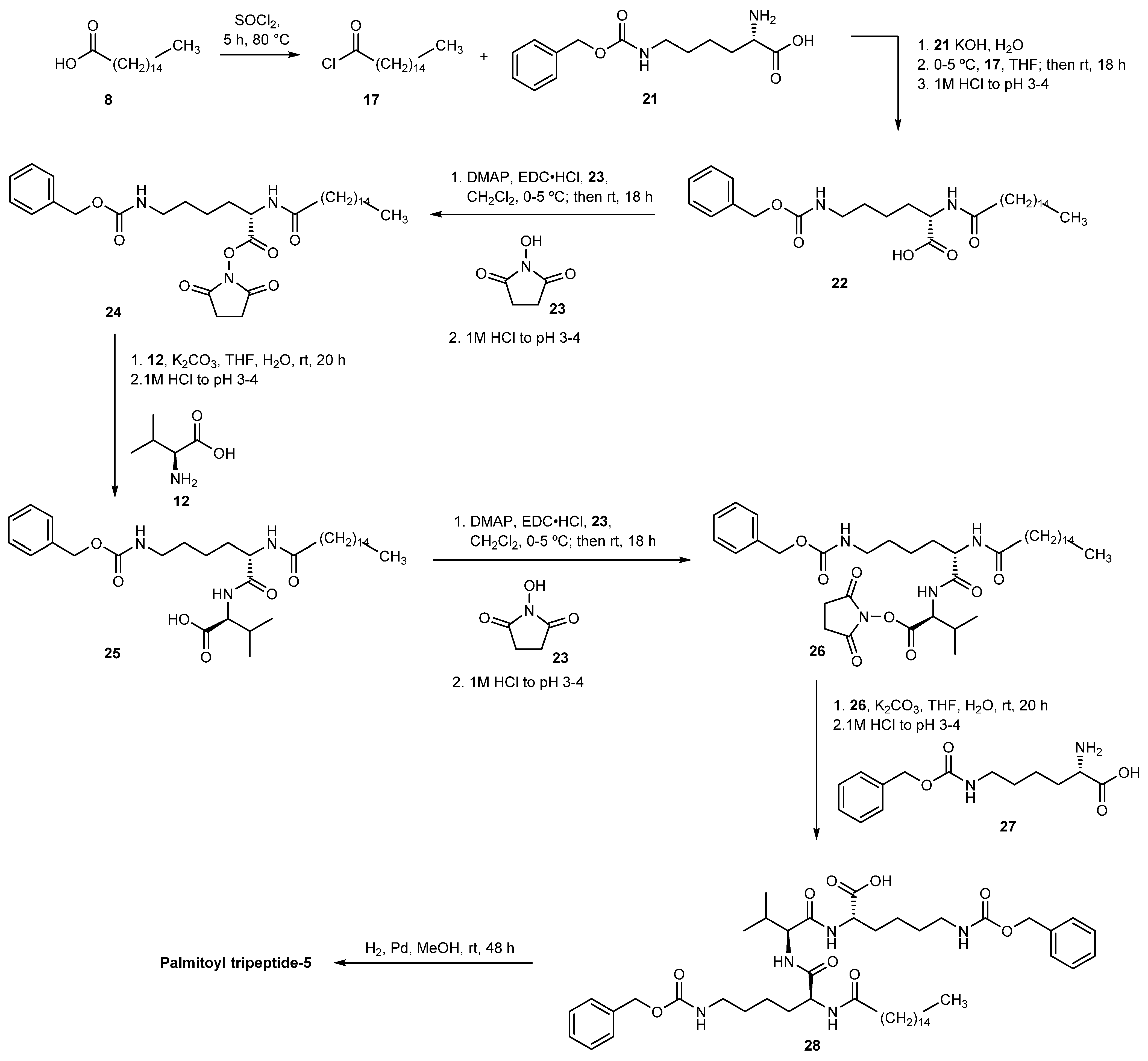
3.2.4. Palmitoyl Tripeptide-5
3.2.5. Acetyl Hexapeptide-49
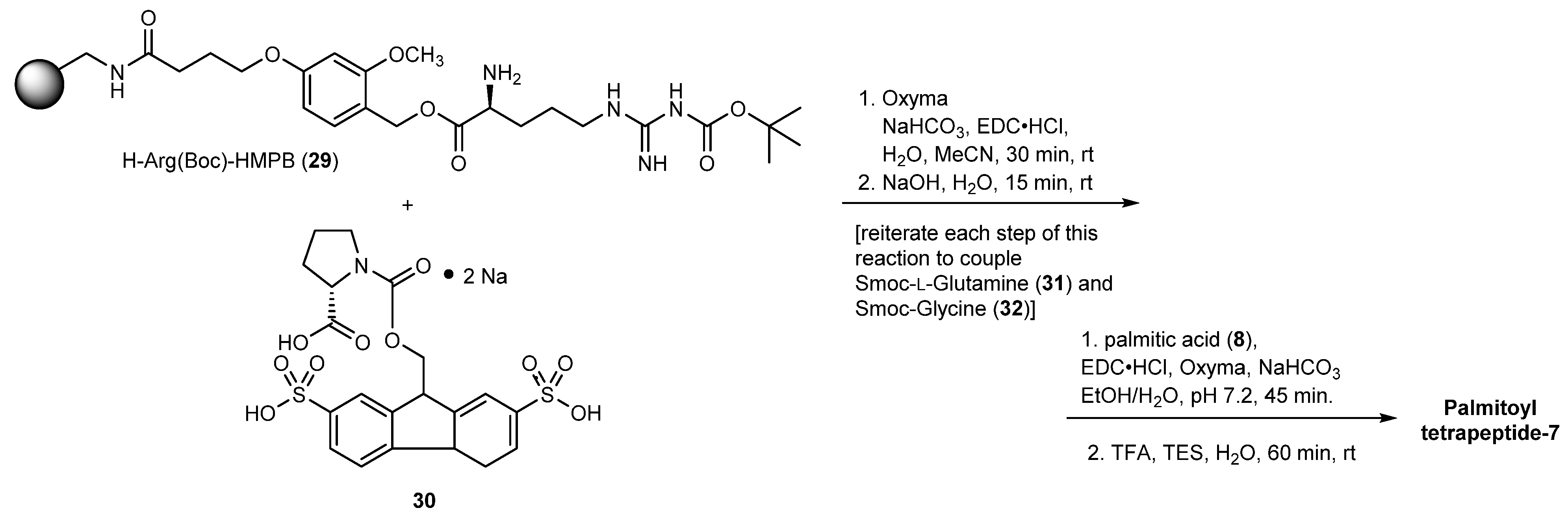
3.2.6. Palmitoyl Tetrapeptide-7
3.2.7. Palmitoyl Oligopeptide
3.2.8. Highlights in the Usage of Synthetic Peptides in Cosmetics for Sensitive Skin
3.3. Applicability of the Described Synthetic Peptides in Pharmeceuticals
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Limitations
Abbreviations and Acronyms
| α-MSH | α-melanocyte stimulating hormone |
| ACTH | adrenocorticotropin |
| ADC | adenylate cyclase |
| AQP3 | aquaporin 3 |
| Boc | tert-Butyloxycarbonyl |
| Bu | Butyl |
| Bz | benzoyl |
| Cbz | benzyloxycarbonyl |
| CLA | conjugated linoleic acid |
| DCC | N,N’-dicyclohexylcarbodiimide |
| DIC | N,N′-diisopropylcarbodiimide |
| DIPEA | N,N-diisopropylethylamineDMAP |
| DMAP | 4-(dimethylamino)pyridine |
| DMF | N,N-dimethylformamide |
| EDC | 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide |
| FLG | filaggrin |
| Fmoc | fluorenylmethyloxycarbonyl |
| h | hours |
| HBTU | N,N,N′,N′-tetramethyl-O-(1H-benzotriazol-1-yl)uronium hexafluorophosphate |
| HOBt | hydroxybenzotriazole |
| INCI | international nomenclature of cosmetic ingredients |
| MMP’s | metalloproteases |
| MSHs | melanocyte-stimulating hormones |
| NF-κβ | nuclear factor κ-β |
| NHS | N-hydroxysuccinimide |
| NMP | N-methyl-2-pyrrolidone |
| Pal | Palmitic acid |
| PAR-2 | proteinase activated receptor 2 |
| PKA | protein kinase A |
| POMC | pro-opiomelanocortin |
| PTSA | p-toluenesulfonic acid |
| RAM | Rink amide |
| rt | room temperature |
| SDS | sodium dodecyl sulfate |
| Smoc | 2,7-disulfo-9-fluorenylmethoxycarbonyl |
| Su | succinimide |
| TEA | triethylamine |
| TFA | trifluoroacetic acid |
| THF | tetrahydrofuran |
| TIS | triisopropylsilane |
| Trt | Trityl |
| TRPV | Transient Receptor Potential Cation Channel Subfamily V |
| TPPA | Transient Receptor Potential Cation Channel Subfamily A |
| TSP-1 | thrombospondin I |
References
- Misery, L. Sensitive skin, reactive skin. Ann. Dermatol. Venereol. 2019, 146, 585–591. [Google Scholar] [CrossRef] [PubMed]
- Berardesca, E.; Farage, M.; Maibach, H. Sensitive skin: An overview. Int. J. Cosmet. Sci. 2013, 35, 2–8. [Google Scholar] [CrossRef]
- Chen, W.; Dai, R.; Li, L. The prevalence of self-declared sensitive skin: A systematic review and meta-analysis. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 1779–1788. [Google Scholar] [CrossRef]
- Farage, M.A. The Prevalence of Sensitive Skin. Front. Med. (Lausanne) 2019, 6, 98. [Google Scholar] [CrossRef]
- Farage, M.A.; Jiang, Y.; Tiesman, J.P.; Fontanillas, P.; Osborne, R. Genome-Wide Association Study Identifies Loci Associated with Sensitive Skin. Cosmetics 2020, 7, 49. [Google Scholar] [CrossRef]
- Verhoeven, E.W.; de Klerk, S.; Kraaimaat, F.W.; van de Kerkhof, P.C.; de Jong, E.M.; Evers, A.W. Biopsychosocial mechanisms of chronic itch in patients with skin diseases: A review. Acta Derm. Venereol. 2008, 88, 211–218. [Google Scholar] [CrossRef]
- Zheng, Y.; Liang, H.; Li, Z.; Tang, M.; Song, L. Skin microbiome in sensitive skin: The decrease of Staphylococcus epidermidis seems to be related to female lactic acid sting test sensitive skin. J. Dermatol. Sci. 2020, 97, 225–228. [Google Scholar] [CrossRef]
- Misery, L.; Weisshaar, E.; Brenaut, E.; Evers, A.W.M.; Huet, F.; Stander, S.; Reich, A.; Berardesca, E.; Serra-Baldrich, E.; Wallengren, J.; et al. Pathophysiology and management of sensitive skin: Position paper from the special interest group on sensitive skin of the International Forum for the Study of Itch (IFSI). J. Eur. Acad. Dermatol. Venereol. 2020, 34, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Misery, L. Neuropsychiatric factors in sensitive skin. Clin. Dermatol. 2017, 35, 281–284. [Google Scholar] [CrossRef]
- Misery, L.; Loser, K.; Stander, S. Sensitive skin. J. Eur. Acad. Dermatol. Venereol. 2016, 30 (Suppl. 1), 2–8. [Google Scholar] [CrossRef]
- Buhe, V.; Vie, K.; Guere, C.; Natalizio, A.; Lheritier, C.; Le Gall-Ianotto, C.; Huet, F.; Talagas, M.; Lebonvallet, N.; Marcorelles, P.; et al. Pathophysiological Study of Sensitive Skin. Acta Derm. Venereol. 2016, 96, 314–318. [Google Scholar] [CrossRef] [Green Version]
- Richters, R.; Falcone, D.; Uzunbajakava, N.; Verkruysse, W.; van Erp, P.; van de Kerkhof, P. What is sensitive skin? A systematic literature review of objective measurements. Skin Pharmacol. Physiol. 2015, 28, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Ferrer-Montiel, A.; Camprubí-Robles, M.; García-Sanz, N.; Sempere, A.; Valente, P.; Nest, W.V.D.; Carreño, C. The contribution of neurogenic inflammation to sensitive skin: Concepts, mechanisms and cosmeceutical intervention. Int. J. Cosmet. Sci. 2009, 11, 311–315. [Google Scholar] [CrossRef]
- Gutte, B. Peptides: Synthesis, Structures, and Applications; Elsevier: Amsterdam, The Netherlands, 1995. [Google Scholar]
- Lintner, K.; Peschard, O. Biologically active peptides: From a laboratory bench curiosity to a functional skin care product. Int. J. Cosmet. Sci. 2000, 22, 207–218. [Google Scholar] [CrossRef] [Green Version]
- Sato, A.K.; Viswanathan, M.; Kent, R.B.; Wood, C.R. Therapeutic peptides: Technological advances driving peptides into development. Curr. Opin. Biotechnol. 2006, 17, 638–642. [Google Scholar] [CrossRef]
- Gorouhi, F.; Maibach, H.I. Role of topical peptides in preventing or treating aged skin. Int. J. Cosmet. Sci. 2009, 31, 327–345. [Google Scholar] [CrossRef]
- Ahsan, H. The biomolecules of beauty: Biochemical pharmacology and immunotoxicology of cosmeceuticals. J. Immunoass. Immunochem. 2019, 40, 91–108. [Google Scholar] [CrossRef]
- Hruby, V.J. Designing peptide receptor agonists and antagonists. Nat. Rev. Drug Discov. 2002, 1, 847–858. [Google Scholar] [CrossRef]
- Kobiela, T.; Milner-Krawczyk, M.; Pasikowska-Piwko, M.; Bobecka-Wesolowska, K.; Eris, I.; Swieszkowski, W.; Dulinska-Molak, I. The Effect of Anti-aging Peptides on Mechanical and Biological Properties of HaCaT Keratinocytes. Int. J. Pept. Res. Ther. 2018, 24, 577–587. [Google Scholar] [CrossRef] [Green Version]
- Schagen, S.K. Topical Peptide Treatments with Effective Anti-Aging Results. Cosmetics 2017, 4, 16. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, M.S.; Magalhães, M.C.; Sousa-Lobo, J.M.; Almeida, I.F. Trending Anti-Aging Peptides. Cosmetics 2020, 7, 91. [Google Scholar] [CrossRef]
- Greff, D. Synthetic Peptides and Their Use in Cosmetic or Dermopharmaceutical Compositions. WO9807744A1, 26 February 1998. [Google Scholar]
- Millington, G.W. Proopiomelanocortin (POMC): The cutaneous roles of its melanocortin products and receptors. Clin. Exp. Dermatol. 2006, 31, 407–412. [Google Scholar] [CrossRef]
- Harno, E.; Gali Ramamoorthy, T.; Coll, A.P.; White, A. POMC: The Physiological Power of Hormone Processing. Physiol. Rev. 2018, 98, 2381–2430. [Google Scholar] [CrossRef]
- Loing, E. Reaching a Zen-Like State in Skin: Biomimetic Peptide to Balance Skin. Available online: https://www.cosmeticsandtoiletries.com/testing/sensory/Reaching-a-Zen-like-State-in-Skin-Biomimetic-Peptide-to-Balance-Sensitivity-420538914.html (accessed on 7 May 2021).
- Calmosensine Skin Pacified, Face Relaxed. Available online: https://www.ulprospector.com/documents/1003852.pdf?bs=11024&b=335122&st=20&r=la&ind=personalcare (accessed on 25 November 2020).
- Khmaladze, I.; Österlund, C.; Smiljanic, S.; Hrapovic, N.; Lafon-Kolb, V.; Amini, N.; Xi, L.; Fabre, S. A novel multifunctional skin care formulation with a unique blend of antipollution, brightening and antiaging active complexes. J. Cosmet. Dermatol. 2020, 19, 1415–1425. [Google Scholar] [CrossRef]
- Sulzberger, M.; Worthmann, A.C.; Holtzmann, U.; Buck, B.; Jung, K.A.; Schoelermann, A.M.; Rippke, F.; Stäb, F.; Wenck, H.; Neufang, G.; et al. Effective treatment for sensitive skin: 4-t-butylcyclohexanol and licochalcone A. J. Eur. Acad. Dermatol. Venereol. 2016, 30 (Suppl. 1), 9–17. [Google Scholar] [CrossRef] [PubMed]
- Calmosensine Sensual Healing. Available online: https://www.ulprospector.com/documents/1003854.pdf?bs=1240&b=44014&st=20&r=na&ind=personalcare (accessed on 7 May 2021).
- Schoelermann, A.M.; Jung, K.A.; Buck, B.; Grönniger, E.; Conzelmann, S. Comparison of skin calming effects of cosmetic products containing 4-t-butylcyclohexanol or acetyl dipeptide-1 cetyl ester on capsaicin-induced facial stinging in volunteers with sensitive skin. J. Eur. Acad. Dermatol. Venereol. 2016, 30 (Suppl. 1), 18–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Archambault, J.-C.; Franchi, J.; Korichi, R. Cosmetic Composition Containing an Extract from Lotus and Method of Cosmetic Care Using said Composition. U.S. 20090148544A1, 11 June 2009. [Google Scholar]
- Liu, Y. Nano-Encapsulated Skin Repair Agent Containing Blue Copper Peptide and its Preparation Method. CN111840125A, 30 October 2020. [Google Scholar]
- Ding, W.; Lv, Q. Polypeptide Composition with Antiallergic Effect. CN106176274A, 7 December 2016. [Google Scholar]
- Potin, A. Use of a Combination of Tyrosine-Arginine Dipeptide and Niacinamide as a Substance P Antagonist. FR2894142A1, 12 June 2009. [Google Scholar]
- Potin, A. Use of Tyrosine-Arginine Dipeptide in a Cosmetic Composition for the Treatment of Cutaneous Redness. FR2894144A1, 12 June 2009. [Google Scholar]
- Martinez, J.; Verdie, P.; Dubs, P.; Pinel, A.M.; Subra, G. Tripeptide-Carboxylic acid Conjugates as α-MSH Agonists and Their Therapeutic and Cosmetic Use. FR2870243A1, 19 November 2010. [Google Scholar]
- NEUTRAZEN™ Active Ingredients Soothing Neurocosmetic. Available online: https://www.ulprospector.com/documents/1045285.pdf?bs=4499&b=125061&st=20&r=eu&ind=personalcare (accessed on 10 May 2021).
- Baldwin, H.; Berson, D.; Vitale, M.; Yatskayer, M.; Chen, N.; Oresajo, C. Clinical effects of a novel topical composition on persistent redness observed in patients who had been successfully treated with topical or oral therapy for papulopustular rosacea. J. Drugs Dermatol. 2014, 13, 326–331. [Google Scholar]
- Zhao, C.; Zhu, W.; Song, X.; Hui, Y.; Sun, L. Anti-Allergic Repair Mask Containing Polypeptide. CN110251415A, 20 September 2019. [Google Scholar]
- Zhang, J.; Zhi, Q.; Yue, Z.; Song, X.; Liao, M.; Zhu, W. Polypeptide Composition Granules for Cosmetics with Anti-Inflammatory and Repairing Effects and Preparation Method Thereof. CN111514055A, 11 August 2020. [Google Scholar]
- Liu, S.; Li, N.; Qiu, J.; Yin, Q.; Xiang, W.; Xiao, X. Preparation of Soothing and Anti-Allergic Cosmetic Composition. CN111494266A, 26 January 2021. [Google Scholar]
- Chenevard, Y.; Fargeon, V. Soothing Cosmetic Composition Helicrysum Italicum and Glycyrrhizinic Acid Derivative. FR2965729A1, 13 April 2012. [Google Scholar]
- BASF. Skinasensyl™ The Neurocosmeceutical Soother. Available online: https://www.carecreations.basf.com/product-formulations/products/products-detail/SKINASENSYL%20PW%20LS%209852/30537033 (accessed on 13 May 2021).
- Yang, F.; Zheng, J. Understand spiciness: Mechanism of TRPV1 channel activation by capsaicin. Protein Cell 2017, 8, 169–177. [Google Scholar] [CrossRef] [Green Version]
- Kapuscinska, A.; Olejnik, A.; Nowak, I. The conjugate of jasmonic acid and tetrapeptide as a novel promising biologically active compound. New J. Chem. 2016, 40, 9007–9011. [Google Scholar] [CrossRef] [Green Version]
- Improving Skin Comfort Via Nervous System Modulation. Available online: https://personalcaremagazine.com/story/6258/improving-skin-comfort-via-nervous-system-modulation (accessed on 13 May 2021).
- Tetrapeptide for Neurosensitive Skin. Available online: https://www.cosmeticsandtoiletries.com/formulating/function/antiirritant/35799934.html (accessed on 13 May 2021).
- Zhang, L.; Falla, T.J. Cosmeceuticals and peptides. Clin. Dermatol. 2009, 27, 485–494. [Google Scholar] [CrossRef]
- Yu, G.; Li, J.; Lin, Z.; Bian, F.; Si, C.; Liu, C. Liquid Phase Synthesis Method of Palmitoyl Tripeptide-5. CN111004306A, 25 September 2020. [Google Scholar]
- Tao, Y.; Chen, J.; Wang, X. Method for Efficiently Preparing Palmitoyl Tripeptide-5 Based on Activated Ester. CN110423264A, 1 December 2020. [Google Scholar]
- Ziegler, H.; Heidl, M.; Imfeld, D. Tripeptides and their derivatives for cosmetic applications for improving skin structure. WO2004099237A1, 18 November 2004. [Google Scholar]
- DSM Launches Regu-Cea. Available online: https://www.happi.com/contents/view_breaking-news/2009-04-22/dsm-launches-regu-cea/ (accessed on 7 May 2021).
- Milanello, S. REGU®-CEA: Approccio multifunzionale contro i sintomi della rosacea. Kosmetica 2009, 56–58. [Google Scholar]
- Liao, Y.; He, L.; Liu, X.; Liu, Y. Composition for Repairing Sensitive Skin, and its Application in Cosmetic. CN109010113A, 18 December 2018. [Google Scholar]
- Lu, X.; Dai, C.; He, H.; Liang, J.; Tang, Z. Multifunctional Toning Lotion and its Preparation Method. CN111632001A, 8 September 2020. [Google Scholar]
- Ji, X.; Lin, J.; Wang, L. Preparation Method of Dried Facial Mask Comprising Ceramide-2 and Traditional Chinese Medicine Extract for Caring Skin. CN109350591A, 25 May 2021. [Google Scholar]
- Avcil, M.; Akman, G.; Klokkers, J.; Jeong, D.; Çelik, A. Efficacy of bioactive peptides loaded on hyaluronic acid microneedle patches: A monocentric clinical study. J. Cosmet. Dermatol. 2020, 19, 328–337. [Google Scholar] [CrossRef]
- Delisens: Protect Skin, Reduce Discomfort. Available online: https://www.happi.com/issues/2013-10/view_features/protect-skin-reduce-discomfort/ (accessed on 7 May 2021).
- Ding, W.; Peng, Y.; Huang, C. Polypeptide Composition with Soothing and Anti-Allergic Effects Containing Palmitoyl Tripeptide-8 In Water-In-Oil System for Preparing Skin Care Product. CN110833515A, 25 February 2020. [Google Scholar]
- Ding, W. Polypeptide for Repairing Facial Steroid Dependent Dermatitis. CN109125107A, 4 January 2019. [Google Scholar]
- Mondon, P.; Hillion, M.; Peschard, O.; Andre, N.; Marchand, T.; Doridot, E.; Feuilloley, M.G.; Pionneau, C.; Chardonnet, S. Evaluation of dermal extracellular matrix and epidermal-dermal junction modifications using matrix-assisted laser desorption/ionization mass spectrometric imaging, in vivo reflectance confocal microscopy, echography, and histology: Effect of age and peptide applications. J. Cosmet. Dermatol. 2015, 14, 152–160. [Google Scholar] [CrossRef]
- Knauer, S.; Koch, N.; Uth, C.; Meusinger, R.; Avrutina, O.; Kolmar, H. Sustainable peptide synthesis enabled by a transient protecting group. Angew. Chem., Int. Ed. 2020, 59, 12984–12990. [Google Scholar] [CrossRef] [PubMed]
- Mi, P.; Pan, J.; Liu, J. Preparation Method of Polypeptide. CN112110984A, 22 December 2020. [Google Scholar]
- Hahn, H.J.; Jung, H.J.; Schrammek-Drusios, M.C.; Lee, S.N.; Kim, J.H.; Kwon, S.B.; An, I.S.; An, S.; Ahn, K.J. Instrumental evaluation of anti-aging effects of cosmetic formulations containing palmitoyl peptides, Silybum marianum seed oil, vitamin E and other functional ingredients on aged human skin. Exp. Ther. Med. 2016, 12, 1171–1176. [Google Scholar] [CrossRef] [Green Version]
- Draelos, Z.D.; Kononov, T.; Fox, T. An open label clinical trial of a peptide treatment serum and supporting regimen designed to improve the appearance of aging facial skin. J. Drugs Dermatol. 2016, 15, 1100–1106. [Google Scholar] [PubMed]
- Johnson, B.Z.; Stevenson, A.W.; Prele, C.M.; Fear, M.W.; Wood, F.M. The Role of IL-6 in Skin Fibrosis and Cutaneous Wound Healing. Biomedicines 2020, 8, 101. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y. A Mild Polypeptide Repairing and Lightening Facial Mask for Facial Ulcers and Sensitive Skin. CN110302124A, 8 October 2019. [Google Scholar]
- Zhang, X.; Zhong, W. Skin Care Composition Containing Plant Extract and Preparation Method Thereof. CN110279646A, 14 August 2020. [Google Scholar]
- Husein El Hadmed, H.; Castillo, R.F. Cosmeceuticals: Peptides, proteins, and growth factors. J. Cosmet. Dermatol. 2016, 15, 514–519. [Google Scholar] [CrossRef] [PubMed]
- Lintner, K. Cosmetic or Dermopharmaceutical Compositions Containing the n-palmytoyl-gly-hys-lys Tripeptide. WO2001043701A2, 21 June 2001. [Google Scholar]
- Huang, Y.; Xing, H.; Wang, Z.; Yu, X. Liquid Phase Synthesis Method of Palmitoyl Tripeptide-1. CN 112409444A, 26 February 2021. [Google Scholar]
- Zheng, Q. Preparation of Palmitoyl Tripeptide-1 by Liquid-Phase Peptide Synthesis Method. CN 108218956 A, 29 June 2018. [Google Scholar]
- Su, X.; Yang, Y.; Bian, Y.; Cui, Y. Preparation of Palmitoyl Hexapeptide with Microchannel Modular Reaction Device. CN 109879936A, 14 June 2019. [Google Scholar]
- Escelsior, A.; Sterlini, B.; Murri, M.B.; Serafini, G.; Aguglia, A.; da Silva, B.P.; Corradi, A.; Valente, P.; Amore, M. Red-hot chili receptors: A systematic review of TRPV1 antagonism in animal models of psychiatric disorders and addiction. Behav. Brain Res. 2020, 393, 112734. [Google Scholar] [CrossRef] [PubMed]
- Caterina, M.J.; Pang, Z. TRP Channels in Skin Biology and Pathophysiology. Pharmaceuticals (Basel) 2016, 9, 77. [Google Scholar] [CrossRef] [Green Version]
- Xie, Z.; Hu, H. TRP Channels as Drug Targets to Relieve Itch. Pharmaceuticals (Basel) 2018, 11, 100. [Google Scholar] [CrossRef] [Green Version]
- Sulk, M.; Seeliger, S.; Aubert, J.; Schwab, V.D.; Cevikbas, F.; Rivier, M.; Nowak, P.; Voegel, J.J.; Buddenkotte, J.; Steinhoff, M. Distribution and expression of non-neuronal transient receptor potential (TRPV) ion channels in rosacea. J. Investig. Dermatol. 2012, 132, 1253–1262. [Google Scholar] [CrossRef] [Green Version]
- Johnson, W., Jr.; Bergfeld, W.F.; Belsito, D.V.; Hill, R.A.; Klaassen, C.D.; Liebler, D.C.; Marks, J.G., Jr.; Shank, R.C.; Slaga, T.J.; Snyder, P.W.; et al. Safety Assessment of Tripeptide-1, Hexapeptide-12, Their Metal Salts and Fatty Acyl Derivatives, and Palmitoyl Tetrapeptide-7 as Used in Cosmetics. Int. J. Toxicol. 2018, 37, 90S–102S. [Google Scholar] [CrossRef]
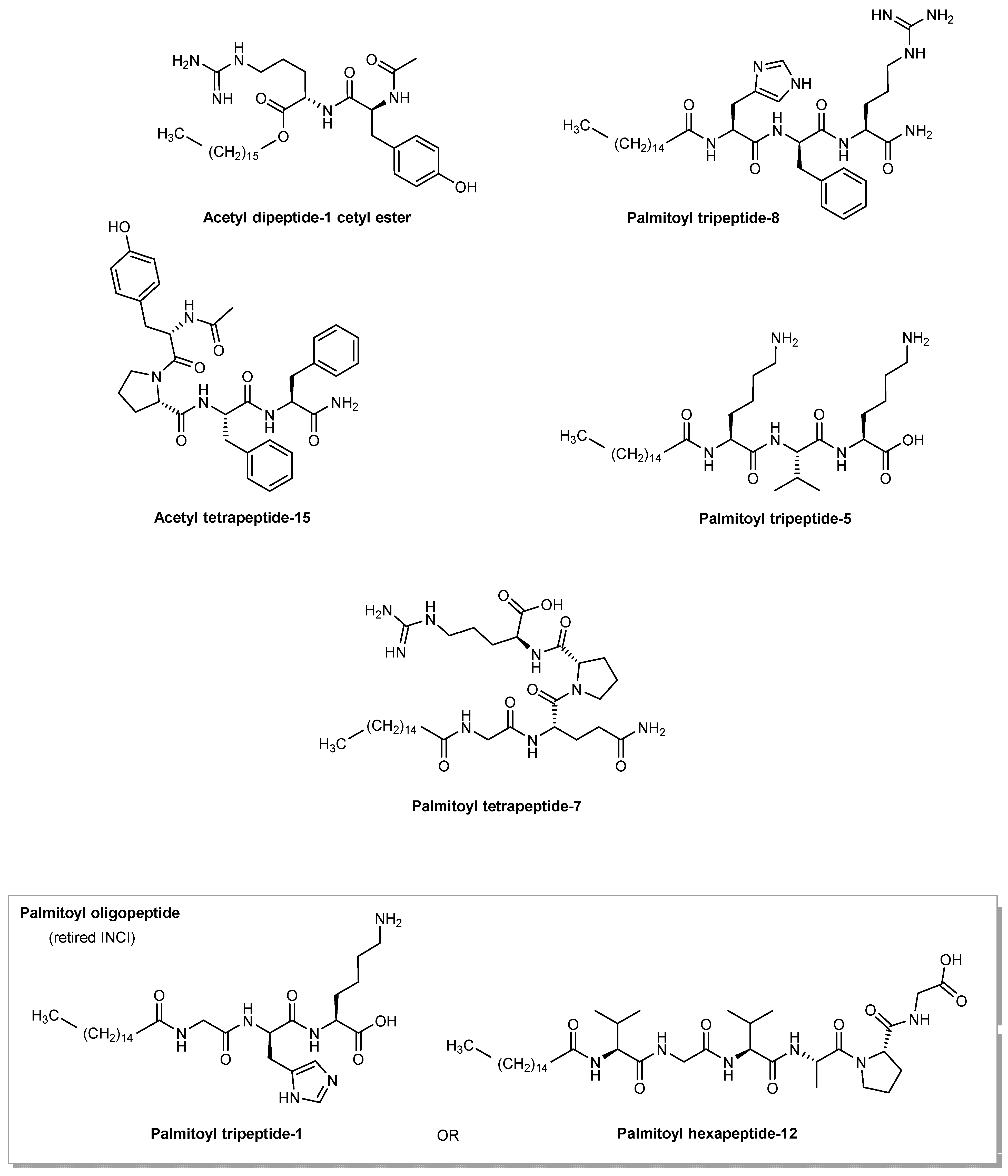



| INCI | Classification | Relative Usage (%) |
|---|---|---|
| Acetyl Dipeptide-1 Cetyl Ester | Neurotransmitter-inhibiting | 5.7 |
| Palmitoyl Tripeptide-8 | Neurotransmitter-inhibiting | 4.5 |
| Acetyl Tetrapeptide-15 | Neurotransmitter-inhibiting | 2.3 |
| Palmitoyl Tripeptide-5 | Signal | 2.3 |
| Acetyl Hexapeptide-49 | Unknown | 1.1 |
| Palmitoyl Tetrapeptide-7 | Signal | 1.1 |
| Palmitoyl Oligopeptide | Signal | 1.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Resende, D.I.S.P.; Ferreira, M.S.; Sousa-Lobo, J.M.; Sousa, E.; Almeida, I.F. Usage of Synthetic Peptides in Cosmetics for Sensitive Skin. Pharmaceuticals 2021, 14, 702. https://doi.org/10.3390/ph14080702
Resende DISP, Ferreira MS, Sousa-Lobo JM, Sousa E, Almeida IF. Usage of Synthetic Peptides in Cosmetics for Sensitive Skin. Pharmaceuticals. 2021; 14(8):702. https://doi.org/10.3390/ph14080702
Chicago/Turabian StyleResende, Diana I. S. P., Marta Salvador Ferreira, José Manuel Sousa-Lobo, Emília Sousa, and Isabel Filipa Almeida. 2021. "Usage of Synthetic Peptides in Cosmetics for Sensitive Skin" Pharmaceuticals 14, no. 8: 702. https://doi.org/10.3390/ph14080702
APA StyleResende, D. I. S. P., Ferreira, M. S., Sousa-Lobo, J. M., Sousa, E., & Almeida, I. F. (2021). Usage of Synthetic Peptides in Cosmetics for Sensitive Skin. Pharmaceuticals, 14(8), 702. https://doi.org/10.3390/ph14080702










