Photodynamic Therapy Combined with Antibiotics or Antifungals against Microorganisms That Cause Skin and Soft Tissue Infections: A Planktonic and Biofilm Approach to Overcome Resistances
Abstract
1. Introduction
1.1. The Problem of Skin and Soft Tissue Infections
1.2. Antimicrobial Resistance in Skin and Soft Tissue Infections Causal Agents
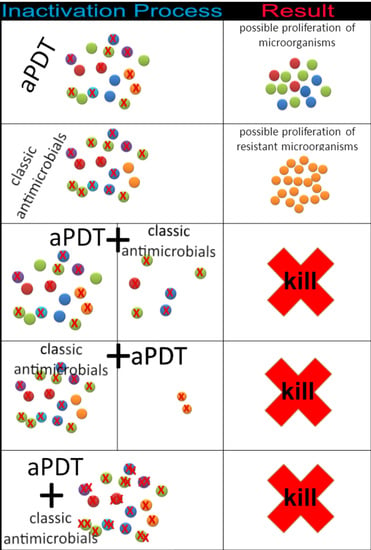
1.3. Antimicrobial Photodynamic Therapy Combined with Antibiotics or Antifungals to Treat SSTIs
1.4. Objective
2. Methodology
2.1. Eligibility Criteria
2.2. Study Selection, Data Collection Process, and Characteristics
3. Results of Studies on In Vitro aPDT Combined with Antimicrobial Agents against Infectious Microorganism of Skin and Soft Tissues
3.1. Staphylococcus spp.
3.1.1. Staphylococcus aureus
Porphycene and Porphyrin Studies
Phenothiazine Studies
Xanthene Studies
3.1.2. Staphylococcus epidermidis and Staphylococcus haemolyticus
3.2. Mycobacterium fortuitum
3.3. Escherichia coli
3.3.1. Porphycene Study
3.3.2. Phenothiazine and Porphyrin Studies
3.3.3. Chlorophyll Study
3.4. Pseudomonas aeruginosa
3.4.1. Porphyrin Studies
3.4.2. Phenothiazine Studies
3.4.3. Xanthene Studies
3.5. Acinetobacter baumannii
3.6. Candida spp.
3.7. Dermatophytes and Moulds
4. Summary of Evidence and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ki, V.; Rotstein, C. Bacterial skin and soft tissue infections in adults: A review of their epidemiology, pathogenesis, diagnosis, treatment and site of care. Can. J. Infect. Dis. Med. Microbiol. 2008, 19, 173–184. [Google Scholar] [CrossRef]
- Russo, A.; Concia, E.; Cristini, F.; De Rosa, F.G.; Esposito, S.; Menichetti, F.; Petrosillo, N.; Tumbarello, M.; Venditti, M.; Viale, P.; et al. Current and future trends in antibiotic therapy of acute bacterial skin and skin-structure infections. Clin. Microbiol. Infect. 2016, 22 (Suppl. 2), S27–S36. [Google Scholar] [CrossRef]
- Sartelli, M.; Guirao, X.; Hardcastle, T.C.; Kluger, Y.; Boermeester, M.A.; Raşa, K.; Ansaloni, L.; Coccolini, F.; Montravers, P.; Abu-Zidan, F.M.; et al. 2018 WSES/SIS-E consensus conference: Recommendations for the management of skin and soft-tissue infections. World J. Emerg. Surg. 2018, 13, 58. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, F.; Khan, T.; Pujalte, G.G.A. Bacterial Skin Infections. Prim. Care 2015, 42, 485–499. [Google Scholar] [CrossRef] [PubMed]
- Moffarah, A.S.; Mohajer, M.A.; Hurwitz, B.L.; Armstrong, D.G. Skin and soft tissue infections. Microbiol. Spectr. 2016, 4, 4. [Google Scholar] [CrossRef]
- Polk, C.; Sampson, M.M.; Roshdy, D.; Davidson, L.E. Skin and Soft Tissue Infections in Patients with Diabetes Mellitus. Infect. Dis. Clin. N. Am. 2021, 35, 183–197. [Google Scholar] [CrossRef] [PubMed]
- Esposito, S.; Noviello, S.; de Caro, F.; Boccia, G. New insights into classification, epidemiology and microbiology of SSTIs, including diabetic foot infections. Infez. Med. 2018, 26, 3–14. [Google Scholar] [PubMed]
- Leong, H.N.; Kurup, A.; Tan, M.Y.; Kwa, A.L.H.; Liau, K.H.; Wilcox, M.H. Management of complicated skin and soft tissue infections with a special focus on the role of newer antibiotics. Infect. Drug Resist. 2018, 11, 1959–1974. [Google Scholar] [CrossRef] [PubMed]
- Stevens, D.L.; Bisno, A.L.; Chambers, H.F.; Dellinger, E.P.; Goldstein, E.J.; Gorbach, S.L.; Hirschmann, J.V.; Kaplan, S.L.; Montoya, J.G.; Wade, J.C.; et al. Practice Guidelines for the Diagnosis and Management of Skin and Soft Tissue Infections: 2014 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2014, 59, e10–e52. [Google Scholar] [CrossRef]
- Dadgostar, P. Antimicrobial Resistance: Implications and Costs. Infect. Drug Resist. 2019, 12, 3903–3910. [Google Scholar] [CrossRef]
- Tacconelli, E. Magrani Global Priority List of Antibiotic-Resistant Bacteria to Guide Research, Discovery, and Development of New Antibiotics; WHO: Geneva, Switzerland, 2017; Available online: https://www.who.int/medicines/publications/WHO-PPL-Short_Summary_25Feb-ET_NM_WHO.pdf?ua=1 (accessed on 20 June 2021).
- Sebeny, P.J.; Riddle, M.S.; Petersen, K. Acinetobacter baumannii skin and soft-tissue infection associated with war trauma. Clin. Infect. Dis. 2008, 47, 444–449. [Google Scholar] [CrossRef]
- Berman, J.; Krysan, D.J. Drug resistance and tolerance in fungi. Nat. Rev. Microbiol. 2020, 18, 319–331. [Google Scholar] [CrossRef] [PubMed]
- Pappas, P.G.; Rex, J.H.; Sobel, J.D.; Filler, S.G.; Dismukes, W.E.; Walsh, T.J.; Edwards, J.E. Infectious Diseases Society of America Guidelines for treatment of candidiasis. Clin. Infect. Dis. 2004, 38, 161–189. [Google Scholar] [CrossRef]
- Gao, L.; Jiang, S.; Sun, Y.; Deng, M.; Wu, Q.; Li, M.; Zeng, T. Evaluation of the Effects of Photodynamic Therapy Alone and Combined with Standard Antifungal Therapy on Planktonic Cells and Biofilms of Fusarium spp. and Exophiala spp. Front. Microbiol. 2016, 7, 617. [Google Scholar] [CrossRef]
- Tainwala, R.; Sharma, Y. Pathogenesis of Dermatophytoses. Indian J. Dermatol. 2011, 56, 259–261. [Google Scholar] [CrossRef] [PubMed]
- Kharkwal, G.B.; Sharma, S.K.; Huang, Y.-Y.; Dai, T.; Hamblin, M.R. Photodynamic therapy for infections: Clinical applications. Lasers Surg. Med. 2011, 43, 755–767. [Google Scholar] [CrossRef]
- Pérez-Laguna, V.; García-Malinis, A.J.; Aspiroz, C.; Rezusta, A.; Gilaberte, Y. Antimicrobial effects of photodynamic therapy: An overview. G. Ital. Dermatol. Venereol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Cieplik, F.; Deng, D.; Crielaard, W.; Buchalla, W.; Hellwig, E.; Al-Ahmad, A.; Maisch, T. Antimicrobial photodynamic therapy—What we know and what we don’t. Crit. Rev. Microbiol. 2018, 44, 571–589. [Google Scholar] [CrossRef] [PubMed]
- O’Riordan, K.; Akilov, O.E.; Hasan, T. The potential for photodynamic therapy in the treatment of localized infections. Photodiagn. Photodyn. Ther. 2005, 2, 247–262. [Google Scholar] [CrossRef]
- Dai, T.; Huang, Y.-Y.; Hamblin, M.R. Photodynamic therapy for localized infections--state of the art. Photodiagn. Photodyn. Ther. 2009, 6, 170–188. [Google Scholar] [CrossRef]
- Nisnevitch, M.; Valkov, A.; Nakonechny, F.; Gutterman, M.; Nitzan, Y. Antibiotics Combined with Photosensitizers: A Novel Approach to Antibacterial Treatment. In Antibiotic Therapy: New Developments; Turner, A., Hall, J., Eds.; Nova Science Inc.: New York, NY, USA, 2013; pp. 63–88. ISBN 978-1-62808-171-8. [Google Scholar]
- Pérez-Laguna, V.; Gilaberte, Y.; Millán-Lou, M.I.; Agut, M.; Nonell, S.; Rezusta, A.; Hamblin, M.R. A combination of photodynamic therapy and antimicrobial compounds to treat skin and mucosal infections: A systematic review. Photochem. Photobiol. Sci. 2019, 18, 1020–1029. [Google Scholar] [CrossRef] [PubMed]
- Wozniak, A.; Grinholc, M. Combined Antimicrobial Activity of Photodynamic Inactivation and Antimicrobials-State of the Art. Front. Microbiol. 2018, 9, 930. [Google Scholar] [CrossRef] [PubMed]
- Bassetti, M.; Baguneid, M.; Bouza, E.; Dryden, M.; Nathwani, D.; Wilcox, M. European perspective and update on the management of complicated skin and soft tissue infections due to methicillin-resistant Staphylococcus aureus after more than 10 years of experience with linezolid. Clin. Microbiol. Infect. 2014, 20 (Suppl. 4), 3–18. [Google Scholar] [CrossRef] [PubMed]
- Ilizirov, Y.; Formanovsky, A.; Mikhura, I.; Paitan, Y.; Nakonechny, F.; Nisnevitch, M. Effect of Photodynamic Antibacterial Chemotherapy Combined with Antibiotics on Gram-Positive and Gram-Negative Bacteria. Molecules 2018, 23, 3152. [Google Scholar] [CrossRef] [PubMed]
- Nieves, I.; Hally, C.; Viappiani, C.; Agut, M.; Nonell, S. A porphycene-gentamicin conjugate for enhanced photodynamic inactivation of bacteria. Bioorganic Chem. 2020, 97, 103661. [Google Scholar] [CrossRef]
- Barra, F.; Roscetto, E.; Soriano, A.A.; Vollaro, A.; Postiglione, I.; Pierantoni, G.M.; Palumbo, G.; Catania, M.R. Photodynamic and Antibiotic Therapy in Combination to Fight Biofilms and Resistant Surface Bacterial Infections. Int. J. Mol. Sci. 2015, 16, 20417–20430. [Google Scholar] [CrossRef] [PubMed]
- Iluz, N.; Maor, Y.; Keller, N.; Malik, Z. The synergistic effect of PDT and oxacillin on clinical isolates of Staphylococcus aureus. Lasers Surg. Med. 2018, 50, 535–551. [Google Scholar] [CrossRef]
- Zhang, Q.-Z.; Zhao, K.-Q.; Wu, Y.; Li, X.-H.; Yang, C.; Guo, L.-M.; Liu, C.-H.; Qu, D.; Zheng, C.-Q. 5-aminolevulinic acid-mediated photodynamic therapy and its strain-dependent combined effect with antibiotics on Staphylococcus aureus biofilm. PLoS ONE 2017, 12, e0174627. [Google Scholar] [CrossRef]
- Di Poto, A.; Sbarra, M.S.; Provenza, G.; Visai, L.; Speziale, P. The effect of photodynamic treatment combined with antibiotic action or host defence mechanisms on Staphylococcus aureus biofilms. Biomaterials 2009, 30, 3158–3166. [Google Scholar] [CrossRef]
- Dastgheyb, S.S.; Eckmann, D.M.; Composto, R.J.; Hickok, N.J. Photo-activated porphyrin in combination with antibiotics: Therapies against Staphylococci. J. Photochem. Photobiol. B 2013, 129, 27–35. [Google Scholar] [CrossRef]
- Branco, T.M.; Valério, N.C.; Jesus, V.I.R.; Dias, C.J.; Neves, M.G.P.M.S.; Faustino, M.A.F.; Almeida, A. Single and combined effects of photodynamic therapy and antibiotics to inactivate Staphylococcus aureus on skin. Photodiagn. Photodyn. Ther. 2018, 21, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Pereira, N.L.F.; Aquino, P.E.A.; Júnior, J.G.A.S.; Cristo, J.S.; Vieira Filho, M.A.; Moura, F.F.; Ferreira, N.M.N.; Silva, M.K.N.; Nascimento, E.M.; Correia, F.M.A.; et al. Antibacterial activity and antibiotic modulating potential of the essential oil obtained from Eugenia jambolana in association with led lights. J. Photochem. Photobiol. B 2017, 174, 144–149. [Google Scholar] [CrossRef]
- Ronqui, M.R.; de Aguiar Coletti, T.M.S.F.; de Freitas, L.M.; Miranda, E.T.; Fontana, C.R. Synergistic antimicrobial effect of photodynamic therapy and ciprofloxacin. J. Photochem. Photobiol. B 2016, 158, 122–129. [Google Scholar] [CrossRef]
- Kashef, N.; Akbarizare, M.; Razzaghi, M.R. In vitro Activity of Linezolid in Combination with Photodynamic Inactivation Against Staphylococcus aureus Biofilms. Avicenna J. Med. Biotechnol. 2017, 9, 44–48. [Google Scholar] [PubMed]
- Liu, S.; Mai, B.; Jia, M.; Lin, D.; Zhang, J.; Liu, Q.; Wang, P. Synergistic antimicrobial effects of photodynamic antimicrobial chemotherapy and gentamicin on Staphylococcus aureus and multidrug-resistant Staphylococcus aureus. Photodiagn. Photodyn. Ther. 2020, 30, 101703. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Laguna, V.; García-Luque, I.; Ballesta, S.; Pérez-Artiaga, L.; Lampaya-Pérez, V.; Rezusta, A.; Gilaberte, Y. Photodynamic therapy using methylene blue, combined or not with gentamicin, against Staphylococcus aureus and Pseudomonas aeruginosa. Photodiagn. Photodyn. Ther. 2020, 31, 101810. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Laguna, V.; Pérez-Artiaga, L.; Lampaya-Pérez, V.; García-Luque, I.; Ballesta, S.; Nonell, S.; Paz-Cristobal, M.P.; Gilaberte, Y.; Rezusta, A. Bactericidal Effect of Photodynamic Therapy, Alone or in Combination with Mupirocin or Linezolid, on Staphylococcus aureus. Front. Microbiol. 2017, 8, 1002. [Google Scholar] [CrossRef]
- Pérez, M.; Robres, P.; Moreno, B.; Bolea, R.; Verde, M.T.; Pérez-Laguna, V.; Aspiroz, C.; Gilaberte, Y.; Rezusta, A. Comparison of Antibacterial Activity and Wound Healing in a Superficial Abrasion Mouse Model of Staphylococcus aureus Skin Infection Using Photodynamic Therapy Based on Methylene Blue or Mupirocin or Both. Front. Med. 2021, 8. [Google Scholar] [CrossRef]
- Pérez-Laguna, V.; García-Luque, I.; Ballesta, S.; Pérez-Artiaga, L.; Lampaya-Pérez, V.; Samper, S.; Soria-Lozano, P.; Rezusta, A.; Gilaberte, Y. Antimicrobial photodynamic activity of Rose Bengal, alone or in combination with Gentamicin, against planktonic and biofilm Staphylococcus aureus. Photodiagn. Photodyn. Ther. 2018, 21, 211–216. [Google Scholar] [CrossRef] [PubMed]
- de Allori, M.C.G.; Jure, M.A.; Romero, C.; de Castillo, M.E.C. Antimicrobial resistance and production of biofilms in clinical isolates of coagulase-negative Staphylococcus strains. Biol. Pharm. Bull. 2006, 29, 1592–1596. [Google Scholar] [CrossRef]
- Fey, P.D.; Olson, M.E. Current concepts in biofilm formation of Staphylococcus epidermidis. Future Microbiol. 2010, 5, 917–933. [Google Scholar] [CrossRef]
- Foster, T. Staphylococcus. In Medical Microbiology, 4th ed.; Baron, S., Ed.; University of Texas Medical Branch at Galveston: Galveston, TX, USA, 1996. Available online: http://www.ncbi.nlm.nih.gov/books/NBK8448/ (accessed on 10 July 2018).
- Viale, P.; Stefani, S. Vascular catheter-associated infections: A microbiological and therapeutic update. J. Chemother. Florence Italy 2006, 18, 235–249. [Google Scholar] [CrossRef]
- Sbarra, M.S.; Arciola, C.R.; Di Poto, A.; Saino, E.; Rohde, H.; Speziale, P.; Visai, L. The photodynamic effect of tetra-substituted N-methyl-pyridyl-porphine combined with the action of vancomycin or host defense mechanisms disrupts Staphylococcus epidermidis biofilms. Int. J. Artif. Organs 2009, 32, 574–583. [Google Scholar] [CrossRef] [PubMed]
- Gong, N.; Tan, Y.; Li, M.; Lu, W.; Lei, X. ALA-PDT combined with antibiotics for the treatment of multiple skin abscesses caused by Mycobacterium fortuitum. Photodiagn. Photodyn. Ther. 2016, 15, 70–72. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; Yang, H.; Huang, X.; Gong, N.; Qin, Q.; Lu, W.; Lei, X. ALA-PDT combined with antibiotics for the treatment of atypical mycobacterial skin infections: Outcomes and safety. Photodiagn. Photodyn. Ther. 2017, 19, 274–277. [Google Scholar] [CrossRef] [PubMed]
- Shih, M.-H.; Huang, F.-C. Effects of photodynamic therapy on rapidly growing nontuberculous mycobacteria keratitis. Investig. Ophthalmol. Vis. Sci. 2011, 52, 223–229. [Google Scholar] [CrossRef]
- Boucher, H.W.; Talbot, G.H.; Bradley, J.S.; Edwards, J.E.; Gilbert, D.; Rice, L.B.; Scheld, M.; Spellberg, B.; Bartlett, J. Bad bugs, no drugs: No ESKAPE! An update from the Infectious Diseases Society of America. Clin. Infect. Dis. 2009, 48, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Tichaczek-Goska, D.; Wojnicz, D.; Symonowicz, K.; Ziółkowski, P.; Hendrich, A.B. Photodynamic enhancement of the activity of antibiotics used in urinary tract infections. Lasers Med. Sci. 2019. [Google Scholar] [CrossRef] [PubMed]
- Almeida, J.; Tomé, J.P.C.; Neves, M.G.P.M.S.; Tomé, A.C.; Cavaleiro, J.A.S.; Cunha, Â.; Costa, L.; Faustino, M.A.F.; Almeida, A. Photodynamic inactivation of multidrug-resistant bacteria in hospital wastewaters: Influence of residual antibiotics. Photochem. Photobiol. Sci. 2014, 13, 626–633. [Google Scholar] [CrossRef]
- Magacho, C.C.; Pinto, J.G.; Souza, B.M.N.; Pereira, A.H.C.; Ferreira-Strixino, J. Comparison of photodynamic therapy with methylene blue associated with ceftriaxone in gram-negative bacteria; an in vitro study. Photodiagn. Photodyn. Ther. 2020, 30, 101691. [Google Scholar] [CrossRef]
- Collins, T.L.; Markus, E.A.; Hassett, D.J.; Robinson, J.B. The effect of a cationic porphyrin on Pseudomonas aeruginosa biofilms. Curr. Microbiol. 2010, 61, 411–416. [Google Scholar] [CrossRef] [PubMed]
- Reznick, Y.; Banin, E.; Lipovsky, A.; Lubart, R.; Polak, P.; Zalevsky, Z. The synergistic effect of visible light and gentamycin on Pseudomona aeruginosa microorganisms. J. Vis. Exp. JoVE 2013, e4370. [Google Scholar] [CrossRef] [PubMed]
- Fila, G.; Kawiak, A.; Grinholc, M.S. Blue light treatment of Pseudomonas aeruginosa: Strong bactericidal activity, synergism with antibiotics and inactivation of virulence factors. Virulence 2017, 8, 938–958. [Google Scholar] [CrossRef] [PubMed]
- Oppezzo, O.J.; Forte Giacobone, A.F. Lethal Effect of Photodynamic Treatment on Persister Bacteria. Photochem. Photobiol. 2018, 94, 186–189. [Google Scholar] [CrossRef] [PubMed]
- Nakonieczna, J.; Wolnikowska, K.; Ogonowska, P.; Neubauer, D.; Bernat, A.; Kamysz, W. Rose Bengal-Mediated Photoinactivation of Multidrug Resistant Pseudomonas aeruginosa Is Enhanced in the Presence of Antimicrobial Peptides. Front. Microbiol. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Wozniak, A.; Rapacka-Zdonczyk, A.; Mutters, N.T.; Grinholc, M. Antimicrobials Are a Photodynamic Inactivation Adjuvant for the Eradication of Extensively Drug-Resistant Acinetobacter baumannii. Front. Microbiol. 2019, 10, 229. [Google Scholar] [CrossRef]
- Kashem, S.W.; Kaplan, D.H. Skin Immunity to Candida albicans. Trends Immunol. 2016, 37, 440–450. [Google Scholar] [CrossRef]
- Lyon, J.P.; Carvalho, C.R.; Rezende, R.R.; Lima, C.J.; Santos, F.V.; Moreira, L.M. Synergism between fluconazole and methylene blue-photodynamic therapy against fluconazole-resistant Candida strains. Indian J. Med. Microbiol. 2016, 34, 506–508. [Google Scholar] [CrossRef]
- Snell, S.B.; Foster, T.H.; Haidaris, C.G. Miconazole induces fungistasis and increases killing of Candida albicans subjected to photodynamic therapy. Photochem. Photobiol. 2012, 88, 596–603. [Google Scholar] [CrossRef]
- Morton, C.O.; Chau, M.; Stack, C. In vitro combination therapy using low dose clotrimazole and photodynamic therapy leads to enhanced killing of the dermatophyte Trichophyton rubrum. BMC Microbiol. 2014, 14, 261. [Google Scholar] [CrossRef]
- Hu, Y.; Qi, X.; Sun, H.; Lu, Y.; Hu, Y.; Chen, X.; Liu, K.; Yang, Y.; Mao, Z.; Wu, Z.; et al. Photodynamic therapy combined with antifungal drugs against chromoblastomycosis and the effect of ALA-PDT on Fonsecaea in vitro. PLoS Negl. Trop. Dis. 2019, 13, e0007849. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Huang, X.; Lu, S.; Hamblin, M.R.; Mylonakis, E.; Zhang, J.; Xi, L. Photodynamic therapy combined with terbinafine against chromoblastomycosis and the effect of PDT on Fonsecaea monophora in vitro. Mycopathologia 2015, 179, 103–109. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yang, Y.; Hu, Y.; Zhang, J.; Li, X.; Lu, C.; Liang, Y.; Xi, L. A refractory case of chromoblastomycosis due to Fonsecaea monophora with improvement by photodynamic therapy. Med. Mycol. 2012, 50, 649–653. [Google Scholar] [CrossRef] [PubMed]
- Guarro, J. Fusariosis, a complex infection caused by a high diversity of fungal species refractory to treatment. Eur. J. Clin. Microbiol. Infect. Dis. 2013, 32, 1491–1500. [Google Scholar] [CrossRef]
- Giroldo, L.M.; Felipe, M.P.; de Oliveira, M.A.; Munin, E.; Alves, L.P.; Costa, M.S. Photodynamic antimicrobial chemotherapy (PACT) with methylene blue increases membrane permeability in Candida albicans. Lasers Med. Sci. 2009, 24, 109–112. [Google Scholar] [CrossRef]
- Li, J.; Zhu, M.; An, L.; Chen, F.; Zhang, X. Fungicidal efficacy of photodynamic therapy using methylene blue against Sporothrix globosa in vitro and in vivo. Eur. J. Dermatol. EJD 2019, 29, 160–166. [Google Scholar] [CrossRef]
- Gilaberte, Y.; Aspiroz, C.; Alejandre, M.C.; Andres-Ciriano, E.; Fortuño, B.; Charlez, L.; Revillo, M.J.; Hamblin, M.R.; Rezusta, A. Cutaneous Sporotrichosis Treated with Photodynamic Therapy: An in Vitro and in Vivo Study. Photomed. Laser Surg. 2014, 32, 54–57. [Google Scholar] [CrossRef]

| Priority 1: CRITICAL |
| -Acinetobacter baumannii, carbapenem-resistant -Pseudomonas aeruginosa, carbapenem-resistant -Enterobacteriaceae (Klebsiella pneumonia, Escherichia coli, Enterobacter spp., Serratia spp., Proteus spp., and Providencia spp., Morganella spp.), carbapenem-resistant, 3rd generation cephalosporin-resistant |
| Priority 2: HIGH |
| -Enterococcus faecium, vancomycin-resistant -Staphylococcus aureus, methicillin-resistant, vancomycin intermediate and resistant -Helicobacter pylori, clarithromycin-resistant -Campylobacter, fluoroquinolone-resistant -Salmonella spp., fluoroquinolone-resistant -Neisseria gonorrhoeae, 3rd generation cephalosporin-resistant, fluoroquinolone-resistant |
| Priority 3: MEDIUM |
| -Streptococcus pneumoniae, penicillin-non-susceptible -Haemophilus influenzae, ampicillin-resistant -Shigella spp., fluoroquinolone-resistant |
| Biofilm Ttudies | |||||||||||
| Target | PS | PS Concentration (μM) | Antibiotic | Antibiotic Concentration (μg/mL) | Source Type | Wavelength (nm) | Intensity (mw/cm2) | Fluence (J/cm2) | Inactivation Fraction (%) | Synergistic Observed Effect (*) | Reference |
| S. haemolyticus clinical isolate | 5-ALA | 40 | gentamicin | 2 | 50-LED | 630 ± 15 | 83 | 250 | ~70 | >inactivation | [28] |
| S. epidermidis clinical isolate | 5-ALA | 40 | gentamicin | 2 | 50-LED | 630 ± 15 | 83 | 250 | ~75 | >inactivation | [28] |
| S. epidermidis RP62A & 5179R | TMP | 10 | vancomycin | 200 | tungsten lamp | 400–800 | 166 | 300 | ~99.9999 | >inactivation | [46] |
| Planktonic Cell Studies | |||||||||||
| Target | PS | PS Concentration (μM) | Antibiotic | Antibiotic Concentration (mg/L) | Preincubation Time (h) | Irradiation Time (h) | Source Type | Wavelength | Media | CFU/200 μL Well | Log10 Reduction |
| S. epidermidis ATCC 35984 | TAPP | 5 | chloramphenicol | 2 | 19 | 5 | 100 W, 120 V Sylvania white light | Broad spectrum | TSB | ~106 | ~2 |
| S. epidermidis ATCC 35984 | TAPP | 5 | tobramycin | 4.5 | 19 | 5 | 100 W, 120 V Sylvania white light | Broad spectrum | TSB | ~106 | ~3 |
| Strain | PS | PS Concentration (mg/mL) | Antibiotic | Antibiotic Concentration (mg/mL) | Preincubation Time (h) | Source Type | Wavelength (nm) | Intensity (mw/cm2) | Fluence (J/cm2) | Media/Culture | CFU/200μL Well | Synergistic Observed Effect (*) | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M. fortuitum clinical isolate | MB | 50 | amikacin | 0–0.5 | 0 + 72 with antib | metal halogen lamp | 560–780 | 100 | 100 | PBS with 0.02% Tween 80 / Muller Hilton | ~108 | >inactivation (≥2 Log10 reduction) | [49] |
| ciprofloxacin hydrochloride | 0–0.06 | ||||||||||||
| moxifloxacin hydrochloride | 0–0.06 |
| Strain | PS | Antibiotic | Phase | Source Type | Wavelength (nm) | Intensity (mw/cm2) | Fluence (J/cm2) | Synergy | Observed Effect (*) | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| E. coli ATCC 25922 | ATAZTMPo | gentamicin | planktonic | LED (Sorisa Photocare) | 638 ± 9 nm | 17 | 45 | yes | >inactivation | [27] |
| E. coli ATCC 25922 | TAPP | tobramycin or chloramphenicol | planktonic | 100 W, 120 V Sylvania white light | Broad spectrum | ND | ND | no | additivity | [32] |
| E. coli ATCC 25922 | MB | ciprofloxacin | planktonic | IrradLED® biopdi, São Carlos, SP, Brazil | ~660 | ND | 2.8 and 5.6 | yes | >inactivation | [35] |
| E. coli ATCC 25922 | MB | ciprofloxacin | biofilm | IrradLED® biopdi, São Carlos, SP, Brazil | 660 | ND | 11.2 and 22 | yes | >inactivation | [35] |
| E. coli ATCC 9027 and MDR clinical isolates | endogenous porphyrins | ciprofloxacin or norfloxacin n | planktonic | LED Dermaled® | ~470 and ~625 | ND | ND | yes | incrase in halo | [34] |
| E. coli | Chlorin e6 | colistin, ciprofloxacin or amikacin | planktonic | diode laser, Laser Coupler 635 (Wroclaw, Poland) | 635 | 290 | 120 | yes | >inactivation | [51] |
| E. coli | Tetra-Py+-Me | ampicillin or chloramphenicol | planktonic | white light lamps (13 lamps OSRAM 21 of 18 W each) | Broad spectrum 380 to 700 | 40 | - | yes | >inactivation | [52] |
| E. coli, E. aerogenes, and K. pneumoniae resistant to 3rd-cephalosporins, clinical isolates | MB | ceftriaxone | planktonic | LED (Biopdi/Irrad-Led 660) | 660n ± 5 | 25 | 25 | no | indifference | [53] |
| P. aeruginosa PAO1 | TMP | tobramycin | biofilm | mercury vapor lamp | Broad spectrum | - | 220–240 | yes | >inactivation & tobramycin MIC decreased | [54] |
| P. aeruginosa PAO1 | endogenous porphyrins | gentamicin | planktonic | Nd:YAG laser continuous / Pulsed-Q switched | 532 | 106 | yes | >inactivation | [55] | |
| P. aeruginosa PAO1and others MDR and XDR | endogenous porphyrins | gentamicin, meropenem or ceftazidime | planktonic | Single-emitter diode lamp | 410 | 15.7 | 50 | yes | >inactivation & antibiotic MIC decreased | [56] |
| ATCC 27853 P. aeruginosa ATCC 27853 | MB | ofloxacin | planktonic | LED | ~637 | 44 | yes | >inactivation | [57] | |
| P. aeruginosa ATCC 27853 | MB | gentamicin | planktonic | LED lamp (Showtec LED Par 64 Short 18 x RGB 3-in-1 LED, Highlite International B.V. Spain) | 625 ± 10 | 7 | 18 | yes | bactericidal effect with lower MB-PDT dose | [38] |
| P. aeruginosa ATCC 27853 | MB | gentamicin | biofilms | LED lamp (Showtec LED Par 64 Short 18 x RGB 3-in-1 LED, Highlite International B.V. Spain) | 625 ± 10 | 7 | 18 | yes | bactericidal effect with lower MB-PDT dose | [38] |
| P. aeruginosa ATCC 10145 and 35 clinical isolates including MDR and XDR | RB | camel or pexiganan | planktonic | LED lamps (SecureMedia, Poland) | ~514 | 23 | 60 | yes | >inactivation | [58] |
| P. aeruginosa ATCC 25668 and sensitive and resistant clinical isolates | RB | sulfanilamide | planktonic | 18 W white luminescent lamp | Broad spectrum 400–700 | 1.25 | - | no | indifference | [26] |
| A. baumannii 2 XDR clinical isolates | RB | gentamycin, doxycicline, trimethoprim-sulfamethoxazole, ciprofloxacin, imipenem, piperacillin-tazobactam, ceftazidime, ampicillin-sulbactam, colistin | planktonic | LED | 515 | 70 | 300 | yes | >inactivation & antibiotic MIC decreased | [59] |
| A. baumannii 2 XDR clinical isolates | endogenous porphyrins | gentamycin, doxycicline, trimethoprim-sulfamethoxazole, ciprofloxacin, imipenem, piperacillin-tazobactam, ceftazidime, ampicillin-sulbactam, colistin | planktonic | LED | 411 | 130 | 109.1 | yes | >inactivation & antibiotic MIC decreased | [59] |
| S. aureus | ||||||
| PS Group | PS | Antibiotic | Phase | Synergy | Observed Effect (*) | Reference |
| Porphycene and Porphyrin | ATAZTMPo | gentamicin | planktonic | yes | >inactivation | [27] |
| 5-ALA | gentamicin | biofilm | yes | >inactivation | [28] | |
| DP | gentamicin, vancomycin, rifampin, fusidic acid | planktonic | no | additivity | [29] | |
| DP | oxacillin | planktonic | yes | oxacillin MIC decreased | [29] | |
| 5-ALA | netilmicin, cefaclor, vancomycin | biofilm | yes | >inactivation | [30] | |
| TMP | vancomycin | biofilm | yes | >inactivation | [31] | |
| TAPP | vancomycin, ceftriaxone | planktonic | no | indifference | [32] | |
| TAPP | chloramphenicol, tobramycin | planktonic | yes-no | >inactivation MRSA; additive MSSA | [32] | |
| Tetra-Py+-Me | ampicillin | planktonic | yes | faster bactericidal effect | [33] | |
| Tetra-Py+-Me | ampicillin | pork skin (ex vivo) | yes | >inactivation | [33] | |
| (endogenous) | ciprofloxacin, norfloxacin | planktonic | yes | >inactivation | [34] | |
| Phenothiazines | MB | chloramphenicol | planktonic | no | additivity | [32] |
| MB | ciprofloxacin | planktonic | yes | >inactivation | [35] | |
| MB | ciprofloxacin | biofilm | yes | >inactivation | [35] | |
| TBO | linezolid | biofilm | yes | >inactivation | [35] | |
| MB | linezolid | biofilm | no | indifference | [35] | |
| TBO | gentamicin | planktonic | yes | >inactivation | [37] | |
| MB | gentamicin | planktonic | yes | bactericidal effect with lower MB-PDT dose | [38] | |
| MB | gentamicin | biofilm | no | no significant > inactivation | [38] | |
| MB | linezolid, mupirocin | planktonic | yes | bactericidal effect with lower MB-PDT dose | [39] | |
| Xanthenes | RB | linezolid, mupirocin | planktonic | yes | bactericidal effect with lower RB-PDT dose | [39] |
| RB | gentamicin | planktonic | yes | bactericidal effect with lower RB-PDT dose | [41] | |
| RB | gentamicin | biofilm | yes | >inactivation | [41] | |
| RB | methicillin | planktonic | yes | methicillin MIC decreased | [26] | |
| S. haemolyticus | ||||||
| PS Group | PS | Antibiotic | Phase | Synergy | Observed Effect (*) | Reference |
| Porphycene and Porphyrin | 5-ALA | gentamicin | biofilm | yes | >inactivation | [28] |
| S. epidermidis | ||||||
| PS group | PS | Antibiotic | Phase | Synergy | Observed effect (*) | Reference |
| Porphycene and Porphyrin | 5-ALA | gentamicin | biofilm | yes | >inactivation | [28] |
| TMP | vancomycin | biofilm | yes | >inactivation | [31] | |
| TAPP | chloramphenicol, tobramycin | planktonic | yes | >inactivation | [32] | |
| M. fortuitum | ||||||
| PS Group | PS | Antibiotic | Phase | Synergy | Observed Effect (*) | Reference |
| Phenothiazines | MB | ciprofloxacin, moxifloxacin or amikacin | planktonic | yes | >inactivation | [49] |
| E. coli | ||||||
| PS Group | PS | Antibiotic | Phase | Synergy | Observed Effect (*) | Reference |
| Porphycene andPorphyrin | ATAZTMPo | gentamicin | planktonic | yes | >inactivation | [27] |
| TAPP | tobramycin or chloramphenicol | planktonic | no | additivity | [32] | |
| endogenous porphyrins | ciprofloxacin or norfloxacin n | planktonic | yes | incrase in halo | [34] | |
| Tetra-Py+-Me | ampicillin or chloramphenicol | planktonic | yes | >inactivation | [52] | |
| Phenothiazines | MB | ciprofloxacin | planktonic | yes | >inactivation | [35] |
| MB | ciprofloxacin | biofilm | yes | >inactivation | [35] | |
| MB | ceftriaxone | planktonic | no | indifference | [52] | |
| Chlorophylls | Chlorin e6 | colistin, ciprofloxacin or amikacin | planktonic | yes | >inactivation | [51] |
| P. aeruginosa | ||||||
| PS Group | PS | Antibiotic | Phase | Synergy | Observed Effect (*) | Reference |
| Porphycene and Porphyrin | TMP | tobramycin | biofilm | yes | >inactivation & tobramycin MIC decreased | [54] |
| endogenous porphyrins | gentamicin | planktonic | yes | >inactivation | [55] | |
| endogenous porphyrins | gentamicin, meropenem or ceftazidime | planktonic | yes | >inactivation & antibiotic MIC decreased | [56] | |
| Phenothiazines | MB | ofloxacin | planktonic | yes | >inactivation | [57] |
| MB | gentamicin | planktonic | yes | bactericidal effect with lower MB-PDT dose | [38] | |
| MB | gentamicin | biofilm | yes | bactericidal effect with lower MB-PDT dose | [38] | |
| Xanthenes | RB | camel or pexiganan | planktonic | yes | >inactivation | [58] |
| RB | sulfanilamide | planktonic | no | indifference | [26] | |
| A. baumannii | ||||||
| PS Group | PS | Antibiotic | Phase | Synergy | Observed Effect (*) | Reference |
| Xanthenes | RB | gentamycin, doxycicline, trimethoprim-sulfamethoxazole, ciprofloxacin, imipenem, piperacillin-tazobactam, ceftazidime, ampicillin-sulbactam, colistin | planktonic | yes | >inactivation & antibiotic MIC decreased | [59] |
| Porphycene and Porphyrin | endogenous porphyrins | gentamycin, doxycicline, trimethoprim-sulfamethoxazole, ciprofloxacin, imipenem, piperacillin-tazobactam, ceftazidime, ampicillin-sulbactam, colistin | planktonic | yes | >inactivation & antibiotic MIC decreased | [59] |
| Candida spp. | ||||||
| PS Group | PS | Antibiotic | Phase | Synergy | Observed Effect (*) | Reference |
| Phenothiazines | MB | fluconazole | planktonic | yes/no | ≥inactivation | [61] |
| MB | miconazole | planktonic | yes | >inactivation | [62] | |
| Porphycene and Porphyrin | TMP | miconazole | planktonic | yes | >inactivation | [62] |
| TMP | fluconazole | planktonic | no | indifference | [62] | |
| Dermatophytes and moulds | ||||||
| PS Group | PS | Antibiotic | Phase | Synergy | Observed Effect (*) | Reference |
| Xanthenes | RB | clotrimazole | Planktonic (in vitro: spores) | yes | ≥inactivation | [63] |
| Porphycene and Porphyrin | 5-ALA | itraconazole | planktonic | yes | ≥inactivation | [64] |
| Phenothiazines | MB | itraconazole, voriconazole, posaconazole, amphotericin | planktonic and biofilms | yes | MIC decreased | [15] |
| MB | itraconazole | planktonic | yes | >inactivation | [69] | |
| Strain | PS | Antifungal | Phase | Source Type | Wavelength (nm) | Intensity (mw/cm2) | Fluence (J/cm2) | Synergy | Observed Effect (*) | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| fluconazole-resistant C. albicans and C. glabratai | MB | fluconazole | planktonic | InGaAlP LED | nd | 200 | - | yes | >inactivation | [61] |
| fluconazole-resistant C. krusei | MB | fluconazole | planktonic | InGaAlP LED | nd | 200 | - | no | indifference | [61] |
| C. albicans SC5314 | TMP | miconazole | planktonic | broadband visible light (Sylvania GRO-LUX, 15 W, part no. F15T8/GRO) | 575–700 | 4 | 1 | yes | >inactivation | [62] |
| C. albicans SC5314 | TMP | fluconazole | planktonic | broadband visible light (Sylvania GRO-LUX, 15 W, part no. F15T8/GRO) | 575–700 | 4 | 1 | no | indifference | [62] |
| C. albicans SC5314 | MB | miconazole | planktonic | broadband visible light (Sylvania GRO-LUX, 15 W, part no. F15T8/GRO) | 575–700 | 4 | 7.2 | yes | >inactivation | [62] |
| T. rubrum clinical isolate | RB | clotrimazole | Planktonic (in vitro: spores) | LED | 530 | 13.4 | 12 | yes | ≥inactivation | [63] |
| F. monophora clinical isolates | 5-ALA | itraconazole | planktonic | Zeiss KL 2500 LED | 635 | 36.8 | 10 | yes | ≥inactivation | [64] |
| E. dermatitidis, F. solani, F. oxysporum clinical isolates | MB | itraconazole, voriconazole, posaconazole, amphotericin | planktonic and biofilms | LED | 635 ± 10 | 100 | 12-24 | yes | MIC decreased | [15] |
| S. globosa 5 clinical isolates | MB | itraconazole | planktonic | LED | 640 ± 10 | 22.2 | 40 | yes | >inactivation | [69] |
|
|
|
|
|
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez-Laguna, V.; García-Luque, I.; Ballesta, S.; Rezusta, A.; Gilaberte, Y. Photodynamic Therapy Combined with Antibiotics or Antifungals against Microorganisms That Cause Skin and Soft Tissue Infections: A Planktonic and Biofilm Approach to Overcome Resistances. Pharmaceuticals 2021, 14, 603. https://doi.org/10.3390/ph14070603
Pérez-Laguna V, García-Luque I, Ballesta S, Rezusta A, Gilaberte Y. Photodynamic Therapy Combined with Antibiotics or Antifungals against Microorganisms That Cause Skin and Soft Tissue Infections: A Planktonic and Biofilm Approach to Overcome Resistances. Pharmaceuticals. 2021; 14(7):603. https://doi.org/10.3390/ph14070603
Chicago/Turabian StylePérez-Laguna, Vanesa, Isabel García-Luque, Sofía Ballesta, Antonio Rezusta, and Yolanda Gilaberte. 2021. "Photodynamic Therapy Combined with Antibiotics or Antifungals against Microorganisms That Cause Skin and Soft Tissue Infections: A Planktonic and Biofilm Approach to Overcome Resistances" Pharmaceuticals 14, no. 7: 603. https://doi.org/10.3390/ph14070603
APA StylePérez-Laguna, V., García-Luque, I., Ballesta, S., Rezusta, A., & Gilaberte, Y. (2021). Photodynamic Therapy Combined with Antibiotics or Antifungals against Microorganisms That Cause Skin and Soft Tissue Infections: A Planktonic and Biofilm Approach to Overcome Resistances. Pharmaceuticals, 14(7), 603. https://doi.org/10.3390/ph14070603