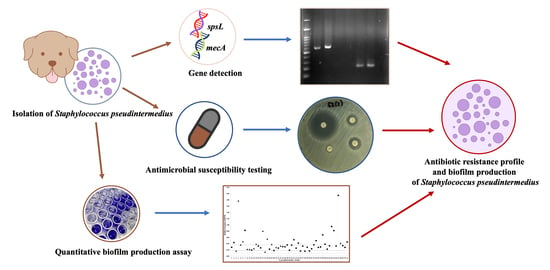
Antibiotic Resistance Profile and Biofilm Production of Staphylococcus pseudintermedius Isolated from Dogs in Thailand
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Isolation of Staphylococcus pseudintermedius
4.2. Antimicrobial Susceptibility Testing
4.3. DNA Extraction
4.4. Gene Detection
4.5. Quantitative Biofilm Production Assay
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Devriese, L.A.; Vancanneyt, M.; Baele, M.; Vaneechoutte, M.; De Graef, E.; Snauwaert, C.; Cleenwerck, I.; Dawyndt, P.; Swings, J.; Decostere, A.; et al. Staphylococcus pseudintermedius sp. nov., a coagulase-positive species from animals. Int. J. Syst. Evol. Microbiol. 2005, 55, 1569–1573. [Google Scholar] [CrossRef]
- Becker, K.; Skov, R.L.; von Eiff, C. Staphylococcus, Micrococcus, and other catalase-positive cocci. In Manual of Clinical Microbiology; ASM Press: Washington, DC, USA, 2015; pp. 354–382. [Google Scholar]
- Somayaji, R.; Priyantha, M.A.; Rubin, J.E.; Church, D. Human infections due to Staphylococcus pseudintermedius, an emerging zoonosis of canine origin: Report of 24 cases. Diagn. Microbiol. Infect. Dis. 2016, 85, 471–476. [Google Scholar] [CrossRef]
- Starlander, G.; Börjesson, S.; Grönlund-Andersson, U.; Tellgren-Roth, C.; Melhus, A. Cluster of infections caused by methicillin-resistant Staphylococcus pseudintermedius in humans in a tertiary hospital. J. Clin. Microbiol. 2014, 52, 3118–3120. [Google Scholar] [CrossRef]
- Kadlec, K.; Weiß, S.; Wendlandt, S.; Schwarz, S.; Tonpitak, W. Characterization of canine and feline methicillin-resistant Staphylococcus pseudintermedius (MRSP) from Thailand. Vet. Microbiol. 2016, 194, 93–97. [Google Scholar] [CrossRef]
- Chanchaithong, P.; Perreten, V.; Schwendener, S.; Tribuddharat, C.; Chongthaleong, A.; Niyomtham, W.; Prapasarakul, N. Strain typing and antimicrobial susceptibility of methicillin-resistant coagulase-positive staphylococcal species in dogs and people associated with dogs in Thailand. J. Appl. Microbiol. 2014, 117, 572–586. [Google Scholar] [CrossRef] [PubMed]
- Phumthanakorn, N.; Fungwithaya, P.; Chanchaithong, P.; Prapasarakul, N. Enterotoxin gene profile of methicillin-resistant Staphylococcus pseudintermedius isolates from dogs, humans and the environment. J. Med. Microbiol. 2018, 67, 866–873. [Google Scholar] [CrossRef] [PubMed]
- Zur, G.; Gurevich, B.; Elad, D. Prior antimicrobial use as a risk factor for resistance in selected Staphylococcus pseudintermedius isolates from the skin and ears of dogs. Vet. Dermatol. 2016, 27, 468–e125. [Google Scholar] [CrossRef]
- Grönthal, T.; Eklund, M.; Thomson, K.; Piiparinen, H.; Sironen, T.; Rantala, M. Antimicrobial resistance in Staphylococcus pseudintermedius and the molecular epidemiology of methicillin-resistant S. pseudintermedius in small animals in Finland. J. Antimicrob. Chemother. 2017, 72, 1021–1030. [Google Scholar] [CrossRef]
- Silva, V.; Oliveira, A.; Manageiro, V.; Caniça, M.; Contente, D.; Capita, R.; Alonso-Calleja, C.; Carvalho, I.; Capelo, J.L.; Igrejas, G.; et al. Clonal diversity and antimicrobial resistance of methicillin-resistant Staphylococcus pseudintermedius isolated from canine pyoderma. Microorganisms 2021, 9, 482. [Google Scholar] [CrossRef] [PubMed]
- Kadlec, K.; Schwarz, S. Antimicrobial resistance of Staphylococcus pseudintermedius. Vet. Dermatol. 2012, 23, 276–282, e255. [Google Scholar] [CrossRef]
- Flemming, H.C.; Wingender, J.; Szewzyk, U.; Steinberg, P.; Rice, S.A.; Kjelleberg, S. Biofilms: An emergent form of bacterial life. Nat. Rev. Microbiol. 2016, 14, 563–575. [Google Scholar] [CrossRef]
- Otto, M. Staphylococcal biofilms. Curr. Top. Microbiol. Immunol. 2008, 322, 207–228. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Walker, M.; Rousseau, J.; Weese, J.S. Characterization of the biofilm forming ability of Staphylococcus pseudintermedius from dogs. BMC Vet. Res. 2013, 9, 93. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Agarwal, A. Biofilm production, a marker of pathogenic potential of colonizing and commensal staphylococci. J. Microbiol. Methods 2009, 76, 88–92. [Google Scholar] [CrossRef]
- Pompilio, A.; De Nicola, S.; Crocetta, V.; Guarnieri, S.; Savini, V.; Carretto, E.; Di Bonaventura, G. New insights in Staphylococcus pseudintermedius pathogenicity: Antibiotic-resistant biofilm formation by a human wound-associated strain. BMC Microbiol. 2015, 15, 109. [Google Scholar] [CrossRef]
- Arima, S.; Ochi, H.; Mitsuhashi, M.; Kibe, R.; Takahashi, K.; Kataoka, Y. Staphylococcus pseudintermedius biofilms secrete factors that induce inflammatory reactions in vitro. Lett. Appl. Microbiol. 2018, 67, 214–219. [Google Scholar] [CrossRef]
- Han, J.I.; Yang, C.H.; Park, H.M. Emergence of biofilm-producing Staphylococcus pseudintermedius isolated from healthy dogs in South Korea. Vet. Q. 2015, 35, 207–210. [Google Scholar] [CrossRef]
- Tonpitak, W.; Sornklien, C. Prevalence of methicillin-resistant Staphylococcus pseudintermedius isolates from healthy dogs and healthy cats in a small animal teaching hospital. Vet. Integr. Sci 2014, 12, 95–105. [Google Scholar]
- Clinical and Laboratory Standards Institute. M100: Performance Standards for Antimicrobial Susceptibility Testing, 30th ed.; CLSI: Wayne, PA, USA, 2020. [Google Scholar]
- Bhooshan, S.; Negi, V.; Khatri, P.K. Staphylococcus pseudintermedius: An undocumented, emerging pathogen in humans. GMS Hyg. Infect. Control 2020, 15, 1–11. [Google Scholar] [CrossRef]
- Darlow, C.A.; Paidakakos, N.; Sikander, M.; Atkins, B. A spinal infection with Staphylococcus pseudintermedius. BMJ Case Rep. 2017, 2017. [Google Scholar] [CrossRef]
- Botoni, L.S.; Scherer, C.B.; Silva, R.O.; Coura, F.M.; Heinemann, M.B.; Paes-Leme, F.O.; Costa-Val, A.P. Prevalence and in vitro susceptibility of methicillin-resistant Staphylococcus pseudintermedius (MRSP) from skin and nostrils of dogs with superficial pyoderma. Pesq. Vet. Bras. 2016, 36, 1178–1180. [Google Scholar] [CrossRef]
- Nocera, F.P.; Meroni, G.; Fiorito, F.; De Martino, L.; Martino, P.A. Occurrence and antimicrobial susceptibility patterns of canine Staphylococcus pseudintermedius strains isolated from two different Italian university veterinary hospitals. Vet. Ital. 2020, 56, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Nisa, S.; Bercker, C.; Midwinter, A.C.; Bruce, I.; Graham, C.F.; Venter, P.; Bell, A.; French, N.P.; Benschop, J.; Bailey, K.M.; et al. Combining MALDI-TOF and genomics in the study of methicillin resistant and multidrug resistant Staphylococcus pseudintermedius in New Zealand. Sci. Rep. 2019, 9, 1271–1273. [Google Scholar] [CrossRef]
- Elhassan, M.M.; Ozbak, H.A.; Hemeg, H.A.; Elmekki, M.A.; Ahmed, L.M. Absence of the mecA gene in methicillin resistant Staphylococcus aureus isolated from different clinical specimens in Shendi City, Sudan. Biomed. Res. Int. 2015, 2015, 895860. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.J.; Huang, I.W.; Wang, C.H.; Chen, P.C.; Wang, H.Y.; Lai, J.F.; Shiau, Y.R.; Lauderdale, T.L. mecA-positive Staphylococcus aureus with low-level oxacillin MIC in Taiwan. J. Clin. Microbiol. 2012, 50, 1679–1683. [Google Scholar] [CrossRef]
- Cho, J.-K.; Lee, M.-R.; Kim, J.-M.; Kim, H.-D. Methicillin-resistant or susceptible Staphylococcus pseudintermedius isolates from dogs and cats. Korean J. Vet. Serv. 2016, 39, 175–181. [Google Scholar] [CrossRef][Green Version]
- Sekiguchi, J.; Tharavichitkul, P.; Miyoshi-Akiyama, T.; Chupia, V.; Fujino, T.; Araake, M.; Irie, A.; Morita, K.; Kuratsuji, T.; Kirikae, T. Cloning and characterization of a novel trimethoprim-resistant dihydrofolate reductase from a nosocomial isolate of Staphylococcus aureus CM.S2 (IMCJ1454). Antimicrob. Agents. Chemother. 2005, 49, 3948–3951. [Google Scholar] [CrossRef]
- Meroni, G.; Soares Filipe, J.F.; Drago, L.; Martino, P.A. Investigation on antibiotic-resistance, biofilm formation and virulence factors in multi drug resistant and non multi drug resistant Staphylococcus pseudintermedius. Microorganisms 2019, 7, 702. [Google Scholar] [CrossRef]
- Senobar Tahaei, S.A.; Stájer, A.; Barrak, I.; Ostorházi, E.; Szabó, D.; Gajdács, M. Correlation between biofilm-formation and the antibiotic resistant phenotype in Staphylococcus aureus isolates: A laboratory-based study in Hungary and a review of the literature. Infect. Drug Resist. 2021, 14, 1155–1168. [Google Scholar] [CrossRef]
- Behzadi, P.; Urbán, E.; Gajdács, M. Association between biofilm-production and antibiotic resistance in uropathogenic Escherichia coli (UPEC): An in vitro study. Diseases 2020, 8, 17. [Google Scholar] [CrossRef]
- Saxena, P.; Joshi, Y.; Rawat, K.; Bisht, R. Biofilms: Architecture, resistance, quorum sensing and control mechanisms. Indian J. Microbiol. 2019, 59, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Maali, Y.; Martins-Simões, P.; Valour, F.; Bouvard, D.; Rasigade, J.P.; Bes, M.; Haenni, M.; Ferry, T.; Laurent, F.; Trouillet-Assant, S. Pathophysiological mechanisms of Staphylococcus non-aureus bone and joint infection: Interspecies homogeneity and specific behavior of S. pseudintermedius. Front. Microbiol. 2016, 7, 1063–1072. [Google Scholar] [CrossRef] [PubMed]
- Stegger, Á.; Andersen, P.; Kearns, A.; Pichon, B.; Holmes, M.; Edwards, G.; Laurent, F.; Teale, C.; Skov, R.; Larsen, A. Rapid detection, differentiation and typing of methicillin-resistant Staphylococcus aureus harbouring either mecA or the new mecA homologue mecALGA251. Clin. Microbiol. Infect. 2012, 18, 395–400. [Google Scholar] [CrossRef]
- Stepanović, S.; Vuković, D.; Hola, V.; Di Bonaventura, G.; Djukić, S.; Cirković, I.; Ruzicka, F. Quantification of biofilm in microtiter plates: Overview of testing conditions and practical recommendations for assessment of biofilm production by staphylococci. APMIS 2007, 115, 891–899. [Google Scholar] [CrossRef] [PubMed]

| Results Obtained by Oxacillin Disk Diffusion Method | mecA Gene Detection |
|---|---|
| MSSP (38/53; 71.70%) | 7/38 (18.42%) |
| MRSP (15/53; 28.30%) | 14/15 (93.33%) |
| Antibiotic Resistance Profile | MRSP (n = 15 isolates) | |
| % Resistant | Number of Isolates | |
| C-DA-TE-CRT-CIP-SXT | 20 | 3 |
| C-DA-TE-CRT-CIP | 6.67 | 1 |
| C-DA-TE-CRT-SXT | 6.67 | 1 |
| C-TE-CRT-CIP | 20 | 3 |
| DA-TE-CRT-CIP-SXT | 26.67 | 4 |
| DA-TE-CIP-SXT | 13.33 | 2 |
| TE-CIP-SXT | 6.67 | 1 |
| Total | 100 | 15 |
| Antibiotic Resistance Profile | MSSP (n = 38 isolates) | |
| % Resistant | Number of Isolates | |
| C-DA-TE-CRT-CIP-SXT | 2.63 | 1 |
| C-DA-TE-CRT-SXT | 2.63 | 1 |
| C | 2.63 | 1 |
| DA-TE-CRT-CIP-SXT | 2.63 | 1 |
| DA-TE-CIP | 2.63 | 1 |
| DA | 2.63 | 1 |
| TE-SXT | 2.63 | 1 |
| Total | 18.41 | 7 |
| Antibiotics | % Biofilm Producer (Isolates) | p Value | |||||
|---|---|---|---|---|---|---|---|
| Resistant | Susceptible | ||||||
| Weak | Moderate | Strong | Weak | Moderate | Strong | ||
| OX | 13.33% (2) | 26.67% (4) | 60.00% (9) | 7.89% (3) | 57.89% (22) | 34.21% (13) | 0.12 |
| C | 9.09% (1) | 45.45% (5) | 45.45% (5) | 9.52% (4) | 50.00% (21) | 40.48% (17) | 0.96 |
| DA | 6.25% (1) | 43.75% (7) | 50.00% (8) | 10.81% (4) | 51.35% (19) | 37.84% (14) | 0.68 |
| TE | 10.00% (2) | 40.00% (8) | 50.00% (10) | 9.09% (3) | 54.55% (18) | 36.36% (12) | 0.58 |
| CRT | 13.33% (2) | 40.00% (6) | 46.67% (7) | 7.89% (3) | 52.63% (20) | 39.47% (15) | 0.66 |
| CIP | 11.76% (2) | 35.29% (6) | 52.94% (9) | 8.33% (3) | 55.56% (20) | 36.11% (13) | 0.39 |
| SXT | 6.67% (1) | 40.00% (6) | 53.33% (8) | 10.53% (4) | 52.63% (20) | 36.84% (14) | 0.54 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jantorn, P.; Heemmamad, H.; Soimala, T.; Indoung, S.; Saising, J.; Chokpaisarn, J.; Wanna, W.; Tipmanee, V.; Saeloh, D. Antibiotic Resistance Profile and Biofilm Production of Staphylococcus pseudintermedius Isolated from Dogs in Thailand. Pharmaceuticals 2021, 14, 592. https://doi.org/10.3390/ph14060592
Jantorn P, Heemmamad H, Soimala T, Indoung S, Saising J, Chokpaisarn J, Wanna W, Tipmanee V, Saeloh D. Antibiotic Resistance Profile and Biofilm Production of Staphylococcus pseudintermedius Isolated from Dogs in Thailand. Pharmaceuticals. 2021; 14(6):592. https://doi.org/10.3390/ph14060592
Chicago/Turabian StyleJantorn, Pavarish, Hawaree Heemmamad, Tanawan Soimala, Saowakon Indoung, Jongkon Saising, Julalak Chokpaisarn, Warapond Wanna, Varomyalin Tipmanee, and Dennapa Saeloh. 2021. "Antibiotic Resistance Profile and Biofilm Production of Staphylococcus pseudintermedius Isolated from Dogs in Thailand" Pharmaceuticals 14, no. 6: 592. https://doi.org/10.3390/ph14060592
APA StyleJantorn, P., Heemmamad, H., Soimala, T., Indoung, S., Saising, J., Chokpaisarn, J., Wanna, W., Tipmanee, V., & Saeloh, D. (2021). Antibiotic Resistance Profile and Biofilm Production of Staphylococcus pseudintermedius Isolated from Dogs in Thailand. Pharmaceuticals, 14(6), 592. https://doi.org/10.3390/ph14060592