Dextran Sodium Sulphate-Induced Gastrointestinal Injury Further Aggravates the Impact of Galantamine on the Gastric Myoelectric Activity in Experimental Pigs
Abstract
1. Introduction
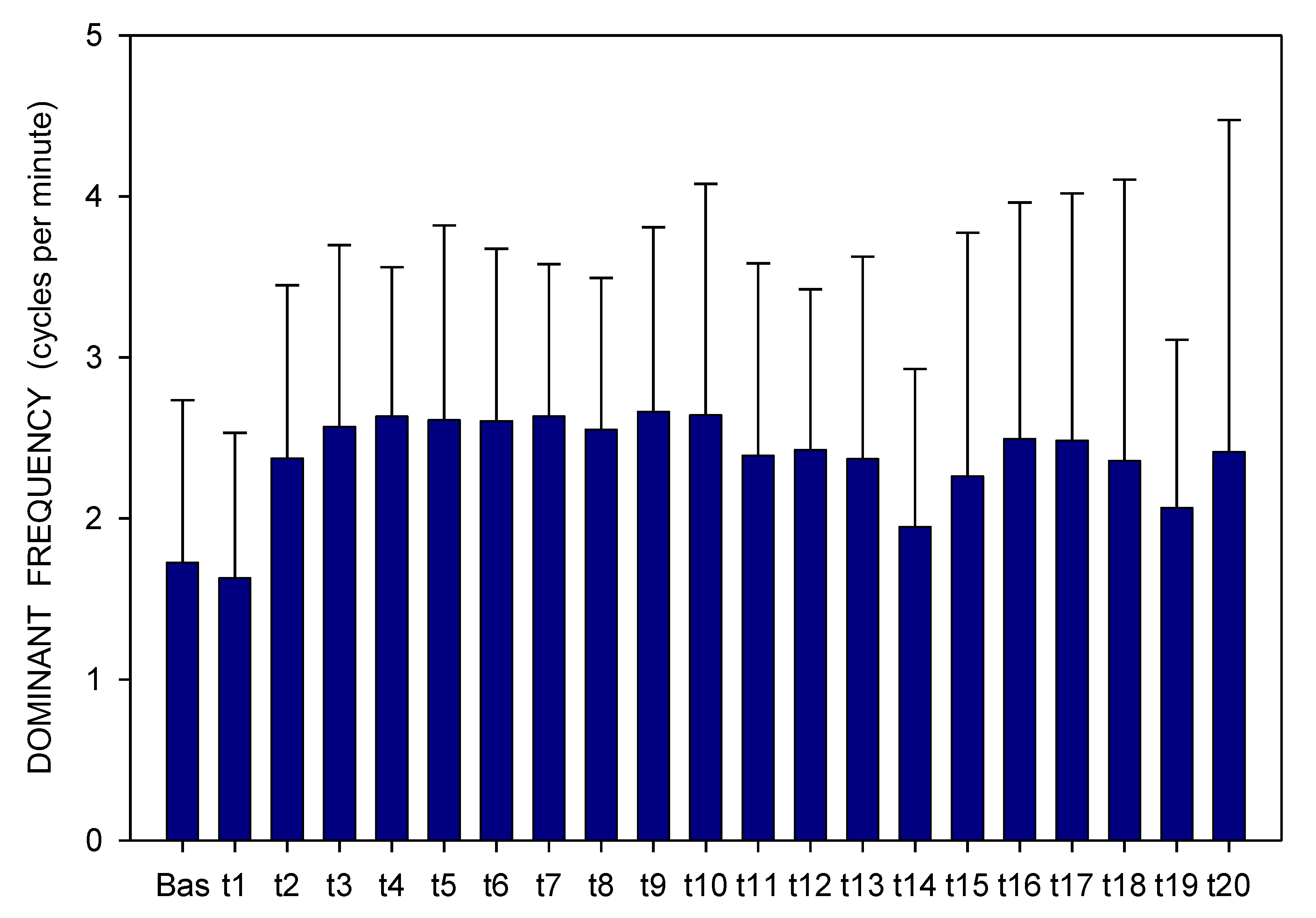
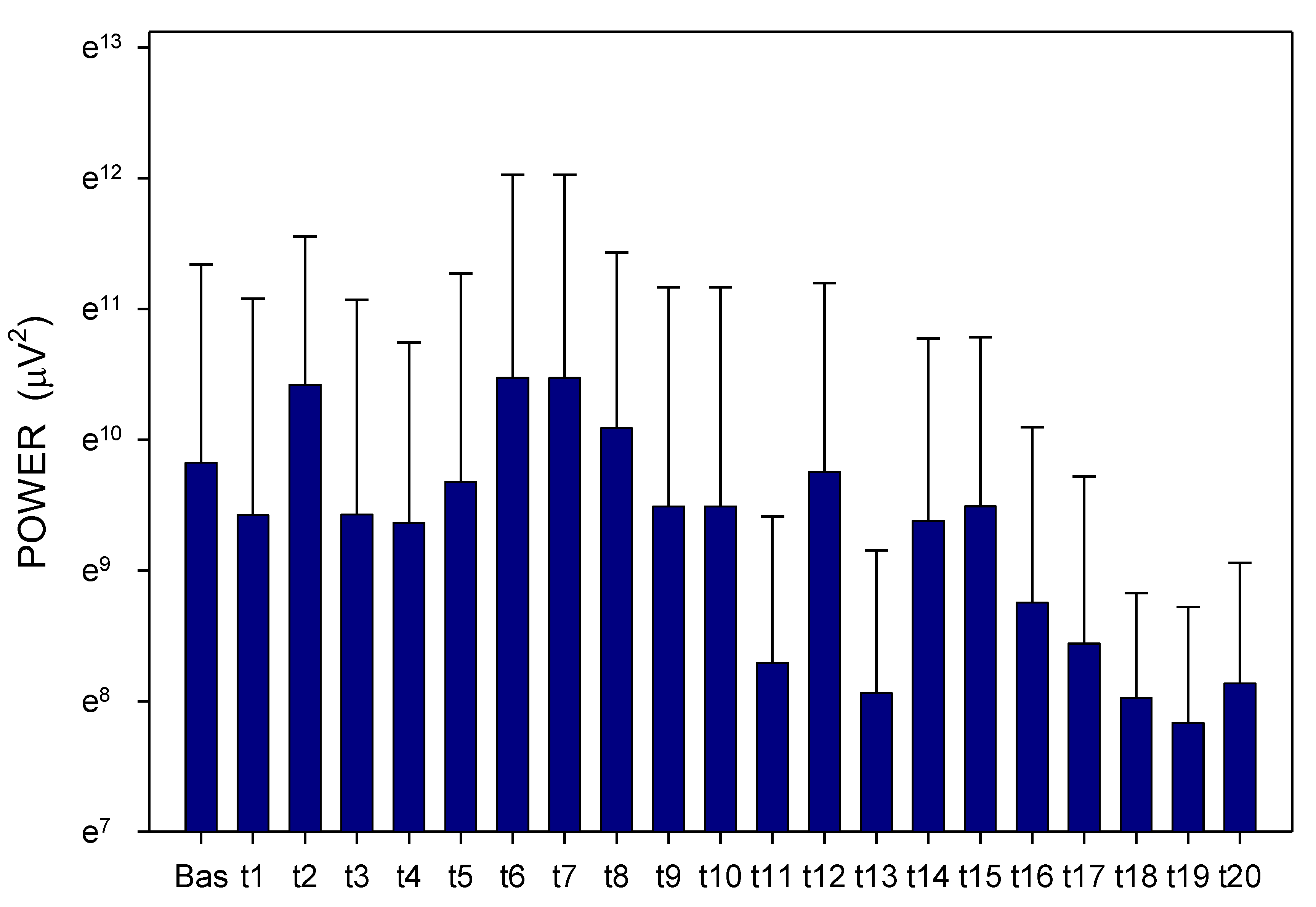
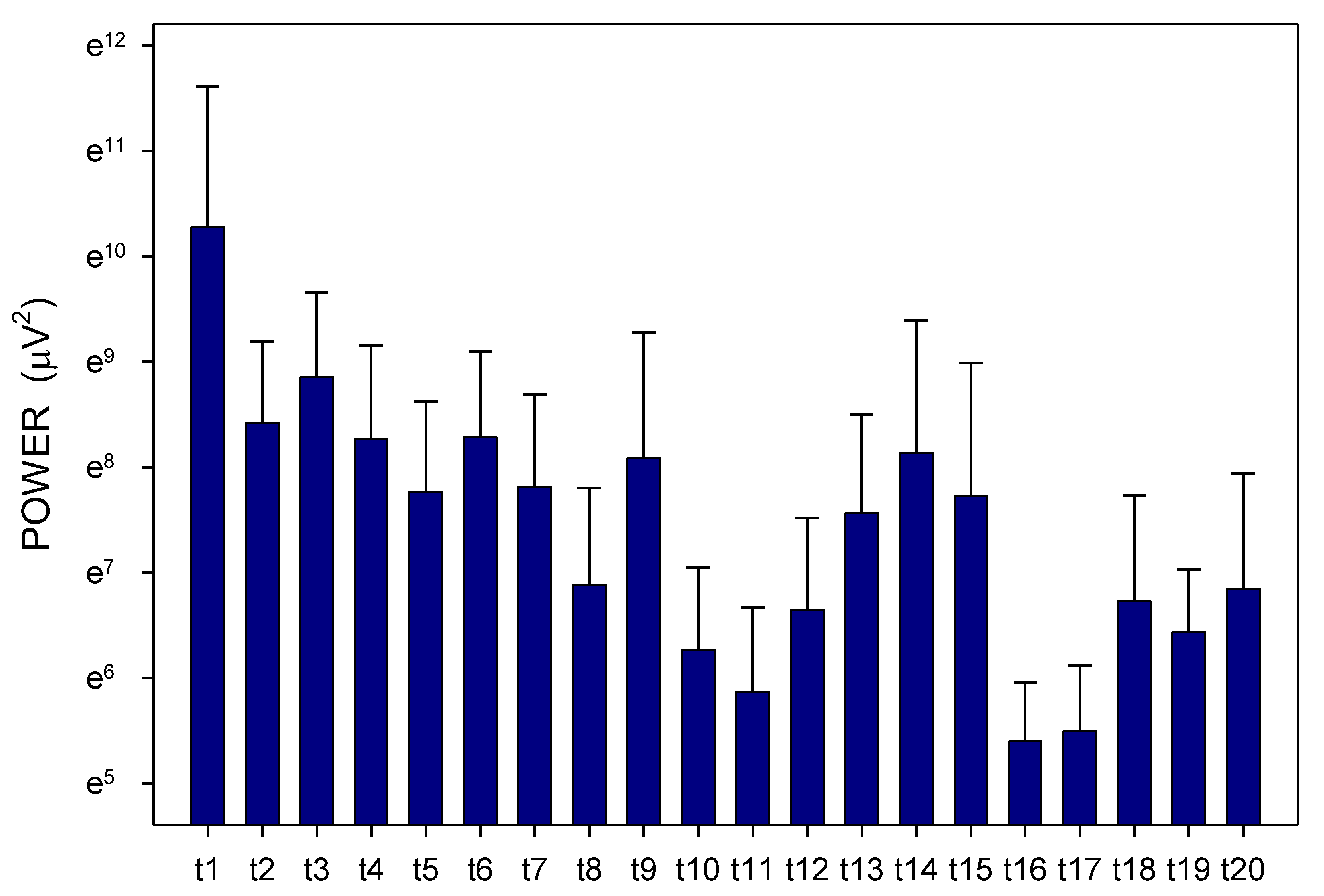
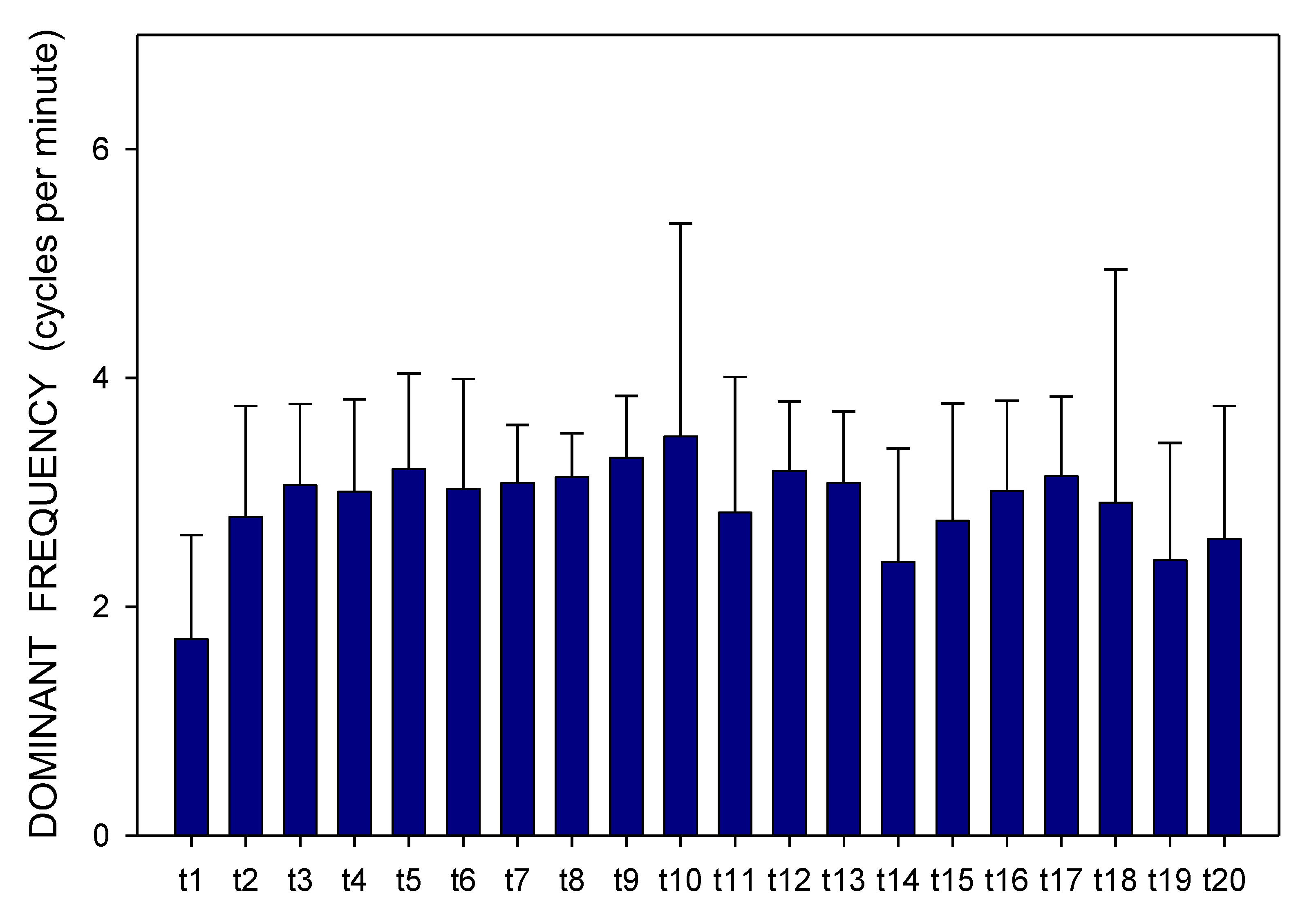
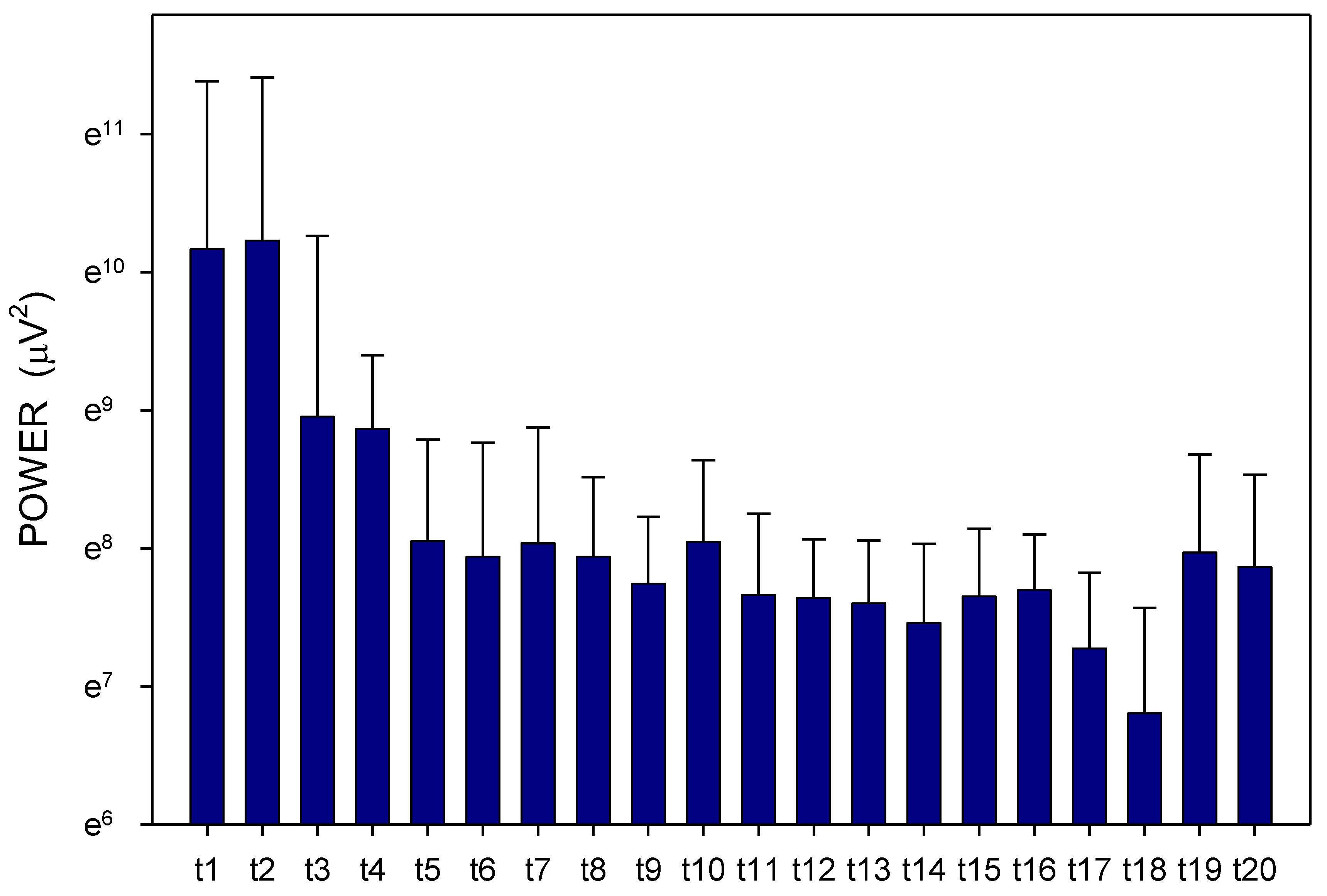
2. Results
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Design of the Study
4.3. Statistical Analysis
4.4. Ethics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tan, C.C.; Yu, J.T.; Wang, H.F.; Tan, M.S.; Meng, X.F.; Wang, C.; Jiang, T.; Zhu, X.C.; Tan, L. Efficacy and safety of donepezil, galantamine, rivastigmine, and memantine for the treatment of Alzheimer’s disease: A systematic review and meta-analysis. J. Alzheimers Dis. 2014, 41, 615–631. [Google Scholar] [CrossRef]
- Hager, K.; Baseman, A.S.; Nye, J.S.; Brashear, H.R.; Han, J.; Sano, M.; Davis, B.; Richards, H.M. Effects of galantamine in a 2-year, randomized, placebo-controlled study in Alzheimer’s disease. Neuropsychiatr. Dis. Treat. 2014, 10, 391–401. [Google Scholar] [PubMed]
- Korabecny, J.; Nepovimova, E.; Cikankova, T.; Spilovska, K.; Vaskova, L.; Mezeiova, E.; Kuca, K.; Hroudova, J. Newly developed drugs for Alzheimer’s disease in relation to energy metabolism, cholinergic and monoaminergic neurotransmission. Neuroscience 2018, 370, 191–206. [Google Scholar] [CrossRef] [PubMed]
- Tricco, A.C.; Ashoor, H.M.; Soobiah, C.; Rios, P.; Veroniki, A.A.; Hamid, J.S.; Ivory, J.D.; Khan, P.A.; Yazdi, F.; Ghassemi, M.; et al. Comparative effectiveness and safety of cognitive enhancers for treating Alzheimer’s disease: Systematic review and network metaanalysis. J. Am. Geriatr. Soc. 2018, 66, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Pohanka, M. Inhibitors of cholinesterases in pharmacology: The current trends. Mini Rev. Med. Chem. 2020, 20, 1532–1542. [Google Scholar] [CrossRef] [PubMed]
- Lilienfeld, S. Galantamine: A novel cholinergic drug with a unique dual mode of action for the treatment of patients with Alzheimer’s disease. CNS Drug Rev. 2002, 8, 159–176. [Google Scholar] [CrossRef]
- Scott, L.J.; Goa, K.L. Galantamine: A review of its use in Alzheimer’s disease. Drugs 2000, 60, 1095–1122. [Google Scholar] [CrossRef] [PubMed]
- Zarotsky, V.; Sramek, J.J.; Cutler, N.R. Galantamine hydrobromide: An agent for Alzheimer’s disease. Am. J. Health Syst. Pharm. 2003, 60, 446–452. [Google Scholar] [CrossRef] [PubMed]
- Hughes, A.; Musher, J.; Thomas, S.K.; Beusterien, K.M.; Strunk, B.; Arcona, S. Gastrointestinal adverse events in a general population sample of nursing home residents taking cholinesterase inhibitors. Consult. Pharm. 2004, 19, 713–720. [Google Scholar] [CrossRef]
- Loy, C.; Schneider, L. Galantamine for Alzheimer’s disease. Cochrane Database Syst. Rev. 2004, CD001747. [Google Scholar] [CrossRef]
- Orgogozo, J.M.; Small, G.W.; Hammond, G.; Van Baelen, B.; Schwalen, S. Effects of galantamine in patients with mild Alzheimer’s disease. Curr. Med. Res. Opin. 2004, 20, 1815–1820. [Google Scholar] [CrossRef]
- Birks, J.; Craig, D. Galantamine for vascular cognitive impairment. Cochrane Database Syst. Rev. 2006, CD004746. [Google Scholar] [CrossRef]
- Prvulovic, D.; Hampel, H.; Pantel, J. Galantamine for Alzheimer’s disease. Expert Opin. Drug Metab. Toxicol. 2010, 6, 345–354. [Google Scholar] [CrossRef] [PubMed]
- Haake, A.; Nguyen, K.; Friedman, L.; Chakkamparambil, B.; Grossberg, G.T. An update on the utility and safety of cholinesterase inhibitors for the treatment of Alzheimer’s disease. Expert Opin. Drug Saf. 2020, 19, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Tamaru, T.; Kobayashi, H.; Kishimoto, S.; Kajiyama, G.; Shimamoto, F.; Brown, W.R. Histochemical study of colonic cancer in experimental colitis of rats. Dig. Dis. Sci. 1993, 38, 529–537. [Google Scholar] [CrossRef] [PubMed]
- Dieleman, L.A.; Ridwan, B.U.; Tennyson, G.S.; Beagley, K.W.; Bucy, R.P.; Elson, C.O. Dextran sulfate sodium-induced colitis occurs in severe combined immunodeficient mice. Gastroenterology 1994, 107, 1643–1652. [Google Scholar] [CrossRef]
- Ni, J.; Chen, S.F.; Hollander, D. Effects of dextran sulphate sodium on intestinal epithelial cells and intestinal lymphocytes. Gut 1996, 39, 234–241. [Google Scholar] [CrossRef]
- Kim, C.J.; Kovacs-Nolan, J.A.; Yang, C.; Archbold, T.; Fan, M.Z.; Mine, Y. l-Tryptophan exhibits therapeutic function in a porcine model of dextran sodium sulfate (DSS)-induced colitis. J. Nutr. Biochem. 2010, 21, 468–475. [Google Scholar] [CrossRef]
- Lackeyram, D.; Mine, Y.; Archbold, T.; Fan, M.Z. The small intestinal apical hydrolase activities are decreased in the piglet with bowel inflammation induced by dextran sodium sulfate. J. Anim. Sci. 2012, 90 (Suppl. 4), 287–289. [Google Scholar] [CrossRef]
- Ibuki, M.; Fukui, K.; Kanatani, H.; Mine, Y. Anti-inflammatory effects of mannanase-hydrolyzed copra meal in a porcine model of colitis. J. Vet. Med. Sci. 2014, 76, 645–651. [Google Scholar] [CrossRef]
- Chassaing, B.; Aitken, J.D.; Malleshappa, M.; Vijay-Kumar, M. Dextran sulfate sodium (DSS)-induced colitis in mice. Curr. Protoc. Immunol. 2014, 104, 15.25.1–15.25.14. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.C.; Bériou, G.; Josien, R. Dextran sulfate sodium (DSS)-induced acute colitis in the rat. Methods Mol. Biol. 2016, 1371, 197–203. [Google Scholar]
- Parang, B.; Barrett, C.W.; Williams, C.S. AOM/DSS model of colitis-associated cancer. Methods Mol. Biol. 2016, 1422, 297–307. [Google Scholar] [PubMed]
- Lackeyram, D.; Young, D.; Kim, C.J.; Yang, C.; Archbold, T.L.; Mine, Y.; Fan, M.Z. Interleukin-10 is differentially expressed in the small intestine and the colon experiencing chronic inflammation and ulcerative colitis induced by dextran sodium sulfate in young pigs. Physiol. Res. 2017, 66, 147–162. [Google Scholar] [CrossRef]
- Nielsen, T.S.; Fredborg, M.; Theil, P.K.; Yue, Y.; Bruhn, L.V.; Andersen, V.; Purup, S. Dietary red meat adversely affects disease severity in a pig model of DSS-induced colitis despite reduction in colonic pro-inflammatory gene expression. Nutrients 2020, 12, 1728. [Google Scholar] [CrossRef]
- Tacheci, I.; Kvetina, J.; Kunes, M.; Edakkanambeth Varayil, J.; Ali, S.M.; Pavlik, M.; Kopacova, M.; Rejchrt, S.; Bures, J.; Pleskot, M. Electrogastrography in experimental pigs: The influence of gastrointestinal injury induced by dextran sodium sulphate on porcine gastric erythromycin-stimulated myoelectric activity. Neuro Endocrinol. Lett. 2011, 32 (Suppl. 1), 131–136. [Google Scholar] [PubMed]
- Chen, J.Z.; McCallum, R.W. (Eds.) Electrogastrography. In Principles and Applications; Raven Press: New York, NY, USA, 1994. [Google Scholar]
- Parkman, H.P.; Hasler, W.L.; Barnett, J.L.; Eaker, E.Y.; American Motility Society Clinical GI Motility Testing Task Force. Electrogastrography: A document prepared by the gastric section of the American Motility Society Clinical GI Motility Testing Task Force. Neurogastroenterol. Motil. 2003, 15, 89–102. [Google Scholar] [CrossRef]
- Koch, K.L.; Stern, R.M. Handbook of Electrogastrography; Oxford University Press: Oxford, UK, 2004. [Google Scholar]
- Bures, J.; Kabelac, K.; Kopacova, M.; Vorisek, V.; Siroky, M.; Palicka, V.; Rejchrt, S. Electrogastrography in patients with Roux-en-Y reconstruction after previous Billroth gastrectomy. Hepatogastroenterology 2008, 55, 1492–1496. [Google Scholar] [PubMed]
- Murakami, H.; Matsumoto, H.; Ueno, D.; Kawai, A.; Ensako, T.; Kaida, Y.; Abe, T.; Kubota, H.; Higashida, M.; Nakashima, H.; et al. Current status of multichannel electrogastrography and examples of its use. J. Smooth Muscle Res. 2013, 49, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Wolpert, N.; Rebollo, I.; Tallon-Baudry, C. Electrogastrography for psychophysiological research: Practical considerations, analysis pipeline, and normative data in a large sample. Psychophysiology 2020, 57, e13599. [Google Scholar] [CrossRef]
- Bures, J.; Kvetina, J.; Radochova, V.; Tacheci, I.; Peterova, E.; Herman, D.; Dolezal, R.; Kopacova, M.; Rejchrt, S.; Douda, T.; et al. The pharmacokinetic parameters and the effect of a single and repeated doses of memantine on gastric myoelectric activity in experimental pigs. PLoS ONE 2020, 15, e0227781. [Google Scholar] [CrossRef] [PubMed]
- Bures, J.; Kvetina, J.; Tacheci, I.; Pavlik, M.; Kunes, M.; Rejchrt, S.; Kuca, K.; Kopacova, M. The effect of different doses of atropine on gastric myoelectrical activity in fasting experimental pigs. J. Appl. Biomed. 2015, 13, 273–277. [Google Scholar] [CrossRef]
- Bures, J.; Kvetina, J.; Pavlik, M.; Kunes, M.; Kopacova, M.; Rejchrt, S.; Jun, D.; Hrabinova, M.; Kuca, K.; Tachecí, I. Impact of paraoxon followed by acetylcholinesterase reactivator HI-6 on gastric myoelectric activity in experimental pigs. Neuro Endocrinol. Lett. 2013, 34 (Suppl. 2), 79–83. [Google Scholar]
- Bures, J.; Jun, D.; Hrabinova, M.; Tacheci, I.; Kvetina, J.; Pavlik, M.; Rejchrt, S.; Douda, T.; Kunes, M.; Kuca, K.; et al. Impact of tacrine and 7-methoxytacrine on gastric myoelectrical activity assessed using electrogastrography in experimental pigs. Neuro Endocrinol. Lett. 2015, 36 (Suppl. 1), 150–155. [Google Scholar]
- Kararli, T.T. Comparison of the gastrointestinal anatomy, physiology, and biochemistry of humans and commonly used laboratory animals. Biopharm. Drug Dispos. 1995, 16, 351–380. [Google Scholar] [CrossRef]
- Gonzalez, L.M.; Moeser, A.J.; Blikslager, A.T. Porcine models of digestive disease: The future of large animal translational research. Transl. Res. 2015, 166, 12–27. [Google Scholar] [CrossRef]
- Xiao, Y.; Yan, H.; Diao, H.; Yu, B.; He, J.; Yu, J.; Zheng, P.; Mao, X.; Luo, Y.; Chen, D. Early gut microbiota intervention suppresses DSS-induced inflammatory responses by deactivating TLR/NLR signalling in pigs. Sci. Rep. 2017, 7, 3224. [Google Scholar] [CrossRef]
- Bures, J.; Tacheci, I.; Kvetina, J.; Radochova, V.; Prchal, L.; Kohoutova, D.; Valis, M.; Novak, M.; Dolezal, R.; Kopacova, M.; et al. The impact of dextran sodium sulfate-induced gastrointestinal injury on the pharmacokinetic parameters of donepezil and its active metabolite 6-O-desmethyldonepezil, and gastric myoelectric activity in experimental pigs. Molecules 2021, 26, 2160. [Google Scholar] [CrossRef] [PubMed]
- Parkman, H.P.; Trate, D.M.; Knight, L.C.; Brown, K.L.; Maurer, A.H.; Fisher, R.S. Cholinergic effects on human gastric motility. Gut 1999, 45, 346–354. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.H.; Taylor, P. Muscarinic receptor agonists and antagonists. In Goodman & Gilman’s Pharmacological Basis of Therapeutics; Brunton, L., Lazo, J.S., Parker, K.L., Eds.; McGraw-Hill: New York, NY, USA, 2006; pp. 183–200. [Google Scholar]
- Kvetina, J.; Tacheci, I.; Pavlik, M.; Kopacova, M.; Rejchrt, S.; Douda, T.; Kunes, M.; Bures, J. Use of electrogastrography in preclinical studies of cholinergic and anticholinergic agents in experimental pigs. Physiol. Res. 2015, 64 (Suppl. 5), S647–S652. [Google Scholar] [CrossRef] [PubMed]
- Turiiski, V.I.; Krustev, A.D.; Sirakov, V.N.; Getova, D.P. In vivo and in vitro study of the influence of the anticholinesterase drug galantamine on motor and evacuative functions of rat gastrointestinal tract. Eur. J. Pharmacol. 2004, 498, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Vigneault, P.; Bourgault, S.; Kaddar, N.; Caillier, B.; Pilote, S.; Patoine, D.; Simard, C.; Drolet, B. Galantamine (Reminyl) delays cardiac ventricular repolarization and prolongs the QT interval by blocking the HERG current. Eur. J. Pharmacol. 2012, 681, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Tacheci, I.; Radochova, V.; Kvetina, J.; Rejchrt, S.; Kopacova, M.; Bures, J. Oesophageal manometry in experimental pigs: Methods and initial experience. Acta Med. 2015, 58, 131–134. [Google Scholar] [CrossRef] [PubMed]
- Tveden-Nyborg, P.; Bergmann, T.K.; Lykkesfeldt, J. Basic & clinical pharmacology & toxicology policy for experimental and clinical studies. Basic Clin. Pharmacol. Toxicol. 2018, 123, 233–235. [Google Scholar]
- Explanatory Report on the European Convention for the Protection of Vertebrate Animals Used for Experimental and Other Scientific Purposes (ETS 123); Council of Europe: Strasbourg, France, 2009.




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bures, J.; Tacheci, I.; Kvetina, J.; Radochova, V.; Kohoutova, D.; Valis, M.; Rejchrt, S.; Knoblochova, V.; Zdarova Karasova, J. Dextran Sodium Sulphate-Induced Gastrointestinal Injury Further Aggravates the Impact of Galantamine on the Gastric Myoelectric Activity in Experimental Pigs. Pharmaceuticals 2021, 14, 590. https://doi.org/10.3390/ph14060590
Bures J, Tacheci I, Kvetina J, Radochova V, Kohoutova D, Valis M, Rejchrt S, Knoblochova V, Zdarova Karasova J. Dextran Sodium Sulphate-Induced Gastrointestinal Injury Further Aggravates the Impact of Galantamine on the Gastric Myoelectric Activity in Experimental Pigs. Pharmaceuticals. 2021; 14(6):590. https://doi.org/10.3390/ph14060590
Chicago/Turabian StyleBures, Jan, Ilja Tacheci, Jaroslav Kvetina, Vera Radochova, Darina Kohoutova, Martin Valis, Stanislav Rejchrt, Veronika Knoblochova, and Jana Zdarova Karasova. 2021. "Dextran Sodium Sulphate-Induced Gastrointestinal Injury Further Aggravates the Impact of Galantamine on the Gastric Myoelectric Activity in Experimental Pigs" Pharmaceuticals 14, no. 6: 590. https://doi.org/10.3390/ph14060590
APA StyleBures, J., Tacheci, I., Kvetina, J., Radochova, V., Kohoutova, D., Valis, M., Rejchrt, S., Knoblochova, V., & Zdarova Karasova, J. (2021). Dextran Sodium Sulphate-Induced Gastrointestinal Injury Further Aggravates the Impact of Galantamine on the Gastric Myoelectric Activity in Experimental Pigs. Pharmaceuticals, 14(6), 590. https://doi.org/10.3390/ph14060590





