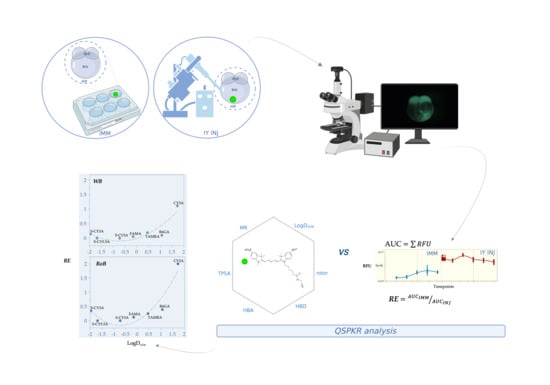
Pharmacokinetics in Zebrafish Embryos (ZFE) Following Immersion and Intrayolk Administration: A Fluorescence-Based Analysis
Abstract
1. Introduction
2. Results
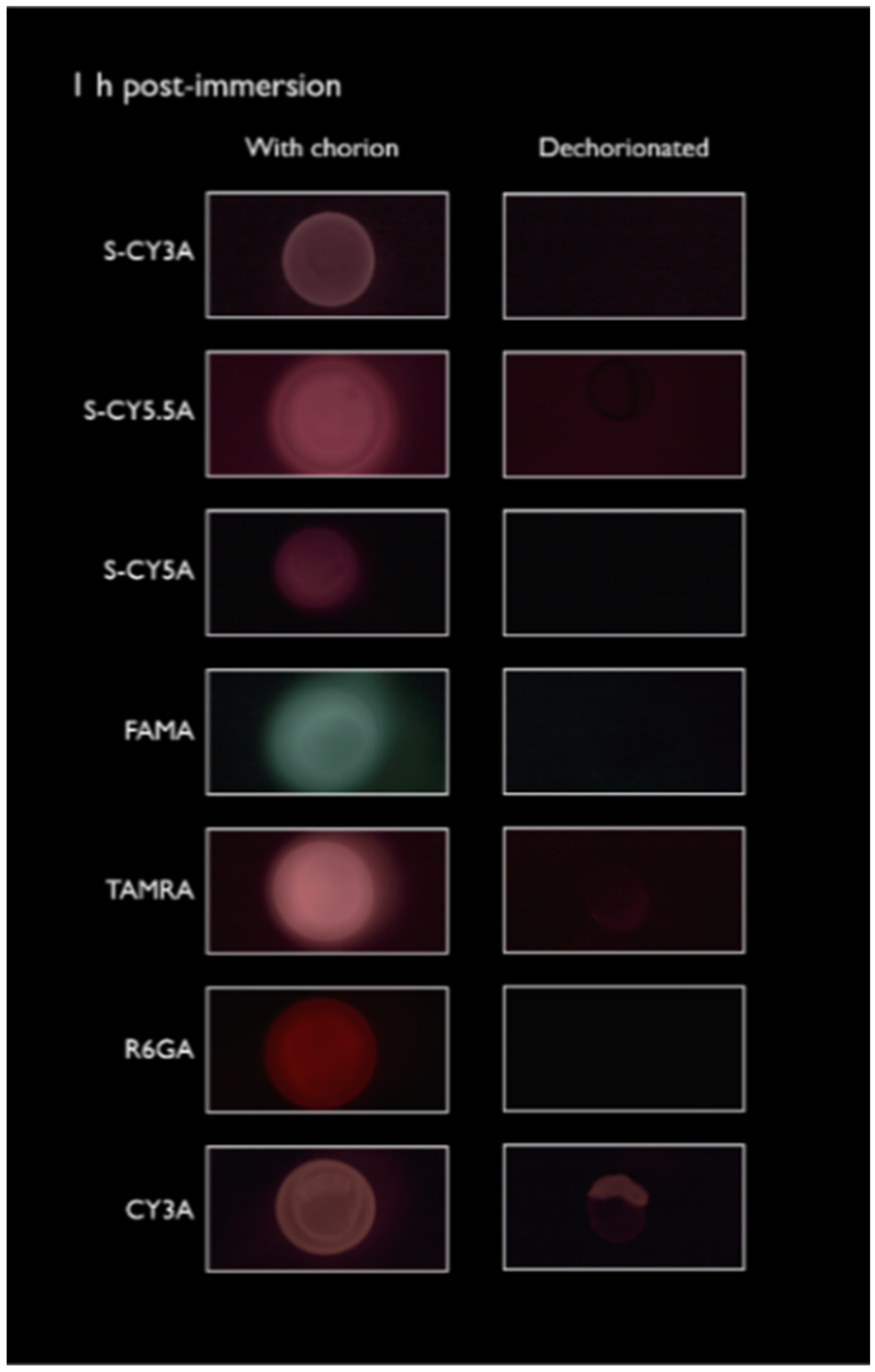
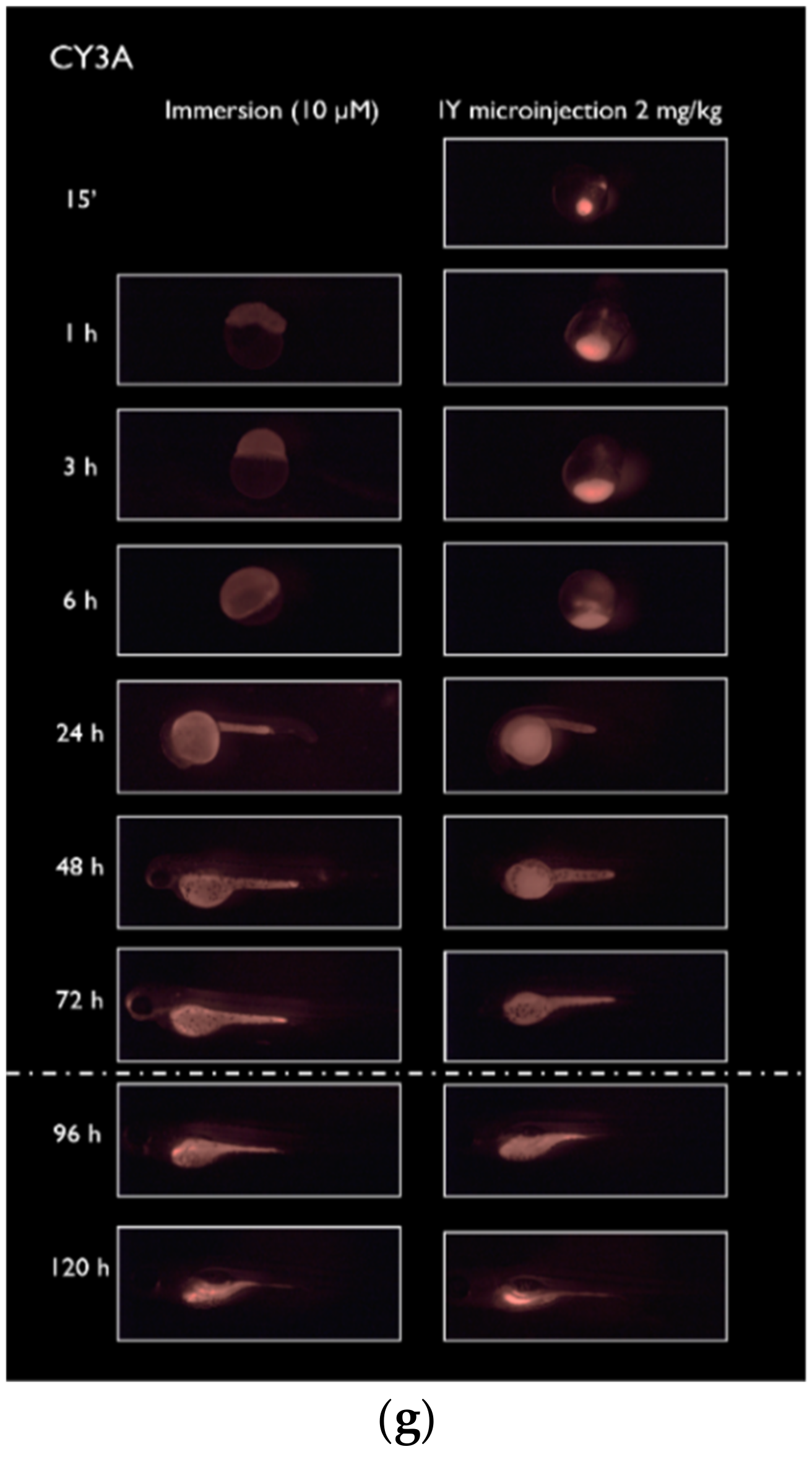
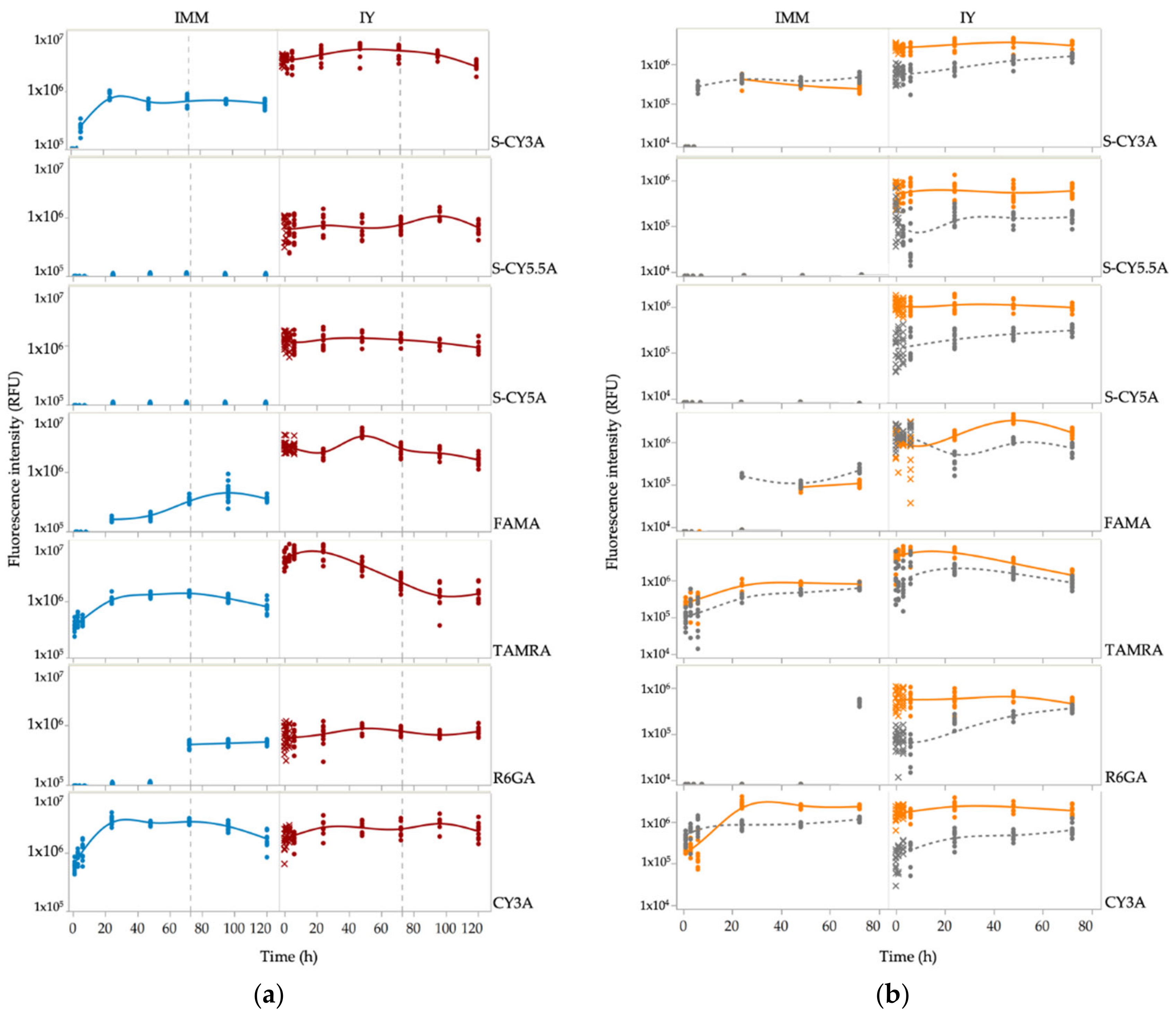
2.1. Spatiotemporal Imaging Following Immersion and IY Microinjection
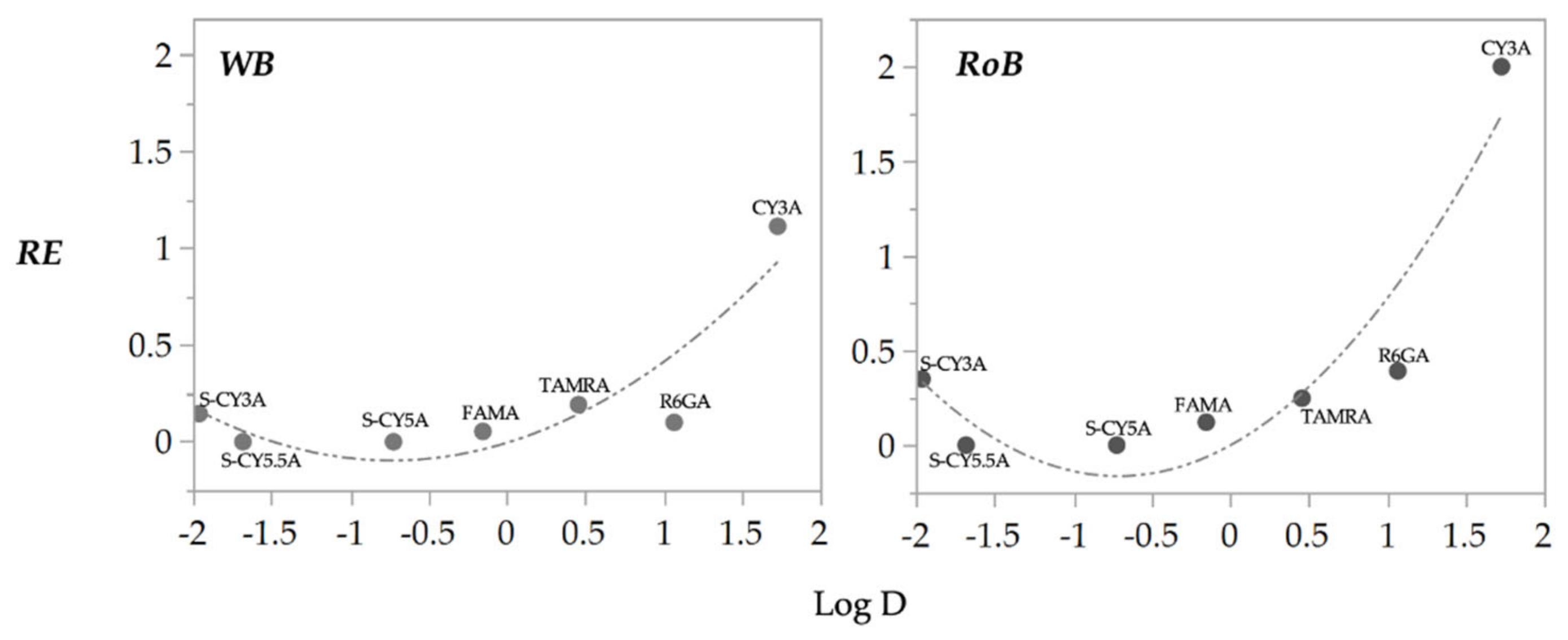
2.2. Non-Compartmental Pharmacokinetic Analysis
2.3. QSPkR Analysis
3. Discussion
4. Materials and Methods
4.1. Zebrafish
4.2. Fluorescent Compounds
4.3. Fluorescent Compound Treatments
4.4. Spatiotemporal Fluorescence Imaging
4.5. Non-Compartmental Pharmacokinetic Analysis
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sewell, F.; Edwards, J.; Prior, H.; Robinson, S. Opportunities to Apply the 3Rs in Safety Assessment Programs. ILAR J. 2016, 57, 234–245. [Google Scholar] [CrossRef]
- Cox, A.G.; Goessling, W. The lure of zebrafish in liver research: Regulation of hepatic growth in development and regeneration. Curr. Opin. Genet. Dev. 2015, 32, 153–161. [Google Scholar] [CrossRef]
- OECD. Test No. 236: Fish Embryo Acute Toxicity (FET) Test; OECD: Paris, France, 2013. [Google Scholar]
- Busquet, F.; Strecker, R.; Rawlings, J.M.; Belanger, S.E.; Braunbeck, T.; Carr, G.J.; Cenijn, P.; Fochtman, P.; Gourmelon, A.; Hübler, N.; et al. OECD validation study to assess intra- and inter-laboratory reproducibility of the zebrafish embryo toxicity test for acute aquatic toxicity testing. Regul. Toxicol. Pharmacol. 2014, 69, 496–511. [Google Scholar] [CrossRef]
- Rihel, J.; Ghosh, M. Zebrafish. In Drug Discovery and Evaluation: Pharmacological Assays; Hock, F., Ed.; Springer International Publishing: Cham, Switzerland, 2016; pp. 4071–4155. [Google Scholar]
- Hisaoka, K.K. Microscopic Studies of the Teleost Chorion. Microsc. Soc. 1958, 77, 240–243. [Google Scholar] [CrossRef]
- Brox, S.; Ritter, A.P.; Küster, E.; Reemtsma, T. Influence of the perivitelline space on the quantification of internal concentrations of chemicals in eggs of zebrafish embryos (Danio rerio). Aquat. Toxicol. 2014, 157, 134–140. [Google Scholar] [CrossRef]
- Hamm, J.T.; Ceger, P.; Allen, D.; Stout, M.; Maull, E.A.; Baker, G.; Zmarowski, A.; Padilla, S.; Perkins, E.; Planchart, A.; et al. Characterizing sources of variability in zebrafish embryo screening protocols. ALTEX 2019, 36, 103–120. [Google Scholar] [CrossRef]
- Henn, K.; Braunbeck, T. Dechorionation as a tool to improve the fish embryo toxicity test (FET) with the zebrafish (Danio rerio). Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2011, 153, 91–98. [Google Scholar] [CrossRef]
- De Koning, C.; Beekhuijzen, M.; Tobor-Kapłon, M.; de Vries-Buitenweg, S.; Schoutsen, D.; Leeijen, N.; van de Waart, B.; Emmen, H. Visualizing Compound Distribution during Zebrafish Embryo Development: The Effects of Lipophilicity and DMSO. Birth Defects Res. B Dev. Reprod. Toxicol. 2015, 104, 253–272. [Google Scholar] [CrossRef] [PubMed]
- Rawson, D.M.; Zhang, T.; Kalicharan, D.; Jongebloed, W.L. Field emission scanning electron microscopy and transmission electron microscopy studies of the chorion, plasma membrane and syncytial layers of the gastrula-stage embryo of the zebrafish Brachydanio rerio: A consideration of the structural and functional relationships with respect to cryoprotectant penetration. Aquac. Res. 2000, 31, 325–336. [Google Scholar] [CrossRef]
- Spaink, H.P.; Cui, C.; Wiweger, M.I.; Jansen, H.J.; Veneman, W.J.; Marín-Juez, R.; de Sonneville, J.; Ordas, A.; Torraca, V.; van der Ent, W.; et al. Robotic injection of zebrafish embryos for high-throughput screening in disease models. Methods 2013, 62, 246–254. [Google Scholar] [CrossRef]
- Ng, A.N.; de Jong-Curtain, T.A.; Mawdsley, D.J.; White, S.J.; Shin, J.; Appel, B.; Dong, P.D.; Stainier, D.Y.; Heath, J.K. Formation of the digestive system in zebrafish: III. Intestinal epithelium morphogenesis. Dev. Biol. 2005, 286, 114–135. [Google Scholar] [CrossRef]
- Silfvast, W.T. Laser Fundamentals, 2nd ed.; Cambridge University Press: Cambridge, UK, 2004. [Google Scholar]
- Fraher, D.; Sanigorski, A.; Mellett, N.A.; Meikle, P.J.; Sinclair, A.J.; Gibert, Y. Zebrafish Embryonic Lipidomic Analysis Reveals that the Yolk Cell Is Metabolically Active in Processing Lipid. Cell Rep. 2016, 14, 1317–1329. [Google Scholar] [CrossRef] [PubMed]
- Guarin, M.; Faelens, R.; Giusti, A.; De Croze, N.; Léonard, M.; Cabooter, D.; Annaert, P.; de Witte, P.; Ny, A. Spatiotemporal imaging and pharmacokinetics of fluorescent compounds in zebrafish eleuthero-embryos after different routes of administration. Sci. Rep. 2021, 11, 12229. [Google Scholar] [CrossRef] [PubMed]
- Shamipour, S.; Kardos, R.; Xue, S.L.; Hof, B.; Hannezo, E.; Heisenberg, C.P. Bulk Actin Dynamics Drive Phase Segregation in Zebrafish Oocytes. Cell 2019, 177, 1463–1479. [Google Scholar] [CrossRef] [PubMed]
- Padilla, S.; Corum, D.; Padnos, B.; Hunter, D.L.; Beam, A.; Houck, K.A.; Sipes, N.; Kleinstreuer, N.; Knudsen, T.; Dix, D.J.; et al. Zebrafish developmental screening of the ToxCast™ Phase I chemical library. Reprod. Toxicol. 2012, 33, 174–187. [Google Scholar] [CrossRef]
- Long, K.; Kostman, S.J.; Fernandez, C.; Burnett, J.C.; Huryn, D.M. Do Zebrafish Obey Lipinski Rules? ACS Med. Chem. Lett. 2019, 10, 1002–1006. [Google Scholar] [CrossRef]
- Winiwarter, S.; Ridderström, M.; Ungell, A.L.; Andersson, T.B.; Zamora, I. Use of Molecular Descriptors for Absorption, Distribution, Metabolism, and Excretion Predictions. In Comprehensive Medicinal Chemistry II.; Triggle, D.J., Taylor, J.B., Eds.; Elsevier Ltd.: Amsterdam, The Netherlands, 2007; Volume 5, pp. 531–554. [Google Scholar]
- Tandon, H.; Ranjan, P.; Chakraborty, T.; Suhag, V. Polarizability: A promising descriptor to study chemical-biological interactions. Mol. Divers. 2021, 25, 249–262. [Google Scholar] [CrossRef]
- Wang, W.; Liu, X.; Gelinas, D.; Ciruna, B.; Sun, Y. A fully automated robotic system for microinjection of zebrafish embryos. PLoS ONE 2007, 2, e862. [Google Scholar] [CrossRef]
- Cordero-Maldonado, M.L.; Perathoner, S.; van der Kolk, K.J.; Boland, R.; Heins-Marroquin, U.; Spaink, H.P.; Meijer, A.H.; Crawford, A.D.; de Sonneville, J. Deep learning image recognition enables efficient genome editing in zebrafish by automated injections. PLoS ONE 2019, 14, e0202377. [Google Scholar] [CrossRef]
- Avdesh, A.; Chen, M.; Martin-Iverson, M.T.; Mondal, A.; Ong, D.; Rainey-Smith, S.; Taddei, K.; Lardelli, M.; Groth, D.M.; Verdile, G.; et al. Regular care and maintenance of a zebrafish (Danio rerio) laboratory: An introduction. J. Vis. Exp. 2012, e4196. [Google Scholar] [CrossRef]
- Danieau’s Solution (30×). Cold Spring Harbor Protocols. 2011. Available online: http://cshprotocols.cshlp.org/content/2011/7/pdb.rec12467.full (accessed on 13 June 2021).
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef] [PubMed]
- Gabrielsson, J.; Weiner, D. Non-compartmental analysis. Methods Mol. Biol. 2012, 929, 377–389. [Google Scholar] [CrossRef]

| Compound | AUC-WB | AUC-Yolk | AUC-RoB | |||
|---|---|---|---|---|---|---|
| IMM | IY | IMM | IY | IMM | IY | |
| S-CY3A | 4.56 | 31.4 | 1.89 | 23.8 | 2.67 | 7.65 |
| S-CY5.5A | 0 | 5.45 | 0 | 4.42 | 0 | 1.02 |
| S-CY5A | 0 | 9.49 | 0 | 7.82 | 0 | 1.67 |
| FAMA | 1.17 | 27.8 | 0.36 | 14.9 | 0.84 | 6.93 |
| TAMRA | 7.76 | 40.4 | 4.98 | 29.1 | 2.78 | 11.2 |
| R6GA | 0.58 | 5.76 | 0 | 4.29 | 0.58 | 1.48 |
| CY3A | 20.9 | 18.9 | 14.6 | 15.7 | 6.41 | 3.21 |
| Compound | RE-WB | RE-RoB | RE-Yolk | RD (IY) |
|---|---|---|---|---|
| S-CY3A | 0.15 | 0.35 | 0.08 | 0.24 |
| S-CY5.5A | 0 | 0 | 0 | 0.19 |
| S-CY5A | 0 | 0 | 0 | 0.18 |
| FAMA | 0.05 | 0.12 | 0.02 | 0.32 |
| TAMRA | 0.19 | 0.25 | 0.17 | 0.28 |
| R6GA | 0.10 | 0.39 | 0 | 0.26 |
| CY3A | 1.11 | 1.99 | 0.93 | 0.17 |
| PK. Parameter | Model | R2 adj | RMSE | p-Value |
|---|---|---|---|---|
| REWB10/2/72h | =−0.011 + 0.187(Log D) + (Log D + 0.179)2 × 0.169 | 0.81 | 0.213 | 0.037 |
| RERoB10/2/72h | =−0.013 + 0.340(Log D) + (Log D + 0.179)2 × 0.320 | 0.82 | 0.299 | 0.015 |
| REYolk10/2/72h | =−1.473 + 0.313(Log D) + 0.006(TPSA) + 0.009(MR) + (Log D + 0.179) (MR − 166.591) × 0.015 | 0.99 | 0.043 | 0.011 |
| Compound NO. | S-Cyanine 3 (S-CY3A) 1 | S-Cyanine 5.5 (S-CY5.5A) 2 | S-Cyanine 5A (S-CY5A) 3 | Fam A, 5-Isomer (FAMA) 4 | Tamra A 5-Isomer (TAMRA) 5 | Rhodamine 6g 6-Isomer (R6GA) 6 | Cyanine 3 (CY3A) 7 |
|---|---|---|---|---|---|---|---|
| MW g/mol | 691.9 | 1054.36 | 547.79 | 413.38 | 467.52 | 462.6 | 530.14 |
| Rotor | 13 | 18 | 11 | 3 | 6 | 7 | 10 |
| HBA | 7 | 13 | 1 | 6 | 4 | 2 | 1 |
| HBD | 1 | 1 | 0 | 3 | 1 | 1 | 1 |
| MR | 180.42 | 241.21 | 185.18 | 109.52 | 135.27 | 144.59 | 169.95 |
| TPSA Å2 | 152.68 | 256.18 | 23.32 | 105.09 | 88.62 | 38.33 | 35.35 |
| Log D | −1.96 | −1.68 | −0.72 | −0.14 | 0.46 | 1.07 | 1.73 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guarin, M.; Ny, A.; De Croze, N.; Maes, J.; Léonard, M.; Annaert, P.; de Witte, P.A.M. Pharmacokinetics in Zebrafish Embryos (ZFE) Following Immersion and Intrayolk Administration: A Fluorescence-Based Analysis. Pharmaceuticals 2021, 14, 576. https://doi.org/10.3390/ph14060576
Guarin M, Ny A, De Croze N, Maes J, Léonard M, Annaert P, de Witte PAM. Pharmacokinetics in Zebrafish Embryos (ZFE) Following Immersion and Intrayolk Administration: A Fluorescence-Based Analysis. Pharmaceuticals. 2021; 14(6):576. https://doi.org/10.3390/ph14060576
Chicago/Turabian StyleGuarin, Marlly, Annelii Ny, Noémie De Croze, Jan Maes, Marc Léonard, Pieter Annaert, and Peter A. M. de Witte. 2021. "Pharmacokinetics in Zebrafish Embryos (ZFE) Following Immersion and Intrayolk Administration: A Fluorescence-Based Analysis" Pharmaceuticals 14, no. 6: 576. https://doi.org/10.3390/ph14060576
APA StyleGuarin, M., Ny, A., De Croze, N., Maes, J., Léonard, M., Annaert, P., & de Witte, P. A. M. (2021). Pharmacokinetics in Zebrafish Embryos (ZFE) Following Immersion and Intrayolk Administration: A Fluorescence-Based Analysis. Pharmaceuticals, 14(6), 576. https://doi.org/10.3390/ph14060576