PESIN Conjugates for Multimodal Imaging: Can Multimerization Compensate Charge Influences on Cell Binding Properties? A Case Study †
Abstract
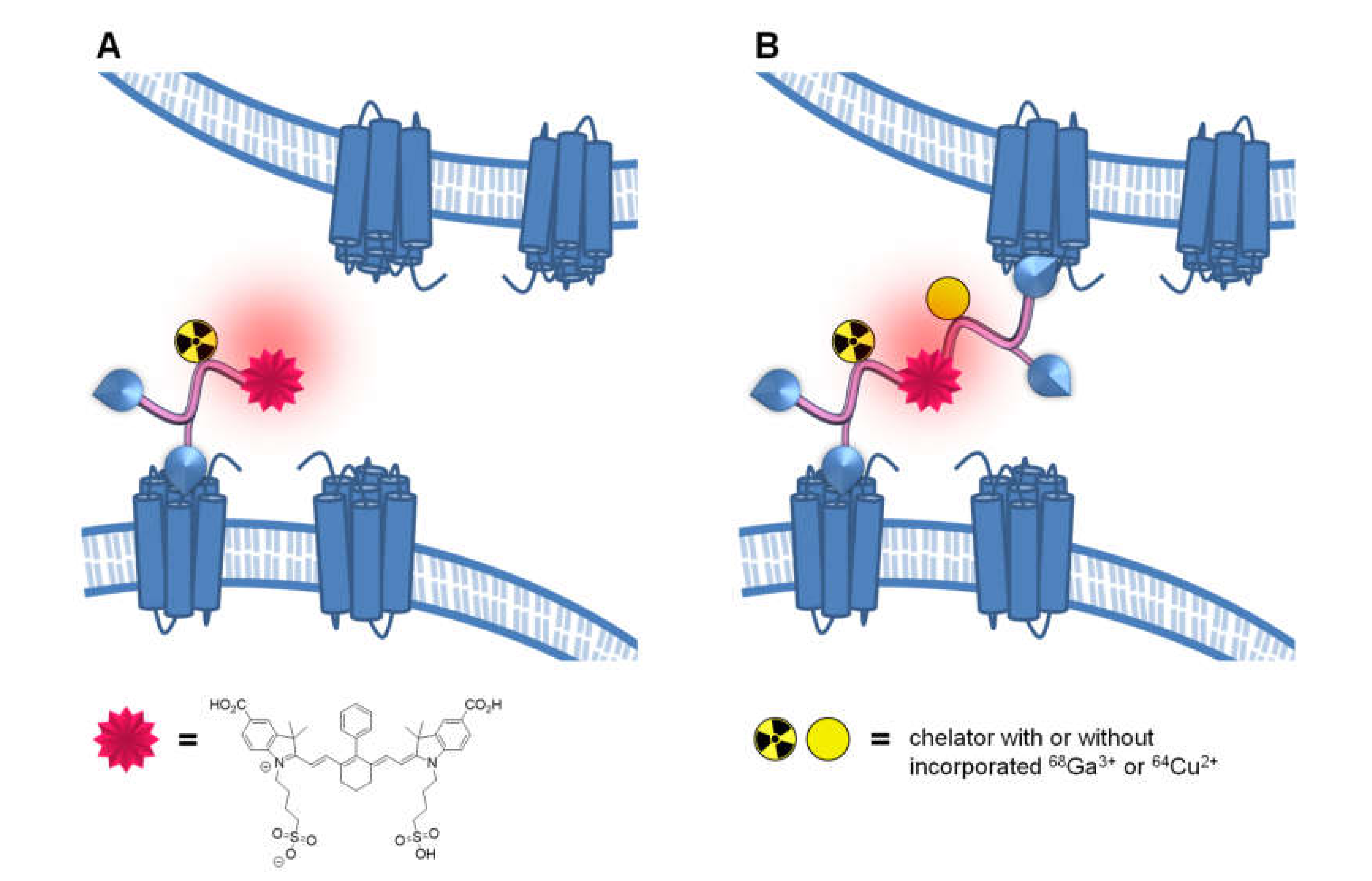
1. Introduction
2. Results and Discussion
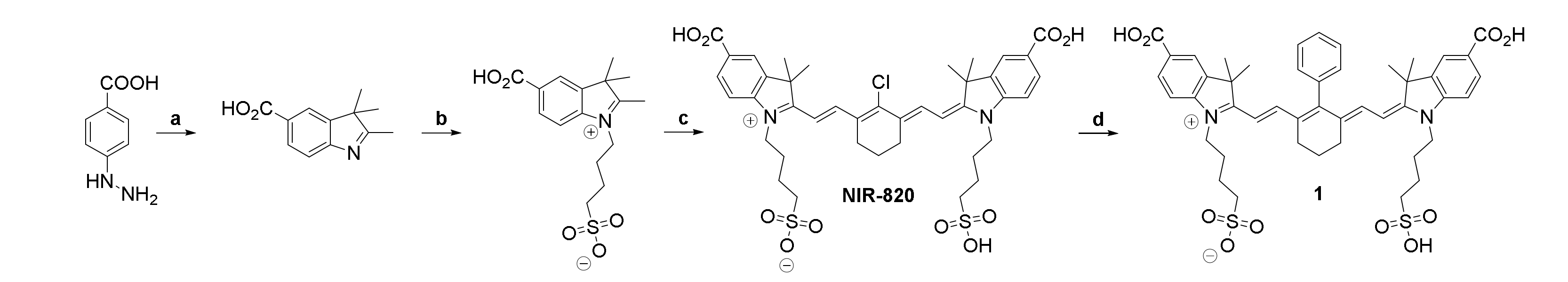
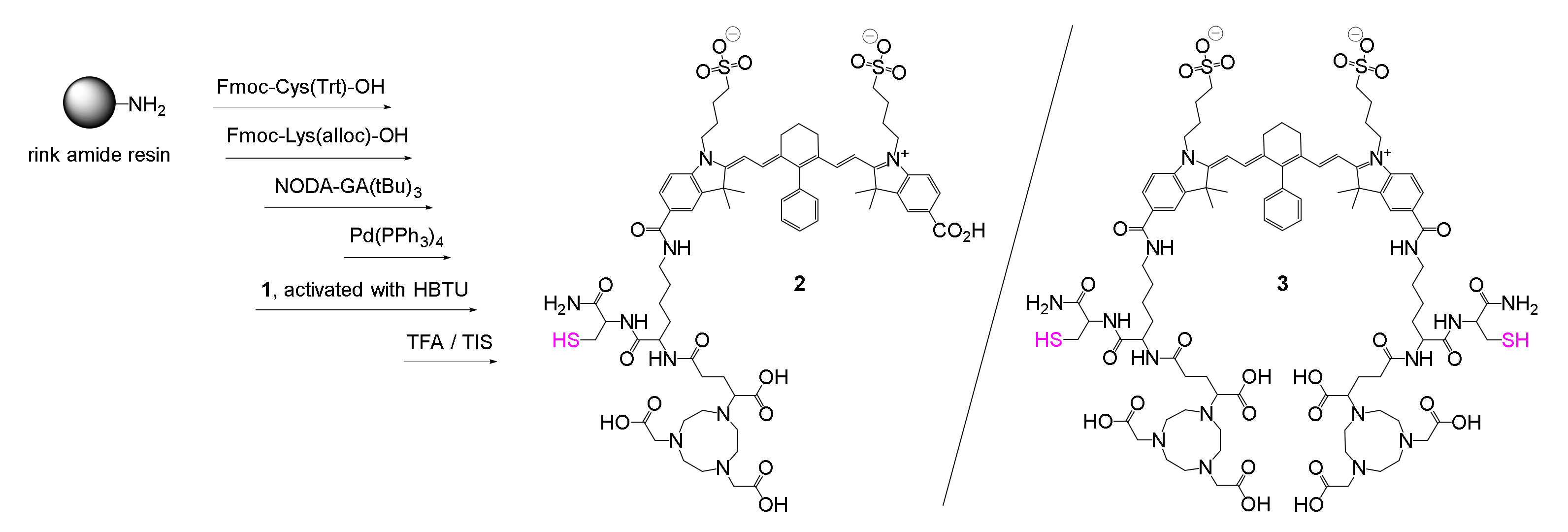
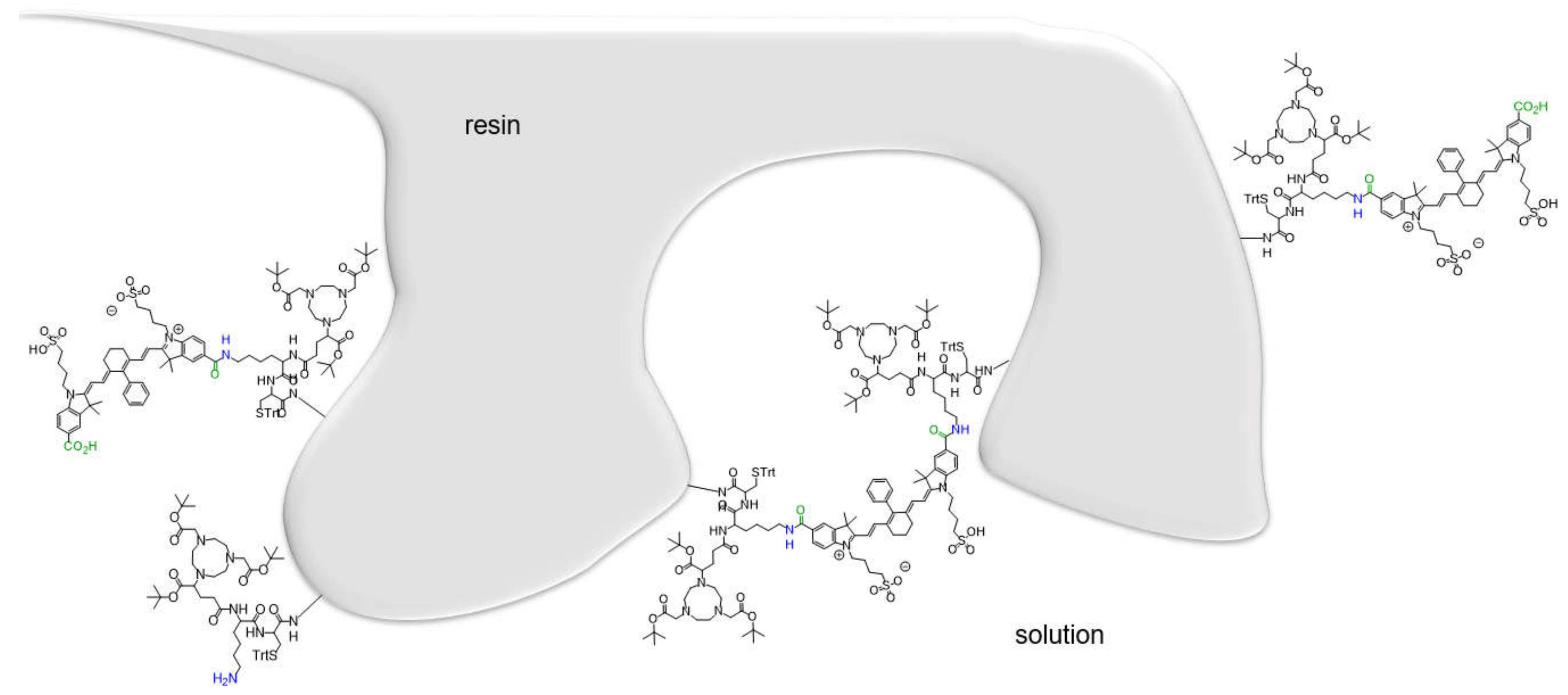
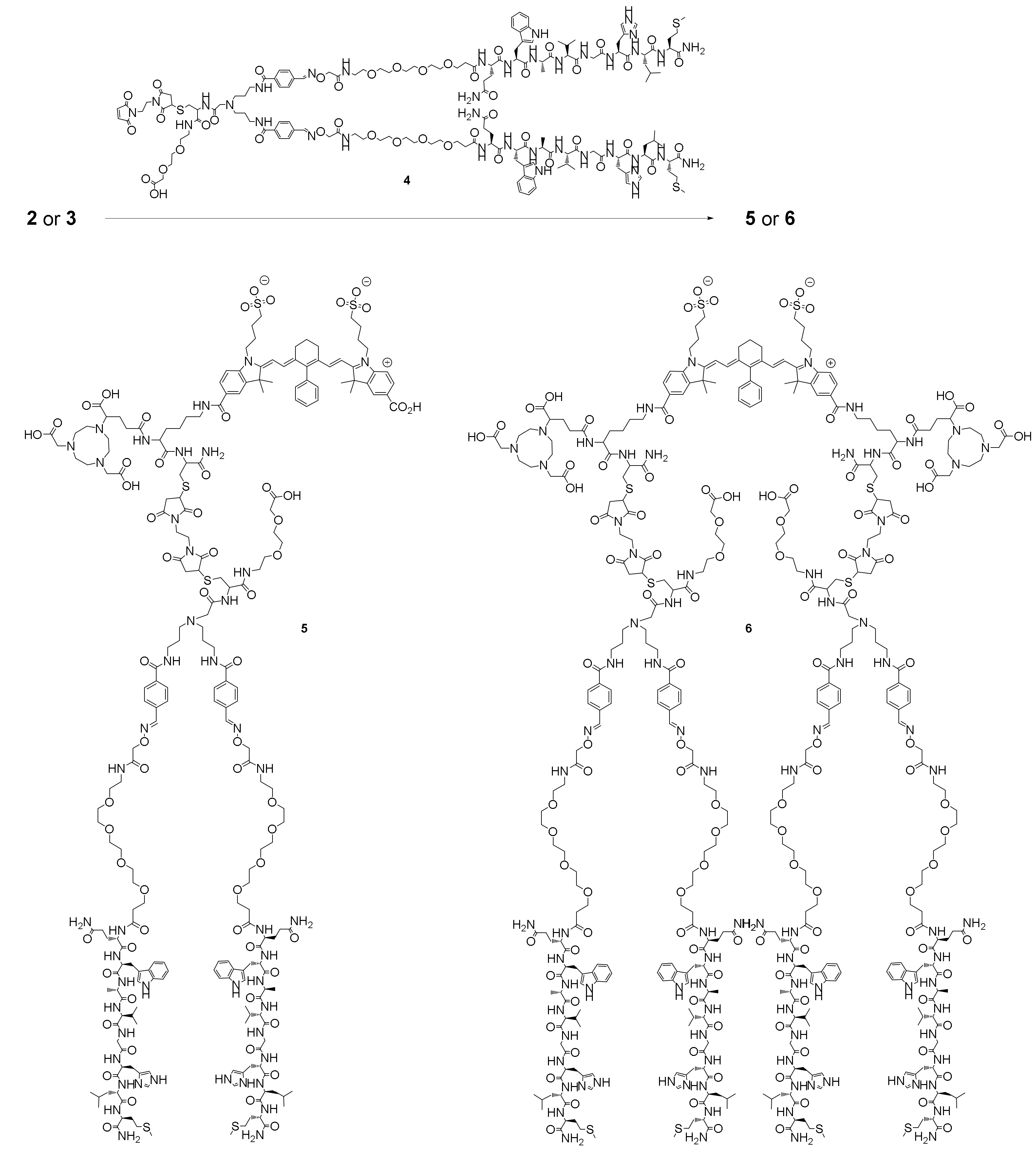
2.1. Synthesis of the Hybrid Multimodal Imaging Units 2 and 3 and Their Peptide Multimer Conjugates 5 and 6
2.2. Determination of Photophysical Properties of Dyes NIR-820 and 1, MIUs 2 and 3, and Their Peptide Multimer Conjugates 5 and 6
2.3. Radiolabeling of 2, 3, 5, and 6 and Determination of Lipophilicity/Hydrophilicity of [68Ga]Ga-2, [68Ga]Ga-3, [68Ga]Ga-5, and [68Ga]Ga-6
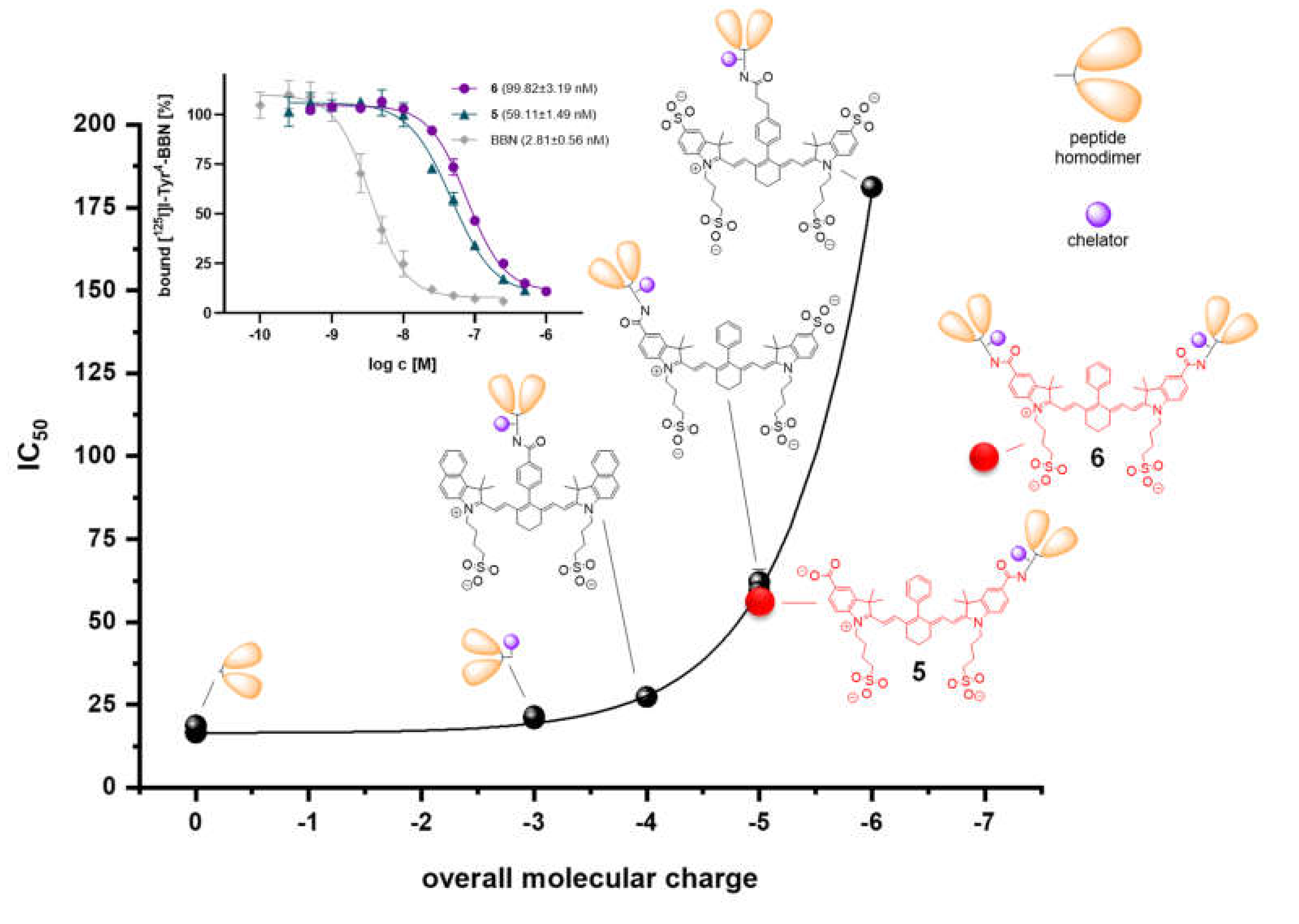
2.4. Determination of In Vitro GRPR Binding Affinities of 5 and 6
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Boros, E.; Packard, A.B. Radioactive Transition Metals for Imaging and Therapy. Chem. Rev. 2019, 119, 870–901. [Google Scholar] [CrossRef]
- Badawi, R.D.; Shi, H.; Hu, P.; Chen, S.; Xu, T.; Price, P.M.; Ding, Y.; Spencer, B.A.; Nardo, L.; Liu, W.; et al. First Human Imaging Studies with the EXPLORER Total-Body PET Scanner. J. Nucl. Med. 2019, 60, 299–303. [Google Scholar] [CrossRef]
- Forshew, T.; Murtaza, M.; Parkinson, C.; Gale, D.; Tsui, D.W.Y.; Kaper, F.; Dawson, S.-J.; Piskorz, A.M.; Jimenez-Linan, M.; Bentley, D.; et al. Noninvasive Identification and Monitoring of Cancer Mutations by Targeted Deep Sequencing of Plasma DNA. Sci. Transl. Med. 2012, 4, 1–14. [Google Scholar] [CrossRef]
- Achilefu, S. Introduction to Concepts and Strategies for Molecular Imaging. Chem. Rev. 2010, 110, 2575–2578. [Google Scholar] [CrossRef]
- Liu, Y.; Solomon, M.; Achilefu, S. Perspectives and potential applications of nanomedicine in breast and prostate cancer. Med. Res. Rev. 2013, 33, 3–32. [Google Scholar] [CrossRef]
- O’Farrell, A.C.; Shnyder, S.D.; Marston, G.; Coletta, P.L.; Gill, J.H. Non-invasive molecular imaging for preclinical cancer therapeutic development. Br. J. Pharmacol. 2013, 169, 719–735. [Google Scholar] [CrossRef] [PubMed]
- MacPherson, D.S.; Fung, K.; Cook, B.E.; Francesconi, L.C.; Zeglis, B.M. A brief overview of metal complexes as nuclear imaging agents. Dalton Trans. 2019, 48, 14547–14565. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, Q.T.; Tsien, R.Y. Fluorescence-guided surgery with live molecular navigation--a new cutting edge. Nat. Rev. Cancer 2013, 13, 653–662. [Google Scholar] [CrossRef] [PubMed]
- Culver, J.; Akers, W.; Achilefu, S. Multimodality molecular imaging with combined optical and SPECT/PET modalities. J. Nucl. Med. 2008, 49, 169–172. [Google Scholar] [CrossRef] [PubMed]
- Ni, D.; Ehlerding, E.B.; Cai, W. Multimodality Imaging Agents with PET as the Fundamental Pillar. Angew. Chem. Int. Ed. Engl. 2019, 58, 2570–2579. [Google Scholar] [CrossRef]
- Pandey, S.K.; Gryshuk, A.L.; Sajjad, M.; Zheng, X.; Chen, Y.; Abouzeid, M.M.; Morgan, J.; Charamisinau, I.; Nabi, H.A.; Oseroff, A.; et al. Multimodality Agents for Tumor Imaging (PET, Fluorescence) and Photodynamic Therapy. A Possible “See and Treat” Approach. J. Med. Chem. 2005, 48, 6286–6295. [Google Scholar] [CrossRef]
- Ghosh, S.C.; Ghosh, P.; Wilganowski, N.; Robinson, H.; Hall, M.A.; Dickinson, G.; Pinkston, K.L.; Harvey, B.R.; Sevick-Muraca, E.M.; Azhdarinia, A. Multimodal chelation platform for near-infrared fluorescence/nuclear imaging. J. Med. Chem. 2013, 56, 406–416. [Google Scholar] [CrossRef]
- Christensen, A.; Juhl, K.; Persson, M.; Charabi, B.W.; Mortensen, J.; Kiss, K.; Lelkaitis, G.; Rubek, N.; Von Buchwald, C.; Kjær, A. uPAR-targeted optical near-infrared (NIR) fluorescence imaging and PET for image-guided surgery in head and neck cancer: Proof-of-concept in orthotopic xenograft model. Oncotarget 2017, 8, 15407–15419. [Google Scholar] [CrossRef]
- Becker, A.; Hessenius, C.; Licha, K.; Ebert, B.; Sukowski, U.; Semmler, W.; Wiedenmann, B.; Grötzinger, C. Receptor-targeted optical imaging of tumors with near-infrared fluorescent ligands. Nat. Biotechnol. 2001, 19, 327–332. [Google Scholar] [CrossRef]
- Renard, E.; Dancer, P.A.; Portal, C.; Denat, F.; Prignon, A.; Goncalves, V. Design of Bimodal Ligands of Neurotensin Receptor 1 for Positron Emission Tomography Imaging and Fluorescence-Guided Surgery of Pancreatic Cancer. J. Med. Chem. 2020, 63, 2426–2433. [Google Scholar] [CrossRef]
- Kang, N.Y.; Lee, J.Y.; Lee, S.H.; Song, I.H.; Hwang, Y.H.; Kim, M.J.; Phue, W.H.; Agrawalla, B.K.; Wan, S.Y.D.; Lalic, J.; et al. Multimodal Imaging Probe Development for Pancreatic beta Cells: From Fluorescence to PET. J. Am. Chem. Soc. 2020, 142, 3430–3439. [Google Scholar] [CrossRef]
- Van Dam, G.M.; Themelis, G.; Crane, L.M.; Harlaar, N.J.; Pleijhuis, R.G.; Kelder, W.; Sarantopoulos, A.; De Jong, J.S.; Arts, H.J.; Van der Zee, A.G.; et al. Intraoperative tumor-specific fluorescence imaging in ovarian cancer by folate receptor-alpha targeting: First in-human results. Nat. Med. 2011, 17, 1315–1319. [Google Scholar] [CrossRef] [PubMed]
- Brouwer, O.R.; Buckle, T.; Bunschoten, A.; Kuil, J.; Vahrmeijer, A.L.; Wendler, T.; Valdés-Olmos, R.A.; Van der Poel, H.G.; Van Leeuwen, F.W. Image navigation as a means to expand the boundaries of fluorescence-guided surgery. Phys. Med. Biol. 2012, 57, 3123–3136. [Google Scholar] [CrossRef]
- Mondal, S.B.; O’Brien, C.M.; Bishop, K.; Fields, R.C.; Margenthaler, J.A.; Achilefu, S. Repurposing Molecular Imaging and Sensing for Cancer Image-Guided Surgery. J. Nucl. Med. 2020, 61, 1113–1122. [Google Scholar] [CrossRef] [PubMed]
- Achilefu, S. Lighting up Tumors with Receptor-specific Optical Molecular Probes. Technol. Cancer Res. Treat. 2004, 3, 393–410. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.H.; Thach, D.; Vaughn, B.A.; Alford, V.M.; Preston, A.N.; Laughlin, S.T.; Boros, E. Linear Desferrichrome-Linked Silicon-Rhodamine Antibody Conjugate Enables Targeted Multimodal Imaging of HER2 in Vitro and in Vivo. Mol. Pharm. 2019, 16, 1412–1420. [Google Scholar] [CrossRef]
- Lee, S.; Xie, J.; Chen, X. Peptide-based probes for targeted molecular imaging. Biochemistry 2010, 49, 1364–1376. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Xie, J.; Chen, X. Peptides and Peptide Hormones for Molecular Imaging and Disease Diagnosis. Chem. Rev. 2010, 110, 3087–3111. [Google Scholar] [CrossRef] [PubMed]
- Louie, A. Multimodality Imaging Probes: Design and Challenges. Chem. Rev. 2010, 110, 3146–3195. [Google Scholar] [CrossRef]
- Chen, J.; Gao, Z.; Li, G.; Wang, T.D. Dual-modal in vivo fluorescence and photoacoustic imaging using a heterodimeric peptide. Chem. Commun. 2018, 54, 13196–13199. [Google Scholar] [CrossRef]
- Hübner, R.; Cheng, X.; Wängler, B.; Wängler, C. Functional Hybrid Molecules for the Visualization of Cancer: PESIN-Homodimers Combined with Multimodal Molecular Imaging Probes for Positron Emission Tomography and Optical Imaging: Suited for Tracking of GRPR-Positive Malignant Tissue. Chem. Eur. J. 2020, 26, 16349–16356. [Google Scholar] [CrossRef] [PubMed]
- Hübner, R.; Von Kiedrowski, V.; Benkert, V.; Wängler, B.; Schirrmacher, R.; Krämer, R.; Wängler, C. Hybrid Multimodal Imaging Synthons for Chemoselective and Efficient Biomolecule Modification with Chelator and Near-Infrared Fluorescent Cyanine Dye. Pharmaceuticals 2020, 13, 250. [Google Scholar] [CrossRef]
- Achilefu, S.; Jimenez, H.N.; Dorshow, R.B.; Bugaj, J.E.; Webb, E.G.; Wilhelm, R.R.; Rajagopalan, R.; Johler, J.; Erion, J.L. Synthesis, in vitro receptor binding, and in vivo evaluation of fluorescein and carbocyanine peptide-based optical contrast agents. J. Med. Chem. 2002, 45, 2003–2015. [Google Scholar] [CrossRef]
- König, S.G.; Krämer, R. Accessing Structurally Diverse Near-Infrared Cyanine Dyes for Folate Receptor-Targeted Cancer Cell Staining. Chem. Eur. J. 2017, 23, 9306–9312. [Google Scholar] [CrossRef]
- König, S.G.; Krämer, R. Polyamine-modified near-infrared cyanine dyes for targeting the nuclei and nucleoli of cells. Dyes Pigment. 2017, 145, 80–94. [Google Scholar] [CrossRef]
- Von Kiedrowski, V.; Hübner, R.; Kail, D.; Cheng, X.; Schirrmacher, R.; Wängler, C.; Wängler, B. Synthesis, characterization and optimization of in vitro properties of NIR-fluorescent cyclic alpha-MSH peptides for melanoma imaging. J. Mater. Chem. B 2020, 8, 10602–10608. [Google Scholar] [CrossRef]
- Exner, R.M.; Cortezon-Tamarit, F.; Pascu, S.I. Explorations into the Effect of meso-Substituents in Tricarbocyanine Dyes: A Path to Diverse Biomolecular Probes and Materials. Angew. Chem. Int. Ed. 2021, 60, 6230–6241. [Google Scholar] [CrossRef]
- Lee, H.; Mason, J.C.; Achilefu, S. Heptamethine Cyanine Dyes with a Robust C-C Bond at the Central Position of the Chromophore. J. Org. Chem. 2006, 71, 7862–7865. [Google Scholar] [CrossRef]
- Lee, D.; Jeong, K.; Luo, X.; Kim, G.; Yang, Y.; Chen, X.; Kim, S.; Yoon, J. Near-infrared fluorescent probes for the detection of glutathione and their application in the fluorescence imaging of living cells and tumor-bearing mice. J. Mater. Chem. B 2018, 6, 2541–2546. [Google Scholar] [CrossRef]
- Usama, S.M.; Park, G.K.; Nomura, S.; Baek, Y.; Choi, H.S.; Burgess, K. Role of Albumin in Accumulation and Persistence of Tumor-Seeking Cyanine Dyes. Bioconjug. Chem. 2020, 31, 248–259. [Google Scholar] [CrossRef] [PubMed]
- Hübner, R.; Benkert, V.; Cheng, X.; Wängler, B.; Krämer, R.; Wängler, C. Probing two PESIN-indocyanine-dye-conjugates: Significance of the used fluorophore. J. Mater. Chem. B 2020, 8, 1302–1309. [Google Scholar] [CrossRef]
- Usama, S.M.; Lin, C.M.; Burgess, K. On the Mechanisms of Uptake of Tumor-Seeking Cyanine Dyes. Bioconjug. Chem. 2018, 29, 3886–3895. [Google Scholar] [CrossRef] [PubMed]
- Nair, D.P.; Podgórski, M.; Chatani, S.; Gong, T.; Xi, W.; Fenoli, C.R.; Bowman, C.N. The Thiol-Michael Addition Click Reaction: A Powerful and Widely Used Tool in Materials Chemistry. Chem. Mater. 2013, 26, 724–744. [Google Scholar] [CrossRef]
- Mohammad, I.; Stanford, C.; Morton, M.D.; Zhu, Q.; Smith, M.B. Structurally modified indocyanine green dyes. Modification of the polyene linker. Dyes Pigment. 2013, 99, 275–283. [Google Scholar] [CrossRef]
- Wängler, C.; Wängler, B.; Lehner, S.; Elsner, A.; Todica, A.; Bartenstein, P.; Hacker, M.; Schirrmacher, R. A universally applicable 68Ga-labeling technique for proteins. J. Nucl. Med. 2011, 52, 586–591. [Google Scholar] [CrossRef]
- Litau, S.; Seibold, U.; Vall-Sagarra, A.; Fricker, G.; Wängler, B.; Wängler, C. Comparative Assessment of Complex Stabilities of Radiocopper Chelating Agents by a Combination of Complex Challenge and in vivo Experiments. ChemMedChem 2015, 10, 1200–1208. [Google Scholar] [CrossRef]
- Cabelll, D.E.; Bielski, B.H.J. Kinetics and Mechanism for the Oxidation of Ascorbic Acid/Ascorbate by H02/02 Radicals. A Pulse Radiolysis and Stopped-Flow Photolysis Study. J. Phys. Chem. 1983, 87, 1809–1812. [Google Scholar] [CrossRef]
- Nakagawa, T.; Hocart, S.J.; Schumann, M.; Tapia, J.A.; Mantey, S.A.; Coy, D.H.; Tokita, K.; Katsuno, T.; Jensen, R.T. Identification of key amino acids in the gastrin-releasing peptide receptor (GRPR) responsible for high affinity binding of gastrin-releasing peptide (GRP). Biochem. Pharmacol. 2005, 69, 579–593. [Google Scholar] [CrossRef]
- Lindner, S.; Michler, C.; Wängler, B.; Bartenstein, P.; Fischer, G.; Schirrmacher, R.; Wängler, C. PESIN multimerization improves receptor avidities and in vivo tumor targeting properties to GRPR-overexpressing tumors. Bioconjug. Chem. 2014, 25, 489–500. [Google Scholar] [CrossRef] [PubMed]
- Edgcomb, S.P.; Murphy, K.P. Variability in the pKa of histidine side-chains correlates with burial within proteins. Proteins 2002, 49, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Chaudhury, S.; Ripoll, D.R.; Wallqvist, A. Structure-based pKa prediction provides a thermodynamic basis for the role of histidines in pH-induced conformational transitions in dengue virus. Biochem. Biophys. Rep. 2015, 4, 375–385. [Google Scholar] [CrossRef][Green Version]
- Pham, W.; Lai, W.-F.; Weissleder, R.; Tung, C.-H. High Efficiency Synthesis of a Bioconjugatable Near-Infrared Fluorochrome. Bioconjug. Chem. 2003, 14, 1048–1051. [Google Scholar] [CrossRef]
- Ravasco, J.; Faustino, H.; Trindade, A.; Gois, P.M.P. Bioconjugation with Maleimides: A Useful Tool for Chemical Biology. Chem. Eur. J. 2019, 25, 43–59. [Google Scholar] [CrossRef]
| Compound | λmax (abs) (nm) | log ε (M−1 cm−1) | λmax(em) (nm) | Stokes Shift (nm) |
|---|---|---|---|---|
| NIR-820 | 785 [30,39] | 5.3 | 805 [30,39] | 20 |
| 1 | 770 [30,31] | 5.4 | 790 [30,31] | 20 |
| 2 | 695/770 | 4.7/4.6 | 790 | 20 |
| 3 | 695/770 | 4.9/4.9 | 795 | 25 |
| 5 | 710/770 | 4.6/5.0 | 790 | 20 |
| 6 | 711/775 | 4.5/5.0 | 795 | 20 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hübner, R.; Paretzki, A.; von Kiedrowski, V.; Maspero, M.; Cheng, X.; Davarci, G.; Braun, D.; Damerow, H.; Judmann, B.; Filippou, V.; et al. PESIN Conjugates for Multimodal Imaging: Can Multimerization Compensate Charge Influences on Cell Binding Properties? A Case Study. Pharmaceuticals 2021, 14, 531. https://doi.org/10.3390/ph14060531
Hübner R, Paretzki A, von Kiedrowski V, Maspero M, Cheng X, Davarci G, Braun D, Damerow H, Judmann B, Filippou V, et al. PESIN Conjugates for Multimodal Imaging: Can Multimerization Compensate Charge Influences on Cell Binding Properties? A Case Study. Pharmaceuticals. 2021; 14(6):531. https://doi.org/10.3390/ph14060531
Chicago/Turabian StyleHübner, Ralph, Alexa Paretzki, Valeska von Kiedrowski, Marco Maspero, Xia Cheng, Güllü Davarci, Diana Braun, Helen Damerow, Benedikt Judmann, Vasileios Filippou, and et al. 2021. "PESIN Conjugates for Multimodal Imaging: Can Multimerization Compensate Charge Influences on Cell Binding Properties? A Case Study" Pharmaceuticals 14, no. 6: 531. https://doi.org/10.3390/ph14060531
APA StyleHübner, R., Paretzki, A., von Kiedrowski, V., Maspero, M., Cheng, X., Davarci, G., Braun, D., Damerow, H., Judmann, B., Filippou, V., Dallanoce, C., Schirrmacher, R., Wängler, B., & Wängler, C. (2021). PESIN Conjugates for Multimodal Imaging: Can Multimerization Compensate Charge Influences on Cell Binding Properties? A Case Study. Pharmaceuticals, 14(6), 531. https://doi.org/10.3390/ph14060531








