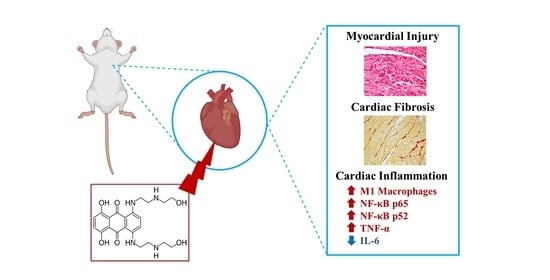
Inflammation as a Possible Trigger for Mitoxantrone-Induced Cardiotoxicity: An In Vivo Study in Adult and Infant Mice
Abstract
1. Introduction
2. Results
- -
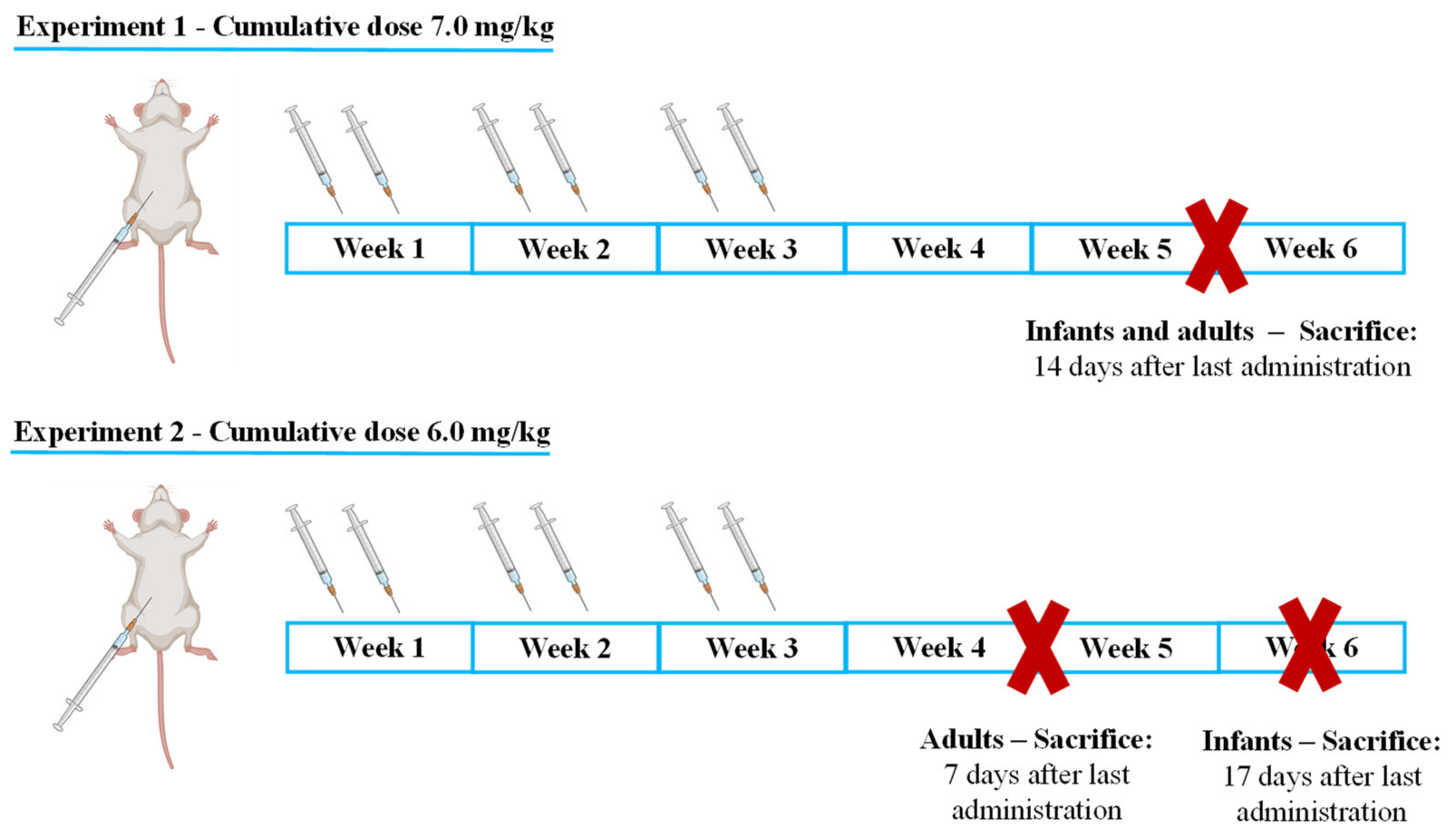
- Infant mice were sacrificed 17 days after the last administration to determine if, with increased lag time, infants would present signs of cardiac damage or still maintained higher cardiac resilience;
- -
- Adults were sacrificed earlier, namely after 7 days to determine early markers of heart damage that may reveal subtle cardiotoxic mechanisms before unacceptable damage occurred.
2.1. MTX Caused Significant Effects on Body Weight and Food/Water Consumption
2.2. Plasma Levels of Total-CK and AST/ALT Ratio Were Increased in the 7.0 mg/kg MTX-Treated Adult Mice
2.3. The Ratio of Heart/Body Weight Decreased in Adults after the Highest Dose of MTX
2.4. Histological Damage Occurred in Cardiac Tissue after MTX-Treatment, Adult Population Being More Susceptible to Cardiac Damage
2.5. Myocardial Fibrosis Was Significantly Increased in MTX-Treated Adult Mice at Both Doses
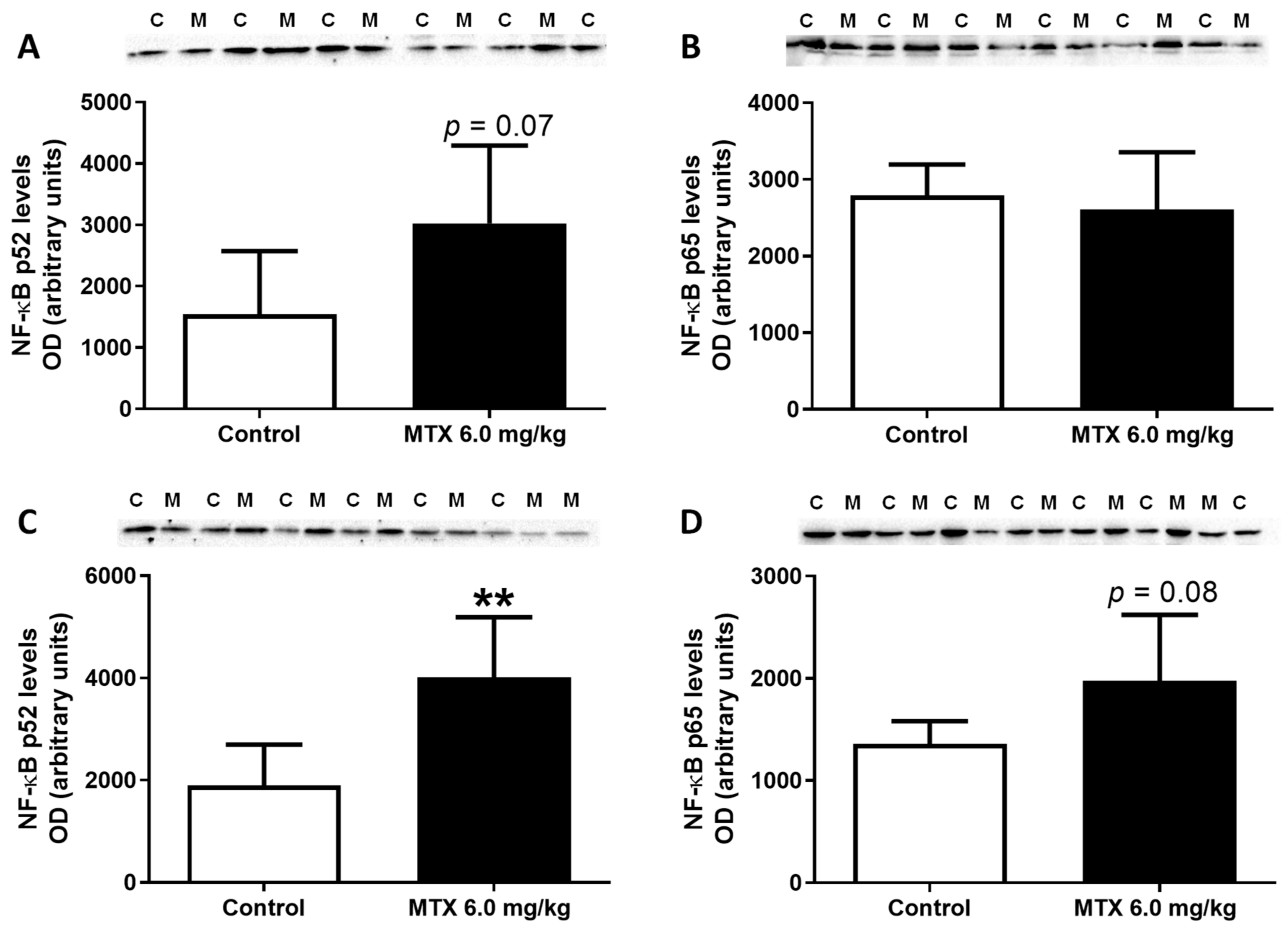
2.6. The Highest Cumulative Dose of MTX Increased Cardiac Total Glutathione in Infants, While Decreasing Noradrenaline Cardiac Levels in Adults
2.7. The Expression of GAPDH Increased in the 6.0 mg/kg MTX-Treated Infant Mice
2.8. At the Lower Cumulative Dose, Protein Carbonylation Increased in Adult Mice
2.9. MTX Increased Catalase Expression Both in Adults and Infants, While in Infants’ Glutathione Peroxidase Expression Increased
2.10. The Heart of MTX-Treated Adult Mice Showed a Higher Density of Infiltrating M1 Macrophages
2.11. In Adults, the Lower Cumulative Dose of MTX Significantly Increased the Expression of NF-ĸB p65
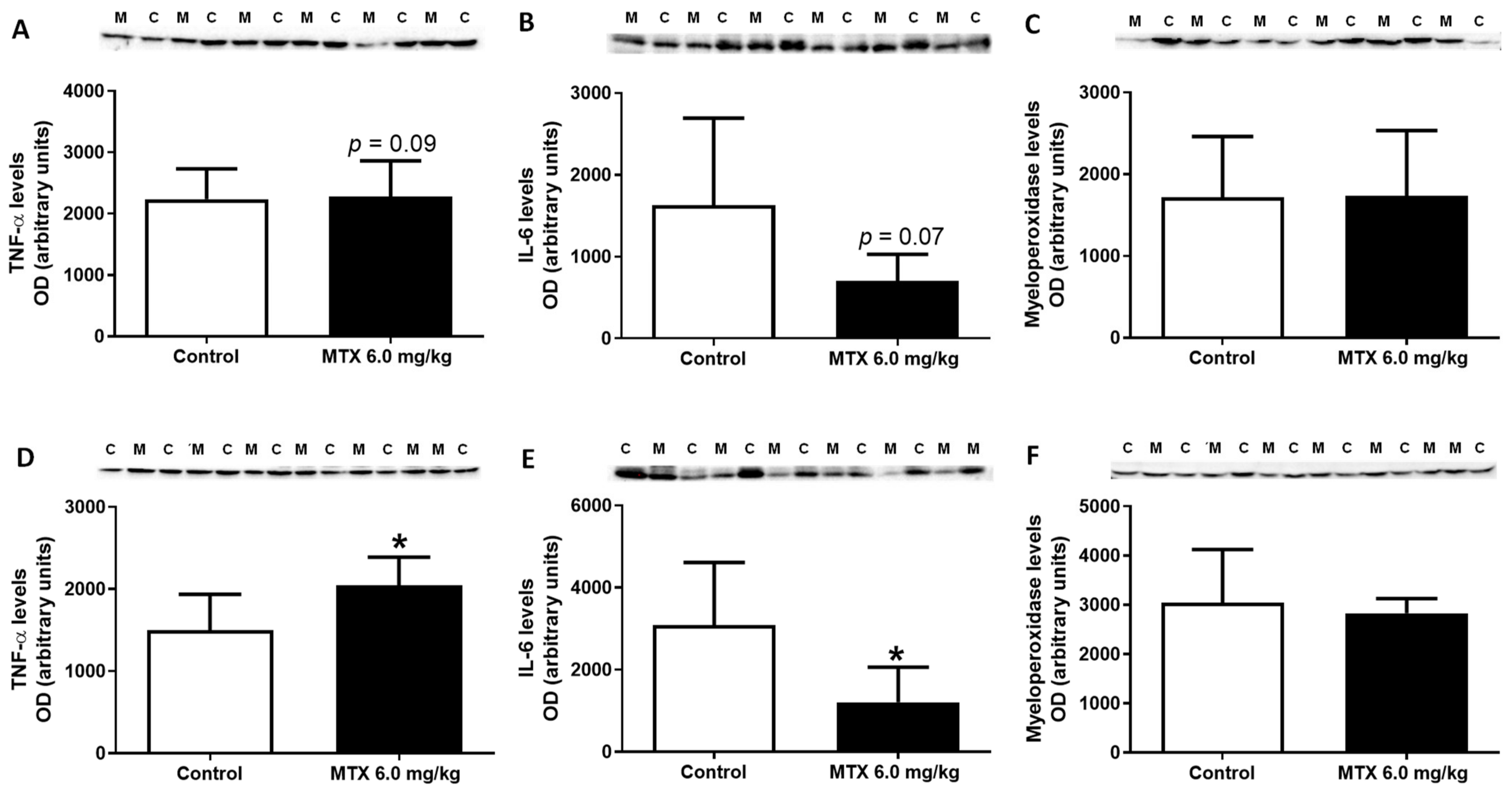
2.12. MTX Increased TNF-α Cardiac Expression while it Decreased IL-6
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Experimental Protocol
4.3. Administration Schedule
4.4. Blood Collection and Plasma Biomarkers
4.5. Tissue Collection
4.6. Histological Analysis of Heart Tissue
4.7. Determination of Cardiac Noradrenaline Levels
4.8. Measurement of ATP Levels
4.9. Determination of Cardiac Creatine and Phosphocreatine Levels
4.10. Measurement of tGSH, GSH, and GSSG
4.11. Assessment of Lipid Peroxidation
4.12. Protein Carbonylation by Slot Blot Analysis
4.13. Western Blotting Analysis
4.14. Immunohistochemistry
4.15. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Reis-Mendes, A.F.; Sousa, E.; de Lourdes Bastos, M.; Costa, V.M. The Role of the Metabolism of Anticancer Drugs in Their Induced-Cardiotoxicity. Curr. Drug Metab. 2015, 17, 75–90. [Google Scholar] [CrossRef]
- Doroshow, J.H. Topoisomerase II inhibitors. In Cancer Chemotherapy and Biotherapy: Principles and Practice; Chabner, B.A., Long, D.L., Eds.; Lippincot Williams and Wilkins: Philadelphia, PA, USA, 2011; pp. 356–391. [Google Scholar]
- Fox, E.J. Mechanism of action of mitoxantrone. Neurology 2004, 63, S15–S18. [Google Scholar] [CrossRef]
- Scott, L.J.; Figgitt, D.P. Mitoxantrone: A review of its use in multiple sclerosis. CNS Drugs 2004, 18, 379–396. [Google Scholar] [CrossRef] [PubMed]
- Costa, V.M.; Carvalho, F.; Duarte, J.A.; de Lourdes Bastos, M.; Remião, F. The heart as a target for xenobiotic toxicity: The cardiac susceptibility to oxidative stress. Chem. Res. Toxicol. 2013, 26, 1285–1311. [Google Scholar] [CrossRef] [PubMed]
- Seiter, K. Toxicity of the topoisomerase II inhibitors. Expert Opin. Drug Saf. 2005, 4, 219–234. [Google Scholar] [CrossRef] [PubMed]
- Fleischer, V.; Salmen, A.; Kollar, S.; Weyer, V.; Siffrin, V.; Chan, A.; Zipp, F.; Luessi, F. Cardiotoxicity of mitoxantrone treatment in a german cohort of 639 multiple sclerosis patients. J. Clin. Neurol. 2014, 10, 289–295. [Google Scholar] [CrossRef]
- Rivera, V.M.; Jeffery, D.R.; Weinstock-Guttman, B.; Bock, D.; Dangond, F. Results from the 5-year, phase IV RENEW (Registry to Evaluate Novantrone Effects in Worsening Multiple Sclerosis) study. BMC Neurol. 2013, 13, 80. [Google Scholar] [CrossRef]
- Frieler, R.A.; Mortensen, M.R. Immune cell and other noncardiomyocyte regulation of cardiac hypertrophy and remodeling. Circulation 2015, 131, 1019–1030. [Google Scholar] [CrossRef] [PubMed]
- Mann, D.L. Innate immunity and the failing heart: The cytokine hypothesis revisited. Circ. Res. 2015, 116, 1254–1268. [Google Scholar] [CrossRef]
- Prabhu, S.D.; Frangogiannis, N.G. The Biological Basis for Cardiac Repair after Myocardial Infarction: From Inflammation to Fibrosis. Circ. Res. 2016, 119, 91–112. [Google Scholar] [CrossRef]
- Zhou, B.; Rao, L.; Peng, Y.; Wang, Y.; Li, Y.; Gao, L.; Chen, Y.; Xue, H.; Song, Y.; Liao, M.; et al. Functional polymorphism of the NFKB1 gene promoter is related to the risk of dilated cardiomyopathy. BMC Med. Genet. 2009, 10, 47. [Google Scholar] [CrossRef] [PubMed]
- Santos, D.G.; Resende, M.F.; Mill, J.G.; Mansur, A.J.; Krieger, J.E.; Pereira, A.C. Nuclear Factor (NF) kappaB polymorphism is associated with heart function in patients with heart failure. BMC Med. Genet. 2010, 11, 89. [Google Scholar] [CrossRef] [PubMed]
- Tsutsui, H.; Kinugawa, S.; Matsushima, S. Oxidative stress and heart failure. Am. J. Physiol. Heart Circ. Physiol. 2011, 301, H2181–H2190. [Google Scholar] [CrossRef] [PubMed]
- Voigt, A.; Rahnefeld, A.; Kloetzel, P.M.; Krüger, E. Cytokine-induced oxidative stress in cardiac inflammation and heart failure-how the ubiquitin proteasome system targets this vicious cycle. Front. Physiol. 2013, 4, 42. [Google Scholar] [CrossRef]
- Nakamura, M.; Sadoshima, J. Mechanisms of physiological and pathological cardiac hypertrophy. Nat. Rev. Cardiol. 2018, 15, 387–407. [Google Scholar] [CrossRef]
- Travers, J.G.; Kamal, F.A.; Robbins, J.; Yutzey, K.E.; Blaxall, B.C. Cardiac Fibrosis: The Fibroblast Awakens. Circ. Res. 2016, 118, 1021–1040. [Google Scholar] [CrossRef]
- Kurrelmeyer, K.M.; Michael, L.H.; Baumgarten, G.; Taffet, G.E.; Peschon, J.J.; Sivasubramanian, N.; Entman, M.L.; Mann, D.L. Endogenous tumor necrosis factor protects the adult cardiac myocyte against ischemic-induced apoptosis in a murine model of acute myocardial infarction. Proc. Natl. Acad. Sci. USA 2000, 97, 5456–5461. [Google Scholar] [CrossRef]
- Nakano, M.; Knowlton, A.A.; Dibbs, Z.; Mann, D.L. Tumor necrosis factor-alpha confers resistance to hypoxic injury in the adult mammalian cardiac myocyte. Circulation 1998, 97, 1392–1400. [Google Scholar] [CrossRef]
- Krown, K.A.; Page, M.T.; Nguyen, C.; Zechner, D.; Gutierrez, V.; Comstock, K.L.; Glembotski, C.C.; Quintana, P.J.; Sabbadini, R.A. Tumor necrosis factor alpha-induced apoptosis in cardiac myocytes. Involvement of the sphingolipid signaling cascade in cardiac cell death. J. Clin. Investig. 1996, 98, 2854–2865. [Google Scholar] [CrossRef]
- Kubota, T.; McTiernan, C.F.; Frye, C.S.; Slawson, S.E.; Lemster, B.H.; Koretsky, A.P.; Demetris, A.J.; Feldman, A.M. Dilated cardiomyopathy in transgenic mice with cardiac-specific overexpression of tumor necrosis factor-alpha. Circ. Res. 1997, 81, 627–635. [Google Scholar] [CrossRef]
- Craig, R.; Larkin, A.; Mingo, A.M.; Thuerauf, D.J.; Andrews, C.; McDonough, P.M.; Glembotski, C. C p38 MAPK and NF-kappa B collaborate to induce interleukin-6 gene expression and release. Evidence for a cytoprotective autocrine signaling pathway in a cardiac myocyte model system. J. Biol. Chem. 2000, 275, 23814–23824. [Google Scholar] [CrossRef]
- Terrell, A.M.; Crisostomo, P.R.; Wairiuko, G.M.; Wang, M.; Morrell, E.D.; Meldrum, D.R. Jak/STAT/SOCS signaling circuits and associated cytokine-mediated inflammation and hypertrophy in the heart. Shock 2006, 26, 226–234. [Google Scholar] [CrossRef]
- Rigacci, L.; Carpaneto, A.; Alterini, R.; Carrai, V.; Bernardi, F.; Bellesi, G.; Longo, G.; Bosi, A.; Rossi Ferrini, P. Treatment of large cell lymphoma in elderly patients with a mitoxantrone, cyclophosphamide, etoposide, and prednisone regimen: Long-term follow-up results. Cancer 2003, 97, 97–104. [Google Scholar] [CrossRef]
- Tan, R.M.; Quah, T.C.; Aung, L.; Liang, S.; Kirk, R.C.; Yeoh, A.E. Improved outcome in childhood acute myeloid leukemia in Singapore with the MRC AML 10 protocol. Pediatr. Blood Cancer 2007, 48, 262–267. [Google Scholar] [CrossRef]
- Pratt, C.B.; Vietti, T.J.; Etcubanas, E.; Sexauer, C.; Krance, R.A.; Mahoney, D.H.; Patterson, R.B. Novantrone for childhood malignant solid tumors. A pediatric oncology group phase II study. Investig. New Drugs 1986, 4, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Dahl, G.V.; Lacayo, N.J.; Brophy, N.; Dunussi-Joannopoulos, K.; Weinstein, H.J.; Chang, M.; Sikic, B.I.; Arceci, R.J. Mitoxantrone, etoposide, and cyclosporine therapy in pediatric patients with recurrent or refractory acute myeloid leukemia. J. Clin. Oncol. 2000, 18, 1867–1875. [Google Scholar] [CrossRef][Green Version]
- Van der Pal, H.J.; van Dalen, E.C.; Hauptmann, M.; Kok, W.E.; Caron, H.N.; van den Bos, C.; Oldenburger, F.; Koning, C.C.; van Leeuwen, F.E.; Kremer, L.C. Cardiac function in 5-year survivors of childhood cancer: A long-term follow-up study. Arch. Intern. Med. 2010, 170, 1247–1255. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, G.T.; Oeffinger, K.C.; Chen, Y.; Kawashima, T.; Yasui, Y.; Leisenring, W.; Stovall, M.; Chow, E.J.; Sklar, C.A.; Mulrooney, D.A.; et al. Modifiable risk factors and major cardiac events among adult survivors of childhood cancer. J. Clin. Oncol. 2013, 31, 3673–3680. [Google Scholar] [CrossRef] [PubMed]
- Mulrooney, D.A.; Armstrong, G.T.; Huang, S.; Ness, K.K.; Ehrhardt, M.J.; Joshi, V.M.; Plana, J.C.; Soliman, E.Z.; Green, D.M.; Srivastava, D.; et al. Cardiac Outcomes in Adult Survivors of Childhood Cancer Exposed to Cardiotoxic Therapy: A Cross-sectional Study. Ann. Intern. Med. 2016, 164, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Wise, J. Adult survivors of childhood cancer are at high risk of cardiac abnormalities, study finds. BMJ 2016, 352, h7026. [Google Scholar] [CrossRef]
- Scully, R.; Lipshultz, S.E. Cardiovasclular toxicity of antitumor drugs: Dimension of the problem in children. In Cardiotoxicity of Non-Cardiovascular Drugs; Minotti, G., Ed.; John Wiley & Sons, Ltd.: New York, NY, USA, 2010; pp. 97–126. [Google Scholar]
- Howlader, N.N.A.; Krapcho, M.; Miller, D.; Brest, A.; Yu, M.; Ruhl, J.; Tatalovich, Z.; Mariotto, A.; Lewis, D.R.; Chen, H.S.; et al. (Eds.) SEER Cancer Statistics Review 1975–2017; National Cancer Institute: Bethesda, MD, USA, 2019. Available online: https://seer.cancer.gov/csr/1975_2017/ (accessed on 9 April 2020).
- Dores-Sousa, J.L.; Duarte, J.A.; Seabra, V.; de Lourdes Bastos, M.; Carvalho, F.; Costa, V.M. The age factor for mitoxantrone’s cardiotoxicity: Multiple doses render the adult mouse heart more susceptible to injury. Toxicology 2015, 329, 106–119. [Google Scholar] [CrossRef] [PubMed]
- Levi, F.; Tampellini, M.; Metzger, G.; Bizi, E.; Lemaigre, G.; Hallek, M. Circadian changes in mitoxantrone toxicity in mice: Relationship with plasma pharmacokinetics. Int. J. Cancer 1994, 59, 543–547. [Google Scholar] [CrossRef] [PubMed]
- Subramaniam, V.; Chuang, G.; Xia, H.; Burn, B.; Bradley, J.; Maderdrut, J.L.; Coy, D.H.; Varner, K.J. Pituitary adenylate cyclase-activating polypeptide (PACAP) protects against mitoxantrone-induced cardiac injury in mice. Peptides 2017, 95, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Rossato, L.G.; Costa, V.M.; Dallegrave, E.; Arbo, M.; Silva, R.; Ferreira, R.; Amado, F.; Dinis-Oliveira, R.J.; Duarte, J.A.; de Lourdes Bastos, M.; et al. Mitochondrial cumulative damage induced by mitoxantrone: Late onset cardiac energetic impairment. Cardiovasc. Toxicol. 2014, 14, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Rej, R. Aminotransferases in disease. Clin. Lab. Med. 1989, 9, 667–687. [Google Scholar] [CrossRef]
- Rossato, L.G.; Costa, V.M.; Dallegrave, E.; Arbo, M.; Dinis-Oliveira, R.J.; Santos-Silva, A.; Duarte, J.A.; de Lourdes Bastos, M.; Palmeira, C.; Remião, F. Cumulative mitoxantrone-induced haematological and hepatic adverse effects in a subchronic in vivo study. Basic Clin. Pharmacol. Toxicol. 2014, 114, 254–262. [Google Scholar] [CrossRef]
- Anghel, N.; Cotoraci, C.; Ivan, A.; Suciu, M.; Herman, H.; Balta, C.; Nicolescu, L.; Olariu, T.; Galajda, Z.; Ardelean, A.; et al. Chrysin attenuates cardiomyocyte apoptosis and loss of intermediate filaments in a mouse model of mitoxantrone cardiotoxicity. Histol. Histopathol. 2015, 30, 1465–1475. [Google Scholar]
- Alderton, P.M.; Gross, J.; Green, M.D. Comparative study of doxorubicin, mitoxantrone, and epirubicin in combination with ICRF-187 (ADR-529) in a chronic cardiotoxicity animal model. Cancer Res. 1992, 52, 194–201. [Google Scholar]
- Cavalletti, E.; Crippa, L.; Mainardi, P.; Oggioni, N.; Cavagnoli, R.; Bellini, O.; Sala, F. Pixantrone (BBR 2778) has reduced cardiotoxic potential in mice pretreated with doxorubicin: Comparative studies against doxorubicin and mitoxantrone. Investig. New Drugs 2007, 25, 187–195. [Google Scholar] [CrossRef]
- Unverferth, D.V.; Unverferth, B.J.; Balcerzak, S.P.; Bashore, T.A.; Neidhart, J.A. Cardiac evaluation of mitoxantrone. Cancer Treat. Rep. 1983, 67, 343–350. [Google Scholar]
- Santulli, G.; Iaccarino, G. Adrenergic signaling in heart failure and cardiovascular aging. Maturitas 2016, 93, 65–72. [Google Scholar] [CrossRef]
- Strumia, E.; Pelliccia, F.; D’Ambrosio, G. Creatine phosphate: Pharmacological and clinical perspectives. Adv. Ther. 2012, 29, 99–123. [Google Scholar] [CrossRef]
- Clark, J.F. Creatine and phosphocreatine: A review of their use in exercise and sport. J. Athl. Train. 1997, 32, 45–51. [Google Scholar]
- Bertero, E.; Maack, C. Metabolic remodelling in heart failure. Nat. Rev. Cardiol. 2018, 15, 457–470. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, R.A.; Sousa, R.P.; Cadete, V.J.; Lopaschuk, G.D.; Palmeira, C.M.; Bjork, J.A.; Wallace, K.B. Metabolic remodeling associated with subchronic doxorubicin cardiomyopathy. Toxicology 2010, 270, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Brandão, S.; Reis-Mendes, A.; Domingues, P.; Alberto Duarte, J.; Lourdes Bastos, M.; Carvalho, F.; Ferreira, R.; Costa, V.M. Exploring the effects of the anticancer drugs doxorubicin and mitoxantrone on cardiac mitochondrial proteome using a murine model. Toxicology 2021. Submitted. [Google Scholar]
- Lores Arnaiz, S.; Llesuy, S. Oxidative stress in mouse heart by antitumoral drugs: A comparative study of doxorubicin and mitoxantrone. Toxicology 1993, 77, 31–38. [Google Scholar] [CrossRef]
- Barbosa, M.R.; Sampaio, I.H.; Teodoro, B.G.; Sousa, T.A.; Zoppi, C.C.; Queiroz, A.L.; Passos, M.A.; Alberici, L.C.; Teixeira, F.R.; Manfiolli, A.O.; et al. Hydrogen peroxide production regulates the mitochondrial function in insulin resistant muscle cells: Effect of catalase overexpression. Biochim. Biophys. Acta 2013, 1832, 1591–1604. [Google Scholar] [CrossRef]
- Koutinos, G.; Stathopoulos, G.P.; Dontas, I.; Perrea-Kotsarelis, D.; Couris, E.; Karayannacos, P.E.; Deliconstantinos, G. The effect of doxorubicin and its analogue mitoxantrone on cardiac muscle and on serum lipids: An experimental study. Anticancer Res. 2002, 22, 815–820. [Google Scholar]
- Costa, V.M.; Capela, J.P.; Sousa, J.R.; Eleutério, R.P.; Rodrigues, P.R.S.; Dores-Sousa, J.L.; Carvalho, R.A.; de Lourdes Bastos, M.; Duarte, J.A.; Remião, F.; et al. Mitoxantrone impairs proteasome activity and prompts early energetic and proteomic changes in HL-1 cardiomyocytes at clinically relevant concentrations. Arch. Toxicol. 2020, 94, 4067–4084. [Google Scholar] [CrossRef] [PubMed]
- Aldini, G.; Dalle-Donne, I.; Facino, R.M.; Milzani, A.; Carini, M. Intervention strategies to inhibit protein carbonylation by lipoxidation-derived reactive carbonyls. Med. Res. Rev. 2007, 27, 817–868. [Google Scholar] [CrossRef] [PubMed]
- Evans, S.; Tzeng, H.P.; Veis, D.J.; Matkovich, S.; Weinheimer, C.; Kovacs, A.; Barger, P.M.; Mann, D.L. TNF receptor-activated factor 2 mediates cardiac protection through noncanonical NF-κB signaling. JCI Insight 2018, 3, e98278. [Google Scholar] [CrossRef] [PubMed]
- Yunna, C.; Mengru, H.; Lei, W.; Weidong, C. Macrophage M1/M2 polarization. Eur. J. Pharmacol. 2020, 877, 173090. [Google Scholar] [CrossRef]
- Moore, K.J.; Sheedy, F.; Fisher, E.A. Macrophages in atherosclerosis: A dynamic balance. Nat. Rev. Immunol. 2013, 13, 709–721. [Google Scholar] [CrossRef] [PubMed]
- Van Dijk, R.A.; Rijs, K.; Wezel, A.; Hamming, J.F.; Kolodgie, F.D.; Virmani, R.; Schaapherder, A.F.; Lindeman, J.H. Systematic Evaluation of the Cellular Innate Immune Response during the Process of Human Atherosclerosis. J. Am. Heart Assoc. 2016, 5, e002860. [Google Scholar] [CrossRef]
- Ponnappan, S.; Ponnappan, U. Aging and immune function: Molecular mechanisms to interventions. Antioxid. Redox Signal. 2011, 14, 1551–1585. [Google Scholar] [CrossRef]
- Khaper, N.; Bryan, S.; Dhingra, S.; Singal, R.; Bajaj, A.; Pathak, C.M.; Singal, P.K. Targeting the vicious inflammation-oxidative stress cycle for the management of heart failure. Antioxid. Redox Signal. 2010, 13, 1033–1049. [Google Scholar] [CrossRef] [PubMed]
- Wollert, K.C.; Drexler, H. The role of interleukin-6 in the failing heart. Heart Fail. Rev. 2001, 6, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Palmieri, E.A.; Benincasa, G.; Di Rella, F.; Casaburi, C.; Monti, M.G.; De Simone, G.; Chiariotti, L.; Palombini, L.; Bruni, C.B.; Saccà, L.; et al. Differential expression of TNF-alpha, IL-6, and IGF-1 by graded mechanical stress in normal rat myocardium. Am. J. Physiol. Heart Circ. Physiol. 2002, 282, H926–H934. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Quagliariello, V.; Coppola, C.; Mita, D.G.; Piscopo, G.; Iaffaioli, R.V.; Botti, G.; Maurea, N. Low doses of Bisphenol A have pro-inflammatory and pro-oxidant effects, stimulate lipid peroxidation and increase the cardiotoxicity of Doxorubicin in cardiomyoblasts. Environ. Toxicol. Pharmacol. 2019, 69, 1–8. [Google Scholar] [CrossRef]
- Quagliariello, V.; Vecchione, R.; Coppola, C.; Di Cicco, C.; De Capua, A.; Piscopo, G.; Paciello, R.; Narciso, V.; Formisano, C.; Taglialatela-Scafati, O.; et al. Cardioprotective Effects of Nanoemulsions Loaded with Anti-Inflammatory Nutraceuticals against Doxorubicin-Induced Cardiotoxicity. Nutrients 2018, 10, 1304. [Google Scholar] [CrossRef] [PubMed]
- Quagliariello, V.; Passariello, M.; Rea, D.; Barbieri, A.; Iovine, M.; Bonelli, A.; Caronna, A.; Botti, G.; De Lorenzo, C.; Maurea, N. Evidences of CTLA-4 and PD-1 Blocking Agents-Induced Cardiotoxicity in Cellular and Preclinical Models. J. Pers. Med. 2020, 10, 179. [Google Scholar] [CrossRef]
- Angelucci, F.; Batocchi, A.P.; Caggiula, M.; Frisullo, G.; Patanella, K.; Sancricca, C.; Nociti, V.; Tonali, P.A.; Mirabella, M. In vivo effects of mitoxantrone on the production of pro- and anti-inflammatory cytokines by peripheral blood mononuclear cells of secondary progressive multiple sclerosis patients. Neuroimmunomodulation 2006, 13, 76–81. [Google Scholar] [CrossRef]
- Dutta, S.; Sengupta, P. Men and mice: Relating their ages. Life Sci. 2016, 152, 244–248. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Lai, X.; Deng, Y.; Song, Y. Correlation between mouse age and human age in anti-tumor research: Significance and method establishment. Life Sci. 2020, 242, 117242. [Google Scholar] [CrossRef] [PubMed]
- Curry, S.H.; DeCory, H.H.; Gabrielsson, J. Phase I: The first opportunity for extrapolation from animal data to human exposure. In Principles and Practice of Pharmaceutical Medicine; Edwards, L.D., Fox, A.W., Stonier, P.D., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2010; pp. 84–106. [Google Scholar]
- Reagan-Shaw, S.; Nihal, M.; Ahmad, N. Dose translation from animal to human studies revisited. FASEB J. 2008, 22, 659–661. [Google Scholar] [CrossRef] [PubMed]
- Al Shoyaib, A.; Archie, S.R.; Karamyan, V.T. Intraperitoneal Route of Drug Administration: Should it Be Used in Experimental Animal Studies? Pharm. Res. 2019, 37, 12. [Google Scholar] [CrossRef] [PubMed]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- DeLuca, M.; McElroy, W.D. Kinetics of the firefly luciferase catalyzed reactions. Biochemistry 1974, 13, 921–925. [Google Scholar] [CrossRef]
- Carvalho, M.; Milhazes, N.; Remiao, F.; Borges, F.; Fernandes, E.; Amado, F.; Monks, T.J.; Carvalho, F.; de Lourdes Bastos, M. Hepatotoxicity of 3,4-methylenedioxyamphetamine and alpha-methyldopamine in isolated rat hepatocytes: Formation of glutathione conjugates. Arch. Toxicol. 2004, 78, 16–24. [Google Scholar] [PubMed]
- Padrão, A.I.; Oliveira, P.; Vitorino, R.; Colaço, B.; Pires, M.J.; Márquez, M.; Castellanos, E.; Neuparth, M.J.; Teixeira, C.; Costa, C.; et al. Bladder cancer-induced skeletal muscle wasting: Disclosing the role of mitochondria plasticity. Int. J. Biochem. Cell Biol. 2013, 45, 1399–1409. [Google Scholar] [CrossRef] [PubMed]
- Garcia, J.; Costa, V.M.; Carvalho, A.T.; Silvestre, R.; Duarte, J.A.; Dourado, D.F.; Arbo, M.D.; Baltazar, T.; Dinis-Oliveira, R.J.; Baptista, P.; et al. A breakthrough on Amanita phalloides poisoning: An effective antidotal effect by polymyxin B. Arch. Toxicol. 2015, 89, 2305–2323. [Google Scholar] [CrossRef] [PubMed]
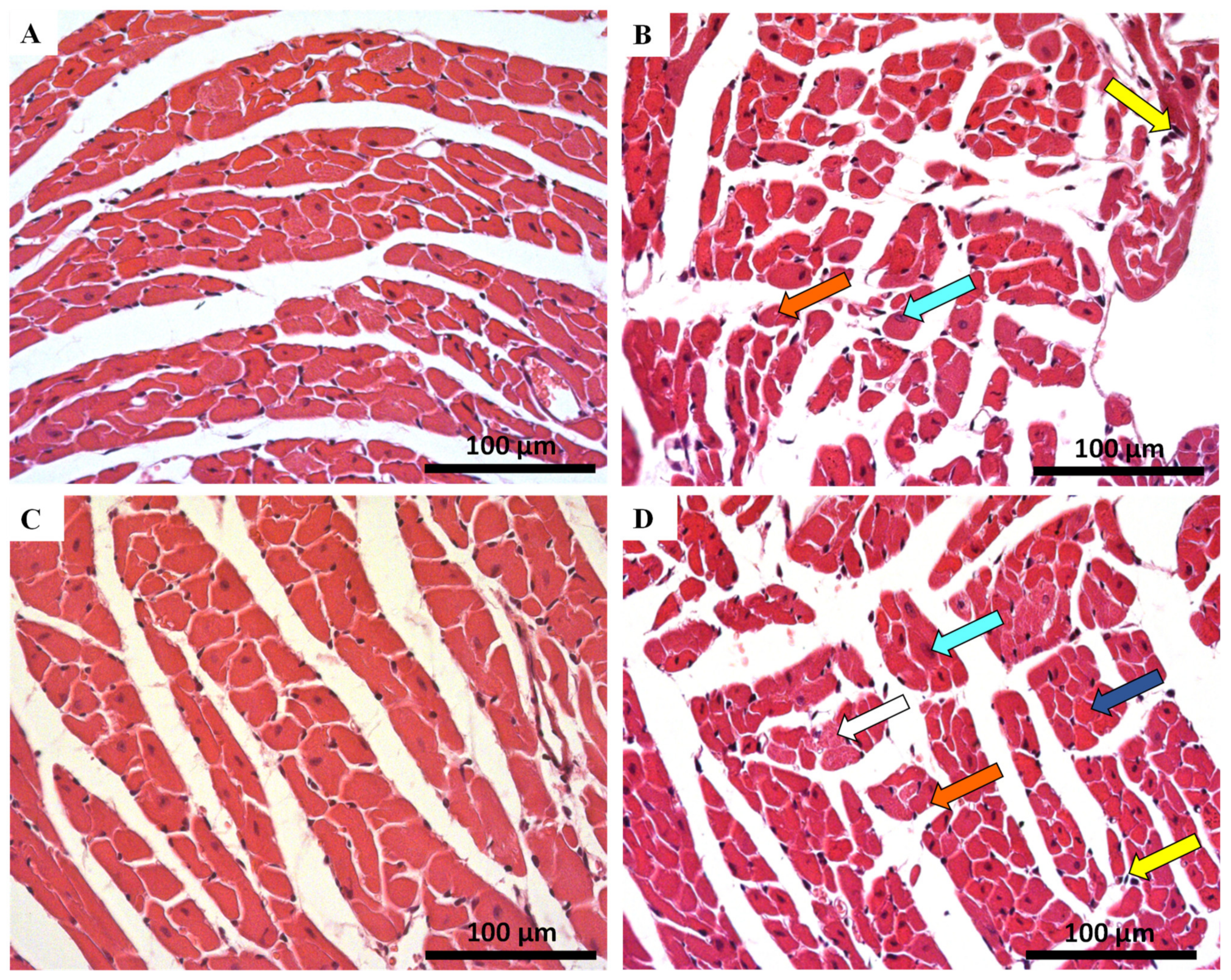
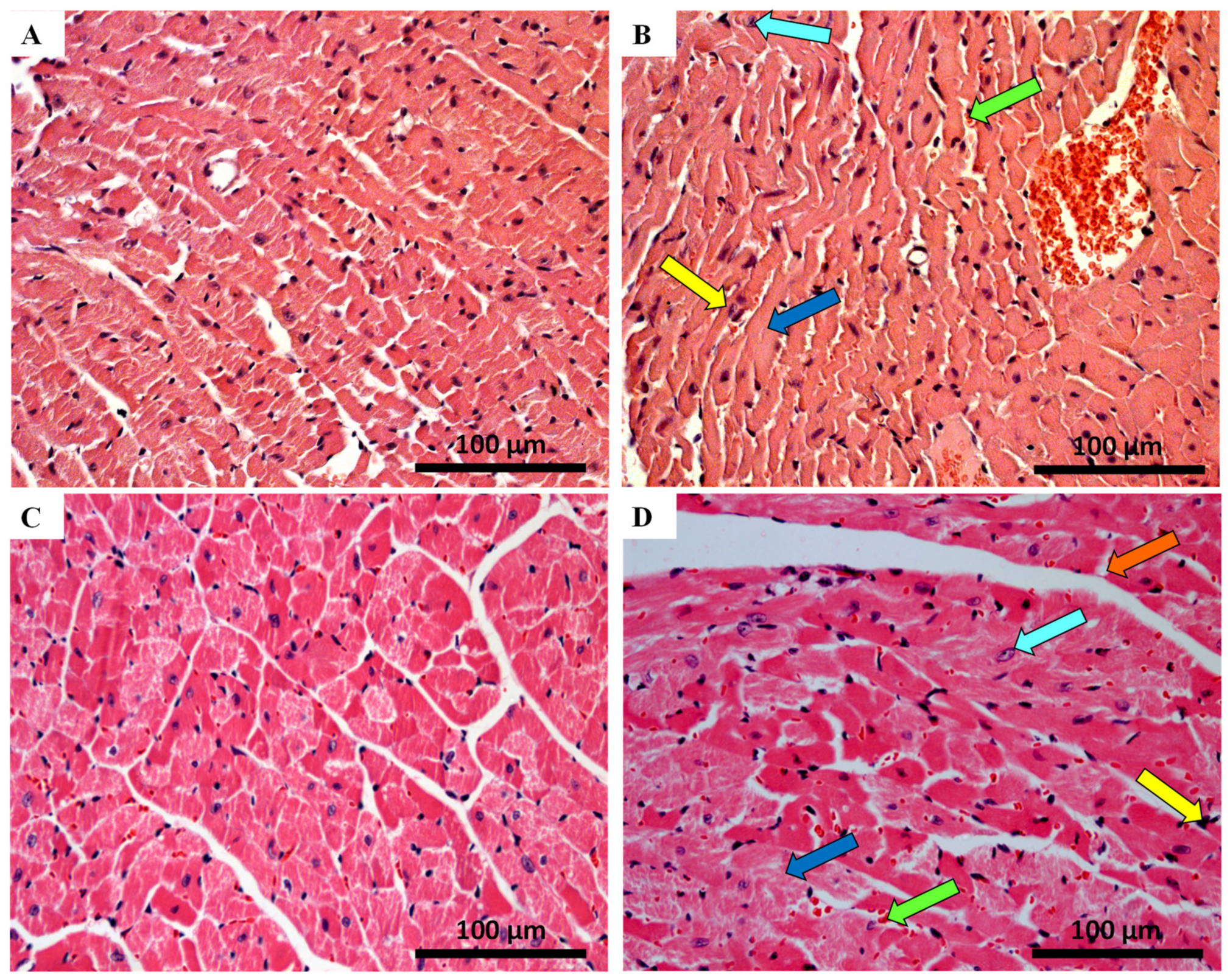
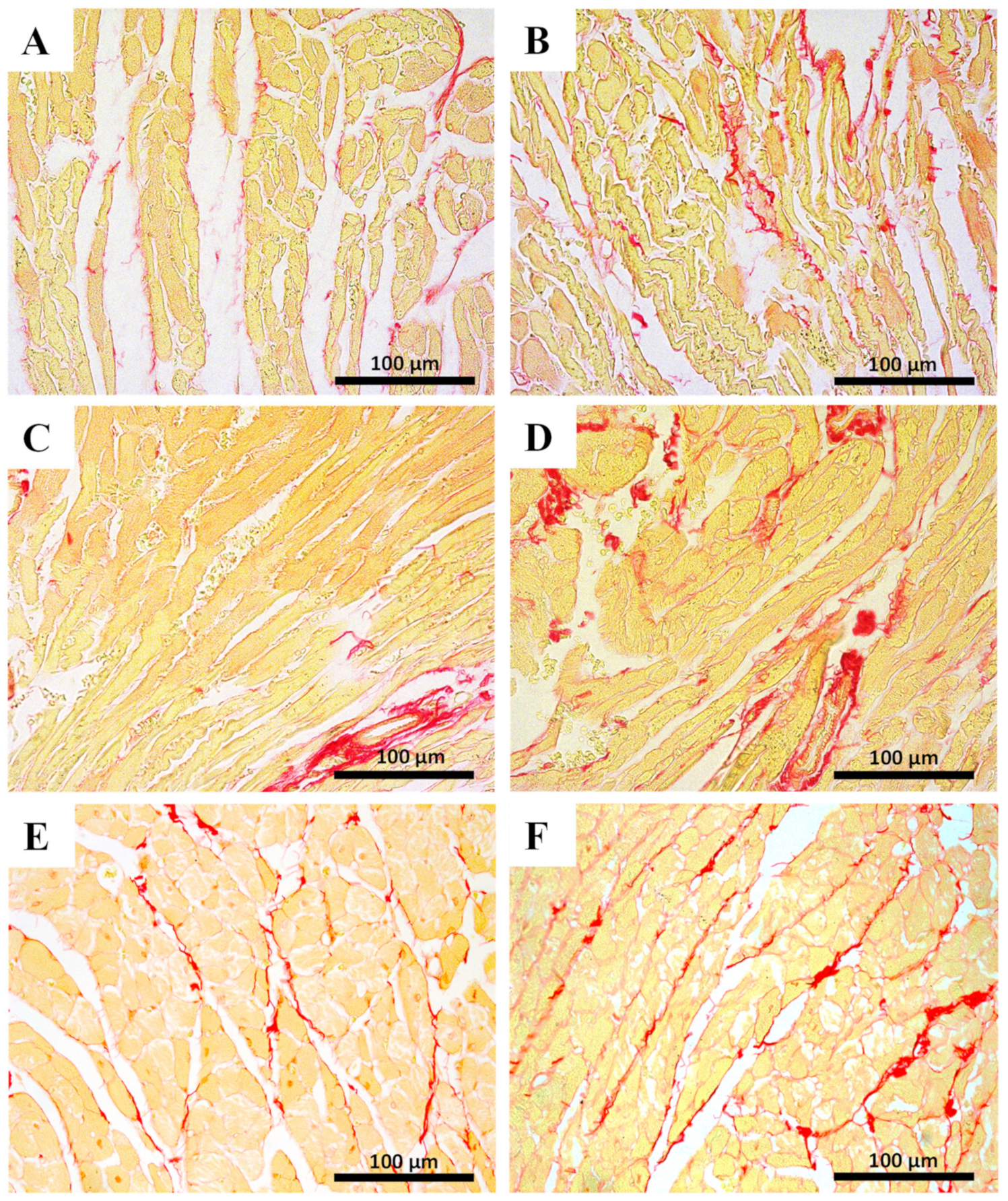
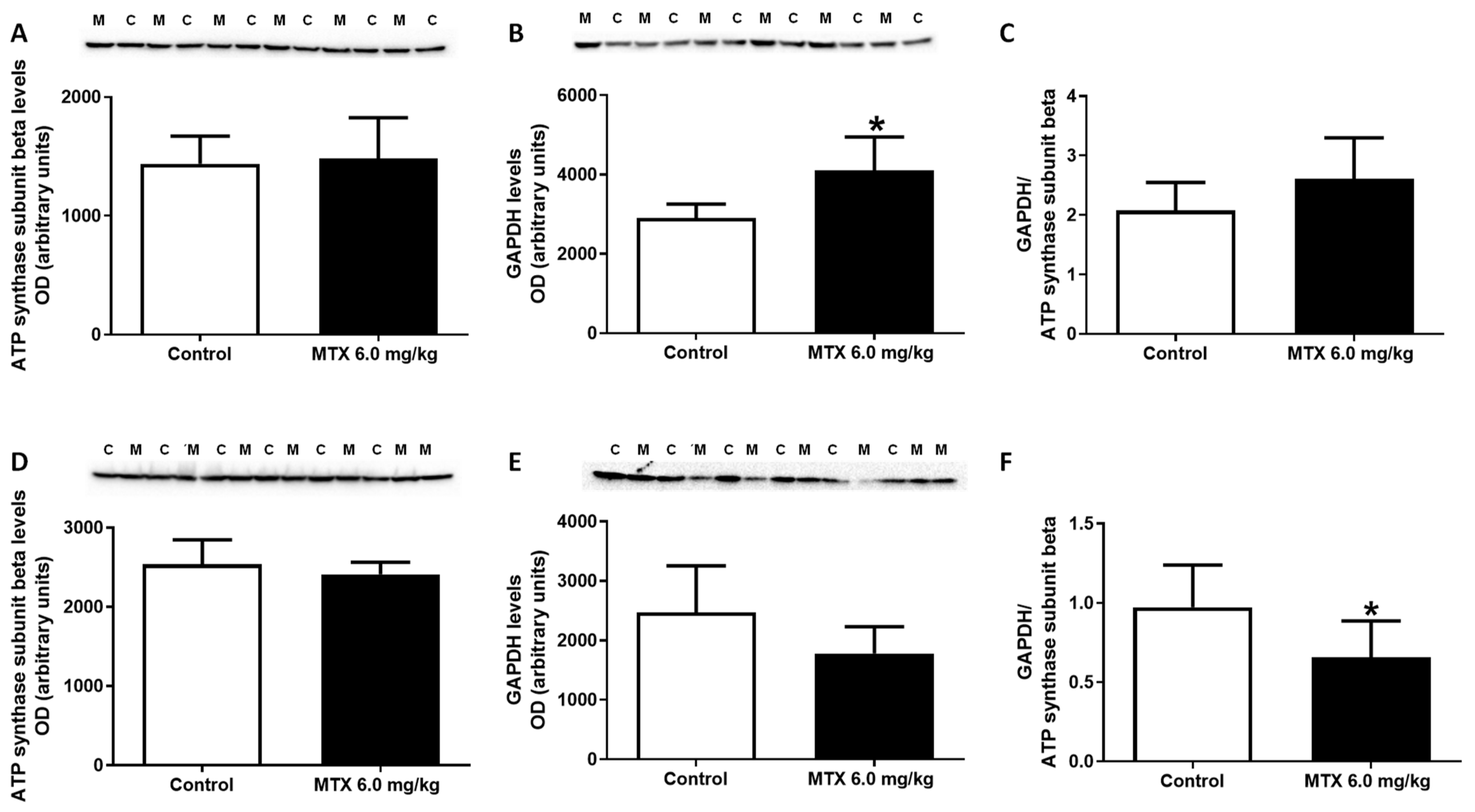
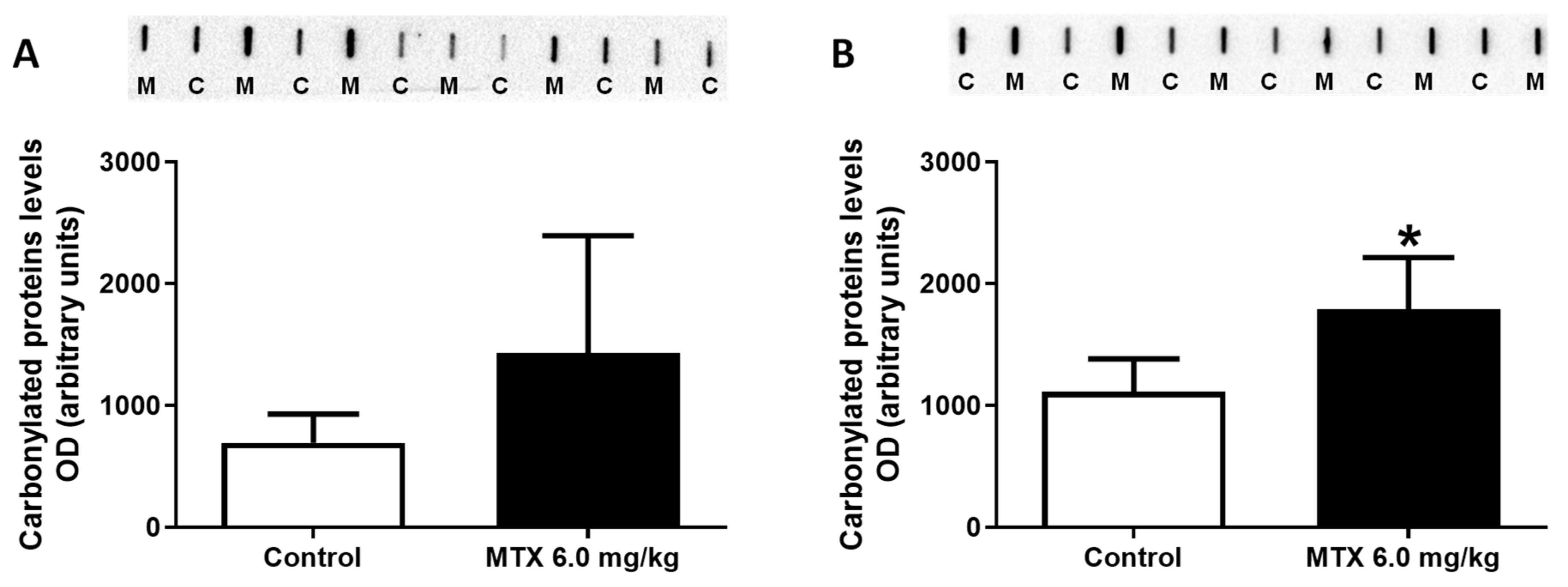
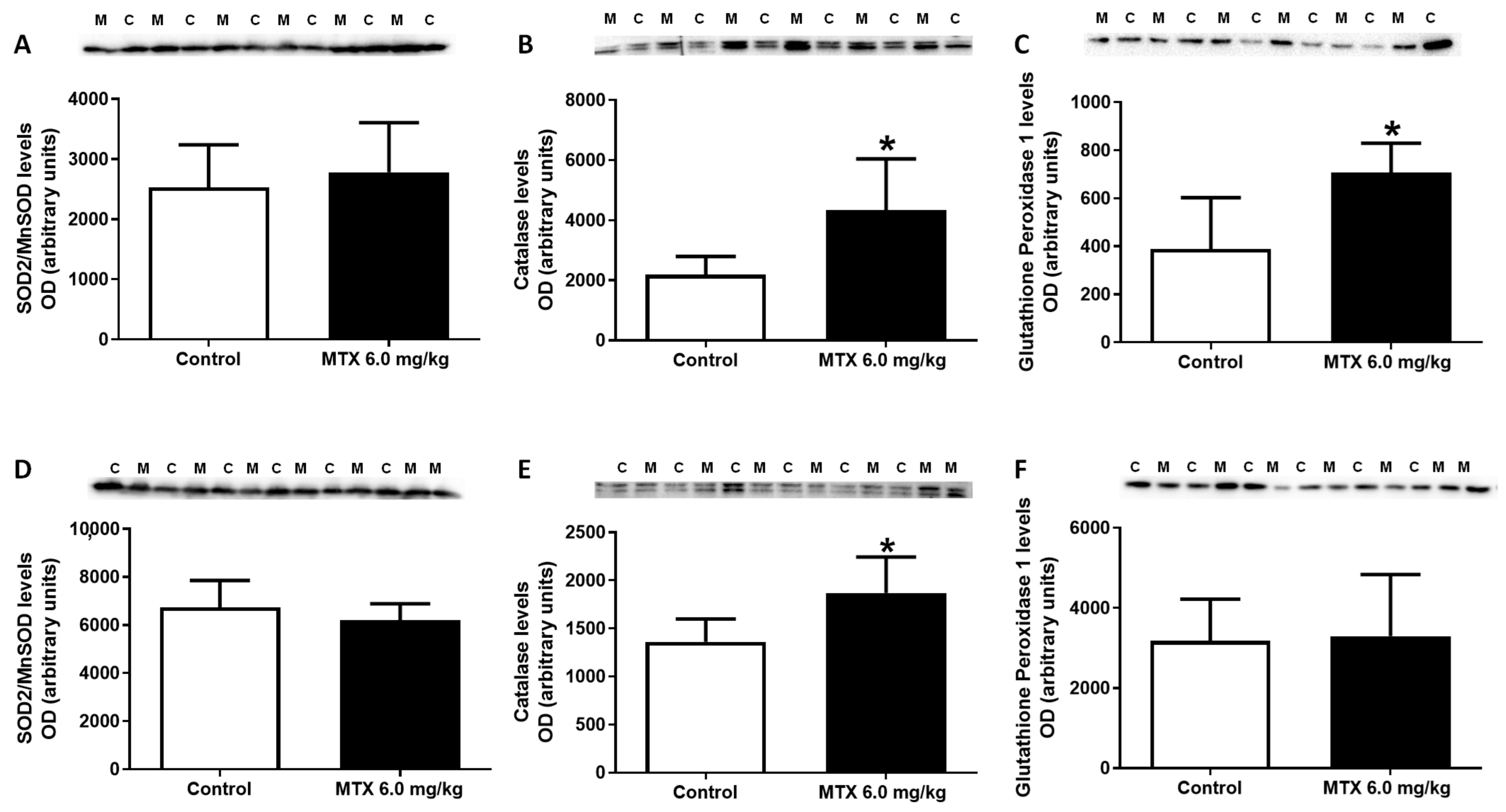
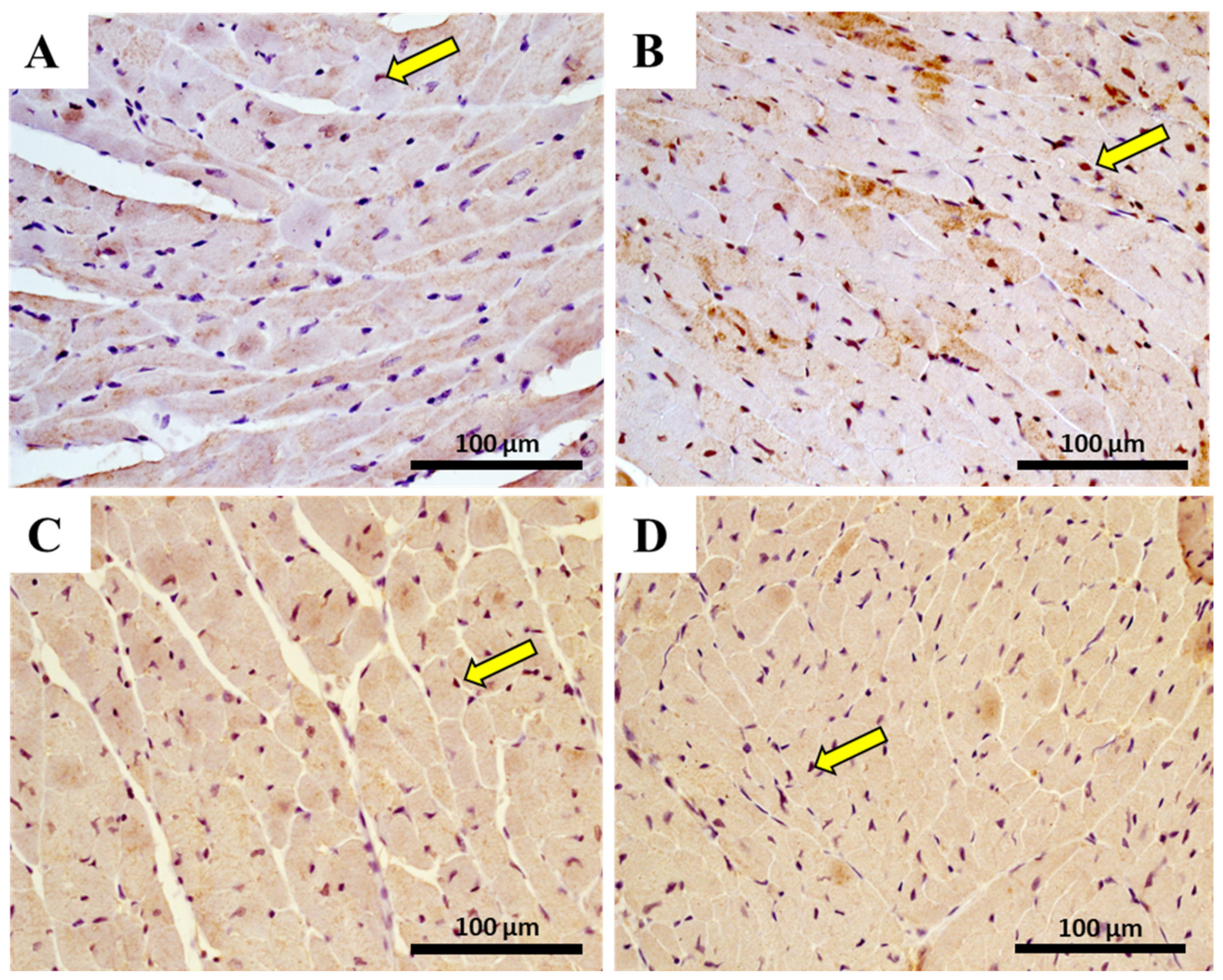
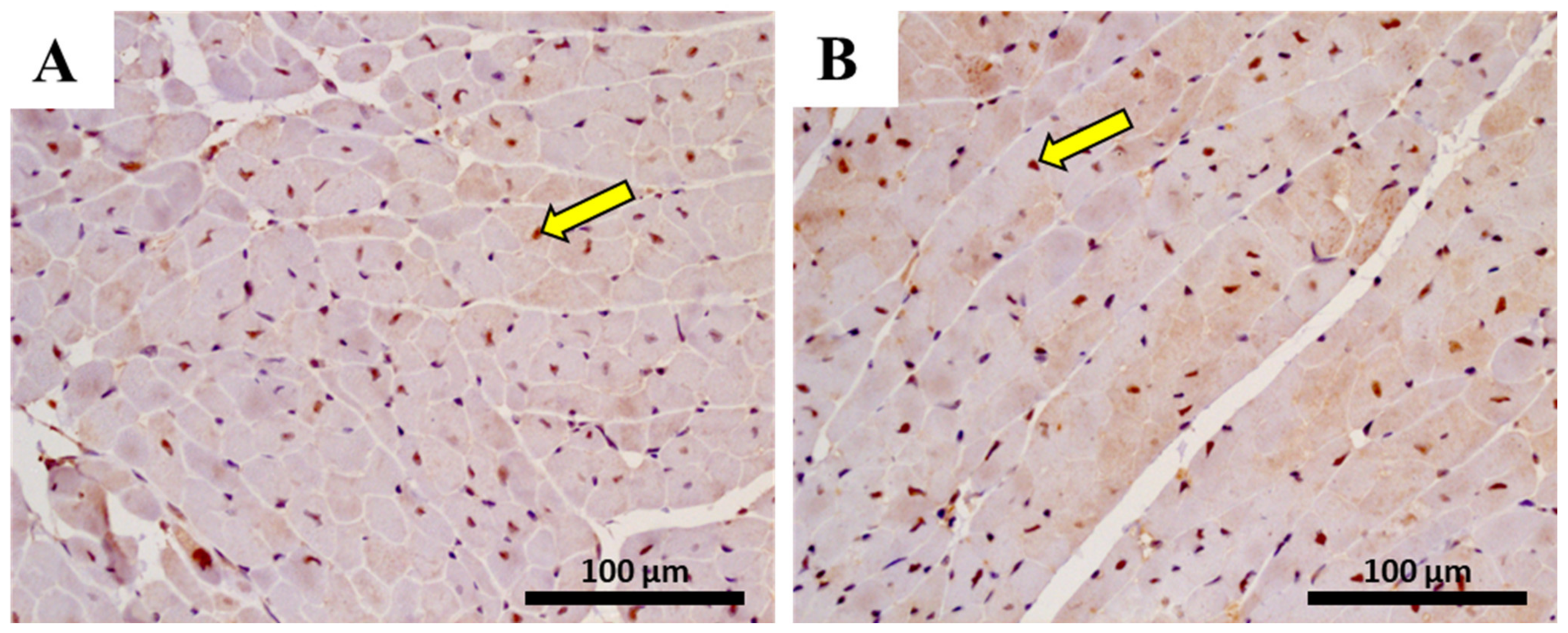
| Hematoxylin-eosin staining | ||||
| INFANT | ADULT | |||
| Control | MTX 7.0 mg/kg | Control | MTX 7.0 mg/kg | |
| Cellular Degeneration | 0.50 ± 0.54 | 0.82 ± 0.63 ** | 0.64 ± 0.63 | 1.42 ± 0.71 **** |
| Necrosis | 0.00 ± 0.00 | 0.04 ± 0.20 | 0.00 ± 0.00 | 0.23 ± 0.43 *** |
| Inflammatory Activity | 0.22 ± 0.42 | 0.85 ± 0.52 **** | 0.30 ± 0.46 | 1.02 ± 0.49 **** |
| Control | MTX 6.0 mg/kg | Control | MTX 6.0 mg/kg | |
| Cellular Degeneration | 0.07 ± 0.25 | 0.47 ± 0.51 *** | 0.02 ± 0.13 | 1.00 ± 0.00 **** |
| Necrosis | 0.00 ± 0.00 | 0.27 ± 0.45 ** | 0.00 ± 0.00 | 1.02 ± 0.14 **** |
| Inflammatory Activity | 0.08 ± 0.27 | 0.50 ± 0.51 *** | 0.05 ± 0.22 | 1.65 ± 0.49 **** |
| Sirius Red Staining | ||||
| INFANT | ADULT | |||
| Control | MTX 7.0 mg/kg | Control | MTX 7.0 mg/kg | |
| % area ratio of collagen/skeletal muscle | 0.14 ± 0.07 | 0.17 ± 0.05 | 0.14 ± 0.02 | 0.24 ± 0.06 **** |
| Control | MTX 6.0 mg/kg | Control | MTX 6.0 mg/kg | |
| % area ratio of collagen/skeletal muscle | 0.10 ± 0.03 | 0.15 ± 0.03 | 0.10 ± 0.02 | 0.16 ± 0.05 ** |
| INFANT | ADULT | |||
|---|---|---|---|---|
| Control | MTX 7.0 mg/kg | Control | MTX 7.0 mg/kg | |
| tGSH (nmol/mg protein) | 7.72 ± 1.18 | 9.41 ± 1.63 * | 8.01 ± 0.77 | 8.57 ± 2.33 |
| GSSG (nmol/mg protein) | 0.62 ± 0.19 | 0.77 ± 0.15 | 0.60 ± 0.10 | 0.60 ± 0.34 |
| GSH/GSSG ratio | 11.94 ± 5.23 | 10.62 ± 2.01 | 11.84 ± 3.52 | 15.40 ± 8.63 |
| MDA (nmol/g protein) | 27.23 ± 9.23 | 28.42 ± 7.32 | 30.65 ± 7.59 | 34.83 ± 12.44 |
| ATP (nmol/mg protein) | 3.94 ± 1.97 | 3.77± 1.42 | 3.92 ± 1.34 | 3.23 ± 1.39 |
| Creatine (nmol/mg protein) | 74.77 ± 16.25 | 78.94 ± 11.09 | 82.17 ± 13.24 | 84.13 ± 25.30 |
| Phosphocreatine (nmol/mg protein) | 41.06 ± 9.71 | 43.08 ± 6.68 | 37.25 ± 8.11 | 49.10 ± 14.36 (p = 0.06) |
| Noradrenaline (nmol/g protein) | 8.26 ± 5.18 | 9.03 ± 4.63 | 9.79 ± 1.99 | 4.64 ± 5.16 * |
| INFANT | ADULT | |||
|---|---|---|---|---|
| Immunohistochemistry | ||||
| Control | MTX 6.0 mg/kg | Control | MTX 6.0 mg/kg | |
| M1 macrophage | 4.28 ± 3.03 | 9.33 ± 7.70 | 12.83 ± 15.37 | 68.11 ± 22.77 **** |
| M2 macrophage | 66.61 ± 43.40 | 62.56 ± 24.92 | 113.70 ± 23.10 | 96.56 ± 28.33 |
| INFANT | ADULT | |||
|---|---|---|---|---|
| Immunohistochemistry | ||||
| Control | MTX 6.0 mg/kg | Control | MTX 6.0 mg/kg | |
| NF-ĸB p65 cells | 28.00 ± 12.35 | 26.00 ± 8.62 | 56.28 ± 15.69 | 75.17 ± 17.05 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reis-Mendes, A.; Dores-Sousa, J.L.; Padrão, A.I.; Duarte-Araújo, M.; Duarte, J.A.; Seabra, V.; Gonçalves-Monteiro, S.; Remião, F.; Carvalho, F.; Sousa, E.; et al. Inflammation as a Possible Trigger for Mitoxantrone-Induced Cardiotoxicity: An In Vivo Study in Adult and Infant Mice. Pharmaceuticals 2021, 14, 510. https://doi.org/10.3390/ph14060510
Reis-Mendes A, Dores-Sousa JL, Padrão AI, Duarte-Araújo M, Duarte JA, Seabra V, Gonçalves-Monteiro S, Remião F, Carvalho F, Sousa E, et al. Inflammation as a Possible Trigger for Mitoxantrone-Induced Cardiotoxicity: An In Vivo Study in Adult and Infant Mice. Pharmaceuticals. 2021; 14(6):510. https://doi.org/10.3390/ph14060510
Chicago/Turabian StyleReis-Mendes, Ana, José Luís Dores-Sousa, Ana Isabel Padrão, Margarida Duarte-Araújo, José Alberto Duarte, Vítor Seabra, Salomé Gonçalves-Monteiro, Fernando Remião, Félix Carvalho, Emília Sousa, and et al. 2021. "Inflammation as a Possible Trigger for Mitoxantrone-Induced Cardiotoxicity: An In Vivo Study in Adult and Infant Mice" Pharmaceuticals 14, no. 6: 510. https://doi.org/10.3390/ph14060510
APA StyleReis-Mendes, A., Dores-Sousa, J. L., Padrão, A. I., Duarte-Araújo, M., Duarte, J. A., Seabra, V., Gonçalves-Monteiro, S., Remião, F., Carvalho, F., Sousa, E., Bastos, M. L., & Costa, V. M. (2021). Inflammation as a Possible Trigger for Mitoxantrone-Induced Cardiotoxicity: An In Vivo Study in Adult and Infant Mice. Pharmaceuticals, 14(6), 510. https://doi.org/10.3390/ph14060510