Synthesis of Multifunctional Nanoparticles for the Combination of Photodynamic Therapy and Immunotherapy
Abstract
1. Introduction
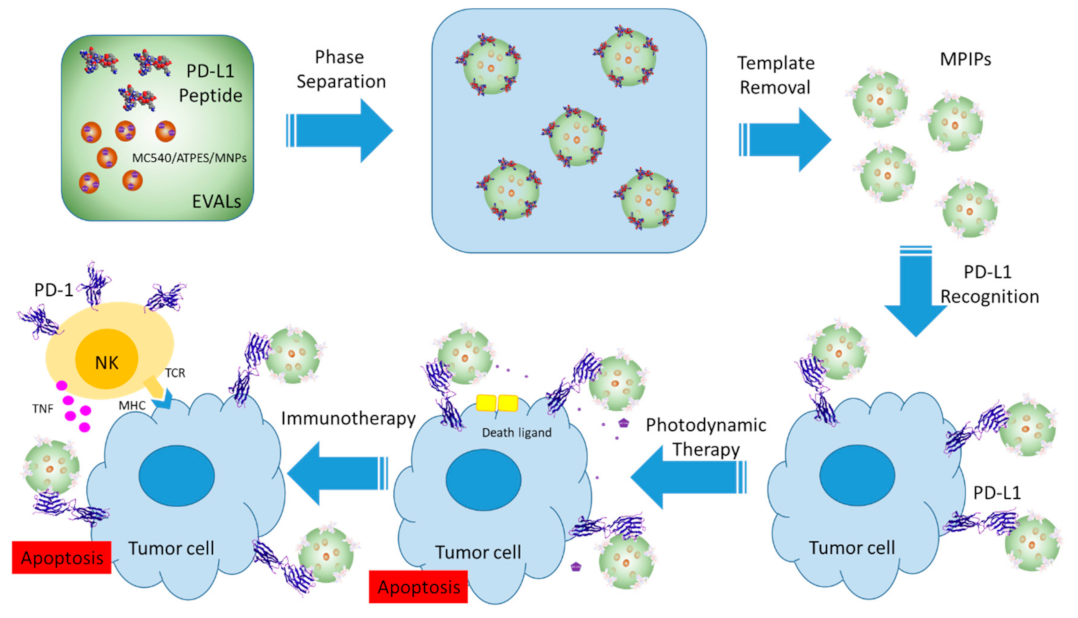
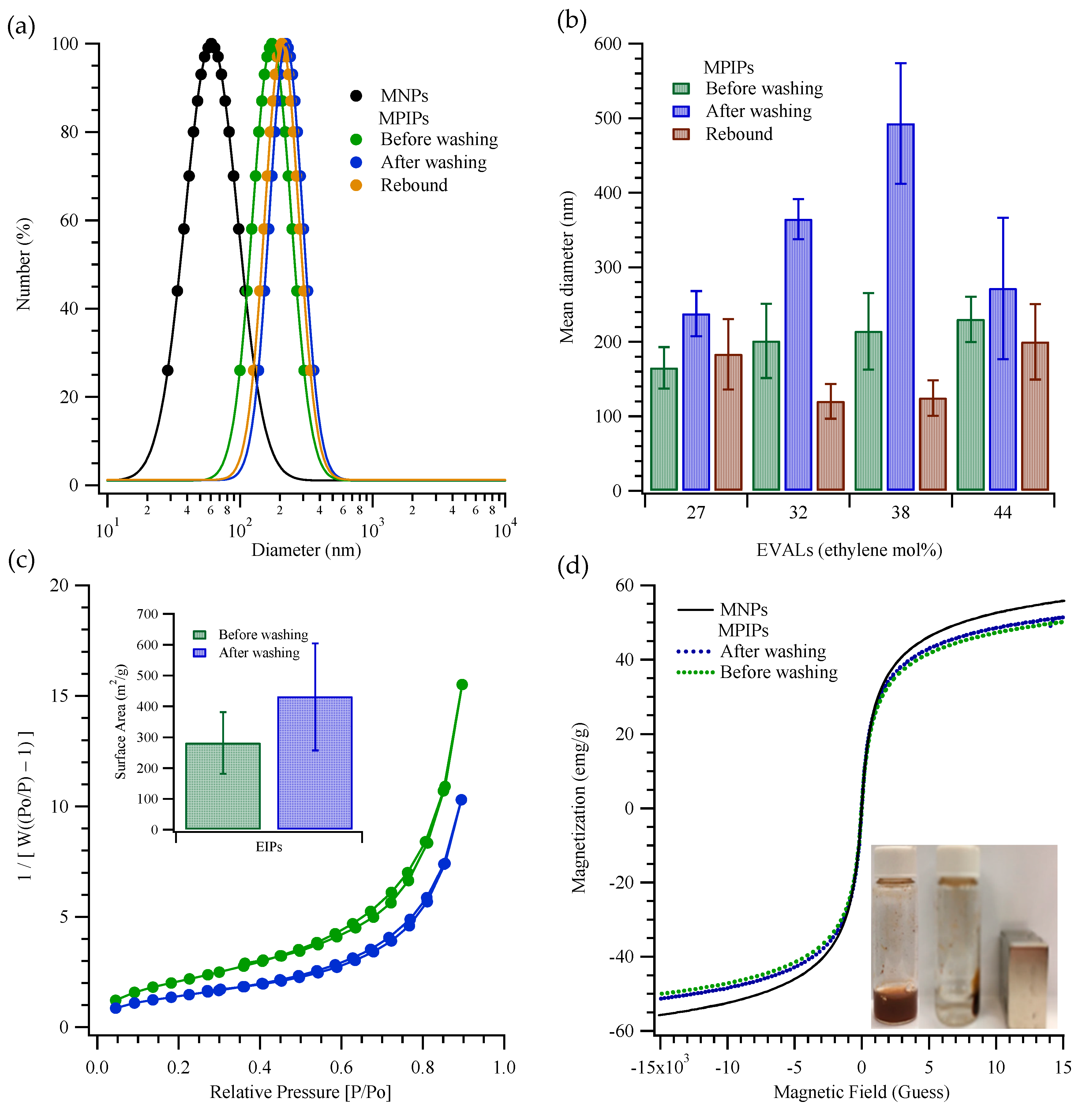
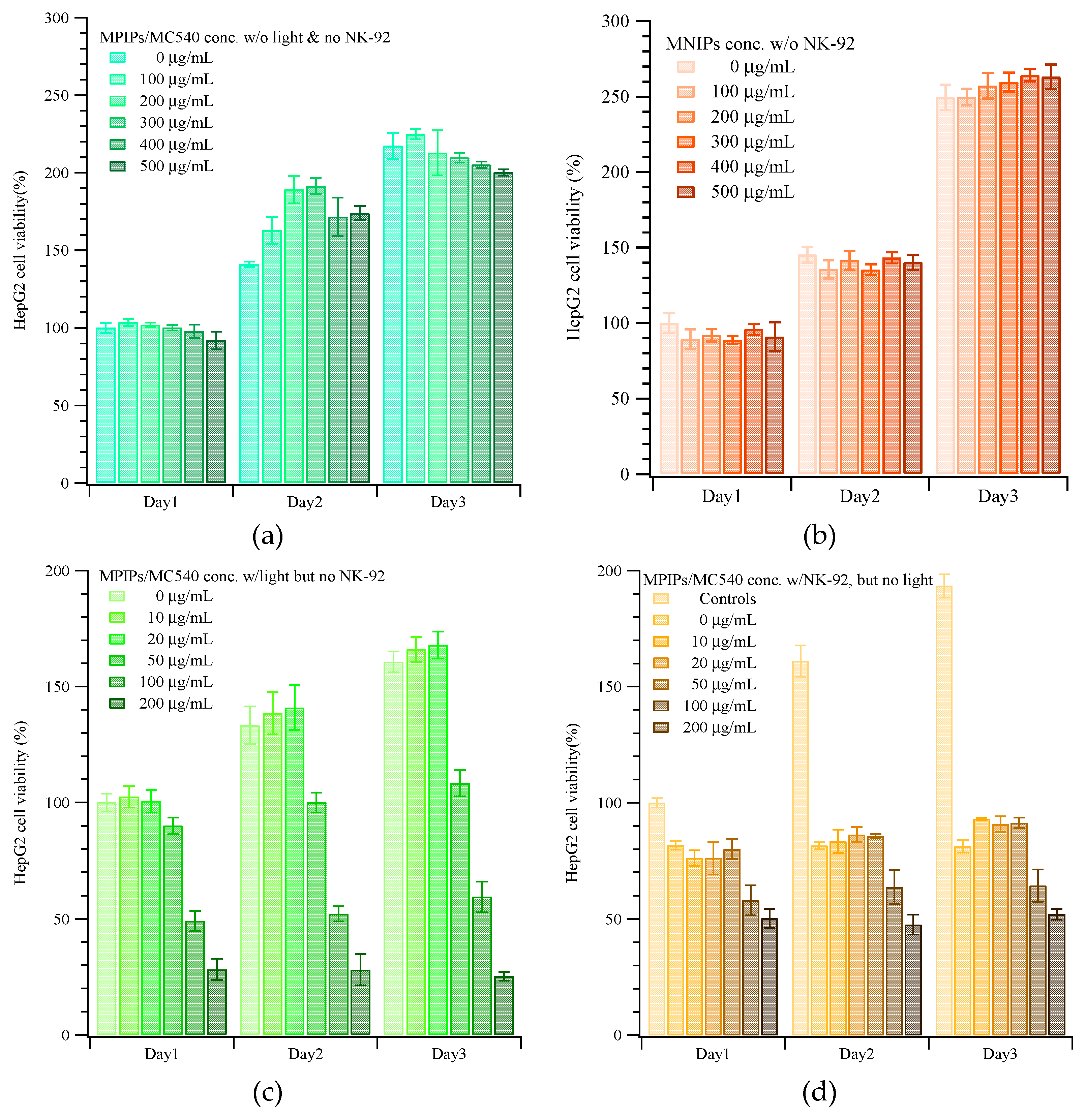
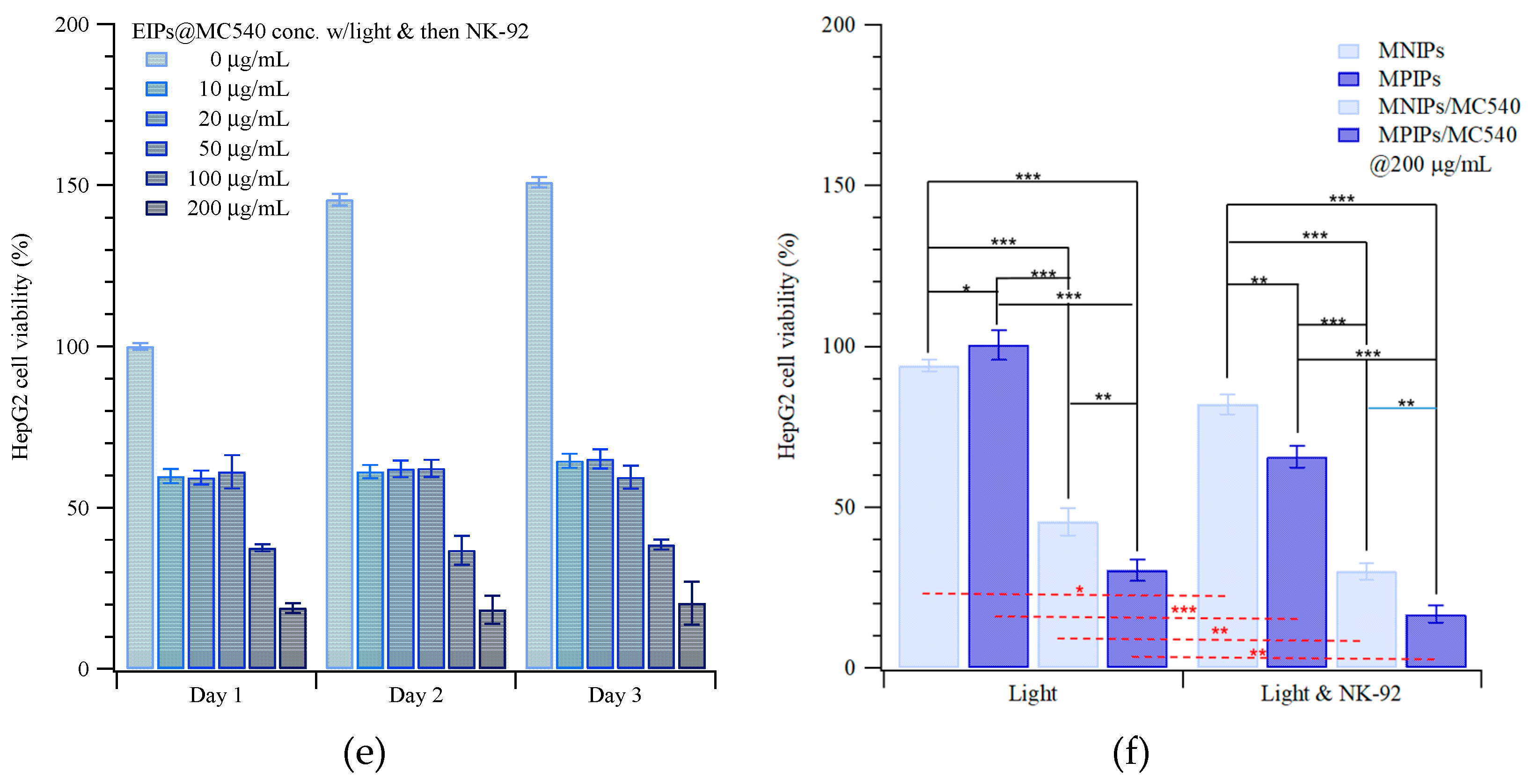
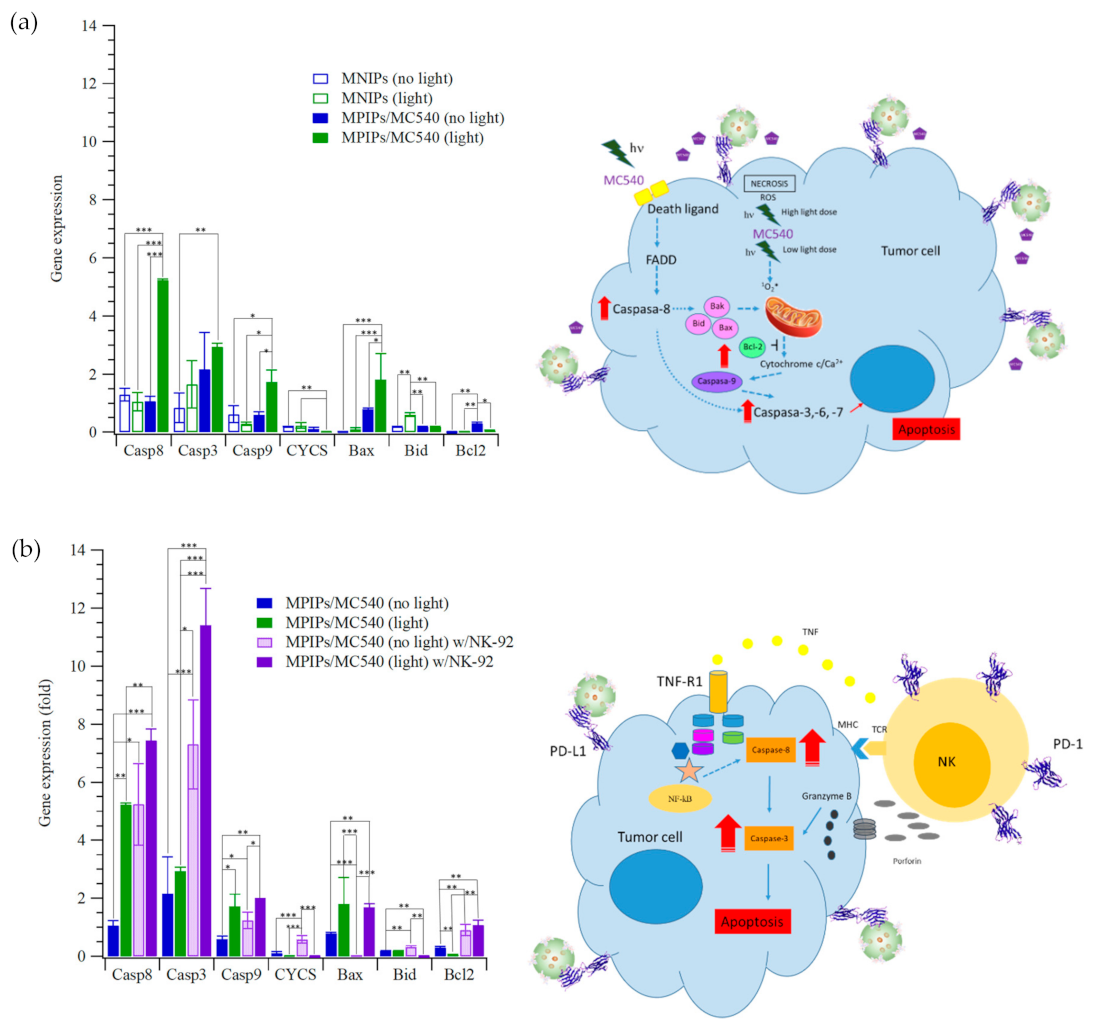
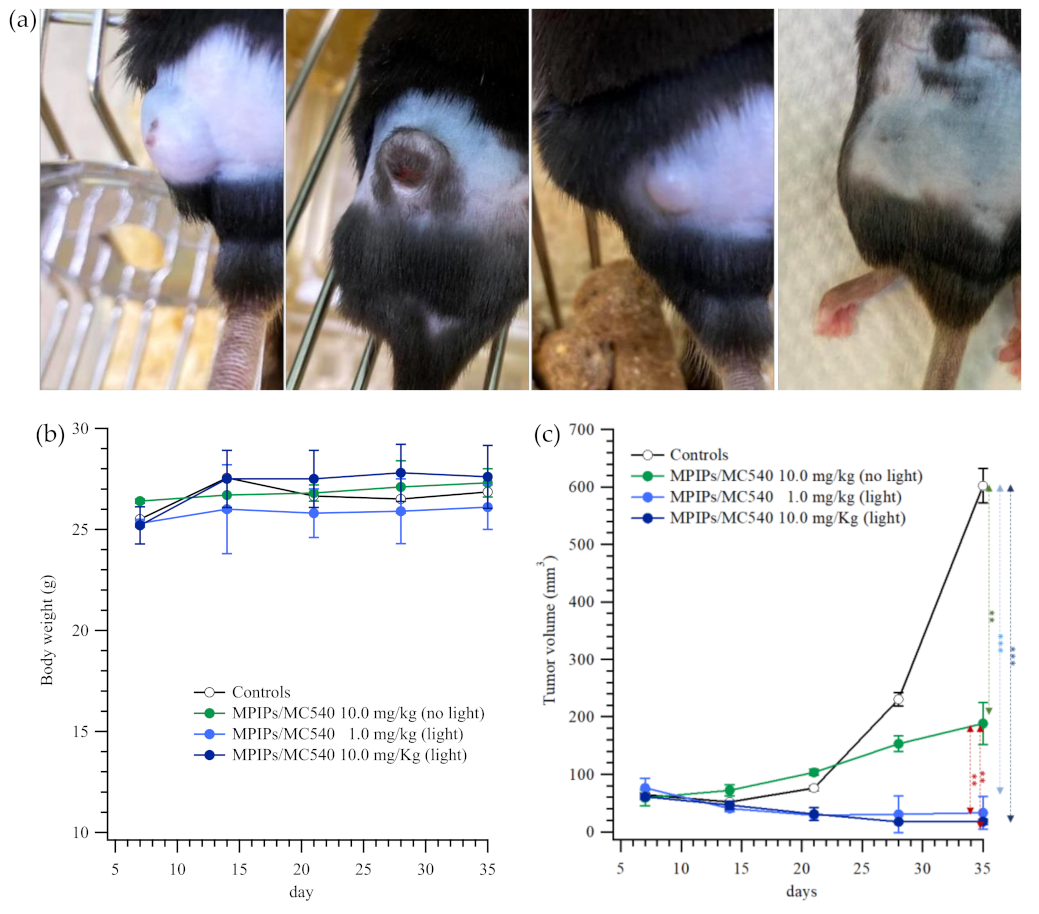
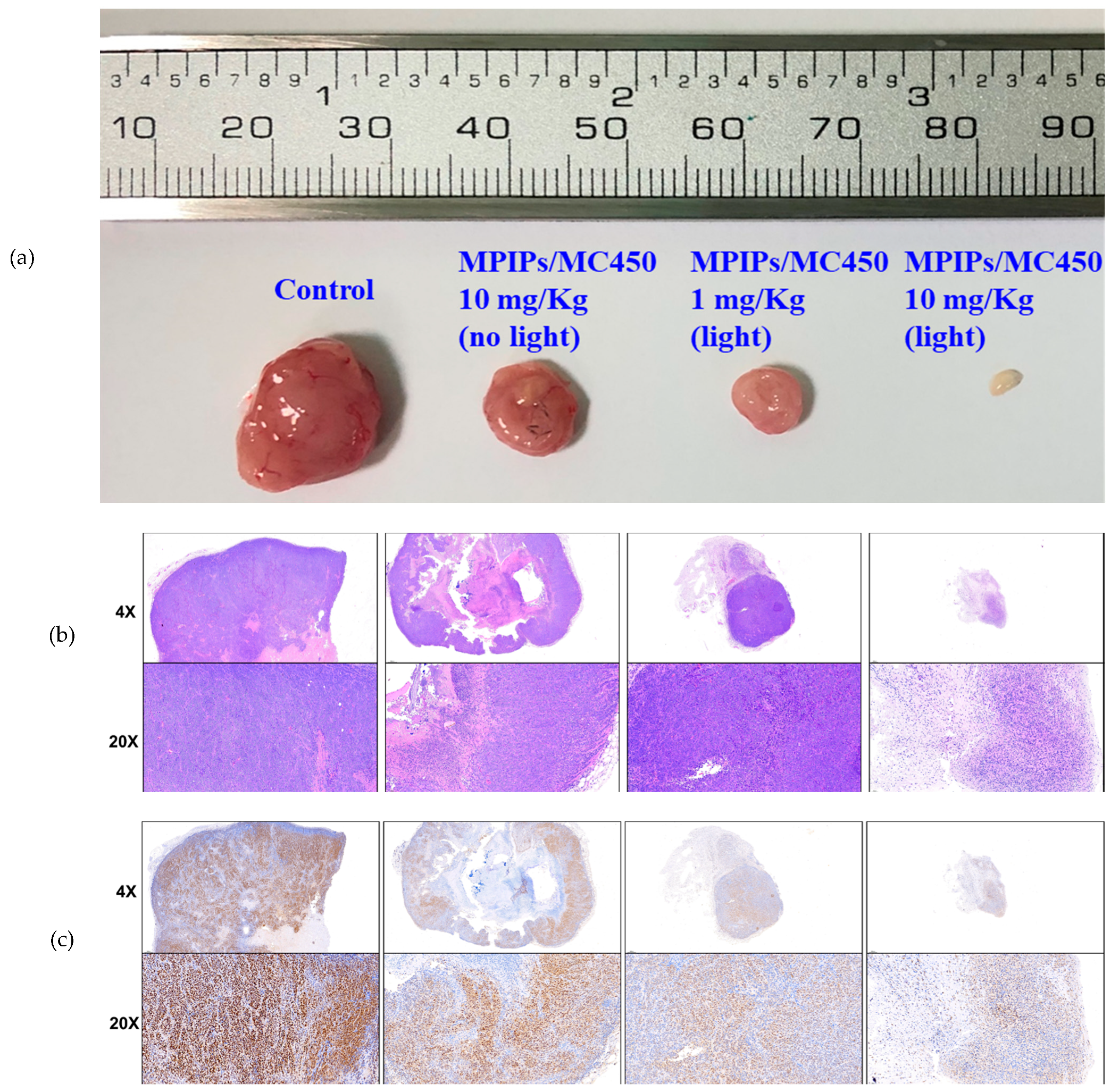
2. Results and Discussion
3. Materials and Methods
3.1. Reagents and Chemicals
3.2. Formation of Multifunctional Magnetic Peptide-Imprinted Composite Nanoparticles
3.3. Cytotoxicity Test of HepG2 Cells with Magnetic Peptide-Imprinted Composite Nanoparticles and NK-92 Cells
3.3.1. MTT Assay for Cell Viability
3.3.2. Photodynamic Therapy (PDT)
3.3.3. Immunotherapy with NK-92 Cells
3.4. Gene Expression of HepG2 Cells Treated with MPIPs, NK92 and PDT
3.5. Animal Model and Immunohistochemical Staining
3.6. Data Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lee, M.-H.; Thomas, J.L.; Wang, H.-Y.; Chang, C.-C.; Lin, C.-C.; Lin, H.-Y. Extraction of resveratrol from polygonum cuspidatum with magnetic orcinol-imprinted poly(ethylene-co-vinyl alcohol) composite particles and their in vitro suppression of human osteogenic sarcoma (HOS) cell line. J. Mater. Chem. 2012, 22, 24644–24651. [Google Scholar] [CrossRef]
- Lee, M.-H.; Thomas, J.L.; Chen, Y.-C.; Wang, H.-Y.; Lin, H.-Y. Hydrolysis of Magnetic Amylase-Imprinted Poly(ethylene-co-vinyl alcohol) Composite Nanoparticles. ACS Appl. Mater. Interfaces 2012, 4, 916–921. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.-Y.; Ho, M.-S.; Lee, M.-H. Instant formation of molecularly imprinted poly(ethylene-co-vinyl alcohol)/quantum dot composite nanoparticles and their use in one-pot urinalysis. Biosens. Bioelectron. 2009, 25, 579–586. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-Y.; Tsai, T.-C.; Thomas, J.L.; Lee, M.-H.; Liu, B.-D.; Lin, H.-Y. Urinalysis with molecularly imprinted poly(ethylene-co-vinyl alcohol) potentiostat sensors. Biosens. Bioelectron. 2009, 24, 2611–2617. [Google Scholar] [CrossRef]
- Lee, M.H.; Thomas, J.L.; Su, Z.L.; Yeh, W.K.; Monzel, A.S.; Bolognin, S.; Schwamborn, J.C.; Yang, C.H.; Lin, H.Y. Epitope imprinting of alpha-synuclein for sensing in Parkinson’s brain organoid culture medium. Biosens Bioelectron. 2020. [Google Scholar] [CrossRef]
- Lee, M.-H.; Lin, C.-C.; Thomas, J.L.; Li, J.-A.; Lin, H.-Y. Cellular reprogramming with multigene activation by the delivery of CRISPR/dCas9 ribonucleoproteins via magnetic peptide-imprinted chitosan nanoparticles. Mater. Today Bio 2021, 9, 100091. [Google Scholar] [CrossRef]
- Lee, M.-H.; Lin, C.-C.; Thomas, J.L.; Chan, C.-K.; Lin, H.-Y. Epitope recognition of magnetic peptide-imprinted chitosan composite nanoparticles for the extraction of CRISPR/dCas9a proteins from transfected cells. Nanotechnology 2021, 32, 18LT02. [Google Scholar] [CrossRef]
- Lee, M.-H.; Thomas, J.L.; Chang, Y.-C.; Tsai, Y.-S.; Liu, B.-D.; Lin, H.-Y. Electrochemical sensing of nuclear matrix protein 22 in urine with molecularly imprinted poly(ethylene-co-vinyl alcohol) coated zinc oxide nanorod arrays for clinical studies of bladder cancer diagnosis. Biosens. Bioelectron. 2016, 79, 789–795. [Google Scholar] [CrossRef]
- Chen, W.-J.; Lee, M.-H.; Thomas, J.L.; Lu, P.-H.; Li, M.-H.; Lin, H.-Y. Microcontact Imprinting of Algae on Poly (ethylene-co-vinyl alcohol) for Biofuel Cells. ACS Appl. Mater. Interfaces 2013, 5, 11123–11128. [Google Scholar] [CrossRef]
- Lee, M.-H.; Thomas, J.L.; Chen, W.-J.; Li, M.-H.; Shih, C.-P.; Lin, H.-Y. Fabrication of bacteria-imprinted polymer coated electrodes for microbial fuel cells. ACS Sustain. Chem. Eng. 2015, 3, 1190–1196. [Google Scholar] [CrossRef]
- Lin, H.-Y.; Hsu, C.-Y.; Thomas, J.L.; Wang, S.-E.; Chen, H.-C.; Chou, T.-C. The microcontact imprinting of proteins: The effect of cross-linking monomers for lysozyme, ribonuclease A and myoglobin. Biosens. Bioelectron. 2006, 22, 534–543. [Google Scholar] [CrossRef]
- Lee, M.-H.; Liu, K.-T.; Thomas, J.L.; Su, Z.-L.; O’Hare, D.; van Wuellen, T.; Chamarro, J.M.; Bolognin, S.; Luo, S.-C.; Schwamborn, J.C.; et al. Peptide-Imprinted Poly(hydroxymethyl 3,4-ethylenedioxythiophene) Nanotubes for Detection of α Synuclein in Human Brain Organoids. ACS Appl. Nano Mater. 2020, 3, 8027–8036. [Google Scholar] [CrossRef]
- Lee, M.-H.; Thomas, J.L.; Liao, C.-L.; Jurcevic, S.; Crnogorac-Jurcevic, T.; Lin, H.-Y. Epitope recognition of peptide-imprinted polymers for Regenerating protein 1 (REG1). Sep. Purif. Technol. 2018, 192, 213–219. [Google Scholar] [CrossRef]
- Refaat, D.; Aggour, M.G.; Farghali, A.A.; Mahajan, R.; Wiklander, J.G.; Nicholls, I.A.; Piletsky, S.A. Strategies for molecular imprinting and the evolution of MIP nanoparticles as plastic antibodies—Synthesis and applications. Int. J. Mol. Sci. 2019, 20, 6304. [Google Scholar] [CrossRef]
- Nishino, H.; Huang, C.S.; Shea, K.J. Selective protein capture by epitope imprinting. Angew. Chem. Int. Ed. 2006, 45, 2392–2396. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; Hoshino, Y.; Rodriguez, A.; Yoo, H.; Shea, K.J. Synthetic polymer nanoparticles with antibody-like affinity for a hydrophilic peptide. ACS Nano 2009, 4, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.-L.; Li, D.-Y.; He, X.-W.; Li, W.-Y.; Zhang, Y.-K. An epitope imprinting method on the surface of magnetic nanoparticles for specific recognition of bovine serum album. J. Mater. Chem. B 2014, 2, 7575–7582. [Google Scholar] [CrossRef]
- Qin, Y.-P.; Jia, C.; He, X.-W.; Li, W.-Y.; Zhang, Y.-K. Thermosensitive Metal Chelation Dual-Template Epitope Imprinting Polymer Using Distillation–Precipitation Polymerization for Simultaneous Recognition of Human Serum Albumin and Transferrin. ACS Appl. Mater. Interfaces 2018, 10, 9060–9068. [Google Scholar] [CrossRef]
- Bossi, A.M.; Sharma, P.S.; Montana, L.; Zoccatelli, G.; Laub, O.; Levi, R. Fingerprint-Imprinted Polymer: Rational Selection of Peptide Epitope Templates for the Determination of Proteins by Molecularly Imprinted Polymers. Anal. Chem. 2012, 84, 4036–4041. [Google Scholar] [CrossRef] [PubMed]
- Iskierko, Z.; Sharma, P.S.; Noworyta, K.R.; Borowicz, P.; Cieplak, M.; Kutner, W.; Bossi, A.M. Selective PQQPFPQQ Gluten Epitope Chemical Sensor with a Molecularly Imprinted Polymer Recognition Unit and an Extended-Gate Field-Effect Transistor Transduction Unit. Anal. Chem. 2019, 91, 4537–4543. [Google Scholar] [CrossRef] [PubMed]
- Lach, P.; Cieplak, M.; Majewska, M.; Noworyta, K.R.; Sharma, P.S.; Kutner, W. “Gate Effect” in p-Synephrine Electrochemical Sensing with a Molecularly Imprinted Polymer and Redox Probes. Anal. Chem. 2019, 91, 7546–7553. [Google Scholar] [CrossRef]
- Lee, M.-H.; Thomas, J.L.; Liao, C.-L.; Jurcevic, S.; Crnogorac-Jurcevic, T.; Lin, H.-Y. Polymers imprinted with three REG1B peptides for electrochemical determination of Regenerating Protein 1B, a urinary biomarker for pancreatic ductal adenocarcinoma. Microchim. Acta 2017, 184, 1773–1780. [Google Scholar] [CrossRef]
- Pan, J.; Chen, W.; Ma, Y.; Pan, G. Molecularly imprinted polymers as receptor mimics for selective cell recognition. Chem. Soc. Rev. 2018, 47, 5574–5587. [Google Scholar] [CrossRef]
- Piletsky, S.; Canfarotta, F.; Poma, A.; Bossi, A.M.; Piletsky, S. Molecularly Imprinted Polymers for Cell Recognition. Trends Biotechnol. 2020, 38, 368–387. [Google Scholar] [CrossRef]
- Guan, J.; Lim, K.S.; Mekhail, T.; Chang, C.-C. Programmed death ligand-1 (PD-L1) expression in the programmed death receptor-1 (PD-1)/PD-L1 blockade: A key player against various cancers. Arch. Pathol. Lab. Med. 2017, 141, 851–861. [Google Scholar] [CrossRef] [PubMed]
- Lau, J.; Cheung, J.; Navarro, A.; Lianoglou, S.; Haley, B.; Totpal, K.; Sanders, L.; Koeppen, H.; Caplazi, P.; McBride, J.; et al. Tumour and host cell PD-L1 is required to mediate suppression of anti-tumour immunity in mice. Nat. Commun. 2017, 8, 14572. [Google Scholar] [CrossRef] [PubMed]
- Zou, W.; Wolchok, J.D.; Chen, L. PD-L1 (B7-H1) and PD-1 pathway blockade for cancer therapy: Mechanisms, response biomarkers, and combinations. Sci. Transl. Med. 2016, 8, rv324–rv328. [Google Scholar] [CrossRef]
- Milling, L.; Zhang, Y.; Irvine, D.J. Delivering safer immunotherapies for cancer. Adv. Drug Deliv. Rev. 2017, 114, 79–101. [Google Scholar] [CrossRef] [PubMed]
- Jo, S.D.; Nam, G.-H.; Kwak, G.; Yang, Y.; Kwon, I.C. Harnessing designed nanoparticles: Current strategies and future perspectives in cancer immunotherapy. Nano Today 2017, 17, 23–37. [Google Scholar] [CrossRef]
- Qian, H.; Liu, B.; Jiang, X. Application of nanomaterials in cancer immunotherapy. Mater. Today Chem. 2018, 7, 53–64. [Google Scholar] [CrossRef]
- Duan, X.; Chan, C.; Lin, W. Nanoparticle-Mediated Immunogenic Cell Death Enables and Potentiates Cancer Immunotherapy. Angew. Chem. Int. Ed. 2019, 58, 670–680. [Google Scholar] [CrossRef]
- Liu, Q.; Tian, J.; Tian, Y.; Sun, Q.; Sun, D.; Wang, F.; Xu, H.; Ying, G.; Wang, J.; Yetisen, A.K.; et al. Near-Infrared-II Nanoparticles for Cancer Imaging of Immune Checkpoint Programmed Death-Ligand 1 and Photodynamic/Immune Therapy. ACS Nano 2021, 15, 515–525. [Google Scholar] [CrossRef]
- Robertson, C.A.; Evans, D.H.; Abrahamse, H. Photodynamic therapy (PDT): A short review on cellular mechanisms and cancer research applications for PDT. J. Photochem. Photobiol. B Biol. 2009, 96, 1–8. [Google Scholar] [CrossRef]
- Hoebeke, M.; Piette, J.; van de Vorst, A. Photosensitized production of singlet oxygen by merocyanine 540 bound to liposomes. J. Photochem. Photobiol. B Biol. 1991, 9, 281–294. [Google Scholar] [CrossRef]
- Firczuk, M.; Nowis, D.; Gołąb, J. PDT-induced inflammatory and host responses. Photochem. Photobiol. Sci. 2011, 10, 653–663. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liu, Z.; Wang, S.; Dong, C.; Gong, X.; Zhao, P.; Chang, J. MC540 and upconverting nanocrystal coloaded polymeric liposome for near-infrared light-triggered photodynamic therapy and cell fluorescent imaging. ACS Appl. Mater. Interfaces 2014, 6, 3219–3225. [Google Scholar] [CrossRef]
- Idris, N.M.; Gnanasammandhan, M.K.; Zhang, J.; Ho, P.C.; Mahendran, R.; Zhang, Y. In vivo photodynamic therapy using upconversion nanoparticles as remote-controlled nanotransducers. Nat. Med. 2012, 18, 1580. [Google Scholar] [CrossRef]
- Xu, J.; Yang, P.; Sun, M.; Bi, H.; Liu, B.; Yang, D.; Gai, S.; He, F.; Lin, J. Highly emissive dye-sensitized upconversion nanostructure for dual-photosensitizer photodynamic therapy and bioimaging. ACS Nano 2017, 11, 4133–4144. [Google Scholar] [CrossRef] [PubMed]
- Trinchet, J.-C.; Beaugrand, M. Treatment of hepatocellular carcinoma in patients with cirrhosis. J. Hepatol. 1997, 27, 756–765. [Google Scholar] [CrossRef]
- Singh, A.K.; Kumar, R.; Pandey, A.K. Hepatocellular carcinoma: Causes, mechanism of progression and biomarkers. Curr. Chem. Genom. Transl. Med. 2018, 12, 9. [Google Scholar] [CrossRef] [PubMed]
- Bruix, J.; Reig, M.; Sherman, M. Evidence-based diagnosis, staging, and treatment of patients with hepatocellular carcinoma. Gastroenterology 2016, 150, 835–853. [Google Scholar] [CrossRef] [PubMed]
- Heimbach, J.K.; Kulik, L.M.; Finn, R.S.; Sirlin, C.B.; Abecassis, M.M.; Roberts, L.R.; Zhu, A.X.; Murad, M.H.; Marrero, J.A. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology 2018, 67, 358–380. [Google Scholar] [CrossRef]
- Lee, M.-H.; Liu, K.-H.; Thomas, J.L.; Chen, J.-R.; Lin, H.-Y. Immunotherapy of Hepatocellular Carcinoma with Magnetic PD-1 Peptide-Imprinted Polymer Nanocomposite and Natural Killer Cells. Biomolecules 2019, 9, 651. [Google Scholar] [CrossRef] [PubMed]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, M.-H.; Thomas, J.L.; Li, J.-A.; Chen, J.-R.; Wang, T.-L.; Lin, H.-Y. Synthesis of Multifunctional Nanoparticles for the Combination of Photodynamic Therapy and Immunotherapy. Pharmaceuticals 2021, 14, 508. https://doi.org/10.3390/ph14060508
Lee M-H, Thomas JL, Li J-A, Chen J-R, Wang T-L, Lin H-Y. Synthesis of Multifunctional Nanoparticles for the Combination of Photodynamic Therapy and Immunotherapy. Pharmaceuticals. 2021; 14(6):508. https://doi.org/10.3390/ph14060508
Chicago/Turabian StyleLee, Mei-Hwa, James L. Thomas, Jin-An Li, Jyun-Ren Chen, Tzong-Liu Wang, and Hung-Yin Lin. 2021. "Synthesis of Multifunctional Nanoparticles for the Combination of Photodynamic Therapy and Immunotherapy" Pharmaceuticals 14, no. 6: 508. https://doi.org/10.3390/ph14060508
APA StyleLee, M.-H., Thomas, J. L., Li, J.-A., Chen, J.-R., Wang, T.-L., & Lin, H.-Y. (2021). Synthesis of Multifunctional Nanoparticles for the Combination of Photodynamic Therapy and Immunotherapy. Pharmaceuticals, 14(6), 508. https://doi.org/10.3390/ph14060508





