Zebrafish, an In Vivo Platform to Screen Drugs and Proteins for Biomedical Use
Abstract
1. Introduction
Advantages of Zebrafish for Drug Screening
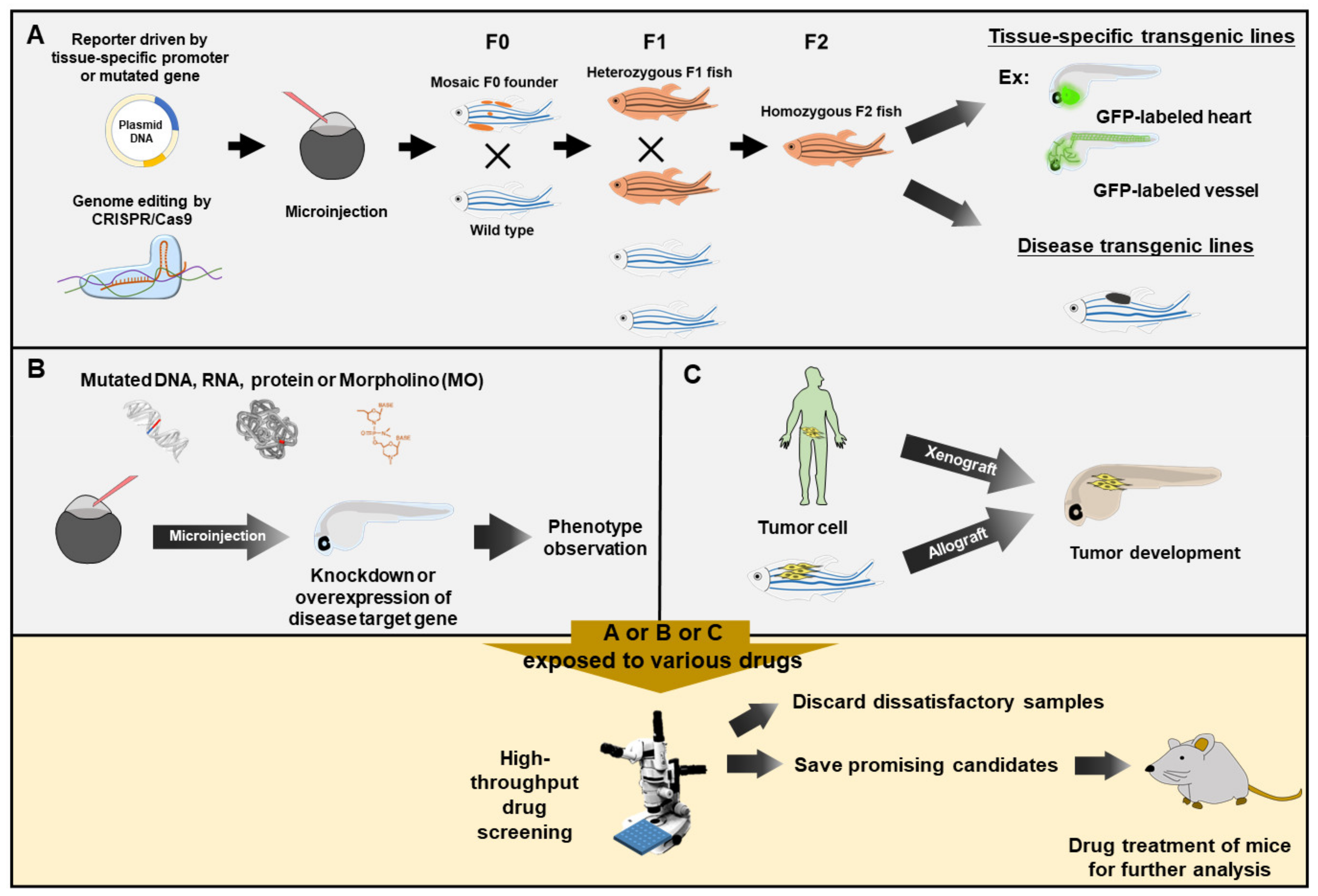
2. Embodiments
2.1. Using the MO-Knockdown Approach to Silence Genes in the Search for Curative Chemical(s)
2.2. Using CRISPR/Cas9 Editing to Mutate Gene(s) in the Search for Compounds for Symptomatic and Pain Relief
2.3. Using Wild-Type Strain to Search for Compounds to Alleviate Clinical Symptoms and Pain
3. Therapeutic Strategy Can Be Found from Phenotypes or Expression Patterns Induced by Chemical Exposure
4. Screening for Potential Cancer Drugs
5. Social Behavior and Screening for Potential Drugs
6. Rare Disease and Screening for Potential Drugs or Proteins
6.1. Introduction
6.2. Mucopolysaccharidosis (MPS)
6.3. Amyotrophic Lateral Sclerosis (ALS)
7. New Compounds Identified from the Zebrafish Screening Platform Have Advanced to Early Clinical Trials
7.1. Homeostasis
7.2. Diamond-Blackfan Anemia
- (i)
- Payne et al. [109] found that L-leucine treatment of zebrafish embryos could improve anemia and developmental defects associated with DBA. A pilot phase I/II study of L-leucine in the treatment of patients with transfusion-dependent DBA is in progress (ClinicalTrials.gov with the number of NCT01362595).
- (ii)
- Ear et al. [110] reported that treatment with RAP-011(Sotatercept) could dramatically restore the hemoglobin level reduced by ribosomal stress in zebrafish. Sotatercept testing in adults with transfusion-dependent DBA is also in progress (ClinicalTrials.gov with the number of NCT01464164).
- (iii)
- Macari et al. [111] employed a mutant zebrafish, Rps29−/−, which carries a mutated ribosomal gene found in DBA patients, to screen novel compounds. They found several calmodulin (CaM) inhibitors that could successfully rescue the hemoglobin level in the mutant embryos. Subsequently, they studied the effect of the CaM inhibitor trifluoperazine (TFP) in human and murine models. The results supported that TFP treatment may be a very effective therapy for DBA patients. Now, this treatment has been permitted to enter a phase I/II clinical trial (ClinicalTrials.gov with the number of NCT03966053).
7.3. Dravet Syndrome
7.4. Congestive Heart Failure
8. Pollutant and Ecotoxic Study and Screening for Potential Drugs
9. Finding the Effect of Prescribed Medicines on Developing Embryos
10. Zebrafish Serves an In Vivo Platform to Study the Properties of Nanoparticles
11. Zebrafish Serves as an Excellent Alternative to Screen the QT Prolongation of Drugs
12. The Limitations and Challenges of Zebrafish Model for Drug Screening
13. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Postlethwait, J.H.; Yan, Y.L.; Gates, M.A.; Horne, S.; Amores, A.; Brownlie, A.; Donovan, A.; Egan, E.S.; Force, A.; Gong, Z.; et al. Vertebrate genome evolution and the zebrafish gene map. Nat. Genet. 1998, 18, 345–349. [Google Scholar] [CrossRef] [PubMed]
- Knapik, E.W.; Goodman, A.; Ekker, M.; Chevrette, M.; Delgado, J.; Neuhauss, S.; Shimoda, N.; Driever, W.; Fishman, M.C.; Jacob, H.J. A microsatellite genetic linkage map for zebrafish. Nat. Genet. 1998, 18, 338–343. [Google Scholar] [CrossRef] [PubMed]
- Gates, M.A.; Kim, L.; Egan, E.S.; Cardozo, T.; Sirotkin, H.I.; Dougan, S.T.; Laskari, D.; Abagyan, R.; Schier, A.F.; Talbot, W.S. A genetic linkage map for zebrafish: Comparative analysis of genes and expressed sequences. Genome Res. 1999, 9, 334–347. [Google Scholar] [PubMed]
- Shimoda, N.; Knapik, E.W.; Ziniti, J.; Sim, C.; Yamada, E.; Kaplan, S.; Jackson, D.; de Sauvage, F.; Jacob, H.; Fishman, M.C. Zebrafish genetic map with 2000 microsatellite markers. Genomics 1999, 58, 219–232. [Google Scholar] [CrossRef] [PubMed]
- Howe, K.; Clark, M.D.; Torroja, C.F.; Torrance, J.; Berthelot, C.; Muffato, M.; Collins, J.E.; Humphray, S.; McLaren, K.; Matthews, L.; et al. The zebrafish reference genome sequence and its relationship to the human genome. Nature 2013, 496, 498–503. [Google Scholar] [CrossRef]
- Haesemeyer, M.; Robson, D.N.; Li, J.M.; Schier, A.F.; Engert, F. The structure and timescales of heat perception in larval zebrafish. Cell Syst. 2015, 1, 338–348. [Google Scholar] [CrossRef]
- Bedell, V.M.; Westcot, S.E.; Ekker, S.C. Lessons from morpholino-based screening in zebrafish. Brief. Funct. Genom. 2011, 10, 181–188. [Google Scholar] [CrossRef]
- Chou, C.Y.; Horng, L.S.; Tsai, H.J. Uniform GFP-expression in transgenic medaka (Oryzias latipes) at the F0 generation. Transgenic Res. 2001, 10, 303–315. [Google Scholar] [CrossRef]
- Kawakami, K.; Shima, A. Identification of the Tol2 transposase of the medaka fish Oryzias latipes that catalyzes excision of a nonautonomous Tol2 element in zebrafish Danio rerio. Gene 1999, 240, 239–244. [Google Scholar] [CrossRef]
- Kawakami, K.; Takeda, H.; Kawakami, N.; Kobayashi, M.; Matsuda, N.; Mishina, M. A transposon-mediated gene trap approach identifies developmentally regulated genes in zebrafish. Dev. Cell 2004, 7, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Halpern, M.E.; Rhee, J.; Goll, M.G.; Akitake, C.M.; Parsons, M.; Leach, S.D. Gal4/UAS transgenic tools and their application to zebrafish. Zebrafish 2008, 5, 97–110. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.J.; Jou, T.S.; Ho, Y.L.; Lee, W.H.; Jeng, Y.T.; Hsieh, F.J.; Tsai, H.J. Conditional expression of a myocardium-specific transgene in zebrafish transgenic lines. Dev. Dyn. 2005, 233, 1294–1303. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Wan, H.; Chia, W.; Tong, Y.; Gong, Z. Demonstration of site-directed recombination in transgenic zebrafish using the Cre/loxP system. Transgenic Res. 2005, 14, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Xiao, A.; Zhou, M.; Zhu, Z.; Lin, S.; Zhang, B. Heritable gene targeting in zebrafish using customized TALENs. Nat. Biotechnol. 2011, 29, 699–700. [Google Scholar] [CrossRef] [PubMed]
- Hwang, W.Y.; Fu, Y.; Reyon, D.; Maeder, M.L.; Tsai, S.Q.; Sander, J.D.; Peterson, R.T.; Yeh, J.R.; Joung, J.K. Efficient genome editing in zebrafish using a CRISPR-Cas system. Nat. Biotechnol. 2013, 31, 227–229. [Google Scholar] [CrossRef]
- Auer, T.O.; Duroure, K.; De Cian, A.; Concordet, J.P.; Del Bene, F. Highly efficient CRISPR/Cas9-mediated knock-in in zebrafish by homology-independent DNA repair. Genome Res. 2014, 24, 142–153. [Google Scholar] [CrossRef]
- Strynatka, K.A.; Gurrola-Gal, M.C.; Berman, J.N.; McMaster, C.R. How Surrogate and Chemical Genetics in Model Organisms Can Suggest Therapies for Human Genetic Diseases. Genetics 2018, 208, 833–851. [Google Scholar] [CrossRef]
- Phillips, J.B.; Westerfield, M. Zebrafish models in translational research: Tipping the scales toward advancements in human health. Dis. Models Mech. 2014, 7, 739–743. [Google Scholar] [CrossRef]
- Kok, F.O.; Shin, M.; Ni, C.W.; Gupta, A.; Grosse, A.S.; van Impel, A.; Kirchmaier, B.C.; Peterson-Maduro, J.; Kourkoulis, G.; Male, I.; et al. Reverse genetic screening reveals poor correlation between morpholino-induced and mutant phenotypes in zebrafish. Dev. Cell 2015, 32, 97–108. [Google Scholar] [CrossRef]
- Fernández-Murray, J.P.; Prykhozhij, S.V.; Dufay, J.N.; Steele, S.L.; Gaston, D.; Nasrallah, G.K.; Coombs, A.J.; Liwski, R.S.; Fernandez, C.V.; Berman, J.N.; et al. Glycine and Folate Ameliorate Models of Congenital Sideroblastic Anemia. PLoS Genet. 2016, 12, e1005783. [Google Scholar] [CrossRef]
- Van Karnebeek, C.D.; Bonafé, L.; Wen, X.Y.; Tarailo-Graovac, M.; Balzano, S.; Royer-Bertrand, B.; Ashikov, A.; Garavelli, L.; Mammi, I.; Turolla, L.; et al. NANS-mediated synthesis of sialic acid is required for brain and skeletal development. Nat. Genet. 2016, 48, 777–784. [Google Scholar] [CrossRef]
- Adamson, K.I.; Sheridan, E.; Grierson, A.J. Use of zebrafish models to investigate rare human disease. J. Med. Genet. 2018, 55, 641–649. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Petree, C.; Requena, T.; Varshney, P.; Varshney, G.K. Expanding the CRISPR Toolbox in Zebrafish for Studying Development and Disease. Front. Cell Dev. Biol. 2019, 7, 13. [Google Scholar] [CrossRef] [PubMed]
- Tuschl, K.; Meyer, E.; Valdivia, L.E.; Zhao, N.; Dadswell, C.; Abdul-Sada, A.; Hung, C.Y.; Simpson, M.A.; Chong, W.K.; Jacques, T.S.; et al. Mutations in SLC39A14 disrupt manganese homeostasis and cause childhood-onset parkinsonism-dystonia. Nat. Commun. 2016, 7, 11601. [Google Scholar] [CrossRef] [PubMed]
- Griffin, A.; Hamling, K.R.; Knupp, K.; Hong, S.; Lee, L.P.; Baraban, S.C. Clemizole and modulators of serotonin signalling suppress seizures in Dravet syndrome. Brain 2017, 140, 669–683. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Kim, Y.S.; Kim, J.; Pattison, J.; Kamaid, A.; Miller, Y.I. Modeling hypercholesterolemia and vascular lipid accumulation in LDL receptor mutant zebrafish. J. Lipid Res. 2018, 59, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Wu, Z.; Sui, X. Biotransformation of ginsenoside Rb1 with wild Cordyceps sinensis and Ascomycota sp. and its antihyperlipidemic effects on the diet-induced cholesterol of zebrafish. J. Food Biochem. 2020, 44, e13192. [Google Scholar] [CrossRef] [PubMed]
- Zielonka, M.; Breuer, M.; Okun, J.G.; Carl, M.; Hoffmann, G.F.; Kölker, S. Pharmacologic rescue of hyperammonemia-induced toxicity in zebrafish by inhibition of ornithine aminotransferase. PLoS ONE 2018, 13, e0203707. [Google Scholar] [CrossRef] [PubMed]
- Mandelbaum, J.; Shestopalov, I.A.; Henderson, R.E.; Chau, N.G.; Knoechel, B.; Wick, M.J.; Zon, L.I. Zebrafish blastomere screen identifies retinoic acid suppression of MYB in adenoid cystic carcinoma. J. Exp. Med. 2018, 215, 2673–2685. [Google Scholar] [CrossRef]
- Huth, M.E.; Ricci, A.J.; Cheng, A.G. Mechanisms of aminoglycoside ototoxicity and targets of hair cell protection. Int. J. Otolaryngol. 2011, 2011, 937861. [Google Scholar] [CrossRef]
- Chowdhury, S.; Owens, K.N.; Herr, R.J.; Jiang, Q.; Chen, X.; Johnson, G.; Groppi, V.E.; Raible, D.W.; Rubel, E.W.; Simon, J.A. Phenotypic Optimization of Urea-Thiophene Carboxamides To Yield Potent, Well Tolerated, and Orally Active Protective Agents against Aminoglycoside-Induced Hearing Loss. J. Med. Chem. 2018, 61, 84–97. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.Y.; Chiang, C.Y.; Tsai, H.J. Zebrafish and Medaka: New model organisms for modern biomedical research. J. Biomed. Sci. 2016, 23, 19. [Google Scholar] [CrossRef] [PubMed]
- Hason, M.; Bartůněk, P. Zebrafish models of cancer-new insights on modeling human cancer in a non-mammalian vertebrate. Genes 2019, 10, 935. [Google Scholar] [CrossRef] [PubMed]
- Lam, S.H.; Gong, Z. Modeling liver cancer using zebrafish: A comparative oncogenomics approach. Cell Cycle 2006, 5, 573–577. [Google Scholar] [CrossRef] [PubMed]
- Amatruda, J.F.; Shepard, J.L.; Stern, H.M.; Zon, L.I. Zebrafish as a cancer model system. Cancer Cell 2002, 1, 229–231. [Google Scholar] [CrossRef]
- Tran, T.C.; Sneed, B.; Haider, J.; Blavo, D.; White, A.; Aiyejorun, T.; Baranowski, T.C.; Rubinstein, A.L.; Doan, T.N.; Dingledine, R.; et al. Automated, quantitative screening assay for antiangiogenic compounds using transgenic zebrafish. Cancer Res. 2007, 67, 11386–11392. [Google Scholar] [CrossRef]
- García-Caballero, M.; Quesada, A.R.; Medina, M.A.; Marí-Beffa, M. Fishing anti(lymph)angiogenic drugs with zebrafish. Drug Discov. Today 2018, 23, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Camus, S.; Quevedo, C.; Menéndez, S.; Paramonov, I.; Stouten, P.F.; Janssen, R.A.; Rueb, S.; He, S.; Snaar-Jagalska, B.E.; Laricchia-Robbio, L.; et al. Identification of phosphorylase kinase as a novel therapeutic target through high-throughput screening for anti-angiogenesis compounds in zebrafish. Oncogene 2012, 31, 4333–4342. [Google Scholar] [CrossRef]
- Nathan, J.; Kannan, R.R. Antiangiogenic molecules from marine actinomycetes and the importance of using zebrafish model in cancer research. Heliyon 2020, 6, e05662. [Google Scholar] [CrossRef]
- Yang, B.; Wang, N.; Wang, S.; Li, X.; Zheng, Y.; Li, M.; Song, J.; Zhang, F.; Mei, W.; Lin, Y.; et al. Network-pharmacology-based identification of caveolin-1 as a key target of Oldenlandia diffusa to suppress breast cancer metastasis. Biomed. Pharmacother. 2019, 112, 108607. [Google Scholar] [CrossRef]
- Beckwith, L.G.; Moore, J.L.; Tsao-Wu, G.S.; Harshbarger, J.C.; Cheng, K.C. Ethylnitrosourea induces neoplasia in zebrafish (Danio rerio). Lab. Investig. 2000, 80, 379–385. [Google Scholar] [CrossRef]
- Berghmans, S.; Murphey, R.D.; Wienholds, E.; Neuberg, D.; Kutok, J.L.; Fletcher, C.D.; Morris, J.P.; Liu, T.X.; Schulte-Merker, S.; Kanki, J.P.; et al. p53 mutant zebrafish develop malignant peripheral nerve sheath tumors. Proc. Natl. Acad. Sci. USA 2005, 102, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.H.; Chen, Y.H.; Wu, T.N.; Lin, Y.J.; Tsai, H.J. A keratin 18 transgenic zebrafish Tg(k18(2.9):RFP) treated with inorganic arsenite reveals visible overproliferation of epithelial cells. Toxicol. Lett. 2006, 163, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Patton, E.E.; Widlund, H.R.; Kutok, J.L.; Kopani, K.R.; Amatruda, J.F.; Murphey, R.D.; Berghmans, S.; Mayhall, E.A.; Traver, D.; Fletcher, C.D.; et al. BRAF mutations are sufficient to promote nevi formation and cooperate with p53 in the genesis of melanoma. Curr. Biol. 2005, 15, 249–254. [Google Scholar] [CrossRef]
- Nguyen, A.T.; Emelyanov, A.; Koh, C.H.; Spitsbergen, J.M.; Parinov, S.; Gong, Z. An inducible kras(V12) transgenic zebrafish model for liver tumorigenesis and chemical drug screening. Dis. Models Mech. 2012, 5, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zheng, W.; Wang, Z.; Zeng, Z.; Zhan, H.; Li, C.; Zhou, L.; Yan, C.; Spitsbergen, J.M.; Gong, Z. A transgenic zebrafish liver tumor model with inducible Myc expression reveals conserved Myc signatures with mammalian liver tumors. Dis. Models Mech. 2013, 6, 414–423. [Google Scholar] [CrossRef]
- Benjamin, D.C.; Hynes, R.O. Intravital imaging of metastasis in adult Zebrafish. BMC Cancer 2017, 17, 660. [Google Scholar] [CrossRef]
- Goyama, S.; Wunderlich, M.; Mulloy, J.C. Xenograft models for normal and malignant stem cells. Blood 2015, 125, 2630–2640. [Google Scholar] [CrossRef]
- Zhao, C.; Wang, X.; Zhao, Y.; Li, Z.; Lin, S.; Wei, Y.; Yang, H. A novel xenograft model in zebrafish for high-resolution investigating dynamics of neovascularization in tumors. PLoS ONE 2011, 6, e21768. [Google Scholar] [CrossRef]
- Jung, D.W.; Oh, E.S.; Park, S.H.; Chang, Y.T.; Kim, C.H.; Choi, S.Y.; Williams, D.R. A novel zebrafish human tumor xenograft model validated for anti-cancer drug screening. Mol. Biosyst. 2012, 8, 1930–1939. [Google Scholar] [CrossRef]
- Brown, H.K.; Schiavone, K.; Tazzyman, S.; Heymann, D.; Chico, T.J. Zebrafish xenograft models of cancer and metastasis for drug discovery. Expert. Opin. Drug Discov. 2017, 12, 379–389. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.H.; Qu, W.; Xu, J.; Feng, F.; He, M.F. 1-Methoxycarbony-β-carboline from Picrasma quassioides exerts anti-angiogenic properties in HUVECs in vitro and zebrafish embryos in vivo. Chin. J. Nat. Med. 2018, 16, 599–609. [Google Scholar] [CrossRef]
- Fior, R.; Povoa, V.; Mendes, R.V.; Carvalho, T.; Gomes, A.; Figueiredo, N.; Ferreira, M.G. Single-cell functional and chemosensitive profiling of combinatorial colorectal therapy in zebrafish xenografts. Proc. Natl. Acad. Sci. USA 2017, 114, E8234–E8243. [Google Scholar] [CrossRef] [PubMed]
- Letrado, P.; de Miguel, I.; Lamberto, I.; Díez-Martínez, R.; Oyarzabal, J. Zebrafish: Speeding up the Cancer Drug Discovery Process. Cancer Res. 2018, 78, 6048–6058. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, J.; Makinoshima, H. Zebrafish-Based Screening Models for the Identification of Anti-Metastatic Drugs. Molecules 2020, 25, 2407. [Google Scholar] [CrossRef]
- Lin, H.S.; Huang, Y.L.; Wang, Y.S.; Hsiao, E.; Hsu, T.A.; Shiao, H.Y.; Jiaang, W.T.; Sampurna, B.P.; Lin, K.H.; Wu, M.S.; et al. Identification of Novel Anti-Liver Cancer Small Molecules with Better Therapeutic Index than Sorafenib via Zebrafish Drug Screening Platform. Cancers 2019, 11, 739. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.; Pu, J.; Sun, J.; Tan, B.; Wang, W.; Wang, L.; Cheng, J.; Zuo, Y. Zebrafish xenograft model of human lung cancer for studying the function of LINC00152 in cell proliferation and invasion. Cancer Cell Int. 2020, 20, 376. [Google Scholar] [CrossRef]
- Yan, C.; Brunson, D.C.; Tang, Q.; Do, D.; Iftimia, N.A.; Moore, J.C.; Hayes, M.N.; Welker, A.M.; Garcia, E.G.; Dubash, T.D.; et al. Visualizing Engrafted Human Cancer and Therapy Responses in Immunodeficient Zebrafish. Cell 2019, 177, 1903–1914. [Google Scholar] [CrossRef]
- Jeong, J.Y.; Kwon, H.B.; Ahn, J.C.; Kang, D.; Kwon, S.H.; Park, J.A.; Kim, K.W. Functional and developmental analysis of the blood-brain barrier in zebrafish. Brain Res. Bull. 2008, 75, 619–628. [Google Scholar] [CrossRef]
- Quiñonez-Silvero, C.; Hübner, K.; Herzog, W. Development of the brain vasculature and the blood-brain barrier in zebrafish. Dev. Biol. 2020, 457, 181–190. [Google Scholar] [CrossRef]
- Yang, T.; Martin, P.; Fogarty, B.; Brown, A.; Schurman, K.; Phipps, R.; Yin, V.P.; Lockman, P.; Bai, S. Exosome delivered anticancer drugs across the blood-brain barrier for brain cancer therapy in Danio rerio. Pharm. Res. 2015, 32, 2003–2014. [Google Scholar] [CrossRef]
- Zeng, A.; Ye, T.; Cao, D.; Huang, X.; Yang, Y.; Chen, X.; Xie, Y.; Yao, S.; Zhao, C. Identify a Blood-Brain Barrier Penetrating Drug-TNB using Zebrafish Orthotopic Glioblastoma Xenograft Model. Sci. Rep. 2017, 7, 14372. [Google Scholar] [CrossRef] [PubMed]
- Geng, Y.; Peterson, R.T. The zebrafish subcortical social brain as a model for studying social behavior disorders. Dis. Models Mech. 2019, 12, dmm039446. [Google Scholar] [CrossRef]
- Bölte, S.; Girdler, S.; Marschik, P.B. The contribution of environmental exposure to the etiology of autism spectrum disorder. Cell Mol. Life Sci. 2019, 76, 1275–1297. [Google Scholar] [CrossRef] [PubMed]
- Courchesne, E.; Pramparo, T.; Gazestani, V.H.; Lombardo, M.V.; Pierce, K.; Lewis, N.E. The ASD Living Biology: From cell proliferation to clinical phenotype. Mol. Psychiatry 2019, 24, 88–107. [Google Scholar] [CrossRef] [PubMed]
- Hulbert, S.W.; Jiang, Y.H. Monogenic mouse models of autism spectrum disorders: Common mechanisms and missing links. Neuroscience 2016, 321, 3–23. [Google Scholar] [CrossRef] [PubMed]
- Sgritta, M.; Dooling, S.W.; Buffington, S.A.; Momin, E.N.; Francis, M.B.; Britton, R.A.; Costa-Mattioli, M. Mechanisms Underlying Microbial-Mediated Changes in Social Behavior in Mouse Models of Autism Spectrum Disorder. Neuron 2019, 101, 246–259. [Google Scholar] [CrossRef]
- Dwivedi, S.; Medishetti, R.; Rani, R.; Sevilimedu, A.; Kulkarni, P.; Yogeeswari, P. Larval zebrafish model for studying the effects of valproic acid on neurodevelopment: An approach towards modeling autism. J. Pharmacol. Toxicol. Methods. 2019, 95, 56–65. [Google Scholar] [CrossRef]
- Strauss, K.A.; Puffenberger, E.G.; Huentelman, M.J.; Gottlieb, S.; Dobrin, S.E.; Parod, J.M.; Stephan, D.A.; Morton, D.H. Recessive symptomatic focal epilepsy and mutant contactin-associated protein-like 2. N. Engl. J. Med. 2006, 354, 1370–1377. [Google Scholar] [CrossRef]
- Hoffman, E.J.; Turner, K.J.; Fernandez, J.M.; Cifuentes, D.; Ghosh, M.; Ijaz, S.; Jain, R.A.; Kubo, F.; Bill, B.R.; Baier, H.; et al. Estrogens Suppress a Behavioral Phenotype in Zebrafish Mutants of the Autism Risk Gene, CNTNAP2. Neuron 2016, 89, 725–733. [Google Scholar] [CrossRef]
- Baron-Cohen, S.; Lombardo, M.V.; Auyeung, B.; Ashwin, E.; Chakrabarti, B.; Knickmeyer, R. Why are autism spectrum conditions more prevalent in males? PLoS Biol. 2011, 9, e1001081. [Google Scholar] [CrossRef] [PubMed]
- Schaafsma, S.M.; Pfaff, D.W. Etiologies underlying sex differences in Autism Spectrum Disorders. Front. Neuroendocrinol. 2014, 35, 255–271. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, F.F.; Gaspary, K.V.; Siebel, A.M.; Bonan, C.D. Oxytocin reversed MK-801-induced social interaction and aggression deficits in zebrafish. Behav. Brain Res. 2016, 311, 368–374. [Google Scholar] [CrossRef]
- Landin, J.; Hovey, D.; Xu, B.; Lagman, D.; Zettergren, A.; Larhammar, D.; Kettunen, P.; Westberg, L. Oxytocin Receptors Regulate Social Preference in Zebrafish. Sci. Rep. 2020, 10, 5435. [Google Scholar] [CrossRef] [PubMed]
- Dodge, J.A.; Chigladze, T.; Donadieu, J.; Grossman, Z.; Ramos, F.; Serlicorni, A.; Siderius, L.; Stefanidis, C.J.; Tasic, V.; Valiulis, A. The importance of rare diseases: From the gene to society. Arch. Dis. Child. 2011, 96, 791–792. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Boycott, K.M.; Dyment, D.A.; Sawyer, S.L.; Vanstone, M.R.; Beaulieu, C.L. Identification of genes for childhood heritable diseases. Annu. Rev. Med. 2014, 65, 19–31. [Google Scholar] [CrossRef]
- Lin, H.Y.; Lin, S.P.; Chuang, C.K.; Niu, D.M.; Chen, M.R.; Tsai, F.J.; Chao, M.C.; Chiu, P.C.; Lin, S.J.; Tsai, L.P.; et al. Incidence of the mucopolysaccharidoses in Taiwan, 1984–2004. Am. J. Med. Genet. A 2009, 149A, 960–964. [Google Scholar] [CrossRef]
- D’Avanzo, F.; Rigon, L.; Zanetti, A.; Tomanin, R. Mucopolysaccharidosis Type II: One Hundred Years of Research, Diagnosis, and Treatment. Int. J. Mol. Sci. 2020, 21, 1258. [Google Scholar] [CrossRef]
- Kim, C.H.; Hwang, H.Z.; Song, S.M.; Paik, K.H.; Kwon, E.K.; Bin Moon, K.; Yoon, J.H.; Han, C.K.; Jin, D.K. Mutational spectrum of the iduronate 2 sulfatase gene in 25 unrelated Korean Hunter syndrome patients: Identification of 13 novel mutations. Hum. Mutat. 2003, 21, 449–450. [Google Scholar] [CrossRef]
- Kato, T.; Kato, Z.; Kuratsubo, I.; Tanaka, N.; Ishigami, T.; Kajihara, J.I.; Sukegawa-Hayasaka, K.; Orii, K.; Isogai, K.; Fukao, T.; et al. Mutational and structural analysis of Japanese patients with mucopolysaccharidosis type II. J. Hum. Genet. 2005, 50, 395–402. [Google Scholar] [CrossRef]
- Neufield, E.F.; Muenzer, J. The mucopolysaccharidoses. In The Metabolic and Molecular Bases of Inherited Disease, 8th ed.; Scriver, C., Beaudet, A.L., Valle, D., Sly, W.S., Eds.; McGraw-Hill: New York, NY, USA, 2001; Volume 136, pp. 3421–3452. [Google Scholar]
- Moro, E.; Tomanin, R.; Friso, A.; Modena, N.; Tiso, N.; Scarpa, M.; Argenton, F. A novel functional role of iduronate-2-sulfatase in zebrafish early development. Matrix Biol. 2010, 29, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Costa, R.; Urbani, A.; Salvalaio, M.; Bellesso, S.; Cieri, D.; Zancan, I.; Filocamo, M.; Bonaldo, P.; Szabò, I.; Tomanin, R.; et al. Perturbations in cell signaling elicit early cardiac defects in mucopolysaccharidosis type II. Hum. Mol. Genet. 2017, 26, 1643–1655. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.Y.; Lee, C.L.; Chang, C.Y.; Chiu, P.C.; Chien, Y.H.; Niu, D.M.; Tsai, F.J.; Hwu, W.L.; Lin, S.J.; Lin, J.L.; et al. Survival and diagnostic age of 175 Taiwanese patients with mucopolysaccharidoses (1985–2019). Orphanet J. Rare Dis. 2020, 15, 314. [Google Scholar] [CrossRef] [PubMed]
- Chuang, C.K.; Lin, H.Y.; Wang, T.J.; Huang, Y.H.; Chan, M.J.; Liao, H.C.; Lo, Y.T.; Wang, L.Y.; Tu, R.Y.; Fang, Y.Y.; et al. Status of newborn screening and follow up investigations for Mucopolysaccharidoses I and II in Taiwan. Orphanet J. Rare Dis. 2018, 13, 84. [Google Scholar] [CrossRef] [PubMed]
- Chan, M.J.; Liao, H.C.; Gelb, M.H.; Chuang, C.K.; Liu, M.Y.; Chen, H.J.; Kao, S.M.; Lin, H.Y.; Huang, Y.H.; Kumar, A.B.; et al. Taiwan National Newborn Screening Program by Tandem Mass Spectrometry for Mucopolysaccharidoses Types I, II, and VI. J. Pediatr. 2019, 205, 176–182. [Google Scholar] [CrossRef]
- Arunkumar, N.; Langan, T.J.; Stapleton, M.; Kubaski, F.; Mason, R.W.; Rajendra Singh, R.; Kobayashi, H.; Yamaguchi, S.; Suzuki, Y.; Orii, K.; et al. Newborn screening of mucopolysaccharidoses: Past, present, and future. J. Hum. Genet. 2020, 65, 557–567. [Google Scholar] [CrossRef]
- Lin, C.Y.; Lin, H.Y.; Chuang, C.K.; Zhang, P.H.; Tu, R.Y.; Lin, S.P.; Tsai, H.J. Effect of Mutated ids Overexpression on IDS Enzyme Activity and Developmental Phenotypes in Zebrafish Embryos: A Valuable Index for Assessing Critical Point-Mutations Associated with Mucopolysaccharidosis Type II Occurrence in Humans. Diagnostics 2020, 10, 854. [Google Scholar] [CrossRef]
- Rowland, L.P.; Shneider, N.A. Amyotrophic lateral sclerosis. N. Engl. J. Med. 2001, 344, 1688–1700. [Google Scholar] [CrossRef]
- Johnston, C.A.; Stanton, B.R.; Turner, M.R.; Gray, R.; Blunt, A.H.; Butt, D.; Ampong, M.A.; Shaw, C.E.; Leigh, P.N.; Al-Chalabi, A. Amyotrophic lateral sclerosis in an urban setting: A population based study of inner city London. J. Neurol. 2006, 253, 1642–1643. [Google Scholar] [CrossRef]
- Logroscino, G.; Traynor, B.J.; Hardiman, O.; Chiò, A.; Mitchell, D.; Swingler, R.J.; Millul, A.; Benn, E.; Beghi, E.; EURALS. Incidence of amyotrophic lateral sclerosis in Europe. J. Neurol. Neurosurg. Psychiatry 2010, 81, 385–390. [Google Scholar] [CrossRef]
- Al-Chalabi, A.; Hardiman, O. The epidemiology of ALS: A conspiracy of genes, environment and time. Nat. Rev. Neurol. 2013, 9, 617–628. [Google Scholar] [CrossRef] [PubMed]
- Morrice, J.R.; Gregory-Evans, C.Y.; Shaw, C.A. Animal models of amyotrophic lateral sclerosis: A comparison of model validity. Neural Regen. Res. 2018, 13, 2050–2054. [Google Scholar] [PubMed]
- Fortier, G.; Butti, Z.; Patten, S.A. Modelling C9orf72-Related Amyotrophic Lateral Sclerosis in Zebrafish. Biomedicines 2020, 8, 440. [Google Scholar] [CrossRef] [PubMed]
- Lemmens, R.; van Hoecke, A.; Hersmus, N.; Geelen, V.; D’Hollander, I.; Thijs, V.; van den Bosch, L.; Carmeliet, P.; Robberecht, W. Overexpression of mutant superoxide dismutase 1 causes a motor axonopathy in the zebrafish. Hum. Mol. Genet. 2007, 16, 2359–2365. [Google Scholar] [CrossRef] [PubMed]
- Kabashi, E.; Lin, L.; Tradewell, M.L.; Dion, P.A.; Bercier, V.; Bourgouin, P.; Rochefort, D.; Bel Hadj, S.; Durham, H.D.; Vande Velde, C.; et al. Gain and loss of function of ALS-related mutations of TARDBP (TDP-43) cause motor deficits in vivo. Hum. Mol. Genet. 2010, 19, 671–683. [Google Scholar] [CrossRef] [PubMed]
- Kabashi, E.; Bercier, V.; Lissouba, A.; Liao, M.; Brustein, E.; Rouleau, G.A.; Drapeau, P. FUS and TARDBP but not SOD1 interact in genetic models of amyotrophic lateral sclerosis. PLoS Genet. 2011, 7, e1002214. [Google Scholar] [CrossRef] [PubMed]
- Butti, Z.; Giacomotto, J.; Patten, S. Reduced C9orf72 function leads to defective synaptic vesicle release and neuromuscular dysfunction in zebrafish. Res. Sq. 2020. [Google Scholar] [CrossRef]
- Vaccaro, A.; Patten, S.A.; Ciura, S.; Maios, C.; Therrien, M.; Drapeau, P.; Kabashi, E.; Parker, J.A. Methylene blue protects against TDP-43 and FUS neuronal toxicity in C. elegans and D. rerio. PLoS ONE 2012, 7, e42117. [Google Scholar] [CrossRef]
- Vaccaro, A.; Patten, S.A.; Aggad, D.; Julien, C.; Maios, C.; Kabashi, E.; Drapeau, P.; Parker, J.A. Pharmacological reduction of ER stress protects against TDP-43 neuronal toxicity in vivo. Neurobiol. Dis. 2013, 55, 64–75. [Google Scholar] [CrossRef]
- Maselli, R.A.; Wollman, R.L.; Leung, C.; Distad, B.; Palombi, S.; Richman, D.P.; Salazar-Grueso, E.F.; Roos, R.P. Neuromuscular transmission in amyotrophic lateral sclerosis. Muscle Nerve 1993, 16, 1193–1203. [Google Scholar] [CrossRef]
- Tremblay, E.; Martineau, É.; Robitaille, R. Opposite Synaptic Alterations at the Neuromuscular Junction in an ALS Mouse Model: When Motor Units Matter. J. Neurosci. 2017, 37, 8901–8918. [Google Scholar] [CrossRef] [PubMed]
- Chand, K.K.; Lee, K.M.; Lee, J.D.; Qiu, H.; Willis, E.F.; Lavidis, N.A.; Hilliard, M.A.; Noakes, P.G. Defects in synaptic transmission at the neuromuscular junction precede motor deficits in a TDP-43Q331K transgenic mouse model of amyotrophic lateral sclerosis. FASEB J. 2018, 32, 2676–2689. [Google Scholar] [CrossRef] [PubMed]
- Bose, P.; Tremblay, E.; Maois, C.; Narasimhan, V.; Armstrong, G.A.B.; Liao, M.; Parker, J.A.; Robitaille, R.; Wen, X.Y.; Barden, C.; et al. The Novel Small Molecule TRVA242 Stabilizes Neuromuscular Junction Defects in Multiple Animal Models of Amyotrophic Lateral Sclerosis. Neurotherapeutics 2019, 16, 1149–1166. [Google Scholar] [CrossRef] [PubMed]
- Bruneteau, G.; Bauché, S.; Gonzalez de Aguilar, J.L.; Brochier, G.; Mandjee, N.; Tanguy, M.L.; Hussain, G.; Behin, A.; Khiami, F.; Sariali, E.; et al. Endplate denervation correlates with Nogo-A muscle expression in amyotrophic lateral sclerosis patients. Ann. Clin. Transl. Neurol. 2015, 2, 362–372. [Google Scholar] [CrossRef]
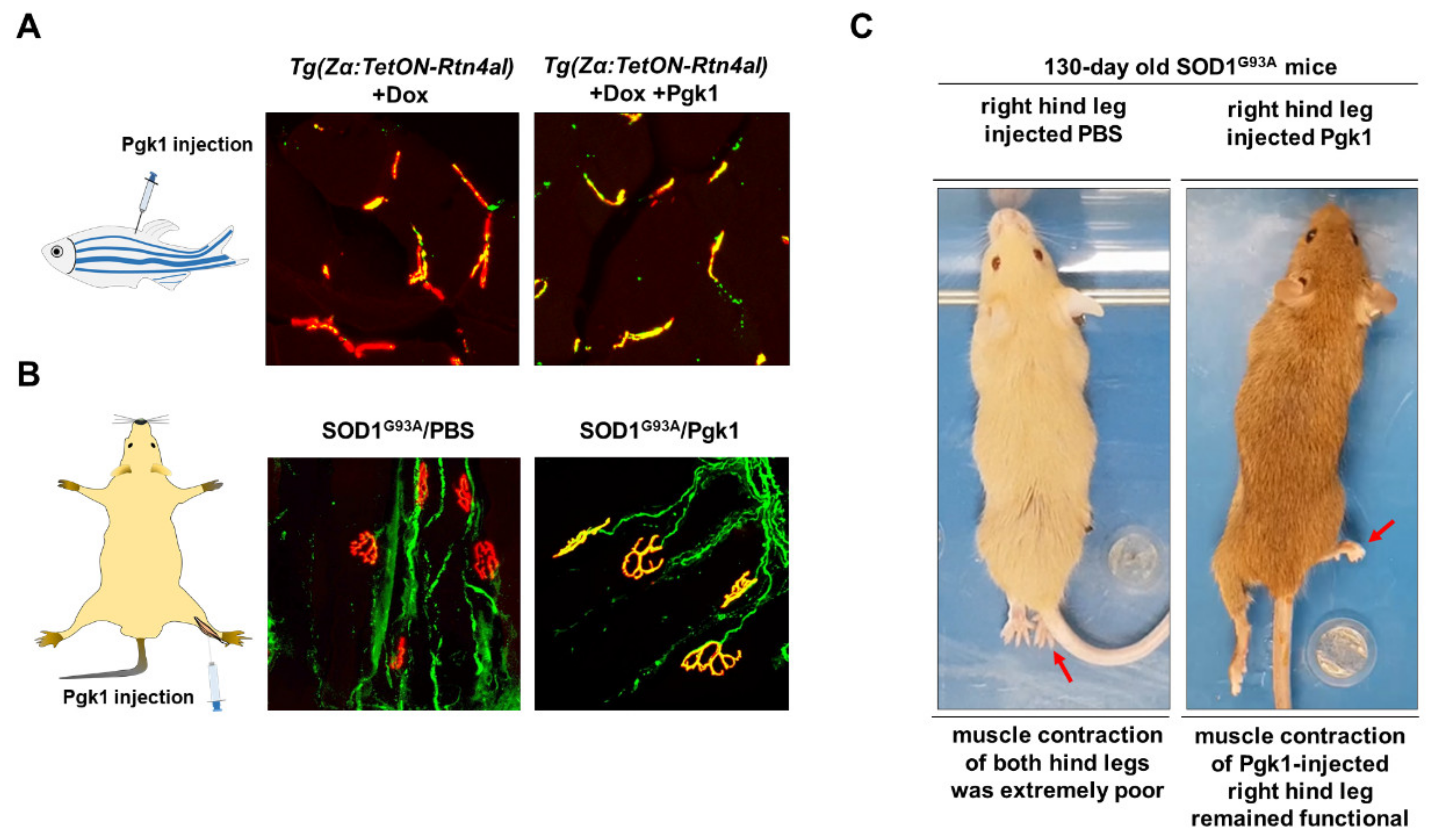
- Lin, C.Y.; Wu, C.L.; Lee, K.Z.; Chen, Y.J.; Zhang, P.H.; Chang, C.Y.; Harn, H.J.; Lin, S.Z.; Tsai, H.J. Extracellular Pgk1 enhances neurite outgrowth of motoneurons through Nogo66/NgR-independent targeting of NogoA. Elife 2019, 8, e49175. [Google Scholar] [CrossRef]
- Lin, C.Y.; Zhang, P.H.; Chen, Y.J.; Wu, C.L.; Tsai, H.J. Conditional Overexpression of rtn4al in Muscle of Adult Zebrafish Displays Defects Similar to Human Amyotrophic Lateral Sclerosis. Mar. Biotechnol. 2019, 21, 52–64. [Google Scholar] [CrossRef]
- North, T.E.; Goessling, W.; Walkley, C.R.; Lengerke, C.; Kopani, K.R.; Lord, A.M.; Weber, G.J.; Bowman, T.V.; Jang, I.H.; Grosser, T.; et al. Prostaglandin E2 regulates vertebrate haematopoietic stem cell homeostasis. Nature 2007, 447, 1007–1011. [Google Scholar] [CrossRef]
- Payne, E.M.; Virgilio, M.; Narla, A.; Sun, H.; Levine, M.; Paw, B.H.; Berliner, N.; Look, A.T.; Ebert, B.L.; Khanna-Gupta, A. L-Leucine improves the anemia and developmental defects associated with Diamond-Blackfan anemia and del(5q) MDS by activating the mTOR pathway. Blood 2012, 120, 2214–2224. [Google Scholar] [CrossRef] [PubMed]
- Ear, J.; Huang, H.; Wilson, T.; Tehrani, Z.; Lindgren, A.; Sung, V.; Laadem, A.; Daniel, T.O.; Chopra, R.; Lin, S. RAP-011 improves erythropoiesis in zebrafish model of Diamond-Blackfan anemia through antagonizing lefty1. Blood 2015, 126, 880–890. [Google Scholar] [CrossRef]
- Macari, E.R.; Taylor, A.M.; Raiser, D.M.; Siva, K.; McGrath, K.; Humphries, J.M.; Flygare, J.; Ebert, B.L.; Zon, L.I. Calmodulin inhibition rescues DBA models with ribosomal protein deficiency through reduction of RSK signaling. Blood 2016, 128, 332. [Google Scholar] [CrossRef]
- Baraban, S.C.; Dinday, M.T.; Hortopan, G.A. Drug screening in Scn1a zebrafish mutant identifies clemizole as a potential Dravet syndrome treatment. Nat. Commun. 2013, 4, 2410. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.J.; Tu, C.T.; Hsiao, C.D.; Hsieh, F.J.; Tsai, H.J. Germ-line transmission of a myocardium-specific GFP transgene reveals critical regulatory elements in the cardiac myosin light chain 2 promoter of zebrafish. Dev. Dyn. 2003, 228, 30–40. [Google Scholar] [CrossRef] [PubMed]
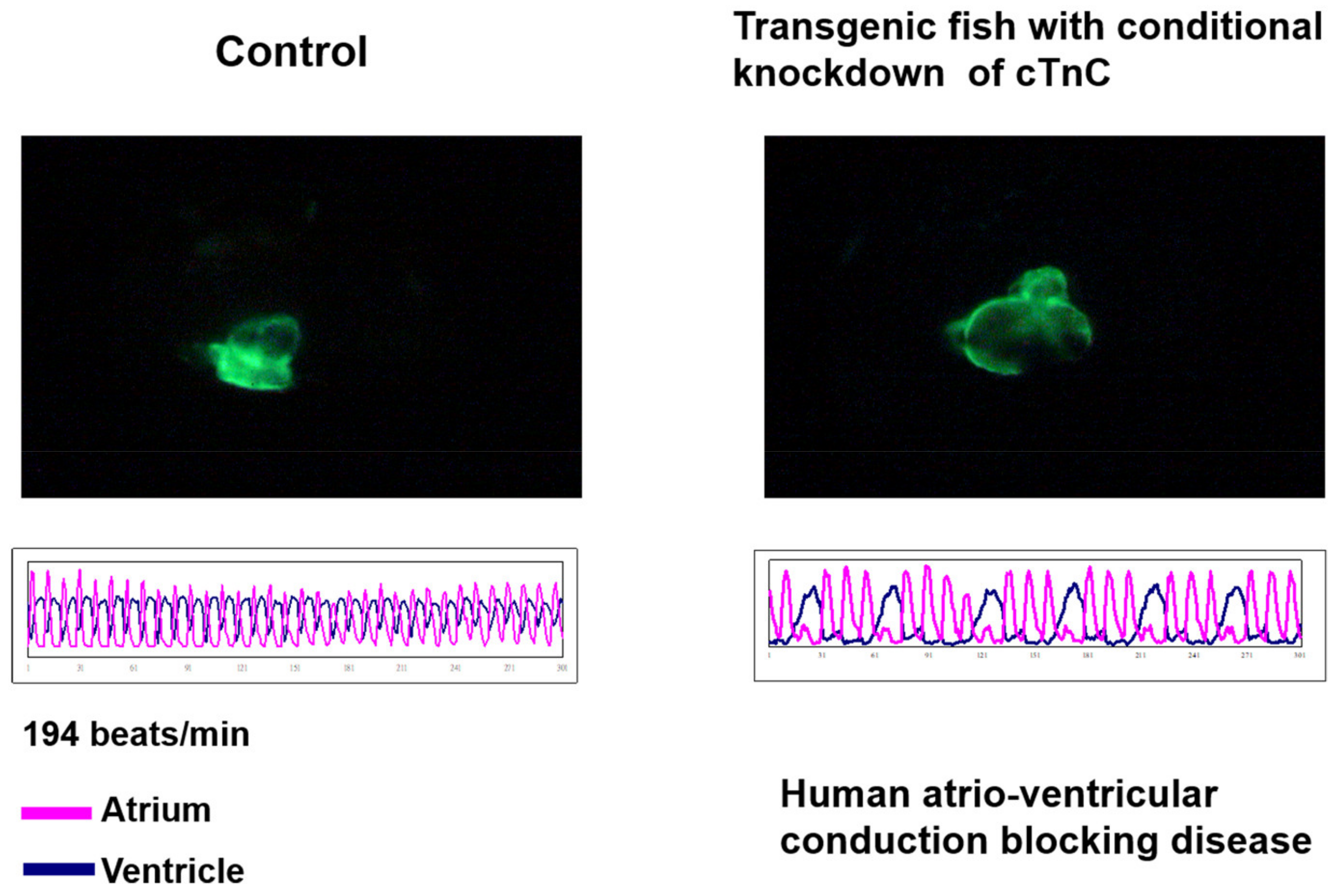
- Ho, Y.L.; Lin, Y.H.; Tsai, W.Y.; Hsieh, F.J.; Tsai, H.J. Conditional antisense-knockdown of zebrafish cardiac troponin C as a new animal model for dilated cardiomyopathy. Circ. J. 2009, 73, 1691–1697. [Google Scholar] [CrossRef] [PubMed]
- Bambino, K.; Chu, J. Zebrafish in Toxicology and Environmental Health. Curr. Top. Dev. Biol. 2017, 124, 331–367. [Google Scholar]
- Xu, H.; Li, C.; Li, Y.; Ng, G.H.; Liu, C.; Zhang, X.; Gong, Z. Generation of Tg(cyp1a:gfp) Transgenic Zebrafish for Development of a Convenient and Sensitive In Vivo Assay for Aryl Hydrocarbon Receptor Activity. Mar. Biotechnol. 2015, 17, 831–840. [Google Scholar] [CrossRef]
- Lee, H.C.; Chen, Y.J.; Liu, Y.W.; Lin, K.Y.; Chen, S.W.; Lin, C.Y.; Lu, Y.C.; Hsu, P.C.; Lee, S.C.; Tsai, H.J. Transgenic zebrafish model to study translational control mediated by upstream open reading frame of human chop gene. Nucleic Acids Res. 2011, 39, e139. [Google Scholar] [CrossRef][Green Version]
- Lee, H.C.; Lu, P.N.; Huang, H.L.; Chu, C.; Li, H.P.; Tsai, H.J. Zebrafish transgenic line huORFZ is an effective living bioindicator for detecting environmental toxicants. PLoS ONE 2014, 9, e90160. [Google Scholar] [CrossRef]
- Ignotti, E.; Hacon Sde, S.; Junger, W.L.; Mourão, D.; Longo, K.; Freitas, S.; Artaxo, P.; Leon, A.C. Air pollution and hospital admissions for respiratory diseases in the subequatorial Amazon: A time series approach. Cad. Saude Publica 2010, 26, 747–761. [Google Scholar] [CrossRef]
- Sigsgaard, T.; Forsberg, B.; Annesi-Maesano, I.; Blomberg, A.; Bølling, A.; Boman, C.; Bønløkke, J.; Brauer, M.; Bruce, N.; Héroux, M.E.; et al. Health impacts of anthropogenic biomass burning in the developed world. Eur. Respir. J. 2015, 46, 1577–1588. [Google Scholar] [CrossRef]
- Babić, S.; Čižmek, L.; Maršavelski, A.; Malev, O.; Pflieger, M.; Strunjak-Perović, I.; Popović, N.T.; Čož-Rakovac, R.; Trebše, P. Utilization of the zebrafish model to unravel the harmful effects of biomass burning during Amazonian wildfires. Sci. Rep. 2021, 11, 2527. [Google Scholar] [CrossRef]
- Chen, Y.H.; Lee, H.C.; Hsu, R.J.; Chen, T.Y.; Huang, Y.K.; Lo, H.C.; Hu, S.C.; Harn, H.J.; Jeng, J.R.; Sun, C.K.; et al. The toxic effect of Amiodarone on valve formation in the developing heart of zebrafish embryos. Reprod. Toxicol. 2012, 33, 233–244. [Google Scholar] [CrossRef]
- Hoppstädter, J.; Perez, J.V.V.; Linnenberger, R.; Dahlem, C.; Legroux, T.M.; Hecksteden, A.; Tse, W.K.F.; Flamini, S.; Andreas, A.; Herrmann, J.; et al. The glucocorticoid-induced leucine zipper mediates statin-induced muscle damage. FASEB J. 2020, 34, 4684–4701. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Zhao, Y.; Nel, A.E.; Lin, S. Zebrafish: An in vivo model for nano EHS studies. Small 2013, 9, 1608–1618. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, C.; Sharma, A.R.; Sharma, G.; Lee, S.S. Zebrafish: A complete animal model to enumerate the nanoparticle toxicity. J. Nanobiotechnol. 2016, 14, 65. [Google Scholar] [CrossRef]
- Jia, H.R.; Zhu, Y.X.; Duan, Q.Y.; Chen, Z.; Wu, F.G. Nanomaterials meet zebrafish: Toxicity evaluation and drug delivery applications. J. Control Release 2019, 311–312, 301–318. [Google Scholar] [CrossRef]
- Sieber, S.; Grossen, P.; Bussmann, J.; Campbell, F.; Kros, A.; Witzigmann, D.; Huwyler, J. Zebrafish as a preclinical in vivo screening model for nanomedicines. Adv. Drug Deliv. Rev. 2019, 151–152, 152–168. [Google Scholar] [CrossRef] [PubMed]
- Javed, I.; Peng, G.; Xing, Y.; Yu, T.; Zhao, M.; Kakinen, A.; Faridi, A.; Parish, C.L.; Ding, F.; Davis, T.P.; et al. Inhibition of amyloid beta toxicity in zebrafish with a chaperone-gold nanoparticle dual strategy. Nat. Commun. 2019, 10, 3780. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, Y.; Takamiya, M.; Jensen, P.B.; Ojea-Jiménez, I.; Claude, H.; Antony, C.; Kjaer-Sorensen, K.; Grabher, C.; Boesen, T.; Gilliland, D.; et al. Differential Nanoparticle Sequestration by Macrophages and Scavenger Endothelial Cells Visualized in Vivo in Real-Time and at Ultrastructural Resolution. ACS Nano. 2020, 14, 1665–1681. [Google Scholar] [CrossRef]
- Chen, Z.Y.; Li, N.J.; Cheng, F.Y.; Hsueh, J.F.; Huang, C.C.; Lu, F.I.; Fu, T.F.; Yan, S.J.; Lee, Y.H.; Wang, Y.J. The Effect of the Chorion on Size-Dependent Acute Toxicity and Underlying Mechanisms of Amine-Modified Silver Nanoparticles in Zebrafish Embryos. Int. J. Mol. Sci. 2020, 21, 2864. [Google Scholar] [CrossRef]
- Alders, M.; Bikker, H.; Christiaans, I. Long QT syndrome. In GeneReviews®; Adam, M.P., Ardinger, H.H., Pagon, R.A., Wallace, S.E., Bean, L.J., Stephens, K., et al., Eds.; University of Washington: Seattle, DC, USA, 1993. [Google Scholar]
- Roden, D.M. Long-QT Syndrome. 2008. Available online: https://www-nejm-org.proxy.lib.sfu.ca/doi/10.1056/NEJMcp0706513 (accessed on 23 October 2020).
- Schwartz, P.J.; Crotti, L.; Insolia, R. Long-QT syndrome from genetics to management. Circ. Arrhythm. Electrophysiol. 2012, 5, 868–877. [Google Scholar] [CrossRef]
- Hammond, T.G.; Pollard, C.E. Use of in vitro methods to predict QT prolongation. Toxicol. Appl. Pharmacol. 2005, 207, 446–450. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.R.; Vlaminckx, E.; Hermans, A.N.; Rohrbacher, J.; van Ammel, K.; Towart, R.; Pugsley, M.; Gallacher, D.J. Predicting drug-induced changes in QT interval and arrhythmias: QT-shortening drugs point to gaps in the ICHS7B Guidelines. Br. J. Pharmacol. 2008, 154, 1427–1438. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.T.; Wu, C.K.; Chiang, F.T.; Tseng, C.D.; Lee, J.K.; Yu, C.C.; Wang, Y.C.; Lai, L.P.; Lin, J.L.; Hwang, J.J. In-vitro recording of adult zebrafish heart electrocardiogram—A platform for pharmacological testing. Clin. Chim. Acta. 2011, 412, 1963–1967. [Google Scholar] [CrossRef] [PubMed]
- Vornanen, M.; Hassinen, M. Zebrafish heart as a model for human cardiac electrophysiology. Channels 2016, 10, 101–110. [Google Scholar] [CrossRef]
- Lin, M.H.; Chou, H.C.; Chen, Y.F.; Liu, W.; Lee, C.C.; Liu, L.Y.; Chuang, Y.J. Development of a rapid and economic in vivo electrocardiogram platform for cardiovascular drug assay and electrophysiology research in adult zebrafish. Sci. Rep. 2018, 8, 15986. [Google Scholar] [CrossRef]
- Zhao, Y.; Yun, M.; Nguyen, S.A.; Tran, M.; Nguyen, T.P. In Vivo Surface Electrocardiography for Adult Zebrafish. J. Vis. Exp. 2019, 150. [Google Scholar] [CrossRef]
- Danik, S.; Cabo, C.; Chiello, C.; Kang, S.; Wit, A.L.; Coromilas, J. Correlation of repolarization of ventricular monophasic action potential with ECG in the murine heart. Am. J. Physiol. Heart Circ. Physiol. 2002, 283, H372–H381. [Google Scholar] [CrossRef]
- Liu, G.; Iden, J.B.; Kovithavongs, K.; Gulamhusein, R.; Duff, H.J.; Kavanagh, K.M. In vivo temporal and spatial distribution of depolarization and repolarization and the illusive murine T wave. J. Physiol. 2004, 555, 267–279. [Google Scholar] [CrossRef]
- Speerschneider, T.; Thomsen, M.B. Physiology and analysis of the electrocardiographic T wave in mice. Acta Physiol. 2013, 209, 262–271. [Google Scholar] [CrossRef]
- Hull, C.M.; Genge, C.E.; Hobbs, Y.; Rayani, K.; Lin, E.; Marvin Gunawan, M.; Shafaattalab, S.; Glen, F.; Tibbits, G.F.; Claydon, T.W. Investigating the utility of adult zebrafish ex vivo whole hearts to pharmacologically screen hERG channel activator compounds. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2019, 317, R921–R931. [Google Scholar] [CrossRef]
- Arnaout, R.; Ferrer, T.; Huisken, J.; Spitzer, K.; Stainier, D.Y.; Tristani-Firouzi, M.; Chi, N.C. Zebrafish model for human long QT syndrome. Proc. Natl. Acad. Sci. USA 2007, 104, 11316–11321. [Google Scholar] [CrossRef] [PubMed]
- Milan, D.J.; Kim, A.M.; Winterfield, J.R.; Jones, I.L.; Pfeufer, A.; Sanna, S.; Arking, D.E.; Amsterdam, A.H.; Sabeh, K.M.; Mably, J.D.; et al. Drug-sensitized zebrafish screen identifies multiple genes, including GINS3, as regulators of myocardial repolarization. Circulation 2009, 120, 553–559. [Google Scholar] [CrossRef] [PubMed]
- Leong, I.U.; Skinner, J.R.; Shelling, A.N.; Love, D.R. Zebrafish as a model for long QT syndrome: The evidence and the means of manipulating zebrafish gene expression. Acta. Physiol. 2010, 199, 257–276. [Google Scholar] [CrossRef] [PubMed]
- Jou, C.J.; Barnett, S.M.; Bian, J.T.; Weng, H.C.; Sheng, X.; Tristani-Firouzi, M. An in vivo cardiac assay to determine the functional consequences of putative long QT syndrome mutations. Circ. Res. 2013, 112, 826–830. [Google Scholar] [CrossRef] [PubMed]
- Postlethwait, J.H.; Woods, I.G.; Ngo-Hazelett, P.; Yan, Y.L.; Kelly, P.D.; Chu, F.; Huang, H.; Hill-Force, A.; Talbot, W.S. Zebrafish comparative genomics and the origins of vertebrate chromosomes. Genome Res. 2000, 10, 1890–1902. [Google Scholar] [CrossRef] [PubMed]
- Morgan, P.; van der Graaf, P.H.; Arrowsmith, J.; Feltner, D.E.; Drummond, K.S.; Wegner, C.D.; Street, S.D.A. Can the flow of medicines be improved? Fundamental pharmacokinetic and pharmacological principles toward improving phase II survival. Drug Discov. Today 2012, 17, 419–424. [Google Scholar] [CrossRef]
- Van Wijk, R.C.; Krekels, E.H.J.; Hankemeier, T.; Spaink, H.P.; van der Graaf, P.H. Systems pharmacology of hepatic metabolism in zebrafish larvae. Drug Discov. Today Dis. Models 2016, 22, 27–34. [Google Scholar] [CrossRef]
- Hu, G.; Siu, S.O.; Li, S.; Chu, I.K.; Kwan, Y.W.; Chan, S.W.; Leung, G.P.; Yan, R.; Lee, S.M. Metabolism of calycosin, an isoflavone from Astragali radix, in zebrafish larvae. Xenobiotica 2012, 42, 294–303. [Google Scholar] [CrossRef]
- Li, Z.H.; Alex, D.; Siu, S.O.; Chu, I.K.; Renn, J.; Winkler, C.; Lou, S.; Tsui, S.K.; Zhao, H.Y.; Yan, W.R.; et al. Combined in vivo imaging and omics approaches reveal metabolism of icaritin and its glycosides in zebrafish larvae. Mol. Biosyst. 2011, 7, 2128–2138. [Google Scholar] [CrossRef]
- Chng, H.T.; Ho, H.K.; Yap, C.W.; Lam, S.H.; Chan, E.C.Y. An investigation of the bioactivation potential and metabolism profile of zebrafish versus human. J. Biomol. Screen 2012, 17, 974–986. [Google Scholar] [CrossRef]
- Kantae, V.; Krekels, E.H.; Ordas, A.; González, O.; van Wijk, R.C.; Harms, A.C.; Racz, P.I.; van der Graaf, P.H.; Spaink, H.P.; Hankemeier, T. Pharmacokinetic modeling of paracetamol uptake and clearance in zebrafish larvae: Expanding the allometric scale in vertebrates with five orders of magnitude. Zebrafish 2016, 13, 504–510. [Google Scholar] [CrossRef] [PubMed]

| Disease | Mutant/Transgenic Line | Size of Screened Library | Identified Drug | Ref. |
|---|---|---|---|---|
| Adenoid cystic carcinoma | Tg(c-myb:GFP) | 3840 | ATRA | [29] |
| Amyotrophic lateral sclerosis | UAS:GR transgenic line crossed with Tg(Hb9) | 3765 | TRVA242 | [104] |
| Aminoglycoside antibiotics | Wild-type adult zebrafish | 99 | ORC-13661 | [31] |
| Dravet syndrome | scn1Lab mutant zebrafish | 370 | Lorcaserin | [25] |
| Dravet syndrome | scn1Lab mutant zebrafish | 320 | Clemizole | [112] |
| Hematologic malignancy | Wild-type embryos | 2480 | ProHema | [108] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.-C.; Lin, C.-Y.; Tsai, H.-J. Zebrafish, an In Vivo Platform to Screen Drugs and Proteins for Biomedical Use. Pharmaceuticals 2021, 14, 500. https://doi.org/10.3390/ph14060500
Lee H-C, Lin C-Y, Tsai H-J. Zebrafish, an In Vivo Platform to Screen Drugs and Proteins for Biomedical Use. Pharmaceuticals. 2021; 14(6):500. https://doi.org/10.3390/ph14060500
Chicago/Turabian StyleLee, Hung-Chieh, Cheng-Yung Lin, and Huai-Jen Tsai. 2021. "Zebrafish, an In Vivo Platform to Screen Drugs and Proteins for Biomedical Use" Pharmaceuticals 14, no. 6: 500. https://doi.org/10.3390/ph14060500
APA StyleLee, H.-C., Lin, C.-Y., & Tsai, H.-J. (2021). Zebrafish, an In Vivo Platform to Screen Drugs and Proteins for Biomedical Use. Pharmaceuticals, 14(6), 500. https://doi.org/10.3390/ph14060500






