Comparison of the Safety and Efficacy between Preserved and Preservative-Free Latanoprost and Preservative-Free Tafluprost
Abstract
1. Introduction
2. Results
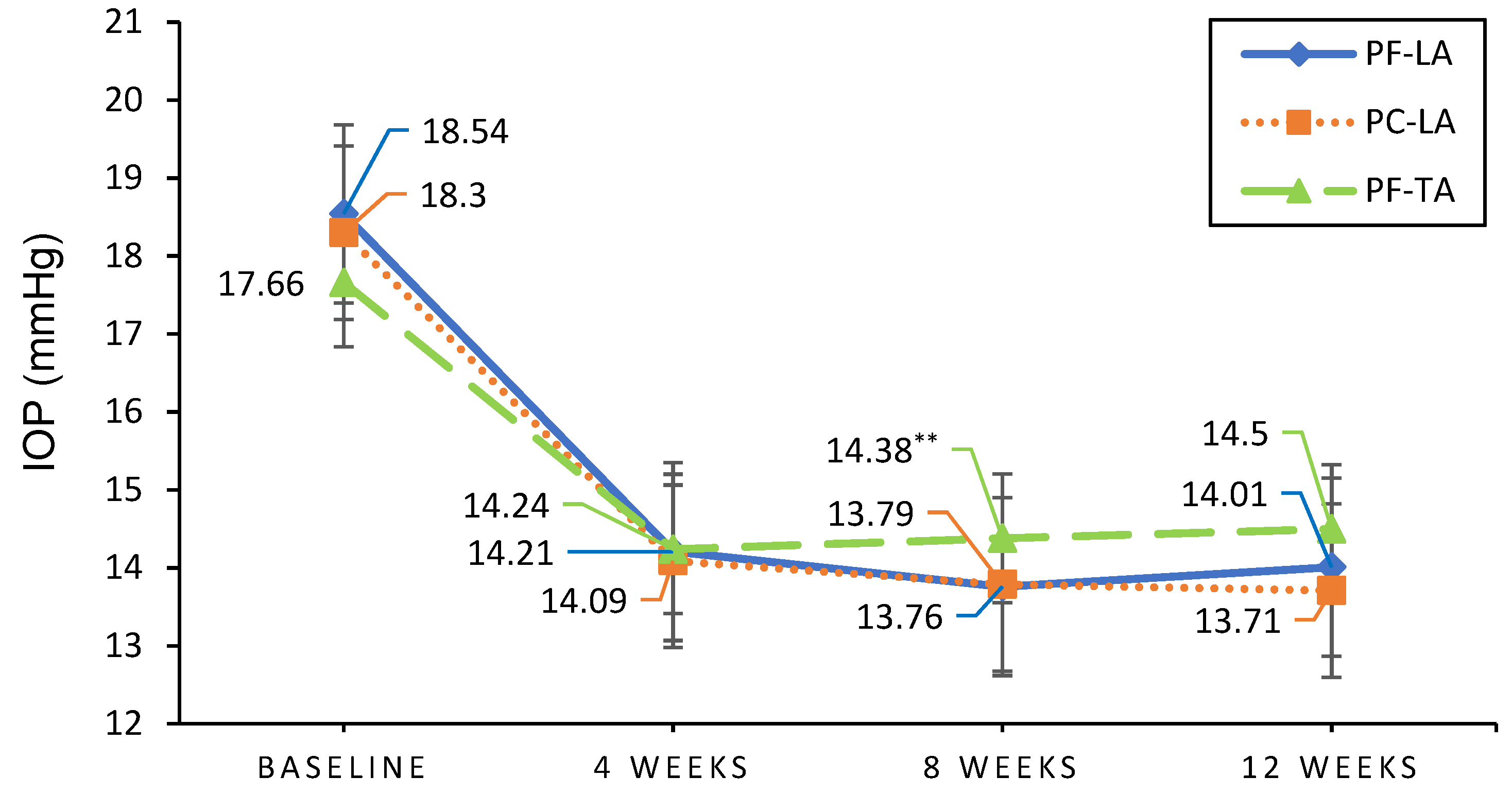
2.1. Efficacy Evaluation
2.2. Safety Evaluation for Ocular Surface
2.2.1. Corneal Staining Scores
2.2.2. Change in Hyperemia Scores (Bulbar)
2.2.3. Changes in Tear Break-Up Time (BUT) Scores
2.2.4. Changes in OSDI (Ocular Surface Diseases Index)
2.3. Adverse Events
3. Discussion
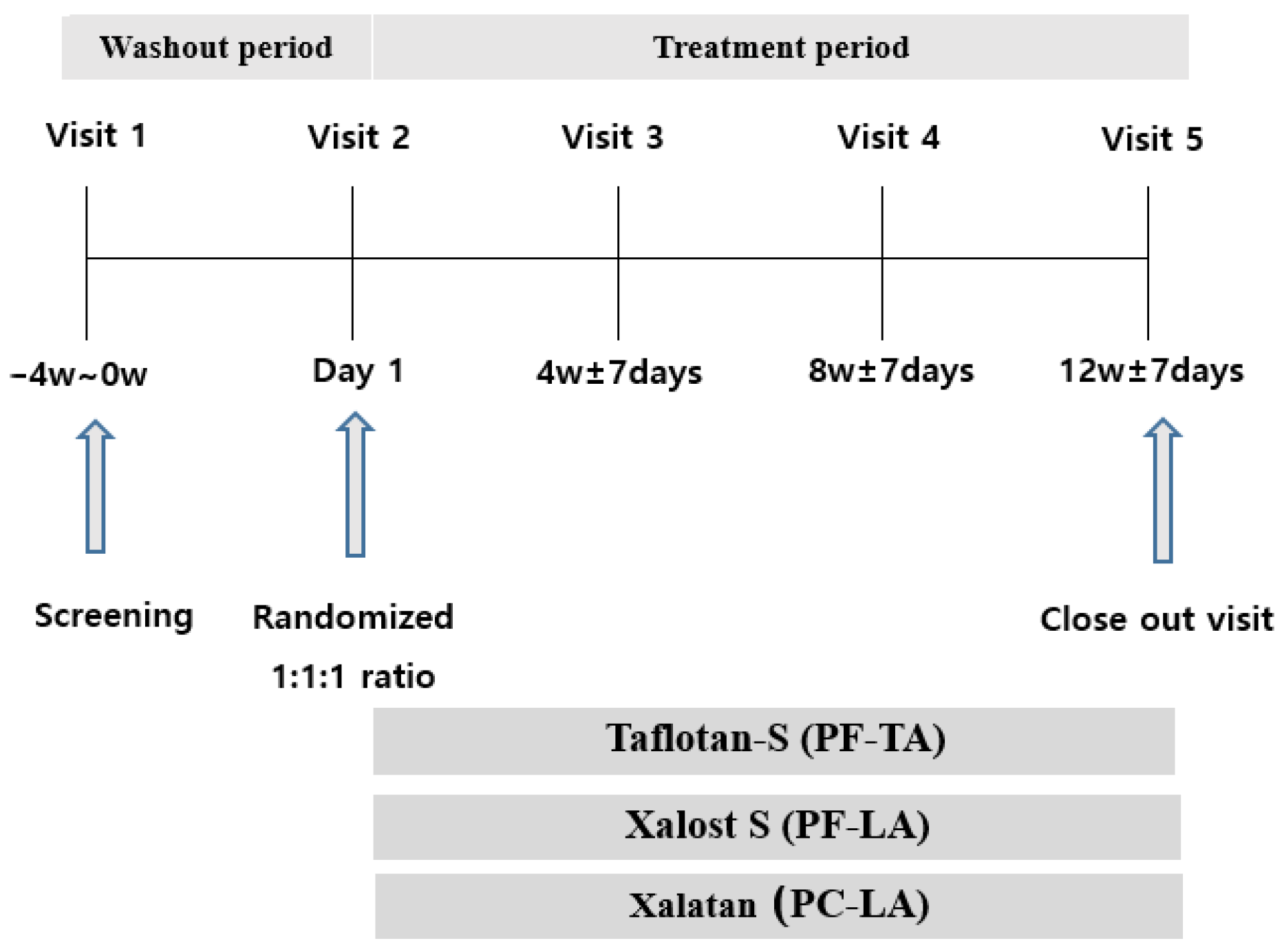
4. Materials and Methods
Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tham, Y.C.; Li, X.; Wong, T.Y.; Quigley, H.A.; Aung, T.; Cheng, C.Y. Global prevalence of glaucoma and projections of glaucoma burden through 2040: A systematic review and meta-analysis. Ophthalmology 2014, 121, 2081–2090. [Google Scholar] [CrossRef] [PubMed]
- Garway-Heath, D.F.; Crabb, D.P.; Bunce, C.; Lascaratos, G.; Amalfitano, F.; Anand, N.; Azuara-Blanco, A.; Bourne, R.R.; Broadway, D.C.; Cunliffe, I.A.; et al. Latanoprost for open-angle glaucoma (UKGTS): A randomised, multicentre, placebo-controlled trial. Lancet 2015, 385, 1295–1304. [Google Scholar] [CrossRef]
- Shukla, A.G.; De Moraes, C.G.; Cioffi, G.A.; Girkin, C.A.; Weinreb, R.N.; Zangwill, L.M.; Liebmann, J.M. The Relationship Between Intraocular Pressure and Rates of Central Versus Peripheral Visual Field Progression. J. Glaucoma 2020, 29, 435–440. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.S.; Ha, A.; Kim, Y.K.; Jeoung, J.W.; Park, K.H. Normal-tension Glaucoma Management: A Survey of Glaucoma Sub-specialists in Korea. Korean J. Ophthalmol. 2020, 34, 425–431. [Google Scholar] [CrossRef]
- Mursch-Edlmayr, A.-S.; Bolz, M.; Strohmaier, C. Vascular Aspects in Glaucoma: From Pathogenesis to Therapeutic Approaches. Int. J. Mol. Sci. 2021, 22, 4662. [Google Scholar] [CrossRef]
- Adornetto, A.; Rombolà, L.; Morrone, L.A.; Nucci, C.; Corasaniti, M.T.; Bagetta, G.; Russo, R. Natural Products: Evidence for Neuroprotection to Be Exploited in Glaucoma. Nutrients 2020, 12, 3158. [Google Scholar] [CrossRef]
- Shim, S.H.; Kim, J.M.; Choi, C.Y.; Kim, C.Y.; Park, K.H. Ginkgo biloba Extract and Bilberry Anthocyanins Improve Visual Function in Patients with Normal Tension Glaucoma. J. Med. Food 2012, 15, 818–823. [Google Scholar] [CrossRef]
- Lemij, H.G.; Hoevenaars, J.G.; van der Windt, C.; Baudouin, C. Patient satisfaction with glaucoma therapy: Reality or myth? Clin. Ophthalmol. 2015, 9, 785–793. [Google Scholar]
- Weinreb, R.N.; Robinson, M.R.; Dibas, M.; Stamer, W.D. Matrix Metalloproteinases and Glaucoma Treatment. J. Ocul. Pharmacol. Ther. 2020, 36, 208–228. [Google Scholar] [CrossRef]
- Alm, A.; Grierson, I.; Shields, M.B. Side Effects Associated with Prostaglandin Analog Therapy. Surv. Ophthalmol. 2008, 53, S93–S105. [Google Scholar] [CrossRef]
- Pérez-Bartolomé, F.; Martínez-De-La-Casa, J.M.; Arriola-Villalobos, P.; Fernández-Pérez, C.; Polo, V.; García-Feijoó, J. Ocular Surface Disease in Patients under Topical Treatment for Glaucoma. Eur. J. Ophthalmol. 2017, 27, 694–704. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Kim, E.J.; Kim, Y.-H.; Kim, Y.I.; Lee, S.-H.; Jung, J.-C.; Lee, K.W.; Park, Y.J. In Vivo Effects of Preservative-free and Preserved Prostaglandin Analogs: Mouse Ocular Surface Study. Korean J. Ophthalmol. 2015, 29, 270–279. [Google Scholar] [CrossRef] [PubMed]
- Misiuk-Hojlo, M.; Pomorska, M.; Mulak, M.; Rekas, M.; Wierzbowska, J.; Prost, M.; Wasyluk, J.; Lubinski, W.; Podboraczynska-Jodko, K.; Romaniuk, W.; et al. The RELIEF study: Tolerability and efficacy of preservative-free latanoprost in the treatment of glaucoma or ocular hypertension. Eur. J. Ophthalmol. 2019, 29, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Hommer, A.; Schmidl, D.; Kromus, M.; Bata, A.M.; Fondi, K.; Werkmeister, R.M.; Baar, C.; Schmetterer, L.; Garhöfer, G. Effect of changing from preserved prostaglandins to preservative-free tafluprost in patients with glaucoma on tear film thickness. Eur. J. Ophthalmol. 2018, 28, 385–392. [Google Scholar] [CrossRef]
- Ussa, F.; Fernandez, I.; Brion, M.; Carracedo, A.; Blazquez, F.; Garcia, M.T.; Sanchez-Jara, A.; De Juan-Marcos, L.; Jimenez-Carmona, S.; Juberias, J.R.; et al. Association between SNPs of Metalloproteinases and Prostaglandin F2α Receptor Genes and Latanoprost Response in Open-Angle Glaucoma. Ophthalmology 2015, 122, 1040–1048.e4. [Google Scholar] [CrossRef]
- Rouland, J.-F.; Traverso, C.E.; Stalmans, I.; El Fekih, L.; Delval, L.; Renault, D.; Baudouin, C. Efficacy and safety of preservative-free latanoprost eyedrops, compared with BAK-preserved latanoprost in patients with ocular hypertension or glaucoma. Br. J. Ophthalmol. 2012, 97, 196–200. [Google Scholar] [CrossRef]
- Aptel, F.; Choudhry, R.; Stalmans, I. Preservative-free versus preserved latanoprost eye drops in patients with open-angle glaucoma or ocular hypertension. Curr. Med. Res. Opin. 2016, 32, 1457–1463. [Google Scholar] [CrossRef]
- Tokuda, N.; Kitaoka, Y.; Matsuzawa, A.; Tsukamoto, A.; Sase, K.; Sakae, S.; Takagi, H. Changes in Ocular Surface Characteristics after Switching from Benzalkonium Chloride-Preserved Latanoprost to Preservative-Free Tafluprost or Benzalkonium Chloride-Preserved Tafluprost. J. Ophthalmol. 2017, 2017, 3540749. [Google Scholar] [CrossRef]
- Uusitalo, H.; Chen, E.; Pfeiffer, N.; Brignole-Baudouin, F.; Kaarniranta, K.; Leino, M.; Puska, P.; Palmgren, E.; Hamacher, T.; Hofmann, G.; et al. Switching from a preserved to a preservative-free prostaglandin preparation in topical glaucoma medication. Acta Ophthalmol. 2009, 88, 329–336. [Google Scholar] [CrossRef]
- Rasmussen, C.A.; Kaufman, P.L.; Kiland, J.A. Benzalkonium Chloride and Glaucoma. J. Ocul. Pharmacol. Ther. 2014, 30, 163–169. [Google Scholar] [CrossRef]
- Janson, B.; Alward, W.L.; Kwon, Y.H.; Bettis, D.I.; Fingert, J.H.; Provencher, L.M.; Goins, K.M.; Wagoner, M.D.; Greiner, M.A. Glaucoma-associated corneal endothelial cell damage: A review. Surv. Ophthalmol. 2018, 63, 500–506. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Huang, Y.; Xie, H.; Pan, J.; Liu, F.; Li, X.; Chen, W.; Hu, J.; Liu, Z. Benzalkonium Chloride Suppresses Rabbit Corneal Endothelium Intercellular Gap Junction Communication. PLoS ONE 2014, 9, e109708. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.; Heo, J.H.; Kim, H.M.; Song, J.S. Comparison of Cytotoxic Effects on Rabbit Corneal Endothelium between Preservative-free and Preservative-containing Dorzolamide/timolol. Korean J. Ophthalmol. 2015, 29, 344–350. [Google Scholar] [CrossRef] [PubMed]
- Boimer, C.; Birt, C.M. Preservative exposure and surgical outcomes in glaucoma patients: The PESO study. J. Glaucoma 2013, 22, 730–735. [Google Scholar] [CrossRef] [PubMed]
- Wilson, C.G.; Zhu, Y.P.; Frier, M.; Rao, L.S.; Gilchrist, P.; Perkins, A.C. Ocular contact time of a carbomer gel (GelTears) in humans. Br. J. Ophthalmol. 1998, 82, 1131–1134. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.-H.; Lim, S.A.; Tchach, H. Efficacy and Safety of Carbomer-Based Lipid-Containing Artificial Tear Formulations in Patients with Dry Eye Syndrome. Cornea 2016, 35, 181–186. [Google Scholar] [CrossRef]
- Marquardt, R.; Christ, T. Corneal contact time of artificial tear solutions. Klinische Monatsblatter Augenheilkunde 1986, 189, 254–257. [Google Scholar] [CrossRef]
- Xiao, Q.; Hu, Y.; Chen, F.; Chen, X. A comparative assessment of the efficacy of carbomer gel and carboxymethyl cellulose containing artificial tears in dry eyes. Acta Acad. Med. Wuhan 2008, 28, 592–595. [Google Scholar] [CrossRef]
- Johnson, M.E.; Murphy, P.J.; Boulton, M. Carbomer and Sodium Hyaluronate Eyedrops for Moderate Dry Eye Treatment. Optom. Vis. Sci. 2008, 85, 750–757. [Google Scholar] [CrossRef]
- Yamada, M.; Kawai, M.; Mochizuki, H.; Hata, Y.; Mashima, Y. Fluorophotometric measurement of the buffering action of human tears in vivo. Curr. Eye Res. 1998, 17, 1005–1009. [Google Scholar] [CrossRef]
- Moussa, W.G.E.H.; Farhat, R.G.; Nehme, J.C.; Sahyoun, M.A.; Schakal, A.R.; Jalkh, A.E.; Karam, M.P.A.; Azar, G.G. Comparison of Efficacy and Ocular Surface Disease Index Score between Bimatoprost, Latanoprost, Travoprost, and Tafluprost in Glaucoma Patients. J. Ophthalmol. 2018, 2018, 1319628. [Google Scholar] [CrossRef]
- Konstas, A.-G.; Boboridis, K.G.; Kapis, P.; Marinopoulos, K.; Voudouragkaki, I.C.; Panayiotou, D.; Mikropoulos, D.G.; Pagkalidou, E.; Haidich, A.-B.; Katsanos, A.; et al. 24-Hour Efficacy and Ocular Surface Health with Preservative-Free Tafluprost Alone and in Conjunction with Preservative-Free Dorzolamide/Timolol Fixed Combination in Open-Angle Glaucoma Patients Insufficiently Controlled with Preserved Latanoprost Monotherapy. Adv. Ther. 2017, 34, 221–235. [Google Scholar] [CrossRef] [PubMed]
- Hodapp, E.; Parrish, R.I.; Anderson, D. Clinical Decisions in Glaucoma; Mosby: St. Louis, MO, USA, 1993. [Google Scholar]
- Diaconita, V.; Quinn, M.; Jamal, D.; Dishan, B.; Malvankar-Mehta, M.S.; Hutnik, C. Washout Duration of Prostaglandin Analogues: A Systematic Review and Meta-analysis. J. Ophthalmol. 2018, 2018, 3190684. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Bartolomé, F.; Sanz-Pozo, C.; la Casa, J.M.-D.; Arriola-Villalobos, P.; Fernández-Pérez, C.; García-Feijoó, J. Assessment of ocular redness measurements obtained with keratograph 5M and correlation with subjective grading scales. J. Fr. Ophtalmol. 2018, 41, 836–846. [Google Scholar] [CrossRef] [PubMed]
- Yoon, K.-C.; Im, S.-K.; Kim, H.-G.; You, I.-C. Usefulness of Double Vital Staining With 1% Fluorescein and 1% Lissamine Green in Patients with Dry Eye Syndrome. Cornea 2011, 30, 972–976. [Google Scholar] [CrossRef] [PubMed]
- Bağbaba, A.; Şen, B.; Delen, D.; Uysal, B.S. An Automated Grading and Diagnosis System for Evaluation of Dry Eye Syndrome. J. Med. Syst. 2018, 42, 227. [Google Scholar] [CrossRef]
- Wong, T.T.; Aung, T.; Ho, C.L. Ocular surface status in glaucoma and ocular hypertension patients with existing corneal disorders switched from latanoprost 0.005% to tafluprost 0.0015%: Comparison of two prostaglandin analogues with different concentrations of benzalkonium chloride. Clin. Exp. Ophthalmol. 2018, 46, 1028–1034. [Google Scholar] [CrossRef]
- Schiffman, R.M.; Christianson, M.D.; Jacobsen, G.; Hirsch, J.D.; Reis, B.L. Reliability and Validity of the Ocular Surface Disease Index. Arch. Ophthalmol. 2000, 118, 615–621. [Google Scholar] [CrossRef]
| Subjects | PF-LA | PC-LA | PF-TA | Total | p-Value |
|---|---|---|---|---|---|
| Randomized | 46 | 46 | 45 | 137 | |
| Completed | 44 | 42 | 40 | 126 | |
| Withdrawal | 2 | 4 | 5 | 11 | |
| Withdrawal reason | |||||
| Protocol violation | 1 | 0 | 0 | 1 | |
| Withdrawal of consent | 1 | 2 | 3 | 6 | |
| Failure to follow-up | 0 | 1 | 0 | 1 | |
| Advance events | 0 | 0 | 1 | 1 | |
| Non-compliance of inclusion/exclusion criteria | 0 | 1 | 1 | 2 | |
| FAS (full analysis set) | 45 | 43 | 43 | 131 | |
| PPS (per-protocol set) | 42 | 40 | 40 | 122 | |
| Gender, female, N (%) * | 21 (46.67) | 20 (46.51) | 17 (39.53) | 58 (44.27) | 0.7471 † |
| Age (years), Mean (SD) | 56.69 (12.96) | 57.44 (11.78) | 56.81 (13.78) | 56.98 (12.77) | 0.9532 ‡ |
| Duration of glaucoma (years (y)/months (m)), Mean (SD) | 3y 9m (4y 3m) | 3y 2m (4y 9m) | 2y 6m (3y 6m) | 3y 3m (4y 4m) | 0.3231 ‡ |
| Hypertension, N (%) | 16 (35.56) | 15 (34.88) | 12 (27.91) | 43 (32.82) | |
| Diabetes mellitus, N (%) | 11 (24.44) | 7 (16.28) | 4 (9.30) | 22 (16.79) |
| PF-LA | PC-LA | PF-TA | p Value § | p Value * | p Value † | p Value ‡ | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | 12 Weeks | Baseline | 12 Weeks | Baseline | 12 Weeks | |||||
| PPS | 18.54 ± 2.76 | 14.01 ± 2.86 | 18.30 ± 2.92 | 13.71 ± 2.81 | 17.66 ± 2.33 | 14.53 ± 2.71 | 0.3651 | <0.0001 | 0.7527 | 0.0515 |
| FAS | 18.54 ± 2.77 | 13.92 ± 2.86 | 18.28 ± 2.82 | 13.86 ± 2.80 | 18.19 ± 3.53 | 15.19 ± 4.25 | 0.5700 | <0.0001 | 0.7948 | 0.0043 |
| PPS Responder | 18.57 (2.89) | 13.54 (2.70) | 18.61 (3.01) | 13.20 (2.72) | 17.75 (2.57) | 13.23 (1.92) | 0.4591 | <0.0001 | 0.3767 | 0.6230 |
| FAS Responder | 18.58 (2.89) | 13.48 (2.70) | 18.60 (2.93) | 13.29 (2.67) | 17.72 (2.53) | 13.26 (1.89) | 0.4199 | <0.0001 | 0.6069 | 0.4335 |
| Baseline | 4 Weeks | p Value * | p Value † | 8 Weeks | p Value * | p Value ‡ | 12 Weeks | p Value * | p Value § | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| PPS | PF-LA | 0.81 ± 0.99 | 0.36 ± 0.53 | 0.0004 | 0.52 ± 0.63 | 0.0559 | 0.48 ± 0.74 | 0.0309 | |||
| PC-LA | 0.58 ± 0.78 | 0.83 ± 0.96 | 0.1196 | 0.0006 | 0.53 ± 0.64 | 0.8560 | 0.6438 | 0.73 ± 0.82 | 0.332 | 0.0431 | |
| PF-TA | 0.70 ± 0.79 | 0.73 ± 0.88 | 0.8909 | 0.0003 | 0.60 ± 0.67 | 0.3930 | 0.6956 | 0.58 ± 0.59 | 0.3111 | 0.0666 | |
| FAS | PF-LA | 0.80 ± 0.99 | 0.36 ± 0.53 | 0.0002 | 0.49 ± 0.63 | 0.0295 | 0.47 ± 0.73 | 0.0311 | |||
| PC-LA | 0.56 ± 0.77 | 0.81 ± 0.93 | 0.0960 | 0.0107 | 0.51 ± 0.63 | 0.8601 | 0.4382 | 0.67 ± 0.81 | 0.4300 | 0.3996 | |
| PF-TA | 0.67 ± 0.78 | 0.72 ± 0.85 | 0.7816 | 0.0065 | 0.60 ± 0.66 | 0.4997 | 0.5379 | 0.58 ± 0.59 | 0.4095 | 0.3132 |
| Analysis | Group | Baseline | 4 Weeks | p Value * | p Value † | 8 Weeks | p Value * | p Value ‡ | 12 Weeks | p Value * | p Value § |
|---|---|---|---|---|---|---|---|---|---|---|---|
| PPS | PF-LA | 0.67 ± 0.69 | 0.98 ± 0.81 | 0.0049 | 0.86 ± 0.81 | 0.1396 | 0.88 ± 0.80 | 0.0649 | |||
| PC-LA | 0.73 ± 0.75 | 0.75 ± 0.67 | 1.0000 | 0.0343 | 0.78 ± 0.70 | 0.7813 | 0.3946 | 0.75 ± 0.71 | 1.0000 | 0.2022 | |
| PF-TA | 0.78 ± 0.77 | 0.95 ± 0.64 | 0.1907 | 0.4507 | 0.88 ± 0.69 | 0.5034 | 0.7479 | 0.80 ± 0.65 | 1.0000 | 0.2646 | |
| FAS | PF-LA | 0.67 ± 0.67 | 1.02 ± 0.87 | 0.0013 | 0.84 ± 0.80 | 0.1396 | 0.89 ± 0.78 | 0.0437 | |||
| PC-LA | 0.74 ± 0.76 | 0.74 ± 0.66 | 1.0000 | 0.0079 | 0.77 ± 0.68 | 1.0000 | 0.0899 | 0.72 ± 0.70 | 1.0000 | 0.0899 | |
| PF-TA | 0.79 ± 0.77 | 0.95 ± 0.65 | 0.1907 | 0.2264 | 0.88 ± 0.70 | 0.5034 | 0.2433 | 0.81 ± 0.66 | 1.0000 | 0.2433 |
| Analysis | Group | Baseline | 4 Weeks | p Value * | p Value † | 8 Weeks | p Value * | p Value ‡ | 12 Weeks | p Value * | p Value § |
|---|---|---|---|---|---|---|---|---|---|---|---|
| PPS | PF-LA | 6.44 ± 2.47 | 6.83 ± 2.53 | 0.3160 | 6.63 ± 3.39 | 0.7106 | 6.14 ± 2.45 | 0.8213 | |||
| PC-LA | 6.06 ± 2.73 | 5.55 ± 2.27 | 0.1464 | 0.0234 | 6.72 ± 3.01 | 0.3900 | 0.6662 | 6.16 ± 2.86 | 0.9182 | 0.7603 | |
| PF-TA | 6.07 ± 2.88 | 5.89 ± 2.67 | 0.7117 | 0.1138 | 5.71 ± 3.00 | 0.4790 | 0.2507 | 5.75 ± 2.86 | 0.2266 | 0.6726 | |
| FAS | PF-LA | 6.29 ± 2.46 | 6.75 ± 2.49 | 0.2133 | 6.58 ± 3.27 | 0.5297 | 6.10 ± 2.40 | 0.9510 | |||
| PC-LA | 6.00 ± 2.68 | 5.53 ± 2.20 | 0.1575 | 0.0183 | 6.61 ± 2.94 | 0.4016 | 0.7828 | 6.13 ± 2.76 | 0.7727 | 0.7776 | |
| PF-TA | 6.00 ± 2.85 | 5.90 ± 2.68 | 0.8172 | 0.1143 | 5.73 ± 2.99 | 0.6356 | 0.2350 | 5.77 ± 2.86 | 0.3488 | 0.6829 |
| PF-LA | PC-LA | PF-TA | ||||
|---|---|---|---|---|---|---|
| p Value * | p Value † | |||||
| Stinging/burning | 4 Weeks | 0.33 ± 0.69 | 0.95 ± 0.81 | 0.0001 | 0.58 ± 0.75 | 0.0770 |
| 8 Weeks | 0.33 ± 0.69 | 0.74 ± 0.79 | 0.0044 | 0.50 ± 0.72 | 0.1507 | |
| 12 Weeks | 0.17± 0.44 | 0.75 ± 0.71 | <0.0001 | 0.45 ± 0.78 | 0.0787 | |
| Sticky eye sensation | 4 Weeks | 0.21 ± 0.52 | 0.25 ± 0.63 | 0.9153 | 0.20 ± 0.46 | 0.9660 |
| 8 Weeks | 0.21 ± 0.56 | 0.33 ± 0.58 | 0.1700 | 0.15 ± 0.43 | 0.7658 | |
| 12 Weeks | 0.15 ± 0.36 | 0.35 ± 0.62 | 0.0924 | 0.20 ± 0.41 | 0.5304 | |
| Itching | 4 Weeks | 0.33 ± 0.61 | 0.40 ± 0.50 | 0.3245 | 0.45 ± 0.75 | 0.5767 |
| 8 Weeks | 0.24 ± 0.48 | 0.33 ± 0.58 | 0.4614 | 0.58 ± 0.90 | 0.1018 | |
| 12 Weeks | 0.22 ± 0.47 | 0.45 ± 0.64 | 0.0691 | 0.55 ± 0.78 | 0.0336 | |
| Blurred vision | 4 Weeks | 0.43 ± 0.70 | 0.40 ± 0.63 | 0.9418 | 0.55 ± 0.81 | 0.5646 |
| 8 Weeks | 0.38 ± 0.73 | 0.56 ± 0.88 | 0.2697 | 0.70 ± 0.94 | 0.0945 | |
| 12 Weeks | 0.34 ± 0.69 | 0.50 ± 0.64 | 0.1209 | 0.53 ± 0.75 | 0.1951 | |
| Sandiness/grittiness | 4 Weeks | 0.38 ± 0.66 | 0.43 ± 0.59 | 0.5650 | 0.53 ± 0.82 | 0.5267 |
| 8 Weeks | 0.36 ± 0.62 | 0.41 ± 0.64 | 0.6660 | 0.60 ± 0.84 | 0.2031 | |
| 12 Weeks | 0.32 ± 0.57 | 0.45 ± 0.68 | 0.3850 | 0.50 ± 0.60 | 0.1117 | |
| Dryness | 4 Weeks | 0.29 ± 0.71 | 0.35 ± 0.70 | 0.4273 | 0.58 ± 0.81 | 0.0443 |
| 8 Weeks | 0.36 ± 0.58 | 0.38 ± 0.63 | 0.9488 | 0.40 ± 0.71 | 1.0000 | |
| 12 Weeks | 0.29 ± 0.51 | 0.53 ± 0.78 | 0.2153 | 0.45 ± 0.78 | 0.5699 | |
| Light sensitivity | 4 Weeks | 0.14 ± 0.42 | 0.35 ± 0.62 | 0.0746 | 0.50 ± 0.88 | 0.0341 |
| 8 Weeks | 0.31 ± 0.72 | 0.31 ± 0.52 | 0.4739 | 0.43 ± 0.71 | 0.2291 | |
| 12 Weeks | 0.17 ± 0.50 | 0.45 ± 0.75 | 0.0327 | 0.33 ± 0.69 | 0.2305 | |
| Pain or soreness | 4 Weeks | 0.12 ± 0.50 | 0.43 ± 0.68 | 0.0048 | 0.33 ± 0.62 | 0.0311 |
| 8 Weeks | 0.24 ± 0.48 | 0.36 ± 0.58 | 0.3279 | 0.28 ± 0.51 | 0.7137 | |
| 12 Weeks | 0.02 ± 0.16 | 0.48 ± 0.68 | 0.0001 | 0.18 ± 0.59 | 0.1569 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.M.; Park, S.W.; Seong, M.; Ha, S.J.; Lee, J.W.; Rho, S.; Lee, C.E.; Kim, K.N.; Kim, T.-W.; Sung, K.R.; et al. Comparison of the Safety and Efficacy between Preserved and Preservative-Free Latanoprost and Preservative-Free Tafluprost. Pharmaceuticals 2021, 14, 501. https://doi.org/10.3390/ph14060501
Kim JM, Park SW, Seong M, Ha SJ, Lee JW, Rho S, Lee CE, Kim KN, Kim T-W, Sung KR, et al. Comparison of the Safety and Efficacy between Preserved and Preservative-Free Latanoprost and Preservative-Free Tafluprost. Pharmaceuticals. 2021; 14(6):501. https://doi.org/10.3390/ph14060501
Chicago/Turabian StyleKim, Joon Mo, Sang Woo Park, Mincheol Seong, Seung Joo Ha, Ji Woong Lee, Seungsoo Rho, Chong Eun Lee, Kyoung Nam Kim, Tae-Woo Kim, Kyung Rim Sung, and et al. 2021. "Comparison of the Safety and Efficacy between Preserved and Preservative-Free Latanoprost and Preservative-Free Tafluprost" Pharmaceuticals 14, no. 6: 501. https://doi.org/10.3390/ph14060501
APA StyleKim, J. M., Park, S. W., Seong, M., Ha, S. J., Lee, J. W., Rho, S., Lee, C. E., Kim, K. N., Kim, T.-W., Sung, K. R., & Kim, C. Y. (2021). Comparison of the Safety and Efficacy between Preserved and Preservative-Free Latanoprost and Preservative-Free Tafluprost. Pharmaceuticals, 14(6), 501. https://doi.org/10.3390/ph14060501






