Human Salivary Histatin-1-Functionalized Gelatin Methacrylate Hydrogels Promote the Regeneration of Cartilage and Subchondral Bone in Temporomandibular Joints
Abstract
1. Introduction
2. Results
2.1. Postoperative Course
2.2. Selection of Hst1 Dosage
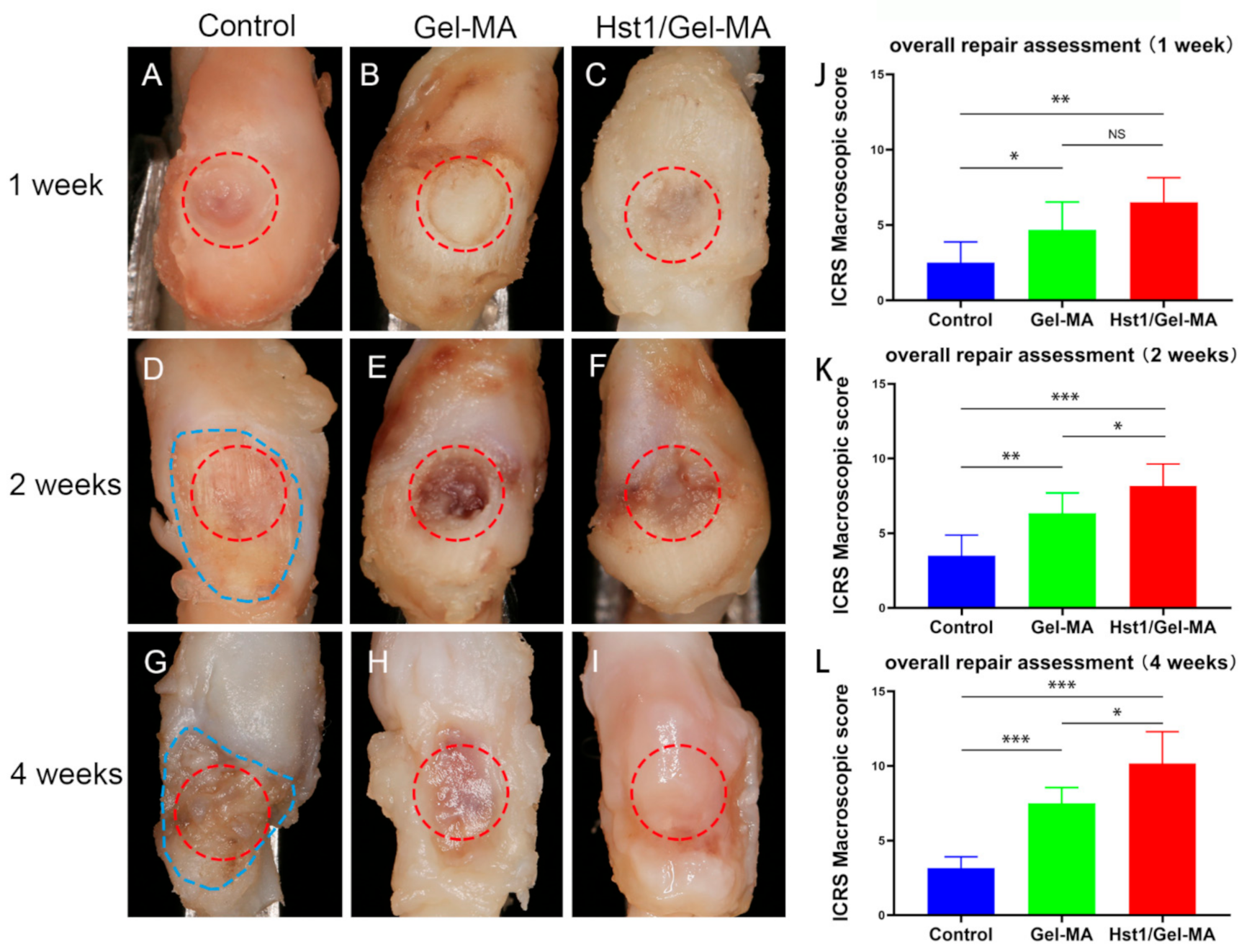
2.3. Macroscopic Evaluation
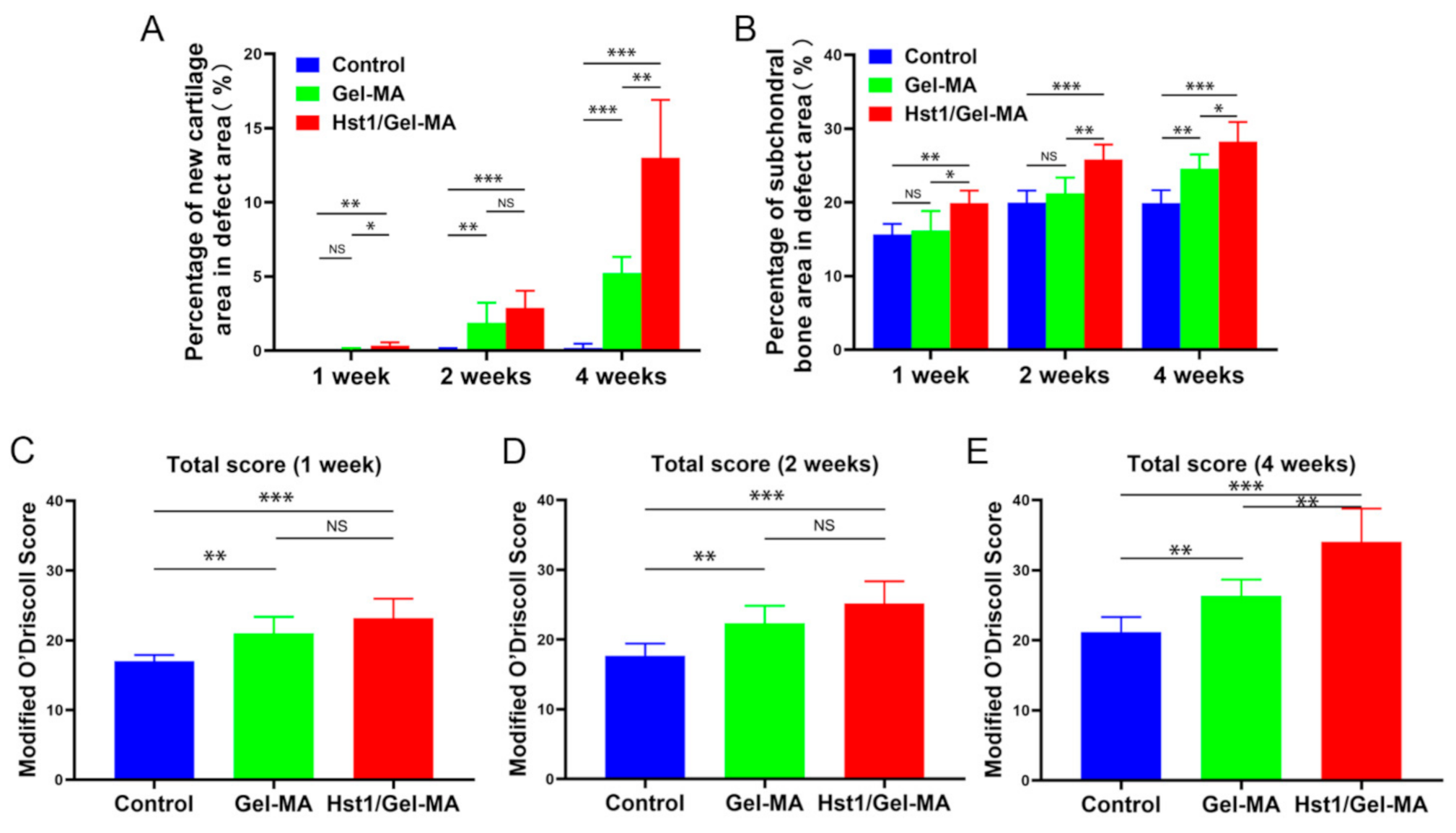
2.4. Histologic Observation and Histomorphometric Analysis on HE-Stained Tissue Sections
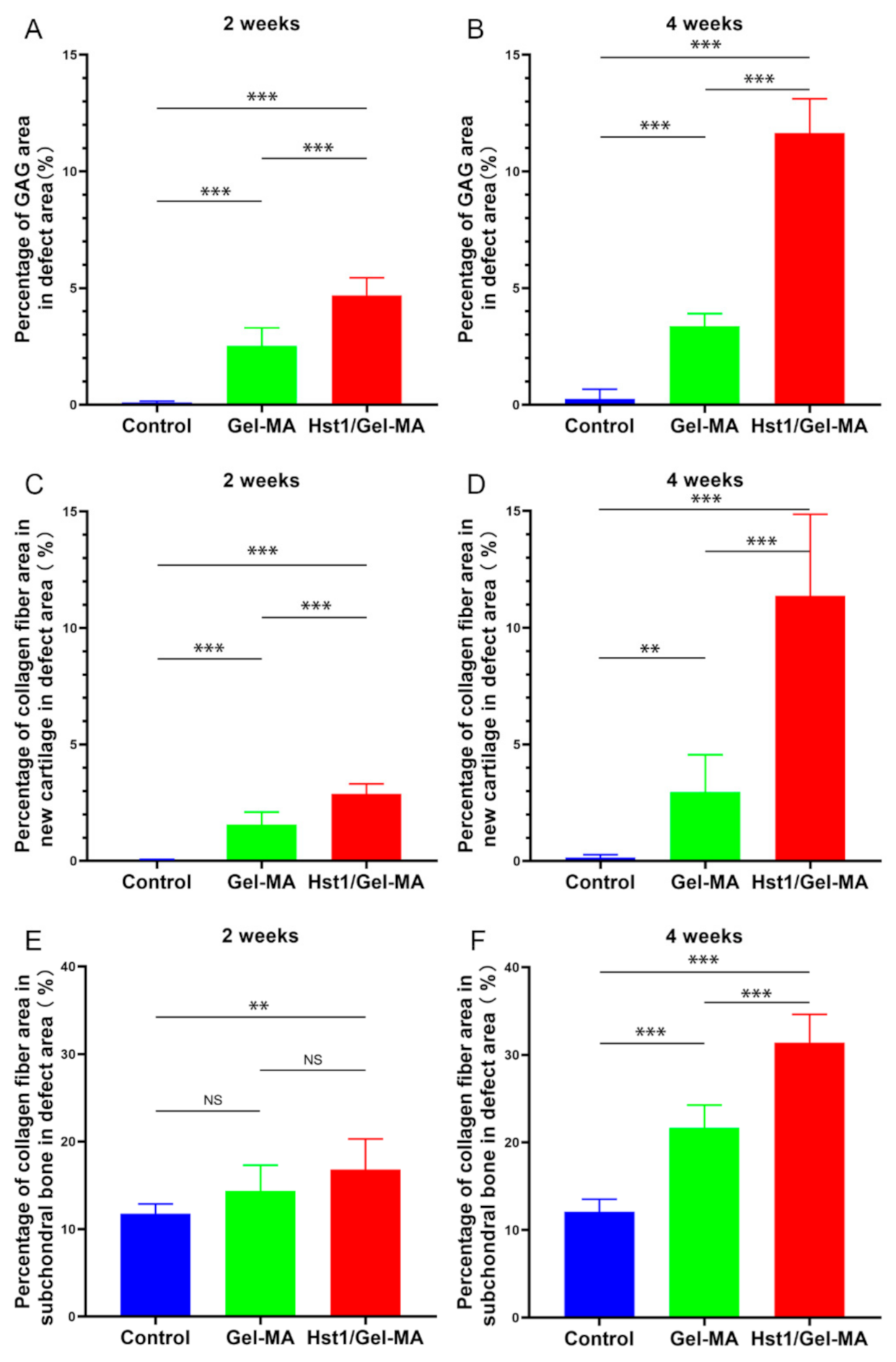
2.5. Histologic Observation and Histomorphometric Analysis on Sections with Alcian Blue Staining or Masson’s Trichrome Staining
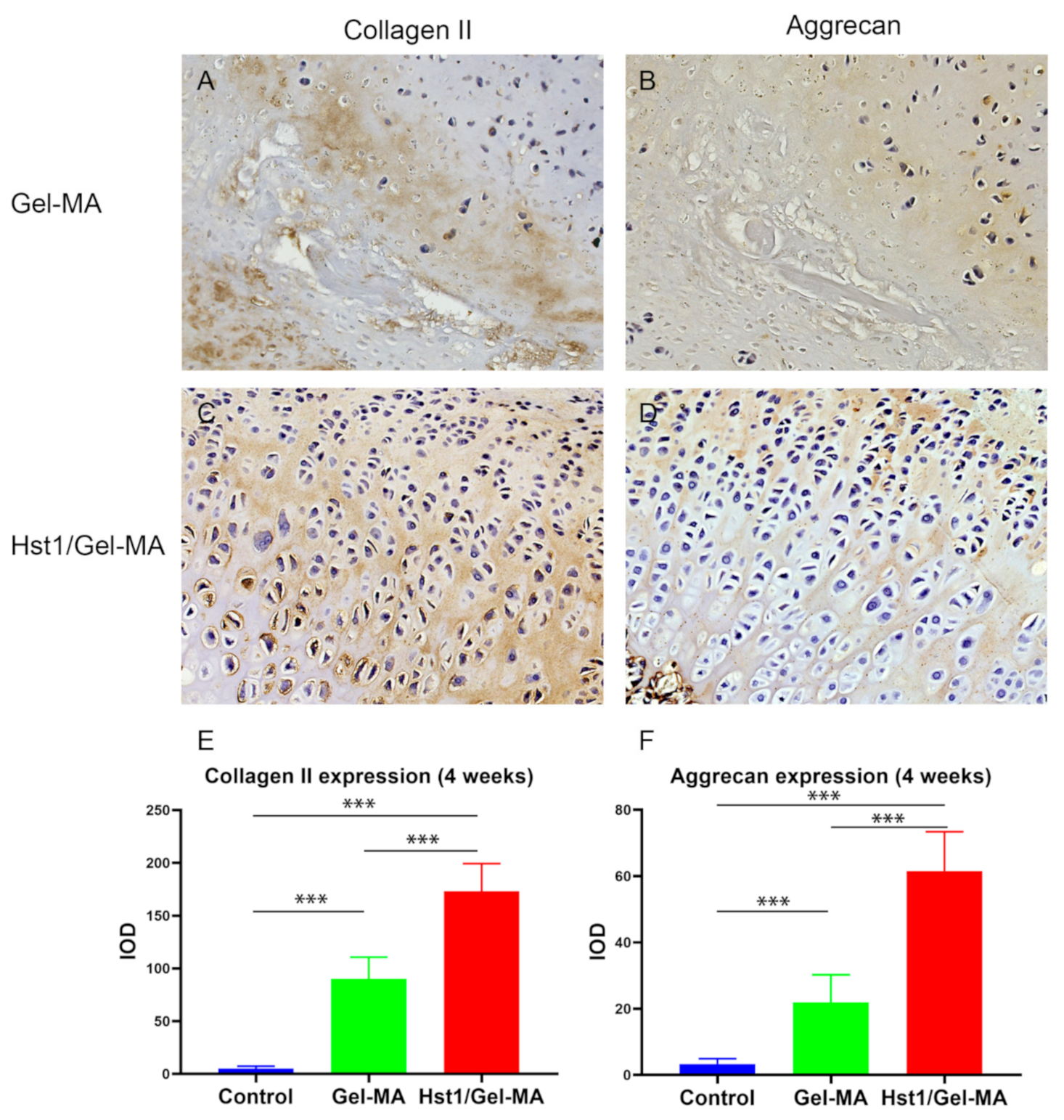
2.6. Immunohistochemical Evaluation
3. Discussion
4. Materials and Methods
4.1. The Preparation of Hydrogel Prepolymer Solution and Hst1
4.2. Group Set-Up
4.3. Animal Surgery
4.4. Macroscopic Score Evaluation
4.5. Histological Examination
4.6. Histomorphometry Analysis
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kuroda, S.; Tanimoto, K.; Izawa, T.; Fujihara, S.; Koolstra, J.H.; Tanaka, E. Biomechanical and biochemical characteristics of the mandibular condylar cartilage. Osteoarthr. Cartil. 2009, 17, 1408–1415. [Google Scholar] [CrossRef]
- LeResche, L. Epidemiology of temporomandibular disorders: Implications for the investigation of etiologic factors. Crit. Rev. Oral. Biol. Med. 1997, 8, 291–305. [Google Scholar] [CrossRef]
- Dormer, N.H.; Busaidy, K.; Berkland, C.J.; Detamore, M.S. Osteochondral interface regeneration of rabbit mandibular condyle with bioactive signal gradients. J. Oral. Maxillofac. Surg. 2011, 69, e50–e57. [Google Scholar] [CrossRef]
- Newman, A.P. Articular Cartilage Repair. Am. J. Sports Med. 1998, 26, 309–324. [Google Scholar] [CrossRef]
- Hunziker, E.B. Articular cartilage repair: Basic science and clinical progress. A review of the current status and prospects. Osteoarthr. Cartil. 2002, 10, 432–463. [Google Scholar] [CrossRef]
- Buckwalter, J. Mechanical Injuries of Articular Cartilage. Iowa Orthop. J. 1992, 12, 50–57. [Google Scholar]
- Mithoefer, K.; McAdams, T.; Williams, R.J.; Kreuz, P.C.; Mandelbaum, B.R. Clinical Efficacy of the Microfracture Technique for Articular Cartilage Repair in the Knee. Am. J. Sports Med. 2009, 37, 2053–2063. [Google Scholar] [CrossRef]
- Harris, J.D.; Siston, R.A.; Pan, X.; Flanigan, D.C. Autologous chondrocyte implantation: A systematic review. J. Bone Joint Surg. Am. 2010, 92, 2220–2233. [Google Scholar] [CrossRef] [PubMed]
- Bridwell, K.H.; Anderson, P.A.; Boden, S.D.; Vaccaro, A.R.; Wang, J.C. What’s New in Spine Surgery. J. Bone Joint Surg. Am. 2012, 94, 1140–1146. [Google Scholar] [CrossRef]
- Abdel-Sayed, P.; Pioletti, D.P. Strategies for improving the repair of focal cartilage defects. Nanomedicine 2015, 10, 2893–2905. [Google Scholar] [CrossRef]
- Gracitelli, G.C.; Moraes, V.Y.; Franciozi, C.E.; Luzo, M.V.; Belloti, J.C. Surgical interventions (microfracture, drilling, mosaicplasty, and allograft transplantation) for treating isolated cartilage defects of the knee in adults. Cochrane Database Syst Rev. 2016, 9, CD010675. [Google Scholar] [CrossRef]
- Bao, W.; Li, M.; Yang, Y.; Wan, Y.; Wang, X.; Bi, N.; Li, C. Advancements and Frontiers in the High Performance of Natural Hydrogels for Cartilage Tissue Engineering. Front Chem. 2020, 8, 53. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, H.; Feng, Y.; Jiang, P.; Su, J.; Huang, C. Dual micelles-loaded gelatin nanofibers and their application in lipopolysaccharide-induced periodontal disease. Int. J. Nanomed. 2019, 14, 963–976. [Google Scholar] [CrossRef]
- Puckert, C.; Tomaskovic-Crook, E.; Gambhir, S.; Wallace, G.G.; Crook, J.M.; Higgins, M.J. Molecular interactions and forces of adhesion between single human neural stem cells and gelatin methacrylate hydrogels of varying stiffness. Acta. Biomater. 2020, 106, 156–169. [Google Scholar] [CrossRef]
- Choi, J.R.; Yong, K.W.; Choi, J.Y.; Cowie, A.C. Recent advances in photo-crosslinkable hydrogels for biomedical applications. BioTechniques 2019, 66, 40–53. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Xie, J.; Zhong, L.; Li, J.; Rong, D.; Li, X.; Ouyang, J. Biomimetic gelatin methacrylamide hydrogel scaffolds for bone tissue engineering. J. Mater. Chem. B 2016, 4, 1070–1080. [Google Scholar] [CrossRef]
- Han, M.-E.; Kang, B.J.; Kim, S.-H.; Kim, H.D.; Hwang, N.S. Gelatin-based extracellular matrix cryogels for cartilage tissue engineering. J. Ind. Eng. Chem. 2017, 45, 421–429. [Google Scholar] [CrossRef]
- Zhu, W.; Cui, H.; Boualam, B.; Masood, F.; Flynn, E.; Rao, R.D.; Zhang, Z.Y.; Zhang, L.G. 3D bioprinting mesenchymal stem cell-laden construct with core-shell nanospheres for cartilage tissue engineering. Nanotechnology 2018, 29, 185101. [Google Scholar] [CrossRef]
- Irmak, G.; Gümüşderelioğlu, M. Photo-activated platelet-rich plasma (PRP)-based patient-specific bio-ink for cartilage tissue engineering. Biomed. Mater. 2020, 15. [Google Scholar] [CrossRef] [PubMed]
- Madry, H.; Alini, M.; Stoddart, M.J.; Evans, C.; Miclau, T.; Steiner, S. Barriers and strategies for the clinical translation of advanced orthopaedic tissue engineering protocols. Eur. Cells Mater. 2014, 27, 17–21. [Google Scholar] [CrossRef]
- Lee, Y.R.; Lee, Y.H.; Kim, K.H.; Im, S.A.; Lee, C.K. Induction of Potent Antigen-specific Cytotoxic T Cell Response by PLGA-nanoparticles Containing Antigen and TLR Agonist. Immune. Netw. 2013, 13, 30–33. [Google Scholar] [CrossRef]
- Torres, P.; Castro, M.; Reyes, M.; Torres, V.A. Histatins, wound healing, and cell migration. Oral Diseases. 2018, 24, 1150–1160. [Google Scholar] [CrossRef]
- Oudhoff, M.J.; Bolscher, J.G.; Nazmi, K.; Kalay, H.; van ‘t Hof, W.; Amerongen, A.V.; Veerman, E.C. Histatins are the major wound-closure stimulating factors in human saliva as identified in a cell culture assay. FASEB J. 2008, 22, 3805–3812. [Google Scholar] [CrossRef]
- van Dijk, I.A.; Nazmi, K.; Bolscher, J.G.; Veerman, E.C.; Stap, J. Histatin-1, a histidine-rich peptide in human saliva, promotes cell-substrate and cell-cell adhesion. FASEB J. 2015, 29, 3124–3132. [Google Scholar] [CrossRef] [PubMed]
- van Dijk, I.A.; Beker, A.F.; Jellema, W.; Nazmi, K.; Wu, G.; Wismeijer, D.; Krawczyk, P.M.; Bolscher, J.G.M.; Veerman, E.C.I.; Stap, J. Histatin 1 Enhances Cell Adhesion to Titanium in an Implant Integration Model. J. Dent. Res. 2017, 96, 430–436. [Google Scholar] [CrossRef] [PubMed]
- Shah, D.; Ali, M.; Shukla, D.; Jain, S.; Aakalu, V.K. Effects of histatin-1 peptide on human corneal epithelial cells. PLoS ONE. 2017, 12, e0178030. [Google Scholar] [CrossRef]
- Oudhoff, M.J.; van den Keijbus, P.A.; Kroeze, K.L.; Nazmi, K.; Gibbs, S.; Bolscher, J.G.; Veerman, E.C.I. Histatins enhance wound closure with oral and non-oral cells. J. Dent. Res. 2009, 88, 846–850. [Google Scholar] [CrossRef] [PubMed]
- Castro, M.; Torres, P.; Solano, L.; Cordova, L.A.; Torres, V.A. Histatin-1 counteracts the cytotoxic and antimigratory effects of zoledronic acid in endothelial and osteoblast-like cells. J. Periodontol. 2019, 90, 766–774. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.Q.; Yi, G.G.; Wu, L.W.; Feng, S.F.; Wu, W.; Peng, L.; Yi, R.W.; Ma, W.; Lu, X. Protective effect of histatin 1 against ultraviolet-induced damage to human corneal epithelial cells. Exp. Ther. Med. 2018, 15, 679–884. [Google Scholar] [CrossRef]
- Torres, P.; Diaz, J.; Arce, M.; Silva, P.; Mendoza, P.; Lois, P.; Molina-Berríos, A.; Owen, G.I.; Palma, V.; Torres, V.A. The salivary peptide histatin-1 promotes endothelial cell adhesion, migration, and angiogenesis. FASEB J. 2017, 31, 4946–4958. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Shi, A.; Shen, C.; Liu, Y.; Wu, G.; Feng, J. Human salivary histatin-1 (Hst1) promotes bone morphogenetic protein 2 (BMP2)-induced osteogenesis and angiogenesis. FEBS Open Bio. 2020, 10, 1503–1515. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Cui, Z.; Urban, J.P. Factors influencing the oxygen concentration gradient from the synovial surface of articular cartilage to the cartilage-bone interface: A modeling study. Arthritis Rheum. 2004, 50, 3915–3924. [Google Scholar] [CrossRef]
- Suzuki, T.; Bessho, K.; Fujimura, K.; Okubo, Y.; Segami, N.; Iizuka, T. Regeneration of defects in the articular cartilage in rabbit temporomandibular joints by bone morphogenetic protein-2. Br. J. Oral. Maxillofac. Surg. 2002, 40, 201–206. [Google Scholar] [CrossRef]
- Haleem, A.M.; Chu, C.R. Advances in Tissue Engineering Techniques for Articular Cartilage Repair. Oper. Tech. Orthop. 2010, 20, 76–89. [Google Scholar] [CrossRef]
- Chu, C.R.; Szczodry, M.; Bruno, S. Animal models for cartilage regeneration and repair. Tissue Eng. Part B Rev. 2010, 16, 105. [Google Scholar] [CrossRef]
- Frassica, M.T.; Grunlan, M.A. Perspectives on Synthetic Materials to Guide Tissue Regeneration for Osteochondral Defect Repair. ACS Biomater. Sci. Eng. 2020, 6, 4324–4336. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.; Zhao, T.; Wang, J.; Wang, C.; Du, J.; Ying, L.; Lin, J.; Zhang, C.; Hu, W.; Wang, L.; et al. Gelatin Methacrylate (GelMA)-Based Hydrogels for Cell Transplantation: An Effective Strategy for Tissue Engineering. Stem. Cell Rev. Rep. 2019, 15, 664–679. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Sun, X.; Wang, Z.; Guo, S.; Yu, G.; Yang, H. Synthesis and Properties of Gelatin Methacryloyl (GelMA) Hydrogels and Their Recent Applications in Load-Bearing Tissue. Polymers 2018, 10, 1290. [Google Scholar] [CrossRef]
- Lam, T.; Dehne, T.; Kruger, J.P.; Hondke, S.; Endres, M.; Thomas, A.; Lauster, R.; Sittinger, M.; Kloke, L. Photopolymerizable gelatin and hyaluronic acid for stereolithographic 3D bioprinting of tissue-engineered cartilage. J. Biomed. Mater. Res. B Appl. Biomater. 2019, 107, 2649–2657. [Google Scholar] [CrossRef]
- Ducy, P.; Karsenty, G. The family of bone morphogenetic proteins. Kidney Int. 2000, 57, 2207–2214. [Google Scholar] [CrossRef]
- Urist, M.R. Bone formation by autoinduction. Science 1965, 150, 893–899. [Google Scholar] [CrossRef]
- Wang, E.A.; Rosen, V.; Cordes, P.; Hewick, R.M.; Kriz, M.J.; Luxenberg, D.P.; Sibley, B.S.; Wozney, J.M. Purification and characterization of other distinct bone-inducing factors. Proc. Natl. Acad. Sci. USA 1988, 85, 9484–9488. [Google Scholar] [CrossRef] [PubMed]
- Bessa, P.C.; Casal, M.; Reis, R.L. Bone morphogenetic proteins in tissue engineering: The road from laboratory to clinic, part II (BMP delivery). J. Tissue Eng. Regen. Med. 2008, 2, 81–96. [Google Scholar] [CrossRef] [PubMed]
- van der Kraan, P.M.; Blaney Davidson, E.N.; van den Berg, W.B. Bone morphogenetic proteins and articular cartilage: To serve and protect or a wolf in sheep clothing’s? Osteoarthr. Cartil. 2010, 18, 735–741. [Google Scholar] [CrossRef] [PubMed]
- Andrades, J.A.; Motaung, S.C.; Jimenez-Palomo, P.; Claros, S.; Lopez-Puerta, J.M.; Becerra, J.; Schmid, T.M.; Reddi, A.H. Induction of superficial zone protein (SZP)/lubricin/PRG 4 in muscle-derived mesenchymal stem/progenitor cells by transforming growth factor-beta1 and bone morphogenetic protein-7. Arthritis Res. Ther. 2012, 14, R72. [Google Scholar] [CrossRef]
- Darling, E.M.; Athanasiou, K.A. Growth factor impact on articular cartilage subpopulations. Cell Tissue Res. 2005, 322, 463–473. [Google Scholar] [CrossRef]
- Eleswarapu, S.V.; Leipzig, N.D.; Athanasiou, K.A. Gene expression of single articular chondrocytes. Cell Tissue Res. 2007, 327, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Coates, E.; Fisher, J.P. Gene expression of alginate-embedded chondrocyte subpopulations and their response to exogenous IGF-1 delivery. J. Tissue Eng. Regen. Med. 2012, 6, 179–192. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Willers, C.; Xu, J.; Zheng, M.-H. The chondrocyte: Biology and clinical application. Tissue Eng. 2006, 12, 1971–1984. [Google Scholar] [CrossRef]
- Sueyoshi, T.; Yamamoto, K.; Akiyama, H. Conditional deletion of Tgfbr2 in hypertrophic chondrocytes delays terminal chondrocyte differentiation. Matrix Biol. 2012, 31, 352–359. [Google Scholar] [CrossRef]
- Niikura, T.; Reddi, A.H. Differential regulation of lubricin/superficial zone protein by transforming growth factor beta/bone morphogenetic protein superfamily members in articular chondrocytes and synoviocytes. Arthritis Rheum. 2007, 56, 2312–2321. [Google Scholar] [CrossRef] [PubMed]
- Mousavizadeh, A.; Jabbari, A.; Akrami, M.; Bardania, H. Cell targeting peptides as smart ligands for targeting of therapeutic or diagnostic agents: A systematic review. Colloids Surf. B Biointerfaces 2017, 158, 507–517. [Google Scholar] [CrossRef]
- Oudhoff, M.J.; Kroeze, K.L.; Nazmi, K.; van den Keijbus, P.A.; van ‘t Hof, W.; Fernandez-Borja, M.; Hordijk, P.L.; Gibbs, S.; Bolscher, J.G.; Veerman, E.C. Structure-activity analysis of histatin, a potent wound healing peptide from human saliva: Cyclization of histatin potentiates molar activity 1000-fold. FASEB J. 2009, 23, 3928–3935. [Google Scholar] [CrossRef] [PubMed]
- van Dijk, I.A.; Ferrando, M.L.; van der Wijk, A.E.; Hoebe, R.A.; Nazmi, K.; de Jonge, W.J.; Krawczyk, P.M.; Bolscher, J.G.; Veerman, E.C.; Stap, J. Human salivary peptide histatin-1 stimulates epithelial and endothelial cell adhesion and barrier function. FASEB J. 2017, 31, 3922–3933. [Google Scholar] [CrossRef]
- Ma, D.; Sun, W.; Nazmi, K.; Veerman, E.C.I.; Bikker, F.J.; Jaspers, R.T.; Jaspers, R.T.; Bolscher, J.G.; Wu, G. Salivary Histatin 1 and 2 Are Targeted to Mitochondria and Endoplasmic Reticulum in Human Cells. Cells 2020, 9, 795. [Google Scholar] [CrossRef] [PubMed]
- Harper, J.; Klagsbrun, M. Cartilage to bone—Angiogenesis leads the way. Nat Med. 1999, 5, 617–618. [Google Scholar] [CrossRef]
- Sun, W.; Ma, D.; Bolscher, J.G.M.; Nazmi, K.; Veerman, E.C.I.; Bikker, F.J.; Sun, P.; Lin, H.; Wu, G. Human Salivary Histatin-1 Promotes Osteogenic Cell Spreading on Both Bio-Inert Substrates and Titanium SLA Surfaces. Front Bioeng Biotechnol. 2020, 8, 584410. [Google Scholar] [CrossRef] [PubMed]
- Torres, P.; Hernandez, N.; Mateluna, C.; Silva, P.; Reyes, M.; Solano, L.; Venegas, S.; Criollo, A.; Nazmi, K.; Bikker, F.J.; et al. Histatin-1 is a novel osteogenic factor that promotes bone cell adhesion, migration, and differentiation. J. Tissue Eng. Regen. Med. 2021, 15, 336–346. [Google Scholar] [CrossRef]
- van den Borne, M.P.; Raijmakers, N.J.; Vanlauwe, J.; Victor, J.; de Jong, S.N.; Bellemans, J.; Saris, D.B.F. International Cartilage Repair Society (ICRS) and Oswestry macroscopic cartilage evaluation scores validated for use in Autologous Chondrocyte Implantation (ACI) and microfracture. Osteoarthr. Cartil. 2007, 15, 1397–1402. [Google Scholar] [CrossRef]
- Bonasia, D.E.; Marmotti, A.; Massa, A.D.; Ferro, A.; Blonna, D.; Castoldi, F.; Rossi, R. Intra- and inter-observer reliability of ten major histological scoring systems used for the evaluation of in vivo cartilage repair. Knee Surg. Sports Traumatol. Arthrosc. 2015, 23, 2484–2493. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, C.; Yao, Y.; Wang, L.; Sun, P.; Feng, J.; Wu, G. Human Salivary Histatin-1-Functionalized Gelatin Methacrylate Hydrogels Promote the Regeneration of Cartilage and Subchondral Bone in Temporomandibular Joints. Pharmaceuticals 2021, 14, 484. https://doi.org/10.3390/ph14050484
Shi C, Yao Y, Wang L, Sun P, Feng J, Wu G. Human Salivary Histatin-1-Functionalized Gelatin Methacrylate Hydrogels Promote the Regeneration of Cartilage and Subchondral Bone in Temporomandibular Joints. Pharmaceuticals. 2021; 14(5):484. https://doi.org/10.3390/ph14050484
Chicago/Turabian StyleShi, Changjing, Yu Yao, Lei Wang, Ping Sun, Jianying Feng, and Gang Wu. 2021. "Human Salivary Histatin-1-Functionalized Gelatin Methacrylate Hydrogels Promote the Regeneration of Cartilage and Subchondral Bone in Temporomandibular Joints" Pharmaceuticals 14, no. 5: 484. https://doi.org/10.3390/ph14050484
APA StyleShi, C., Yao, Y., Wang, L., Sun, P., Feng, J., & Wu, G. (2021). Human Salivary Histatin-1-Functionalized Gelatin Methacrylate Hydrogels Promote the Regeneration of Cartilage and Subchondral Bone in Temporomandibular Joints. Pharmaceuticals, 14(5), 484. https://doi.org/10.3390/ph14050484







