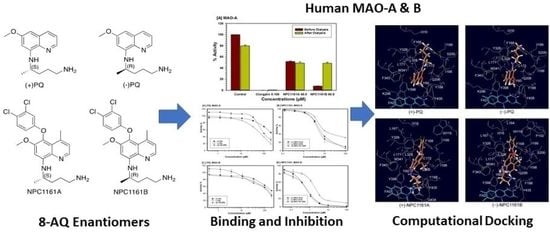
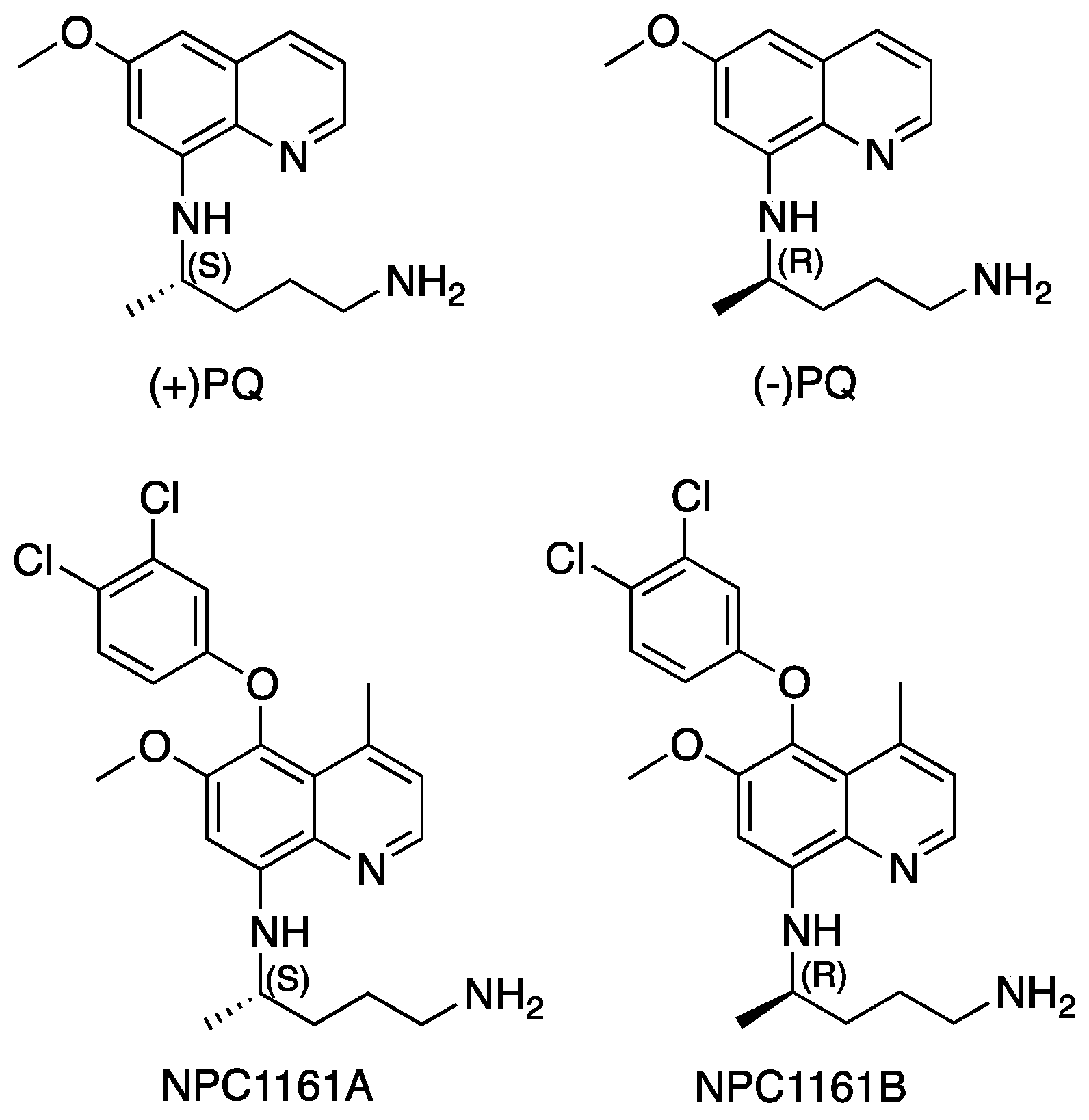
Enantioselective Interactions of Anti-Infective 8-Aminoquinoline Therapeutics with Human Monoamine Oxidases A and B †
Abstract
1. Introduction
2. Results
2.1. Determination of Inhibitory Effects of 8-AQs Analogs on MAO-A and -B
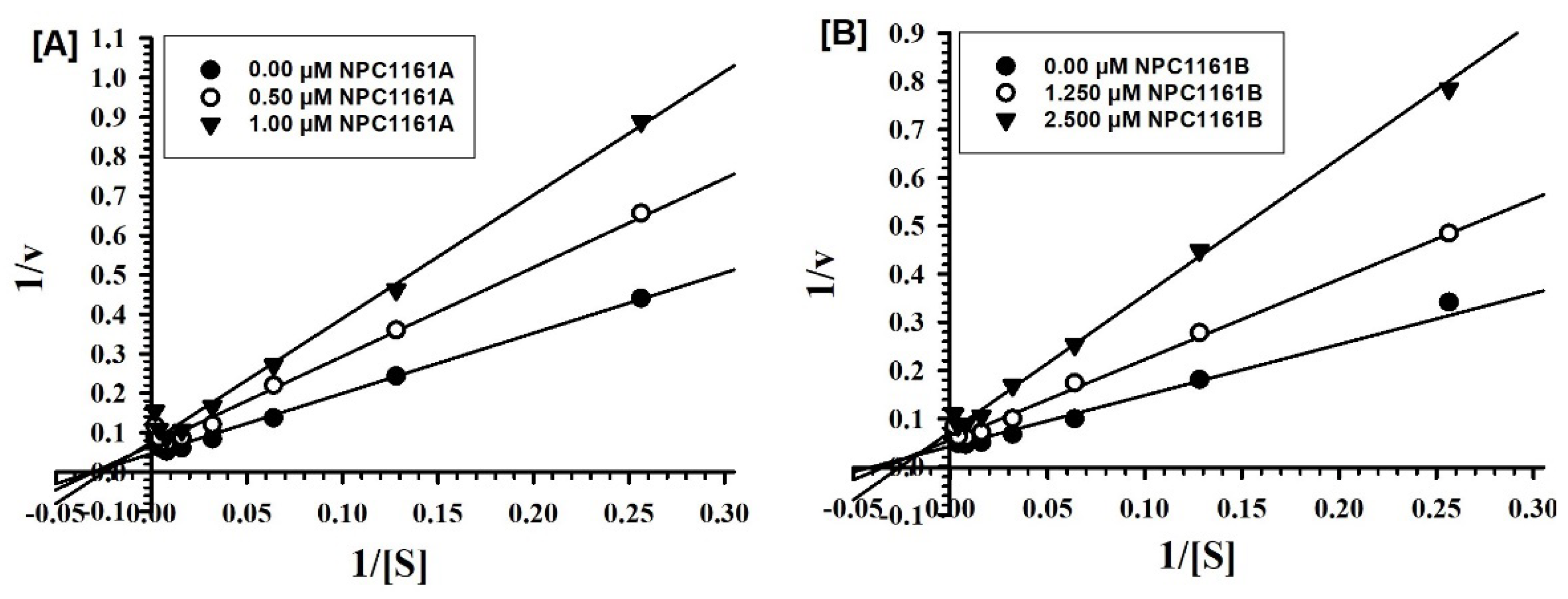
2.2. Enzyme-Inhibition Kinetics for Interaction of 8-AQs with MAO-A and -B
2.3. Enzyme-Inhibition Kinetics for Interaction of 8-AQs with MAO-A and -B
2.4. Computational Analysis of Binding of (S)-(+)-PQ, (R)-(−)-PQ, (S)-(+)-NPC1161A and (R)-(−)-NPC1161B with MAO-A and MAO-B
3. Discussion
4. Materials and Methods
4.1. Reagents and Chemicals
4.2. In Vitro MAO Inhibition Assay
4.3. Determination of IC50 Values
4.4. Enzyme Kinetics and Mechanism Studies
4.5. Analysis of Binding of Inhibitors with the Enzymes
4.6. Computational Docking Methods for PQ, NPC1161 and Their Enantiomers with MAO-A and -B
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Meltzer, E.; Schwartz, E. Utility of 8-Aminoquinolines in Malaria Prophylaxis in Travelers. Curr. Infect. Dis. Rep. 2019, 21, 43. [Google Scholar] [CrossRef]
- Baird, J.K. 8-Aminoquinoline Therapy for Latent Malaria. Clin. Microbiol. Rev. 2019, 32. [Google Scholar] [CrossRef]
- Graves, P.M.; Choi, L.; Gelband, H.; Garner, P. Primaquine or other 8-aminoquinolines for reducing Plasmodium falciparum transmission. Cochrane Database Syst. Rev. 2018, 2, Cd008152. [Google Scholar] [CrossRef]
- Myint, H.Y.; Berman, J.; Walker, L.; Pybus, B.; Melendez, V.; Baird, J.K.; Ohrt, C. Review: Improving the therapeutic index of 8-aminoquinolines by the use of drug combinations: Review of the literature and proposal for future investigations. Am. J. Trop. Med. Hyg. 2011, 85, 1010–1014. [Google Scholar] [CrossRef]
- Tekwani, B.L.; Walker, L.A. 8-Aminoquinolines: Future role as antiprotozoal drugs. Curr. Opin. Infect. Dis. 2006, 19, 623–631. [Google Scholar] [CrossRef]
- Shaw, M.M.; McChesney, J.D.; Nanayakkara, D.; Dias, L.R.; Lee, C.H.; Durant, P.J.; Ball, M.D.; Smith, J.W.; Bartlett, M.S. Modified 8-aminoquinolines cure mouse Pneumocystis carinii infection. J. Eukaryot. Microbiol. 1997, 44, 40s. [Google Scholar] [CrossRef]
- Vale, N.; Collins, M.S.; Gut, J.; Ferraz, R.; Rosenthal, P.J.; Cushion, M.T.; Moreira, R.; Gomes, P. Anti-Pneumocystis carinii and antiplasmodial activities of primaquine-derived imidazolidin-4-ones. Bioorg. Med. Chem. Lett. 2008, 18, 485–488. [Google Scholar] [CrossRef]
- Vela Casasempere, P.; Ruiz Torregrosa, P.; García Sevila, R. Pneumocystis Jirovecii in Immunocompromised Patients with Rheumatic Diseases. Reumatol. Clin. 2020, in press. [Google Scholar] [CrossRef]
- Pavic, K.; Perkovic, I.; Pospisilova, S.; Machado, M.; Fontinha, D.; Prudencio, M.; Jampilek, J.; Coffey, A.; Endersen, L.; Rimac, H.; et al. Primaquine hybrids as promising antimycobacterial and antimalarial agents. Eur. J. Med. Chem. 2018, 143, 769–779. [Google Scholar] [CrossRef]
- Pavic, K.; Rajic, Z.; Michnova, H.; Jampilek, J.; Perkovic, I.; Zorc, B. Second generation of primaquine ureas and bis-ureas as potential antimycobacterial agents. Mol. Divers. 2019, 23, 657–667. [Google Scholar] [CrossRef]
- Ganesan, S.; Tekwani, B.L.; Sahu, R.; Tripathi, L.M.; Walker, L.A. Cytochrome P(450)-dependent toxic effects of primaquine on human erythrocytes. Toxicol. Appl. Pharmacol. 2009, 241, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, S.; Chaurasiya, N.D.; Sahu, R.; Walker, L.A.; Tekwani, B.L. Understanding the mechanisms for metabolism-linked hemolytic toxicity of primaquine against glucose 6-phosphate dehydrogenase deficient human erythrocytes: Evaluation of eryptotic pathway. Toxicology 2012, 294, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Hamerly, T.; Tweedell, R.E.; Hritzo, B.; Nyasembe, V.O.; Tekwani, B.L.; Nanayakkara, N.P.D.; Walker, L.A.; Dinglasan, R.R. NPC1161B, an 8-Aminoquinoline Analog, Is Metabolized in the Mosquito and Inhibits Plasmodium falciparum Oocyst Maturation. Front. Pharmacol. 2019, 10, 1265. [Google Scholar] [CrossRef] [PubMed]
- Camarda, G.; Jirawatcharadech, P.; Priestley, R.S.; Saif, A.; March, S.; Wong, M.H.L.; Leung, S.; Miller, A.B.; Baker, D.A.; Alano, P.; et al. Antimalarial activity of primaquine operates via a two-step biochemical relay. Nat. Commun. 2019, 10, 3226. [Google Scholar] [CrossRef] [PubMed]
- Avula, B.; Tekwani, B.L.; Chaurasiya, N.D.; Fasinu, P.; Dhammika Nanayakkara, N.P.; Bhandara Herath, H.M.T.; Wang, Y.H.; Bae, J.Y.; Khan, S.I.; Elsohly, M.A.; et al. Metabolism of primaquine in normal human volunteers: Investigation of phase I and phase II metabolites from plasma and urine using ultra-high performance liquid chromatography-quadrupole time-of-flight mass spectrometry. Malar. J. 2018, 17, 294. [Google Scholar] [CrossRef] [PubMed]
- Chaves, L.F.; Huber, J.H.; Rojas Salas, O.; Ramírez Rojas, M.; Romero, L.M.; Gutiérrez Alvarado, J.M.; Perkins, T.A.; Prado, M.; Rodríguez, R.M. Malaria Elimination in Costa Rica: Changes in Treatment and Mass Drug Administration. Microorganisms 2020, 8, 984. [Google Scholar] [CrossRef]
- Phommasone, K.; van Leth, F.; Peto, T.J.; Landier, J.; Nguyen, T.N.; Tripura, R.; Pongvongsa, T.; Lwin, K.M.; Kajeechiwa, L.; Thwin, M.M.; et al. Mass drug administrations with dihydroartemisinin-piperaquine and single low dose primaquine to eliminate Plasmodium falciparum have only a transient impact on Plasmodium vivax: Findings from randomised controlled trials. PLoS ONE 2020, 15, e0228190. [Google Scholar] [CrossRef]
- Wickham, K.S.; Baresel, P.C.; Marcsisin, S.R.; Sousa, J.; Vuong, C.T.; Reichard, G.A.; Campo, B.; Tekwani, B.L.; Walker, L.A.; Rochford, R. Single-Dose Primaquine in a Preclinical Model of Glucose-6-Phosphate Dehydrogenase Deficiency: Implications for Use in Malaria Transmission-Blocking Programs. Antimicrob. Agents Chemother. 2016, 60, 5906–5913. [Google Scholar] [CrossRef]
- Chu, C.S.; Bancone, G.; Nosten, F.; White, N.J.; Luzzatto, L. Primaquine-induced haemolysis in females heterozygous for G6PD deficiency. Malar. J. 2018, 17, 101. [Google Scholar] [CrossRef]
- Belfield, K.D.; Tichy, E.M. Review and drug therapy implications of glucose-6-phosphate dehydrogenase deficiency. Am. J. Health Syst. Pharm. 2018, 75, 97–104. [Google Scholar] [CrossRef]
- Uthman, O.A.; Graves, P.M.; Saunders, R.; Gelband, H.; Richardson, M.; Garner, P. Safety of primaquine given to people with G6PD deficiency: Systematic review of prospective studies. Malar. J. 2017, 16, 346. [Google Scholar] [CrossRef] [PubMed]
- Avula, B.; Tekwani, B.L.; Chaurasiya, N.D.; Nanayakkara, N.P.; Wang, Y.H.; Khan, S.I.; Adelli, V.R.; Sahu, R.; Elsohly, M.A.; McChesney, J.D.; et al. Profiling primaquine metabolites in primary human hepatocytes using UHPLC-QTOF-MS with 13C stable isotope labeling. J. Mass Spectrom. 2013, 48, 276–285. [Google Scholar] [CrossRef] [PubMed]
- Fasinu, P.S.; Nanayakkara, N.P.D.; Wang, Y.H.; Chaurasiya, N.D.; Herath, H.M.B.; McChesney, J.D.; Avula, B.; Khan, I.; Tekwani, B.L.; Walker, L.A. Formation primaquine-5,6-orthoquinone, the putative active and toxic metabolite of primaquine via direct oxidation in human erythrocytes. Malar. J. 2019, 18, 30. [Google Scholar] [CrossRef] [PubMed]
- Pybus, B.S.; Marcsisin, S.R.; Jin, X.; Deye, G.; Sousa, J.C.; Li, Q.; Caridha, D.; Zeng, Q.; Reichard, G.A.; Ockenhouse, C.; et al. The metabolism of primaquine to its active metabolite is dependent on CYP 2D6. Malar. J. 2013, 12, 212. [Google Scholar] [CrossRef] [PubMed]
- Constantino, L.; Paixão, P.; Moreira, R.; Portela, M.J.; Do Rosario, V.E.; Iley, J. Metabolism of primaquine by liver homogenate fractions. Evidence for monoamine oxidase and cytochrome P450 involvement in the oxidative deamination of primaquine to carboxyprimaquine. Exp. Toxicol. Pathol. 1999, 51, 299–303. [Google Scholar] [CrossRef]
- Frischer, H.; Mellovitz, R.L.; Ahmad, T.; Nora, M.V. The conversion of primaquine into primaquine-aldehyde, primaquine-alcohol, and carboxyprimaquine, a major plasma metabolite. J. Lab. Clin. Med. 1991, 117, 468–476. [Google Scholar]
- Ariffin, N.M.; Islahudin, F.; Kumolosasi, E.; Makmor-Bakry, M. Effects of MAO-A and CYP450 on primaquine metabolism in healthy volunteers. Parasitol. Res. 2019, 118, 1011–1018. [Google Scholar] [CrossRef]
- Mello, A.; Vieira, M.; Sena, L.W.P.; Paixão, T.P.D.; Pinto, A.C.G.; Grisólia, D.P.A.; Silva, M.T.; Vieira, J.L.F. Levels of primaquine and carboxyprimaquine in patients with malaria vivax from the Brazilian Amazon basin. Rev. Inst. Med. Trop. Sao Paulo 2018, 60, e66. [Google Scholar] [CrossRef]
- Pukrittayakamee, S.; Tarning, J.; Jittamala, P.; Charunwatthana, P.; Lawpoolsri, S.; Lee, S.J.; Hanpithakpong, W.; Hanboonkunupakarn, B.; Day, N.P.; Ashley, E.A.; et al. Pharmacokinetic interactions between primaquine and chloroquine. Antimicrob. Agents Chemother. 2014, 58, 3354–3359. [Google Scholar] [CrossRef]
- Taylor, W.R.J.; Thriemer, K.; von Seidlein, L.; Yuentrakul, P.; Assawariyathipat, T.; Assefa, A.; Auburn, S.; Chand, K.; Chau, N.H.; Cheah, P.Y.; et al. Short-course primaquine for the radical cure of Plasmodium vivax malaria: A multicentre, randomised, placebo-controlled non-inferiority trial. Lancet 2019, 394, 929–938. [Google Scholar] [CrossRef]
- Baird, J.K. Still defining optimal primaquine therapy against relapse after 63 years of continuous use. Travel Med. Infect. Dis. 2015, 13, 215–216. [Google Scholar] [CrossRef] [PubMed]
- Avula, B.; Khan, S.I.; Tekwani, B.L.; Nanayakkara, N.P.; McChesney, J.D.; Walker, L.A.; Khan, I.A. Analysis of primaquine and its metabolite carboxyprimaquine in biological samples: Enantiomeric separation, method validation and quantification. Biomed. Chromatogr. 2011, 25, 1010–1017. [Google Scholar] [CrossRef]
- Leven, M.; Held, J.; Duffy, S.; Alves Avelar, L.A.; Meister, S.; Delves, M.; Plouffe, D.; Kuna, K.; Tschan, S.; Avery, V.M.; et al. 8-Aminoquinolines with an Aminoxyalkyl Side Chain Exert in vitro Dual-Stage Antiplasmodial Activity. ChemMedChem 2019, 14, 501–511. [Google Scholar] [CrossRef] [PubMed]
- Vandekerckhove, S.; Van Herreweghe, S.; Willems, J.; Danneels, B.; Desmet, T.; de Kock, C.; Smith, P.J.; Chibale, K.; D’Hooghe, M. Synthesis of functionalized 3-, 5-, 6- and 8-aminoquinolines via intermediate (3-pyrrolin-1-yl)- and (2-oxopyrrolidin-1-yl)quinolines and evaluation of their antiplasmodial and antifungal activity. Eur. J. Med. Chem. 2015, 92, 91–102. [Google Scholar] [CrossRef]
- Kaur, K.; Jain, M.; Khan, S.I.; Jacob, M.R.; Tekwani, B.L.; Singh, S.; Singh, P.P.; Jain, R. Synthesis, antiprotozoal, antimicrobial, β-hematin inhibition, cytotoxicity and methemoglobin (MetHb) formation activities of bis(8-aminoquinolines). Bioorgan. Med. Chem. 2011, 19, 197–210. [Google Scholar] [CrossRef]
- Nanayakkara, N.P.; Ager, A.L., Jr.; Bartlett, M.S.; Yardley, V.; Croft, S.L.; Khan, I.A.; McChesney, J.D.; Walker, L.A. Antiparasitic activities and toxicities of individual enantiomers of the 8-aminoquinoline 8-[(4-amino-1-methylbutyl)amino]-6-methoxy-4-methyl-5-[3,4-dichlorophenoxy]quinoline succinate. Antimicrob. Agents Chemother. 2008, 52, 2130–2137. [Google Scholar] [CrossRef]
- Nan, A.; Nanayakkara, N.P.; Walker, L.A.; Yardley, V.; Croft, S.L.; Ghandehari, H. N-(2-hydroxypropyl)methacrylamide (HPMA) copolymers for targeted delivery of 8-aminoquinoline antileishmanial drugs. J. Control Release 2001, 77, 233–243. [Google Scholar] [CrossRef]
- Nanayakkara, N.P.; Tekwani, B.L.; Herath, H.M.; Sahu, R.; Gettayacamin, M.; Tungtaeng, A.; van Gessel, Y.; Baresel, P.; Wickham, K.S.; Bartlett, M.S.; et al. Scalable preparation and differential pharmacologic and toxicologic profiles of primaquine enantiomers. Antimicrob. Agents Chemother. 2014, 58, 4737–4744. [Google Scholar] [CrossRef]
- Brocks, D.R.; Mehvar, R. Stereoselectivity in the pharmacodynamics and pharmacokinetics of the chiral antimalarial drugs. Clin. Pharmacokinet. 2003, 42, 1359–1382. [Google Scholar] [CrossRef]
- Agarwal, S.; Gupta, U.R.; Daniel, C.S.; Gupta, R.C.; Anand, N.; Agarwal, S.S. Susceptibility of glucose-6-phosphate dehydrogenase deficient red cells to primaquine, primaquine enantiomers, and its two putative metabolites. II. Effect on red blood cell membrane, lipid peroxidation, MC-540 staining, and scanning electron microscopic studies. Biochem. Pharmacol. 1991, 41, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Saunders, D.; Vanachayangkul, P.; Imerbsin, R.; Khemawoot, P.; Siripokasupkul, R.; Tekwani, B.L.; Sampath, A.; Nanayakkara, N.P.; Ohrt, C.; Lanteri, C.; et al. Pharmacokinetics and pharmacodynamics of (+)-primaquine and (-)-primaquine enantiomers in rhesus macaques (Macaca mulatta). Antimicrob. Agents Chemother. 2014, 58, 7283–7291. [Google Scholar] [CrossRef]
- Fasinu, P.S.; Avula, B.; Tekwani, B.L.; Nanayakkara, N.P.; Wang, Y.H.; Bandara Herath, H.M.; McChesney, J.D.; Reichard, G.A.; Marcsisin, S.R.; Elsohly, M.A.; et al. Differential kinetic profiles and metabolism of primaquine enantiomers by human hepatocytes. Malar. J. 2016, 15, 224. [Google Scholar] [CrossRef]
- Fasinu, P.S.; Tekwani, B.L.; Nanayakkara, N.P.; Avula, B.; Herath, H.M.; Wang, Y.H.; Adelli, V.R.; Elsohly, M.A.; Khan, S.I.; Khan, I.A.; et al. Enantioselective metabolism of primaquine by human CYP2D6. Malar. J. 2014, 13, 507. [Google Scholar] [CrossRef] [PubMed]
- Fasinu, P.S.; Tekwani, B.L.; Avula, B.; Chaurasiya, N.D.; Nanayakkara, N.P.; Wang, Y.H.; Khan, I.A.; Walker, L.A. Pathway-specific inhibition of primaquine metabolism by chloroquine/quinine. Malar. J. 2016, 15, 466. [Google Scholar] [CrossRef] [PubMed]
- Tekwani, B.L.; Avula, B.; Sahu, R.; Chaurasiya, N.D.; Khan, S.I.; Jain, S.; Fasinu, P.S.; Herath, H.M.; Stanford, D.; Nanayakkara, N.P.; et al. Enantioselective pharmacokinetics of primaquine in healthy human volunteers. Drug Metab. Dispos. 2015, 43, 571–577. [Google Scholar] [CrossRef]
- Chairat, K.; Jittamala, P.; Hanboonkunupakarn, B.; Pukrittayakamee, S.; Hanpithakpong, W.; Blessborn, D.; White, N.J.; Day, N.P.J.; Tarning, J. Enantiospecific pharmacokinetics and drug-drug interactions of primaquine and blood-stage antimalarial drugs. J. Antimicrob. Chemother. 2018, 73, 3102–3113. [Google Scholar] [CrossRef] [PubMed]
- Edmondson, D.E.; Binda, C. Monoamine Oxidases. Subcell. Biochem. 2018, 87, 117–139. [Google Scholar] [CrossRef]
- Ramsay, R.R. Molecular aspects of monoamine oxidase B. Prog. Neuropsychopharmacol. Biol. Psychiatry 2016, 69, 81–89. [Google Scholar] [CrossRef]
- Wang, C.C.; Billett, E.; Borchert, A.; Kuhn, H.; Ufer, C. Monoamine oxidases in development. Cell. Mol. Life Sci. 2013, 70, 599–630. [Google Scholar] [CrossRef]
- Benedetti, M.S. Biotransformation of xenobiotics by amine oxidases. Fundam. Clin. Pharmacol. 2001, 15, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Gong, B.; Boor, P.J. The role of amine oxidases in xenobiotic metabolism. Expert Opin. Drug Metab. Toxicol. 2006, 2, 559–571. [Google Scholar] [CrossRef] [PubMed]
- Strolin Benedetti, M.; Whomsley, R.; Baltes, E. Involvement of enzymes other than CYPs in the oxidative metabolism of xenobiotics. Expert Opin. Drug Metab. Toxicol. 2006, 2, 895–921. [Google Scholar] [CrossRef]
- Imamura, Y.; Wu, X.; Noda, A.; Noda, H. Side-chain metabolism of propranolol: Involvement of monoamine oxidase and mitochondrial aldehyde dehydrogenase in the metabolism of N-desisopropylpropranolol to naphthoxylactic acid in rat liver. Life Sci. 2002, 70, 2687–2697. [Google Scholar] [CrossRef]
- Obach, R.S.; Cox, L.M.; Tremaine, L.M. Sertraline is metabolized by multiple cytochrome P450 enzymes, monoamine oxidases, and glucuronyl transferases in human: An in vitro study. Drug Metab. Dispos. 2005, 33, 262–270. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, S.; Ito, Y.; Fukushima, T.; Sugawara, Y.; Ohashi, M. Metabolism of diltiazem. III. Oxidative deamination of diltiazem in rat liver microsomes. J. Pharmacobiodyn. 1990, 13, 612–621. [Google Scholar] [CrossRef]
- Sanchez-Spitman, A.B.; Swen, J.J.; Dezentje, V.O.; Moes, D.; Gelderblom, H.; Guchelaar, H.J. Clinical pharmacokinetics and pharmacogenetics of tamoxifen and endoxifen. Expert Rev. Clin. Pharmacol. 2019, 12, 523–536. [Google Scholar] [CrossRef]
- Pybus, B.S.; Sousa, J.C.; Jin, X.; Ferguson, J.A.; Christian, R.E.; Barnhart, R.; Vuong, C.; Sciotti, R.J.; Reichard, G.A.; Kozar, M.P.; et al. CYP450 phenotyping and accurate mass identification of metabolites of the 8-aminoquinoline, anti-malarial drug primaquine. Malar. J. 2012, 11, 259. [Google Scholar] [CrossRef]
- Brossi, A.; Millet, P.; Landau, I.; Bembenek, M.E.; Abell, C.W. Antimalarial activity and inhibition of monoamine oxidases A and B by exo-erythrocytic antimalarials. Optical isomers of primaquine, N-acylated congeners, primaquine metabolites and 5-phenoxy-substituted analogues. FEBS Lett. 1987, 214, 291–294. [Google Scholar] [CrossRef]
- Chaurasiya, N.D.; Ganesan, S.; Nanayakkara, N.P.; Dias, L.R.; Walker, L.A.; Tekwani, B.L. Inhibition of human monoamine oxidase A and B by 5-phenoxy 8-aminoquinoline analogs. Bioorg. Med. Chem. Lett. 2012, 22, 1701–1704. [Google Scholar] [CrossRef]
- Robinson, J.B. Stereoselectivity and isoenzyme selectivity of monoamine oxidase inhibitors. Enantiomers of amphetamine, N-methylamphetamine and deprenyl. Biochem. Pharmacol. 1985, 34, 4105–4108. [Google Scholar] [CrossRef]
- Kosel, M.; Gnerre, C.; Voirol, P.; Amey, M.; Rochat, B.; Bouras, C.; Testa, B.; Baumann, P. In vitro biotransformation of the selective serotonin reuptake inhibitor citalopram, its enantiomers and demethylated metabolites by monoamine oxidase in rat and human brain preparations. Mol. Psychiatry 2002, 7, 181–188. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Knez, D.; Colettis, N.; Iacovino, L.G.; Sova, M.; Pislar, A.; Konc, J.; Lesnik, S.; Higgs, J.; Kamecki, F.; Mangialavori, I.; et al. Stereoselective Activity of 1-Propargyl-4-styrylpiperidine-like Analogues That Can Discriminate between Monoamine Oxidase Isoforms A and B. J. Med. Chem. 2020, 63, 1361–1387. [Google Scholar] [CrossRef] [PubMed]
- Pandey, P.; Chaurasiya, N.D.; Tekwani, B.L.; Doerksen, R.J. Interactions of endocannabinoid virodhamine and related analogs with human monoamine oxidase-A and -B. Biochem. Pharmacol. 2018, 155, 82–91. [Google Scholar] [CrossRef]
- De Colibus, L.; Li, M.; Binda, C.; Lustig, A.; Edmondson, D.E.; Mattevi, A. Three-dimensional structure of human monoamine oxidase A (MAO A): Relation to the structures of rat MAO A and human MAO B. Proc. Natl. Acad. Sci. USA 2005, 102, 12684–12689. [Google Scholar] [CrossRef]
- Jin, X.; Pybus, B.S.; Marcsisin, R.; Logan, T.; Luong, T.L.; Sousa, J.; Matlock, N.; Collazo, V.; Asher, C.; Carroll, D.; et al. An LC-MS based study of the metabolic profile of primaquine, an 8-aminoquinoline antiparasitic drug, with an in vitro primary human hepatocyte culture model. Eur. J. Drug Metab. Pharmacokinet. 2014, 39, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Zottig, V.E.; Carr, K.A.; Clarke, J.G.; Shmuklarsky, M.J.; Kreishman-Deitrick, M. Army Antimalarial Drug Development: An Advanced Development Case Study for Tafenoquine. Mil. Med. 2020, 185, 617–623. [Google Scholar] [CrossRef]
- Berman, J.D. Approval of Tafenoquine for Malaria Chemoprophylaxis. Am. J. Trop. Med. Hyg. 2019, 100, 1301–1304. [Google Scholar] [CrossRef]
- Commons, R.J.; McCarthy, J.S.; Price, R.N. Tafenoquine for the radical cure and prevention of malaria: The importance of testing for G6PD deficiency. Med. J. Aust. 2020, 212, 152–153. [Google Scholar] [CrossRef]
- Matsumoto, T.; Suzuki, O.; Furuta, T.; Asai, M.; Kurokawa, Y.; Nimura, Y.; Katsumata, Y.; Takahashi, I. A sensitive fluorometric assay for serum monoamine oxidase with kynuramine as substrate. Clin. Biochem. 1985, 18, 126–129. [Google Scholar] [CrossRef]
- Chaurasiya, N.D.; Zhao, J.; Pandey, P.; Doerksen, R.J.; Muhammad, I.; Tekwani, B.L. Selective Inhibition of Human Monoamine Oxidase B by Acacetin 7-Methyl Ether Isolated from Turnera diffusa (Damiana). Molecules 2019, 24, 810. [Google Scholar] [CrossRef]
- Glide; Version 5.7; Schrödinger LLC: New York, NY, USA, 2011.
- Son, S.Y.; Ma, J.; Kondou, Y.; Yoshimura, M.; Yamashita, E.; Tsukihara, T. Structure of human monoamine oxidase A at 2.2-A resolution: The control of opening the entry for substrates/inhibitors. Proc. Natl. Acad. Sci. USA 2008, 105, 5739–5744. [Google Scholar] [CrossRef]
- Binda, C.; Li, M.; Hubalek, F.; Restelli, N.; Edmondson, D.E.; Mattevi, A. Insights into the mode of inhibition of human mitochondrial monoamine oxidase B from high-resolution crystal structures. Proc. Natl. Acad. Sci. USA 2003, 100, 9750–9755. [Google Scholar] [CrossRef]
- Gaspar, A.; Teixeira, F.; Uriarte, E.; Milhazes, N.; Melo, A.; Cordeiro, M.N.; Ortuso, F.; Alcaro, S.; Borges, F. Towards the discovery of a novel class of monoamine oxidase inhibitors: Structure-property-activity and docking studies on chromone amides. ChemMedChem 2011, 6, 628–632. [Google Scholar] [CrossRef] [PubMed]
- Chimenti, F.; Secci, D.; Bolasco, A.; Chimenti, P.; Bizzarri, B.; Granese, A.; Carradori, S.; Yanez, M.; Orallo, F.; Ortuso, F.; et al. Synthesis, molecular modeling, and selective inhibitory activity against human monoamine oxidases of 3-carboxamido-7-substituted coumarins. J. Med. Chem. 2009, 52, 1935–1942. [Google Scholar] [CrossRef] [PubMed]
- Gokhan-Kelekci, N.; Simsek, O.O.; Ercan, A.; Yelekci, K.; Sahin, Z.S.; Isik, S.; Ucar, G.; Bilgin, A.A. Synthesis and molecular modeling of some novel hexahydroindazole derivatives as potent monoamine oxidase inhibitors. Bioorganic Med. Chem. 2009, 17, 6761–6772. [Google Scholar] [CrossRef] [PubMed]
- Harkcom, W.T.; Bevan, D.R. Molecular docking of inhibitors into monoamine oxidase B. Biochem. Biophys. Res. Commun. 2007, 360, 401–406. [Google Scholar] [CrossRef]





| 8-Aminoquinolines | MAO -A IC50 (µM) ± SD | MAO -B IC50 (µM) ± SD |
|---|---|---|
| (RS)-(±)-Primaquine | 87.83. ± 3.049 | 106.75 ± 1.181 |
| (S)-(+)-Primaquine | >200.00 | 170.00 ± 15.27 |
| (R)-(−)-Primaquine | 74.33 ± 2.96 | 116.66 ± 8.819 |
| (RS)-(±)-NPC1161 | 6.18 ± 0.361 | 1.68 ± 0.024 |
| (S)-(+)-NPC1161A | 5.24 ± 0.112 | 0.54 ± 0.012 |
| (R)-(−)-NPC1161B | 9.70 ± 0.178 | 2.93 ± 0.017 |
| Harmine | 0.0051 ± 0.0001 | 53.08 ± 3.915 |
| Safinamide | 88.00 ± 1.414 | 0.058 ± 0.002 |
| Clorgyline [63] | 0.004 ± 0.0005 | - |
| Deprenyl [63] | - | 0.49 ± 0.0036 |
| Compound | MAO-A | MAO-B | ||
|---|---|---|---|---|
| Ki (µM) ± SD | Type of Inhibition | Ki (µM) ± SD | Type of Inhibition | |
| (S)-(+)-NPC1161A | 3.298± 0.508 | Mixed type/ irreversible | 0.294 ± 0.080 | Mixed type/ irreversible |
| (R)-(−)-NPC1161B | 3.993± 0.640 | Mixed type/ reversible | 1.385± 0.284 | Mixed type/ reversible |
| Compound | Docking Scores | |
|---|---|---|
| MAO-A | MAO-B | |
| (S)-(+)-PQ | −8.523 | −8.220 |
| (R)-(−)-PQ | −8.822 | −8.565 |
| (S)-(+)-NPC1161A | −8.983 | −9.518 |
| (R)-(−)-NPC1161B | −8.968 | −9.234 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chaurasiya, N.D.; Liu, H.; Doerksen, R.J.; Nanayakkara, N.P.D.; Walker, L.A.; Tekwani, B.L. Enantioselective Interactions of Anti-Infective 8-Aminoquinoline Therapeutics with Human Monoamine Oxidases A and B. Pharmaceuticals 2021, 14, 398. https://doi.org/10.3390/ph14050398
Chaurasiya ND, Liu H, Doerksen RJ, Nanayakkara NPD, Walker LA, Tekwani BL. Enantioselective Interactions of Anti-Infective 8-Aminoquinoline Therapeutics with Human Monoamine Oxidases A and B. Pharmaceuticals. 2021; 14(5):398. https://doi.org/10.3390/ph14050398
Chicago/Turabian StyleChaurasiya, Narayan D., Haining Liu, Robert J. Doerksen, N. P. Dhammika Nanayakkara, Larry A. Walker, and Babu L. Tekwani. 2021. "Enantioselective Interactions of Anti-Infective 8-Aminoquinoline Therapeutics with Human Monoamine Oxidases A and B" Pharmaceuticals 14, no. 5: 398. https://doi.org/10.3390/ph14050398
APA StyleChaurasiya, N. D., Liu, H., Doerksen, R. J., Nanayakkara, N. P. D., Walker, L. A., & Tekwani, B. L. (2021). Enantioselective Interactions of Anti-Infective 8-Aminoquinoline Therapeutics with Human Monoamine Oxidases A and B. Pharmaceuticals, 14(5), 398. https://doi.org/10.3390/ph14050398