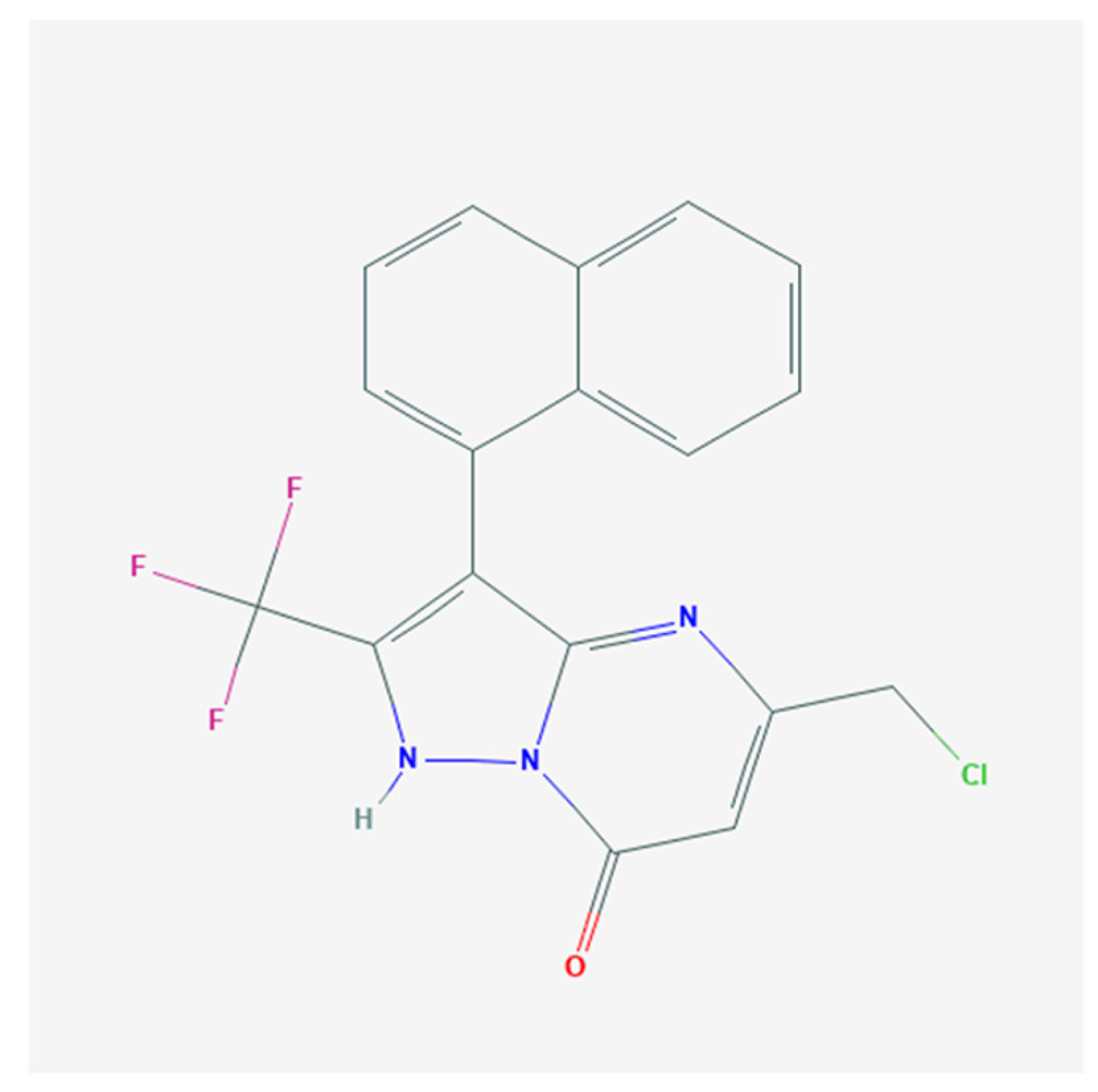
Effective Activation of BKCa Channels by QO-40 (5-(Chloromethyl)-3-(Naphthalen-1-yl)-2-(Trifluoromethyl)Pyrazolo [1,5-a]pyrimidin-7(4H)-one), Known to Be an Opener of KCNQ2/Q3 Channels
Abstract
1. Introduction
2. Results
2.1. Stimulatory Effect of QO-40 on IK(Ca) Recorded from Pituitary GH3 Lactotrophs
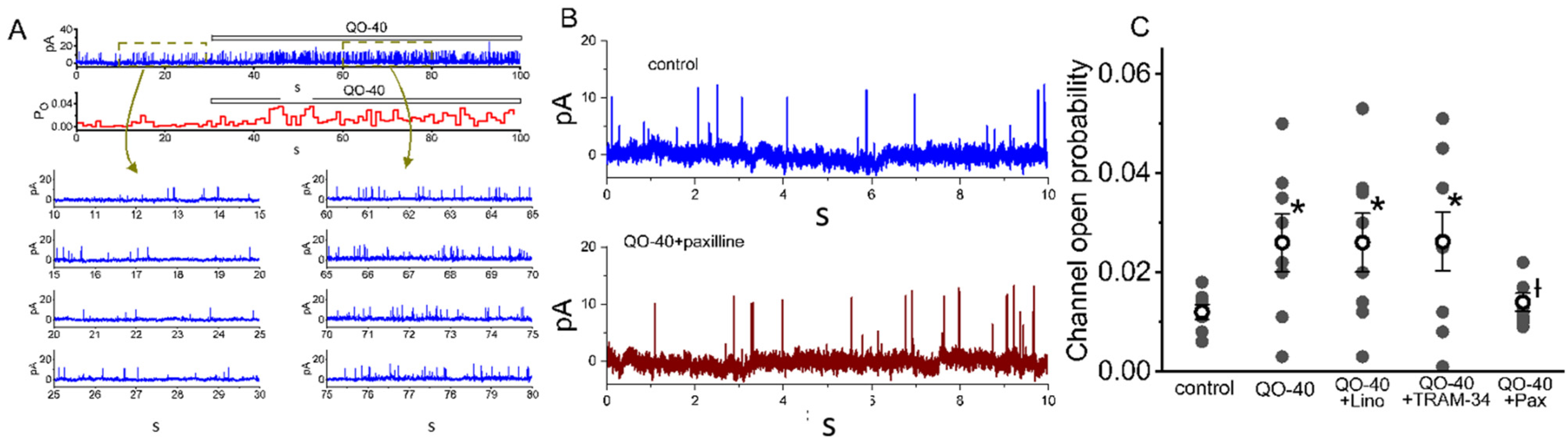
2.2. Comparisons among the Effects of QO-40, QO-40 Plus Linopirdine, QO-40 Plus TRAM-34, QO-40 Plus GAL-021 and QO-40 Plus Paxilline on IK(Ca) Amplitude in GH3 Cells
2.3. Stimulatory Effect of QO-40 on Large-Conductance Ca2+-Activated K+ (BKCa) Channels in GH3 Cells
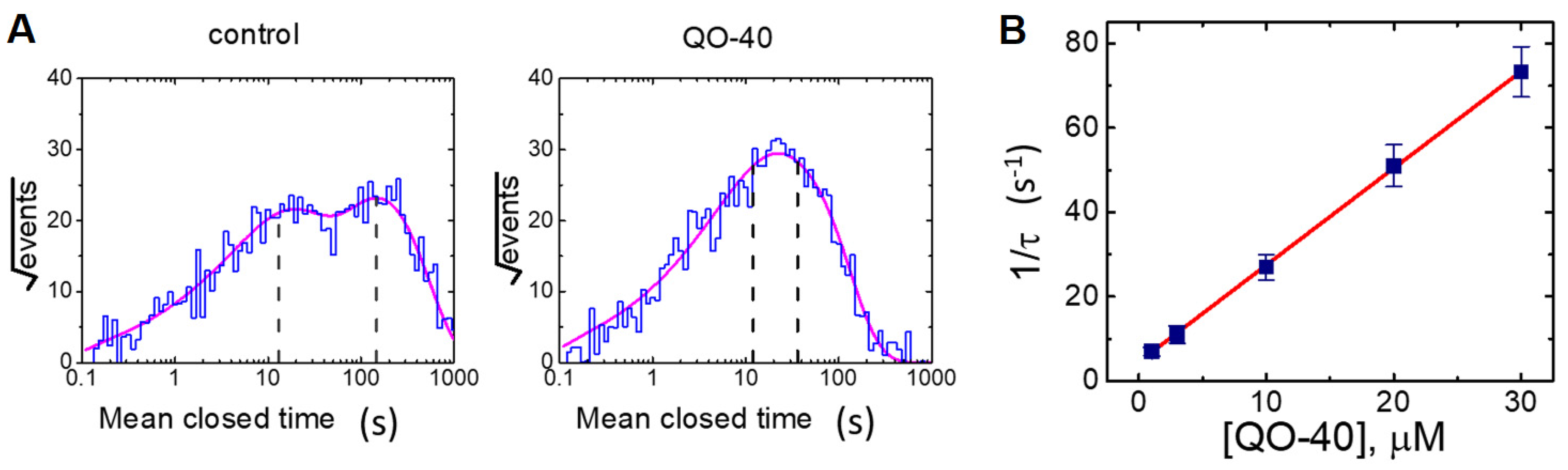
2.4. Effect of QO-40 on Kinetic Behavior of BKCa Channels
2.5. Effect of QO-40 on the Steady-State Activation Curve of BKCa Channels in GH3 Cells
2.6. Effect of QO-40 on the Voltage-Dependent Hysteresis of BK-Channel Activity Elicited in Response to a Long-Lasting Isosceles-Triangular Ramp Pulse
3. Discussion
4. Materials and Methods
4.1. Chemicals and Solutions Used in This Work
4.2. Cell Preparations
4.3. Electrophysiological Recordings
4.4. Data Analyses
4.5. Single-Channel Analyses
4.6. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Jia, C.; Qi, J.; Zhang, F.; Mi, Y.; Zhang, X.; Chen, X.; Liu, L.; Du, X.; Zhang, H. Activation of KCNQ2/3 potassium channels by novel pyrazolo[1,5-a]pyrimidin-7(4H)-one derivatives. Pharmacology 2011, 87, 297–310. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, Y.; Hou, P.; Yan, Z.; Kong, W.; Liu, B.; Li, X.; Yao, J.; Zhang, Y.; Qin, F.; et al. TRPV1 channels are functionally coupled with BK(mSlo1) channels in rat dorsal root ganglion (DRG) neurons. PLoS ONE 2013, 8, e78203. [Google Scholar] [CrossRef]
- Teng, B.C.; Song, Y.; Zhang, F.; Ma, T.Y.; Qi, J.L.; Zhang, H.L.; Li, G.; Wang, K. Activation of neuronal Kv7/KCNQ/M-channels by the opener QO58-lysine and its anti-nociceptive effects on inflammatory pain in rodents. Acta Pharmacol. Sin. 2016, 37, 1054–1062. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Mi, Y.; Qi, J.L.; Li, J.W.; Si, M.; Guan, B.C.; Du, X.N.; An, H.L.; Zhang, H.L. Modulation of Kv7 potassium channels by a novel opener pyrazolo[1,5-a]pyrimidin-7(4H)-one compound QO-58. Br. J. Pharmacol. 2013, 168, 1030–1042. [Google Scholar] [CrossRef]
- Zhang, F.; Liu, Y.; Zhang, D.; Fan, X.; Shao, D.; Li, H. Suppression of KCNQ/M potassium channel in dorsal root ganglia neurons contributes to the development of osteoarthritic pain. Pharmacology 2019, 103, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Gao, H.; Jaffe, D.; Zhang, H.; Gamper, N. M-type K+ channels in peripheral nociceptive pathways. Br. J. Pharmacol. 2018, 175, 2158–2172. [Google Scholar] [CrossRef]
- Wu, S.N.; Wang, Y.J.; Lin, M.W. Potent stimulation of large-conductance Ca2+-activated K+ channels by rottlerin, an inhibitor of protein kinase C-δ, in pituitary tumor (GH3) cells and in cortical neuronal (HCN-1A) cells. J. Cell Physiol. 2007, 210, 655–666. [Google Scholar] [CrossRef]
- Gold, M.S.; Gebhart, G.F. Nociceptor sensitization in pain pathogenesis. Nat. Med. 2010, 16, 1248–1257. [Google Scholar] [CrossRef]
- Bentzen, B.H.; Olesen, S.P.; Ronn, L.C.; Grunnet, M. BK channel activators and their therapeutic perspectives. Front. Physiol. 2014, 5, 389. [Google Scholar] [CrossRef] [PubMed]
- Morera, F.J.; Saravia, J.; Pontigo, J.P.; Vargas-Chacoff, L.; Contreras, G.F.; Pupo, A.; Lorenzo, Y.; Castillo, K.; Tilegenova, C.; Cuello, L.G.; et al. Voltage-dependent BK and Hv1 channels expressed in non-excitable tissues: New therapeutics opportunities as targets in human diseases. Pharmacol. Res. 2015, 101, 56–64. [Google Scholar] [CrossRef]
- Kshatri, A.S.; Gonzalez-Hernandez, A.; Giraldez, T. Physiological roles and therapeutic potential of Ca2+ activated potassium channels in the nervous system. Front. Mol. Neurosci. 2018, 11, 258. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.N. Large-conductance Ca2+- activated K+ channels: Physiological role and pharmacology. Curr. Med. Chem. 2003, 10, 649–661. [Google Scholar] [CrossRef]
- Hewawasam, P.; Ding, M.; Chen, N.; King, D.; Knipe, J.; Pajor, L.; Ortiz, A.; Gribkoff, V.K.; Starrett, J. Synthesis of water-soluble prodrugs of BMS-191011: A maxi-K channel opener targeted for post-stroke neuroprotection. Bioorg. Med. Chem. Lett. 2003, 13, 1695–1698. [Google Scholar] [CrossRef]
- Korsgaard, M.P.; Hartz, B.P.; Brown, W.D.; Ahring, P.K.; Strobaek, D.; Mirza, N.R. Anxiolytic effects of Maxipost (BMS-204352) and retigabine via activation of neuronal Kv7 channels. J. Pharmacol. Exp. Ther. 2005, 314, 282–292. [Google Scholar] [CrossRef] [PubMed]
- Hsu, H.T.; Tseng, Y.T.; Lo, Y.C.; Wu, S.N. Ability of naringenin, a bioflavonoid, to activate M-type potassium current in motor neuron-like cells and to increase BKCa-channel activity in HEK293T cells transfected with α-hSlo subunit. BMC Neurosci. 2014, 15, 135. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.; Zhang, F.; Mi, Y.; Fu, Y.; Xu, W.; Zhang, D.; Wu, Y.; Du, X.; Jia, Q.; Wang, K.; et al. Design, synthesis and biological activity of pyrazolo[1,5-a]pyrimidin-7(4H)-ones as novel Kv7/KCNQ potassium channel activators. Eur. J. Med. Chem. 2011, 46, 934–943. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.N.; Chern, J.H.; Shen, S.; Chen, H.H.; Hsu, Y.T.; Lee, C.C.; Chan, M.H.; Lai, M.C.; Shie, F.S. Stimulatory actions of a novel thiourea derivative on large-conductance, calcium-activated potassium channels. J. Cell Physiol. 2017, 232, 3409–3421. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.N.; Hwang, T.; Teng, C.M.; Li, H.F.; Jan, C.R. The mechanism of actions of 3-(5’-(hydroxymethyl-2’-furyl)-1-benzyl indazole (YC-1) on Ca2+-activated K+ currents in GH3 lactotrophs. Neuropharmacology 2000, 39, 1788–1799. [Google Scholar] [CrossRef]
- Wu, S.N.; Lo, Y.K.; Li, H.F.; Shen, A.Y. Functional coupling of voltage-dependent L-type Ca2+ current to Ca2+-activated K+ current in pituitary GH3 cells. Chin. J. Physiol. 2001, 44, 161–167. [Google Scholar]
- Olesen, S.P.; Munch, E.; Moldt, P.; Drejer, J. Selective activation of Ca2+-dependent K+ channels by novel benzimidazolone. Eur. J. Pharmacol. 1994, 251, 53–59. [Google Scholar] [CrossRef]
- Knaus, H.G.; McManus, O.B.; Lee, S.H.; Schmalhofer, W.A.; Garcia-Calvo, M.; Helms, L.M.; Sanchez, M.; Giangiacomo, K.; Reuben, J.P.; Smith, A.B., 3rd; et al. Tremorgenic indole alkaloids potently inhibit smooth muscle high-conductance calcium-activated potassium channels. Biochemistry 1994, 33, 5819–5828. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.C.; Ruan, J.S.; Wu, S.N. Evidence of dcreased ativity in itermediate-cnductance clcium-ativated ptassium cannels during retinoic acid-induced differentiation in motor neuron-like NSC-34 cells. Cell Physiol. Biochem. 2018, 48, 2374–2388. [Google Scholar] [CrossRef] [PubMed]
- Yeh, P.S.; Wu, S.J.; Hung, T.Y.; Huang, Y.M.; Hsu, C.W.; Sze, C.I.; Hsieh, Y.J.; Huang, C.W.; Wu, S.N. Evidence for the inhibition by temozolomide, an imidazotetrazine family alkylator, of intermediate-conductance Ca2+-activated K+ channels in glioma cells. Cell Physiol. Biochem. 2016, 38, 1727–1742. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.L.; Gao, Z.H.; Li, S.W.; Wu, S.N. High efficacy by GAL-021: A known intravenous peripheral chemoreceptor modulator that suppresses BKCa-channel activity and inhibits IK(M) or Ih. Biomolecules 2020, 10, 188. [Google Scholar] [CrossRef]
- Dallas, M.L.; Peers, C.; Golder, F.J.; Baby, S.; Gruber, R.; MacIntyre, D.E. GAL-021 and GAL-160 are efficacious in rat models of obstructive and central sleep apnea and inhibit BKCa in isolated rat carotid body glomus cells. Adv. Exp. Med. Biol. 2015, 860, 361–370. [Google Scholar] [CrossRef]
- Wu, S.N.; Li, H.F.; Jan, C.R.; Shen, A.Y. Inhibition of Ca2+-activated K+ current by clotrimazole in rat anterior pituitary GH3 cells. Neuropharmacology 1999, 38, 979–989. [Google Scholar] [CrossRef]
- Mannikko, R.; Pandey, S.; Larsson, H.P.; Elinder, F. Hysteresis in the voltage dependence of HCN channels: Conversion between two modes affects pacemaker properties. J. Gen. Physiol. 2005, 125, 305–326. [Google Scholar] [CrossRef]
- Villalba-Galea, C.A. Hysteresis in voltage-gated channels. Channels 2017, 11, 140–155. [Google Scholar] [CrossRef]
- Wu, S.N.; Liu, S.I.; Huang, M.H. Cilostazol, an inhibitor of type 3 phosphodiesterase, stimulates large-conductance, calcium-activated potassium channels in pituitary GH3 cells and pheochromocytoma PC12 cells. Endocrinology 2004, 145, 1175–1184. [Google Scholar] [CrossRef]
- Liu, C.; Jinlong, Q.I.; Zhang, H.; Jia, Q. Pharmacokinetic study of QO-58: A new potassium channel opener. Chin. Pharmacol. Bull. 2014, 30, 574–577. [Google Scholar]
- Zhang, X.L.; Mok, L.P.; Lee, K.Y.; Charbonnet, M.; Gold, M.S. Inflammation-induced changes in BKCa currents in cutaneous dorsal root ganglion neurons from the adult rat. Mol. Pain 2012, 8, 37. [Google Scholar] [CrossRef]
- Yu, W.; Lin, X.; Gao, S.; Li, C. Age-related changes of inactivating BK channels in rat dorsal root ganglion neurons. J. Neurol. Sci. 2015, 358, 138–145. [Google Scholar] [CrossRef]
- Noh, M.C.; Stemkowski, P.L.; Smith, P.A. Long-term actions of interleukin-1beta on K+, Na+ and Ca2+ channel currents in small, IB4-positive dorsal root ganglion neurons; possible relevance to the etiology of neuropathic pain. J. Neuroimmunol. 2019, 332, 198–211. [Google Scholar] [CrossRef]
- Wu, S.N.; Hsu, M.C.; Liao, Y.K.; Wu, F.T.; Jong, Y.J.; Lo, Y.C. Evidence for inhibitory effects of flupirtine, a centrally acting analgesic, on delayed rectifier K+ currents in motor neuron-like cells. Evid Based Complement. Alternat Med. 2012, 2012, 148403. [Google Scholar] [CrossRef]
- Huang, C.W.; Hung, T.Y.; Liao, Y.K.; Hsu, M.C.; Wu, S.N. Underlying mechanism of regulatory actions of diclofenac, a nonsteroidal anti-inflammatory agent, on neuronal potassium channels and firing: An experimental and theoretical study. J. Physiol. Pharmacol. 2013, 64, 269–280. [Google Scholar]
- Liu, X.; Chang, Y.; Reinhart, P.H.; Sontheimer, H.; Chang, Y. Cloning and characterization of glioma BK, a novel BK channel isoform highly expressed in human glioma cells. J. Neurosci. 2002, 22, 1840–1849. [Google Scholar] [CrossRef] [PubMed]
- Wrzosek, A.; Augustynek, B.; Zochowska, M.; Szewczyk, A. Mitochondrial potassium channels as druggable targets. Biomolecules 2020, 10, 1200. [Google Scholar] [CrossRef] [PubMed]
- Villalba-Galea, C.A.; Chiem, A.T. Hysteretic behavior in voltage-gated channels. Front. Physiol. 2020, 11, 579596. [Google Scholar]
- Carl, A.; Sanders, K.M. Measurement of single channel open probability with voltage ramps. J. Neurosci. Methods 1990, 33, 157–163. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, W.-T.; Wu, S.-N. Effective Activation of BKCa Channels by QO-40 (5-(Chloromethyl)-3-(Naphthalen-1-yl)-2-(Trifluoromethyl)Pyrazolo [1,5-a]pyrimidin-7(4H)-one), Known to Be an Opener of KCNQ2/Q3 Channels. Pharmaceuticals 2021, 14, 388. https://doi.org/10.3390/ph14050388
Chang W-T, Wu S-N. Effective Activation of BKCa Channels by QO-40 (5-(Chloromethyl)-3-(Naphthalen-1-yl)-2-(Trifluoromethyl)Pyrazolo [1,5-a]pyrimidin-7(4H)-one), Known to Be an Opener of KCNQ2/Q3 Channels. Pharmaceuticals. 2021; 14(5):388. https://doi.org/10.3390/ph14050388
Chicago/Turabian StyleChang, Wei-Ting, and Sheng-Nan Wu. 2021. "Effective Activation of BKCa Channels by QO-40 (5-(Chloromethyl)-3-(Naphthalen-1-yl)-2-(Trifluoromethyl)Pyrazolo [1,5-a]pyrimidin-7(4H)-one), Known to Be an Opener of KCNQ2/Q3 Channels" Pharmaceuticals 14, no. 5: 388. https://doi.org/10.3390/ph14050388
APA StyleChang, W.-T., & Wu, S.-N. (2021). Effective Activation of BKCa Channels by QO-40 (5-(Chloromethyl)-3-(Naphthalen-1-yl)-2-(Trifluoromethyl)Pyrazolo [1,5-a]pyrimidin-7(4H)-one), Known to Be an Opener of KCNQ2/Q3 Channels. Pharmaceuticals, 14(5), 388. https://doi.org/10.3390/ph14050388




