The Immunomodulatory and Anti-Inflammatory Effect of Curcumin on Immune Cell Populations, Cytokines, and In Vivo Models of Rheumatoid Arthritis
Abstract
1. Introduction
2. Understanding the Pathogenesis of RA and the Effects of Curcumin
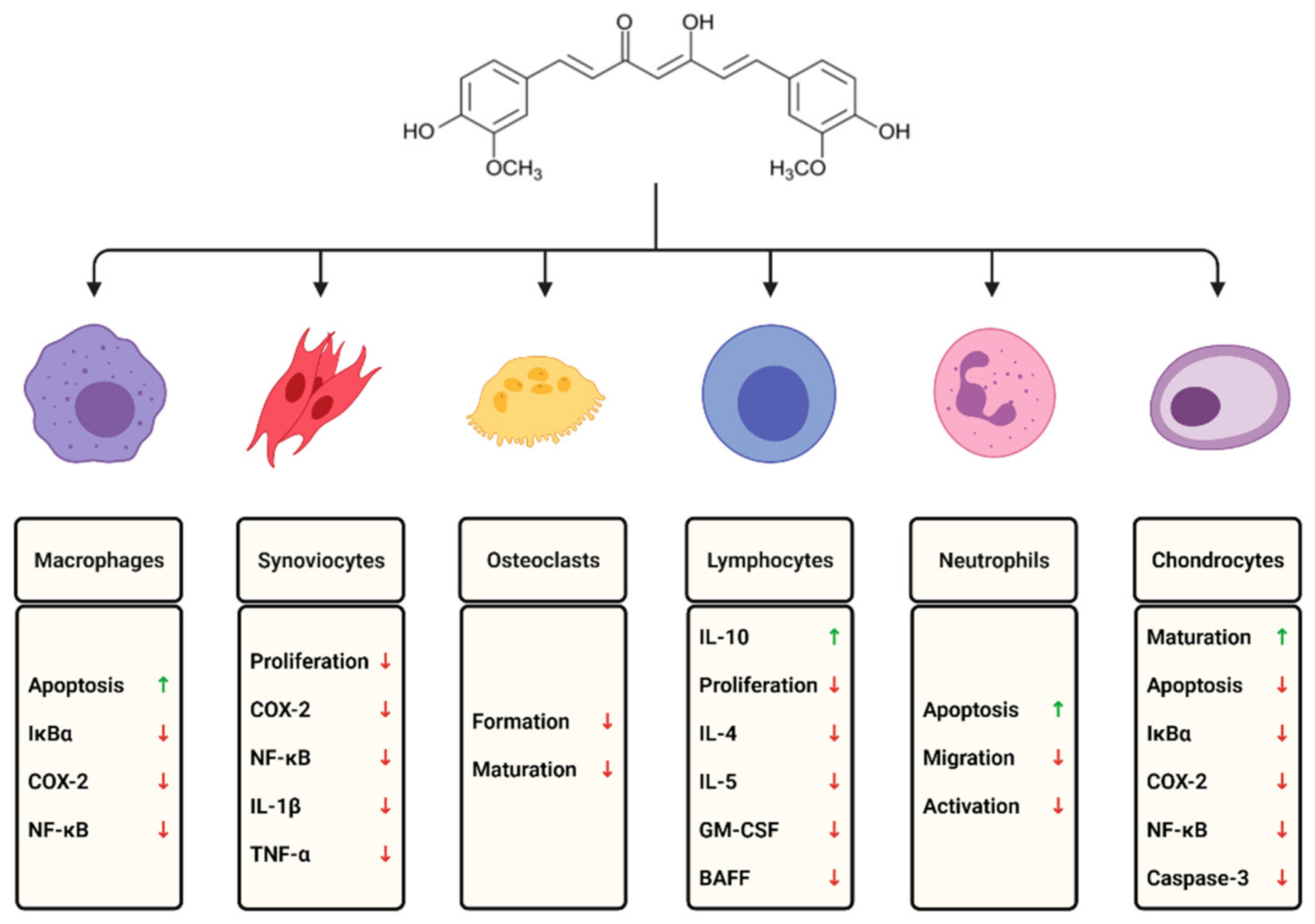
3. Cells Involved in the Course of RA and the Modulatory Role of Curcumin
3.1. Macrophages and Monocytes
3.2. Lymphocytes
3.3. Neutrophils
3.4. Natural Killer (NK) Cells
3.5. Fibroblast-Like Synoviocytes
3.6. Mast Cells
3.7. Chondrocytes
3.8. Osteoclasts
4. RA Markers and Most Common Cytokines—Potential Therapeutic Targets
4.1. IL-1 Family
4.2. IL-1
4.3. IL-18
4.4. IL-33
4.5. IL-6 and IL-27
4.6. IL-23 and IL-17 Axis
4.7. Selected Cytokines of IL-2R Group
4.8. IL-8
4.9. GM-CSF
4.10. IFN-γ
4.11. TNF-α
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kumar, V.; Kanwar, J.R.; Verma, A.K. Rheumatoid arthritis: Basic pathophysiology and role of chitosan nanoparticles in therapy. In Advances and Avenues in the Development of Novel Carriers for Bioactives and Biological Agents; Singh, M.R., Singh, D., Kanwar, J.R., Chauhan, N.S., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 481–507. ISBN 978-0-12-819666-3. [Google Scholar]
- Turesson, C.; O’Fallon, W.M.; Crowson, C.S.; Gabriel, S.E.; Matteson, E.L. Extra-articular disease manifestations in rheumatoid arthritis: Incidence trends and risk factors over 46 years. Ann. Rheum. Dis. 2003, 62, 722–727. [Google Scholar] [CrossRef]
- Sebbag, M.; Chapuy-Regaud, S.; Auger, I.; Petit-Texeira, E.; Clavel, C.; Nogueira, L.; Vincent, C.; Cornélis, F.; Roudier, J.; Serre, G. Clinical and pathophysiological significance of the autoimmune response to citrullinated proteins in rheumatoid arthritis. Jt. Bone Spine 2004, 71, 493–502. [Google Scholar] [CrossRef]
- Waugh, A.; Grant, A. Ross and Wilson: Anatomy and Physiology in Health and Illness, 12th ed.; Elsevier: Amsterdam, The Netherland, 2013; pp. 297–301. [Google Scholar]
- Aletaha, D.; Neogi, T.; Silman, A.J.; Funovits, J.; Felson, D.T.; Bingham, C.O., III; Birnbaum, N.S.; Burmester, G.R.; Bykerk, V.P.; Cohen, M.D.; et al. 2010 Rheumatoid arthritis classification criteria: An American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheumatol. 2010, 62, 2569–2581. [Google Scholar] [CrossRef]
- Smolen, J.S.; Aletaha, D.; McInnes, I.B. Rheumatoid arthritis. Lancet 2016, 388, 2023–2038. [Google Scholar] [CrossRef]
- O’Dell, J.R. Rheumatoid Arthritis: The Clinical Picture. In Arthritis and Allied Conditions: A Textbook of Rheumatology; Isenberg, D.A., Madison, P.J., Woo, P., Klars, D., Breedveld, F.C., Eds.; Oxford University Press: Oxford, UK, 2005; pp. 1165–1194. [Google Scholar]
- Elshabrawy, H.A.; Chen, Z.; Volin, M.V.; Ravella, S.; Virupannavar, S.; Shahrara, S. The pathogenic role of angiogenesis in rheumatoid arthritis. Angiogenesis 2015, 18, 433–448. [Google Scholar] [CrossRef] [PubMed]
- Neumann, E.; Lefèvre, S.; Zimmermann, B.; Gay, S.; Müller-Ladner, U. Rheumatoid arthritis progression mediated by activated synovial fibroblasts. Trends Mol. Med. 2010, 16, 458–468. [Google Scholar] [CrossRef] [PubMed]
- Bartok, B.; Firestein, G.S. Fibroblast-like synoviocytes: Key effector cells in rheumatoid arthritis. Immunol. Rev. 2009, 233, 233–255. [Google Scholar] [CrossRef]
- Ouboussad, L.; Burska, A.N.; Melville, A.; Buch, M.H. Synovial Tissue Heterogeneity in Rheumatoid Arthritis and Changes With Biologic and Targeted Synthetic Therapies to Inform Stratified Therapy. Front. Med. 2019, 6, 45. [Google Scholar] [CrossRef] [PubMed]
- Derksen, V.F.A.M.; Huizinga, T.W.J.; Van Der Woude, D. The role of autoantibodies in the pathophysiology of rheumatoid arthritis. Semin. Immunopathol. 2017, 39, 437–446. [Google Scholar] [CrossRef]
- Conigliaro, P.; Triggianese, P.; De Martino, E.; Fonti, G.L.; Chimenti, M.S.; Sunzini, F.; Viola, A.; Canofari, C.; Perricone, R. Challenges in the treatment of Rheumatoid Arthritis. Autoimmun. Rev. 2019, 18, 706–713. [Google Scholar] [CrossRef]
- Nemtsova, M.V.; Zaletaev, D.V.; Bure, I.V.; Mikhaylenko, D.S.; Kuznetsova, E.B.; Alekseeva, E.A.; Beloukhova, M.I.; Deviatkin, A.A.; Lukashev, A.N.; Zamyatnin, A.A.J. Epigenetic Changes in the Pathogenesis of Rheumatoid Arthritis. Front. Genet. 2019, 10, 570. [Google Scholar] [CrossRef] [PubMed]
- Mazzone, R.; Zwergel, C.; Artico, M.; Taurone, S.; Ralli, M.; Greco, A.; Mai, A. The emerging role of epigenetics in human autoimmune disorders. Clin. Epigenet. 2019, 11, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Wada, T.T.; Araki, Y.; Sato, K.; Aizaki, Y.; Yokota, K.; Kim, Y.T.; Oda, H.; Kurokawa, R.; Mimura, T. Aberrant histone acetylation contributes to elevated interleukin-6 production in rheumatoid arthritis synovial fibroblasts. Biochem. Biophys. Res. Commun. 2014, 444, 682–686. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhou, M.; Lv, X.; Song, L.; Zhang, D.; He, Y.; Wang, M.; Zhao, X.; Yuan, X.; Shi, G.; et al. Reduced Activity of HDAC3 and Increased Acetylation of Histones H3 in Peripheral Blood Mononuclear Cells of Patients with Rheumatoid Arthritis. J. Immunol. Res. 2018, 2018, 1–10. [Google Scholar] [CrossRef]
- Lin, Y.-C.; Lin, Y.-C.; Wu, C.-C.; Huang, M.-Y.; Tsai, W.-C.; Hung, C.-H.; Kuo, P.-L. The immunomodulatory effects of TNF-α inhibitors on human Th17 cells via RORγt histone acetylation. Oncotarget 2016, 8, 7559–7571. [Google Scholar] [CrossRef]
- Glossop, J.R.; Emes, R.D.; Nixon, N.B.; Packham, J.C.; Fryer, A.A.; Mattey, D.L.; Farrell, W.E. Genome-wide profiling in treatment-naive early rheumatoid arthritis reveals DNA methylome changes in T and B lymphocytes. Epigenomics 2016, 8, 209–224. [Google Scholar] [CrossRef]
- Hassan, F.-U.; Rehman, M.S.-U.; Khan, M.S.; Ali, M.A.; Javed, A.; Nawaz, A.; Yang, C. Curcumin as an Alternative Epigenetic Modulator: Mechanism of Action and Potential Effects. Front. Genet. 2019, 10, 514. [Google Scholar] [CrossRef]
- Boyanapalli, S.S.S.; Kong, A.-N.T. “Curcumin, the King of Spices”: Epigenetic Regulatory Mechanisms in the Prevention of Cancer, Neurological, and Inflammatory Diseases. Curr. Pharmacol. Rep. 2015, 1, 129–139. [Google Scholar] [CrossRef]
- He, Y.; Yue, Y.; Zheng, X.; Zhang, K.; Chen, S.; Du, Z. Curcumin, Inflammation, and Chronic Diseases: How Are They Linked? Molecules 2015, 20, 9183–9213. [Google Scholar] [CrossRef]
- Bright, J.J. Curcumin and Autoimmune Disease. Chem. Biol. Pteridines Folates 2007, 595, 425–451. [Google Scholar] [CrossRef]
- Chandran, B.; Goel, A. A Randomized, Pilot Study to Assess the Efficacy and Safety of Curcumin in Patients with Active Rheumatoid Arthritis. Phytother. Res. 2012, 26, 1719–1725. [Google Scholar] [CrossRef]
- Nelson, K.M.; Dahlin, J.L.; Bisson, J.; Graham, J.; Pauli, G.F.; Walters, M.A. The Essential Medicinal Chemistry of Curcumin: Miniperspective. J. Med. Chem. 2017, 60, 1620–1637. [Google Scholar] [CrossRef] [PubMed]
- Kotha, R.R.; Luthria, D.L. Curcumin: Biological, Pharmaceutical, Nutraceutical, and Analytical Aspects. Molecules 2019, 24, 2930. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.A.; Steward, W.P.; Gescher, A.J. Pharmacokinetics and pharmacodynamics of curcumin. Adv. Exp. Med. Biol. 2007, 595, 453–470. [Google Scholar] [PubMed]
- Cheng, D.; Li, W.; Wang, L.; Lin, T.; Poiani, G.; Wassef, A.; Hudlikar, R.; Ondar, P.; Brunetti, L.; Kong, A.-N. Pharmacokinetics, Pharmacodynamics, and PKPD Modeling of Curcumin in Regulating Antioxidant and Epigenetic Gene Expression in Healthy Human Volunteers. Mol. Pharm. 2019, 16, 1881–1889. [Google Scholar] [CrossRef] [PubMed]
- Ambreen, G.; Duse, L.; Tariq, I.; Ali, U.; Ali, S.; Pinnapireddy, S.R.; Bette, M.; Bakowsky, U.; Mandic, R. Sensitivity of Papilloma Virus-Associated Cell Lines to Photodynamic Therapy with Curcumin-Loaded Liposomes. Cancers 2020, 12, 3278. [Google Scholar] [CrossRef] [PubMed]
- Amalraj, A.; Varma, K.; Jacob, J.; Divya, C.; Kunnumakkara, A.B.; Stohs, S.J.; Gopi, S. A Novel Highly Bioavailable Curcumin Formulation Improves Symptoms and Diagnostic Indicators in Rheumatoid Arthritis Patients: A Randomized, Double-Blind, Placebo-Controlled, Two-Dose, Three-Arm, and Parallel-Group Study. J. Med. Food 2017, 20, 1022–1030. [Google Scholar] [CrossRef]
- Alam, J.; Jantan, I.; Bukhari, S.N.A. Rheumatoid arthritis: Recent advances on its etiology, role of cytokines and pharmacotherapy. Biomed. Pharmacother. 2017, 92, 615–633. [Google Scholar] [CrossRef]
- Bugatti, S.; Bozzalla Cassione, E.; De Stefano, L.; Manzo, A. Established rheumatoid arthritis. The pathogenic aspects. Best Pract. Res. Clin. Rheumatol. 2019, 33, 101478. [Google Scholar] [CrossRef]
- Firestein, G.S.; McInnes, I.B. Immunopathogenesis of Rheumatoid Arthritis. Immunity 2017, 46, 183–196. [Google Scholar] [CrossRef]
- Wang, Q.; Ye, C.; Sun, S.; Li, R.; Shi, X.; Wang, S.; Zeng, X.; Kuang, N.; Liu, Y.; Shi, Q.; et al. Curcumin attenuates collagen-induced rat arthritis via anti-inflammatory and apoptotic effects. Int. Immunopharmacol. 2019, 72, 292–300. [Google Scholar] [CrossRef] [PubMed]
- Murakami, Y.; Kawata, A.; Fujisawa, S. Expression of Cyclooxygenase-2, Nitric Oxide Synthase 2 and Heme Oxygenase-1 mRNA Induced by Bis-Eugenol in RAW264.7 Cells and their Antioxidant Activity Determined Using the Induction Period Method. In Vivo 2018, 31, 819–831. [Google Scholar] [CrossRef]
- Mollazadeh, H.; Cicero, A.F.G.; Blesso, C.N.; Pirro, M.; Majeed, M.; Sahebkar, A. Immune modulation by curcumin: The role of interleukin-10. Crit. Rev. Food Sci. Nutr. 2019, 59, 89–101. [Google Scholar] [CrossRef]
- Huang, G.; Xu, Z.; Huang, Y.; Duan, X.; Gong, W.; Zhang, Y.; Fan, J.; He, F. Curcumin Protects Against Collagen-Induced Arthritis via Suppression of BAFF Production. J. Clin. Immunol. 2012, 33, 550–557. [Google Scholar] [CrossRef]
- Harada, Y.; Kato, Y.; Miyaji, T.; Omote, H.; Moriyama, Y.; Hiasa, M. Vesicular nucleotide transporter mediates ATP release and migration in neutrophils. J. Biol. Chem. 2018, 293, 3770–3779. [Google Scholar] [CrossRef]
- Da Silva, J.L.G.; Passos, D.F.; Bernardes, V.M.; Cabral, F.L.; Schimites, P.G.; Manzoni, A.G.; De Oliveira, E.G.; Silva, C.D.B.D.; Beck, R.C.R.; Jantsch, M.H.; et al. Co-Nanoencapsulation of Vitamin D3 and Curcumin Regulates Inflammation and Purine Metabolism in a Model of Arthritis. Inflammation 2019, 42, 1595–1610. [Google Scholar] [CrossRef] [PubMed]
- Jackson, J.K.; Higo, T.; Hunter, W.L.; Burt, H.M. The antioxidants curcumin and quercetin inhibit inflammatory processes associated with arthritis. Inflamm. Res. 2006, 55, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Gianchecchi, E.; Delfino, D.V.; Fierabracci, A. NK cells in autoimmune diseases: Linking innate and adaptive immune responses. Autoimmun. Rev. 2018, 17, 142–154. [Google Scholar] [CrossRef]
- Chalan, P.; Bijzet, J.; Kroesen, B.-J.; Boots, A.M.; Brouwer, E. Altered Natural Killer Cell Subsets in Seropositive Arthralgia and Early Rheumatoid Arthritis Are Associated with Autoantibody Status. J. Rheumatol. 2016, 43, 1008–1016. [Google Scholar] [CrossRef]
- Daïen, C.I.; Gailhac, S.; Audo, R.; Mura, T.; Hahne, M.; Combe, B.; Morel, J. High levels of natural killer cells are associated with response to tocilizumab in patients with severe rheumatoid arthritis. Rheumatology 2014, 54, 601–608. [Google Scholar] [CrossRef]
- Fang, W.; Zhang, Y.; Chen, Z. Innate lymphoid cells in inflammatory arthritis. Arthritis Res. 2020, 22, 1–7. [Google Scholar] [CrossRef]
- Abdollahi, E.; Momtazi, A.A.; Johnston, T.P.; Sahebkar, A. Therapeutic effects of curcumin in inflammatory and immune-mediated diseases: A nature-made jack-of-all-trades? J. Cell. Physiol. 2018, 233, 830–848. [Google Scholar] [CrossRef]
- Lee, H.H.; Cho, H. Improved Anti-Cancer Effect of Curcumin on Breast Cancer Cells by Increasing the Activity of Natural Killer Cells. J. Microbiol. Biotechnol. 2018, 28, 874–882. [Google Scholar] [CrossRef]
- Zhang, H.-G.; Kim, H.; Liu, C.; Yu, S.; Wang, J.; Grizzle, W.E.; Kimberly, R.P.; Barnes, S. Curcumin reverses breast tumor exosomes mediated immune suppression of NK cell tumor cytotoxicity. Biochim. Biophys. Acta BBA Bioenerg. 2007, 1773, 1116–1123. [Google Scholar] [CrossRef]
- Wang, S.; Li, H.; Zhang, M.; Yue, L.-T.; Wang, C.-C.; Zhang, P.; Liu, Y.; Duan, R.-S. Curcumin ameliorates experimental autoimmune myasthenia gravis by diverse immune cells. Neurosci. Lett. 2016, 626, 25–34. [Google Scholar] [CrossRef]
- Guo, Q.; Wang, Y.; Xu, D.; Nossent, J.; Pavlos, N.J.; Xu, J. Rheumatoid arthritis: Pathological mechanisms and modern pharmacologic therapies. Bone Res. 2018, 6, 1–14. [Google Scholar] [CrossRef]
- Shehzad, A.; Rehman, G.; Lee, Y.S. Curcumin in inflammatory diseases. BioFactors 2012, 39, 69–77. [Google Scholar] [CrossRef]
- Kloesch, B.; Becker, T.; Dietersdorfer, E.; Kiener, H.; Steiner, G. Anti-inflammatory and apoptotic effects of the polyphenol curcumin on human fibroblast-like synoviocytes. Int. Immunopharmacol. 2013, 15, 400–405. [Google Scholar] [CrossRef]
- Manca, M.L.; Lattuada, D.; Valenti, D.; Marelli, O.; Corradini, C.; Fernàndez-Busquets, X.; Zaru, M.; Maccioni, A.M.; Fadda, A.M.; Manconi, M. Potential therapeutic effect of curcumin loaded hyalurosomes against inflammatory and oxidative processes involved in the pathogenesis of rheumatoid arthritis: The use of fibroblast-like synovial cells cultured in synovial fluid. Eur. J. Pharm. Biopharm. 2019, 136, 84–92. [Google Scholar] [CrossRef]
- Moon, D.-O.; Kim, M.-O.; Choi, Y.H.; Park, Y.-M.; Kim, G.-Y. Curcumin attenuates inflammatory response in IL-1β-induced human synovial fibroblasts and collagen-induced arthritis in mouse model. Int. Immunopharmacol. 2010, 10, 605–610. [Google Scholar] [CrossRef]
- Dai, Q.; Zhou, D.; Xu, L.; Song, X. Curcumin alleviates rheumatoid arthritis-induced inflammation and synovial hyperplasia by targeting mTOR pathway in rats. Drug Des. Dev. Ther. 2018, 12, 4095–4105. [Google Scholar] [CrossRef]
- Cai, H.; Zheng, Z.; Sun, Y.; Liu, Z.; Zhang, M.; Li, C. The effect of curcumin and its nanoformulation on adjuvant-induced arthritis in rats. Drug Des. Dev. Ther. 2015, 9, 4931–4942. [Google Scholar] [CrossRef]
- Rivellese, F.; Mauro, D.; Nerviani, A.; Pagani, S.; Fossati-Jimack, L.; Messemaker, T.; Kurreeman, F.A.S.; Toes, R.E.M.; Ramming, A.; Rauber, S.; et al. Mast cells in early rheumatoid arthritis associate with disease severity and support B cell autoantibody production. Ann. Rheum. Dis. 2018, 77, 1773–1781. [Google Scholar] [CrossRef]
- Min, H.K.; Kim, K.-W.; Lee, S.-H.; Kim, H.-R. Roles of mast cells in rheumatoid arthritis. Korean J. Intern. Med. 2020, 35, 12–24. [Google Scholar] [CrossRef]
- Ragipoglu, D.; Dudeck, A.; Haffner-Luntzer, M.; Voss, M.; Kroner, J.; Ignatius, A.; Fischer, V. The Role of Mast Cells in Bone Metabolism and Bone Disorders. Front. Immunol. 2020, 11, 163. [Google Scholar] [CrossRef]
- Rivellese, F.; Rossi, F.W.; Galdiero, M.R.; Pitzalis, C.; de Paulis, A. Mast cells in early rheumatoid arthritis. Int. J. Mol. Sci. 2019, 20, 2040. [Google Scholar] [CrossRef]
- Rivellese, F.; Nerviani, A.; Rossi, F.W.; Marone, G.; Matucci-Cerinic, M.; de Paulis, A.; Pitzalis, C. Mast cells in rheumatoid arthritis: Friends or foes? Autoimmun. Rev. 2017, 16, 557–563. [Google Scholar] [CrossRef]
- Kong, R.; Kang, O.H.; Seo, Y.S.; Zhou, T.; Kim, S.A.; Shin, D.W.; Kwon, D.Y. MAPKs and NF-κB pathway inhibitory effect of bisdemethoxycurcumin on phorbol-12-myristate-13-acetate and A23187-induced inflammation in human mast cells. Mol. Med. Rep. 2018, 17, 630–635. [Google Scholar] [CrossRef]
- Nishikawa, H.; Tsutsumi, J.; Kitani, S. Anti-inflammatory and anti-oxidative effect of curcumin in connective tissue type mast cell. J. Funct. Foods 2013, 5, 763–772. [Google Scholar] [CrossRef]
- Ota, M.; Tanaka, Y.; Nakagawa, I.; Jiang, J.J.; Arima, Y.; Kamimura, D.; Onodera, T.; Iwasaki, N.; Murakami, M. Role of Chondrocytes in the Development of Rheumatoid Arthritis via Transmembrane Protein 147–Mediated NF-κB Activation. Arthritis Rheumatol. 2020, 72, 931–942. [Google Scholar] [CrossRef]
- Harre, U.; Schett, G. Cellular and molecular pathways of structural damage in rheumatoid arthritis. Semin. Immunopathol. 2017, 39, 355–363. [Google Scholar] [CrossRef]
- McInnes, I.B.; Schett, G. Mechanism of Disease The Pathogenesis of Rheumatoid Arthritis. N. Engl. J. Med. 2011, 365, 2205–2219. [Google Scholar] [CrossRef] [PubMed]
- Buhrmann, C.; Mobasheri, A.; Matis, U.; Shakibaei, M. Curcumin mediated suppression of nuclear factor-κB promotes chondrogenic differentiation of mesenchymal stem cells in a high-density co-culture microenvironment. Arthritis Res. Ther. 2010, 12, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Shakibaei, M.; John, T.; Schulze-Tanzil, G.; Lehmann, I.; Mobasheri, A. Suppression of NF-κB activation by curcumin leads to inhibition of expression of cyclo-oxygenase-2 and matrix metalloproteinase-9 in human articular chondrocytes: Implications for the treatment of osteoarthritis. Biochem. Pharmacol. 2007, 73, 1434–1445. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, S. Emerging anti-osteoclast therapy for rheumatoid arthritis. J. Orthop. Sci. 2018, 23, 717–721. [Google Scholar] [CrossRef] [PubMed]
- Shim, J.-H.; Stavre, Z.; Gravallese, E.M. Bone Loss in Rheumatoid Arthritis: Basic Mechanisms and Clinical Implications. Calcif. Tissue Int. 2017, 102, 533–546. [Google Scholar] [CrossRef]
- Shang, W.; Zhao, L.-J.; Dong, X.-L.; Zhao, Z.-M.; Li, J.; Zhang, B.-B.; Cai, H. Curcumin inhibits osteoclastogenic potential in PBMCs from rheumatoid arthritis patients via the suppression of MAPK/RANK/c-Fos/NFATc1 signaling pathways. Mol. Med. Rep. 2016, 14, 3620–3626. [Google Scholar] [CrossRef]
- Von Metzler, I.; Krebbel, H.; Kuckelkorn, U.; Heider, U.; Jakob, C.; Kaiser, M.; Fleissner, C.; Terpos, E.; Sezer, O. Curcumin diminishes human osteoclastogenesis by inhibition of the signalosome-associated IκB kinase. J. Cancer Res. Clin. Oncol. 2008, 135, 173–179. [Google Scholar] [CrossRef]
- Furst, D.E.; Emery, P. Rheumatoid arthritis pathophysiology: Update on emerging cytokine and cytokine-associated cell targets. Rheumatology 2014, 53, 1560–1569. [Google Scholar] [CrossRef]
- Fields, J.K.; Günther, S.; Sundberg, E.J. Structural Basis of IL-1 Family Cytokine Signaling. Front. Immunol. 2019, 10, 1412. [Google Scholar] [CrossRef]
- Dinarello, C.A. Overview of the IL-1 family in innate inflammation and acquired immunity. Immunol. Rev. 2018, 281, 8–27. [Google Scholar] [CrossRef] [PubMed]
- Boissier, M.-C.; Semerano, L.; Challal, S.; Saidenberg-Kermanac’H, N.; Falgarone, G. Rheumatoid arthritis: From autoimmunity to synovitis and joint destruction. J. Autoimmun. 2012, 39, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Noack, M.; Miossec, P. Selected cytokine pathways in rheumatoid arthritis. Semin. Immunopathol. 2017, 39, 365–383. [Google Scholar] [CrossRef] [PubMed]
- Liu, E.; Perl, A. Pathogenesis and treatment of autoimmune rheumatic diseases. Curr. Opin. Rheumatol. 2019, 31, 307–315. [Google Scholar] [CrossRef]
- Mateen, S.; Zafar, A.; Moin, S.; Khan, A.Q.; Zubair, S. Understanding the role of cytokines in the pathogenesis of rheumatoid arthritis. Clin. Chim. Acta 2016, 455, 161–171. [Google Scholar] [CrossRef]
- Cici, D.; Corrado, A.; Rotondo, C.; Cantatore, F.P. Wnt Signaling and Biological Therapy in Rheumatoid Arthritis and Spondyloarthritis. Int. J. Mol. Sci. 2019, 20, 5552. [Google Scholar] [CrossRef]
- Krumm, B.; Meng, X.; Xiang, Y.; Deng, J. Identification of small molecule inhibitors of Interleukin-18. Sci. Rep. 2017, 7, 483. [Google Scholar] [CrossRef] [PubMed]
- Maczynska, I.; Millo, B.; Ratajczak-Stefańska, V.; Maleszka, R.; Szych, Z.; Kurpisz, M.; Giedrys-Kalemba, S. Proinflammatory cytokine (IL-1β, IL-6, IL-12, IL-18 and TNF-α) levels in sera of patients with subacute cutaneous lupus erythematosus (SCLE). Immunol. Lett. 2006, 102, 79–82. [Google Scholar] [CrossRef] [PubMed]
- Kaplanski, G. Interleukin-18: Biological properties and role in disease pathogenesis. Immunol. Rev. 2018, 281, 138–153. [Google Scholar] [CrossRef]
- Nozaki, Y.; Ri, J.; Sakai, K.; Niki, K.; Kinoshita, K.; Funauchi, M.; Matsumura, I. Inhibition of the IL-18 Receptor Signaling Pathway Ameliorates Disease in a Murine Model of Rheumatoid Arthritis. Cells 2019, 9, 11. [Google Scholar] [CrossRef]
- Yin, H.; Guo, Q.; Li, X.; Tang, T.; Li, C.; Wang, H.; Sun, Y.; Feng, Q.; Ma, C.; Gao, C.; et al. Curcumin Suppresses IL-1β Secretion and Prevents Inflammation through Inhibition of the NLRP3 Inflammasome. J. Immunol. 2018, 200, 2835–2846. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Liu, W.; Zhang, H.; Li, H.; Liu, J.; Zhang, F.; Jiang, T.; Jiang, S. Curcumin Prevents Osteoarthritis by Inhibiting the Activation of Inflammasome NLRP3. J. Interf. Cytokine Res. 2017, 37, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Yadav, R.; Jee, B.; Awasthi, S.K. Curcumin Suppresses the Production of Pro-inflammatory Cytokine Interleukin-18 in Lipopolysaccharide Stimulated Murine Macrophage-Like Cells. Indian J. Clin. Biochem. 2014, 30, 109–112. [Google Scholar] [CrossRef]
- Pinto, S.M.; Subbannayya, Y.; Rex, D.A.B.; Raju, R.; Chatterjee, O.; Advani, J.; Radhakrishnan, A.; Prasad, T.S.K.; Wani, M.R.; Pandey, A. A network map of IL-33 signaling pathway. J. Cell Commun. Signal. 2018, 12, 615–624. [Google Scholar] [CrossRef]
- Macedo, R.B.V.; Kakehasi, A.M.; de Andrade, M.V.M. IL33 in rheumatoid arthritis: Potential contribution to pathogenesis. Rev. Bras. Reum. 2016, 56, 451–457. [Google Scholar] [CrossRef]
- Chen, Z.; Bozec, A.; Ramming, A.; Schett, G. Anti-inflammatory and immune-regulatory cytokines in rheumatoid arthritis. Nat. Rev. Rheumatol. 2019, 15, 9–17. [Google Scholar] [CrossRef]
- Braun, H.; Afonina, I.S.; Mueller, C.; Beyaert, R. Dichotomous function of IL-33 in health and disease: From biology to clinical implications. Biochem. Pharmacol. 2018, 148, 238–252. [Google Scholar] [CrossRef]
- Sharma, S.; Sethi, G.S.; Naura, A.S. Curcumin Ameliorates Ovalbumin-Induced Atopic Dermatitis and Blocks the Progression of Atopic March in Mice. Inflammation 2019, 43, 358–369. [Google Scholar] [CrossRef] [PubMed]
- Pandolfi, F.; Franza, L.; Carusi, V.; Altamura, S.; Andriollo, G.; Nucera, E. Interleukin-6 in Rheumatoid Arthritis. Int. J. Mol. Sci. 2020, 21, 5238. [Google Scholar] [CrossRef] [PubMed]
- Avci, A.B.; Feist, E.; Burmester, G.R. Targeting IL-6 or IL-6 Receptor in Rheumatoid Arthritis: What’s the Difference? BioDrugs 2018, 32, 531–546. [Google Scholar] [CrossRef]
- Atzeni, F.; Nucera, V.; Masala, I.F.; Sarzi-Puttini, P.; Bonitta, G. Il-6 Involvement in pain, fatigue and mood disorders in rheumatoid arthritis and the effects of Il-6 inhibitor sarilumab. Pharmacol. Res. 2019, 149, 104402. [Google Scholar] [CrossRef] [PubMed]
- Jones, G.W.; Hill, D.G.; Cardus, A.; A Jones, S. IL-27: A double agent in the IL-6 family. Clin. Exp. Immunol. 2018, 193, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Choy, E.H.; De Benedetti, F.; Takeuchi, T.; Hashizume, M.; John, M.R.; Kishimoto, T. Translating IL-6 biology into effective treatments. Nat. Rev. Rheumatol. 2020, 16, 335–345. [Google Scholar] [CrossRef]
- Millier, M.J.; Lazaro, K.; Stamp, L.K.; Hessian, P.A. The contribution from interleukin-27 towards rheumatoid inflammation: Insights from gene expression. Genes Immun. 2020, 21, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Shahi, A.; Afzali, S.; Salehi, S.; Aslani, S.; Mahmoudi, M.; Jamshidi, A.; Amirzargar, A. IL-27 and autoimmune rheumatologic diseases: The good, the bad, and the ugly. Int. Immunopharmacol. 2020, 84, 106538. [Google Scholar] [CrossRef]
- Yuan, N.; Yu, G.; Liu, D.; Wang, X.; Zhao, L. An emerging role of interleukin-23 in rheumatoid arthritis. Immunopharmacol. Immunotoxicol. 2019, 41, 185–191. [Google Scholar] [CrossRef]
- Abdo, A.I.K.; Tye, G.J. Interleukin 23 and autoimmune diseases: Current and possible future therapies. Inflamm. Res. 2020, 69, 463–480. [Google Scholar] [CrossRef]
- Bunte, K.; Beikler, T. Th17 Cells and the IL-23/IL-17 Axis in the Pathogenesis of Periodontitis and Immune-Mediated Inflammatory Diseases. Int. J. Mol. Sci. 2019, 20, 3394. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, E.; Rogge, L. The IL-23/IL-17 pathway in human chronic inflammatory diseases—New insight from genetics and targeted therapies. Microbes Infect. 2019, 21, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.; Li, B.; Luo, L.; Jiang, W.; Lu, Q.; Rong, M.; Lai, R. Curcumin shows excellent therapeutic effect on psoriasis in mouse model. Biochimie 2016, 123, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Zwicky, P.; Unger, S.; Becher, B. Targeting interleukin-17 in chronic inflammatory disease: A clinical perspective. J. Exp. Med. 2020, 217, 217. [Google Scholar] [CrossRef] [PubMed]
- Taams, L.S. Interleukin-17 in rheumatoid arthritis: Trials and tribulations. J. Exp. Med. 2020, 217. [Google Scholar] [CrossRef]
- Lubberts, E. The IL-23–IL-17 axis in inflammatory arthritis. Nat. Rev. Rheumatol. 2015, 11, 415–429. [Google Scholar] [CrossRef]
- Robert, M.; Miossec, P. IL-17 in Rheumatoid Arthritis and Precision Medicine: From Synovitis Expression to Circulating Bioactive Levels. Front. Med. 2019, 5, 364. [Google Scholar] [CrossRef]
- Kim, E.K.; Kwon, J.E.; Lee, S.Y.; Lee, E.J.; Kim, D.S.; Moon, S.J.; Lee, J.; Kwok, S.K.; Park, S.H.; Cho, M. La IL-17-mediated mitochondrial dysfunction impairs apoptosis in rheumatoid arthritis synovial fibroblasts through activation of autophagy. Cell Death Dis. 2017, 8, e2565. [Google Scholar] [CrossRef] [PubMed]
- Skyvalidas, D.Ν.; Mavropoulos, A.; Tsiogkas, S.; Dardiotis, E.; Liaskos, C.; Mamuris, Z.; Roussaki-Schulze, A.; Sakkas, L.I.; Zafiriou, E.; Bogdanos, D.P. Curcumin mediates attenuation of pro-inflammatory interferon γ and interleukin 17 cytokine responses in psoriatic disease, strengthening its role as a dietary immunosuppressant. Nutr. Res. 2020, 75, 95–108. [Google Scholar] [CrossRef]
- Long, D.; Chen, Y.; Wu, H.; Zhao, M.; Lu, Q. Clinical significance and immunobiology of IL-21 in autoimmunity. J. Autoimmun. 2019, 99, 1–14. [Google Scholar] [CrossRef]
- Kim, S.-J.; Chang, H.J.; Volin, M.V.; Umar, S.; Van Raemdonck, K.; Chevalier, A.; Palasiewicz, K.; Christman, J.W.; Volkov, S.; Arami, S.; et al. Macrophages are the primary effector cells in IL-7-induced arthritis. Cell. Mol. Immunol. 2019, 17, 728–740. [Google Scholar] [CrossRef]
- Cai, L.; Xu, H.; Zhang, H.; Zhang, L.; Wang, G.; Nie, H. Blockade of IL-7Rα alleviates collagen-induced arthritis via inhibiting Th1 cell differentiation and CD4+ T cell migration. Mol. Immunol. 2016, 79, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Hartgring, S.A.; Willis, C.R.; Bijlsma, J.W.; Lafeber, F.P.; Van Roon, J.A. Interleukin-7-aggravated joint inflammation and tissue destruction in collagen-induced arthritis is associated with T-cell and B-cell activation. Arthritis Res. Ther. 2012, 14, R137. [Google Scholar] [CrossRef]
- Reyes-Pérez, I.V.; Sánchez-Hernández, P.E.; Muñoz-Valle, J.F.; Martínez-Bonilla, G.E.; García-Iglesias, T.; González-Díaz, V.; García-Arellano, S.; Cerpa-Cruz, S.; Polanco-Cruz, J.; Ramírez-Dueñas, M.G. Cytokines (IL-15, IL-21, and IFN-γ) in rheumatoid arthritis: Association with positivity to autoantibodies (RF, anti-CCP, anti-MCV, and anti-PADI4) and clinical activity. Clin. Rheumatol. 2019, 38, 3061–3071. [Google Scholar] [CrossRef]
- Allard-Chamard, H.; Mishra, H.K.; Nandi, M.; Mayhue, M.; Menendez, A.; Ilangumaran, S.; Ramanathan, S. Interleukin-15 in autoimmunity. Cytokine 2020, 136, 155258. [Google Scholar] [CrossRef]
- Yang, X.-K.; Xu, W.-D.; Leng, R.-X.; Liang, Y.; Liu, Y.-Y.; Fang, X.-Y.; Feng, C.-C.; Li, R.; Cen, H.; Pan, H.-F.; et al. Therapeutic potential of IL-15 in rheumatoid arthritis. Hum. Immunol. 2015, 76, 812–818. [Google Scholar] [CrossRef] [PubMed]
- Kurowska, W.; Przygodzka, M.; Jakubaszek, M.; Kwiatkowska, B.; Maslinski, W. Interleukin-15 as a Biomarker Candidate of Rheumatoid Arthritis Development. J. Clin. Med. 2020, 9, 1555. [Google Scholar] [CrossRef] [PubMed]
- Dinesh, P.; Rasool, M. Multifaceted role of IL-21 in rheumatoid arthritis: Current understanding and future perspectives. J. Cell. Physiol. 2017, 233, 3918–3928. [Google Scholar] [CrossRef]
- Agonia, I.; Couras, J.; Cunha, A.; Andrade, A.J.; Macedo, J.; Sousa-Pinto, B. IL-17, IL-21 and IL-22 polymorphisms in rheumatoid arthritis: A systematic review and meta-analysis. Cytokine 2020, 125, 154813. [Google Scholar] [CrossRef] [PubMed]
- Forward, N.A.; Conrad, D.M.; Coombs, M.; Doucette, C.D.; Furlong, S.J.; Lin, T.-J.; Hoskin, D.W. Curcumin blocks interleukin (IL)-2 signaling in T-lymphocytes by inhibiting IL-2 synthesis, CD25 expression, and IL-2 receptor signaling. Biochem. Biophys. Res. Commun. 2011, 407, 801–806. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.-M.; Xu, R.; Huang, X.-Y.; Cheng, S.-M.; Huang, M.-F.; Yue, H.-Y.; Wang, X.; Zou, Y.; Lu, A.-P.; Liu, D.-Y. Curcumin Suppressed Activation of Dendritic Cells via JAK/STAT/SOCS Signal in Mice with Experimental Colitis. Front. Pharmacol. 2016, 7, 455. [Google Scholar] [CrossRef] [PubMed]
- Loganes, C.; Lega, S.; Bramuzzo, M.; Brumatti, L.V.; Piscianz, E.; Valencic, E.; Tommasini, A.; Marcuzzi, A. Curcumin Anti-Apoptotic Action in a Model of Intestinal Epithelial Inflammatory Damage. Nutrients 2017, 9, 578. [Google Scholar] [CrossRef]
- Lin, J.; He, Y.; Wang, B.; Xun, Z.; Chen, S.; Zeng, Z.; Ou, Q. Blocking of YY1 reduce neutrophil infiltration by inhibiting IL-8 production via the PI3K-Akt-mTOR signaling pathway in rheumatoid arthritis. Clin. Exp. Immunol. 2019, 195, 226–236. [Google Scholar] [CrossRef]
- Morita, T.; Shima, Y.; Fujimoto, K.; Tsuboi, H.; Saeki, Y.; Narazaki, M.; Ogata, A.; Kumanogoh, A. Anti-receptor activator of nuclear factor κB ligand antibody treatment increases osteoclastogenesis-promoting IL-8 in patients with rheumatoid arthritis. Int. Immunol. 2019, 31, 277–285. [Google Scholar] [CrossRef]
- Kaczyński, T.; Wroński, J.; Głuszko, P.; Kryczka, T.; Miskiewicz, A.; Górski, B.; Radkowski, M.; Strzemecki, D.; Grieb, P.; Górska, R. Salivary interleukin 6, interleukin 8, interleukin 17A, and tumour necrosis factor αlevels in patients with periodontitis and rheumatoid arthritis. Cent. Eur. J. Immunol. 2019, 44, 269–276. [Google Scholar] [CrossRef] [PubMed]
- An, Q.; Yan, W.; Zhao, Y.; Yu, K. Enhanced neutrophil autophagy and increased concentrations of IL-6, IL-8, IL-10 and MCP-1 in rheumatoid arthritis. Int. Immunopharmacol. 2018, 65, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Hidaka, H.; Ishiko, T.; Furuhashi, T.; Kamohara, H.; Suzuki, S.; Miyazaki, M.; Ikeda, O.; Mita, S.; Setoguchi, T.; Ogawa, M. Curcumin inhibits interleukin 8 production and enhances interleukin 8 receptor expression on the cell surface: Impact on human pancreatic carcinoma cell growth by autocrine regulation. Cancer 2002, 95, 1206–1214. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.K.; Rains, J.; Croad, J.; Larson, B.; Jones, K. Curcumin Supplementation Lowers TNF-α, IL-6, IL-8, and MCP-1 Secretion in High Glucose-Treated Cultured Monocytes and Blood Levels of TNF-α, IL-6, MCP-1, Glucose, and Glycosylated Hemoglobin in Diabetic Rats. Antioxidants Redox Signal. 2009, 11, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Avci, A.B.; Feist, E.; Burmester, G.-R. Targeting GM-CSF in rheumatoid arthritis. Clin. Exp. Rheumatol. 2016, 34, 39–44. [Google Scholar]
- Lotfi, N.; Thome, R.; Rezaei, N.; Zhang, G.-X.; Rezaei, A.; Rostami, A.; Esmaeil, N. Roles of GM-CSF in the Pathogenesis of Autoimmune Diseases: An Update. Front. Immunol. 2019, 10, 1265. [Google Scholar] [CrossRef]
- Gertsch, J.; Güttinger, M.; Heilmann, J.; Sticher, O. Curcumin differentially modulates mRNA profiles in Jurkat T and human peripheral blood mononuclear cells. Bioorg. Med. Chem. 2003, 11, 1057–1063. [Google Scholar] [CrossRef]
- Edwards, C.J.; Feldman, J.L.; Beech, J.; Shields, K.M.; Stover, J.A.; Trepicchio, W.L.; Larsen, G.; Foxwell, B.M.J.; Brennan, F.M.; Feldmann, M.; et al. Molecular Profile of Peripheral Blood Mononuclear Cells from Patients with Rheumatoid Arthritis. Mol. Med. 2007, 13, 40–58. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.; Tian, L.; Luo, G.; Yu, X. Interferon-Gamma-Mediated Osteoimmunology. Front. Immunol. 2018, 9, 1508. [Google Scholar] [CrossRef]
- Kato, M. New insights into IFN-γ in rheumatoid arthritis: Role in the era of JAK inhibitors. Immunol. Med. 2020, 43, 72–78. [Google Scholar] [CrossRef]
- Olalekan, S.A.; Cao, Y.; Hamel, K.M.; Finnegan, A. B cells expressing IFN-gamma suppress Treg-cell differentiation and promote autoimmune experimental arthritis. Eur. J. Immunol. 2015, 45, 988–998. [Google Scholar] [CrossRef] [PubMed]
- Blüml, S.; Scheinecker, C.; Smolen, J.S.; Redlich, K. Targeting TNF receptors in rheumatoid arthritis. Int. Immunol. 2012, 24, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B. TNF and Bone Remodeling. Curr. Osteoporos. Rep. 2017, 15, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Radner, H.; Aletaha, D. Anti-TNF Therapie in der Rheumatoiden Arthritis—Ein Überblick. Wien. Med. Wochenschr. 2015, 165, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Bek, S.; Bojesen, A.B.; Nielsen, J.V.; Sode, J.; Bank, S.; Vogel, U.; Andersen, V. Systematic review and meta-analysis: Pharmacogenetics of anti-TNF treatment response in rheumatoid arthritis. Pharmacogenom. J. 2017, 17, 403–411. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Makuch, S.; Więcek, K.; Woźniak, M. The Immunomodulatory and Anti-Inflammatory Effect of Curcumin on Immune Cell Populations, Cytokines, and In Vivo Models of Rheumatoid Arthritis. Pharmaceuticals 2021, 14, 309. https://doi.org/10.3390/ph14040309
Makuch S, Więcek K, Woźniak M. The Immunomodulatory and Anti-Inflammatory Effect of Curcumin on Immune Cell Populations, Cytokines, and In Vivo Models of Rheumatoid Arthritis. Pharmaceuticals. 2021; 14(4):309. https://doi.org/10.3390/ph14040309
Chicago/Turabian StyleMakuch, Sebastian, Kamil Więcek, and Marta Woźniak. 2021. "The Immunomodulatory and Anti-Inflammatory Effect of Curcumin on Immune Cell Populations, Cytokines, and In Vivo Models of Rheumatoid Arthritis" Pharmaceuticals 14, no. 4: 309. https://doi.org/10.3390/ph14040309
APA StyleMakuch, S., Więcek, K., & Woźniak, M. (2021). The Immunomodulatory and Anti-Inflammatory Effect of Curcumin on Immune Cell Populations, Cytokines, and In Vivo Models of Rheumatoid Arthritis. Pharmaceuticals, 14(4), 309. https://doi.org/10.3390/ph14040309





