Overview of Synthetic Cannabinoids ADB-FUBINACA and AMB-FUBINACA: Clinical, Analytical, and Forensic Implications
Abstract
1. Introduction
2. Methodology
3. Chemistry and Chemical Analysis
4. Methods for SC Detection
5. Prevalence and Patterns of Abuse
6. Legal Status
7. Subjective and Other Biological Effects
8. Clinical Toxicology
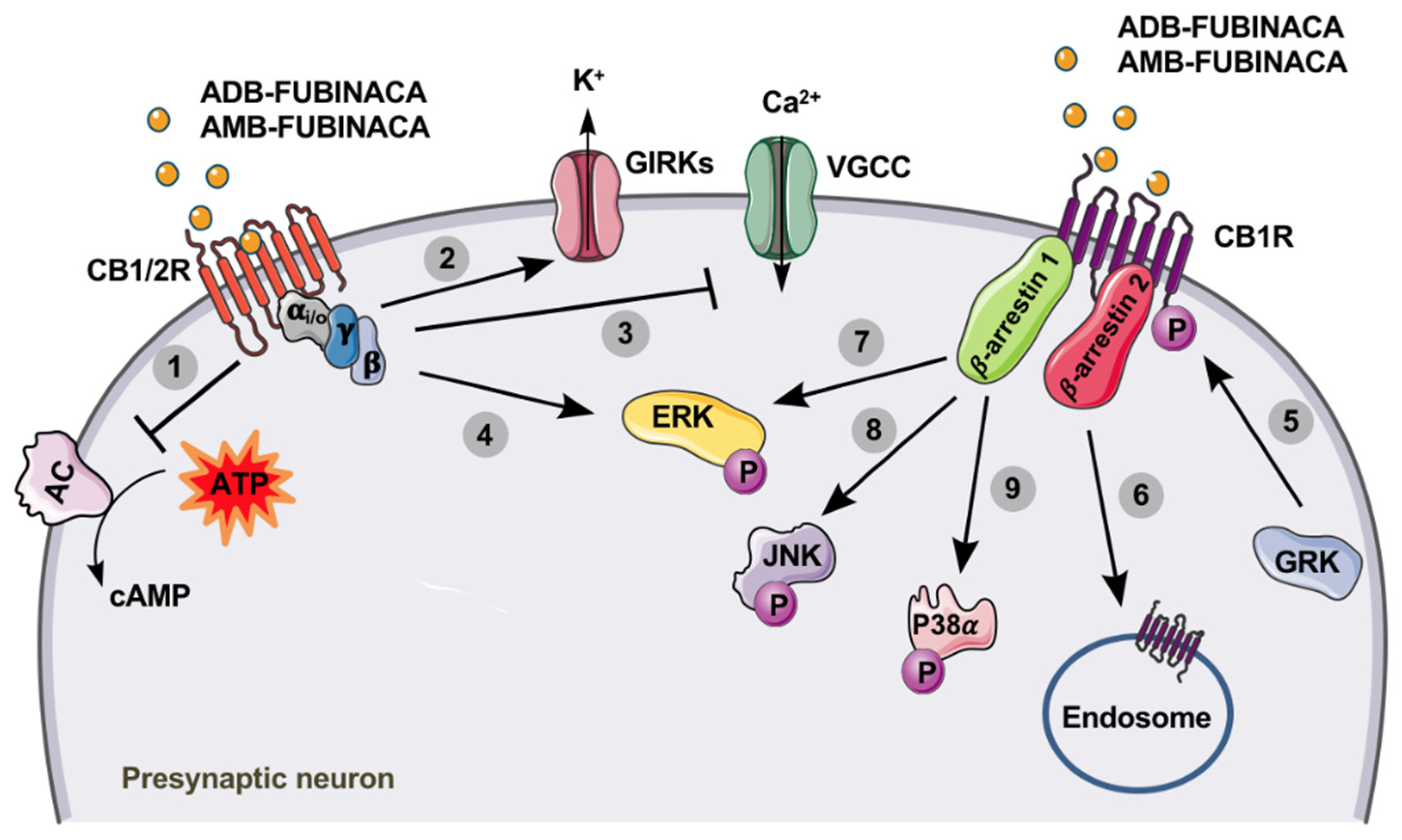
8.1. Mechanisms of Toxicity
8.2. Treatment
9. Pharmacodynamics
10. Pharmacokinetics
10.1. Absorption
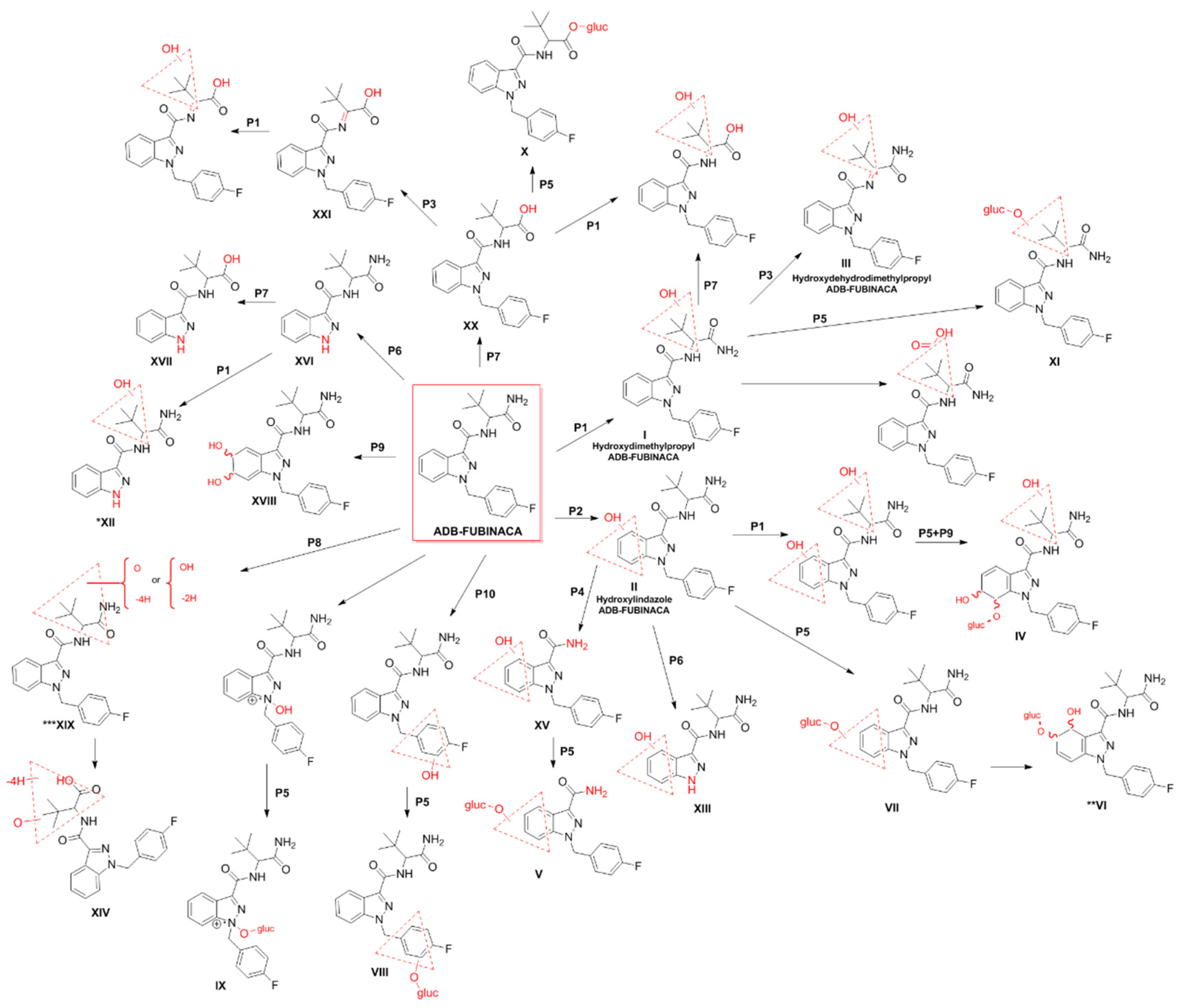
10.2. Metabolism and Elimination
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- EMCDDA. European Drug Report 2020: Trends and Developments; European Union Publications Office: Luxembourg, 2020; Available online: https://www.emcdda.europa.eu/system/files/publications/13236/TDAT20001ENN_web.pdf (accessed on 14 November 2020).
- Adamowicz, P.M.E.; Maslanka, M. Fatal Intoxication with New Synthetic Cannabinoids AMB-FUBINACA and EMB-FUBINACA. Clin. Toxicol. 2019, 57, 1103–1108. [Google Scholar] [CrossRef]
- Adams, A.J.; Banister, S.D.; Irizarry, L.; Trecki, J.; Schwartz, M.; Gerona, R. “Zombie” Outbreak caused by the Synthetic Cannabinoid AMB-FUBINACA in New York. N. Engl. J. Med. 2017, 376, 235–242. [Google Scholar] [CrossRef]
- Lam, R.T.M.; Leung, S.; Chong, Y.; Tsui, M.; Mak, T. Supraventricular Tachycardia and Acute Confusion Following Ingestion of E-cigarette Fluid Containing AB-FUBINACA and ADB-FUBINACA: A Case Report with Quantitative Analysis of Serum Drug Concentrations. Clin. Toxicol. 2017, 55, 662–667. [Google Scholar] [CrossRef]
- Shanks, K.G.; Clark, W.; Behonick, G. Death Associated with the Use of the Synthetic Cannabinoid ADB-FUBINACA. J. Anal. Toxicol. 2016, 236–239. [Google Scholar] [CrossRef]
- EMCDDA. European Drug Report 2018: Trends and Developments; European Union Publications Office: Luxembourg, 2018; Available online: https://www.emcdda.europa.eu/system/files/publications/8585/20181816_TDAT18001ENN_PDF.pdf (accessed on 9 April 2020).
- EMCDDA. Fentanils and Synthetic Cannabinoids: Driving Greater Complexity into the Drug Situation; An update from the EU Early Warning System; Publications Office of the European Union: Luxembourg, 2018; Available online: http://www.emcdda.europa.eu/system/files/publications/8870/2018-2489-td0118414enn.pdf (accessed on 24 September 2020).
- Buchler, I.P.; Hayes, M.J.; Hegde, S.G.; Hockerman, S.L.; Jones, D.E.; Kortum, S.W. Indazole Derivatives. Patent WO 2009/106980-A2, 3 September 2009. [Google Scholar]
- Uchiyama, N.M.S.; Kawamura, M.; Kikura-Hanajiri, R.; Goda, Y. Two New-type Cannabimimetic Quinolinyl Carboxylates, QUPIC and QUCHIC, two New Cannabimimetic Carboxamide Derivatives, ADB-FUBINACA and ADBICA, and five Fynthetic Cannabinoids Detected with a Thiophene Derivative α-PVT and an Opioid Receptor Agonist AH-7921 Identified in Illegal Products. Forensic Sci. Int. 2013, 31, 223–240. [Google Scholar] [CrossRef]
- Carlier, J.D.X.; Wohlfarth, A.; Scheidweiler, K.; Huestis, M.A. In Vitro Metabolite Profiling of ADB-FUBINACA, a New Synthetic Cannabinoid. Curr. Neuropharmacol. 2017, 15, 682–691. [Google Scholar] [CrossRef]
- Banister, S.D.; Longworth, M.; Kevin, R.; Sachdev, S.; Santiago, M.; Stuart, J.; Mack, J.B.C.; Glass, M.; McGregor, I.S.; Connor, M. Pharmacology of Valinate and Tert-leucinate Synthetic Cannabinoids 5F-AMBICA, 5F-AMB, 5F-ADB, AMB-FUBINACA, MDMB-FUBINACA, MDMB-CHMICA, and their Analogues. ACS Chem. Neurosci. 2016, 7, 1241–1254. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Health. AB-FUBINACA and AMB FUBINACA: Report to the Expert Advisory Committee on Drugs. 2019. Available online: https://www.health.govt.nz/our-work/regulation-health-and-disability-system/psychoactive-substances-regulation/synthetic-cannabis (accessed on 16 March 2020).
- DEA. Annual Emerging Threat Report. 2017. Available online: https://publicintelligence.net/dea-emerging-threats-reports-2017/ (accessed on 21 May 2020).
- World Drug Report. SIRIUS—United Nations Publication. 2018. Available online: https://www.unodc.org/wdr2018 (accessed on 24 March 2020).
- Freund, S.A.; Banning, A.S. Synthetic Cannabinoids: A Review of the Clinical Implications of a New Drug of Choice. JAAPA 2017, 30, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Tai, S.; Fantegrossi, W.E. Pharmacological and Toxicological Effects of Synthetic Cannabinoids and their Metabolites. Curr. Top. Behav. Neurosci. 2016, 32, 249–262. [Google Scholar] [CrossRef]
- Gamage, T.F.; Farquhar, C.E.; Lefever, T.W.; Marusich, J.A.; Kevin, R.C.; McGregor, I.S.; Wiley, J.L.; Thomas, B.F. Molecular and Behavioral Pharmacological Characterization of Abused Synthetic Cannabinoids MMB-and MDMB-FUBINACA, MN-18, NNEI, CUMYL-PICA, and 5-Fluoro-CUMYL-PICA. J. Pharm. Exp. 2018, 365, 437–446. [Google Scholar] [CrossRef]
- Castaneto, M.S.; Gorelick, D.A.; Desrosiers, N.A.; Hartman, R.L.; Pirard, S.; Huestis, M.A. Synthetic Cannabinoids: Epidemiology, Pharmacodynamics, and Clinical Implications. Drug Alcohol Depend. 2014, 144, 12–41. [Google Scholar] [CrossRef]
- Noble, C.; Cannaert, A.; Linnet, K.; Stove, C.P. Application of an Activity-based Receptor Bioassay to Investigate the In Vitro Activity of Selected Indole-and Indazole-3-carboxamide-based Synthetic Cannabinoids at CB1 and CB2 Receptors. Drug Test. Anal. 2019, 11, 501–511. [Google Scholar] [CrossRef]
- Banister, S.D.; Moir, M.; Stuart, J.; Kevin, R.C.; Wood, K.E.; Longworth, M.; Wilkinson, S.M.; Beinat, C.; Buchanan, A.S.; Glass, M. Pharmacology of Indole and Indazole Synthetic Cannabinoid Designer Drugs AB-FUBINACA, ADB-FUBINACA, AB-PINACA, ADB-PINACA, 5F-AB-PINACA, 5F-ADB-PINACA, ADBICA, and 5F-ADBICA. ACS Chem. Neurosci. 2015, 6, 1546–1559. [Google Scholar] [CrossRef] [PubMed]
- Zattera, L.; Errasti, J.; Supervía, A. Intoxication by the Synthetic Cannabinoid 5-fluoro-ABD, Acquired as Ketamine. Med. Clin. 2018. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, M.D.; Trecki, J.; Edison, L.A.; Steck, A.R.; Arnold, J.K.; Gerona, R.R. A Common Source Outbreak of Severe Delirium Associated with Exposure to the Novel Synthetic Cannabinoid ADB-PINACA. J. Emerg. Med. 2015, 48, 573–580. [Google Scholar] [CrossRef] [PubMed]
- Trecki, J.; Gerona, R.R.; Schwartz, M.D. Synthetic Cannabinoid-related Illnesses and Deaths. N. Engl. J. Med. 2015, 373, 103–107. [Google Scholar] [CrossRef]
- Tait, R.J.C.D.; Mountain, D.; Hill, S.L.; Lenton, S. A Systematic Review of Adverse Events Arising from the Use of Synthetic Cannabinoids and their Associated Treatment. Clin. Toxicol. 2016, 54, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Adedinsewo, D.A.; Odewole, O.; Todd, T. Acute Rhabdomyolysis Following Synthetic Cannabinoid Ingestion. N. Am. J. Med. Sci. 2016, 8, 256. [Google Scholar] [CrossRef]
- EMCDDA. Perspectives on Drugs: Synthetic Cannabinoids in Europe. 2017. Available online: https://www.emcdda.europa.eu/system/files/publications/2753/POD_Synthetic%20cannabinoids_0.pdf (accessed on 13 May 2020).
- Tamama, K.L. Newly Emerging Drugs of Abuse. Handb. Exp. Pharm. 2019. [Google Scholar] [CrossRef]
- Kumar, K.K.; Shalev-Benami, M.; Robertson, M.J.; Hu, H.; Banister, S.D.; Hollingsworth, S.A.; Latorraca, N.R.; Kato, H.E.; Hilger, D.; Maeda, S. Structure of a Signaling Cannabinoid Receptor 1-G Protein Complex. Cell 2019, 176, 448–458.e12. [Google Scholar] [CrossRef]
- WHO. Critical Review Report: FUB-AMB (MMB-FUBINACA, AMB-FUBINACA). World Health Organ Tech Rep Ser. 2018. Available online: https://www.who.int/medicines/access/controlled-substances/Fub_amb.pdf (accessed on 22 September 2020).
- Doi, T.; Asada, A.; Takeda, A.; Tagami, T.; Katagi, M.; Kamata, H.; Sawabe, Y.J.J.O.C.A. Enantioseparation of the carboxamide-type synthetic cannabinoids N-(1-amino-3-methyl-1-oxobutan-2-yl)-1-(5-fluoropentyl)-1H-indazole-3-carboxamide and methyl [1-(5-fluoropentyl)-1H-indazole-3-carbonyl]-valinate in illicit herbal products. J. Chromatogr. A 2016, 1473, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Antonides, L.H.; Cannaert, A.; Norman, C.; Vives, L.; Harrison, A.; Costello, A.; Nic Daeid, N.; Stove, C.P.; Sutcliffe, O.B.; McKenzie, C.J.F. Enantiospecific synthesis, chiral separation, and biological activity of four indazole-3-carboxamide-type synthetic cannabinoid receptor agonists and their detection in seized drug samples. Front. Chem. 2019, 7, 321. [Google Scholar] [CrossRef]
- Doi, T.; Tagami, T.; Takeda, A.; Asada, A.; Sawabe, Y.J.F.T. Evaluation of carboxamide-type synthetic cannabinoids as CB 1/CB 2 receptor agonists: Difference between the enantiomers. Forensic Toxicol. 2018, 36, 51–60. [Google Scholar] [CrossRef]
- Antonides, L.H.; Cannaert, A.; Norman, C.; NicDáeid, N.; Sutcliffe, O.B.; Stove, C.P.; McKenzie, C.J.D.T. Shape matters: The application of activity-based in vitro bioassays and chiral profiling to the pharmacological evaluation of synthetic cannabinoid receptor agonists in drug-infused papers seized in prisons. Drug Test. Anal. 2020. [Google Scholar] [CrossRef]
- Ametovski, A.; Macdonald, C.; Manning, J.J.; Haneef, S.S.; Santiago, M.; Martin, L.; Sparkes, E.; Reckers, A.; Gerona, R.R.; Connor, M.J.A.C.N. Exploring Stereochemical and Conformational Requirements at Cannabinoid Receptors for Synthetic Cannabinoids Related to SDB-006, 5F-SDB-006, CUMYL-PICA, and 5F-CUMYL-PICA. ACS Chem. Neurosci. 2020, 11, 3672–3682. [Google Scholar] [CrossRef]
- Chemicals, C. ADB-FUBINACA. Available online: www.caymanchem.com/product/142922018 (accessed on 27 March 2020).
- DEA. ADB-FUBINACA. 2017. Available online: http://swgdrug.org/Monographs/ADB-FUBINACA.pdf (accessed on 27 March 2020).
- WHO. Critical Review Report: ADB-FUBINACA. World Health Organ Tech Rep Ser. 2018. Available online: https://www.who.int/medicines/access/controlled-substances/ADB_Fubinaca.pdf (accessed on 22 September 2020).
- Takayama, T.S.M.; Todoroki, K.; Inoue, K.; Min, J.Z.; Kikura-Hanajiri, R.; Goda, Y.; Toyo’oka, T. UPLC/ESI-MS/MS-based Determination of Metabolism of Several New Illicit Drugs, ADB-FUBINACA, AB-FUBINACA, AB-PINACA, QUPIC, 5F-QUPIC and α-PVT, by Human Liver Microsome. Biomed. Chromatogr. 2014, 28, 831–838. [Google Scholar] [CrossRef]
- Xu, D.Z.W.; Lij, J.; Qin, S.; Lu, J. Analysis of AMB-FUBINACA Biotransformation Pathways in Human Liver Microsome and Zebrafish Systems by Liquid Chromatography-High Resolution Mass Spectrometry. Front. Chem. 2019, 7, 240. [Google Scholar] [CrossRef] [PubMed]
- Kevin, R.C.; Kovach, A.L.; Lefever, T.W.; Gamage, T.F.; Wiley, J.L.; McGregor, I.S.; Thomas, B.F. Toxic by Design? Formation of Thermal Degradants and Cyanide from Carboxamide-type Synthetic Cannabinoids CUMYL-PICA, 5F-CUMYL-PICA, AMB-FUBINACA, MDMB-FUBINACA, NNEI, and MN-18 During Exposure to High Temperatures. Forensic Toxicol. 2019, 37, 17–26. [Google Scholar] [CrossRef]
- Hess, C.E.A. Freeze-thaw Stability and Long-term Stability of 84 Synthetic Cannabinoids in Serum. Drug Test. Anal. 2017, 9, 1506–1511. [Google Scholar] [CrossRef] [PubMed]
- Tynon, M.H.J.; Kacinko, S.; Ervin, A.; McMullin, M.; Logan, B.K. Rapid and Sensitive Screening and Confirmation of Thirty-four Aminocarbonyl/Carboxamide (NACA) and Arylindole Synthetic Cannabinoid Drugs in Human Whole Blood. Drug Test. Anal. 2017, 9, 924–934. [Google Scholar] [CrossRef]
- Scheidweiler, K.B.; Jarvis, M.J.; Huestis, M.A. Nontargeted SWATH Acquisition for Identifying 47 Synthetic Cannabinoid Metabolites in Human Urine by Liquid Chromatography-high-resolution Tandem Mass Spectrometry. Anal. Bioanal. Chem. 2015, 407, 883–897. [Google Scholar] [CrossRef] [PubMed]
- Muehlethaler, C.L.M.; Lombardi, J.R. Towards a Validation of Surface-enhanced Raman Scattering (SERS) for use in Forensic Science: Repeatability and Reproducibility Experiments. Forensic Sci. Int. 2016, 268, 1–13. [Google Scholar] [CrossRef]
- Fabregat-Safont, D.M.M.; Noble, C.; Cannaert, A.; Stove, C.P.; Sancho, J.V.; Linnet, K.; Hernández, F.; Ibáñez, M. Comprehensive Investigation on Synthetic Cannabinoids: Metabolic Behavior and Potency Testing, Using 5F-APP-PICA and AMB-FUBINACA as Model Compounds. Drug Test. Anal. 2019, 11, 1358–1368. [Google Scholar] [CrossRef]
- Dinis-Oliveira, R.J. Metabolomics of Δ9-tetrahydrocannabinol: Implications in Toxicity. Drug Metab. Rev. 2016, 48, 80–87. [Google Scholar] [CrossRef]
- Krasowski, M.D.; Ekins, S. Using Cheminformatics to Predict Cross Reactivity of “Designer Drugs” to their Currently Available Immunoassays. J. Cheminform. 2014, 6, 22. [Google Scholar] [CrossRef] [PubMed]
- Spinelli, E.; Barnes, A.J.; Young, S.; Castaneto, M.S.; Martin, T.M.; Klette, K.L.; Huestis, M.A. Performance Characteristics of an ELISA Screening Assay for Urinary Synthetic Cannabinoids. Drug Test. Anal. 2015, 7, 467–474. [Google Scholar] [CrossRef]
- Hamilton, R.J.; Keyfes, V.; Banka, S.S. Synthetic Cannabinoid Abuse Resulting in ST-segment Elevation Myocardial Infarction Requiring Percutaneous Coronary Intervention. J. Emerg. Med. 2017, 52, 496–498. [Google Scholar] [CrossRef]
- Savchuk, S.A.S.; Pechnikov, A.; Rizvanova, L.; Shestakova, K.; Tagliaro, F. In Vivo Metabolism of the New Synthetic Cannabinoid APINAC in Rats by GC–MS and LC–QTOF-MS. Forensic Toxicol. 2017, 35, 359–368. [Google Scholar] [CrossRef]
- Mostowtt, T.M.B. Surface Enhanced Raman Spectroscopy (SERS) as a Method for the Toxicological Analysis of Synthetic Cannabinoids. Talanta 2017, 164, 396–402. [Google Scholar] [CrossRef]
- Kavanagh, P.G.A.; Krupina, N. Detection of Metabolites of Two Synthetic Cannabimimetics, MDMB-FUBINACA and ADB-FUBINACA, in Authentic Human Urine Specimens by Accurate Mass LC–MS: A Comparison of Intersecting Metabolic Patterns. Forensic Toxicol. 2017, 35, 284–300. [Google Scholar] [CrossRef]
- Castaneto, M.S.; Wohlfarth, A.; Pang, S.; Zhu, M.; Scheidweiler, K.B.; Kronstrand, R.; Huestis, M.A. Identification of AB-FUBINACA Metabolites in Human Hepatocytes and Urine Using High-resolution Mass Spectrometry. Forensic Toxicol. 2015, 33, 295–310. [Google Scholar] [CrossRef]
- Carlier, J.D.X.; Scheidweiler, K.B.; Huestis, M.A. Distinguishing Intake of New Synthetic Cannabinoids ADB-PINACA and 5F-ADB-PINACA with Human Hepatocyte Metabolites and High-resolution Mass Spectrometry. Clin. Chem. 2017, 63, 1008–1021. [Google Scholar] [CrossRef]
- Rouxinol, D.; da Silva, D.D.; Silva, J.P.; Carvalho, F.; de Lourdes Bastos, M.; Carmo, H. Biodistribution and Metabolic Profile of 3, 4-dimethylmethcathinone (3, 4-DMMC) in Wistar Rats Through Gas Chromatography–Mass Spectrometry (GC–MS) Analysis. Toxicol. Lett. 2020, 320, 113–123. [Google Scholar] [CrossRef]
- Hasegawa, K.W.A.; Minakata, K.; Gonmori, K.; Nozawa, H.; Yamagishi, I.; Watanabe, K.; Suzuki, O. Postmortem Distribution of AB-CHMINACA, 5-fluoro-AMB, and Diphenidine in Body Fluids and Solid Tissues in a Fatal Poisoning Case: Usefulness of Adipose Tissue for Detection of the Drugs in Unchanged Forms. Forensic Toxicol. 2015, 33, 45–53. [Google Scholar] [CrossRef]
- Islam, S.K.; Cheng, Y.P.; Birke, R.L.; Green, O.; Kubic, T.; Lombardi, J.R. Rapid and Sensitive Detection of Synthetic Cannabinoids AMB-FUBINACA and α-PVP Using Surface Enhanced Raman Scattering (SERS). J. Chem. Phys. 2018, 506, 31–35. [Google Scholar] [CrossRef]
- SNFL. Analytical Report: FUB-AMB. Slovenija National Forensic Laboratory, Ljubljana. 2015. Available online: https://www.policija.si/apps/nfl_response_web/0_Analytical_Reports_final/AMB-FUBICA-ID-1657-16-rpt220916.pdf (accessed on 20 March 2020).
- Göl, E.Ç.İ. Assessment of Types of Synthetic Cannabinoids in Narcotic Cases Assessed by the Council of Forensic Medicine Between 2011–2015, Ankara, Turkey. Forensic Sci. Int. 2017, 280, 124–129. [Google Scholar] [CrossRef]
- Brandehoff, N.A.A.; McDaniel, K.; Banister, S.D.; Gerona, R.; Monte, A.A. Synthetic Cannabinoid “Black Mamba” Infidelity in Patients Presenting for Emergency Stabilization in Colorado: A P SCAN Cohort. Clin. Toxicol. 2018, 56, 193–198. [Google Scholar] [CrossRef]
- Nacca, N.S.R.; Loflin, R.; Weltler, A.; Gorodetsky, R.; Kacinko, S.; Moran, J.; Krotulski, A.; Wiegand, T. Coma, Seizures, Atrioventricular Block, and Hypoglycemia in an ADB-FUBINACA Body-packer. J. Emerg. Med. 2018, 55, 788–791. [Google Scholar] [CrossRef] [PubMed]
- Kovács, K.; Kereszty, É.; Berkecz, R.; Tiszlavicz, L.; Sija, É.; Körmöczi, T.; Jenei, N.; Révész-Schmehl, H.; Institóris, L. Fatal Intoxication of a Regular Drug user Following N-ethyl-hexedrone and ADB-FUBINACA Consumption. J. Forensic Leg. Med. 2019, 65, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Göl, E.Ç.I. New Psychoactive Substances in Turkey: Narcotics Cases Assessed by the Council of Forensic Medicine Between 2016 and 2017 in Ankara, Turkey. Forensic Sci. Int. 2019, 294, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Al-Matrouk, A.; Alqallaf, M.; AlShemmeri, A.; BoJbarah, H. Identification of Synthetic Cannabinoids that Were Seized, Consumed, or Associated With Deaths in Kuwait in 2018 Using GC–MS and LC–MS-MS Analysis. Forensic Sci. Int. 2019, 303, 109960. [Google Scholar] [CrossRef] [PubMed]
- Ong, R.S.; Kappatos, D.C.; Russell, S.G.G.; Poulsen, H.A.; Banister, S.D.; Gerona, R.R.; Glass, M.; Johnson, C.S.; McCarthy, M. Simultaneous Analysis of 29 Synthetic Cannabinoids and Metabolites, Amphetamines, and Cannabinoids in Human Whole Blood by Liquid Chromatography–tandem Mass Spectrometry–A New Zealand Perspective of Use in 2018. Drug Test. Anal. 2020, 12, 195–214. [Google Scholar] [CrossRef] [PubMed]
- Lavado, E.L.J.; Carapinha, L.; Torrado, M.; Frango, P.; Calado, V. New Psychoactive Substances in Portugal: Trendspotter Methodology/Final Report; SICAD: Uboldo, Italy, 2018. [Google Scholar]
- Moeller, S.L.C.; Struffert, T.; Schwarze, B.; Gerner, S.T.; Schwab, S.; Köhrmann, M.; Machold, K.; Philipsen, A.; Müller, H.H. Ischemic Stroke Associated With the Use of a Synthetic Cannabinoid (Spice). Asian J. Psychiatr. 2017, 25, 127–130. [Google Scholar] [CrossRef] [PubMed]
- EMCDDA. European Drug Report 2017: Trends and Developments; European Union Publications Office: Luxembourg, 2017; Available online: https://www.emcdda.europa.eu/system/files/publications/4541/TDAT17001ENN.pdf (accessed on 17 May 2020).
- EMCDDA. European Drug Report 2019: Trends and Developments; European Union Publications Office: Luxembourg, 2019; Available online: https://www.emcdda.europa.eu/system/files/publications/11364/20191724_TDAT19001ENN_PDF.pdf (accessed on 24 May 2020).
- Yin, S. Adolescents and Drug Abuse: 21st Century Synthetic Substances. Clin. Pediatr. Emerg. Med. 2019, 20, 17–24. [Google Scholar] [CrossRef]
- DEA. Schedules of Controlled Substances: Temporary Placement of Six Synthetic Cannabinoids (5F-ADB, 5F-AMB, 5F-APINACA, ADB-FUBINACA, MDMB-CHMICA and MDMB-FUBINACA) into Schedule I. Temporary Scheduling Order. Fed. Regist. 2017, 82, 17119–17124. [Google Scholar]
- DEA. Emerging Threat Report. 2018. Available online: https://ndews.umd.edu/sites/ndews.umd.edu/files/Emerging-Threat-Report-2018-Annual.pdf (accessed on 30 March 2020).
- DEA. Emerging Threat Report. 2019. Available online: https://ndews.umd.edu/sites/ndews.umd.edu/files/DEA-Emerging-Threat-Report-2019-Annual.pdf (accessed on 30 March 2020).
- Decision, C. Proposal on the Position to Be Taken, on Behalf of the European Union, in the Sixty-second Session of the Commission on Narcotic Drugs on the Scheduling of Substances Under the Single Convention on Narcotic Drugs of 1961, as Amended. 2019. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:52018PC0862 (accessed on 1 April 2020).
- DEA. Placement of FUB-AMB in Schedule I. 2020. Available online: https://www.deadiversion.usdoj.gov/fed_regs/rules/2020/fr0330.htm (accessed on 20 March 2020).
- DEA. Placement of 5F-ADB, 5F-AMB, 5F-APINACA, ADB-FUBINACA, MDMB-CHMICA and MDMB-FUBINACA in Schedule I. 2020. Available online: https://www.deadiversion.usdoj.gov/fed_regs/rules/2020/fr0124.htm (accessed on 29 September 2020).
- Government, C. Unauthorized Product Containing Synthetic Cannabinoids Sold from Stores in Edmonton. Recalls and Safety Alerts. 2017. Available online: http://healthycanadians.gc.ca/recall-alert-rappel-avis/hc-sc/2017/64304a-eng.php?_ga=2.231065567.1384206184.1517954225-1412185756.1513370286 (accessed on 15 March 2020).
- Services, A.H. AMB-FUBINACA & Other Synthetic Cannabinoids-Backgrounder. Alberta Addiction e Mental Health Research Partnership Program. 2018. Available online: https://www.albertahealthservices.ca/assets/info/res/mhr/if-res-mhr-amb-fubinaca-backgrounder.pdf (accessed on 15 March 2020).
- 1920/2006, E.n. Regulation of the European Parliament and of the Council of 12 December 2006. EMCDDA, Ed.; 2006. Available online: https://eur-lex.europa.eu/legal-content/pt/TXT/?uri=CELEX%3A32006R1920 (accessed on 30 March 2020).
- BtMG. Drug Trafficking Act (Narcotics Act-BtMG), Appendix II. German Controlled Substances Law. 2019. Available online: https://www.gesetze-im-internet.de/btmg_1981/anlage_ii.html (accessed on 20 March 2020).
- Justice, M.O. Decree-Law n.º 15/93 of 22 January. 1993, pp. 234–252. Available online: https://data.dre.pt/eli/dec-lei/15/1993/01/22/p/dre/pt/html (accessed on 21 March 2020).
- Committee. Craft n.º 71/XIV/1a-CACDLG/2020. Assembly of the Republic Commission for Constitutional Affairs Rights Freedoms and Guarantees. 2020. Available online: http://app.parlamento.pt/webutils/docs/doc.pdfpath6148523063446f764c324679626d56304c334e706447567a4c31684a566b786c5a793944543030764d554e425130524d5279394562324e31625756756447397a5357357059326c6864476c3259554e7662576c7a633246764c3249325a475a6d597a526a4c546b304d4749744e445a6959793035593251354c5745314d6d517a4d44686c5a4459354d4335775a47593d&fich=b6dffc4c-940b-46bc-9cd9-a52d308ed690.pdf&Inline=true&fbclid=IwAR3hGByRFkHzmsrauXgisEcHsHWfuxOqvODXriGrk8rp48W5z_lUTPcKBQE (accessed on 17 March 2020).
- Alipour, A.; Patel, P.B.; Shabbir, Z.; Gabrielson, S. Review of the Many Faces of Synthetic Cannabinoid Toxicities. Ment. Health Clin. 2019, 9, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Cohen, K.; Weinstein, A.M. Synthetic and Non-synthetic Cannabinoid Drugs and Their Adverse Effects-a Review from Public Health Prospective. Front. Public Health 2018, 6, 162. [Google Scholar] [CrossRef] [PubMed]
- Hermanns-Clausen, M.K.S.; Szabo, B.; Auwärter, V. Acute Toxicity Due to the Confirmed Consumption of Synthetic Cannabinoids: Clinical and Laboratory Findings. Addiction 2013, 108, 534–544. [Google Scholar] [CrossRef] [PubMed]
- Tournebize, J.; Gibaja, V.; Kahn, J.P. Acute Effects of Synthetic Cannabinoids: Update 2015. Subst. Abus. 2016, 38, 344–366. [Google Scholar] [CrossRef]
- Finlay, D.B.; Manning, J.J.; Ibsen, M.S.; Macdonald, C.E.; Patel, M.; Javitch, J.A.; Banister, S.D.; Glass, M. Do Toxic Synthetic Cannabinoid Receptor Agonists Have Signature In Vitro Activity Profiles? A Case Study of AMB-FUBINACA. ACS Chem. Neurosci. 2019, 10, 4350–4360. [Google Scholar] [CrossRef]
- Forum, D. FUB-AMB, AMB-FUBINACA. 2016. Available online: https://forum.dopalamy.com/topic/10829-fub-amb-amb-fubinaca/?page=2 (accessed on 20 March 2020).
- Network, R.I. A Look so Far Into my Use of FUBAMB. Reddit Research Chemicals Fórum. 2016. Available online: https://www.reddit.com/r/researchchemicals/comments/4dre6j/a_look_so_far_into_my_use_of_fubamb/ (accessed on 20 March 2020).
- Davidson, C.; Opacka-Juffry, J.; Martin, A.A.; Ovejero, D.G.; Holgado, E.M.; Holgado, F.M. Spicing Up Pharmacology: A Review of Synthetic Cannabinoids from Structure to Adverse Events. Adv. Pharm. 2017, 135–168. [Google Scholar] [CrossRef]
- Thornton, S.L.; Bram, D.; Milligan, D.; Gerona, R. Rhabdomyolysis Associated With Laboratory Confirmed FUB-AMB Use. Clin. Toxicol. 2015, 53, 650–651. [Google Scholar]
- Chan, S.W.J.; Lee, B. Fatalities Related to New Psychoactive Substances in Singapore—A Case Series. Forensic Sci. Int. 2019, 304, 109892. [Google Scholar] [CrossRef]
- Rose, D.Z.; Guerrero, W.R.; Mokin, M.V.; Gooch, C.L.; Bozeman, A.C.; Pearson, J.M.; Burgin, W.S. Hemorrhagic Stroke Following Use of the Synthetic Marijuana “Spice”. Neurology 2015, 85, 1177–1179. [Google Scholar] [CrossRef] [PubMed]
- Freeman, M.J.; Rose, D.Z.; Myers, M.A.; Gooch, C.L.; Bozeman, A.C.; Burgin, W.S. Ischemic Stroke After Use of the Synthetic Marijuana “Spice”. Neurology 2013, 81, 2090–2093. [Google Scholar] [CrossRef]
- Müller, H.H.; Kornhuber, J.; Sperling, W. The Behavioral Profile of Spice and Synthetic Cannabinoids in Humans. Brain Res. Bull. 2016, 126, 3–7. [Google Scholar] [CrossRef]
- Bracewell-Worrall, A. Synthetic Drugs Kill 45 People in One Year—Coroner. 2018. Available online: https://www.newshub.co.nz/home/politics/2018/07/synthetic-drugs-kills-45-people-in-one-year-coroner.html (accessed on 16 March 2020).
- Kappatos, D.J.C.; Ong, R. ‘Border to Grave’ Surveillance of New Psychoactive Substances in New Zealand: The Dramatic Impact of AMB-FUBINACA. In Proceedings of the Flash presentation at 56th TIAFT Annual Meeting, Ghent, Belgium, 26–30 August 2018. [Google Scholar]
- Gatch, M.B.; Forster, M.J. Cannabinoid-like Effects of Five Novel Carboxamide Synthetic Cannabinoids. Neurotoxicology 2019, 70, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Karinen, R.T.S.S.; Oiestad, E.L.; Vindenes, V. Concentrations of APINACA, 5F-APINACA, UR-144 and Its Degradant Product in Blood Samples from Six Impaired Drivers Compared to Previous Reported Concentrations of Other Synthetic Cannabinoids. Forensic Sci. Int. 2015, 246, 98–103. [Google Scholar] [CrossRef]
- Kaizaki-Mitsumoto, A.H.K.; Funada, M.; Odanaka, Y.; Kumamoto, H.; Numazawa, S. Pyrolysis of UR-144, a Synthetic Cannabinoid, Augments an Affinity to Human CB1 Receptor and Cannabimimetic Effects in Mice. J. Toxicol. Sci. 2017, 42, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Thomas, B.F.; Lefever, T.W.; Cortes, R.A.; Grabenauer, M.; Kovach, A.L.; Cox, A.O.; Patel, P.R.; Pollard, G.T.; Marusich, J.A.; Kevin, R.C. Thermolytic Degradation of Synthetic Cannabinoids: Chemical Exposures and Pharmacological Consequences. J. Pharm. Exp. 2017, 361, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Manson, M.M. Epoxides—Is there a Human Health Problem? Br. J. Ind. Med. 1980, 37, 317–336. [Google Scholar] [CrossRef]
- Hughes, T.B.; Miller, G.P.; Swamidass, S.J. Modeling Epoxidation of Drug-like Molecules with a Deep Machine Learning Network. ACS Cent. Sci. 2015, 1, 168–180. [Google Scholar] [CrossRef]
- Besse, P.V.H. Chemical and Biological Synthesis of Chiral Epoxides. Tetrahedron 1994, 50, 8885–8927. [Google Scholar] [CrossRef]
- Cooper, Z.D. Adverse Effects of Synthetic Cannabinoids: Management of Acute Toxicity and Withdrawal. Curr. Psychiatry Rep. 2016, 18, 52. [Google Scholar] [CrossRef]
- Ustundag, M.F.; Ozhan Ibis, E.; Yucel, A.; Ozcan, H. Synthetic Cannabis-induced Mania. Case Rep. Psychiatry 2015, 2015, 310930. [Google Scholar] [CrossRef] [PubMed]
- Wouters, E.M.L.; Cannaert, A.; Auwärter, V.; Stove, C. Functional Evaluation of Carboxy Metabolites of Synthetic Cannabinoid Receptor Agonists Featuring Scaffolds Based on l-valine or l-tert-leucine. Drug Test. Anal. 2019, 11, 1183–1191. [Google Scholar] [CrossRef]
- Kendall, D.A.; Yudowski, G.A. Cannabinoid Receptors in the Central Nervous System: Their Signaling and Roles in Disease. Front. Cell. Neurosci. 2016, 10, 294. [Google Scholar] [CrossRef] [PubMed]
- Nogueras-Ortiz, C.; Yudowski, G.A. The Multiple Waves of Cannabinoid 1 Receptor Signaling. Mol. Pharm. 2016, 90, 620–626. [Google Scholar] [CrossRef]
- Zou, S.; Kumar, U. Cannabinoid Receptors and the Endocannabinoid System: Signaling and Function in the Central Nervous System. Int. J. Mol. Sci. 2018, 19, 833. [Google Scholar] [CrossRef]
- Longworth, M.; Banister, S.D.; Mack, J.B.; Glass, M.; Connor, M.; Kassiou, M. The 2-alkyl-2H-indazole Regioisomers of Synthetic Cannabinoids AB-CHMINACA, AB-FUBINACA, AB-PINACA, and 5F-AB-PINACA are Possible Manufacturing Impurities With Cannabimimetic Activities. Forensic Toxicol. 2016, 34, 286–303. [Google Scholar] [CrossRef]
- Lefever, T.W.; Marusich, J.A.; Thomas, B.F.; Barrus, D.G.; Peiper, N.C.; Kevin, R.C.; Wiley, J.L. Vaping Synthetic Cannabinoids: A Novel Preclinical Model of E-cigarette Use in Mice. Subst. Abus. 2017, 11, 1178221817701739. [Google Scholar] [CrossRef] [PubMed]
- Cannaert, A.S.J.; Franz, F.; Auwärter, V.; Stove, C.P. Detection and Activity Profiling of Synthetic Cannabinoids and Their Metabolites With a Newly Developed Bioassay. Anal. Chem. 2016, 88, 11476–11485. [Google Scholar] [CrossRef] [PubMed]
- Jang, M.; Kim, I.S.; Park, Y.N.; Kim, J.; Han, I.; Baeck, S.; Yang, W.; Yoo, H.H. Determination of Urinary Metabolites of XLR-11 by Liquid Chromatography–quadrupole Time-of-flight Mass Spectrometry. Anal. Bioanal. Chem. 2016, 408, 503–516. [Google Scholar] [CrossRef]
- Vikingsson, S.G.H.; Brinkhagen, L.; Mukhtar, S.; Josefsson, M. Identification of AB-FUBINACA Metabolites in Authentic Urine Samples Suitable as Urinary Markers of Drug Intake Using Liquid Chromatography Quadrupole Tandem Time of Flight Mass Spectrometry. Drug Test. Anal. 2016, 8, 950–956. [Google Scholar] [CrossRef] [PubMed]
- Kevin, R.C.; Lefever, T.W.; Snyder, R.W.; Patel, P.R.; Fennell, T.R.; Wiley, J.L.; McGregor, I.S.; Thomas, B.F. In Vitro and In Vivo Pharmacokinetics and Metabolism of Synthetic Cannabinoids CUMYL-PICA and 5F-CUMYL-PICA. Forensic Toxicol. 2017, 35, 333–347. [Google Scholar] [CrossRef]
- Tebo, C.; Mazer-Amirshahi, M.; DeGeorge, L.; Gelfand, B.; Leak, C.; Tolliver, S.; Sauter, D. Suspected Synthetic Cannabinoid Receptor Agonist Intoxication: Does Analysis of Samples Reflect the Presence of Suspected Agents? Am. J. Emerg. Med. 2019, 37, 1846–1849. [Google Scholar] [CrossRef] [PubMed]
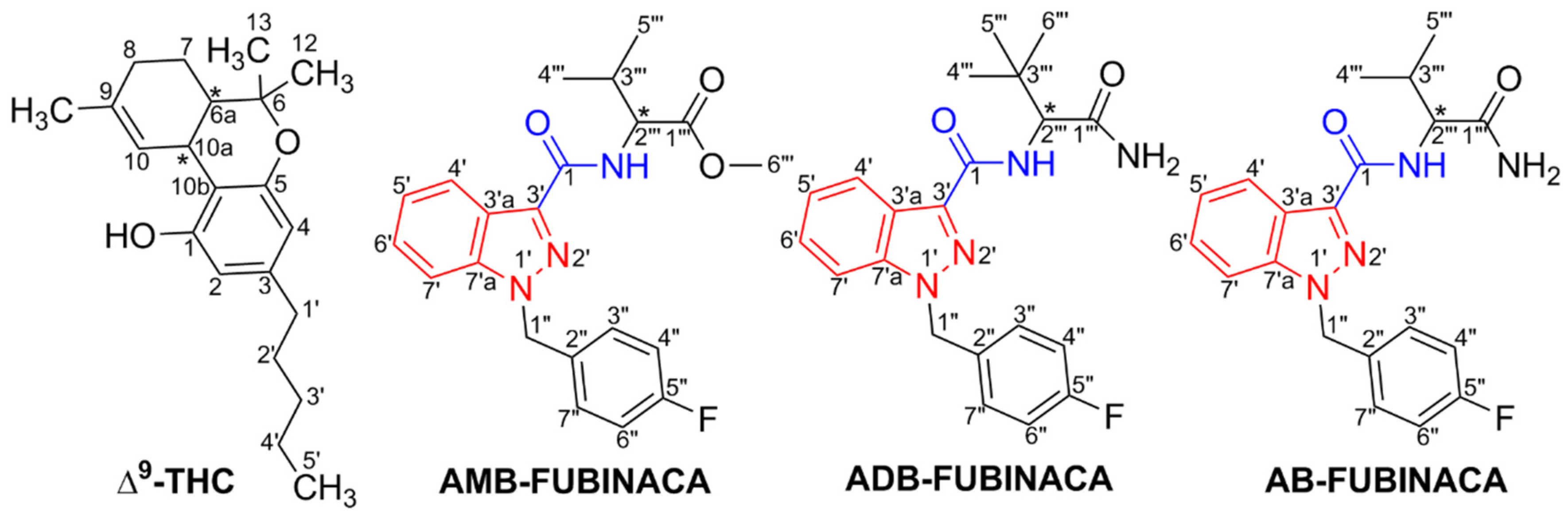

| ADB-FUBINACA | AMB-FUBINACA | |
|---|---|---|
| Chemical Formula | C21H23FN4O2 | C21H22FN3O3 |
| CAS (Chemical Abstract Service) registration number | 1445583-51-6 | 1971007-92-7 |
| Name in the International Union of Pure and Applied Chemistry (IUPAC) | (S)-N-(1-Amino-3,3-dimethyl-1-oxobutane-2-yl)-1-(4-fluorobenzyl)-1H-indazole-3-carboxamide | Methyl (S)-2-[1-(4-fluorobenzyl)-1H-indazole-3-carboxamido]-3-methylbutanoate |
| Other Designations | FUB-AMB, FUB-MMB and MMB-FUBINACA | |
| Molar Mass | 382.4 g/mol | 383.4 g/mol |
| Fusion Point | 135–137 °C | Unknown |
| Solubility | Soluble in dimethyl sulfoxide and ethanol | Soluble in dichloromethane, ethanol and methanol. Low solubility in water |
| Physical Appearance | Crystalline solid; White powder | Crystalline solid; White to yellowish powder; Slightly sweetish to the taste, with a sweet, somewhat pleasant aroma |
| Sample | Other Substance(s) Analysed | Sample Preparation/Extraction | Method for Analysis | Method Validation Parameters | Results From the Study | Reference (Year) |
|---|---|---|---|---|---|---|
| Illicit herbal-type products sold on the internet between 2012 and 2013 | Opioid AH-7921, and other 8 SCs | 10 mg of the herbal matrix crushed to powder were extracted with 1 mL methanol under ultrasonication for 10 min. Following centrifugation (5 min, 3000 rpm), the supernatant was passed through a centrifugal filter | LC-QTOF/MS with a photodiode array detector | n.d. | ADB-FUBINACA was identified for the first time in illicit products (no quantification) | [9] (2013) |
|
Standards from the National Institutes of Health Sciences (Tokyo, Japan) and their metabolites obtained from incubation with human liver microsomes | AB-FUBINACA; AB-PINACA; QUPIC; 5F-QUPIC; α-PVT; and the metabolites produced in vitro | 5 mM ADB-FUBINACA (in DMSO) was diluted 1000× in human liver microsome reaction mixture and incubated for 60 min at 37 °C. An equal volume of acetonitrile was added, and the solution centrifuged (10 min, 26,000 g). A total of 1 mL of the upper layer was diluted with water–acetonitrile containing 0.1% formic acid, and dried under reduced pressure. The residue was dissolved in 100 μL of 0.1% formic acid | UPLC/ESI-MS/MS | n.d. | The ADB-FUBINACA metabolite resulting from methyl hydroxylation at the dimethylpropane chain was disclosed for the first time | [38] (2014) |
| Samples obtained from laboratory synthesis through L-tert-leucinamide | AB-FUBINACA; AB-PINACA; ADB-PINACA; 5F-AB-PINACA; 5F-ADB-PINACA; ADBICA; 5F-ADBICA | - | LRMS-ESI; HPLC; LC-MS | n.d. | Pharmacodynamic parameters of ADB-FUBINACA were elucidated: EC50 1.2 nM at CB1R and EC50 3.5 nM at CB2R | [20] (2015) |
| Human post-mortem blood from a fatal poisoning | ∆9-THC; ∆9-THC-COOH | Blood specimen was collected from the inferior vena cava in a 60 mL polypropylene bottle, added of sodium fluoride and potassium oxalate, and a 500 μL aliquot extracted at pH 10.2 into hexane–ethyl acetate (98:2). The organic supernatant was evaporated to dryness under nitrogen, and the residue was reconstituted in 50% acetonitrile | LC–MS/MS | Internal standard: JWH-122-d9; LOQ: 0.2 ng/mL; LOD: 0.1 ng/mL; Accuracy: at 1.5 ng/mL, intrarun 84.6–106.2% and interrun 93.7%; at 6 ng/mL, intrarun 97.3–111.2% and interrun 101.9%; Precision: at 1.5 ng/mL, Intrarun 3.3–6.7% and interrun 11.4%; at 6 ng/mL, intrarun 4.8–6.3% and interrun 8.6%; Linearity: 0.2–10 ng/mL | ADB-FUBINACA: 7.3 ng/mL; ∆9-THC: 1.1 ng/mL; ∆9-THC-COOH: 4.7 ng/mL | [5] (2016) |
| Herbaceous samples seized by police from users or dealers, in Turkey between 2011 and 2015 | Other 28 SCs | 10 mg of each herbal mixture were extracted for 20 min in 1 mL of chloroform under sonication. Subsequently, 10 μL of the extract was evaporated to dryness and dissolved in 200 μL of methanol before injection | GC-MS | n.d. | Identification with no quantification. ADB-FUBINACA was the substance most identified (27.11%) | [59] (2017) |
| DEA Reference Material Collection | - | NMR: Dilution of the analyte to 7 mg/mL in CDCl3 containing TMS for reference at 0 ppm; GC-MS: Analyte dilution of 4 mg/mL CHCl3 | NMR; FTIR-ATR; GC-MS | Internal standard: Dimethylsulfone (NMR) | - | [13] (2017) |
| Human plasma from real cases | 83 SCs | 1 mL of plasma was fortified with 100 μL of 50 ng/mL of each internal standard and 500 μL of a carbonate buffer (pH 10). The aqueous phase was extracted with 4 mL of a mixture of n-hexane and ethyl acetate (99:1, v/v). After vortex mixing (1 min) and centrifugation (10 min, 4000 rpm), the organic phase was evaporated under nitrogen. The residue was dissolved in 100 μL of methanol: water: isopropanol (50:35:15, v/v) | LC–MS/MS | Internal standards: JWH-073-d7; JWH-200-d5; MAM-2201-N-(5-chloropentyl)analogue-d5 | ADB-FUBINACA remains stable when stored at −20 °C and 4 °C for 105 days; ADB-FUBINACA remains stable when stored at 20 °C for 315 days | [41] (2017) |
| Cayman Chemical standards and their metabolites obtained from incubation with human liver microsomes or human hepatocytes | - | Microssomes: 100 μM ADB-FUBINACA (in methanol) was diluted 100× in reaction mixture and incubated for 0, 3, 8, 13, 20, 45, and 60 min at 37 °C. Samples were then collected and added with an equal volume of cold acetonitrile. The samples were stored at −80 °C until analysis. The samples were thawed and diluted 100× with mobile phase before injection. Hepatocytes: ADB-FUBINACA (methanol) was diluted in hepatocyte suspension to a final 10 μM concentration, and incubated at 37 °C for 0, 1, and 3 h. Reaction was quenched with an equal volume of ice-cold acetonitrile and samples stored at −80 °C until analysis. After thawing, cells were centrifuged (5000 g for 10 min, at 4 °C) to remove cell debris and supernatants diluted 5× with 0.1% formic acid in water (mobile phase) before injection. Note: Samples were not extracted before injection to increase detection of potential metabolites. However, matrix suppression might impede detection of metabolites with low signal intensity | LC–QTOF/MS | n.d. | ADB-FUBINACA hydroxy-alkyl, ADB-FUBINACA hydroxydehydroalkyl and ADB-FUBINACA hydroxylindazole were recommended as biomarkers of exposure | [10] (2017) |
| 1142 blood samples from forensic investigations, including post-mortem examinations and driving impairment cases, between March and September 2015 | 34 SCs | 500 μL of whole blood was added of 0.1 ng/μL internal standard and 500 μL 1.0 M TRIS HCl, pH 10.2. Then, separate extractions were performed to optimise recovery of two classes of SCs: Arylindole compounds: Tubes were vortexed and extracted with 3 mL of 99% hexane/1% ethyl acetate for approximately 15 min, under agitation. Following centrifugation (10 min; 3500 rpm), the organic layer was evaporated to dryness at 30 °C under a gentle stream of nitrogen. Residue was reconstituted by adding 200 μL of methanol with 1% formic acid Aminocarbonyl/carboxamide compounds: Tubes were vortexed and extracted with 3 mL of methyl t-butyl ether for approximately 15 min, under agitation. Following centrifugation (10 min; 3500 rpm), the organic layer was evaporated to dryness at 30 °C under a gentle stream of nitrogen. Residue was reconstituted by adding 200 μL of 50:50 mixture of water with 0.1% formic acid: methanol with 0.1% formic acid | LC–MS/MS | Internal standard: AB-FUBINACA-d4; LOD: 1.0 ng/mL; Recovery: 110 ± 0.268% | ADB-FUBINACA was detected in 34 (2.3%) samples | [42] (2017) |
| AB-FUBINACA | 0.5 mL of the sample was added to a Toxi Tube-A extraction tube and shaken for 30 min and centrifuged (5 min, 2500 g). 1.25 mL of the top phase was dried under compressed air and reconstituted in 0.8 mL of 30% acetonitrile. A 10 µL aliquot was injected | HPLC-DAD; LC-MS/MS; GC-MS; IT-TOF/MS | Internal standards: 5F-AB-PINACA, pinezapam, AB-FUBINACA and ADB-FUBINACA | ADB-FUBINACA: 15.6 ng/mL; AB-FUBINACA: 5.6 ng/mL | [4] (2017) | |
| Human blood and urine from eight real cases of “Black Mamba” use prospectively captured through the Colorado site of the Psychoactive Surveillance Consortium and Analysis Network | - | Blood and/or urine samples were collected at the time of presentation. Any drug or paraphilia found with the patient was confiscated and tested. Samples were stored on ice for less than 12 h. Plasma and urine were then frozen at −80 °C, previous to shipment on dry ice to the reference laboratory at the University of California, San Francisco. No further data are available on sample preparation | LC-QTOF/MS | LOQ: 31.25 ng/mL | Only five patients had SCs found in blood or urine; three patients tested positive for ADB-FUBINACA (<31.25 ng/mL) | [60] (2018) |
| Human blood from an ADB-FUBINACA body packer (non-fatal poisoning) | Cannabis and AB-FUBINACA | - | LC-QTOF/MS | Routine validated method (NMS Labs, Willow Grove, PA) | 34 ng/mL ADB-FUBINACA | [61] (2018) |
| Human blood from a fatal poisoning | N-ethylhexedrone | To 1 mL blood sample, 30 μL of 5% ammonia solution and 2 mL ethyl acetate were added and mixed by vortex for 1 min. 1.5 mL of the upper layer were evaporated to dryness at 50 °C. The residue was dissolved in 200 μL 50:50, 0.1% formic acid: 0.1% formic acid in acetonitrile, and centrifuged at 7900 rpm for 10 min. 20 μL of the supernatant were analysed | LC–MS/MS | LOQ: 10 ng/mL; LDO: 0.01 ng/mL; Recovery: 98.9% at 1.5 ng/mL; Precision: Intraday: 9.23% at 0.1 ng/mL; 3.98% at 1.5 ng/mL; 4.75% at 6 ng/mL; Interday: 3.60% at 0.1 ng/mL; 1.86% at 1.5 ng/mL; 4.69% at 6 ng/mL; Linearity: 0.01–10 ng/mL; r2: 0.9972 | ADB-FUBINACA: 0.08 ng/mL; N-ethylhexedrone: 285 ng/mL | [62] (2019) |
| Seized samples (tablets, herbs, powders of different types and seals) in Turkey between 2016 and 2017 | 79 SCs; 6 cathinones; 3 tryptamines; 2 phenethylamines | Powders and crystals: dissolved in methanol at 1 mg/mL and further 100× diluted prior to injection. Herbal samples: 10 mg were extracted for 20 min in 1 mL of chloroform under sonication. Then, 10 μL of the extract were evaporated to dryness and residue was dissolved in 200 μL of methanol, prior to injection | GC-MS (identification only); LC–MS/MS (in some samples) | n.d. | ADB-FUBINACA was the third most identified NPS in the narcotic samples (8.95%) | [63] (2019) |
| 434 seized samples from the Narcotics and Psychotropic Laboratory; 70 human urine samples from non-fatal cases from the Toxicology Laboratory; 6 post-mortem urine samples from the Forensic Medicine Department, in Kuwait in 2018 | More than 15 SCs; 3 synthetic cathinones | Seized samples: approximately 500 mg of the dried leaves or powder were dissolved in 1 mL of methanol and centrifuged for 10 min at 1253 g at 21 °C. The supernatant was used; Urine: glucuronide conjugates were hydrolysed by adding 2 mL of 100 mM acetate buffer (pH 5.0) and 50 mL of β-glucuronidase to each mL of urine. The samples were vortexed for 30 s, heated to 65 °C for 1 to 2 h, and allowed to cool previous to solid-phase extraction | LC–MS/MS; GC–MS | n.d. | The majority of SCs were indazole-3-carboxamides, such as ADB-FUBINACA and AMB-FUBINACA. The most common SCs were 5F-ADB, AMB-FUBINACA, and 5Cl-AKB-48. Various mixtures of 2, 3, or 4 types of SCs were identified. The most common mixture was AMB-FUBINACA with 5F-ADB. These drugs were mixed, either together or individually, with methamphetamine, tramadol, heroin, ∆9-THC, and ketamine. SCs were associated with six reported deaths | [64] (2019) |
| Human blood from real cases | 29 SCs; 4 amphetamines; ∆9-THC; ∆9-THC-COOH | To 200 μL of sample, 20 μL of internal standard was added for a final concentration of 5 ng/mL. After the addition of 200 μL of 100 mM sodium acetate buffer (pH 5.0), 300 μL of the conditioned blood was loaded onto a supported-liquid-extraction cartridge. Analytes were eluted with 700 μL methyl terc-butyl ether (×2). 20 μL of 0.1 M methanolic HCl was then added to elute SCs, and all extracts were dried at 30 °C under nitrogen flow. Residues were reconstituted in 80 μL of 50:50 (v/v) water: methanol and vortexed prior to centrifugation (3000 rpm, 5 min) | LC–MS/MS | Internal standards: JWH-018; N5HP-d5; LOQ: 1 ng/mL; LOD: 0.1 to 6.0 ng/mL; Accuracy: 5.8% at 1 ng/mL; 19.1% at 5 ng/mL; Precision: 10.5% at 1 ng/mL; 9.6% at 5 ng/mL; Linearity: 1–6 ng/mL; r2: 0.999 | The validated method allowed for the simultaneous confirmation of 29 SCs and metabolites, 4 amphetamines, and 2 phytocannabinoids in human whole blood. The five most commonly detected SCs in toxicological samples in New Zealand in 2018 were AMB-FUBINACA and/or its acid metabolite, 5F-ADB and/or its acid metabolite, ADB-FUBINACA, 5F-MDMB-PICA acid metabolite, and MDMB-FUBINACA acid metabolite | [65] (2020) |
| Sample | Other Substance(s) Analysed | Sample Preparation/Extraction | Method for Analysis | Method Validation Parameters | Results From the Study | Reference (Year) |
|---|---|---|---|---|---|---|
| Serum, whole blood, and urine samples from 8 patients among the 18 who were transported to local hospitals; and a sample of the herbal “incense” product “AK-47 24 Karat Gold”, which was implicated in the so-called “Zombie” Outbreak in New York, 2016 | De-esterified acid metabolite of AMB-FUBINACA | - | LC–QTOF/MS | Internal standard: AMB-FUBINACA | AMB-FUBINACA was identified in AK-47 24 Karat Gold at 16.0 ± 3.9 mg/g. The de-esterified acid metabolite was found in the serum or whole blood of all eight patients, with concentrations ranging from 77 to 636 ng/mL in serum; and in urine of one patient at 165 ng/mL | [3] (2017) |
| Cayman Chemical drug standards | α-PVP and other NPS | 2 µL of 0.1 mM AMB-FUBINACA were mixed with 4 µL of silver nanoparticles and 2 µL of MgCl2 | SERS | LOD: 1 nM | Identification of these drugs in a combination pose a challenge for SERS, however this technique is very useful for detecting individual drugs | [57] (2018) |
| Samples from human liver microsomes in vitro and zebrafish models in vivo | Metabolites of AMB-FUBINACA | Human liver microsomes: AMB-FUBINACA at 5 mM (in methanol) was diluted 200× in microsome suspension and incubated for 1 h at 37 °C. Then, uridine diphosphate glucuronic acid trisodium salt was added and incubated for another half an hour. To terminate the reaction 200 µL of acetonitrile was added, followed by centrifugation (13,000 g; for 10 min). 100 µL of the supernatant was used after membrane filtering for analysis Zebrafish (6–10 months; 0.8–1.2 g): After exposure to 0.1, 0.5, and 1 µg/mL of AMB-FUBINACA (24 °C) for 24 h, zebrafish were removed, cleaned with water and euthanised. The zebrafish were homogenised with a ball mill, and the samples were loaded onto a SPE-Pak@Vac PSA extraction column, which had been conditioned with 1 mL of methanol and 1 mL of water. The column was washed with 1 mL of acetonitrile. The eluent was dried by evaporation at 60 °C under a stream of nitrogen. The residue was reconstituted in 100 µL of flow phase composed of acetonitrile, and 10 µL of the reconstituted solution was injected for analysis | HPLC | n.d. | The precision, simplicity and efficiency of the technique proved advantages for the identification of 17 metabolites, making it a useful tool for the detection of polar metabolites, in clinical and forensic contexts | [39] (2019) |
| Cayman Chemical standards | CUMYL-PICA, 5F-CUMYL-PICA, MDMB-FUBINACA, NNEI and MN-18 | Each SC was dissolved in acetonitrile at 0.5 mg/mL, and 16 µL were added to a quartz capillary tube loaded into the thermolysis autosampler that passed the individual samples to the thermolysis probe equilibrated at 50 °C, which was then rapidly heated (20 °C/second) to the desired temperature. The samples were heated sequentially to 200, 400, 600, and 800 °C | GC-MS; LC-MS/MS | n.d. | SCs heated above 400 °C produce thermolytic, potentially toxic degradants, such as naphthalene, 1-naphthylamine, cyanide and toluene | [40] (2019) |
| Human blood from real cases | 29 SCs; 4 amphetamines; ∆9-THC; ∆9-THC-COOH | To 200 μL of sample, 20 μL of internal standard was added for a final concentration of 5 ng/mL. After the addition of 200 μL of 100 mM sodium acetate buffer (pH 5.0), 300 μL of the conditioned blood was loaded onto a supported-liquid-extraction cartridge. Analytes were eluted with 700 μL methyl terc-butyl ether (× 2). 20 μL of 0.1 M methanolic HCl was then added to elute SCs, and all extracts were dried at 30 °C under nitrogen flow. Residues were reconstituted in 80 μL of 50:50 (v/v) water: methanol and vortexed prior to centrifugation (3000 rpm, 5 min) | LC–MS/MS | Internal standards: JWH-018; N5HP-d5; LOQ: 1 ng/mL; LOD: 0.1 to 6.0 ng/mL; Accuracy: 5.8% at 1 ng/mL; 19.1% at 5 ng/mL; Precision: 10.5% at 1 ng/mL; 9.6% at 5 ng/mL; Linearity: r2: 0.999 with a confirmation ranged between 1 to 6 ng/mL | The validated method allowed for the simultaneous confirmation of 29 SCs and metabolites, 4 amphetamines, and 2 phytocannabinoids in human whole blood. The five most commonly detected SCs in toxicological samples in New Zealand in 2018 were AMB-FUBINACA and/or its acid metabolite, 5F-ADB and/or its acid metabolite, ADB-FUBINACA, 5F-MDMB-PICA acid metabolite, and MDMB-FUBINACA acid metabolite | [65] (2020) |
| Substance | Type and Circumstances of the Intoxication | Matrix for Analytical Confirmation | Concentration | Other Detected Substances | Clinical Observations | Reference (Year) |
|---|---|---|---|---|---|---|
| ADB-FUBINACA (“Mojo”) | Fatal; In 2015, shortly after smoking an SC product, a 41-year-old female became violent and aggressive with her family. She was physically restrained by her children and eventually became unresponsive. She was declared dead by the emergency personnel a short time thereafter | Blood | 7.3 ng/mL (19.1 nM) | ∆9-THC: 1.1 ng/mL; ∆9-THC-COOH: 4.7 ng/mL | Remarkable findings at autopsy included pulmonary oedema, vascular congestion and thrombotic occlusion of the lumen of the left anterior descending coronary artery by haemorrhagic disruption of coronary arterial plaque, as well as ischemia of the anterior left ventricular myocardium | [5] (2016) |
| AMB-FUBINACA (“AK-47 24 Karat Gold”) | Non-fatal; On July 12, 2016, a mass intoxication of 33 persons in a New York City neighbourhood, in an event described in the popular press as a “zombie” outbreak. From the 18 patients transported to local hospitals, samples from eight were analysed, revealing the presence of a metabolite of AMB-FUBINACA. The herbal “incense” product implicated in the outbreak was also analysed revealing the presence of AMB-FUBINACA | Blood; Urine | AMB-FUBINACA de-esterified acid metabolite: 77 to 636 ng/mL (202.37 to 1671.5 nM) in blood; 165 ng/mL (433.7 nM) in urine; no parent compound detected | - | Strong CNS depressant effects that would account for the “zombie-like” behaviour of the users | [3] (2017) |
| ADB-FUBINACA | Non-fatal; A 24-year-old man considered healthy was taken to the medical emergency room due to acute confusion, agitation, visual hallucinations, and palpitations. 30 min before arrival, he had smoked two drops of electronic cigarette fluid from a bottle labelled “VaporFi”, mixed with a transparent liquid from another unlabelled bottle that he found to be “liquid cannabis”. The declared ingredients of the “VaporFi” were propylene glycol, glycerin and natural and artificial flavours; the composition of the unlabelled transparent fluid was unknown. The two bottles were purchased over the Internet and were intended for “vaporization” with an electronic device that aerosolises liquids | Urine | 15.6 ng/mL (41 nM) | AB-FUBINACA: 5.6 ng/mL; lidocaine, clindamycin, and cetirizine | Supraventricular tachycardia, mild hypokalaemia, acute confusion, agitation, visual hallucinations, and palpitations. The patient recovered uneventfully with supportive treatment and was discharged 22 h after admission | [4] (2017) |
| AMB-FUBINACA and ADB-FUBINACA (“Black Mamba”) | Non-fatal; From August 1 to November 30, 2016, eight acute poisoned patients went to the emergency room after consuming “Black Mamba”. There were four men and four women between the ages of 16 and 43 | Serum and urine | Metabolites: 45.3–115.9 ng/mL in serum and <1.599 ng/mL (LOQ) in urine; ADB-FUBINACA < 31.25 ng/mL (LOQ) in serum; AMB-FUBINACA: 58.7–115.9 ng/mL in serum | Cocaine: 7.8 ng/mL in the serum; 59.2, 2203 and 2362 ng/mL in the urine of 3 different patients; Benzodiazepine: 3078 ng/mL in the blood; 25.710 and 29.368 ng/mL in the urine of 2 different patients; ∆9-THC; (Meth) Amphetamine: 59.5, 349.1, 1461 ng/mL in serum of 3 different patients; 177 and 765.2 ng/mL in urine of 2 different patients; Ethanol; NGR-3: <15.6 ng/mL (LOQ) in serum and 11.2 ng/mL in urine; 3-MeO-PCP: 60.3–114.1 ng/mL in urine | Tonic–clonic seizures, elevated blood pressure. Four patients were agitated and/or delirious. Four patients had chest pain and one had T wave inversions on the electrocardiogram | [60] (2018) |
| ADB-FUBINACA | Non-fatal; A 38-year-old male inmate was transferred to a medical centre after a seven-day hospitalization for abnormal behaviour (altered mental status and bradycardia). A computed tomography scan of the abdomen and pelvis revealed multiple packages in the patient’s stomach and rectum. Multiple attempts at gastrointestinal decontamination were unsuccessful. On hospital day eight, the patient developed hypertensive emergency and was taken to the operating room for exploratory laparotomy. Twenty-two poorly wrapped packages were removed from the bowel | Serum and urine | 34 ng/mL (89.36 nM) in serum; 17 ng/mL (44.68 nM) in urine | Benzodiazepine, ∆9-THC-COOH, metoclopramide, atropine, MDMB-FUBINACA, diphenhydramine, metoclopramide, scopolamine and midazolam Cocaine: <20 ng/m (LOQ) | Upon arriving at the treatment unit, the patient was awake, but with inadequate answers to questions, complaining of shortness of breath and staring blankly into space. He demonstrated progressive encephalopathy, second-degree atrioventricular block type I, hypotension, sinus bradycardia, hypoglycaemia, hypothermia, hypopnea, and respiratory failure. Postoperatively, the patient demonstrated both generalised and focal seizure activity. His mental status slowly returned to baseline over the period of about one week and he was ultimately discharged without neurological sequelae after one month | [61] (2018) |
| “Crystal” and ADB-FUBINACA | Fatal; According to the mother, her 23-year-old son consumed “crystal” at night with friends and arrived home at 4 a.m., feeling bad and going to bed. When she entered the room, the son was kneeling on the bed, so he leaned forward, lost consciousness and died at 9:30 am. When the ambulance arrived, they tried to revive him, without success. The general practitioner declared death and suspected poisoning. The police found no illicit or designer drugs in his room | Blood | ADB-FUBINACA: 0.08 ng/mL (0.21 nM) | NEH: 285 ng/mL (749 nM) | Tachycardia, acute heart and pulmonary failure | [62] (2019) |
| AMB-FUBINACA | Fatal; A 27-year-old man was found dead in his bed by a roommate at about 11 a.m. He had been last seen alive at around 5 a.m.. The doctor checked the body at 2:45 p.m. and found no injuries to the corpse. However, vomiting was observed evolving from the oral cavity and nasal passages. The doctor was unable to determine the cause of death at the scene but found that the man had died about 3 to 8 h earlier. Empty alcohol bottles were found in the apartment, and 20 mg omeprazole tablets were found in the corpse. According to the testimonies of family members and roommates, the man had been drinking alcohol daily, for about three years. He had been treated for paranoid schizophrenia. The man started smoking marijuana at age 16 and later became addicted to “legal drugs”. The autopsy was performed five days after death | Blood and urine | In urine: 4.7 ng/mL (12.35 nM); and 8.2 ng/mL (21.55 nM) after hydrolysis of metabolites; In blood: no drug detected (LOQ ≤ 0.1 ng/mL) | EMB-FUBINACA: no drug detected (LOQ ≤ 0.1 ng/mL) in blood: 0.2 ng/mL in urine; Lorazepam: 6 ng/mL in blood and 37 ng/mL in urine; Haloperidol: 11 ng/mL in blood and 4 ng/mL in urine; Lidocaine: 29 ng/mL in blood and 35 ng/mL in urine | Vomit, congestion of internal organs, pulmonary oedema and left-sided pleural adhesions were found in post-mortem examination. The cause of death was acute respiratory failure with an unidentifiable cause | [2] (2019) |
| ADB-FUBINACA | Fatal; A 17-year-old boy died after smoking an unknown product. Soon after consumption, he experienced uncontrollable tremors and vomiting. After 6 h, he entered the emergency room already dead | Peripheral blood (femoral), urine, stomach and biliary contents | In blood: 56 ng/mL (146.5 nM)- the largest documented so far | - | Tremors and vomiting. The cause of death was attributed to the toxicity of ADB-FUBINACA since no other substance was found | [92] (2019) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lobato-Freitas, C.; Brito-da-Costa, A.M.; Dinis-Oliveira, R.J.; Carmo, H.; Carvalho, F.; Silva, J.P.; Dias-da-Silva, D. Overview of Synthetic Cannabinoids ADB-FUBINACA and AMB-FUBINACA: Clinical, Analytical, and Forensic Implications. Pharmaceuticals 2021, 14, 186. https://doi.org/10.3390/ph14030186
Lobato-Freitas C, Brito-da-Costa AM, Dinis-Oliveira RJ, Carmo H, Carvalho F, Silva JP, Dias-da-Silva D. Overview of Synthetic Cannabinoids ADB-FUBINACA and AMB-FUBINACA: Clinical, Analytical, and Forensic Implications. Pharmaceuticals. 2021; 14(3):186. https://doi.org/10.3390/ph14030186
Chicago/Turabian StyleLobato-Freitas, Carolina, Andreia Machado Brito-da-Costa, Ricardo Jorge Dinis-Oliveira, Helena Carmo, Félix Carvalho, João Pedro Silva, and Diana Dias-da-Silva. 2021. "Overview of Synthetic Cannabinoids ADB-FUBINACA and AMB-FUBINACA: Clinical, Analytical, and Forensic Implications" Pharmaceuticals 14, no. 3: 186. https://doi.org/10.3390/ph14030186
APA StyleLobato-Freitas, C., Brito-da-Costa, A. M., Dinis-Oliveira, R. J., Carmo, H., Carvalho, F., Silva, J. P., & Dias-da-Silva, D. (2021). Overview of Synthetic Cannabinoids ADB-FUBINACA and AMB-FUBINACA: Clinical, Analytical, and Forensic Implications. Pharmaceuticals, 14(3), 186. https://doi.org/10.3390/ph14030186











