Evaluation of the Synergistic Antibacterial Effects of Fosfomycin in Combination with Selected Antibiotics against Carbapenem–Resistant Acinetobacter baumannii
Abstract
1. Introduction
2. Results
2.1. Distribution of A. baumannii Isolates
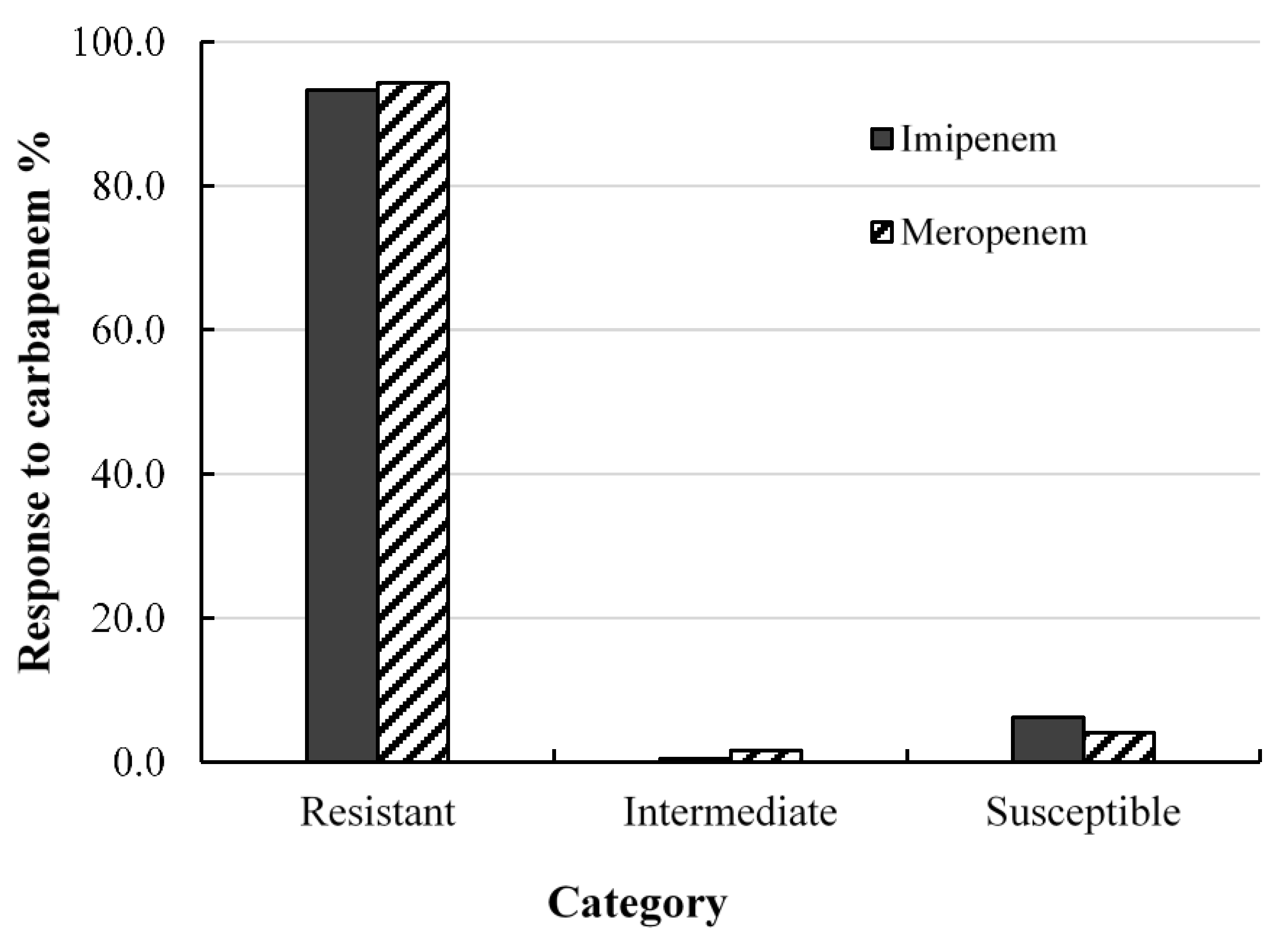
2.2. Antibacterial Effects of Carbapenem on Clinical Isolates of A. baumannii
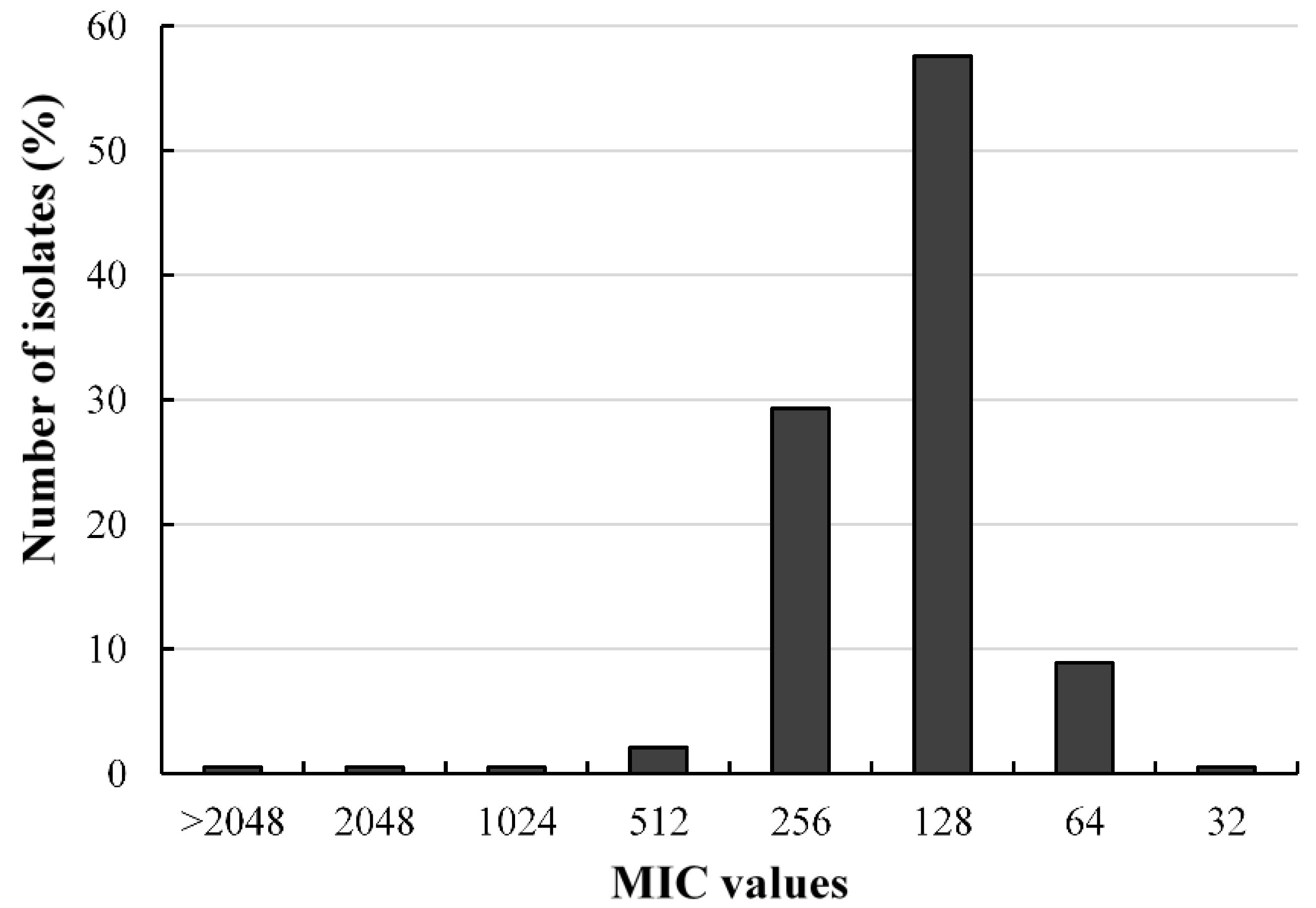
2.3. Antibacterial Activities of Fosfomycin against A. baumannii Isolates
2.4. Antibacterial Effects of Fosfomycin-Resistant Isolates
2.5. Susceptibility Profile of Carbapenem- and Fosfomycin-Resistant A. baumannii Isolates
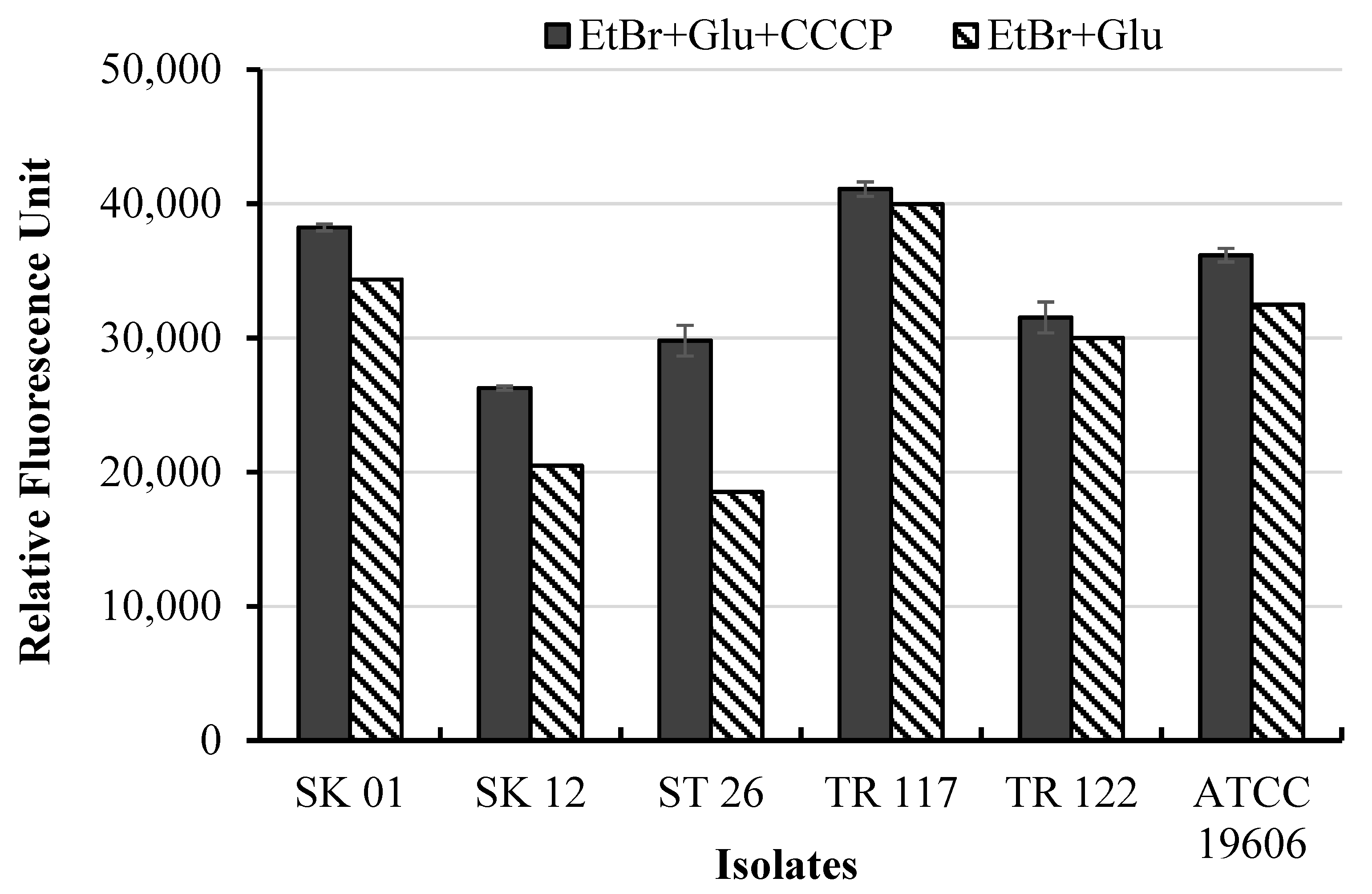
2.6. Ethidium Bromide Uptake
2.7. Synergistic Effects of Fosfomycin-Antibiotics Combination
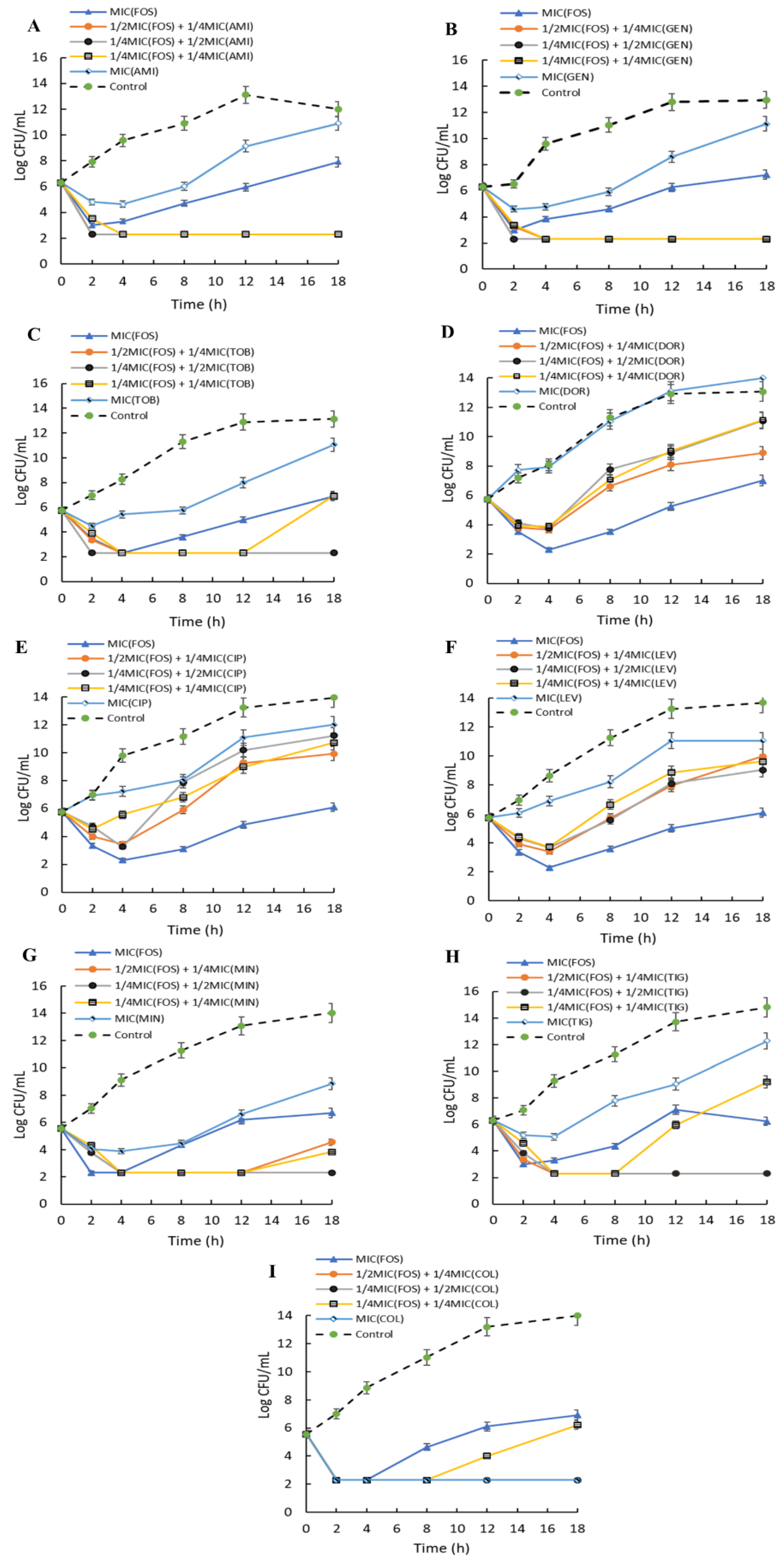
2.8. Time-Kill Kinetics of Combinations of Fosfomycin on A. baumannii
3. Discussion
4. Materials and Methods
4.1. Chemicals and Media
4.2. Bacterial Strains
4.3. Resistance to Carbapenems
4.4. Screening for Fosfomycin Resistance
4.5. Antibiogram of Fosfomycin-Resistant Isolates
4.6. Ethidium Bromide Uptake Assay
4.7. Checkerboard Technique
4.8. Time-Kill Assay
4.9. Ethical Statement
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Worthington, R.J.; Melander, C. Combination approaches to combat multidrug-resistant bacteria. Trends Biotechnol. 2013, 31, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Coates, A.R.; Hu, Y.; Holt, J.; Yeh, P. Antibiotic combination therapy against resistant bacterial infections: Synergy, rejuvenation and resistance reduction. Expert Rev. Anti-Infect. Ther. 2020, 18, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Theuretzbacher, U.; Van Bambeke, F.; Cantón, R.; Giske, C.G.; Mouton, J.W.; Nation, R.L.; Paul, M.; Turnidge, J.D.; Kahlmeter, G. Reviving old antibiotics. J. Antimicrob. Chemother. 2015, 70, 2177–2181. [Google Scholar] [CrossRef]
- Sullivan, G.J.; Delgado, N.N.; Maharjan, R.; Cain, A.K. How antibiotics work together: Molecular mechanisms behind combination therapy. Curr. Opin. Microbiol. 2020, 57, 31–40. [Google Scholar] [CrossRef] [PubMed]
- WHO. Global Priority List of Antibiotic-Resistant Bacteria to Guide Research, Discovery, and Development of New Antibiotics. 2018. Available online: https://www.who.int/medicines/publications/WHO-PPL-Short_Summary_25Feb-ET_NM_WHO.pdf (accessed on 20 January 2021).
- EUCAST. Breakpoint Tables for Interpretation of MICs and Zone Diameters; Version 10.0, 2020; EUCAST: Växjö, Sweden, 2020. [Google Scholar]
- Jin, L.; Li, J.; Nation, R.L.; Nicolazzo, J.A. Brain penetration of colistin in mice assessed by a novel high-performance liquid chromatographic technique. Antimicrob. Agents Chemother. 2009, 53, 4247–4251. [Google Scholar] [CrossRef]
- Lu, Q.; Girardi, C.; Zhang, M.; Bouhemad, B.; Louchahi, K.; Petitjean, O.; Wallet, F.; Becquemin, M.-H.; Le Naour, G.; Marquette, C.-H. Nebulized and intravenous colistin in experimental pneumonia caused by Pseudomonas aeruginosa. Intensive Care Med. 2010, 36, 1147–1155. [Google Scholar] [CrossRef]
- Markantonis, S.; Markou, N.; Fousteri, M.; Sakellaridis, N.; Karatzas, S.; Alamanos, I.; Dimopoulou, E.; Baltopoulos, G. Penetration of colistin into cerebrospinal fluid. Antimicrob. Agents Chemother. 2009, 53, 4907–4910. [Google Scholar] [CrossRef]
- Markou, N.; Markantonis, S.L.; Dimitrakis, E.; Panidis, D.; Boutzouka, E.; Karatzas, S.; Rafailidis, P.; Apostolakos, H.; Baltopoulos, G. Colistin serum concentrations after intravenous administration in critically ill patients with serious multidrug-resistant, Gram-negative bacilli infections: A prospective, open-label, uncontrolled study. Clin. Ther. 2008, 30, 143–151. [Google Scholar] [CrossRef]
- Lengerke, C.; Haap, M.; Mayer, F.; Kanz, L.; Kinzig, M.; Schumacher, U.; Sörgel, F.; Riessen, R. Low tigecycline concentrations in the cerebrospinal fluid of a neutropenic patient with inflamed meninges. Antimicrob. Agents Chemother. 2011, 55, 449–450. [Google Scholar] [CrossRef]
- Kussmann, M.; Baumann, A.; Hauer, S.; Pichler, P.; Zeitlinger, M.; Wiesholzer, M.; Burgmann, H.; Poeppl, W.; Reznicek, G. Compatibility of fosfomycin with different commercial peritoneal dialysis solutions. Eur. J. Clin. Microbiol. Infect. Dis. 2017, 36, 2237–2242. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Monogue, M.L.; Almarzoky Abuhussain, S.S.; Kuti, J.L.; Nicolau, D.P. Physical compatibility of fosfomycin for injection with select iv drugs during simulated Y-site administration. Bull. Am. Soc. Hosp. Pharm. 2018, 75, e36–e44. [Google Scholar]
- Falagas, M.E.; Vouloumanou, E.K.; Samonis, G.; Vardakas, K.Z. Fosfomycin. Clin. Microbiol. Rev. 2016, 29, 321–347. [Google Scholar] [CrossRef] [PubMed]
- Zhanel, G.G.; Zhanel, M.A.; Karlowsky, J.A. Intravenous fosfomycin: An assessment of its potential for use in the treatment of systemic infections in Canada. Can. J. Infect. Dis. Med. Microbiol. 2018, 2018, 8912039. [Google Scholar] [CrossRef]
- Kaye, K.S.; Rice, L.B.; Dane, A.; Stus, V.; Sagan, O.; Fedosiuk, E.; Das, A.; Skarinsky, D.; Eckburg, P.B.; Ellis-Grosse, E.J. Intravenous fosfomycin (ZTI-01) for the treatment of complicated urinary tract infections (cUTI) including acute pyelonephritis (AP): Results from a multi-center, randomized, double-blind phase 2/3 study in hospitalized adults (ZEUS). Open Forum Infect. Dis. 2017, 4, S528–S529. [Google Scholar] [CrossRef]
- Falagas, M.E.; Giannopoulou, K.P.; Kokolakis, G.N.; Rafailidis, P.I. Fosfomycin: Use beyond urinary tract and gastrointestinal infections. Clin. Infect. Dis. 2008, 46, 1069–1077. [Google Scholar] [CrossRef]
- Russo, A.; Bassetti, M.; Bellelli, V.; Bianchi, L.; Cattaneo, F.M.; Mazzocchetti, S.; Paciacconi, E.; Cottini, F.; Schiattarella, A.; Tufaro, G. Efficacy of a Fosfomycin-Containing Regimen for Treatment of Severe Pneumonia Caused by Multidrug-Resistant Acinetobacter baumannii: A Prospective, Observational Study. Infect. Dis. Ther. 2020. [Google Scholar] [CrossRef]
- Ku, N.S.; Lee, S.H.; Lim, Y.S.; Choi, H.; Ahn, J.Y.; Jeong, S.J.; Shin, S.J.; Choi, J.Y.; Choi, Y.H.; Yeom, J.-S. In vivo efficacy of combination of colistin with fosfomycin or minocycline in a mouse model of multidrug-resistant Acinetobacter baumannii pneumonia. Sci. Rep. 2019, 9, 17127. [Google Scholar] [CrossRef] [PubMed]
- Chukamnerd, A.; Pomwised, R.; Phoo, M.T.P.; Terbtothakun, P.; Hortiwakul, T.; Charoenmak, B.; Chusri, S. In vitro synergistic activity of fosfomycin in combination with other antimicrobial agents against carbapenem-resistant Klebsiella pneumoniae isolated from patients in a hospital in Thailand. J. Infect. Chemother. 2020, 27, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Erturk Sengel, B.; Altinkanat Gelmez, G.; Soyletir, G.; Korten, V. In vitro synergistic activity of fosfomycin in combination with meropenem, amikacin and colistin against OXA-48 and/or NDM-producing Klebsiella pneumoniae. J. Chemother. 2020, 32, 237–243. [Google Scholar] [CrossRef] [PubMed]
- El-Wafa, W.M.A.; Ibrahim, Y.M. In Vitro Activity of Fosfomycin in Double and Triple Combination with Imipenem, Ciprofloxacin and Tobramycin Against Multidrug-Resistant Escherichia coli. Curr. Microbiol. 2020, 77, 755–761. [Google Scholar] [CrossRef]
- Seok, H.; Choi, J.Y.; Wi, Y.M.; Park, D.W.; Peck, K.R.; Ko, K.S. Fosfomycin Resistance in Escherichia coli isolates from South Korea and in vitro activity of fosfomycin alone and in combination with other antibiotics. Antibiotics 2020, 9, 112. [Google Scholar] [CrossRef]
- Cuba, G.T.; Rocha-Santos, G.; Cayô, R.; Streling, A.P.; Nodari, C.S.; Gales, A.C.; Pignatari, A.C.; Nicolau, D.P.; Kiffer, C.R. In vitro synergy of ceftolozane/tazobactam in combination with fosfomycin or aztreonam against MDR Pseudomonas aeruginosa. J. Antimicrob. Chemother. 2020, 75, 1874–1878. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing; CLSI: Wayne, PA, USA, 2017. [Google Scholar]
- Leite, G.C.; Oliveira, M.S.; Perdigão-Neto, L.V.; Rocha, C.K.D.; Guimarães, T.; Rizek, C.; Levin, A.S.; Costa, S.F. Antimicrobial combination against pan-resistant Acinetobacter baumannii isolates with different resistance mechanisms. PLoS ONE 2016, 11, e0151270. [Google Scholar] [CrossRef] [PubMed]
- Perdigao-Neto, L.; Oliveira, M.; Rizek, C.; Carrilho, C.; Costa, S.; Levin, A. Susceptibility of multiresistant Gram-negative bacteria to fosfomycin and performance of different susceptibility testing methods. Antimicrob. Agents Chemother. 2014, 58, 1763–1767. [Google Scholar] [CrossRef][Green Version]
- Singkham-in, U.; Chatsuwan, T. In vitro activities of carbapenems in combination with amikacin, colistin, or fosfomycin against carbapenem-resistant Acinetobacter baumannii clinical isolates. Diagn. Microbiol. Infect. Dis. 2018, 91, 169–174. [Google Scholar] [CrossRef]
- Leelasupasri, S.; Santimaleeworagun, W.; Jitwasinkul, T. Antimicrobial susceptibility among colistin, sulbactam, and fosfomycin and a synergism study of colistin in combination with sulbactam or fosfomycin against clinical isolates of carbapenem-resistant Acinetobacter baumannii. J. Pathog. 2018, 2018, 3893492. [Google Scholar] [CrossRef]
- Lu, C.L.; Liu, C.Y.; Huang, Y.T.; Liao, C.H.; Teng, L.J.; Turnidge, J.D.; Hsueh, P.R. Antimicrobial susceptibilities of commonly encountered bacterial isolates to fosfomycin determined by agar dilution and disk diffusion methods. Antimicrob. Agents Chemother. 2011, 55, 4295–4301. [Google Scholar] [CrossRef]
- Sharma, A.; Sharma, R.; Bhattacharyya, T.; Bhando, T.; Pathania, R. Fosfomycin resistance in Acinetobacter baumannii is mediated by efflux through a major facilitator superfamily (MFS) transporter—AbaF. J. Antimicrob. Chemother. 2016, 72, 68–74. [Google Scholar] [CrossRef]
- Siriyong, T.; Chusri, S.; Srimanote, P.; Tipmanee, V.; Voravuthikunchai, S.P. Holarrhena antidysenterica extract and its steroidal alkaloid, conessine, as resistance-modifying agents against extensively drug-resistant Acinetobacter baumannii. Microb. Drug Resist. 2016, 22, 273–282. [Google Scholar] [CrossRef] [PubMed]
- WHO. Antibiotic Resistance Fact Sheets. Available online: https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance (accessed on 20 January 2021).
- Paiboonvong, T.; Rodjun, V.; Houngsaitong, J.; Chomnawang, M.; Montakantikul, P.; Chulavatnatol, S. Comparative in vitro activity of sitafloxacin against multidrug-resistant and carbapenem-resistant Acinetobacter baumannii clinical isolates in Thailand. Sci. Asia 2020, 47, 37–42. [Google Scholar] [CrossRef]
- Xu, X.; Xu, L.; Yuan, G.; Wang, Y.; Qu, Y.; Zhou, M. Synergistic combination of two antimicrobial agents closing each other’s mutant selection windows to prevent antimicrobial resistance. Sci. Rep. 2018, 8, 7237. [Google Scholar] [CrossRef]
- Domalaon, R.; Ammeter, D.; Brizuela, M.; Gorityala, B.K.; Zhanel, G.G.; Schweizer, F. Repurposed antimicrobial combination therapy: Tobramycin-ciprofloxacin hybrid augments activity of the anticancer drug mitomycin C against multidrug-resistant Gram-negative bacteria. Front. Microbiol. 2019, 10, 1556. [Google Scholar] [CrossRef]
- Gorityala, B.K.; Guchhait, G.; Fernando, D.M.; Deo, S.; McKenna, S.A.; Zhanel, G.G.; Kumar, A.; Schweizer, F. Adjuvants based on hybrid antibiotics overcome resistance in Pseudomonas aeruginosa and enhance fluoroquinolone efficacy. Angew. Chem. Int. Ed. 2016, 55, 555–559. [Google Scholar] [CrossRef] [PubMed]
- Aghamali, M.; Sedighi, M.; Mohammadzadeh, N.; Abbasian, S.; Ghafouri, Z.; Kouhsari, E. Fosfomycin: Mechanisms and the increasing prevalence of resistance. J. Med. Microbiol. 2019, 68, 11–25. [Google Scholar] [CrossRef]
- García-de-la-Mària, C.; Gasch, O.; García-Gonzalez, J.; Soy, D.; Shaw, E.; Ambrosioni, J.; Almela, M.; Pericàs, J.M.; Tellez, A.; Falces, C. The combination of daptomycin and fosfomycin has synergistic, potent, and rapid bactericidal activity against methicillin-resistant Staphylococcus aureus in a rabbit model of experimental endocarditis. Antimicrob. Agents Chemother. 2018, 62, e02633-17. [Google Scholar] [CrossRef]
- Simonetti, O.; Morroni, G.; Ghiselli, R.; Orlando, F.; Brenciani, A.; Xhuvelaj, L.; Provinciali, M.; Offidani, A.; Guerrieri, M.; Giacometti, A. In vitro and in vivo activity of fosfomycin alone and in combination with rifampin and tigecycline against Gram-positive cocci isolated from surgical wound infections. J. Med. Microbiol. 2018, 67, 139–143. [Google Scholar] [CrossRef]
- Chavan, R.; Naphade, B.; Waykar, B.; Bhagwat, S. In vitro activity of fosfomycin and nitrofurantoin against contemporary Enterobacterales pathogens isolated from indian tertiary care hospitals. Microb. Drug Resist. 2020. [Google Scholar] [CrossRef]
- Ontong, J.C.; Ozioma, N.F.; Voravuthikunchai, S.P.; Chusri, S. Synergistic antibacterial effects of colistin in combination with aminoglycoside, carbapenems, cephalosporins, fluoroquinolones, tetracyclines, fosfomycin, and piperacillin on multidrug resistant Klebsiella pneumoniae isolates. PLoS ONE 2021, 16, e0244673. [Google Scholar] [CrossRef] [PubMed]
- Gil-Marqués, M.L.; Moreno-Martínez, P.; Costas, C.; Pachón, J.; Blázquez, J.; McConnell, M.J. Peptidoglycan recycling contributes to intrinsic resistance to fosfomycin in Acinetobacter baumannii. J. Antimicrob. Chemother. 2018, 73, 2960–2968. [Google Scholar] [CrossRef] [PubMed]
- Kareem, S.M. Emergence of mcr- and fosA3-mediated colistin and fosfomycin resistance among carbapenem-resistant Acinetobacter baumannii in Iraq. Meta Gene 2020, 25, 100708. [Google Scholar] [CrossRef]
- Ardebili, A.; Talebi, M.; Azimi, L.; Lari, A.R. Effect of efflux pump inhibitor carbonyl cyanide 3-chlorophenylhydrazone on the minimum inhibitory concentration of ciprofloxacin in Acinetobacter baumannii clinical isolates. Jundishapur J. Microbiol. 2014, 7, e8691. [Google Scholar] [CrossRef] [PubMed]
- Baron, S.A.; Rolain, J.M. Efflux pump inhibitor CCCP to rescue colistin susceptibility in mcr-1 plasmid-mediated colistin-resistant strains and Gram-negative bacteria. J. Antimicrob. Chemother. 2018, 73, 1862–1871. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, A.B.; Rhomberg, P.R.; Abuan, T.; Walters, K.-A.; Flamm, R.K. Potentiation effects of amikacin and fosfomycin against selected amikacin-nonsusceptible Gram-negative respiratory tract pathogens. Antimicrob. Agents Chemother. 2014, 58, 3714–3719. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Luo, Q.; Shi, Q.; Huang, C.; Yu, X.; Niu, T.; Zhou, K.; Zhang, J.; Xiao, Y. In vitro antibacterial effect of fosfomycin combination therapy against colistin-resistant Klebsiella pneumoniae. Infect. Drug Resist. 2018, 11, 577–585. [Google Scholar] [CrossRef] [PubMed]
- Krause, K.M.; Serio, A.W.; Kane, T.R.; Connolly, L.E. Aminoglycosides: An overview. Cold Spring Harb. Perspect. Med. 2016, 6, a027029. [Google Scholar] [CrossRef]
- Silver, L.L. Fosfomycin: Mechanism and resistance. Cold Spring Harb. Perspect. Med. 2017, 7, a025262. [Google Scholar] [CrossRef]
- Leite, G.C.; Neto, L.V.P.; Gaudereto, J.J.; de Maio Carrilho, C.M.D.; Rossi, F.; Levin, A.S.; Costa, S. Effect of antibiotics combination and comparison of methods for detection of synergism in multiresistant Gram-negative bacteria. J. Infect. Dis. Ther. 2015, 3, 1000207. [Google Scholar] [CrossRef]
- Petersen, P.J.; Labthavikul, P.; Jones, C.H.; Bradford, P.A. In vitro antibacterial activities of tigecycline in combination with other antimicrobial agents determined by chequerboard and time-kill kinetic analysis. J. Antimicrob. Chemother. 2006, 57, 573–576. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.J.; Yang, H.F. Synergy against extensively drug-resistant Acinetobacter baumannii in vitro by two old antibiotics: Colistin and chloramphenicol. Int. J. Antimicrob. Agents 2017, 49, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Chusri, S.; Chongsuvivatwong, V.; Rivera, J.I.; Silpapojakul, K.; Singkhamanan, K.; McNeil, E.; Doi, Y. Clinical outcomes of hospital-acquired infection with Acinetobacter nosocomialis and Acinetobacter pittii. Antimicrob. Agents. Chemother. 2014, 58, 4172–4179. [Google Scholar] [CrossRef]
- CLSI. Performance Standards for Antibiotics Susceptibility Testing; M100S; CLSI: Wayne, PA, USA, 2016. [Google Scholar]
- Kheshti, R.; Pourabbas, B.; Mosayebi, M.; Vazin, A. In vitro activity of colistin in combination with various antimicrobials against Acinetobacter baumannii species, a report from South Iran. Infect. Drug Resist. 2019, 12, 129. [Google Scholar] [CrossRef] [PubMed]
| Isolates | Broth Dilution µg/mL | Agar Dilution µg/mL | +CCCP (25 µg/mL) | +CCCP (12.5 µg/mL) |
|---|---|---|---|---|
| SK01 | 512 | 512 | 128 | 128 |
| SK12 | 512 | 512 | 128 | 128 |
| ST26 | >2048 | >2048 | ND | ND |
| TR117 | 512 | 512 | 64 | 128 |
| TR122 | 1024 | 512 | 128 | 256 |
| ATCC 19606 | 128 | 128 | 128 | 128 |
| Isolates | Antibiotics (µg/mL) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Carbapenems | Polymyxin | Aminoglycosides | Folate Inhibitor | Fluoroquinolones | Glycylcycline | |||||||
| IMI | MER | DOR | COL | AMI | GEN | TOB | TMS | CIP | LEV | MIN | TIG | |
| SK01 | 32(R) | 32(R) | 16(R) | 1(S) | 4(S) | 128(R) | 32(R) | >16/80(R) | >16(R) | 16(R) | 0.5(S) | 4(S) |
| SK12 | 32(R) | 8(R) | 8(R) | 2(I) | 8(S) | 256(R) | 64(R) | >16/80(R) | 2(I) | 1(S) | 8(I) | 8(I) |
| ST26 | >128(R) | >128(R) | >128(R) | 2(I) | 2(S) | 32(R) | 8(I) | >16/80(R) | >128(R) | 32(R) | 16(R) | 8(S) |
| TR117 | >128(R) | >128(R) | 64(R) | 2(I) | 1(S) | 1(S) | 0.5(S) | >16/80(R) | 32(R) | 4(I) | 1(S) | 4(S) |
| TR122 | >128(R) | >128(R) | 64(R) | 2(I) | 1(S) | 0.5(S) | 0.5(S) | >16/80(R) | 16(R) | 4(I) | 0.25(S) | 4(S) |
| ATCC 19606 | >128(R) | >128(R) | >128(R) | 2(I) | 256(R) | 4(S) | 1(S) | <2/38(S) | > 32(R) | 32(R) | 0.5(S) | 8(I) |
| Isolates | Carbapenems | Aminoglycosides | Glycylcyclines | Fluoroquinolones | Polymyxin | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FOS | µg/mL | FICI | Result | Fold Reduction | FOS | µg/mL | FICI | Result | Fold Reduction | FOS | µg/mL | FICI | Result | Fold Reduction | FOS | µg/mL | FICI | Result | Fold Reduction | FOS | µg/mL | FICI | Result | Fold Reduction | |
| IMI | GEN | TIG | CIP | COL | |||||||||||||||||||||
| SK01 | 256 | 8 | 0.75 | A | 2 | 256 | 0.5 | 0.50 | A | 2 | 256 | 0.5 | 0.63 | A | 2 | ND | ND | ND | ND | ND | 256 | 0.031 | 0.53 | A | 2 |
| 4 | 0.63 | A | 2 | 1 | 0.51 | A | 2 | 128 | 0.5 | 0.38 | S | 4 | 0.062 | 0.56 | A | 2 | |||||||||
| 128 | 16 | 0.75 | A | 4 | 2 | 0.52 | A | 2 | 1 | 0.50 | S | 4 | 128 | 0.25 | 0.50 | S | 4 | ||||||||
| 64 | 16 | 0.63 | A | 8 | 4 | 0.53 | A | 2 | 0.5 | 0.75 | A | 4 | |||||||||||||
| 128 | 8 | 0.31 | S | 4 | 64 | 0.5 | 0.63 | A | 8 | ||||||||||||||||
| 16 | 0.38 | S | 4 | ||||||||||||||||||||||
| 32 | 0.50 | S | 4 | ||||||||||||||||||||||
| DOR | AMI | MIN | LEV | ||||||||||||||||||||||
| 256 | 0.25 | 0.52 | A | 2 | 256 | 0.062 | 0.52 | A | 2 | 256 | 0.015 | 0.53 | A | 2 | 256 | 4 | 0.75 | A | 2 | ||||||
| 0.5 | 0.53 | A | 2 | 128 | 0.125 | 0.28 | S | 4 | 0.031 | 0.56 | A | 2 | 8 | 1 | I | 2 | |||||||||
| 1 | 0.56 | A | 2 | 0.25 | 0.31 | S | 4 | 128 | 0.031 | 0.31 | S | 4 | |||||||||||||
| 128 | 4 | 0.50 | S | 4 | 0.5 | 0.38 | S | 4 | 0.062 | 0.37 | S | 4 | |||||||||||||
| 8 | 0.75 | A | 4 | 1 | 0.50 | S | 4 | 0.125 | 0.50 | S | 4 | ||||||||||||||
| 64 | 1 | 0.38 | S | 8 | 64 | 0.125 | 0.38 | S | 4 | ||||||||||||||||
| 32 | 1 | 0.31 | S | 0.25 | 0.63 | A | 8 | ||||||||||||||||||
| MER | TOB | ||||||||||||||||||||||||
| 256 | 4 | 0.63 | A | 2 | 256 | 0.062 | 0.50 | A | 2 | ||||||||||||||||
| 128 | 16 | 0.75 | A | 4 | 128 | 2 | 0.31 | S | 4 | ||||||||||||||||
| 64 | 16 | 0.63 | A | 8 | 4 | 0.38 | S | 4 | |||||||||||||||||
| 8 | 0.50 | S | 4 | ||||||||||||||||||||||
| SK12 | IMI | GEN | TIG | CIP | COL | ||||||||||||||||||||
| 256 | 4 | 0.63 | A | 2 | 256 | 64 | 0.75 | A | 2 | 128 | 4 | 0.75 | A | 4 | ND | ND | ND | ND | ND | 128 | 1 | 0.75 | A | 4 | |
| 2 | 0.56 | A | 2 | 128 | 128 | 0.75 | A | 4 | 64 | 4 | 0.63 | A | 8 | 64 | 1 | 0.63 | A | 8 | |||||||
| 1 | 0.53 | A | 2 | 64 | 0.50 | S | 4 | 32 | 4 | 0.56 | A | 16 | |||||||||||||
| 128 | 16 | 0.75 | A | 4 | |||||||||||||||||||||
| DOR | AMI | MIN | LEV | ||||||||||||||||||||||
| 256 | 4 | 1.00 | I | 2 | 64 | 4 | 0.63 | A | 8 | 256 | 4 | 1.00 | I | 2 | 256 | 0.5 | 1.00 | I | 2 | ||||||
| 2 | 0.75 | A | 2 | 32 | 4 | 0.56 | A | 16 | 0.25 | 0.75 | A | 2 | |||||||||||||
| 128 | 4 | 0.75 | A | 4 | 128 | 0.5 | 0.75 | A | 4 | ||||||||||||||||
| 2 | 0.50 | S | 4 | 64 | 0.5 | 0.63 | A | 8 | |||||||||||||||||
| 64 | 4 | 0.63 | A | 8 | 32 | 0.5 | 0.56 | A | 16 | ||||||||||||||||
| MER | TOB | ||||||||||||||||||||||||
| 256 | 0.5 | 0.56 | A | 2 | 256 | 32 | 1.00 | I | 2 | ||||||||||||||||
| 128 | 2 | 0.50 | S | 4 | 128 | 32 | 0.75 | A | 4 | ||||||||||||||||
| 1 | 0.38 | S | 4 | ||||||||||||||||||||||
| 64 | 4 | 0.63 | A | 8 | |||||||||||||||||||||
| 32 | 4 | 0.56 | A | 16 | |||||||||||||||||||||
| ST26 | IMI | GEN | TIG | CIP | COL | ||||||||||||||||||||
| ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ||
| DOR | AMI | MIN | LEV | ||||||||||||||||||||||
| ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | |||||||
| MER | TOB | ||||||||||||||||||||||||
| ND | ND | ND | ND | ND | ND | ND | ND | ND | |||||||||||||||||
| TR117 | IMI | GEN | TIG | CIP | COL | ||||||||||||||||||||
| ND | ND | ND | ND | ND | 256 | 0.062 | 0.56 | A | 2 | 256 | 0.5 | 0.63 | A | 2 | 256 | 0.5 | 0.52 | A | 2 | 128 | 0.5 | 0.50 | S | 4 | |
| 0.031 | 0.53 | A | 2 | 0.25 | 0.56 | A | 2 | 0.25 | 0.51 | A | 2 | 64 | 1 | 0.63 | A | 8 | |||||||||
| 128 | 0.25 | 0.50 | S | 4 | 128 | 1 | 0.50 | S | 4 | 128 | 8 | 0.50 | S | 4 | |||||||||||
| 0.125 | 0.38 | S | 4 | 0.5 | 0.38 | S | 4 | 4 | 0.38 | S | 4 | ||||||||||||||
| 64 | 0.25 | 0.38 | S | 8 | 0.25 | 0.31 | S | 4 | 2 | 0.31 | S | 4 | |||||||||||||
| 32 | 0.25 | 0.31 | S | 16 | 64 | 1 | 0.38 | S | 8 | 64 | 8 | 0.38 | S | 8 | |||||||||||
| 0.5 | 0.25 | S | 8 | ||||||||||||||||||||||
| 32 | 2 | 0.56 | A | 16 | |||||||||||||||||||||
| 1 | 0.31 | S | 16 | ||||||||||||||||||||||
| DOR | AMI | MIN | LEV | ||||||||||||||||||||||
| ND | ND | ND | ND | ND | 256 | 0.062 | 0.56 | A | 2 | 256 | 0.062 | 0.56 | A | 2 | 256 | 0.5 | 0.63 | A | 2 | ||||||
| 0.031 | 0.53 | A | 2 | 256 | 0.25 | 0.56 | A | 2 | |||||||||||||||||
| 128 | 0.25 | 0.50 | S | 4 | 128 | 1 | 0.50 | S | 4 | ||||||||||||||||
| TOB | 0.125 | 0.38 | S | 4 | 64 | 1 | 0.38 | S | 8 | ||||||||||||||||
| MER | 256 | 0.062 | 0.62 | A | 2 | 0.062 | 0.31 | S | 4 | ||||||||||||||||
| ND | ND | ND | ND | ND | 128 | 0.125 | 0.50 | S | 4 | 0.031 | 0.28 | S | 4 | ||||||||||||
| 0.062 | 0.37 | S | 4 | 64 | 0.25 | 0.38 | S | 8 | |||||||||||||||||
| 64 | 0.25 | 0.63 | A | 8 | 0.125 | 0.25 | S | 8 | |||||||||||||||||
| 0.125 | 0.38 | S | 8 | 32 | 0.5 | 0.56 | A | 16 | |||||||||||||||||
| 32 | 0.25 | 0.56 | A | 16 | 0.25 | 0.31 | S | 16 | |||||||||||||||||
| TR122 | IMI | GEN | TIG | CIP | COL | ||||||||||||||||||||
| ND | ND | ND | ND | ND | 512 | 0.062 | 0.63 | A | 2 | 512 | 1 | 0.75 | A | 2 | 512 | 4 | 0.75 | A | 2 | 512 | 0.5 | 0.75 | A | 2 | |
| DOR | 0.031 | 0.56 | A | 2 | 0.5 | 0.63 | A | 2 | 2 | 0.63 | A | 2 | 0.25 | 0.63 | A | 2 | |||||||||
| 512 | 4 | 0.56 | A | 2 | 256 | 0.125 | 0.50 | S | 4 | 0.25 | 0.56 | A | 2 | 1 | 0.56 | A | 2 | 0.125 | 0.56 | A | 2 | ||||
| 2 | 0.53 | A | 2 | 0.062 | 0.38 | S | 4 | 0.125 | 0.53 | A | 2 | 0.062 | 0.53 | A | 2 | ||||||||||
| 256 | 16 | 0.50 | S | 4 | 128 | 0.25 | 0.63 | A | 8 | 0.062 | 0.52 | A | 2 | LEV | 256 | 0.5 | 0.50 | S | 4 | ||||||
| 8 | 0.38 | S | 4 | AMI | 256 | 1 | 0.50 | S | 4 | 512 | 1 | 0.75 | A | 2 | 0.25 | 0.38 | S | 4 | |||||||
| 4 | 0.31 | S | 4 | 512 | 0.25 | 0.75 | A | 2 | 0.5 | 0.38 | S | 4 | 0.5 | 0.63 | A | 2 | |||||||||
| 128 | 32 | 0.63 | A | 8 | 0.125 | 0.63 | A | 2 | 0.25 | 0.31 | S | 4 | 0.25 | 0.56 | A | 2 | |||||||||
| MER | 256 | 0.25 | 0.50 | S | 4 | 0.125 | 0.28 | S | 4 | 0.125 | 0.53 | A | 2 | ||||||||||||
| ND | ND | ND | ND | ND | 0.125 | 0.38 | S | 4 | 0.062 | 0.27 | S | 4 | 256 | 1 | 0.50 | S | 4 | ||||||||
| 128 | 0.25 | 0.38 | S | 8 | 128 | 2 | 0.63 | A | 8 | 0.5 | 0.38 | S | 4 | ||||||||||||
| 1 | 0.38 | S | 8 | ||||||||||||||||||||||
| TOB | 0.5 | 0.25 | S | 8 | |||||||||||||||||||||
| 512 | 0.062 | 0.62 | A | 2 | MIN | ||||||||||||||||||||
| 256 | 0.125 | 0.50 | S | 4 | 512 | 0.0312 | 0.63 | A | 2 | ||||||||||||||||
| 128 | 0.125 | 0.38 | S | 8 | 0.0156 | 0.56 | A | 2 | |||||||||||||||||
| 64 | 0.25 | 0.56 | A | 16 | 256 | 0.0625 | 0.50 | S | 4 | ||||||||||||||||
| 0.0312 | 0.37 | S | 4 | ||||||||||||||||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nwabor, O.F.; Terbtothakun, P.; Voravuthikunchai, S.P.; Chusri, S. Evaluation of the Synergistic Antibacterial Effects of Fosfomycin in Combination with Selected Antibiotics against Carbapenem–Resistant Acinetobacter baumannii. Pharmaceuticals 2021, 14, 185. https://doi.org/10.3390/ph14030185
Nwabor OF, Terbtothakun P, Voravuthikunchai SP, Chusri S. Evaluation of the Synergistic Antibacterial Effects of Fosfomycin in Combination with Selected Antibiotics against Carbapenem–Resistant Acinetobacter baumannii. Pharmaceuticals. 2021; 14(3):185. https://doi.org/10.3390/ph14030185
Chicago/Turabian StyleNwabor, Ozioma F., Pawarisa Terbtothakun, Supayang P. Voravuthikunchai, and Sarunyou Chusri. 2021. "Evaluation of the Synergistic Antibacterial Effects of Fosfomycin in Combination with Selected Antibiotics against Carbapenem–Resistant Acinetobacter baumannii" Pharmaceuticals 14, no. 3: 185. https://doi.org/10.3390/ph14030185
APA StyleNwabor, O. F., Terbtothakun, P., Voravuthikunchai, S. P., & Chusri, S. (2021). Evaluation of the Synergistic Antibacterial Effects of Fosfomycin in Combination with Selected Antibiotics against Carbapenem–Resistant Acinetobacter baumannii. Pharmaceuticals, 14(3), 185. https://doi.org/10.3390/ph14030185






