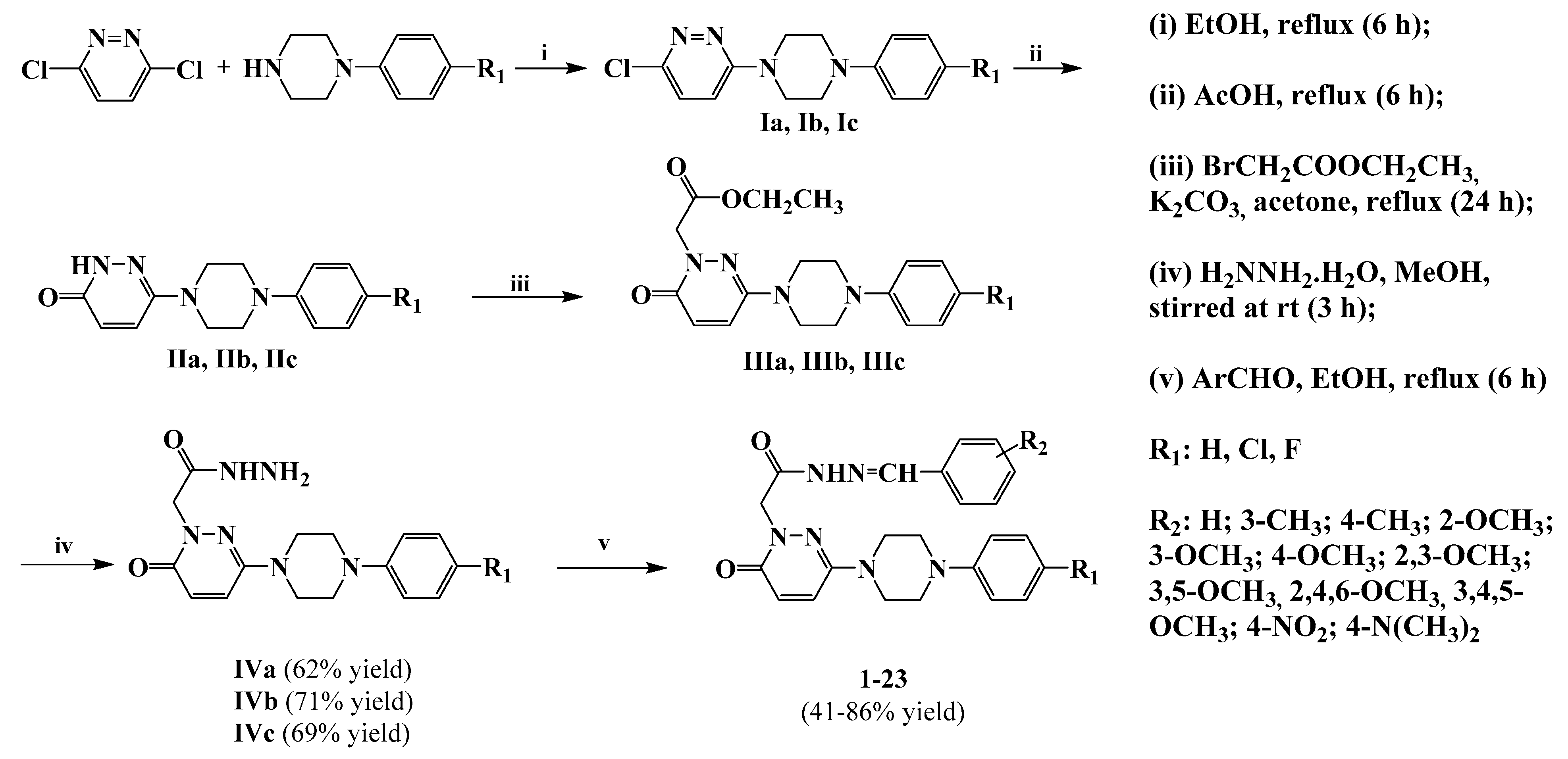
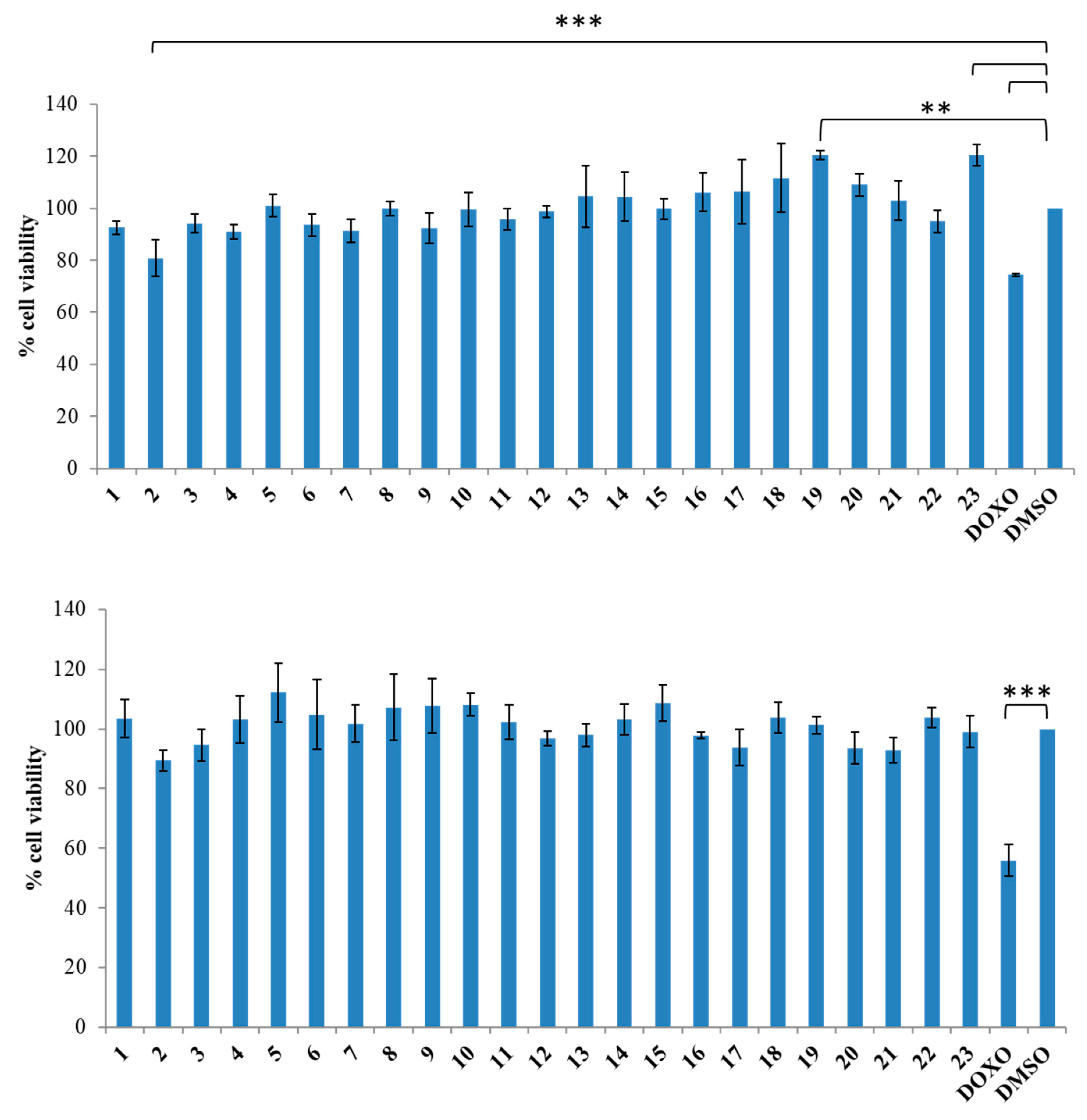
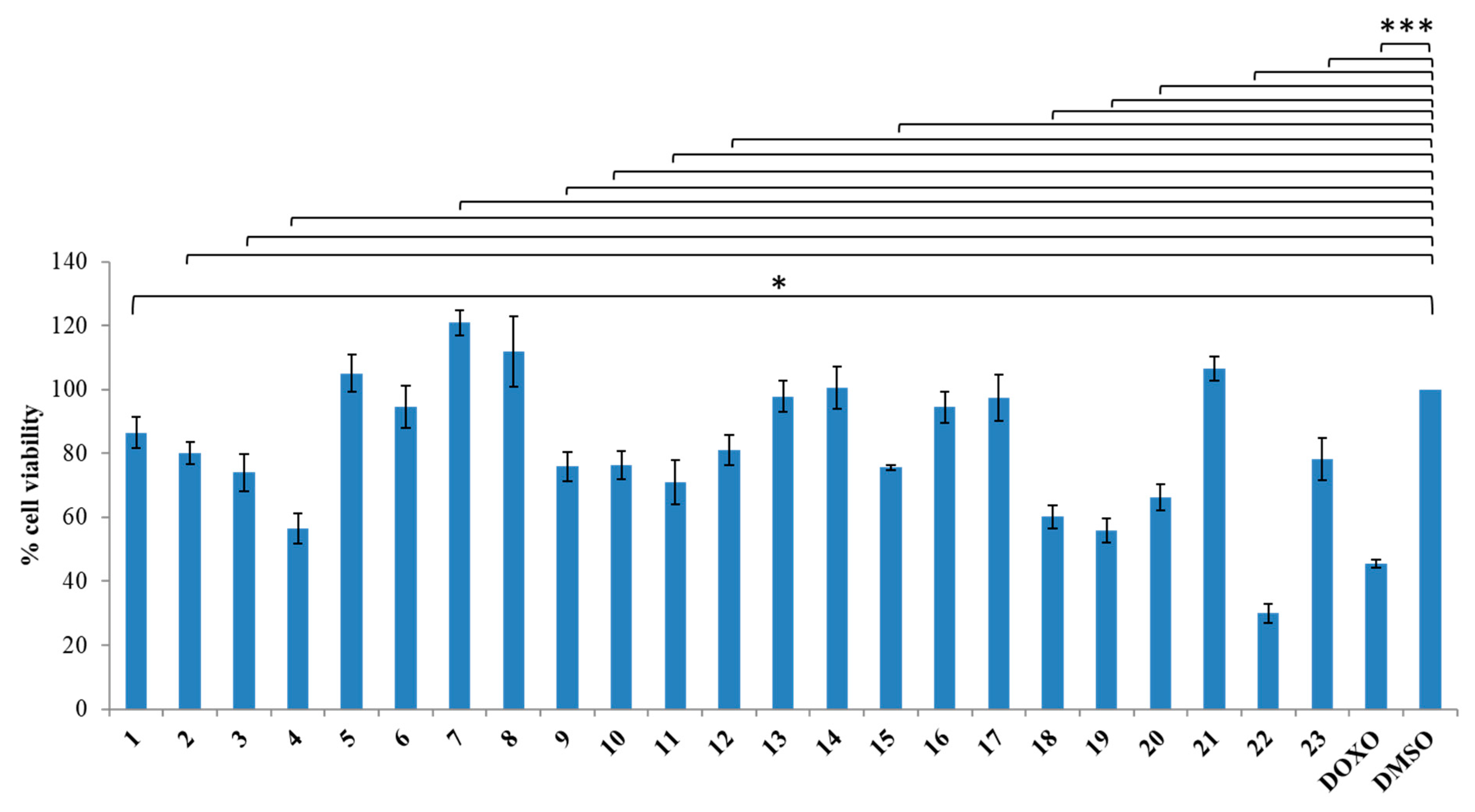
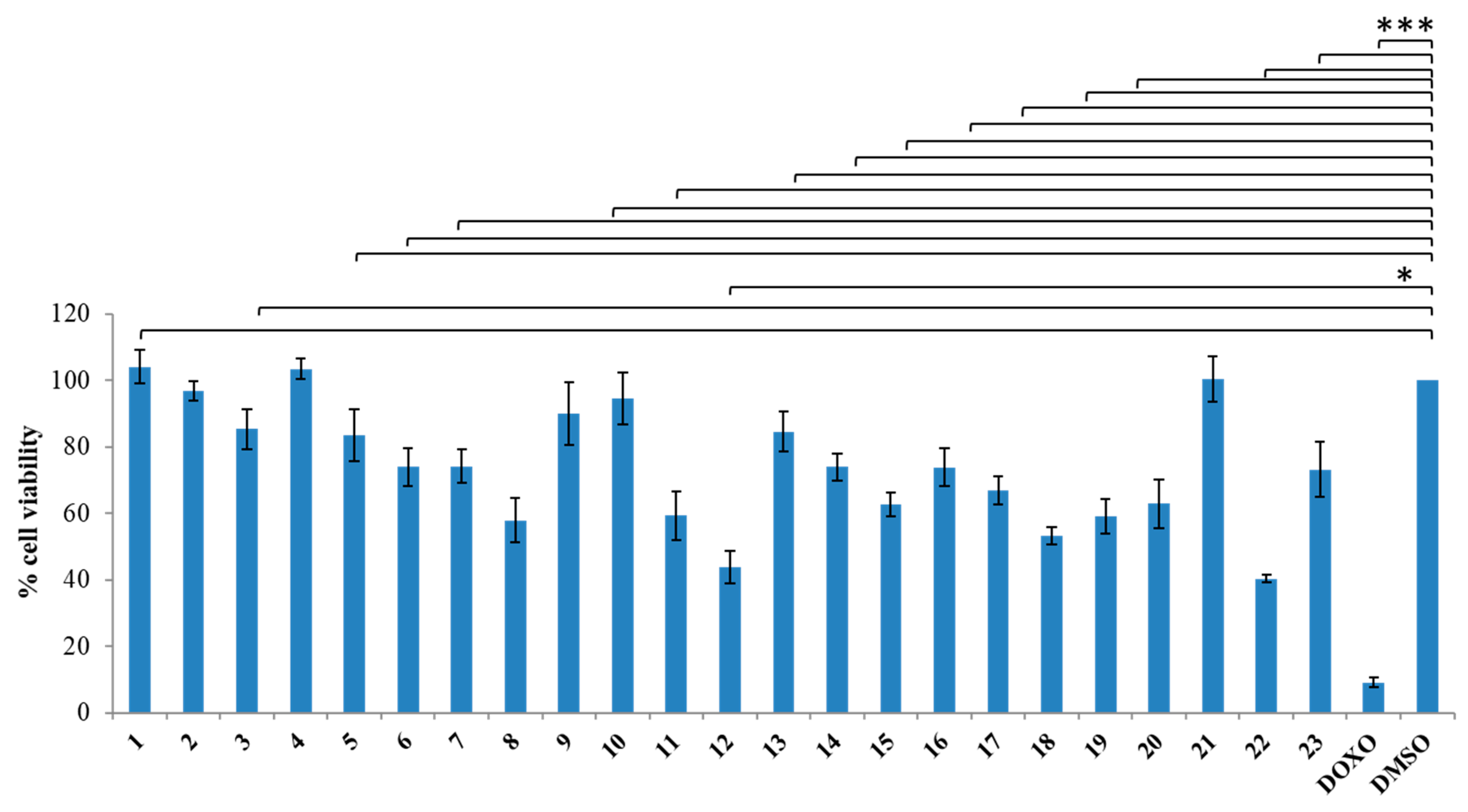
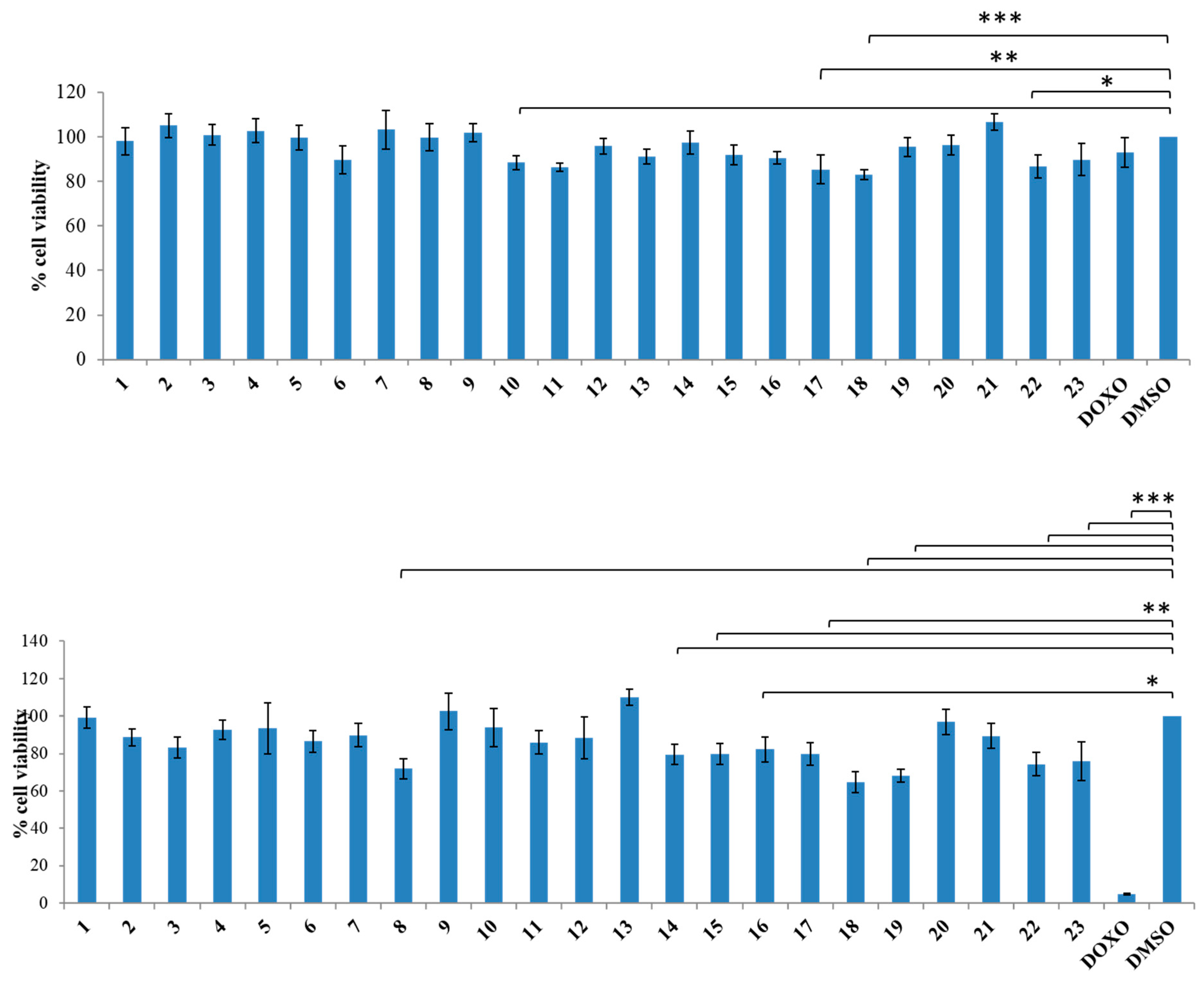
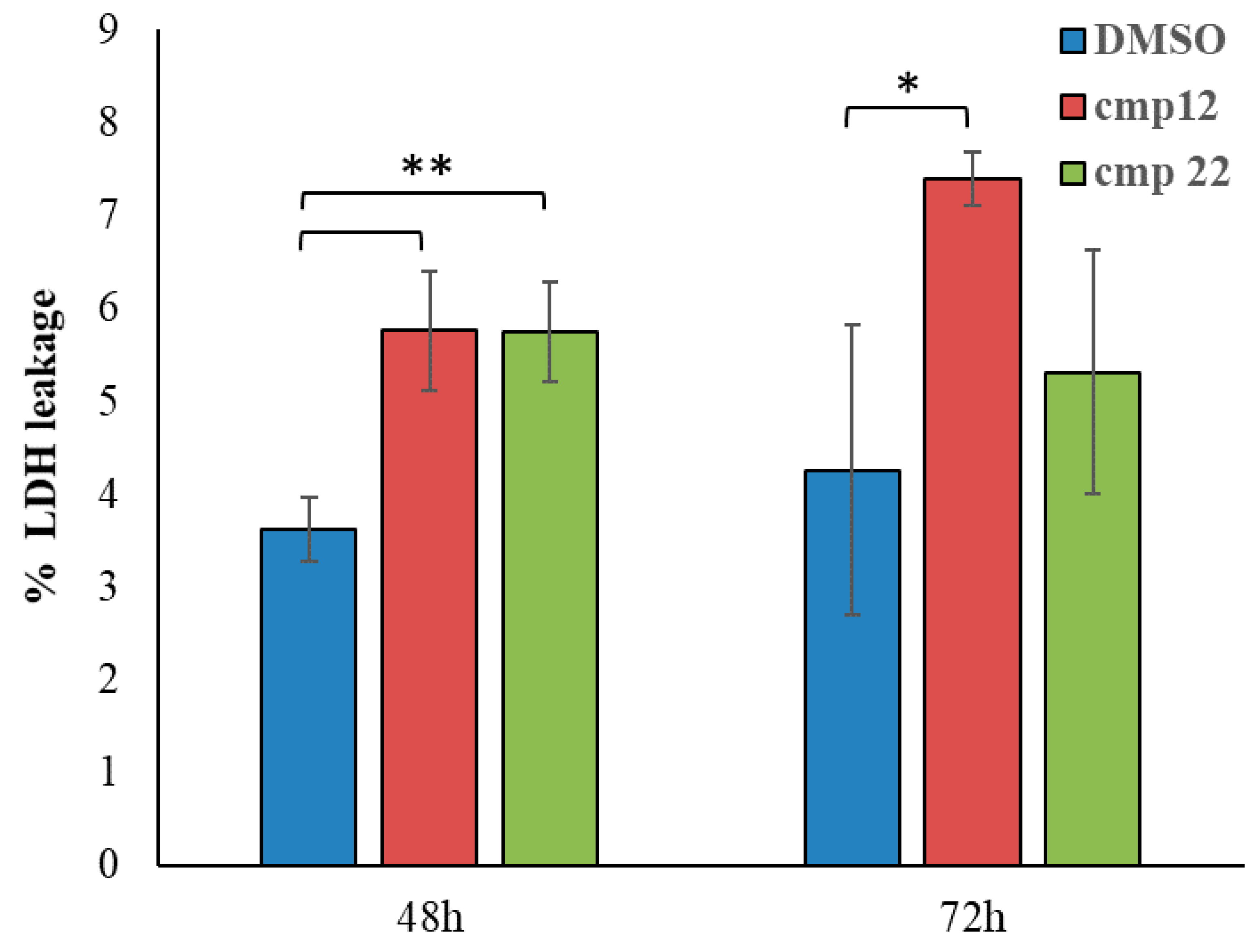
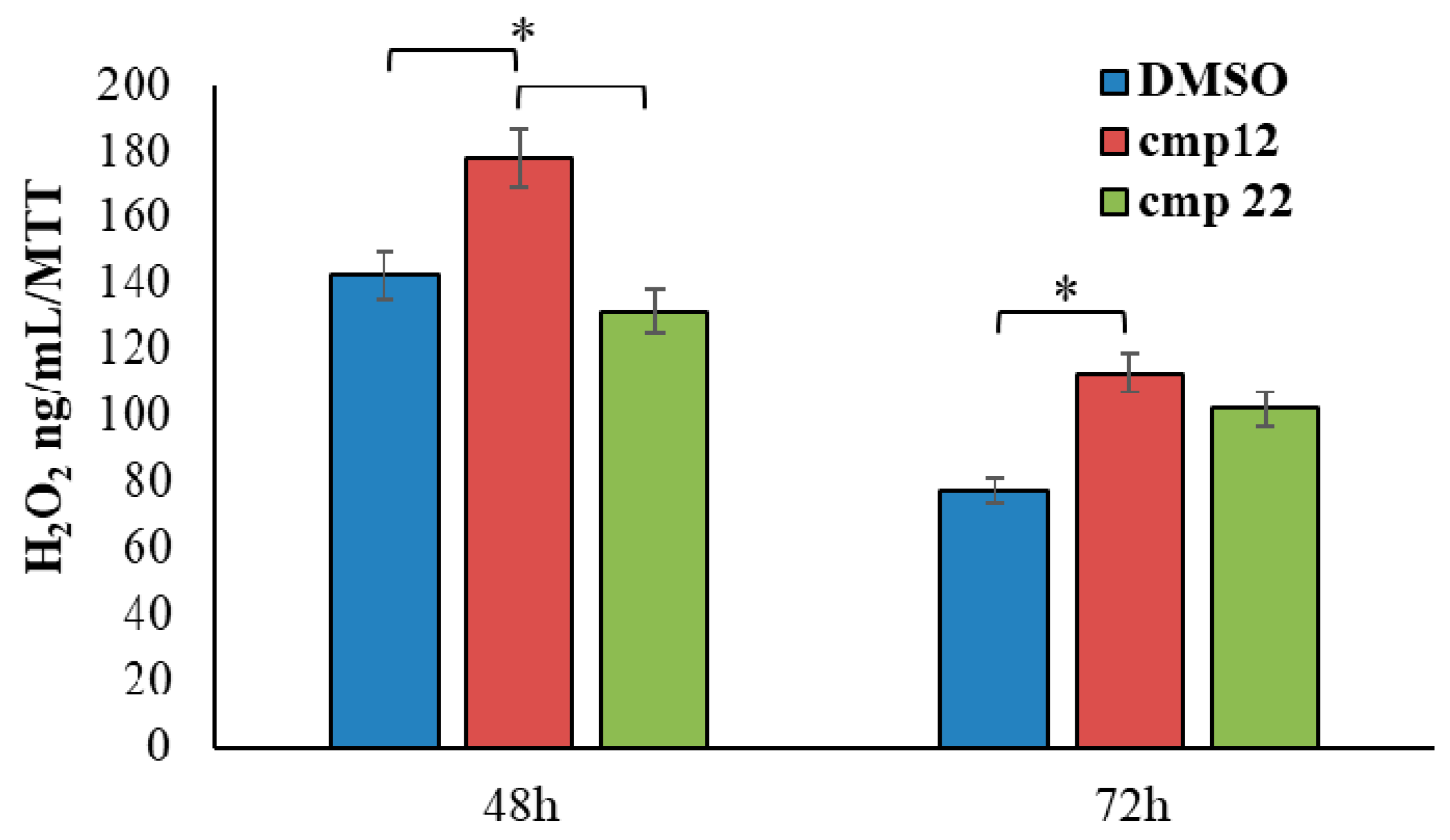
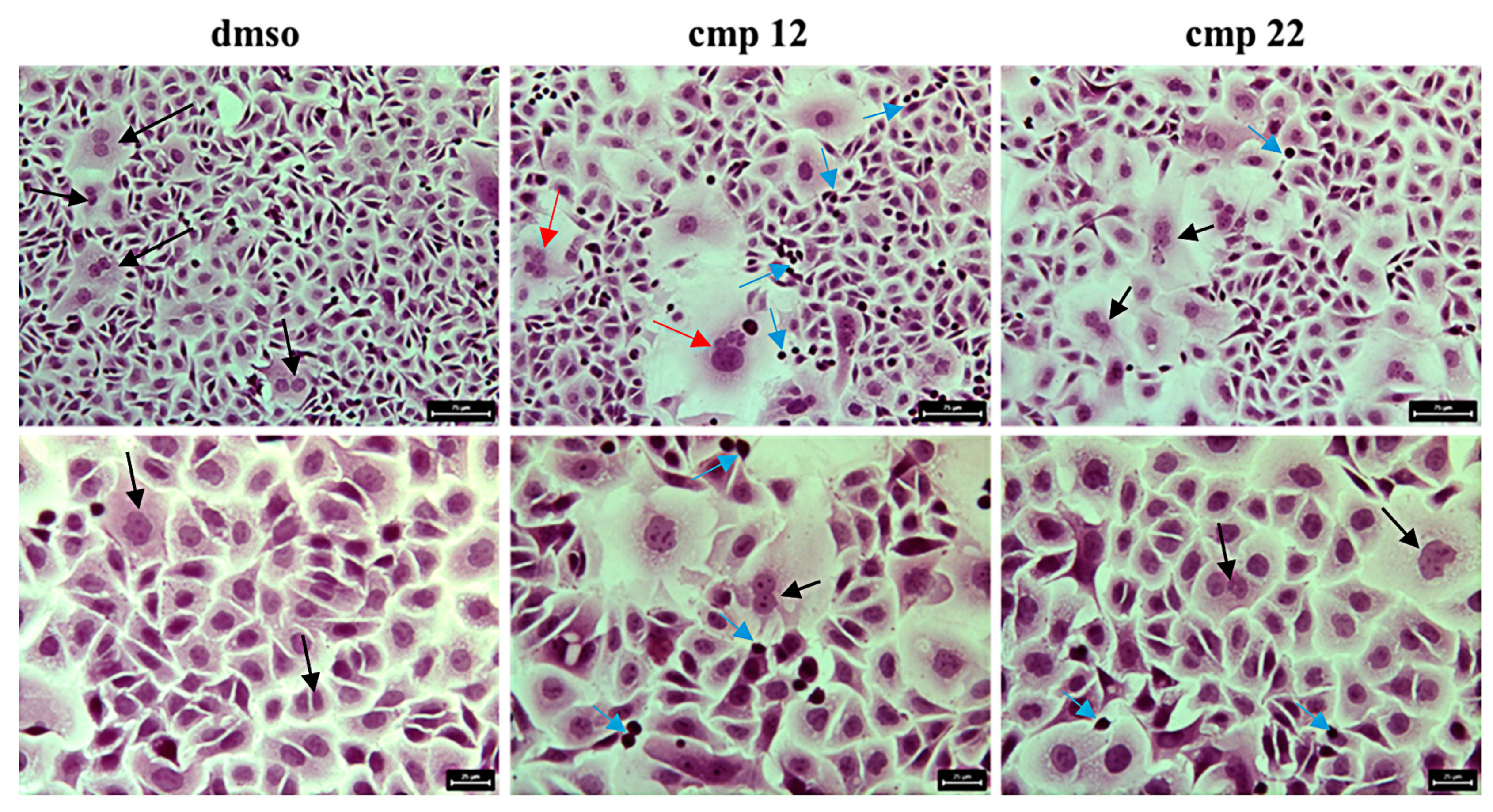
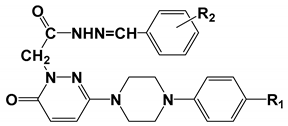
3.1.2. General Synthesis of 6-(4-(4-Chlorophenyl/4-fluorophenyl/phenyl)piperazin-1-yl)pyridazin-3(2H)-one-2-acetyl-2-(substituted/nonsubstituted benzal)hydrazones 1–23
A suitable benzaldehyde derivative (0.01 mol) and the appropriate 6-(4-(4-chlorophenyl/4-fluorophenyl/phenyl)piperazin-1-yl]-3(2H)-pyridazinone-2-yl acetohydrazide IVa–c (0.01 mol) were added to ethanol (15 mL). The mixture was stirred under reflux for 6 h. The reaction mixture was transferred into ice water, filtered and dried. The residue was crystallized from methanol:water mixtures. The structures of the compounds were determined by 1H-NMR, 13C-NMR, IR spectroscopy and elemental analysis. All spectral data of the compounds were in accordance with the assigned structures as given below.
N′-Benzylidene-2-(6-oxo-3-(4-phenylpiperazin-1-yl)pyridazin-1(6H)-yl)acetohydrazide (1). White crystals (methanol/water); yield 83%; m.p. 236–237 °C; IR (ν cm−1, ATR): 3055 (C–H aromatic), 2947 (C–H aliphatic), 1652, 1629 (C=O), 1561 (C=N), 1231 (C–N). 1H-NMR (DMSO-d6, 600 MHz): δ 3.22 (4H; t; CH2N; Hb + Hb’), 3.38 (4H; t; CH2N; Ha + Ha’), 5.07 (2H; s; NCH2CO), 6.92 (1H; d; J = 4.2 Hz, pyridazinone H5), 6.94 (1H; d; J = 4.2 Hz, pyridazinone H4), 6.98–7.72 (10H; m; phenyl protons), 8.02 (1H; s; –N=CH–), 11.70 (1H; s; -NH–N); 13C–NMR (DMSO-d6, 150 MHz): δ 46.5 (2C, CH2–N; b + b’), 48.4 (2C, CH2–N; a + a’), 53.0 (1C; –N–CH2–C=O), 116.2 (1C; =CH), 119.7 (2C; phenyl C2,6), 127.6 (1C; phenyl C4), 129.4 (1C; =CH-phenyl C6), 130.4 (1C; =CH-phenyl C2), 130.6 (2C; phenyl C3,5), 131.1 (1C; =CH–phenyl C1), 134.4 (1C; =CH–phenyl C3), 144.3 (2C; =CH–phenyl C4,5), 147.4 (1C; pyridazinone C5), 149.1 (1C; phenyl C1), 151.3 (1C; pyridazinone C6), 158.2 (1C; pyridazinone C4), 163.8 (1C; CH2–N–C=O), 168.5 (1C; pyridazinone C3); LC/API-ESMS m/z 439 [M + Na]+; Anal. Calcd. for C23H24N6O3.1/4 H2O: C, 65.62; H, 5.87; N, 19.96. Found: C, 65.96; H, 6.103; N, 20.12%.
N’-(3-Methylbenzylidene)-2-(6-oxo-3-(4-phenylpiperazin-1-yl)pyridazin-1(6H)-yl)aceto-hydrazide (2). White crystals (methanol/water); yield 80%; m.p. 224–225 °C; IR (ν cm−1, ATR): 3052 (C–H aromatic), 2949 (C–H aliphatic), 1652 (C=O), 1581 (C=N), 1267 (C–N). 1H-NMR (DMSO-d6, 600 MHz): δ 2.34 (3H; s; CH3), 3.28 (4H; t; CH2N; Hb + Hb’), 3.38 (4H; t; CH2N; Ha + Ha’), 5.07 (2H; s; NCH2CO), 6.92 (1H; d; J = 4.1 Hz, pyridazinone H5), 6.93 (1H; d; J = 4.2 Hz, pyridazinone H4), 7.22–7.65 (9H; m; phenyl protons), 8.19 (1H; s; –N=CH–), 11.64 (1H; s; –NH–N); 13C-NMR (DMSO-d6, 150 MHz): δ 21.4 (1C; CH3), 39.6 (2C, CH2–N; b + b’), 39.7 (2C, CH2–N; a + a’), 53.5 (1C; –N–CH2–C=O), 116.2 (1C; =CH), 119.7 (2C; phenyl C2,6), 124.9 (1C; phenyl C4), 127.7 (1C; 3-methylphenyl C6), 129.4 (1C; 3-methylphenyl C2), 131.0 (2C; phenyl C3,5), 134.4 (1C; 3-methylphenyl C1), 134.6 (1C; 3-methylphenyl C3), 138.5 (2C; 3-methylphenyl C4,5), 138.5 (1C; pyridazinone C5), 144.4 (1C; phenyl C1), 149.0 (1C; pyridazinone C6), 149.1 (1C; pyridazinone C4), 163.7 (1C; CH2–N–C=O), 168.4 (1C; pyridazinone C3); LC/API-ESMS m/z 453 [M + Na]+; Anal. Calcd. for C24H26N6O2.1/3 H2O: C, 66.04; H, 6.16; N, 19.25. Found: C, 66.09; H, 6.10; N, 19.63%.
N’-(4-Methylbenzylidene)-2-(6-oxo-3-(4-phenylpiperazin-1-yl)pyridazin-1(6H)-yl)aceto-hydrazide (3). White crystals (methanol/water); yield 74%; m.p. 210–212 °C; IR (ν cm−1, ATR): 3400 (N–H), 3052 (C–H aromatic), 2949 (C–H aliphatic), 1652 (C=O), 1581 (C=N), 1267 (C–N). 1H-NMR (DMSO-d6, 600 MHz): δ 2.34 (3H; s; CH3), 3.22 (4H; t; CH2N; Hb + Hb’), 3.38 (4H; t; CH2N; Ha + Ha’), 5.05 (2H; s; NCH2CO), 6.90 (1H; d; J = 4.1 Hz, pyridazinone H5), 6.93 (1H; d; J = 4.2 Hz, pyridazinone H4), 7.22–7.65 (9H; m; phenyl protons), 8.18 (1H; s; –N=CH–), 11.59 (1H; s; –NH–N); 13C-NMR (DMSO-d6, 150 MHz): δ 21.5 (1C; CH3), 46.4 (2C, CH2-N; b+b’), 48.4 (2C, CH2-N; a+a’), 53.1 (1C; -N-CH2-C=O), 116.2 (1C; =CH), 119.8 (2C; phenyl C2,6), 126.9 (1C; phenyl C4), 127.6 (1C; 4-methylphenyl C6), 129.8 (1C; 4-methylphenyl C2), 129.9 (2C; phenyl C3,5), 131.0 (1C; 4-methylphenyl C1), 131.9 (1C; 4-methylphenyl C3), 140.2 (2C; 4-methylphenyl C4,5), 140.4 (1C; pyridazinone C5), 144.3 (1C; phenyl C1), 144.4 (1C; pyridazinone C6), 149.1 (1C; pyridazinone C4), 158.2 (1C; CH2–N–C=O), 168.3 (1C; pyridazinone C3); LC/API-ESMS m/z 453 [M + Na]+; Anal. Calcd. for C24H26N6O2.2/3 H2O: C, 65.14; H, 6.23; N, 18.99. Found: C, 64.86; H, 5.99; N, 19.30%.
N’-(3-Methoxybenzylidene)-2-(6-oxo-3-(4-phenylpiperazin-1-yl)pyridazin-1(6H)-yl)acetohydrazide (4). White crystals (methanol/water); yield 68%; m.p. 205–206 °C; IR (ν cm−1, ATR): 3389 (N–H), 3094 (C–H aromatic), 2971 (C–H aliphatic), 1681, 1652 (C=O), 1575 (C=N), 1243 (C–N), 1174 (C–O). 1H-NMR (DMSO-d6, 600 MHz): δ 3.22 (4H; t; CH2N; b + b’), 3.38 (4H; t; CH2N; a + a’), 3.80 (3H; s; OCH3), 5.04 (2H; s; CH2CO), 6.93 (1H; d; J = 4.2 Hz, pyridazinone H5), 6.98 (1H; d; J = 4.2 Hz, pyridazinone H4), 7.01–7.96 (9H; m; phenyl protons), 8.16 (1H; s; –N=CH–), 11.51 (1H; s; –NH–N); 13C-NMR (DMSO-d6, 150 MHz): δ 46.5 (2C, CH2–N; b + b’), 48.4 (2C, CH2–N; a + a’), 52.9 (1C; –N–CH2-C=O), 55.8 (1C, O–CH3), 114.8 (1C; =CH), 116.2 (2C; phenyl C2,6), 127.1 (1C; phenyl C4), 128.9 (1C; 3-methoxyphenyl C5,6), 129.4 (1C; 3-methoxyphenyl C1), 131.0 (1C; phenyl C3,5), 131.1 (1C; 3-methoxyphenyl C4), 144.2 (1C; pyridazinone C4), 147.3 (1C; 3-methoxyphenyl C2), 149.0 (1C; pyridazinone C5), 151.3 (1C; phenyl C1), 158.1 (1C; pyridazinone C6), 158.2 (1C; 3-methoxyphenyl C3), 163.5 (1C; CH2–N–C=O), 168.2 (1C; pyridazinone C3); LC/API-ESMS m/z 469.2 [M + Na]+; Anal. Calcd. for C24H26N6O3.3/2 H2O: C, 60.87; H, 6.17; N, 18.01. Found: C, 61.70; H, 5.74; N, 18.01%.
N’-(2,3-Dimethoxybenzylidene)-2-(6-oxo-3-(4-phenylpiperazin-1-yl)pyridazin-1(6H)-yl)aceto-hydrazide (5). White crystals (methanol/water); yield 86%; m.p. 249–250 °C; IR (ν cm−1, ATR): 3105 (C–H aromatic), 2966 (C–H aliphatic), 1695, 1659 (C=O), 1571 (C=N), 1232 (C–N), 1070 (C–O). 1H-NMR (DMSO-d6, 600 MHz): δ 3.22 (4H; t; CH2N; Hb + Hb’), 3.38 (4H; t; CH2N; Ha + Ha’), 3.78 (3H; s; OCH3), 3.84 (3H; s; OCH3), 5.05 (2H; s; NCH2CO), 6.99 (1H; d; J = 4.2 Hz, pyridazinone H5), 7.10 (1H; d; J = 4.2 Hz, pyridazinone H4), 7.12–7.65 (8H; m; phenyl protons), 8.31 (1H; s; –N=CH–), 11.63 (1H; s; –NH–N); 13C-NMR (DMSO-d6, 150 MHz): δ 46.5 (2C, CH2–N; b + b’), 48.4 (2C, CH2–N; a + a’), 52.9 (1C; –N–CH2–C=O), 56.2 (1C; –O–CH3), 61.7 (1C; –O–CH3), 114.6 (1C; =CH), 116.2 (2C; phenyl C2,6), 119.7 (1C; phenyl C4), 124.8 (1C; 2,3-dimethoxyphenyl C6), 127.9 (1C; 2,3-dimethoxyphenyl C1), 130.9 (2C; phenyl C3,5), 131.1 (2C; 2,3-dimethoxyphenyl C4,5), 140.1 (2C; 2,3-dimethoxyphenyl C3), 142.9 (1C; 2,3-dimethoxyphenyl C2), 148.4 (1C; pyridazinone C5), 149.0 (1C; phenyl C1), 151.3 (1C; pyridazinone C6), 158.2 (1C; pyridazinone C4), 163.7 (1C; CH2–N–C=O), 168.4 (1C; pyridazinone C3); LC/API-ESMS m/z 496 [M]+; Anal. Calcd. for C25H28N6O4.1/3 H2O: C, 62.23; H, 5.99; N, 17.42. Found: C, 62.59; H, 5.77; N, 17.62%.
N’-(3,5-Dimethoxybenzylidene)-2-(6-oxo-3-(4-phenylpiperazin-1-yl)pyridazin-1(6H)-yl)aceto-hydrazide (6). White crystals (methanol/water); yield 57%; m.p. 232–233 °C; IR (ν cm−1, ATR): 3094 (C–H aromatic), 2960 (C–H aliphatic), 1685, 1657 (C=O), 1574 (C=N), 1262 (C–N), 1150 (C–O). 1H–NMR (DMSO-d6, 600 MHz): δ 3.22 (4H; t; CH2N; Hb + Hb’), 3.38 (4H; t; CH2N; Ha + Ha’), 3.78 (6H; s; OCH3), 5.10 (2H; s; NCH2CO), 6.87 (1H; d; J = 4.1 Hz, pyridazinone H5), 6.91 (1H; d; J = 4.2 Hz, pyridazinone H4), 6.99–7.93 (8H; m; phenyl protons), 8.14 (1H; s; –N=CH–), 11.68 (1H; s; –NH–N); 13C-NMR (DMSO-d6, 150 MHz): δ 46.5 (2C, CH2–N; b + b’), 48.4 (2C, CH2–N; a + a’), 53.0 (1C; –N–CH2–C=O), 55.8 (2C; –OCH3), 105.1 (1C; =CH), 116.2 (2C; phenyl C2,6), 119.7 (1C; phenyl C4), 127.1 (1C; 3,5-dimethoxyphenyl C6), 129.3 (1C; 3,5-dimethoxyphenyl C2), 131.0 (1C; phenyl C1), 136.5 (1C; 3,5-dimethoxyphenyl C1), 136.6 (1C; 3,5-dimethoxyphenyl C4), 144.1 (2C; 3,5- dimethoxyphenyl C3,5), 147.3 (1C; pyridazinone C5), 149.0 (1C; phenyl C1), 151.3 (1C; pyridazinone C6), 158.2 (1C; pyridazinone C4), 163.8 (1C; CH2–N–C=O), 168.5 (1C; pyridazinone C3); LC/API-ESMS m/z 468.8 [M]+; Anal. Calcd. for C25H28N6O4.1/3 H2O: C, 62.23; H, 5.99; N, 17.42. Found: C, 62.35; H, 5.71; N, 17.57%.
N’-(2,4,6-Trimethoxybenzylidene)-2-(6-oxo-3-(4-phenylpiperazin-1-yl)pyridazin-1(6H)-yl)aceto-hydrazide (7). White crystals (methanol/water); yield 82%; m.p. 254–255 °C; IR (ν cm−1, ATR): 3232 (N–H), 3086 (C–H aromatic), 2933 (C–H aliphatic), 1697, 1670 (C=O), 1595 (C=N), 1239 (C–N), 1126, 1057 (C–O). 1H-NMR (DMSO-d6, 600 MHz): δ 3.22 (4H; t; CH2N; Hb + Hb’), 3.38 (4H; t; CH2N; Ha + Ha’), 3.81 (6H; s; OCH3), 3.83 (3H; s; OCH3), 4.94 (2H; s; NCH2CO), 6.29 (1H; d; J = 4.2 Hz, pyridazinone H5), 6.94 (1H; d; J = 4.2 Hz, pyridazinone H4), 6.80–7.64 (8H; m; pyridazinone H4 and phenyl protons), 8.19 (1H; s; –N=CH–), 11.26 (1H; s; –NH–N); 13C-NMR (DMSO-d6, 150 MHz): δ 46.5 (2C, CH2–N; b + b’), 48.4 (2C, CH2–N; a + a’), 53.4 (1C; –N–CH2–C=O), 55.89 (1C; –OCH3), 56.53 (2C; –OCH3), 104.3 (1C; =CH), 116.3 (2C; phenyl C2,6), 119.7 (1C; phenyl C4), 126.9 (1C; 2,4,6-trimethoxyphenyl C5), 129.4 (1C; 2,4,6-trimethoxyphenyl C3), 131.0 (2C; phenyl C3,5), 139.6 (1C; 2,4,6-trimethoxyphenyl C1), 143.2 (1C; 2,4,6-trimethoxyphenyl C4), 148.9 (2C; 2,4,6-trimethoxyphenyl C2,6), 151.3 (1C; pyridazinone C5), 158.2 (1C; phenyl C1), 158.3 (1C; pyridazinone C6), 160.3 (1C; pyridazinone C4), 162.7 (1C; CH2–N–C=O), 167.9 (1C; pyridazinone C3); LC/API-ESMS m/z 437 [M-2(OCH3)]+; Anal. Calcd. for C26H30N6O5: C, 61.65; H, 5.97; N, 16.59. Found: C, 61.28; H, 5.82; N, 16.67%.
N’-(3,4,5-Trimethoxybenzylidene)-2-(6-oxo-3-(4-phenylpiperazin-1-yl)pyridazin-1(6H)-yl)aceto-hydrazide (8). White crystals (methanol/water); yield 83%; m.p. 238–239 °C; IR (ν cm−1, ATR): 3216 (N–H), 3065 (C–H aromatic), 2943 (C–H aliphatic), 1669 (C=O), 1578 (C=N), 1231 (C–N), 1124 (C–O). 1H-NMR (DMSO-d6, 600 MHz): δ 3.22 (4H; t; CH2N; Hb + Hb’), 3.38 (4H; t; CH2N; Ha + Ha’), 3.83 (9H; s; OCH3), 5.08 (2H; s; NCH2CO), 6.82 (1H; d; J = 4.1 Hz, pyridazinone H5), 6.92 (1H; d; J = 4.2 Hz, pyridazinone H4), 6.98–7.92 (7H; m; phenyl protons), 8.14 (1H; s; –N=CH–), 11.68 (1H; s; –NH–N); 13C-NMR (DMSO-d6, 150 MHz): δ 46.5 (2C, CH2–N; b + b’), 48.4 (2C, CH2–N; a + a’), 53.5 (1C; –N–CH2–C=O), 56.4 (1C; –OCH3), 60.6 (2C; –OCH3), 104.6 (1C; =CH), 116.3 (2C; phenyl C2,6), 119.8 (1C; phenyl C4), 127.0 (1C; 3,4,5-trimethoxyphenyl C6), 129.4 (1C; 3,4,5-trimethoxyphenyl C2), 129.9 (2C; phenyl C3,5), 131.0 (1C; 3,4,5-trimethoxyphenyl C1), 139.5 (1C; 3,4,5-trimethoxyphenyl C3), 144.1 (2C; 3,4,5-trimethoxyphenyl C4,5), 147.4 (1C; pyridazinone C5), 149.1 (1C; phenyl C1), 151.3 (1C; pyridazinone C6), 153.6 (1C; pyridazinone C4), 163.7 (1C; CH2–N–C=O), 168.5 (1C; pyridazinone C3); LC/API-ESMS m/z 529.2 [M + Na]+; Anal. Calcd. for C26H30N6O5.1/2 H2O: C, 60.57; H, 6.06; N, 16.30. Found: C, 60.92; H, 5.72; N, 16.55%.
N’-(4-Nitrobenzylidene)-2-(6-oxo-3-(4-phenylpiperazin-1-yl)pyridazin-1(6H)-yl)acetohydrazide (9). White crystals (methanol/water); yield 54%; m.p. 239–240 °C; IR (ν cm−1, ATR): 3089 (C–H aromatic), 2967 (C–H aliphatic), 1692, 1660 (C=O), 1574 (C=N), 1339 (aromatic-NO2), 1235 (C–N). 1H-NMR (DMSO-d6, 600 MHz): δ 3.23 (4H; t; CH2N; Hb + Hb’), 3.39 (4H; t; CH2N; Ha + Ha’), 5.11 (2H; s; NCH2CO), 6.82 (1H; d; J = 4.0 Hz, pyridazinone H5), 6.92 (1H; d; J = 4.2 Hz, pyridazinone H4), 7.22–8.33 (9H; m; phenyl protons), 8.30 (1H; s; –N=CH–), 11.96 (1H; s; –NH–N); 13C-NMR (DMSO-d6, 150 MHz): δ 46.5 (2C, CH2–N; b + b’), 48.4 (2C, CH2–N; a+a’), 53.0 (1C; –N–CH2–C=O), 105.1 (1C; =CH), 116.2 (2C; phenyl C2,6), 124.4 (1C; phenyl C4), 124.5 (1C; 4-nitrophenyl C6), 128.3 (1C; 4-nitrophenyl C2), 128.4 (2C; phenyl C3,5), 129.4 (1C; 4-nitrophenyl C1), 140.8 (2C; 4-nitrophenyl C3,5), 141.9 (2C; =CH–phenyl C4), 142.1 (1C; pyridazinone C5), 148.2 (1C; phenyl C1), 149.1 (1C; pyridazinone C6), 151.3 (1C; pyridazinone C4), 158.2 (1C; CH2–N–C=O), 168.9 (1C; pyridazinone C3); LC/API-ESMS m/z 413 [M - NO2]+; Anal. Calcd. for C23H23N7O4.3/2 H2O: C, 56.55; H, 5.36; N, 20.07. Found: C, 56.71; H, 4.99; N, 19.97%.
N’-(2-Methoxybenzylidene)-2-(6-oxo-3-(4-(4-chlorophenyl)piperazin-1-yl)pyridazin-1(6H)-yl)-acetohydrazide (10). White crystals (methanol/water); yield 80%; m.p. 241–242 °C; IR (ν cm−1, ATR): 3236 (N–H), 3074 (C–H aromatic), 2970 (C–H aliphatic), 1683, 1668 (C=O), 1573 (C=N), 1231 (C–N), 1122 (C–O), 837 (C–Cl). 1H-NMR (DMSO-d6, 600 MHz): δ 3.23 (4H; t; CH2N; b + b’), 3.38 (4H; t; CH2N; a + a’), 3.86 (3H; s; OCH3), 5.04 (2H; s; CH2CO), 7.24 (1H; d; J = 4.2 Hz, pyridazinone H5), 7.25 (1H; d; J = 4.2 Hz, pyridazinone H4), 7.26–7.86 (8H; m; phenyl protons), 8.36 (1H; s; –N=CH–), 11.61 (1H; s; –NH–N); 13C-NMR (DMSO-d6, 600 MHz): δ 46.3 (2C, CH2–N; b + b’), 48.2 (2C, CH2–N; a + a’), 53.0 (1C; –N–CH2–C=O), 56.1 (1C; –OCH3), 112.3 (1C; =CH), 117.7 (2C; 4-chlorophenyl C2,6), 121.2 (1C; 2-methoxyphenyl C6), 122.4 (1C; 2-methoxyphenyl C5), 123.2 (2C; 4-chlorophenyl C3,5), 125.9 (2C; 2-methoxyphenyl C3,4), 127.1 (1C; pyridazinone C5), 129.1 (1C; 4-chlorophenyl C1), 131.1 (1C; 2-methoxyphenyl C1), 139.9 (1C; 2-methoxyphenyl C2), 142.9 (1C; 4-chlorophenyl C4), 148.9 (1C; pyridazinone C4), 150.1 (1C; pyridazinone C6), 163.6 (1C; CH2–N–C=O), 166.3 (1C; pyridazinone C3); LC/MSMS (ESI+) m/z 503 [M + Na]+; Anal. Calcd for C24H25ClN6O3.1/5H2O: C, 59.49; H, 5.28; N, 17.34. Found: C, 59.13; H, 5.21; N, 17.93%.
N’-(3-Methoxybenzylidene)-2-(6-oxo-3-(4-(4-chlorophenyl)piperazin-1-yl)pyridazin-1(6H)-yl)-acetohydrazide (11). White crystals (methanol/water); yield 82%; m.p. 214–215 °C; IR (ν cm−1, ATR): 3065 (C–H aromatic), 2943 (C–H aliphatic), 1696, 1656 (C=O), 1567 (C=N), 1231 (C–N), 1124 (C–O), 836 (C–Cl). 1H-NMR (DMSO-d6, 600 MHz): δ 3.23 (4H; t; CH2N; b + b’), 3.38 (4H; t; CH2N; a + a’), 3.80 (3H; s; OCH3), 5.06 (2H; s; CH2CO), 6.99 (1H; d; J = 4.2 Hz, pyridazinone H5), 7.00–7.98 (9H; m; phenyl protons and pyridazinone H4), 8.18 (1H; s; –N=CH–), 11.67 (1H; s; –NH–N); 13C-NMR (DMSO-d6, 150 MHz): δ 46.3 (2C, CH2–N; b + b’), 48.1 (2C, CH2–N; a + a’), 53.0 (1C; –N–CH2–C=O), 55.6 (1C; –OCH3), 111.6 (1C; =CH), 116.6 (2C; 4-chlorophenyl C2,6), 117.7 (1C; 3-methoxyphenyl C6), 123.2 (1C; 3-methoxyphenyl C2), 127.1 (2C; 4-chlorophenyl C3,5), 129.1 (2C; 3-methoxyphenyl C4,5), 130.4 (1C; pyridazinone C5), 131.0 (1C; 4-chlorophenyl C1), 135.9 (1C; 3-methoxyphenyl C1), 144.1 (1C; 3-methoxyphenyl C3), 148.9 (1C; 4-chlorophenyl C4), 150.1 (1C; pyridazinone C4), 158.2 (1C; pyridazinone C6), 160.0 (1C; CH2–N–C=O), 168.5 (1C; pyridazinone C3); LC/MSMS (ESI+) m/z 414 [M-(Cl + OCH3)]+; Anal. Calcd for C24H25ClN6O3.1/9H2O: C, 59.69; H, 5.26; N, 17.40. Found: C, 59.48; H, 5.12; N, 18.23%.
N’-(2,3-Dimethoxybenzylidene)-2-(6-oxo-3-(4-(4-chlorophenyl)piperazin-1-yl)pyridazin-1(6H)-yl)acetohydrazide (12). White crystals (methanol/water); yield 80%; m.p. 252–253 °C; IR (ν cm−1, ATR): 3196 (N–H), 3045 (C–H aromatic), 2968 (C–H aliphatic), 1690, 1667 (C=O), 1561 (C=N), 1230 (C–N), 1065 (C–O), 843 (C–Cl). 1H-NMR (DMSO-d6, 600 MHz): δ 3.23 (4H; t; CH2N; Hb + Hb’), 3.37 (4H; t; CH2N; Ha + Ha’), 3.78 (3H; s; OCH3), 3.84 (3H; s; OCH3), 5.04 (2H; s; NCH2CO), 6.92 (1H; d; J = 4.2 Hz, pyridazinone H5), 6.99 (1H; d; J = 4.2 Hz, pyridazinone H4), 7.10–7.65 (7H; m; phenyl protons), 8.30 (1H; s; –N=CH–), 11.62 (1H; s; –NH–N); 13C-NMR (DMSO-d6, 150 MHz): δ 46.3 (2C, CH2–N; b + b’), 48.1 (2C, CH2–N; a + a’), 52.9 (1C; –N–CH2–C=O), 56.2 (1C; –OCH3), 61.7 (1C; –OCH3), 114.6 (1C; =CH), 117.4 (2C; 4-chlorophenyl C2,6), 123.2 (2C; 4-chlorophenyl C3,5), 127.9 (1C; 2,3-dimethoxyphenyl C6), 129.1 (1C; 2,3-dimethoxyphenyl C1), 131.0 (1C; 4-chlorophenyl C1), 140.1 (2C; 2,3-dimethoxyphenyl C4,5), 148.3 (1C; 2,3-dimethoxyphenyl C2), 148.4 (1C; 2,3-dimethoxyphenyl C3), 148.9 (1C; pyridazinone C5), 150.1 (1C; 4-chlorophenyl C4), 153.1 (1C; pyridazinone C6), 158.2 (1C; pyridazinone C4), 163.7 (1C; CH2–N–C=O), 168.4 (1C; pyridazinone C3); LC/API-ESMS m/z 533 [M + Na]+; Anal. Calcd. for C25H27ClN6O4.1/9 H2O: C, 58.53; H, 5.35; N, 16.38. Found: C, 58.15; H, 5.16; N, 17.30%.
N’-(2,4,6-Trimethoxybenzylidene)-2-(6-oxo-3-(4-(4-chlorophenyl)piperazin-1-yl)pyridazin-1(6H)-yl)acetohydrazide (13). White crystals (methanol/water); yield 86%; m.p. 281–282 °C; IR (ν cm−1, ATR): 3231 (N–H), 3086 (C–H aromatic), 2933 (C–H aliphatic), 1697, 1668 (C=O), 1592 (C=N), 1236 (C–N), 1156 (C–O), 851 (C–Cl). 1H-NMR (DMSO-d6, 600 MHz): δ 3.22 (4H; t; CH2N; Hb + Hb’), 3.38 (4H; t; CH2N; Ha + Ha’), 3.81 (6H; s; OCH3), 3.83 (3H; s; OCH3), 4.93 (2H; s; NCH2CO), 6.29 (1H; d; J = 4.1 Hz, pyridazinone H5), 6.89 (1H; d; J = 4.2 Hz, pyridazinone H4), 6.91–7.64 (6H; m; phenyl protons), 8.18 (1H; s; –N=CH–), 11.25 (1H; s; –NH–N); 13C-NMR (DMSO-d6, 150 MHz): δ 46.4 (2C, CH2–N; b + b’), 48.1 (2C, CH2–N; a + a’), 52.9 (1C; –N–CH2–C=O), 55.9 (1C; –OCH3), 56.5 (2C; –OCH3), 104.3 (1C; =CH), 117.7 (2C; 4-chlorophenyl C2,6), 123.2 (2C; 2,4,6-trimethoxyphenyl C3,5), 126.9 (1C; 4-chlorophenyl C3), 129.1 (1C; 4-chlorophenyl C5), 131.0 (1C; 4-chlorophenyl C1), 139.6 (1C; 2,4,6-trimethoxyphenyl C1), 143.2 (1C; 2,4,6-trimethoxyphenyl C2,4), 148.9 (2C; 2,4,6-trimethoxyphenyl C6), 150.1 (1C; pyridazinone C5), 158.3 (1C; 4-chlorophenyl C4), 160.3 (1C; pyridazinone C6), 160.4 (1C; pyridazinone C4), 162.7 (1C; CH2–N–C=O), 167.9 (1C; pyridazinone C3); LC/API-ESMS m/z 563 [M + Na]+; Anal. Calcd. for C26H29ClN6O5.1/6 H2O: C, 57.40; H, 5.43; N, 15.45. Found: C, 57.01; H, 5.23; N, 16.47%.
N’-(3,4,5-Trimethoxybenzylidene)-2-(6-oxo-3-(4-(4-chlorophenyl)piperazin-1-yl)pyridazin-1(6H)-yl)acetohydrazide (14). White crystals (methanol/water); yield 77%; m.p. 285–286 °C; IR (ν cm−1, ATR): 3213 (N–H), 3062 (C–H aromatic), 2938 (C–H aliphatic), 1681, 1663 (C=O), 1595 (C=N), 1232 (C–N), 1157 (C–O), 851 (C–Cl). 1H-NMR (DMSO-d6, 600 MHz): δ 3.23 (4H; t; CH2N; Hb + Hb’), 3.36 (4H; t; CH2N; Ha + Ha’), 3.70 (6H; s; OCH3), 3.83 (3H; s; OCH3), 5.07 (2H; s; NCH2CO), 6.91 (1H; d; J = 4.2 Hz, pyridazinone H5), 6.93-7.92 (7H; m; phenyl protons and pyridazinone H4), 8.14 (1H; s; –N=CH–), 11.67 (1H; s; –NH–N); 13C-NMR (DMSO-d6, 150 MHz): δ 46.4 (2C, CH2–N; b + b’), 48.1 (2C, CH2–N; a + a’), 53.1 (1C; –N–CH2–C=O), 56.5 (1C; –OCH3), 60.5 (2C; –OCH3), 104.6 (1C; =CH), 117.7 (2C; 4-chlorophenyl C2,6), 119.8 (2C; 2,4,6-trimethoxyphenyl C2,6), 123.2 (1C; 4-chlorophenyl C3), 129.1 (1C; 4-chlorophenyl C5), 129.9 (1C; 4-chlorophenyl C1), 131.1 (1C; 2,4,6-trimethoxyphenyl C1), 139.5 (1C; 2,4,6-trimethoxyphenyl C3,5), 144.0 (2C; 2,4,6-trimethoxyphenyl C4), 148.9 (1C; pyridazinone C5), 150.1 (1C; 4-chlorophenyl C4), 153.6 (1C; pyridazinone C6), 158.2 (1C; pyridazinone C4), 163.7 (1C; CH2–N–C=O), 168.5 (1C; pyridazinone C3); LC/API-ESMS m/z 560 [M]+; Anal. Calcd. for C26H29ClN6O5.1/4 H2O: C, 57.25; H, 5.45; N, 15.41. Found: C, 56.86; H, 5.20; N, 16.63%.
N’-(4-Nitrobenzylidene)-2-(6-oxo-3-(4-(4-chlorophenyl)piperazin-1-yl)pyridazin-1(6H)-yl)acetohydrazide (15). White crystals (methanol/water); yield 86%; m.p. 238–239 °C; IR (ν cm−1, ATR): 3077 (C–H aromatic), 2964 (C–H aliphatic), 1689, 1654 (C=O), 1580 (C=N), 1340 (aromatic NO2), 1236 (C–N), 836 (C–Cl). 1H-NMR (DMSO-d6, 600 MHz): δ 3.23 (4H; t; CH2N; b + b’), 3.38 (4H; t; CH2N; a + a’), 5.10 (2H; s; CH2CO), 6.92 (1H; d; J = 4.2 Hz, pyridazinone H5), 6.99 (1H; d; J = 4.2 Hz, pyridazinone H5), 7.24–8.29 (8H; m; phenyl protons), 8.33 (1H; s; –N=CH–), 11.96 (1H; s; –NH–N); 13C-NMR (DMSO-d6, 150 MHz): δ 46.3 (2C, CH2–N; b + b’), 48.1 (2C, CH2-N; a + a’), 53.0 (1C; –N–CH2–C=O), 105.1 (1C; =CH), 117.7 (2C; 4-chlorophenyl C2,6), 124.5 (1C; 4-nitrophenyl C6), 127.1 (1C; 4-nitrophenyl C2), 128.3 (2C; 4-chlorophenyl C3,5), 129.1 (2C; 4-nitrophenyl C3,5), 131.0 (1C; pyridazinone C5), 141.9 (1C; 4-chlorophenyl C1), 145.0 (1C; 4-nitrophenyl C1), 148.2 (1C; 4-nitrophenyl C4), 149.0 (1C; 4-chlorophenyl C4), 150.1 (1C; pyridazinone C4), 158.2 (1C; pyridazinone C6), 164.3 (1C; CH2–N–C=O), 168.9 (1C; pyridazinone C3); LC/MSMS (ESI+) m/z 414 [M-(Cl + NO2)]+; Anal. Calcd for C23H22ClN7O4.1/9H2O: C, 55.48; H, 4.50; N, 19.69. Found: C, 55.19; H, 4.42; N, 20.76%.
N’-(4-Dimethylaminobenzylidene)-2-(6-oxo-3-(4-(4-chlorophenyl)piperazin-1-yl)pyridazin-1(6H)-yl)acetohydrazide (16). White crystals (methanol/water); yield 68%; m.p. 243–244 °C; IR (ν cm−1, ATR): 3082 (C–H aromatic), 2962 (C–H aliphatic), 1678, 1665 (C=O), 1589 (C=N), 1227 (C–N), 836 (C–Cl). 1H-NMR (DMSO-d6, 600 MHz): δ 2.96 (6H; s; –N(CH3)2), 3.23 (4H; t; CH2N; b + b’), 3.37 (4H; t; CH2N; a + a’), 5.01 (2H; s; CH2CO), 6.72 (1H; d; J = 4.1 Hz, pyridazinone H5), 6.74 (1H; d; J = 4.2 Hz, pyridazinone H5), 6.91–7.88 (8H; m; phenyl protons), 8.06 (1H; s; –N=CH–), 11.36 (1H; s; –NH–N); 13C-NMR (DMSO-d6, 150 MHz): δ 46.4 (2C, CH2–N; b + b’), 46.5 (2C; –N(CH3)2), 48.1 (2C, CH2–N; a+a’), 52.9 (1C; –N–CH2–C=O), 112.3 (1C; =CH), 117.7 (2C; 4-chlorophenyl C2,6), 121.8 (1C; 4-dimethylaminophenyl C6), 127.1 (1C; 4-dimethylaminophenyl C2), 128.9 (2C; 4-chlorophenyl C3,5), 129.1 (2C; 4-dimethylaminophenyl C3,5), 131.1 (1C; pyridazinone C5), 145.1 (1C; 4-chlorophenyl C1), 148.2 (1C; 4-dimethylphenyl C1), 149.0 (1C; 4-dimethylphenyl C4), 150.1 (1C; 4-chlorophenyl C4), 151.9 (1C; pyridazinone C4), 158.2 (1C; pyridazinone C6), 163.1 (1C; CH2–N–C=O), 167.8 (1C; pyridazinone C3); LC/MSMS (ESI+) m/z 516 [M + Na]+; Anal. Calcd for C25H28ClN7O2.1/6 H2O: C, 60.42; H, 5.75; N, 19.73. Found: C, 60.03; H, 5.55; N, 20.48%.
N’-Benzylidene-2-(6-oxo-3-(4-(4-fluorophenyl)piperazin-1-yl)pyridazin-1(6H)-yl)acetohydrazide (17). White crystals (methanol/water); yield 47%; m.p. 207–208 °C; IR (ν cm−1, ATR): 3178 (N–H), 3056 (C–H aromatic), 2960 (C–H aliphatic), 1652 (C=O), 1585 (C=N), 1222 (C–N), 837 (C–F). 1H-NMR (DMSO-d6, 600 MHz): δ 3.17 (4H; t; CH2N; b + b’), 3.38 (4H; t; CH2N; a + a’), 5.06 (2H; s; CH2CO), 7.01 (1H; d; J = 4.2 Hz, pyridazinone H5), 7.02 (1H; d; J = 4.2 Hz, pyridazinone H4), 7.06-7.72 (9H; m; phenyl protons), 8.26 (1H; s; –N=CH–), 11.66 (1H; s; –NH–N); 13C-NMR (DMSO-d6, 150 MHz): δ 46.5 (2C, CH2–N; b + b’), 49.1 (2C, CH2–N; a + a’), 52.9 (1C; –N–CH2-C=O), 115.7 (1C; =CH), 118.1 (2C; phenyl C4), 127.2 (1C; phenyl C3,5), 129.3 (1C; phenyl C2,6), 130.4 (2C; 4-fluorophenyl C3,5), 131.0 (2C; 4-fluorophenyl C2,6), 134.4 (1C; 4-fluorophenyl C3,5), 144.3 (1C; 4-fluorophenyl C1), 147.4 (1C; phenyl C1), 148.2 (1C; pyridazinone C5), 149.1 (1C; 4-fluorophenyl C4), 155.9 (1C; pyridazinone C4), 158.2 (1C; pyridazinone C6), 163.8 (1C; CH2–N–C=O), 168.5 (1C; pyridazinone C3); LC/MSMS (ESI+) m/z 457 [M + Na]+; Anal. Calcd for C23H23FN6O2.1/2H2O: C, 62.29; H, 5.45; N, 18.95. Found: C, 62.21; H, 5.32; N, 18.81%.
N’-(3-M-ethylbenzylidene)-2-(6-oxo-3-(4-(4-fluorophenyl)piperazin-1-yl)pyridazin-1(6H)-yl)acetohydrazide (18). White crystals (methanol/water); yield 51%; m.p. 201–202 °C; IR (ν cm−1, ATR): 3170 (N–H), 3045 (C–H aromatic), 2988 (C–H aliphatic), 1652 (C=O), 1576 (C=N), 1264 (C–N). 1H-NMR (DMSO-d6, 600 MHz): δ 2.51 (3H; s; CH3), 3.17 (4H; t; CH2N; Hb+Hb’), 3.38 (4H; t; CH2N; Ha + Ha’), 5.07 (2H; s; NCH2CO), 7.00 (1H; d; J = 4.2 Hz, pyridazinone H5), 7.02–7.98 (9H; m; phenyl protons and pyridazinone H5), 8.19 (1H; s; –N=CH–), 11.67 (1H; s; –NH–N); 13C-NMR (DMSO-d6, 150 MHz): δ 21.4 (1C; CH3), 46.5 (2C, CH2–N; b + b’), 49.1 (2C, CH2–N; a + a’), 55.6 (1C; –N–CH2–C=O), 111.6 (1C; =CH), 115.9 (2C; 4-fluorophenyl C2,6), 118.0 (2C; 4-fluorophenyl C3,5), 120.2 (1C; 3-methylphenyl C6), 130.4 (1C; 3-methylphenyl C2), 131.0 (1C; 4-fluorophenyl C1), 135.9 (1C; 3-methylphenyl C1), 144.1 (1C; 3-methylphenyl C3), 147.3 (2C; 3-methylphenyl C4,5), 148.2 (1C; pyridazinone C5), 149.0 (1C; 4-fluorophenyl C4), 155.9 (1C; pyridazinone C6), 158.2 (1C; pyridazinone C4), 163.8 (1C; CH2–N–C=O), 168.5 (1C; pyridazinone C3); LC/API-ESMS m/z 414 [M-(F + CH3)]+; Anal. Calcd. for C24H25FN6O2.4/5 H2O: C, 61.20; H, 5.80; N, 17.84. Found: C, 60.85; H, 5.40; N, 17.84%.
N’-(3-Methoxybenzylidene)-2-(6-oxo-3-(4-(4-fluorophenyl)piperazin-1-yl)pyridazin-1(6H)-yl)acetohydrazide (19). White crystals (methanol/water); yield 48%; m.p. 202–203 °C; IR (ν cm−1, ATR): 3069 (C–H aromatic), 2960 (C–H aliphatic), 1698, 1656 (C=O), 1569 (C=N), 1234 (C–N), 1158 (C–O), 836 (C–F). 1H-NMR (DMSO-d6, 600 MHz): δ 3.17 (4H; t; CH2N; b + b’), 3.38 (4H; t; CH2N; a + a’), 3.80 (3H; s; OCH3), 5.06 (2H; s; CH2CO), 7.00 (1H; d; J = 4.2 Hz, pyridazinone H5), 7.01–7.98 (9H; m; phenyl protons and pyridazinone H4), 8.19 (1H; s; –N=CH–), 11.67 (1H; s; –NH–N); 13C-NMR (DMSO-d6, 150 MHz): δ 46.5 (2C, CH2–N; b + b’), 49.1 (2C, CH2–N; a + a’), 53.0 (1C; –N–CH2–C=O), 55.6 (1C; –OCH3), 111.6 (1C; =CH), 115.6 (2C; 4-fluorophenyl C2,6), 118.1 (1C; 3-methoxyphenyl C6), 120.2 (1C; 3-methoxyphenyl C2), 130.4 (2C; 4-fluorophenyl C3,5), 131.0 (2C; 3-methoxyphenyl C4,5), 135.9 (1C; pyridazinone C5), 144.1 (1C; 4-fluorophenyl C1), 147.3 (1C; 3-methoxyphenyl C1), 149.0 (1C; 3-methoxyphenyl C3), 155.9 (1C; 4-fluorophenyl C4), 157.5 (1C; pyridazinone C4), 158.2 (1C; pyridazinone C6), 160.0 (1C; CH2–N–C=O), 168.5 (1C; pyridazinone C3); LC/MSMS (ESI+) m/z 487 [M + Na]+; Anal. Calcd for C24H25FN6O3.2/3H2O: C, 60.49; H, 5.57; N, 17.64. Found: C, 60.06; H, 5.13; N, 17.43%.
N’-(4-Methoxybenzylidene)-2-(6-oxo-3-(4-(4-chlorophenyl)piperazin-1-yl)pyridazin-1(6H)-yl)acetohydrazide (20). White crystals (methanol/water); yield 41%; m.p. 247–248 °C; IR (ν cm−1, ATR): 3065 (C–H aromatic), 2966 (C–H aliphatic), 1686 (C=O), 1570 (C=N), 1247 (C–N), 1024 (C–O), 836 (C–F). 1H-NMR (DMSO-d6, 600 MHz): δ 3.17 (4H; t; CH2N; b + b’), 3.38 (4H; t; CH2N; a + a’), 3.80 (3H; s; OCH3), 5.04 (2H; s; CH2CO), 6.91 (1H; d; J = 4.1 Hz, pyridazinone H5), 6.92-7.96 (9H; m; phenyl protons and pyridazinone H4), 8.16 (1H; s; –N=CH–), 11.52 (1H; s; –NH–N); 13C-NMR (DMSO-d6, 150 MHz): δ 46.5 (2C, CH2–N; b + b’), 49.1 (2C, CH2–N; a + a’), 52.9 (1C; –N–CH2–C=O), 55.8 (1C; –OCH3), 114.8 (1C; =CH), 115.9 (2C; 4-fluorophenyl C2,6), 118.1 (1C; 4-methoxyphenyl C6), 127.0 (1C; 4-methoxyphenyl C2), 128.9 (2C; 4-fluorophenyl C3,5), 129.2 (2C; 4-methoxyphenyl C3,5), 131.0 (1C; pyridazinone C5), 144.1 (1C; 4-fluorophenyl C1), 147.3 (1C; 4-methoxyphenyl C1), 148.2 (1C; 4-methoxyphenyl C4), 149.1 (1C; 4-fluorophenyl C4), 155.9 (1C; pyridazinone C4), 158.2 (1C; pyridazinone C6), 163.5 (1C; CH2–N–C=O), 168.2 (1C; pyridazinone C3); LC/MSMS (ESI+) m/z 487 [M + Na]+; Anal. Calcd for C24H25FN6O3.2/3H2O: C, 60.49; H, 5.57; N, 17.64. Found: C, 60.05; H, 5.24; N, 17.50%.
N’-(2,4,6-Trimethoxybenzylidene)-2-(6-oxo-3-(4-(4-fluorophenyl)piperazin-1-yl)pyridazin-1(6H)-yl)acetohydrazide (21). White crystals (methanol/water); yield 44%; m.p. 275–276 °C; IR (ν cm−1, ATR): 3231 (N–H), 3085 (C–H aromatic), 2963 (C–H aliphatic), 1673, 1668 (C=O), 1595 (C=N), 1240 (C–N), 1128 (C–O), 851 (C–F). 1H-NMR (DMSO-d6, 600 MHz): δ 3.18 (4H; t; CH2N; Hb + Hb’), 3.37 (4H; t; CH2N; Ha + Ha’), 3.82 (6H; s; OCH3), 3.83 (3H; s; OCH3), 4.94 (2H; s; NCH2CO), 6.29 (1H; d; J = 4.2 Hz, pyridazinone H5), 6.90 (1H; d; J = 4.2 Hz, pyridazinone H4), 6.91–7.64 (6H; m; phenyl protons), 8.19 (1H; s; –N=CH–), 11.26 (1H; s; –NH–N); 13C-NMR (DMSO-d6, 150 MHz): δ 46.5 (2C, CH2–N; b + b’), 49.2 (2C, CH2–N; a + a’), 52.9 (1C; –N–CH2–C=O), 55.9 (2C; –OCH3), 56.5 (2C; –OCH3), 104.2 (1C; =CH), 115.9 (2C; 4-fluorophenyl C2,6), 118.1 (2C; 2,4,6-trimethoxyphenyl C3,5), 126.9 (1C; 4-fluorophenyl C3), 127.0 (1C; 4-fluorophenyl C5), 131.0 (1C; 4-fluorophenyl C1), 139.6 (1C; 2,4,6-trimethoxyphenyl C1), 148.2 (3C; 2,4,6-trimethoxyphenyl C2,4,6), 148.9 (1C; pyridazinone C5), 155.9(1C; 4-fluorophenyl C4), 158.3 (1C; pyridazinone C6), 160.4 (1C; pyridazinone C4), 162.6 (1C; CH2–N–C=O) and 167.9 (1C; pyridazinone C3); LC/API-ESMS m/z 524 [M]+; Anal. Calcd. for C26H29FN6O5.H2O: C, 57.56; H, 5.76; N, 15.49. Found: C, 57.75; H, 5.30; N, 15.64%.
N’-(4-Nitrobenzylidene)-2-(6-oxo-3-(4-(4-fluorophenyl)piperazin-1-yl)pyridazin-1(6H)-yl)acetohydrazide (22). White crystals (methanol/water); yield 49%; m.p. 243–244 °C; IR (ν cm−1, ATR): 3077 (C–H aromatic), 2963 (C–H aliphatic), 1690, 1659 (C=O), 1580 (C=N), 1339 (aromatic NO2), 1236 (C–N), 836 (C–F). 1H-NMR (DMSO-d6, 600 MHz): δ 3.17 (4H; t; CH2N; b + b’), 3.39 (4H; t; CH2N; a + a’), 5.11 (2H; s; NCH2CO), 6.94 (1H; d; J = 4.2 Hz, pyridazinone H5), 6.99 (1H; d; J = 4.2 Hz, pyridazinone H5), 7.00-8.39 (8H; m; phenyl protons), 8.88 (1H; s; –N=CH–), 11.96 (1H; s; –NH–N); 13C-NMR (DMSO-d6, 150 MHz): δ 46.5 (2C, CH2–N; b + b’), 49.1 (2C, CH2–N; a+a’), 53.0 (1C; –N–CH2–C=O), 115.9 (1C; =CH), 118.0 (2C; 4-fluorophenyl C2,6), 124.5 (1C; 4-nitrophenyl C6), 127.1 (1C; 4-nitrophenyl C2), 128.4 (2C; 4-fluorophenyl C3,5), 128.5 (2C; 4-nitrophenyl C3,5), 131.0 (1C; pyridazinone C5), 140.8 (1C; 4-fluorophenyl C1), 141.9 (1C; 4-nitrophenyl C1), 148.2 (1C; 4-nitrophenyl C4), 149.1 (1C; 4-fluorophenyl C4), 155.9 (1C; pyridazinone C4), 157.5 (1C; pyridazinone C6), 158.2 (1C; CH2–N–C=O), 168.9 (1C; pyridazinone C3); LC/MSMS (ESI+) m/z 414 [M-(F + NO2)]+; Anal. Calcd for C23H22FN7O4.2/3H2O: C, 56.21; H, 4.79; N, 19.95. Found: C, 56.07; H, 4.89; N, 19.63%.
N’-(4-dimethylaminobenzylidene)-2-(6-oxo-3-(4-(4-fluorophenyl)piperazin-1-yl)pyridazin-1(6H)-yl)acetohydrazide (23). White crystals (methanol/water); yield 45%; m.p. 248–249 °C; IR (ν cm−1, ATR): 3046 (C–H aromatic), 2994 (C–H aliphatic), 1646, 1612 (C=O), 1584 (C=N), 1265 (C–N), 846 (C–F). 1H-NMR (DMSO-d6, 600 MHz): δ 2.97 (6H; s; –N(CH3)2), 3.17 (4H; t; CH2N; b + b’), 3.38 (4H; t; CH2N; a + a’), 5.01 (2H; s; CH2CO), 6.75 (1H; d; J = 4.1 Hz, pyridazinone H5), 6.77 (1H; d; J = 4.2 Hz, pyridazinone H5), 6.91–7.88 (8H; m; phenyl protons), 8.06 (1H; s; –N=CH–), 11.35 (1H; s; –NH–N); 13C-NMR (DMSO-d6, 150 MHz): δ 46.5 (2C, CH2–N; b + b’), 48.4 (2C; –N(CH3)2), 49.1 (2C, CH2–N; a+a’), 52.9 (1C; –N–CH2–C=O), 112.3 (1C; =CH), 115.9 (2C; 4-fluorophenyl C2,6), 118.1 (1C; 4-dimethylaminophenyl C6), 121.8 (1C; 4-dimethylaminophenyl C2), 126.9 (2C; 4-fluorophenyl C3,5), 128.9 (2C; 4-dimethylaminophenyl C3,5), 129.9 (1C; pyridazinone C5), 145.1 (1C; 4-fluorophenyl C1), 148.2 (1C; 4-dimethylphenyl C1), 151.9 (1C; 4-dimethylphenyl C4), 155.9 (1C; 4-fluorophenyl C4), 157.5 (1C; pyridazinone C4), 158.2 (1C; pyridazinone C6), 163.1 (1C; CH2–N–C=O), 167.8 (1C; pyridazinone C3); LC/MSMS (ESI+) m/z 501 [M + Na]+; Anal. Calcd for C25H28FN7O2.1/3 H2O: C, 62.10; H, 5.98; N, 20.28. Found: C, 62.24; H, 5.80; N, 20.37%.