Therapeutic Sequencing in ALK+ NSCLC
Abstract
1. The New Era of Second-Generation ALK Inhibitors
1.1. Best-First for Longer Survival
1.2. Best-First before Chemotherapy
1.3. When Should Brain Radiotherapy Be Offered?
1.4. Safety and Tolerability
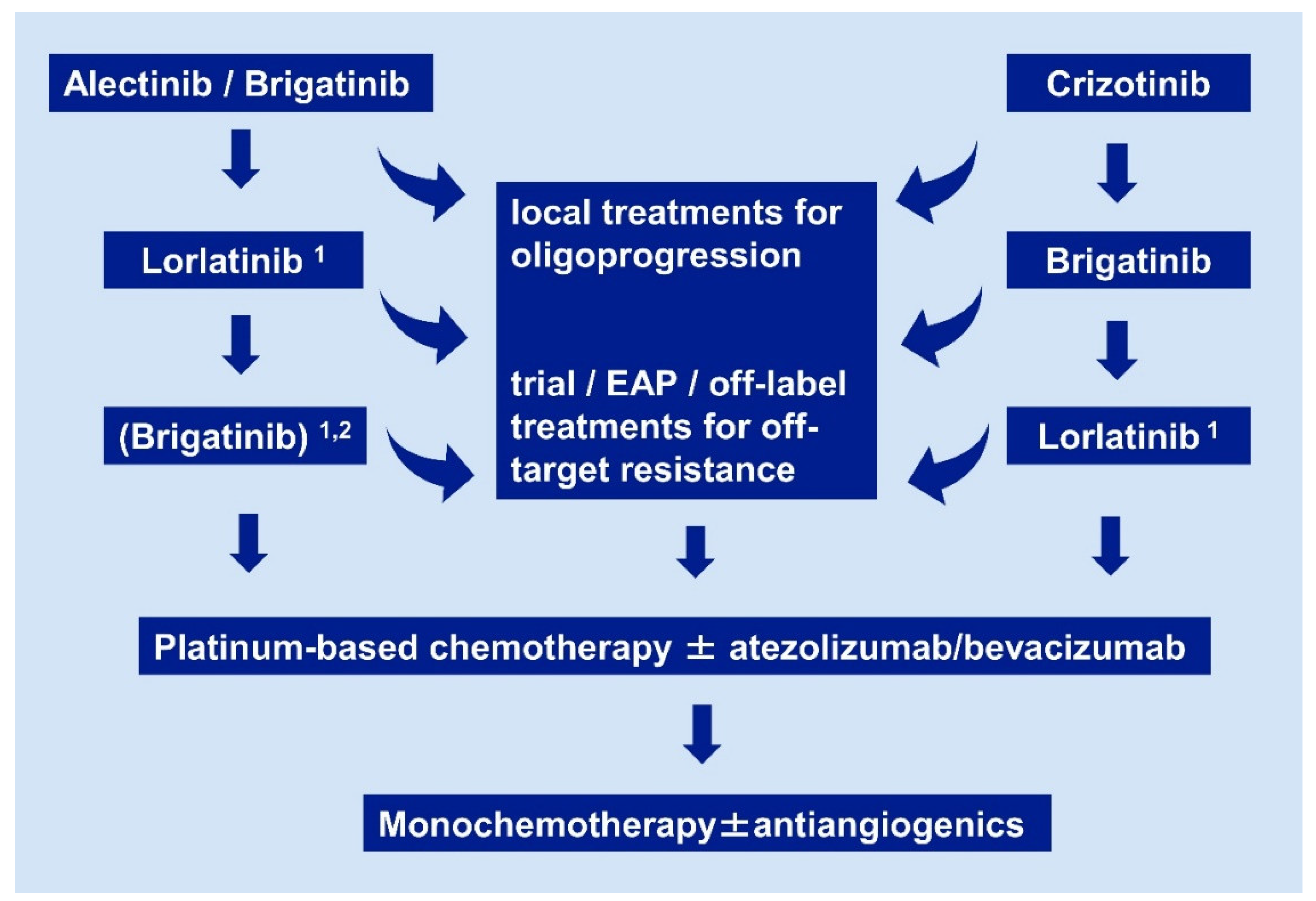
2. State-of-the-Art after the First Line
2.1. Oligoprogressive Patients
2.2. Systemic Treatment after Alectinib or Brigatinib
2.3. Systemic Treatment after Crizotinib
2.4. Treatment with ALK Inhibitors after Lorlatinib
| First Line: | Crizotinib | Ceritinib | Alectinib | Alectinib | Brigatinib | Ensartinib | Lorlatinib | Lorlatinib |
| study [ref.] | PROFILE-1014 [12,29] | ASCEND-4 [46] | J-ALEX [76,77] | ALEX [1,28] | ALTA-1L [5,78] | eXalt3 [4] | CROWN [10] | global phase II [61] (EXP1) |
| comparator | chemo | chemo | crizotinib | crizotinib | crizotinib | crizotinib | crizotinib | single arm |
| patients (n) | 172 | 189 | 103 | 152 | 137 | 143 | 149 | 30 |
| ORR (%) | 74 | 73 | 76 | 83 | 76 | 75 | 76 | 90 |
| mPFS (mo) | 10.9 | 16.6 | 34.1 | 25.7 */34.8 ** | 24.0 */29.4 ** | 25.8* | NR | NR |
| HR | 0.45 | 0.50 | 0.34 | 0.50 | 0.49 | 0.52 | 0.28 | N/A |
| Post Crizotinib: | Ceritinib | Ceritinib | Alectinib | Alectinib | Brigatinib | Brigatinib | Ensartinib | Lorlatinib |
| study [ref.] | ASCEND-1 [31] | ASCEND-2 [79] | global phase II [13] | phase II [14] | phase I/II [15] | ALTA 90/180 mg [16,17] | phase I/II [80] | global phase II [61,62] (EXP2/3A) |
| patients (n) | 163 | 140 | 138 | 87 | 70 | 110 | 29 | 59 |
| ORR (%) | 56 | 38 | 50 | 48 | 71 | 55 | 69 | 70 |
| mPFS (mo) | 6.9 | 5.7 | 8.9 | 8.1 | 13.4 | 12.9/16.7 | 9 | 11.1 |
| Post Alectinib (or other 2G TKI): | Ceritinib | Ceritinib | Brigatinib | Brigatinib | Brigatinib | Brigatinib | Lorlatinib | |
| study [ref.] | phase II [81] (Japan) | retrospective [82] (Japan) | ALTA-2 [83] | Phase II (IIT, USA) [64] | retrospective [65] | EAP (EU) [70] | global phase II [61,62] (EXP3B/4/5) | |
| patients (n) | 20 | 9 | 103 | 19 | 18 | 111 | 139 | |
| ORR (%, (n)) | 25 (4/20) | 44 (4/9) | pending | 47 (9/19) | 17 (3/18) | n/a | 41 (52/127) | |
| mPFS (mo) | 3.7 | 4.4 | 6.4 | 4.4 | n/a | 6.9 | ||
| mTNT (mo) | 8.7 | |||||||
| Post Lorlatinib: | Brigatinib | |||||||
| study [ref.] | EAP (EU) [70] | |||||||
| patients (n) | 37 | |||||||
| ORR (%) | n/a | |||||||
| mPFS (mo) | n/a | |||||||
| mTNT (mo) | 7.5 | |||||||
| ALK+, First Line: | Crizotinib | Ceritinib | Alectinib | Brigatinib | Ensartinib | Lorlatinib |
| study [ref.] | ALTA1L-ALEX (asympt. BM) [1,5] | ASCEND-4 (stable BM) [46] | ALEX (asympt. BM) [1] | ALTA-1L (asympt. BM) [5] | eXalt3 (asympt. BM) [4] | CROWN (asympt. BM) [10] |
| patients (n) for iORR: | 21–22 | 22 | 21 | 18 | 13 | 14 |
| iORR (%) | 29–50 | 73 | 81 | 78 | 75 | 82 |
| patients (n) total: | 138–151 | n/a | 152 | 137 | 143 | 149 |
| brain progression at 1 year: | 19–41 | n/a | 9.4 | 8.8 | 4 | 2.8 |
| ALK+, Post Crizotinib: | Ceritinib | Alectinib | Brigatinib | Ensartinib | Lorlatinib | |
| study [ref.] | ASCEND-2 [79] | phase I/II pooled [84] | ALTA (180mg) [85] | phase I/II [80] | phase II [61] | |
| patients (n) | 20 | 50 | 18 | 6 | 37 | |
| iORR (%) | 45 | 64 | 67 | 83 | 68 | |
| iORR (%) 1L | 73 | 81 | 78 | 75 | 75 | |
| ΔiORR 2L-1L | −29% | −17% | −11% | 8% | −7% | |
| (% of 1L) | (−39%) | (−21%) | (−14%) | (+11%) | (−9%) | |
| EGFR+, First Line and beyond: | Gefitinib/Erlotinib | Osimertinib | Radiotherapy /+ΤΚΙ | |||
| study [ref.] | FLAURA [39] (stable BM) | FLAURA [39] (stable BM) | various studies [86,87,88] | |||
| patients (n) for iORR: | 19 | 22 | ||||
| iORR (%) | 68 | 91 | 50–60 / 85 | |||
| patients (N) total: | 277 | 279 | ||||
| brain progression at 1 year: | 24 | 8 | ||||
2.5. Do We Need Rebiopsies and Next-Generation Sequencing (NGS) Testing?
2.6. The Emerging Concept of Molecular Risk and Value of Disease Monitoring in ALK+ NSCLC
2.7. Treatment after TKI
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Peters, S.; Camidge, D.R.; Shaw, A.T.; Gadgeel, S.; Ahn, J.S.; Kim, D.W.; Ou, S.I.; Perol, M.; Dziadziuszko, R.; Rosell, R.; et al. Alectinib versus Crizotinib in Untreated ALK-Positive Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2017, 377, 829–838. [Google Scholar] [CrossRef] [PubMed]
- Planchard, D.; Popat, S.; Kerr, K.; Novello, S.; Smit, E.F.; Faivre-Finn, C.; Mok, T.S.; Reck, M.; van Schil, P.E.; Hellmann, M.D.; et al. Metastatic non-small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2019, 30, 863–870. [Google Scholar] [CrossRef] [PubMed]
- National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology (NCCN Guidelines). Non-Small Cell Lung Cancer, Version 1.2021. Available online: www.nccn.org (accessed on 14 December 2020).
- Selvaggi, G.; Wakelee, H.A.; Mok, T.; Wu, Y.-L.; Reck, M.; Chiappori, A.; Cicin, I.; Lee, D.H.; Breder, V.; Fan, Y.; et al. Abstract 2: Phase 3 Randomized Study of Ensartinib vs Crizotinib in Anaplastic Lymphoma Kinase (ALK)-Positive NSCLC Patients: eXalt3. In WCLC 2020 Virtual Presidential Symposium; Organized by the International Association for the Study of Lung Cancer (IASLC). Available online: https://wclc2020.iaslc.org/virtual-presidential-symposium (accessed on 14 December 2020).
- Camidge, D.R.; Kim, H.R.; Ahn, M.J.; Yang, J.C.; Han, J.Y.; Lee, J.S.; Hochmair, M.J.; Li, J.Y.; Chang, G.C.; Lee, K.H.; et al. Brigatinib versus Crizotinib in ALK-Positive Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 379, 2027–2039. [Google Scholar] [CrossRef]
- Bayliss, R.; Choi, J.; Fennell, D.A.; Fry, A.M.; Richards, M.W. Molecular mechanisms that underpin EML4-ALK driven cancers and their response to targeted drugs. Cell Mol. Life Sci. 2016, 73, 1209–1224. [Google Scholar] [CrossRef] [PubMed]
- Rodon Ahnert, J.; Gray, N.; Mok, T.; Gainor, J. What It Takes to Improve a First-Generation Inhibitor to a Second- or Third-Generation Small Molecule. ASCO Educ. Book 2019, 196–205. [Google Scholar] [CrossRef]
- De La Bellacasa, R.P.; Karachaliou, N.; Estrada-Tejedor, R.; Teixidó, J.; Costa, C.; Borrell, J.I. ALK and ROS1 as a joint target for the treatment of lung cancer: A review. Transl. Lung Cancer Res. 2013, 2, 72–86. [Google Scholar]
- Wang, W.-C.; Shiao, H.-Y.; Lee, C.-C.; Fung, K.-S.; Hsieh, H.-P. Anaplastic lymphoma kinase (ALK) inhibitors: A review of design and discovery. Med. Chem. Commun. 2014, 5, 1266–1279. [Google Scholar] [CrossRef]
- Shaw, A.T.; Bauer, T.M.; Marinis, F.D.; Felip, E.; Goto, Y.; Liu, G.; Mazieres, J.; Kim, D.-W.; Mok, T.; Polli, A.; et al. First-Line Lorlatinib or Crizotinib in Advanced ALK-Positive Lung Cancer. N. Engl. J. Med. 2020, 383, 2018–2029. [Google Scholar] [CrossRef]
- Soria, J.C.; Ohe, Y.; Vansteenkiste, J.; Reungwetwattana, T.; Chewaskulyong, B.; Lee, K.H.; Dechaphunkul, A.; Imamura, F.; Nogami, N.; Kurata, T.; et al. Osimertinib in Untreated EGFR-Mutated Advanced Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 378, 113–125. [Google Scholar] [CrossRef]
- Solomon, B.J.; Mok, T.; Kim, D.W.; Wu, Y.L.; Nakagawa, K.; Mekhail, T.; Felip, E.; Cappuzzo, F.; Paolini, J.; Usari, T.; et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N. Engl. J. Med. 2014, 371, 2167–2177. [Google Scholar] [CrossRef]
- Ou, S.H.; Ahn, J.S.; de Petris, L.; Govindan, R.; Yang, J.C.; Hughes, B.; Lena, H.; Moro-Sibilot, D.; Bearz, A.; Ramirez, S.V.; et al. Alectinib in Crizotinib-Refractory ALK-Rearranged Non-Small-Cell Lung Cancer: A Phase II Global Study. J. Clin. Oncol. 2016, 34, 661–668. [Google Scholar] [CrossRef] [PubMed]
- Shaw, A.T.; Gandhi, L.; Gadgeel, S.; Riely, G.J.; Cetnar, J.; West, H.; Camidge, D.R.; Socinski, M.A.; Chiappori, A.; Mekhail, T.; et al. Alectinib in ALK-positive, crizotinib-resistant, non-small-cell lung cancer: A single-group, multicentre, phase 2 trial. Lancet Oncol. 2016, 17, 234–242. [Google Scholar] [CrossRef]
- Gettinger, S.N.; Bazhenova, L.A.; Langer, C.J.; Salgia, R.; Gold, K.A.; Rosell, R.; Shaw, A.T.; Weiss, G.J.; Tugnait, M.; Narasimhan, N.I.; et al. Activity and safety of brigatinib in ALK-rearranged non-small-cell lung cancer and other malignancies: A single-arm, open-label, phase 1/2 trial. Lancet Oncol. 2016, 17, 1683–1696. [Google Scholar] [CrossRef]
- Kim, D.-W.; Tiseo, M.; Ahn, M.-J.; Reckamp, K.L.; Hansen, K.H.; Kim, S.-W.; Huber, R.M.; West, H.L.; Groen, H.J.M.; Hochmair, M.J.; et al. Brigatinib in Patients with Crizotinib-Refractory Anaplastic Lymphoma Kinase–Positive Non–Small-Cell Lung Cancer: A Randomized, Multicenter Phase II Trial. J. Clin. Oncol. 2017, 35, 2490–2498. [Google Scholar] [CrossRef]
- Ahn, M.; Camidge, D.R.; Tiseo, M.; Reckamp, K.; Hansen, K.; Kim, S.; Huber, R.; West, H.; Groen, H.; Hochmair, M.; et al. OA 05.05 Brigatinib in Crizotinib-Refractory ALK+ NSCLC: Updated Efficacy and Safety Results From ALTA, a Randomized Phase 2 Trial. J. Thorac. Oncol. 2017, 12, S1755–S1756. [Google Scholar] [CrossRef]
- Hochmair, M.J.; Morabito, A.; Hao, D.; Yang, C.-T.; Soo, R.A.; Yang, J.C.-H.; Gucalp, R.; Halmos, B.; Wang, L.; Märten, A.; et al. Sequential afatinib and osimertinib in patients with EGFR mutation-positive non-small-cell lung cancer: Updated analysis of the observational GioTag study. Future Oncol. 2019, 15, 2905–2914. [Google Scholar] [CrossRef]
- Christopoulos, P.; Budczies, J.; Kirchner, M.; Dietz, S.; Sultmann, H.; Thomas, M.; Stenzinger, A. Defining molecular risk in ALK(+) NSCLC. Oncotarget 2019, 10, 3093–3103. [Google Scholar] [CrossRef]
- Woo, C.G.; Seo, S.; Kim, S.W.; Jang, S.J.; Park, K.S.; Song, J.Y.; Lee, B.; Richards, M.W.; Bayliss, R.; Lee, D.H.; et al. Differential protein stability and clinical responses of EML4-ALK fusion variants to various ALK inhibitors in advanced ALK-rearranged non-small cell lung cancer. Ann. Oncol. 2017, 28, 791–797. [Google Scholar] [CrossRef]
- Sabir, S.R.; Yeoh, S.; Jackson, G.; Bayliss, R. EML4-ALK Variants: Biological and Molecular Properties, and the Implications for Patients. Cancers 2017, 9, 118. [Google Scholar] [CrossRef]
- O’Regan, L.; Barone, G.; Adib, R.; Woo, C.G.; Jeong, H.J.; Richardson, E.L.; Richards, M.W.; Muller, P.A.J.; Collis, S.J.; Fennell, D.A.; et al. EML4-ALK V3 oncogenic fusion proteins promote microtubule stabilization and accelerated migration through NEK9 and NEK7. J. Cell Sci. 2020, 133. [Google Scholar] [CrossRef]
- Christopoulos, P.; Endris, V.; Bozorgmehr, F.; Elsayed, M.; Kirchner, M.; Ristau, J.; Buchhalter, I.; Penzel, R.; Herth Felix, J.; Heussel Claus, P.; et al. EML4-ALK fusion variant V3 is a high-risk feature conferring accelerated metastatic spread, early treatment failure and worse overall survival in ALK+ non-small cell lung cancer. Int. J. Cancer 2018, 142, 2589–2598. [Google Scholar] [CrossRef] [PubMed]
- Noh, K.W.; Lee, M.S.; Lee, S.E.; Song, J.Y.; Shin, H.T.; Kim, Y.J.; Oh, D.Y.; Jung, K.; Sung, M.; Kim, M.; et al. Molecular breakdown: A comprehensive view of anaplastic lymphoma kinase (ALK)-rearranged non-small cell lung cancer. J. Pathol. 2017, 243, 307–319. [Google Scholar] [CrossRef] [PubMed]
- Christopoulos, P. ALK disease: Best first or later, and do we care about variants? Precis. Cancer Med. 2019. [Google Scholar] [CrossRef]
- Doebele, R.C.; Lu, X.; Sumey, C.; Maxson, D.A.; Weickhardt, A.J.; Oton, A.B.; Bunn, P.A., Jr.; Baron, A.E.; Franklin, W.A.; Aisner, D.L.; et al. Oncogene status predicts patterns of metastatic spread in treatment-naive nonsmall cell lung cancer. Cancer 2012, 118, 4502–4511. [Google Scholar] [CrossRef]
- Patil, T.; Smith, D.E.; Bunn, P.A.; Aisner, D.L.; Le, A.T.; Hancock, M.; Purcell, W.T.; Bowles, D.W.; Camidge, D.R.; Doebele, R.C. The Incidence of Brain Metastases in Stage IV ROS1-Rearranged Non-Small Cell Lung Cancer and Rate of Central Nervous System Progression on Crizotinib. J. Thorac. Oncol. 2018, 13, 1717–1726. [Google Scholar] [CrossRef]
- Mok, T.; Camidge, D.R.; Gadgeel, S.M.; Rosell, R.; Dziadziuszko, R.; Kim, D.-W.; Pérol, M.; Ou, S.-H.I.; Ahn, J.S.; Shaw, A.T.; et al. Updated overall survival and final progression-free survival data for patients with treatment-naive advanced ALK-positive non-small-cell lung cancer in the ALEX study. Ann. Oncol. 2020, 31, 1056–1064. [Google Scholar] [CrossRef]
- Solomon, B.J.; Kim, D.-W.; Wu, Y.-L.; Nakagawa, K.; Mekhail, T.; Felip, E.; Cappuzzo, F.; Paolini, J.; Usari, T.; Tang, Y.; et al. Final Overall Survival Analysis from a Study Comparing First-Line Crizotinib Versus Chemotherapy in ALK-Mutation-Positive Non–Small-Cell Lung Cancer. J. Clin. Oncol. 2018, 36, 2251–2258. [Google Scholar] [CrossRef]
- Shaw, A.T.; Kim, D.W.; Mehra, R.; Tan, D.S.; Felip, E.; Chow, L.Q.; Camidge, D.R.; Vansteenkiste, J.; Sharma, S.; de Pas, T.; et al. Ceritinib in ALK-rearranged non-small-cell lung cancer. N. Engl. J. Med. 2014, 370, 1189–1197. [Google Scholar] [CrossRef]
- Kim, D.W.; Mehra, R.; Tan, D.S.W.; Felip, E.; Chow, L.Q.M.; Camidge, D.R.; Vansteenkiste, J.; Sharma, S.; de Pas, T.; Riely, G.J.; et al. Activity and safety of ceritinib in patients with ALK-rearranged non-small-cell lung cancer (ASCEND-1): Updated results from the multicentre, open-label, phase 1 trial. Lancet Oncol. 2016, 17, 452–463. [Google Scholar] [CrossRef]
- Camidge, D.R.; Dziadziuszko, R.; Peters, S.; Mok, T.; Noe, J.; Nowicka, M.; Gadgeel, S.M.; Cheema, P.; Pavlakis, N.; de Marinis, F.; et al. Updated Efficacy and Safety Data and Impact of the EML4-ALK Fusion Variant on the Efficacy of Alectinib in Untreated ALK-positive Advanced Non-small-cell Lung Cancer in the Global Phase III ALEX Study. J. Thorac. Oncol. 2019. [Google Scholar] [CrossRef]
- Shaw, A.T.; Kim, D.W.; Nakagawa, K.; Seto, T.; Crino, L.; Ahn, M.J.; de Pas, T.; Besse, B.; Solomon, B.J.; Blackhall, F.; et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N. Engl. J. Med. 2013, 368, 2385–2394. [Google Scholar] [CrossRef] [PubMed]
- Cheung-Ong, K.; Giaever, G.; Nislow, C. DNA-damaging agents in cancer chemotherapy: Serendipity and chemical biology. Chem. Biol. 2013, 20, 648–659. [Google Scholar] [CrossRef] [PubMed]
- Gainor, J.F.; Dardaei, L.; Yoda, S.; Friboulet, L.; Leshchiner, I.; Katayama, R.; Dagogo-Jack, I.; Gadgeel, S.; Schultz, K.; Singh, M.; et al. Molecular Mechanisms of Resistance to First- and Second-Generation ALK Inhibitors in ALK-Rearranged Lung Cancer. Cancer Discov. 2016, 6, 1118–1133. [Google Scholar] [CrossRef] [PubMed]
- Sotillo, R.; Schvartzman, J.M.; Socci, N.D.; Benezra, R. Mad2-induced chromosome instability leads to lung tumour relapse after oncogene withdrawal. Nature 2010, 464, 436–440. [Google Scholar] [CrossRef] [PubMed]
- Griesinger, F.; Roeper, J.; Pöttgen, C.; Willborn, K.C.; Eberhardt, W.E.E. Brain metastases in ALK-positive NSCLC—Time to adjust current treatment algorithms. Oncotarget 2018, 9, 35181–35194. [Google Scholar] [CrossRef]
- Magnuson, W.J.; Lester-Coll, N.H.; Wu, A.J.; Yang, T.J.; Lockney, N.A.; Gerber, N.K.; Beal, K.; Amini, A.; Patil, T.; Kavanagh, B.D.; et al. Management of Brain Metastases in Tyrosine Kinase Inhibitor–Naïve Epidermal Growth Factor Receptor–Mutant Non–Small-Cell Lung Cancer: A Retrospective Multi-Institutional Analysis. J. Clin. Oncol. 2017, 35, 1070–1077. [Google Scholar] [CrossRef]
- Reungwetwattana, T.; Nakagawa, K.; Cho, B.C.; Cobo, M.; Cho, E.K.; Bertolini, A.; Bohnet, S.; Zhou, C.; Lee, K.H.; Nogami, N.; et al. CNS Response to Osimertinib Versus Standard Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitors in Patients With Untreated EGFR-Mutated Advanced Non–Small-Cell Lung Cancer. J. Clin. Oncol. 2018, 36, 3290–3297. [Google Scholar] [CrossRef]
- Lin, J.J.; Jiang, G.Y.; Joshipura, N.; Ackil, J.; Digumarthy, S.R.; Rincon, S.P.; Yeap, B.Y.; Gainor, J.F.; Shaw, A.T. Efficacy of Alectinib in Patients with ALK-Positive NSCLC and Symptomatic or Large CNS Metastases. J. Thorac. Oncol. 2019, 14, 683–690. [Google Scholar] [CrossRef]
- Stephens, S.J.; Moravan, M.J.; Salama, J.K. Managing Patients with Oligometastatic Non-Small-Cell Lung Cancer. J. Oncol. Pract. 2018, 14, 23–31. [Google Scholar] [CrossRef]
- Yamamoto, M.; Serizawa, T.; Shuto, T.; Akabane, A.; Higuchi, Y.; Kawagishi, J.; Yamanaka, K.; Sato, Y.; Jokura, H.; Yomo, S.; et al. Stereotactic radiosurgery for patients with multiple brain metastases (JLGK0901): A multi-institutional prospective observational study. Lancet Oncol. 2014, 15, 387–395. [Google Scholar] [CrossRef]
- Robin, T.P.; Camidge, D.R.; Stuhr, K.; Nath, S.K.; Breeze, R.E.; Pacheco, J.M.; Liu, A.K.; Gaspar, L.E.; Purcell, W.T.; Doebele, R.C.; et al. Excellent Outcomes with Radiosurgery for Multiple Brain Metastases in ALK and EGFR Driven Non-Small Cell Lung Cancer. J. Thorac. Oncol. 2018, 13, 715–720. [Google Scholar] [CrossRef] [PubMed]
- Tallet, A.V.; Azria, D.; Barlesi, F.; Spano, J.P.; Carpentier, A.F.; Goncalves, A.; Metellus, P. Neurocognitive function impairment after whole brain radiotherapy for brain metastases: Actual assessment. Radiat. Oncol. 2012, 7, 77. [Google Scholar] [CrossRef] [PubMed]
- Ng, T.L.; Narasimhan, N.; Gupta, N.; Venkatakrishnan, K.; Kerstein, D.; Camidge, D.R. Early-Onset Pulmonary Events Associated with Brigatinib Use in Advanced NSCLC. J. Thorac. Oncol. 2020, 15, 1190–1199. [Google Scholar] [CrossRef] [PubMed]
- Soria, J.C.; Tan, D.S.W.; Chiari, R.; Wu, Y.L.; Paz-Ares, L.; Wolf, J.; Geater, S.L.; Orlov, S.; Cortinovis, D.; Yu, C.J.; et al. First-line ceritinib versus platinum-based chemotherapy in advanced ALK-rearranged non-small-cell lung cancer (ASCEND-4): A randomised, open-label, phase 3 study. Lancet 2017, 389, 917–929. [Google Scholar] [CrossRef]
- Shaw, A.T.; Kim, T.M.; Crinò, L.; Gridelli, C.; Kiura, K.; Liu, G.; Novello, S.; Bearz, A.; Gautschi, O.; Mok, T.; et al. Ceritinib versus chemotherapy in patients with ALK-rearranged non-small-cell lung cancer previously given chemotherapy and crizotinib (ASCEND-5): A randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2017, 18, 874–886. [Google Scholar] [CrossRef]
- Cho, B.C.; Kim, D.-W.; Bearz, A.; Laurie, S.A.; McKeage, M.; Borra, G.; Park, K.; Kim, S.-W.; Ghosn, M.; Ardizzoni, A.; et al. ASCEND-8: A Randomized Phase 1 Study of Ceritinib, 450 mg or 600 mg, Taken with a Low-Fat Meal versus 750 mg in Fasted State in Patients with Anaplastic Lymphoma Kinase (ALK)-Rearranged Metastatic Non-Small Cell Lung Cancer (NSCLC). J. Thorac. Oncol. 2017, 12, 1357–1367. [Google Scholar] [CrossRef]
- Schaefer, E.S.; Baik, C. Proactive management strategies for potential gastrointestinal adverse reactions with ceritinib in patients with advanced ALK-positive non-small-cell lung cancer. Cancer Manag. Res. 2016, 8, 33–38. [Google Scholar] [CrossRef]
- Johnson, T.W.; Richardson, P.F.; Bailey, S.; Brooun, A.; Burke, B.J.; Collins, M.R.; Cui, J.J.; Deal, J.G.; Deng, Y.-L.; Dinh, D.; et al. Discovery of (10R)-7-amino-12-fluoro-2,10,16-trimethyl-15-oxo-10,15,16,17-tetrahydro-2H-8,4-(metheno)pyrazolo4,3-h2,5,11-benzoxadiazacyclotetradecine-3-carbonitrile (PF-06463922), a macrocyclic inhibitor of anaplastic lymphoma kinase (ALK) and c-ros oncogene 1 (ROS1) with preclinical brain exposure and broad-spectrum potency against ALK-resistant mutations. J. Med. Chem. 2014, 57, 4720–4744. [Google Scholar] [CrossRef]
- Drilon, A. TRK inhibitors in TRK fusion-positive cancers. Ann. Oncol. 2019, 30, viii23–viii30. [Google Scholar] [CrossRef]
- Rheinheimer, S.; Heussel, C.-P.; Mayer, P.; Gaissmaier, L.; Bozorgmehr, F.; Winter, H.; Herth, F.J.; Muley, T.; Liersch, S.; Bischoff, H.; et al. Oligoprogressive Non-Small-Cell Lung Cancer under Treatment with PD-(L)1 Inhibitors. Cancers 2020, 12, 1046. [Google Scholar] [CrossRef]
- Gan, G.N.; Weickhardt, A.J.; Scheier, B.; Doebele, R.C.; Gaspar, L.E.; Kavanagh, B.D.; Camidge, D.R. Stereotactic radiation therapy can safely and durably control sites of extra-central nervous system oligoprogressive disease in anaplastic lymphoma kinase-positive lung cancer patients receiving crizotinib. Int. J. Radiat. Oncol. Biol. Phys. 2014, 88, 892–898. [Google Scholar] [CrossRef] [PubMed]
- Tumati, V.; Iyengar, P. The current state of oligometastatic and oligoprogressive non-small cell lung cancer. J. Thorac. Dis. 2018, 10, S2537–S2544. [Google Scholar] [CrossRef] [PubMed]
- Schmid, S.; Klingbiel, D.; Aeppli, S.; Britschgi, C.; Gautschi, O.; Pless, M.; Rothschild, S.; Wannesson, L.; Janthur, W.; Foerbs, D.; et al. Patterns of progression on osimertinib in EGFR T790M positive NSCLC: A Swiss cohort study. Lung Cancer 2019, 130, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Weickhardt, A.J.; Scheier, B.; Burke, J.M.; Gan, G.; Lu, X.; Bunn, P.A., Jr.; Aisner, D.L.; Gaspar, L.E.; Kavanagh, B.D.; Doebele, R.C.; et al. Local ablative therapy of oligoprogressive disease prolongs disease control by tyrosine kinase inhibitors in oncogene-addicted non-small-cell lung cancer. J. Thorac. Oncol. 2012, 7, 1807–1814. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Liu, H.; Meng, S.; Jiang, T.; Li, X.; Liang, S.; Ren, S.; Zhou, C. First-line continual EGFR-TKI plus local ablative therapy demonstrated survival benefit in EGFR-mutant NSCLC patients with oligoprogressive disease. J. Cancer 2019, 10, 522–529. [Google Scholar] [CrossRef]
- Yu, H.A.; Sima, C.S.; Huang, J.; Solomon, S.B.; Rimner, A.; Paik, P.; Pietanza, M.C.; Azzoli, C.G.; Rizvi, N.A.; Krug, L.M.; et al. Local therapy with continued EGFR tyrosine kinase inhibitor therapy as a treatment strategy in EGFR-mutant advanced lung cancers that have developed acquired resistance to EGFR tyrosine kinase inhibitors. J. Thorac. Oncol. 2013, 8, 346–351. [Google Scholar] [CrossRef]
- Ng, T.L.; Morgan, R.L.; Patil, T.; Barón, A.E.; Smith, D.E.; Ross Camidge, D. Detection of oligoprogressive disease in oncogene-addicted non-small cell lung cancer using PET/CT versus CT in patients receiving a tyrosine kinase inhibitor. Lung Cancer 2018, 126, 112–118. [Google Scholar] [CrossRef]
- Remon, J.; Besse, B. Brain Metastases in Oncogene-Addicted Non-Small Cell Lung Cancer Patients: Incidence and Treatment. Front. Oncol. 2018, 8, 88. [Google Scholar] [CrossRef]
- Solomon, B.J.; Besse, B.; Bauer, T.M.; Felip, E.; Soo, R.A.; Camidge, D.R.; Chiari, R.; Bearz, A.; Lin, C.C.; Gadgeel, S.M.; et al. Lorlatinib in patients with ALK-positive non-small-cell lung cancer: Results from a global phase 2 study. Lancet Oncol. 2018, 19, 1654–1667. [Google Scholar] [CrossRef]
- Shaw, A.T.; Solomon, B.J.; Besse, B.; Bauer, T.M.; Lin, C.C.; Soo, R.A.; Riely, G.J.; Ou, S.I.; Clancy, J.S.; Li, S.; et al. ALK Resistance Mutations and Efficacy of Lorlatinib in Advanced Anaplastic Lymphoma Kinase-Positive Non-Small-Cell Lung Cancer. J. Clin. Oncol. 2019, JCO1802236. [Google Scholar] [CrossRef]
- Shaw, A.T.; Felip, E.; Bauer, T.M.; Besse, B.; Navarro, A.; Postel-Vinay, S.; Gainor, J.F.; Johnson, M.; Dietrich, J.; James, L.P.; et al. Lorlatinib in non-small-cell lung cancer with ALK or ROS1 rearrangement: An international, multicentre, open-label, single-arm first-in-man phase 1 trial. Lancet Oncol. 2017, 18, 1590–1599. [Google Scholar] [CrossRef]
- Stinchcombe, T.; Doebele, R.C.; Wang, X.F.; Gerber, D.E.; Horn, L.; Camidge, D.R. Preliminary results of single arm phase 2 trial of brigatinib in patients (pts) with progression disease (PD) after next-generation (NG) anaplastic lymphoma kinase (ALK) tyrosine kinase inhibitors (TKIs) in ALK + non-small cell lung cancer (NSCLC). J. Clin. Oncol. 2019, 37, 9027. [Google Scholar] [CrossRef]
- Lin, J.J.; Zhu, V.W.; Schoenfeld, A.J.; Yeap, B.Y.; Saxena, A.; Ferris, L.A.; Dagogo-Jack, I.; Farago, A.F.; Taber, A.; Traynor, A.; et al. Brigatinib in Patients With Alectinib-Refractory ALK-Positive NSCLC. J. Thorac. Oncol. 2018, 13, 1530–1538. [Google Scholar] [CrossRef] [PubMed]
- Horn, L.; Whisenant, J.G.; Wakelee, H.; Reckamp, K.L.; Qiao, H.; Leal, T.A.; Du, L.; Hernandez, J.; Huang, V.; Blumenschein, G.R.; et al. Monitoring Therapeutic Response and Resistance: Analysis of Circulating Tumor DNA in Patients with ALK+ Lung Cancer. J. Thorac. Oncol. 2019, 14, 1901–1911. [Google Scholar] [CrossRef] [PubMed]
- FDA. FDA Approves Lorlatinib for Second- or Third-Line Treatment of ALK-Positive Metastatic NSCLC. Available online: https://www.fda.gov/drugs/fda-approves-lorlatinib-second-or-third-line-treatment-alk-positive-metastatic-nsclc#:~:text=Drugs-,FDA%20approves%20lorlatinib%20for%20second%2D%20or%20third%2Dline%20treatment,of%20ALK%2Dpositive%20metastatic%20NSCLC&text=On%20November%202%2C%202018%2C%20the,LORBRENA%2C%20Pfizer%2C%20Inc (accessed on 15 December 2020).
- EMA. Lorviqua; Product Information. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/lorviqua (accessed on 15 December 2020).
- Lin, J.J.; Riely, G.J.; Shaw, A.T. Targeting ALK: Precision Medicine Takes on Drug Resistance. Cancer Discov. 2017, 7, 137–155. [Google Scholar] [CrossRef]
- Lin, M.M.; Pan, X.; Hou, P.; Allen, S.; Baumann, P.; Hochmair, M.J. Treatment duration of brigatinib in patients enrolled in the international expanded access program (EAP). Ann. Oncol. 2019, 30, ii48. [Google Scholar] [CrossRef]
- Hochmair, M.; Weinlinger, C.; Prosch, H. Intracranial remission with brigatinib rechallenge as fifth-line ALK inhibition therapy in a lung cancer patient. Anti-Cancer Drugs 2019, 30, 1058–1060. [Google Scholar] [CrossRef]
- Dietz, S.; Christopoulos, P.; Yuan, Z.; Angeles, A.K.; Gu, L.; Volckmar, A.-L.; Ogrodnik, S.J.; Janke, F.; Fratte, C.D.; Zemojtel, T.; et al. Longitudinal therapy monitoring of ALK-positive lung cancer by combined copy number and targeted mutation profiling of cell-free DNA. EBioMedicine 2020, 62, 103103. [Google Scholar] [CrossRef]
- Yoda, S.; Lin, J.J.; Lawrence, M.S.; Burke, B.J.; Friboulet, L.; Langenbucher, A.; Dardaei, L.; Prutisto-Chang, K.; Dagogo-Jack, I.; Timofeevski, S.; et al. Sequential ALK Inhibitors Can Select for Lorlatinib-Resistant Compound ALK Mutations in ALK-Positive Lung Cancer. Cancer Discov. 2018, 8, 714–729. [Google Scholar] [CrossRef]
- Dagogo-Jack, I.; Rooney, M.; Lin, J.J.; Nagy, R.J.; Yeap, B.Y.; Hubbeling, H.; Chin, E.; Ackil, J.; Farago, A.F.; Hata, A.N.; et al. Treatment with Next-Generation ALK Inhibitors Fuels Plasma ALK Mutation Diversity. Clin. Cancer Res. 2019, 25, 6662–6670. [Google Scholar] [CrossRef]
- Cui, J.J.; Rogers, E.; Zhai, D.; Deng, W.; Ung, J.; Nguyen, V.; Zhang, H.; Zhang, X.; Parra, A.; Barrera, M.; et al. Abstract 5226: TPX-0131: A next generation macrocyclic ALK inhibitor that overcomes ALK resistant mutations refractory to current approved ALK inhibitors. Experimental and Molecular Therapeutics. In Proceedings of the AACR Annual Meeting 2020, American Association for Cancer Research: 08152020, Philadelphia, PA, USA, 27–28 April and 22–24 June 2020; p. 5226. [Google Scholar]
- Hida, T.; Nokihara, H.; Kondo, M.; Kim, Y.H.; Azuma, K.; Seto, T.; Takiguchi, Y.; Nishio, M.; Yoshioka, H.; Imamura, F.; et al. Alectinib versus crizotinib in patients with ALK-positive non-small-cell lung cancer (J-ALEX): An open-label, randomised phase 3 trial. Lancet 2017, 390, 29–39. [Google Scholar] [CrossRef]
- Nakagawa, K.; Hida, T.; Nokihara, H.; Morise, M.; Azuma, K.; Kim, Y.H.; Seto, T.; Takiguchi, Y.; Nishio, M.; Yoshioka, H.; et al. Final progression-free survival results from the J-ALEX study of alectinib versus crizotinib in ALK-positive non-small-cell lung cancer. Lung Cancer 2020, 139, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Camidge, D.R.; Kim, H.R.; Ahn, M.-J.; Yang, J.C.H.; Han, J.-Y.; Hochmair, M.J.; Lee, K.H.; Delmonte, A.; García Campelo, M.R.; Kim, D.-W.; et al. Brigatinib Versus Crizotinib in Advanced ALK Inhibitor-Naive ALK-Positive Non-Small Cell Lung Cancer: Second Interim Analysis of the Phase III ALTA-1L Trial. J. Clin. Oncol. 2020, 38, 3592–3603. [Google Scholar] [CrossRef] [PubMed]
- Crino, L.; Ahn, M.J.; de Marinis, F.; Groen, H.J.; Wakelee, H.; Hida, T.; Mok, T.; Spigel, D.; Felip, E.; Nishio, M.; et al. Multicenter Phase II Study of Whole-Body and Intracranial Activity with Ceritinib in Patients With ALK-Rearranged Non-Small-Cell Lung Cancer Previously Treated with Chemotherapy and Crizotinib: Results From ASCEND-2. J. Clin. Oncol. 2016, 34, 2866–2873. [Google Scholar] [CrossRef]
- Horn, L.; Infante, J.R.; Reckamp, K.L.; Blumenschein, G.R.; Leal, T.A.; Waqar, S.N.; Gitlitz, B.J.; Sanborn, R.E.; Whisenant, J.G.; Du, L.; et al. Ensartinib (X-396) in ALK-Positive Non-Small Cell Lung Cancer: Results from a First-in-Human Phase I/II, Multicenter Study. Clin. Cancer Res. 2018, 24, 2771–2779. [Google Scholar] [CrossRef] [PubMed]
- Hida, T.; Seto, T.; Horinouchi, H.; Maemondo, M.; Takeda, M.; Hotta, K.; Hirai, F.; Kim, Y.H.; Matsumoto, S.; Ito, M.; et al. Phase II study of ceritinib in alectinib-pretreated patients with anaplastic lymphoma kinase-rearranged metastatic non-small-cell lung cancer in Japan: ASCEND-9. Cancer Sci. 2018, 109, 2863–2872. [Google Scholar] [CrossRef]
- Yoshida, H.; Kim, Y.H.; Ozasa, H.; Sakamori, Y.; Tsuji, T.; Nomizo, T.; Yasuda, Y.; Yamamoto, T.; Ajimizu, H.; Hirai, T. Efficacy of Ceritinib After Alectinib for ALK-positive Non-small Cell Lung Cancer. Vivo 2018, 32, 1587–1590. [Google Scholar] [CrossRef]
- Kim, E.S.; Ou, S.-H.I.; Barlesi, F.; Mok, T.S.K.; Ahn, M.-J.; Bunn, V.; Zhang, P. Phase 2 study of brigatinib in patients (pts) with anaplastic lymphoma kinase (ALK)−positive, advanced non–small cell lung cancer (NSCLC) that progressed on alectinib or ceritinib. J. Clin. Oncol. 2019, 37, TPS9115. [Google Scholar] [CrossRef]
- Gadgeel, S.M.; Shaw, A.T.; Govindan, R.; Gandhi, L.; Socinski, M.A.; Camidge, D.R.; Petris, L.D.; Kim, D.-W.; Chiappori, A.; Moro-Sibilot, D.L.; et al. Pooled Analysis of CNS Response to Alectinib in Two Studies of Pretreated Patients with ALK-Positive Non-Small-Cell Lung Cancer. J. Clin. Oncol. 2016, 34, 4079–4085. [Google Scholar] [CrossRef]
- Camidge, D.R.; Kim, D.-W.; Tiseo, M.; Langer, C.J.; Ahn, M.-J.; Shaw, A.T.; Huber, R.M.; Hochmair, M.J.; Lee, D.H.; Bazhenova, L.A.; et al. Exploratory Analysis of Brigatinib Activity in Patients with Anaplastic Lymphoma Kinase-Positive Non-Small-Cell Lung Cancer and Brain Metastases in Two Clinical Trials. J. Clin. Oncol. 2018, 36, 2693–2701. [Google Scholar] [CrossRef]
- Welsh, J.W.; Komaki, R.; Amini, A.; Munsell, M.F.; Unger, W.; Allen, P.K.; Chang, J.Y.; Wefel, J.S.; McGovern, S.L.; Garland, L.L.; et al. Phase II trial of erlotinib plus concurrent whole-brain radiation therapy for patients with brain metastases from non-small-cell lung cancer. J. Clin. Oncol. 2013, 31, 895–902. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, S.H.; Lin, H.C.; Chou, Y.T.; Lin, S.E.; Kuo, C.C.; Yu, M.C.; Chung, C.L. Impact of epidermal growth factor receptor mutations on intracranial treatment response and survival after brain metastases in lung adenocarcinoma patients. Lung Cancer 2013, 81, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Min, W.; Li, Y.; Yue, Z.; Wu, C.; Zhou, C. Radiotherapy plus EGFR TKIs in non-small cell lung cancer patients with brain metastases: An update meta-analysis. Cancer Med. 2016, 5, 1055–1065. [Google Scholar] [CrossRef] [PubMed]
- Bordi, P.; Tiseo, M.; Rofi, E.; Petrini, I.; Restante, G.; Danesi, R.; Del Re, M. Detection of ALK and KRAS Mutations in Circulating Tumor DNA of Patients with Advanced ALK-Positive NSCLC With Disease Progression During Crizotinib Treatment. Clin. Lung Cancer 2017, 18, 692–697. [Google Scholar] [CrossRef]
- Dagogo-Jack, I.; Yoda, S.; Lennerz, J.K.; Langenbucher, A.; Lin, J.J.; Rooney, M.M.; Prutisto-Chang, K.; Oh, A.; Adams, N.A.; Yeap, B.Y.; et al. MET Alterations Are a Recurring and Actionable Resistance Mechanism in ALK-Positive Lung Cancer. Clin. Cancer Res. 2020, 26, 2535–2545. [Google Scholar] [CrossRef]
- Dietz, S.; Christopoulos, P.; Gu, L.; Volckmar, A.-L.; Endris, V.; Yuan, Z.; Ogrodnik, S.J.; Zemojtel, T.; Heussel, C.-P.; Schneider, M.A.; et al. Serial liquid biopsies for detection of treatment failure and profiling of resistance mechanisms in KLC1-ALK-rearranged lung cancer. Cold Spring Harb. Mol. Case Stud. 2019, 5. [Google Scholar] [CrossRef]
- Minari, R.; Gnetti, L.; Lagrasta, C.A.; Squadrilli, A.; Bordi, P.; Azzoni, C.; Bottarelli, L.; Cosenza, A.; Ferri, L.; Caruso, G.; et al. Emergence of a HER2-amplified clone during disease progression in an ALK-rearranged NSCLC patient treated with ALK-inhibitors: A case report. Transl. Lung Cancer Res. 2020, 9, 787–792. [Google Scholar] [CrossRef]
- Sakakibara-Konishi, J.; Kitai, H.; Ikezawa, Y.; Hatanaka, Y.; Sasaki, T.; Yoshida, R.; Chiba, S.; Matsumoto, S.; Goto, K.; Mizugaki, H.; et al. Response to Crizotinib Re-administration after Progression on Lorlatinib in a Patient With ALK-rearranged Non-small-cell Lung Cancer. Clin. Lung Cancer 2019, 20, e555–e559. [Google Scholar] [CrossRef]
- Yang, C.-Y.; Liao, W.-Y.; Ho, C.-C.; Chen, K.-Y.; Tsai, T.-H.; Hsu, C.-L.; Liu, Y.-N.; Su, K.-Y.; Chang, Y.-L.; Wu, C.-T.; et al. Association of Programmed Death-Ligand 1 Expression with Fusion Variants and Clinical Outcomes in Patients with Anaplastic Lymphoma Kinase-Positive Lung Adenocarcinoma Receiving Crizotinib. Oncologist 2020, 25, 702–711. [Google Scholar] [CrossRef]
- Chang, G.-C.; Yang, T.-Y.; Chen, K.-C.; Hsu, K.-H.; Huang, Y.-H.; Su, K.-Y.; Yu, S.-L.; Tseng, J.-S. ALK variants, PD-L1 expression, and their association with outcomes in ALK-positive NSCLC patients. Sci. Rep. 2020, 10, 21063. [Google Scholar] [CrossRef]
- Su, Y.; Long, X.; Song, Y.; Chen, P.; Li, S.; Yang, H.; Wu, P.; Wang, Y.; Bing, Z.; Cao, Z.; et al. Distribution of ALK Fusion Variants and Correlation with Clinical Outcomes in Chinese Patients with Non-Small Cell Lung Cancer Treated with Crizotinib. Target. Oncol. 2019, 14, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Tao, H.; Shi, L.; Zhou, A.; Li, H.; Gai, F.; Huang, Z.; Che, N.; Liu, Z. Distribution of EML4-ALK fusion variants and clinical outcomes in patients with resected non-small cell lung cancer. Lung Cancer 2020, 149, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Camidge, D.R.; Niu, H.; Kim, H.R.; Yang, J.C.-H.; Ahn, M.-J.; Li, J.Y.-C.; Hochmair, M.; Delmonte, A.; Spira, A.I.; Campelo, R.G.; et al. Correlation of baseline molecular and clinical variables with ALK inhibitor efficacy in ALTA-1L. J. Clin. Oncol. 2020, 38, 9517. [Google Scholar] [CrossRef]
- Christopoulos, P.; Kirchner, M.; Endris, V.; Stenzinger, A.; Thomas, M. EML4-ALK V3, treatment resistance, and survival: Refining the diagnosis of ALK+ NSCLC. J. Thorac. Dis. 2018, 10, S1989–S1991. [Google Scholar] [CrossRef]
- Richards, M.W.; Law, E.W.; Rennalls, L.P.; Busacca, S.; O’Regan, L.; Fry, A.M.; Fennell, D.A.; Bayliss, R. Crystal structure of EML1 reveals the basis for Hsp90 dependence of oncogenic EML4-ALK by disruption of an atypical beta-propeller domain. Proc. Natl. Acad. Sci. USA 2014, 111, 5195–5200. [Google Scholar] [CrossRef]
- Volckmar, A.L.; Leichsenring, J.; Kirchner, M.; Christopoulos, P.; Neumann, O.; Budczies, J.; Morais de Oliveira, C.M.; Rempel, E.; Buchhalter, I.; Brandt, R.; et al. Combined targeted DNA and RNA sequencing of advanced NSCLC in routine molecular diagnostics: Analysis of the first 3,000 Heidelberg cases. Int. J. Cancer 2019, 145, 649–661. [Google Scholar] [CrossRef]
- Benayed, R.; Offin, M.; Mullaney, K.; Sukhadia, P.; Rios, K.; Desmeules, P.; Ptashkin, R.; Won, H.; Chang, J.; Halpenny, D.; et al. High Yield of RNA Sequencing for Targetable Kinase Fusions in Lung Adenocarcinomas with No Mitogenic Driver Alteration Detected by DNA Sequencing and Low Tumor Mutation Burden. Clin. Cancer Res. 2019, 25, 4712–4722. [Google Scholar] [CrossRef]
- Leighl, N.B.; Page, R.D.; Raymond, V.M.; Daniel, D.B.; Divers, S.G.; Reckamp, K.L.; Villalona-Calero, M.A.; Dix, D.; Odegaard, J.I.; Lanman, R.B.; et al. Clinical Utility of Comprehensive Cell-free DNA Analysis to Identify Genomic Biomarkers in Patients with Newly Diagnosed Metastatic Non-small Cell Lung Cancer. Clin. Cancer Res. 2019, 25, 4691–4700. [Google Scholar] [CrossRef]
- Supplee, J.G.; Milan, M.S.D.; Lim, L.P.; Potts, K.T.; Sholl, L.M.; Oxnard, G.R.; Paweletz, C.P. Sensitivity of next-generation sequencing assays detecting oncogenic fusions in plasma cell-free DNA. Lung Cancer 2019, 134, 96–99. [Google Scholar] [CrossRef]
- Christopoulos, P.; Kirchner, M.; Bozorgmehr, F.; Endris, V.; Elsayed, M.; Budczies, J.; Ristau, J.; Penzel, R.; Herth, F.J.; Heussel, C.P.; et al. Identification of a highly lethal V3+TP53+ subset in ALK+ lung adenocarcinoma. Int. J. Cancer 2019, 190–199. [Google Scholar] [CrossRef]
- Kron, A.; Alidousty, C.; Scheffler, M.; Merkelbach-Bruse, S.; Seidel, D.; Riedel, R.; Ihle, M.; Michels, S.; Nogova, L.; Fassunke, J.; et al. Impact of TP53 mutation status on systemic treatment outcome in ALK-rearranged non-small-cell lung cancer. Ann. Oncol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Christopoulos, P.; Dietz, S.; Kirchner, M.; Volckmar, A.L.; Endris, V.; Neumann, O.; Ogrodnik, S.; Heussel, C.P.; Herth, F.J.; Eichhorn, M.; et al. Detection of TP53 Mutations in Tissue or Liquid Rebiopsies at Progression Identifies ALK+ Lung Cancer Patients with Poor Survival. Cancers 2019, 11, 124. [Google Scholar] [CrossRef] [PubMed]
- Socinski, M.A.; Jotte, R.M.; Cappuzzo, F.; Orlandi, F.; Stroyakovskiy, D.; Nogami, N.; Rodriguez-Abreu, D.; Moro-Sibilot, D.; Thomas, C.A.; Barlesi, F.; et al. Atezolizumab for First-Line Treatment of Metastatic Nonsquamous NSCLC. N. Engl. J. Med. 2018, 378, 2288–2301. [Google Scholar] [CrossRef] [PubMed]
- Gettinger, S.; Politi, K. PD-1 axis inhibitors in EGFR and ALK Driven Lung Cancer: Lost cause? Clin. Cancer Res. 2016, 22, 4539–4541. [Google Scholar] [CrossRef] [PubMed]
- Mazieres, J.; Drilon, A.; Lusque, A.; Mhanna, L.; Cortot, A.B.; Mezquita, L.; Thai, A.A.; Mascaux, C.; Couraud, S.; Veillon, R.; et al. Immune checkpoint inhibitors for patients with advanced lung cancer and oncogenic driver alterations: Results from the IMMUNOTARGET registry. Ann. Oncol. 2019, 30, 1321–1328. [Google Scholar] [CrossRef]
- Gaissmaier, L.; Christopoulos, P. Immune Modulation in Lung Cancer: Current Concepts and Future Strategies. Respiration 2020, 1–27. [Google Scholar] [CrossRef]
- Patel, M.; Jabbour, S.K.; Malhotra, J. ALK inhibitors and checkpoint blockade: A cautionary tale of mixing oil with water? J. Thorac. Dis. 2018, 10, S2198–S2201. [Google Scholar] [CrossRef]
- Duchemann, B.; Friboulet, L.; Besse, B. Therapeutic management of ALK+ nonsmall cell lung cancer patients. Eur. Respir. J. 2015, 46, 230–242. [Google Scholar] [CrossRef]
- Pacheco, J.M.; Camidge, D.R. Is long-term survival possible for patients with stage IV ALK+ non-small cell lung cancer? Expert Rev. Respir. Med. 2019, 13, 399–401. [Google Scholar] [CrossRef]
- McCusker, M.G.; Russo, A.; Scilla, K.A.; Mehra, R.; Rolfo, C. How I treat ALK-positive non-small cell lung cancer. Esmo Open 2019, 4, e000524. [Google Scholar] [CrossRef]
- Lin, J.J.; Schoenfeld, A.J.; Zhu, V.W.; Yeap, B.Y.; Chin, E.; Rooney, M.; Plodkowski, A.J.; Digumarthy, S.R.; Dagogo-Jack, I.; Gainor, J.F.; et al. Efficacy of Platinum/Pemetrexed Combination Chemotherapy in ALK-Positive NSCLC Refractory to Second-Generation ALK Inhibitors. J. Thorac. Oncol. 2020, 15, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Duruisseaux, M.; Besse, B.; Cadranel, J.; Perol, M.; Mennecier, B.; Bigay-Game, L.; Descourt, R.; Dansin, E.; Audigier-Valette, C.; Moreau, L.; et al. Overall survival with crizotinib and next-generation ALK inhibitors in ALK-positive non-small-cell lung cancer (IFCT-1302 CLINALK): A French nationwide cohort retrospective study. Oncotarget 2017, 8, 21903–21917. [Google Scholar] [CrossRef] [PubMed]
- Heather, J.M.; Spindler, M.J.; Cobbold, M.; Gainor, J.F.; Johnson, D.S.; Hata, A.N. Anaplastic lymphoma kinase fusions as a target for TCR-directed cellular therapies. J. Immunol. 2020, 204, 239.10. [Google Scholar]
- Wang, L.; Lui, V.W.Y. Emerging Roles of ALK in Immunity and Insights for Immunotherapy. Cancers 2020, 12, 426. [Google Scholar] [CrossRef]
- Gaissmaier, L.; Elshiaty, M.; Christopoulos, P. Breaking Bottlenecks for the TCR Therapy of Cancer. Cells 2020, 9, 2095. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elsayed, M.; Christopoulos, P. Therapeutic Sequencing in ALK+ NSCLC. Pharmaceuticals 2021, 14, 80. https://doi.org/10.3390/ph14020080
Elsayed M, Christopoulos P. Therapeutic Sequencing in ALK+ NSCLC. Pharmaceuticals. 2021; 14(2):80. https://doi.org/10.3390/ph14020080
Chicago/Turabian StyleElsayed, Mei, and Petros Christopoulos. 2021. "Therapeutic Sequencing in ALK+ NSCLC" Pharmaceuticals 14, no. 2: 80. https://doi.org/10.3390/ph14020080
APA StyleElsayed, M., & Christopoulos, P. (2021). Therapeutic Sequencing in ALK+ NSCLC. Pharmaceuticals, 14(2), 80. https://doi.org/10.3390/ph14020080






