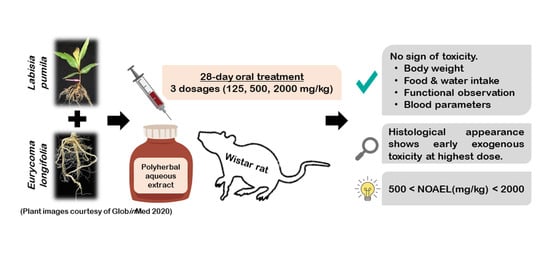
Herbal-Based Formulation Containing Eurycoma longifolia and Labisia pumila Aqueous Extracts: Safe for Consumption?
Abstract
1. Introduction
2. Results
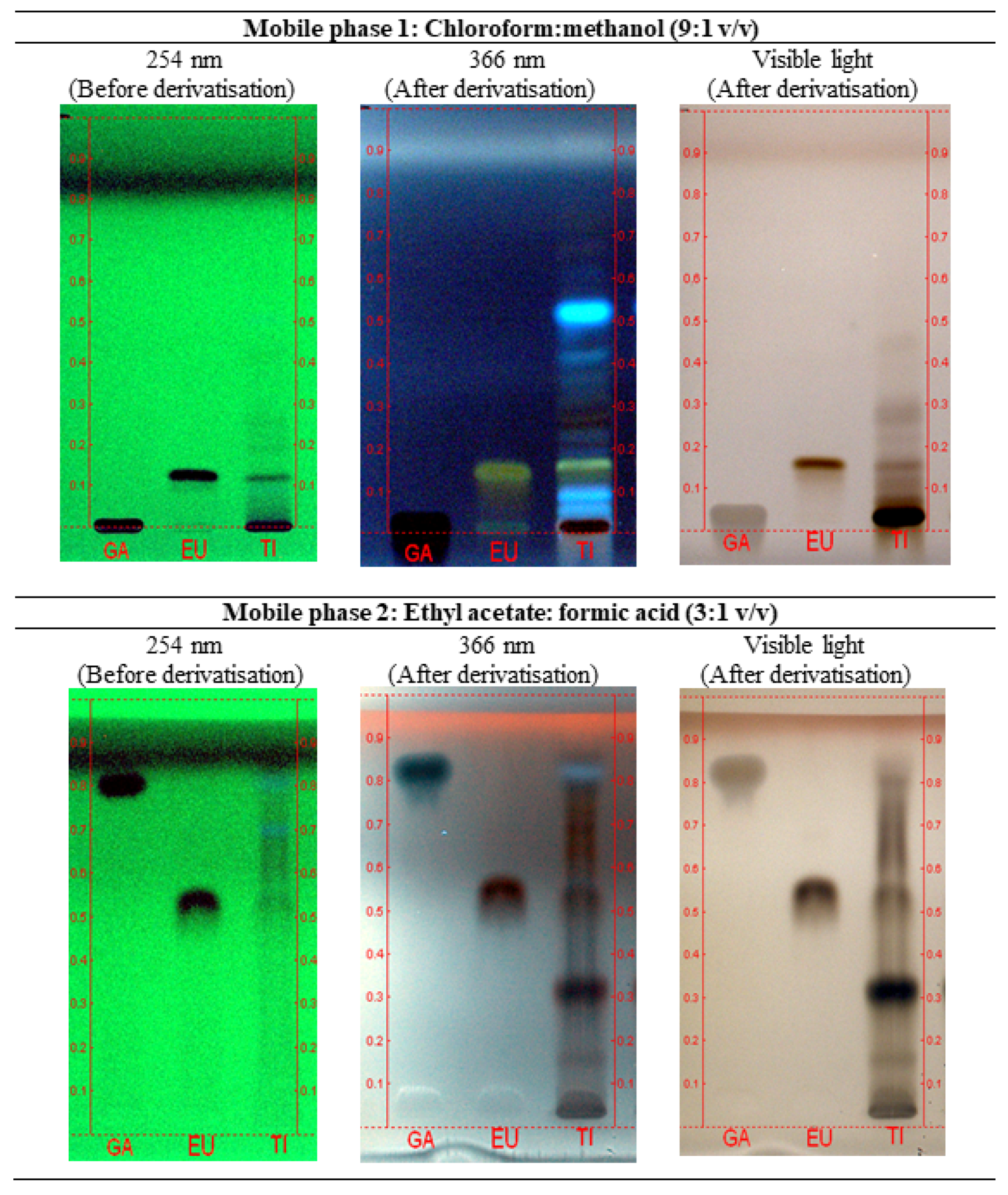
2.1. Chemical Identification of Test Item (P.SLP Formulation)
2.2. Mortality, Clinical and Functional Observations
2.3. Body Weight
2.4. Food and Water Consumption
2.5. Haematology and Clinical Biochemistry
2.6. Gross Pathology Examination
2.7. Relative Organ Weight
2.8. Histopathology
3. Discussion
4. Materials and Methods
4.1. Test Item
4.2. Chemicals and Reagents
4.3. Chemical Identification of the Test Item
4.4. Preparation of Test Item for Animal Study
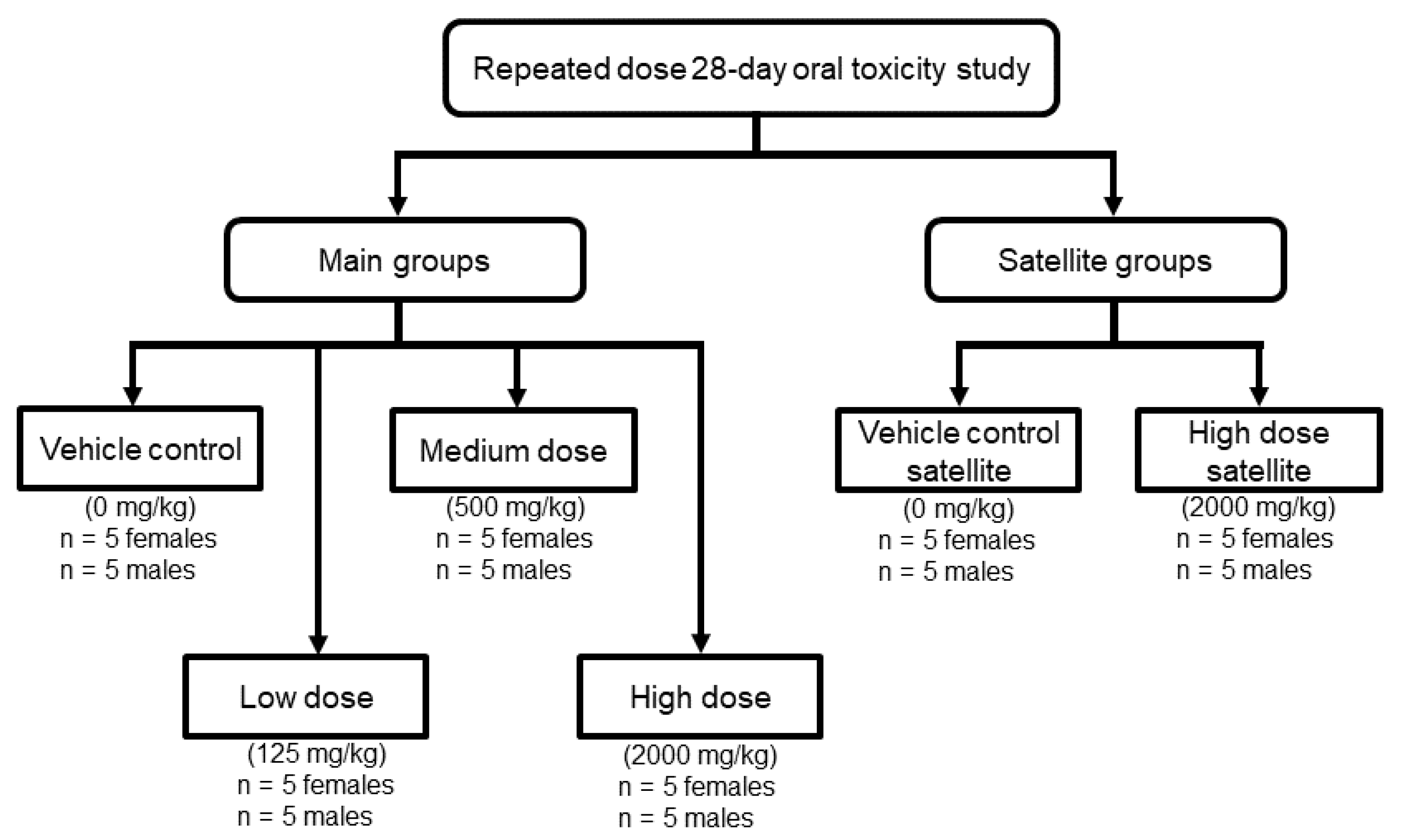
4.5. Experimental Animals
4.6. Animal Experimental Procedures
4.6.1. Mortality, Clinical and Functional Observation
4.6.2. Body Weight
4.6.3. Food and Water Consumption
4.6.4. Haematology and Clinical Biochemistry
4.6.5. Gross Pathology Examination
4.6.6. Relative Organ Weight
4.6.7. Histopathology
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. WHO Traditional Medicine Strategy: 2014–2023. Available online: https://www.who.int/traditional-complementary-integrative-medicine/publications/trm_strategy14_23/en/ (accessed on 1 October 2020).
- Hill, S.; Bero, L.; McColl, G.J.; Roughead, E. Expensive medicines: Ensuring objective appraisal and equitable access. Bull. World Health Organ. 2015, 93, 4. [Google Scholar] [CrossRef]
- Zhang, P.; Wang, F.; Hu, J.; Sorrentino, R. Exploring the Relationship between Drug Side-Effects and Therapeutic Indications. AMIA Ann. Symp. 2013, 2013, 1568–1577. [Google Scholar]
- Jamal, J.A.; Houghton, P.J.; Ridzwan, R. Contact dermatitis caused by kacip Fatimah. In Towards Bridging Science and Herbal Industry; Chang, Y.S., Mastura, M., Vimala, S., Zainon, A.S., Eds.; Forest Research Institute of Malaysia: Kuala Lumpur, Malaysia, 2001. [Google Scholar]
- Faizal, B.; Noormalin, A.; Zailatul, H.M.Y.; Mastuty, S.; Ali, M.; Izzah, A.R.; Shonali, S.; Shahnaz, M. Allergic reaction to Eurycoma longifolia Jack—A case report. Med. J. M. 2010, 65 (Suppl. A), 42. [Google Scholar]
- Salman, S.A.B.; Sulaiman, S.A.; Wahab, M.S.A.; Ismail, Z.; Ismail, R.; Yuen, K.H.; Gan, S.H.; Msc, S.A.B.S.; Mpharm, R.I. Modification of propranolol’s bioavailability by Eurycoma longifolia water-based extract. J. Clin. Pharm. Ther. 2010, 35, 691–696. [Google Scholar] [CrossRef] [PubMed]
- Showande, S.J.; Adegbolagun, O.M.; Igbinoba, S.I.; Fakeye, T.O. In vivo pharmacodynamic and pharmacokinetic interactions of Hibiscus sabdariffa calyces extracts with simvastatin. J. Clin. Pharm. Ther. 2017, 42, 695–703. [Google Scholar] [CrossRef]
- Guo, M.; Wang, T.-Y.; Yang, J.; Chang, H.; Ji, S.; Gao, F. Interaction of clopidogrel and fufang danshen dripping pills assay in coronary heart disease based on non-target metabolomics. J. Ethnopharmacol. 2019, 234, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.C. Liver toxicity related to herbs and dietary supplements: Online table of case reports. Part 2 of 5 series. Food Chem. Toxicol. 2017, 107, 472–501. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.C. Kidney toxicity related to herbs and dietary supplements: Online table of case reports. Part 3 of 5 series. Food Chem. Toxicol. 2017, 107, 502–519. [Google Scholar] [CrossRef]
- Brown, A.C. Heart Toxicity Related to Herbs and Dietary Supplements: Online Table of Case Reports. Part 4 of 5. J. Diet. Suppl. 2017, 15, 516–555. [Google Scholar] [CrossRef]
- Malaysian Herbal Monograph Committee. Malaysian Herbal Monograph 2015; Institute for Medical Research: Kuala Lumpur, Malaysia, 2015.
- Gimlette, J.D.; Burkhill, I.H. The Medical Book of Malayan Medicine; The Gardens’ Bulletin Straits Settlements; Botanic Gardens: Singapore, 1930; Volume 6, p. 329.
- Burkill, I.H.; Haniff, M. Malay Village Medicine; The Gardens’ Bulletin Straits Settlement 2; Botanic Gardens: Singapore, 1930; p. 182.
- Isa, W.A.R.W.M.; Amin, I.M.; Ishak, N. Designing Mobile Information Architecture (IA) M-Health Learning Application for Traditional Malay Medicinal Plants with Medicinal Properties Using User Persona. Adv. Sci. Lett. 2018, 24, 603–607. [Google Scholar] [CrossRef]
- Burkill, I.H. A Dictionary of the Economic Products of the Malay Peninsula; Ministry of Agriculture: Putrajaya, Malaysia, 1966; Volume 2, p. 1311.
- Chen, Y.; Phang, W.-M.; Mu, A.K.-W.; Chan, C.-K.; Low, B.-S.; Sasidharan, S.; Chan, K.-L. Decreased expression of alpha-2-HS glycoprotein in the sera of rats treated with Eurycoma longifolia extract. Front. Pharmacol. 2015, 6. [Google Scholar] [CrossRef] [PubMed]
- Solomon, M.C.; Henkel, R.; Erasmus, N. In vivo effects of Eurycoma longifolia Jack (Tongkat Ali) extract on reproductive functions in the rat. Andrologia 2013, 46, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Low, B.-S.; Choi, S.-B.; Wahab, H.A.; Das, P.K.; Chan, K.-L. Eurycomanone, the major quassinoid in Eurycoma longifolia root extract increases spermatogenesis by inhibiting the activity of phosphodiesterase and aromatase in steroidogenesis. J. Ethnopharmacol. 2013, 149, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Low, B.-S.; Das, P.K.; Chan, K.-L. Standardized quassinoid-rich Eurycoma longifolia extract improved spermatogenesis and fertility in male rats via the hypothalamic–pituitary–gonadal axis. J. Ethnopharmacol. 2013, 145, 706–714. [Google Scholar] [CrossRef] [PubMed]
- Teh, C.-H.; Abdulghani, M.A.; Morita, H.; Shiro, M.; Hussin, A.H.; Chan, K.-L. Comparative X-ray and conformational analysis of a new crystal of 13α,21-dihydroeurycomanone with eurycomanone from Eurycoma longifolia and their anti-estrogenic activity using the uterotrophic assay. Planta Med. 2010, 77, 128–132. [Google Scholar] [CrossRef] [PubMed]
- Wahab, N.A.; Mokhtar, N.M.; Halim, W.N.H.; Das, S. The effect of eurycoma longifolia jack on spermatogenesis in estrogen-treated rats. Clinics 2010, 65, 93–98. [Google Scholar] [CrossRef]
- Zanoli, P.; Zavatti, M.; Montanari, C.; Baraldi, M. Influence of Eurycoma longifolia on the copulatory activity of sexually sluggish and impotent male rats. J. Ethnopharmacol. 2009, 126, 308–313. [Google Scholar] [CrossRef]
- Ang, H.; Lee, K.L.; Kiyoshi, M. Sexual Arousal in Sexually Sluggish Old Male Rats after Oral Administration of Eurycoma longifolia Jack. J. Basic Clin. Physiol. Pharmacol. 2004, 15, 303–309. [Google Scholar] [CrossRef]
- Ridzuan, M.A.R.M.; Sow, A.; Rain, A.N.; Ilham, A.M.; Zakiah, I. Eurycoma longifolia extract-artemisinin combination: Parasitemia suppression of Plasmodium yoelii-infected mice. Trop. Biomed. 2007, 24, 111–118. [Google Scholar]
- Wernsdorfer, W.H.; Ismail, S.; Chan, K.L.; Congpuong, K.; Wernsdorfer, G. Activity of Eurycoma longifolia root extract against Plasmodium falciparum in vitro. Wien. Klin. Wochenschr. 2009, 121, 23–26. [Google Scholar] [CrossRef]
- Ridzuan, M.A.R.M.; Rain, A.N.; Zhari, I.; Zakiah, I. Effect of Eurycoma longifolia extract on the Glutathione level in Plasmodium falciparum infected erythrocytes in vitro. Trop. Biomed. 2005, 22, 155–163. [Google Scholar]
- Ang, H.H.; Chan, K.L.; Mak, J.W. Effect of 7-day daily replacement of culture medium containing Eurycoma longifolia Jack constituents on the Malaysian Plasmodium falciparum isolates. J. Ethnopharmacol. 1995, 49, 171–175. [Google Scholar] [CrossRef]
- Lam, C.K.; Choo, C.; Abdullah, N.R.; Ismail, Z. Antiplasmodial studies of Eurycoma longifolia Jack using the lactate dehydrogenase assay of Plasmodium falciparum. J. Ethnopharmacol. 2004, 92, 223–227. [Google Scholar] [CrossRef]
- Nadia, M.E.; Nazrun, A.S.; Norazlina, M.; Isa, N.M.; Norliza, M.; Nirwana, S.I. The Anti-Inflammatory, Phytoestrogenic, and Antioxidative Role ofLabisia pumilain Prevention of Postmenopausal Osteoporosis. Adv. Pharmacol. Sci. 2012, 2012, 1–7. [Google Scholar] [CrossRef]
- Kadir, A.A.; Hussain, N.H.N.; Bebakar, W.M.W.; Mohd, D.M.; Mohammad, Z.W.; Hassan, I.I.; Shukor, N.; Kamaruddin, N.A.; Mohamud, W.N.W. The Effect ofLabisia pumilavar.alataon Postmenopausal Women: A Pilot Study. Evid. Based Complement. Altern. Med. 2012, 2012, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Manneras, L.; Fazliana, M.; Nazaimoon, W.W.; Lönn, M.; Gu, H.; Östenson, C.; Stener-Victorin, E. Beneficial metabolic effects of the Malaysian herb Labisia pumila var. alata in a rat model of polycystic ovary syndrome. J. Ethnopharmacol. 2010, 127, 346–351. [Google Scholar] [CrossRef]
- Fazliana, M.; Nazaimoon, W.W.; Gu, H.F.; Östenson, C.-G. Labisia pumila extract regulates body weight and adipokines in ovariectomized rats. Maturitas 2009, 62, 91–97. [Google Scholar] [CrossRef]
- Jamal, J.A.; Houghton, P.J.; Milligan, S.R. Testing of Labisia pumila for oestrogenic activity using a recombinant yeast screen. J. Pharm. Pharmacol. 1998, 50, 79. [Google Scholar] [CrossRef]
- Jamal, J.A.; Houghton, P.J.; Milligan, S.R.; Jantan, I. The oestrogenic and cytotoxic effects of the extracts of Labisia pumila var. alata and Labisia pumila var. pumila in vitro. Malays. J. Med. Sci. 2003, 1, 53–60. [Google Scholar]
- Zakaria, Y.; Rahmat, A.; Hawariah, L.P.A.; Abdullah, N.R.; Houghton, P.J. Eurycomanone induce apoptosis in HepG2 cells via up-regulation of p53. Cancer Cell Int. 2009, 9, 16. [Google Scholar] [CrossRef]
- Kong, C.; Khalil, I.; Rahman, N.A.; Tan, M.-W.; Nathan, S. Discovery of potential anti-infectives against Staphylococcus aureus using a Caenorhabditis elegans infection model. BMC Complement. Altern. Med. 2014, 14, 4. [Google Scholar] [CrossRef] [PubMed]
- Karimi, E.; Jaafar, H.Z.E.; Ahmad, S. Phytochemical Analysis and Antimicrobial Activities of Methanolic Extracts of Leaf, Stem and Root from Different Varieties of Labisa pumila Benth. Molecules 2011, 16, 4438–4450. [Google Scholar] [CrossRef] [PubMed]
- Dang, N.H.; Choo, Y.-Y.; Dat, N.T.; Nam, N.H.; Chau, V.M.; Lee, J.-H.; Dang, N.H. 7-Methoxy-(9H-β-Carbolin-1-il)-(E)-1-Propenoic Acid, a β-Carboline Alkaloid FromEurycoma longifolia, Exhibits Anti-Inflammatory Effects by Activating the Nrf2/Heme Oxygenase-1 Pathway. J. Cell. Biochem. 2016, 117, 659–670. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.H.; Jaafar, H.Z.E. The Relationship of Nitrogen and C/N Ratio with Secondary Metabolites Levels and Antioxidant Activities in Three Varieties of Malaysian Kacip Fatimah (Labisia pumila Blume). Molecules 2011, 16, 5514–5526. [Google Scholar] [CrossRef] [PubMed]
- Shuid, A.N.; Abu Bakar, M.F.; Shukor, T.A.A.; Muhammad, N.; Mohamed, N.; Soelaiman, I.N. The anti-osteoporotic effect of Eurycoma Longifolia in aged orchidectomised rat model. Aging Male 2010, 14, 150–154. [Google Scholar] [CrossRef]
- Fathilah, S.N.; Shuid, A.N.; Mohamed, N.; Muhammad, N.; Soelaiman, I.N. Labisia pumila protects the bone of estrogen-deficient rat model: A histomorphometric study. J. Ethnopharmacol. 2012, 142, 294–299. [Google Scholar] [CrossRef]
- Effendy, N.M.; Mohamed, N.; Muhammad, N.; Mohamad, I.N.; Shuid, A.N. Eurycoma longifolia: Medicinal Plant in the Prevention and Treatment of Male Osteoporosis due to Androgen Deficiency. Evid. Based Complement. Altern. Med. 2012, 2012, 1–9. [Google Scholar] [CrossRef]
- Effendy, N.M.; Khamis, M.F.; Shuid, A.N. The effects of Labisia pumila extracts on bone microarchitecture of ovariectomized-induced osteoporosis rats: A micro-CT analysis. J. X-Ray Sci. Technol. 2017, 25, 101–112. [Google Scholar] [CrossRef]
- Kotirum, S.; Ismail, S.B.; Chaiyakunapruk, N. Efficacy of Tongkat Ali (Eurycoma longifolia) on erectile function improvement: Systematic review and meta-analysis of randomized controlled trials. Complement. Ther. Med. 2015, 23, 693–698. [Google Scholar] [CrossRef]
- Thu, H.E.; Mohamed, I.N.; Hussain, Z.; Jayusman, P.A.; Shuid, A.N. Eurycoma Longifolia as a potential adoptogen of male sexual health: A systematic review on clinical studies. Chin. J. Nat. Med. 2017, 15, 71–80. [Google Scholar] [CrossRef]
- Rehman, S.U.; Choe, K.; Yoo, H.H. Review on a Traditional Herbal Medicine, Eurycoma longifolia Jack (Tongkat Ali): Its Traditional Uses, Chemistry, Evidence-Based Pharmacology and Toxicology. Molecules 2016, 21, 331. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, N.A.; Razaly, N.I.; Rani, M.D.M.; Aris, M.S.M.; Effendy, N. An Evidence-Based Review: The Effects of Malaysian Traditional Herbs on Osteoporotic Rat Models. Malays. J. Med. Sci. 2018, 25, 6–30. [Google Scholar] [CrossRef] [PubMed]
- Barik, C.S.; Kanungo, S.K.; Tripathy, N.K.; Panda, J.R.; Padhi, M. A review on therapeutic potential of polyherbal formulation. Int. J. Pharm. Sci. Drug Res. 2015, 7, 211–228. [Google Scholar]
- Che, C.-T.; Wang, Z.J.; Chow, M.S.S.; Lam, C.W.K. Herb-Herb Combination for Therapeutic Enhancement and Advancement: Theory, Practice and Future Perspectives. Molecules 2013, 18, 5125–5141. [Google Scholar] [CrossRef] [PubMed]
- Ekor, M. The growing use of herbal medicines: Issues relating to adverse reactions and challenges in monitoring safety. Front. Pharmacol. 2014, 4, 177. [Google Scholar] [CrossRef]
- Shuid, A.; Siang, L.; Chin, T.; Muhammad, N.; Mohamed, N.; Soelaiman, I. Acute and Subacute Toxicity Studies of Eurycoma longifolia in Male Rats. Int. J. Pharmacol. 2011, 7, 641–646. [Google Scholar] [CrossRef]
- Wan Ezumi, M.F.; Siti Amrah, S.; Suhaimi, A.W.M.; Mohsin, S.S.J. Evaluation of the female reproductive toxicity of aqueous extract of Labisia pumila var. alata in rats. Indian J. Pharmacol. 2007, 39, 30. [Google Scholar] [CrossRef]
- Singh, G.; Ganjoo, M.; Youssouf, M.; Koul, A.; Sharma, R.; Singh, S.; Sangwan, P.; Koul, S.; Ahamad, D.; Johri, R. Sub-acute toxicity evaluation of an aqueous extract of Labisia pumila, a Malaysian herb. Food Chem. Toxicol. 2009, 47, 2661–2665. [Google Scholar] [CrossRef]
- Docea, A.O.; Gofita, E.; Goumenou, M.; Calina, D.; Rogoveanu, O.; Varut, M.; Olaru, C.; Kerasioti, E.; Fountoucidou, P.; Taitzoglou, I.; et al. Six months exposure to a real life mixture of 13 chemicals’ below individual NOAELs induced non monotonic sex-dependent biochemical and redox status changes in rats. Food Chem. Toxicol. 2018, 115, 470–481. [Google Scholar] [CrossRef]
- Tsatsakis, A.; Docea, A.O.; Constantin, C.; Calina, D.; Zlatian, O.; Nikolouzakis, T.K.; Stivaktakis, P.; Kalogeraki, A.; Liesivuori, J.; Tzanakakis, G.; et al. Genotoxic, cytotoxic, and cytopathological effects in rats exposed for 18 months to a mixture of 13 chemicals in doses below NOAEL levels. Toxicol. Lett. 2019, 316, 154–170. [Google Scholar] [CrossRef]
- Fuad, W.E.M.; Sulaiman, S.A.; Islam, M.N.; Wahab, M.S.A.; Jamalullail, S.M.S. Evaluation of the Teratogenicity of Aqueous Extract of Labisia pumila var. alata in rats. Malays. J. Med. Sci. 2005, 12, 13–21. [Google Scholar]
- Choudhary, Y.K.; Bommu, P.; Ming, Y.K.; Zulkawi, N.B. Acute, subacute and subchronic 90-days toxicity of Eurycoma longifolia aqueous extract (PHYSTA) in Wistar rats. J. Pharm. Pharm. Sci. 2012, 4, 232–238. [Google Scholar]
- Li, C.-H.; Liao, J.-W.; Liao, P.-L.; Huang, W.-K.; Tse, L.-S.; Lin, C.-H.; Kang, J.J.; Cheng, Y.-W. Evaluation of Acute 13-Week Subchronic Toxicity and Genotoxicity of the Powdered Root of Tongkat Ali (Eurycoma longifoliaJack). Evid. Based Complement. Altern. Med. 2013, 2013, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Low, B.-S.; Das, P.K.; Lam, C.K. Acute, Reproductive Toxicity and Two-generation Teratology Studies of a Standardized Quassinoid-rich Extract ofEurycoma longifoliaJack in Sprague-Dawley Rats. Phytother. Res. 2013, 28, 1022–1029. [Google Scholar] [CrossRef]
- Chen, C.K.; Mohamad, W.M.Z.W.; Ooi, F.K.; Ismail, S.B.; Abdullah, M.R.; George, A. Supplementation of Eurycoma longifolia Jack Extract for 6 Weeks Does Not Affect Urinary Testosterone: Epitestosterone Ratio, Liver and Renal Functions in Male Recreational Athletes. Int. J. Prev. Med. 2014, 5, 728–733. [Google Scholar]
- Sengupta, P. The laboratory rat: Relating its age with human’s. Int. J. Prev. Med. 2013, 4, 624–630. [Google Scholar]
- U.S. Food and Drug Administration. Guidance for Industry and Other Stakeholders: Redbook 2000 Toxicological Principles for the Safety Assessment of Food Ingredients. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/guidance-industry-and-other-stakeholders-redbook-2000 (accessed on 1 October 2020).
- Damsch, S.; Eichenbaum, G.; Tonelli, A.; Lammens, L.; Bulck, K.V.D.; Feyen, B.; Vandenberghe, J.; Megens, A.; Knight, E.; Kelley, M. Gavage-Related Reflux in Rats. Toxicol. Pathol. 2011, 39, 348–360. [Google Scholar] [CrossRef]
- Ahmad, N.; Samiulla, D.S.; Teh, B.P.; Zainol, M.; Zolkifli, N.A.; Muhammad, A.; Matom, E.; Zulkapli, A.; Abdullah, N.R.; Ismail, Z.; et al. Bioavailability of Eurycomanone in Its Pure Form and in a Standardised Eurycoma longifolia Water Extract. Pharmaceutics 2018, 10, 90. [Google Scholar] [CrossRef]
- Low, B.-S.; Zhang, S.; Choy, W.-P.; Yuen, K.-H.; Chan, K.-L. Bioavailability and Pharmacokinetic Studies of Eurycomanone fromEurycoma longifolia. Planta Med. 2005, 71, 803–807. [Google Scholar] [CrossRef]
- Variya, B.C.; Bakrania, A.K.; Madan, P.; Patel, S.S. Acute and 28-days repeated dose sub-acute toxicity study of gallic acid in albino mice. Regul. Toxicol. Pharmacol. 2019, 101, 71–78. [Google Scholar] [CrossRef]
- OECD Guideline for Testing of Chemicals. Number 407: Repeated Dose 28-Day Oral Toxicity Study in Rodents. Available online: https://ntp.niehs.nih.gov/iccvam/suppdocs/feddocs/oecd/oecdtg407-2008.pdf (accessed on 1 October 2020).
- Voisin, E.M.; Ruthsatz, M.; Collins, J.M.; Hoyle, P.C. Extrapolation of animal toxicity to humans: Interspecies comparisons in drug development. Regul. Toxicol. Pharmacol. 1990, 12, 107–116. [Google Scholar] [CrossRef]
- Zbinden, G. Predictive value of animal studies in toxicology. Regul. Toxicol. Pharmacol. 1991, 14, 167–177. [Google Scholar] [CrossRef]
- ClinicalTrials.Gov. A Clinical Trial on the Efficacy of a Combination Herbal Product, Biotropics’ Nu Femme, on Menopausal Symptoms and Quality of Life in Women (14NMHB). Available online: https://clinicaltrials.gov/ct2/show/study/NCT02269891?term=nu+femme&draw=2&rank=1 (accessed on 1 October 2020).
- Ressel, L.; Hetzel, U.; Ricci, E. Blunt Force Trauma in Veterinary Forensic Pathology. Veter Pathol. 2016, 53, 941–961. [Google Scholar] [CrossRef] [PubMed]
- Jose, M.; Adyanthaya, S. Quality and safety aspects in histopathology laboratory. J. Oral Maxillofac. Pathol. 2013, 17, 402–407. [Google Scholar] [CrossRef]
- Westwood, F.R. The Female Rat Reproductive Cycle: A Practical Histological Guide to Staging. Toxicol. Pathol. 2008, 36, 375–384. [Google Scholar] [CrossRef]
- National Centre for Replacement Refinement & Reduction of Animals in Research. Cardiac Puncture. Available online: https://www.nc3rs.org.uk/rat-cardiac-puncture-terminal (accessed on 1 October 2020).
- Furtado, K.; Andrade, F. Comparison of the beneficial and adverse effects of inhaled and injectable anaesthetics: A mini-review. OA Anaesth. 2013, 1, 20. [Google Scholar] [CrossRef]
- Schober, P.; Schwarte, L.A. From system to organ to cell: Oxygenation and perfusion measurement in anesthesia and critical care. J. Clin. Monit. 2012, 26, 255–265. [Google Scholar] [CrossRef]
- Fleknell, P. Laboratory Animal Anaesthesia, 3rd ed.; Academic Press: Cambridge, MA, USA, 2009; pp. 54–55. [Google Scholar]
- Ludders, J.W. Advantages and Guidelines for Using Isoflurane. Veter Clin. N. Am. Small Anim. Pr. 1992, 22, 328–331. [Google Scholar] [CrossRef]
- Dai, G.; Jiang, Z.; Bai, Y.; Zhang, Q.; Zhu, L.; Bai, X.; Ju, W.; Pan, R. Pharmacokinetic herb-drug interaction of Xuesaitong dispersible tablet and aspirin after oral administration in blood stasis model rats. Phytomedicine 2017, 26, 62–68. [Google Scholar] [CrossRef]
- Ma, S.-T.; Dai, G.; Bi, X.; Gong, M.; Miao, C.; Chen, H.; Gao, L.; Zhao, W.; Liu, T.; Zhang, N.-R. The Herb-Drug Interaction of Clopidogrel and Xuesaitong Dispersible Tablet by Modulation of the Pharmacodynamics and Liver Carboxylesterase 1A Metabolism. Evid. Based Complement. Altern. Med. 2018, 2018, 5651989. [Google Scholar] [CrossRef]
- Global Information Hub on Integrated Medicine. Eurycoma Longifolia Jack. Available online: https://www.globinmed.com/index.php?option=com_content&view=article&id=102022:eurycoma-longifolia-jack-102022&catid=209&Itemid=143 (accessed on 1 October 2020).
- Global Information Hub on Integrated Medicine. Labisia Pumila. Available online: https://www.globinmed.com/index.php?option=com_content&view=article&id=102032:labisia-pumila-102032&catid=209&Itemid=143 (accessed on 1 October 2020).
- Kementerian Kesihatan Malaysia. Principles and Guide to Ethical Use of Laboratory Animals; Ministry of Health Malaysia: Kuala Lumpur, Malaysia, 2002.



| Female Rats | Male Rats | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dose (mg/kg) (Group) | Parameters | Dose (mg/kg) (Group) | ||||||||||
| 0 (Vehicle Control) | 125 (Low Dose) | 500 (Medium Dose) | 2000 (High Dose) | 0 (Vehicle Control Satellite) | 2000 (High-Dose Satellite) | 0 (Vehicle Control) | 125 (Low Dose) | 500 (Medium Dose) | 2000 (High Dose) | 0 (Vehicle Control Satellite) | 2000 (High-Dose Satellite) | |
| 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 1/5 | Mortality (died/dosed) | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 |
| NAD | NAD | NAD | NAD | NAD | NAD | Clinical signs of toxicity | NAD | NAD | NAD | NAD | NAD | NAD |
| 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | Incidence of clinical signs of toxicity | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 |
| N/A | N/A | N/A | N/A | N/A | N/A | Onset of clinical signs of toxicity (day) | N/A | N/A | N/A | N/A | N/A | N/A |
| N/A | N/A | N/A | N/A | N/A | N/A | Duration/severity of clinical signs of toxicity | N/A | N/A | N/A | N/A | N/A | N/A |
| 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | Incidence of lesions/dosed | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 |
| N/A | N/A | N/A | N/A | N/A | N/A | Type of lesions | N/A | N/A | N/A | N/A | N/A | N/A |
| N/A | N/A | N/A | N/A | N/A | N/A | Severity of lesions | N/A | N/A | N/A | N/A | N/A | N/A |
| Female Rats | Male Rats | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dose (mg/kg) (Group) | Parameters | Dose (mg/kg) (Group) | ||||||||||
| 0 (Vehicle Control) | 125 (Low Dose) | 500 (Medium Dose) | 2000 (High Dose) | 0 (Vehicle Control Satellite) | 2000 (High-Dose Satellite) | 0 (Vehicle Control) | 125 (Low Dose) | 500 (Medium Dose) | 2000 (High Dose) | 0 (Vehicle Control Satellite) | 2000 (High-Dose Satellite) | |
| 1335.40 ± 251.54 | 1273.00 ± 314.98 | 1278.00 ± 257.02 | 1029.80 ± 234.67 | 1399.80 ± 197.64 | 1252.00 ± 299.80 | General activity | 1042.80 ± 306.00 | 1072.00 ± 183.94 | 1220.40 ± 61.06 | 1121.20 ± 159.34 | 927.60 ± 352.26 | 790.40 ± 282.33 |
| 91.40 ± 19.31 | 83.80 ± 23.47 | 83.00 ± 11.68 | 75.40 ± 11.95 | 90.00 ± 11.92 | 95.25 ± 19.60 | Stereotyped movement | 84.00 ± 19.96 | 80.80 ± 14.60 | 96.60 ± 16.86 | 85.00 ± 12.31 | 73.60 ± 42.96 | 55.60 ± 19.83 |
| 1244.00 ± 249.15 | 1189.20 ± 294.44 | 1195.00 ± 252.36 | 954.40 ± 226.39 | 1309.80 ± 190.73 | 1156.75 ± 283.99 | Locomotion | 958.80 ± 291.39 | 991.20 ± 177.56 | 1123.80 ± 51.80 | 1036.20 ± 154.56 | 854.00 ± 310.84 | 734.80 ± 263.68 |
| 555.32 ± 144.31 | 683.76 ± 164.89 | 859.74 ± 104.77 * | 857.42 ± 155.00 * | 828.20 ± 120.30 | 891.85 ± 119.11 | Forelimb grip strength (g) | 894.54 ± 143.06 | 873.68 ± 149.43 | 1008.78 ± 160.67 | 943.12 ± 98.40 | 920.84 ± 140.86 | 997.30 ± 66.07 |
| 1002.08 ± 177.52 | 955.72 ± 59.88 | 1013.48 ± 156.98 | 1027.84 ± 74.99 | 947.00 ± 158.82 | 995.08 ± 244.18 | Forelimb and hind limb grip strength (g) | 1068.46 ± 72.42 | 1146.04 ± 227.37 | 1200.40 ± 218.91 | 1154.40 ± 85.66 | 1091.04 ± 133.74 | 1127.62 ± 178.69 |
| Female Rats | Male Rats | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dose (mg/kg) (Group) | Weekly Body Weight Change | Dose (mg/kg) (Group) | ||||||||||
| 0 (Vehicle Control) | 125 (Low Dose) | 500 (Medium Dose) | 2000 (High Dose) | 0 (Vehicle Control Satellite) | 2000 (High-Dose Satellite) | 0 (Vehicle Control) | 125 (Low Dose) | 500 (Medium Dose) | 2000 (High Dose) | 0 (Vehicle Control Satellite) | 2000 (High-Dose Satellite) | |
| 10.41 ± 5.23 | 8.49 ± 1.65 | 9.78 ± 2.34 | 8.89 ± 5.26 | 6.32 ± 2.02 | 7.96 ± 2.89 | Week 1 | 12.92 ± 3.67 | 12.20 ± 1.58 | 13.10 ± 1.57 | 13.74 ± 4.27 | 13.45 ± 2.56 | 12.33 ± 2.04 |
| 2.98 ± 3.22 | 5.78 ± 1.77 | 5.31 ± 1.40 | 5.39 ± 1.90 | 7.19 ± 3.61 | 8.97 ± 3.42 | Week 2 | 6.78 ± 2.58 | 8.34 ± 1.84 | 9.08 ± 1.97 | 8.57 ± 0.74 | 7.61 ± 3.18 | 7.72 ± 0.69 |
| 6.17 ± 5.17 | 2.03 ± 2.31 | 4.14 ± 2.63 | 4.11 ± 3.60 | 2.95 ± 5.17 | 1.43 ± 3.23 | Week 3 | 6.36 ± 1.16 | 6.41 ± 1.15 | 6.90 ± 1.18 | 6.23 ± 1.11 | 7.31 ± 2.14 | 5.09 ± 2.32 |
| 1.81 ± 1.99 | 3.51 ± 3.47 | 4.16 ± 3.55 | 4.39 ± 2.88 | 3.47 ± 4.22 | 4.88 ± 2.69 | Week 4 | 2.96 ± 1.11 | 3.40 ± 1.19 | 4.39 ± 1.65 | 3.13 ± 1.07 | 5.81 ± 0.54 | 4.64 ± 1.47 |
| N/A | N/A | N/A | N/A | 2.73 ± 3.49 | 1.79 ± 0.67 | Week 5 | N/A | N/A | N/A | N/A | 9.35 ± 9.89 | 4.81 ± 1.34 |
| N/A | N/A | N/A | N/A | 0.78 ± 2.42 | 3.45 ± 1.02 | Week 6 | N/A | N/A | N/A | N/A | -0.59 ± 8.95 | 3.02 ± 1.09 |
| Female Rats | Male Rats | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dose (mg/kg) (Group) | Weekly Food Consumption | Dose (mg/kg) (Group) | ||||||||||
| 0 (Vehicle Control) | 125 (Low Dose) | 500 (Medium Dose) | 2000 (High Dose) | 0 (Vehicle Control Satellite) | 2000 (High-Dose Satellite) | 0 (Vehicle Control) | 125 (Low Dose) | 500 (Medium Dose) | 2000 (High Dose) | 0 (Vehicle Control Satellite) | 2000 (High-Dose Satellite) | |
| 118.81 ± 18.27 | 115.09 ± 10.68 | 118.75 ± 4.14 | 118.42 ± 13.48 | 112.01 ± 6.82 | 120.69 ± 7.62 | Week 1 | 170.50 ± 15.83 | 163.38 ± 15.48 | 171.88 ± 8.13 | 162.10 ± 14.83 | 174.30 ± 13.20 | 170.49 ± 12.48 |
| 124.49 ± 12.62 | 118.91 ± 16.79 | 128.31 ± 5.78 | 122.10 ± 12.89 | 118.91 ± 2.43 | 130.12 ± 13.76 | Week 2 | 168.53 ± 18.67 | 166.04 ± 12.46 | 173.39 ± 9.96 | 163.51 ± 14.01 | 176.59 ± 10.77 | 166.94 ± 14.28 |
| 126.75 ± 10.80 | 116.83 ± 10.71 | 127.29 ± 2.60 | 123.48 ± 9.17 | 118.40 ± 6.19 | 122.90 ± 9.07 | Week 3 | 170.66 ± 21.76 | 165.07 ± 16.91 | 174.58 ± 9.19 | 158.75 ± 14.62 | 174.35 ± 12.36 | 165.23 ± 16.63 |
| 110.24 ± 13.49 | 100.46 ± 8.75 | 112.10 ± 11.26 | 108.76 ± 7.43 | 112.00 ± 4.62 | 117.33 ± 7.00 | Week 4 | 147.02 ± 17.50 | 143.65 ± 12.20 | 151.89 ± 7.31 | 136.08 ± 16.14 | 172.85 ± 15.19 | 156.11 ± 16.95 |
| N/A | N/A | N/A | N/A | 116.34 ± 5.88 | 99.49 ± 5.04 | Week 5 | N/A | N/A | N/A | N/A | 172.37 ± 17.74 | 165.34 ± 14.94 |
| N/A | N/A | N/A | N/A | 126.28 ± 8.37 | 113.95 ± 6.51 | Week 6 | N/A | N/A | N/A | N/A | 150.87 ± 13.63 | 153.05 ± 19.47 |
| Female Rats | Male Rats | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dose (mg/kg) (Group) | Weekly Water Consumption | Dose (mg/kg) (Group) | ||||||||||
| 0 (Vehicle Control) | 125 (Low Dose) | 500 (Medium Dose) | 2000 (High Dose) | 0 (Vehicle Control Satellite) | 2000 (High-Dose Satellite) | 0 (Vehicle Control) | 125 (Low Dose) | 500 (Medium Dose) | 2000 (High Dose) | 0 (Vehicle Control Satellite) | 2000 (High-Dose Satellite) | |
| 347.6 ± 99.0 | 292.2 ± 36.3 | 340.0 ± 95.5 | 316.4 ± 85.0 | 321.8 ± 37.0 | 275.0 ± 67.4 | Week 1 | 419.0 ± 89.4 | 371.0 ± 82.1 | 316.2 ± 34.0 | 406.4 ± 122.3 | 358.4 ± 88.9 | 345.6 ± 36.3 |
| 460.6 ± 158.2 | 325.4 ± 33.3 | 378.8 ± 88.3 | 335.2 ± 99.1 | 347.6 ± 60.4 | 326.8 ± 62.1 | Week 2 | 409.8 ± 82.8 | 372.0 ± 80.4 | 334.8 ± 45.3 | 423.2 ± 121.3 | 391.2 ± 96.7 | 374.4 ± 50.9 |
| 497.0 ± 197.8 | 332.0 ± 42.9 | 409.4 ± 71.4 | 342.0 ± 114.7 | 354.2 ± 66.0 | 281.3 ± 49.6 | Week 3 | 407.8 ± 111.2 | 364.0 ± 97.0 | 298.0 ± 55.5 | 393.0 ± 131.6 | 368.0 ± 71.0 | 366.0 ± 46.2 |
| 368.2 ± 129.4 | 288.2 ± 41.5 | 400.6 ± 100.9 | 302.2 ± 69.9 | 366.4 ± 105.2 | 292.3 ± 55.5 | Week 4 | 362.8 ± 104.4 | 330.4 ± 91.0 | 291.6 ± 34.7 | 346.0 ± 89.0 | 354.6 ± 54.6 | 351.8 ± 66.6 |
| N/A | N/A | N/A | N/A | 357.4 ± 117.6 | 266.8 ± 69.6 | Week 5 | N/A | N/A | N/A | N/A | 349.0 ± 78.2 | 334.2 ± 47.4 |
| N/A | N/A | N/A | N/A | 310.8 ± 85.6 | 381.5 ± 277.4 | Week 6 | N/A | N/A | N/A | N/A | 317.0 ± 64.8 | 323.0 ± 78.5 |
| Female Rats | Male Rats | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dose (mg/kg) (Group) | Parameters | Dose (mg/kg) (Group) | ||||||||||
| 0 (Vehicle Control) | 125 (Low Dose) | 500 (Medium Dose) | 2000 (High Dose) | 0 (Vehicle Control Satellite) | 2000 (High-Dose Satellite) | 0 (Vehicle Control) | 125 (Low Dose) | 500 (Medium Dose) | 2000 (High Dose) | 0 (Vehicle Control Satellite) | 2000 (High-Dose Satellite) | |
| 33.0 ± 3.1 | 33.3 ± 1.6 | 33.3 ± 2.5 | 32.6 ± 2.5 | 36.3 ± 2.6 | 35.8 ± 1.1 | HCT (%) | 36.4 ± 2.2 | 36.5 ± 3.2 | 37.6 ± 1.8 | 35.9 ± 2.3 | 38.4 ± 2.2 | 38.4 ± 0.4 |
| 12.6 ± 0.5 | 12.8 ± 0.3 | 12.9 ± 0.9 | 12.7 ± 0.6 | 13.6 ± 0.8 | 13.6 ± 0.4 | HGB (g/dL) | 13.7 ± 0.6 | 14.0 ± 0.8 | 14.2 ± 0.7 | 13.9 ± 0.7 | 14.3 ± 0.7 | 14.5 ± 0.2 |
| 6.27 ± 0.48 | 6.64 ± 0.43 | 6.68 ± 0.64 | 6.70 ± 0.48 | 7.17 ± 0.50 | 7.22 ± 0.27 | RBC (106 cells/mm) | 7.26 ± 0.51 | 7.21 ± 0.59 | 7.61 ± 0.31 | 7.22 ± 0.40 | 7.83 ± 0.47 | 7.90 ± 0.19 |
| 3.6 ± 1.3 | 3.6 ± 1.5 | 3.0 ± 2.0 | 4.4 ± 2.8 | 3.4 ± 1.6 | 3.3 ± 1.0 | WBC (103 cells/mm) | 7.2 ± 1.0 | 8.1 ± 1.8 | 8.5 ± 3.4 | 7.2 ± 4.0 | 6.0 ± 1.3 | 7.4 ± 1.6 |
| 1002 ± 123 | 1132 ± 157 | 1023 ± 119 | 1206 ± 119 | 972 ± 182 | 1017 ± 133 | PLT (103 cells/mm) | 1124 ± 168 | 1134 ± 234 | 1154 ± 188 | 1316 ± 147 | 1208 ± 57 | 1082 ± 134 |
| Female Rats | Male Rats | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dose (mg/kg) (Group) | Parameters | Dose (mg/kg) (Group) | ||||||||||
| 0 (Vehicle Control) | 125 (Low Dose) | 500 (Medium Dose) | 2000 (High Dose) | 0 (Vehicle Control Satellite) | 2000 (High-Dose Satellite) | 0 (Vehicle Control) | 125 (Low Dose) | 500 (Medium Dose) | 2000 (High Dose) | 0 (Vehicle Control Satellite) | 2000 (High-Dose Satellite) | |
| Liver Profile | ||||||||||||
| 62.3 ± 2.1 | 62.8 ± 1.8 | 60.4 ± 2.3 | 60.0 ± 2.9 | 62.0 ± 1.0 | 62.5 ± 3.3 | Total protein (g/L) | 60.2 ± 3.1 | 65.6 ± 3.9 | 61.8 ± 4.2 | 60.4 ± 2.1 | 61.0 ± 3.4 | 60.4 ± 2.6 |
| 14.7 ± 0.5 | 15.0 ± 0.0 | 13.8 ± 1.3 | 14.0 ± 1.2 | 14.4 ± 0.9 | 15.0 ± 0.8 | Albumin (g/L) | 13.2 ± 1.3 | 13.8 ± 0.8 | 13.2 ± 0.8 | 12.6 ± 0.5 | 12.8 ± 0.4 | 12.6 ± 0.5 |
| 32.1 ± 5.9 | 35.0 ± 8.5 | 34.2 ± 4.3 | 28.8 ± 5.9 | 34.4 ± 5.7 | 30.3 ± 3.7 | ALT (U/L) | 40.2 ± 10.1 | 34.4 ± 5.8 | 32.8 ± 6.1 | 31.8 ± 5.1 | 37.8 ± 6.2 | 34.4 ± 7.4 |
| 83.2 ± 14.2 | 79.0 ± 23.5 | 67.2 ± 22.3 | 51.4 ± 9.8 | 67.4 ± 22.5 | 54.8 ± 12.7 | ALP (U/L) | 127.4 ± 24.8 | 108.20 ± 14.8 | 119.0 ± 25.1 | 93.6 ± 36.8 | 91.0 ± 9.2 | 90.6 ± 7.3 |
| 109.8 ± 6.0 | 96.8 ± 14.8 | 100.2 ± 9.7 | 104.6 ± 23.2 | 81.2 ± 11.7 | 78.8 ± 11.7 | AST (U/L) | 80.3 ± 13.0 | 74.6 ± 17.1 | 74.8 ± 7.0 | 76.6 ± 9.0 | 70.8 ± 10.6 | 62.0 ± 3.5 |
| Female Rats | Male Rats | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dose (mg/kg) (Group) | Parameters | Dose (mg/kg) (Group) | ||||||||||
| 0 (Vehicle Control) | 125 (Low Dose) | 500 (Medium Dose) | 2000 (High Dose) | 0 (Vehicle Control Satellite) | 2000 (High-Dose Satellite) | 0 (Vehicle Control) | 125 (Low Dose) | 500 (Medium Dose) | 2000 (High Dose) | 0 (Vehicle Control Satellite) | 2000 (High-Dose Satellite) | |
| Renal Profile | ||||||||||||
| 4.1 ± 0.3 | 3.8 ± 0.2 | 4.2 ± 0.5 | 4.0 ± 0.4 | 3.9 ± 0.6 | 3.7 ± 0.2 | Potassium (mmol/L) | 4.0 ± 0.3 | 4.4 ± 0.4 | 4.1 ± 0.3 | 4.5 ± 0.2 | 4.0 ± 0.4 | 4.0 ± 0.3 |
| 38.4 ± 7.6 | 33.8 ± 2.7 | 39.0 ± 2.5 | 35.2 ± 5.4 | 39.4 ± 7.4 | 41.5 ± 9.3 | Creatinine (µmol/L) | 30.4 ± 3.2 | 32.2 ± 3.6 | 34.0 ± 7.0 | 27.2 ± 6.3 | 37.0 ± 6.1 | 32.4 ± 5.7 |
| 7.1 ± 0.7 | 6.7 ± 0.7 | 7.5 ± 1.0 | 6.1 ± 1.4 | 6.6 ± 1.1 | 7.2 ± 0.7 | Urea (mmol/L) | 6.7 ± 0.4 | 6.9 ± 0.9 | 6.9 ± 0.7 | 6.6 ± 1.1 | 6.5 ± 1.1 | 7.1 ± 1.3 |
| 94.3 ± 9.1 | 78.6 ± 30.5 | 111.8 ± 22.0 | 95.2 ± 25.8 | 77.8 ± 27.5 | 98.8 ± 35.7 | Uric acid (µmol/L) | 77.2 ± 5.8 | 78.80 ± 18.1 | 71.0 ± 28.2 | 68.6 ± 14.3 | 69.6 ± 15.7 | 82.4 ± 48.6 |
| Lipid Profile | ||||||||||||
| 1.9 ± 0.2 | 2.1 ± 0.4 | 2.1 ± 0.2 | 1.7 ± 0.1 | 2.1 ± 0.3 | 2.0 ± 0.2 | Total cholesterol (mmol/L) | 1.7 ± 0.2 | 2.3 ± 0.4 | 1.8 ± 0.2 | 1.4 ± 0.3 | 2.3 ± 0.1 | 2.1 ± 0.3 |
| 0.4 ± 0.1 | 0.5 ± 0.1 | 0.4 ± 0.1 | 0.4 ± 0.2 | 0.7 ± 0.2 | 0.5 ± 0.2 | Triglyceride (mmol/L) | 0.7 ± 0.2 | 1.0 ± 0.4 | 1.0 ± 0.3 | 1.0 ± 0.3 | 0.9 ± 0.2 | 0.9 ± 0.3 |
| 6.71 ± 0.40 | 6.70 ± 0.81 | 6.87 ± 0.71 | 7.26 ± 0.66 | 8.42 ± 1.13 | 7.45 ± 0.78 | Glucose (mmol/L) | 8.70 ± 0.56 | 8.58 ± 0.79 | 8.96 ± 0.90 | 9.64 ± 1.72 | 8.57 ± 0.74 | 9.31 ± 2.05 |
| Female Rats | Male Rats | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dose (mg/kg) (Group) | Organs | Dose (mg/kg) (Group) | ||||||||||
| 0 (Vehicle Control) | 125 (Low Dose) | 500 (Medium Dose) | 2000 (High Dose) | 0 (Vehicle Control Satellite) | 2000 (High-Dose Satellite) | 0 (Vehicle Control) | 125 (Low Dose) | 500 (Medium Dose) | 2000 (High Dose) | 0 (Vehicle Control Satellite) | 2000 (High-Dose Satellite) | |
| 5/5 | 4/5 | 5/5 | 5/5 | 5/5 | 4/5 | Lung | 4/5 | 5/5 | 4/5 | 5/5 | 5/5 | 4/5 |
| 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | Heart | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 |
| 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | Spleen | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 |
| 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | Stomach | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 |
| 1/5 | 3/5 | 3/5 | 3/5 | 0/5 | 0/5 | IT | 0/5 | 2/5 | 1/5 | 1/5 | 0/5 | 5/5 |
| 3/5 | 2/5 | 3/5 | 3/5 | 1/5 | 2/5 | Liver | 1/5 | 2/5 | 2/5 | 1/5 | 1/5 | 1/5 |
| 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | Kidneys | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 5/5 |
| 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | Adrenals | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 |
| 0/5 | 0/5 | 0/5 | 1/5 | 0/5 | 0/5 | Ovaries | N/A | N/A | N/A | N/A | N/A | N/A |
| 0/5 | 0/5 | 1/5 | 0/5 | 0/5 | 1/5 | Uterine horn | N/A | N/A | N/A | N/A | N/A | N/A |
| N/A | N/A | N/A | N/A | N/A | N/A | Testes | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 |
| Female Rats | Male Rats | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dose (mg/kg) (Group) | Organs | Dose (mg/kg) (Group) | ||||||||||
| 0 (Vehicle Control) | 125 (Low Dose) | 500 (Medium Dose) | 2000 (High Dose) | 0 (Vehicle Control Satellite) | 2000 (High-Dose Satellite) | 0 (Vehicle Control) | 125 (Low Dose) | 500 (Medium Dose) | 2000 (High Dose) | 0 (Vehicle Control Satellite) | 2000 (High-Dose Satellite) | |
| 0.4444 ± 0.0454 | 0.4358 ± 0.0209 | 0.4191 ± 0.0330 | 0.3871 ± 0.0310 | 0.3905 ± 0.0135 | 0.3893 ± 0.0216 | Lung | 0.3399 ± 0.0456 | 0.3528 ± 0.0203 | 0.3135 ± 0.04 | 0.3486 ± 0.0432 | 0.3057 ± 0.0318 | 0.3459 ± 0.0890 |
| 0.2890 ± 0.0307 | 0.2850 ± 0.0252 | 0.2876 ± 0.0132 | 0.2930 ± 0.0219 | 0.2729 ± 0.0158 | 0.2769 ± 0.0104 | Heart | 0.2494 ± 0.0331 | 0.2639 ± 0.0231 | 0.2559 ± 0.0132 | 0.2539 ± 0.0357 | 0.2297 ± 0.0185 | 0.2390 ± 0.0229 |
| 0.2332 ± 0.0281 | 0.2292 ± 0.0246 | 0.2282 ± 0.0304 | 0.2180 ± 0.0196 | 0.2028 ± 0.0126 | 0.2000 ± 0.0139 | Spleen | 0.2063 ± 0.0331 | 0.2052 ± 0.0244 | 0.1816 ± 0.0227 | 0.1829 ± 0.0124 | 0.1731 ± 0.0183 | 0.1962 ± 0.0205 |
| 0.5471 ± 0.0688 | 0.5849 ± 0.0919 | 0.5867 ± 0.0651 | 0.5605 ± 0.0247 | 0.5328 ± 0.0269 | 0.5273 ± 0.0155 | Stomach | 0.4714 ± 0.0494 | 0.4856 ± 0.0490 | 0.4743 ± 0.0329 | 0.4823 ± 0.0618 | 0.4533 ± 0.0366 | 0.4418 ± 0.0263 |
| 2.6132 ± 0.6347 | 2.6427 ± 0.5049 | 2.6492 ± 0.7822 | 2.5011 ± 0.3024 | 2.3388 ± 0.4279 | 2.1914 ± 0.1792 | IT | 1.8803 ± 0.3418 | 2.1921 ± 0.2580 | 2.0550 ± 0.2891 | 2.0597 ± 0.2814 | 2.0162 ± 0.2676 | 1.7278 ± 0.0613 |
| 2.7278 ± 0.1933 | 2.5790 ± 0.1221 | 2.5379 ± 0.0668 | 2.6613 ± 0.0964 | 2.4010 ± 0.1312 | 2.5351 ± 0.0276 | Liver | 2.9989 ± 0.4124 | 3.1014 ± 0.3272 | 2.8786 ± 0.1110 | 3.0926 ± 0.1527 | 2.7864 ± 0.2314 | 2.9335 ± 0.1799 |
| 0.3155 ± 0.0174 | 0.3255 ± 0.0291 | 0.3051 ± 0.0061 | 0.3325 ± 0.0233 | 0.2904 ± 0.0300 | 0.3216 ± 0.0228 | Kidney Right | 0.3248 ± 0.0252 | 0.3339 ± 0.0269 | 0.3082 ± 0.0085 | 0.3238 ± 0.0135 | 0.3083 ± 0.0192 | 0.3318 ± 0.0355 |
| 0.3172 ± 0.0171 | 0.3254 ± 0.0198 | 0.2910 ± 0.0119 | 0.3199 ± 0.0316 | 0.2836 ± 0.0300 | 0.3147 ± 0.0230 | Kidney Left | 0.3252 ± 0.0204 | 0.3283 ± 0.0264 | 0.3111 ± 0.0129 | 0.3171 ± 0.0251 | 0.3034 ± 0.0265 | 0.3270 ± 0.0371 |
| Female Rats | Male Rats | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dose (mg/kg) (Group) | Organs | Dose (mg/kg) (Group) | ||||||||||
| 0 (Vehicle Control) | 125 (Low Dose) | 500 (Medium Dose) | 2000 (High Dose) | 0 (Vehicle Control Satellite) | 2000 (High-Dose Satellite) | 0 (Vehicle Control) | 125 (Low Dose) | 500 (Medium Dose) | 2000 (High Dose) | 0 (Vehicle Control Satellite) | 2000 (High-Dose Satellite) | |
| 0.0152 ± 0.0036 | 0.0137 ± 0.0012 | 0.0157 ± 0.0024 | 0.0134 ± 0.0044 | 0.0130 ± 0.0013 | 0.0130 ± 0.0010 | Adrenal Right | 0.0083 ± 0.0016 | 0.0086 ± 0.0012 | 0.0077 ± 0.0009 | 0.0077 ± 0.0015 | 0.0065 ± 0.0013 | 0.0071 ± 0.0012 |
| 0.0168 ± 0.0043 | 0.0148 ± 0.0012 | 0.0149 ± 0.0023 | 0.0161 ± 0.0023 | 0.0143 ± 0.0018 | 0.0130 ± 0.0027 | Adrenal Left | 0.0082 ± 0.0010 | 0.0091 ± 0.0014 | 0.0084 ± 0.0007 | 0.0086 ± 0.0012 | 0.0077 ± 0.0013 | 0.0081 ± 0.0007 |
| 0.0275 ± 0.0035 | 0.0252 ± 0.0083 | 0.0207 ± 0.0042 | 0.0223 ± 0.0065 | 0.0196 ± 0.0024 | 0.0169 ± 0.0028 | Ovary Right | N/A | N/A | N/A | N/A | N/A | N/A |
| 0.0256 ± 0.0029 | 0.0188 ± 0.0036 * | 0.0166 ± 0.0033 * | 0.0202 ± 0.0040 | 0.0205 ± 0.0019 | 0.0183 ± 0.0043 # | Ovary Left | N/A | N/A | N/A | N/A | N/A | N/A |
| 0.0624 ± 0.0864 | 0.0718 ± 0.1002 | 0.1533 ± 0.0964 | 0.1619 ± 0.0969 | 0.1732 ± 0.0535 | 0.2122 ± 0.0421 | Uterine Horn | N/A | N/A | N/A | N/A | N/A | N/A |
| N/A | N/A | N/A | N/A | N/A | N/A | Testes Right | 0.4504 ± 0.0596 | 0.4121 ± 0.0309 | 0.4068 ± 0.0361 | 0.4320 ± 0.0251 | 0.3924 ± 0.0289 | 0.4110 ± 0.0316 |
| N/A | N/A | N/A | N/A | N/A | N/A | Testes Left | 0.4595 ± 0.0874 | 0.4096 ± 0.0272 | 0.4099 ± 0.0366 | 0.4290 ± 0.0238 | 0.3936 ± 0.0303 | 0.4156 ± 0.0480 |
| Female Rats | Male Rats | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dose (mg/kg) (Group) | Organs | Dose (mg/kg) (Group) | ||||||||||
| 0 (Vehicle Control) | 125 (Low Dose) | 500 (Medium Dose) | 2000 (High Dose) | 0 (Vehicle Control Satellite) | 2000 (High-Dose Satellite) | 0 (Vehicle Control) | 125 (Low Dose) | 500 (Medium Dose) | 2000 (High Dose) | 0 (Vehicle Control Satellite) | 2000 (High-Dose Satellite) | |
| 0/5 | 5/5 | 4/5 | 4/5 | 5/5 | 5/5 | Lung | 2/5 | 5/5 | 5/5 | 5/5 | 5/5 | 5/5 |
| 0/5 | 0/5 | 0/5 | 5/5 | 0/5 | 5/5 | Heart | 0/5 | 0/5 | 0/5 | 5/5 | 0/5 | 5/5 |
| 0/5 | 2/5 | 0/5 | 1/5 | 0/5 | 0/5 | Spleen | 0/5 | 0/5 | 0/5 | 1/5 | 0/5 | 0/5 |
| 0/5 | 0/5 | 0/5 | 4/5 | 0/5 | 5/5 | Stomach | 0/5 | 0/5 | 0/5 | 5/5 | 0/5 | 5/5 |
| 0/5 | 3/5 | 0/5 | 4/5 | 0/5 | 5/5 | IT | 0/5 | 2/5 | 1/5 | 5/5 | 0/5 | 5/5 |
| 0/5 | 5/5 | 3/5 | 4/5 | 0/5 | 5/5 | Liver | 0/5 | 2/5 | 1/5 | 5/5 | 0/5 | 5/5 |
| 0/5 | 5/5 | 3/5 | 4/5 | 0/5 | 5/5 | Kidneys | 0/5 | 0/5 | 0/5 | 5/5 | 0/5 | 5/5 |
| 0/5 | 0/5 | 0/5 | 1/5 | 0/5 | 0/5 | Adrenals | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 |
| 0/5 | 0/5 | 0/5 | 1/5 | 0/5 | 0/5 | Ovaries | N/A | N/A | N/A | N/A | N/A | N/A |
| 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | Uterine Horn | N/A | N/A | N/A | N/A | N/A | N/A |
| N/A | N/A | N/A | N/A | N/A | N/A | Testes | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teh, B.P.; Ahmad, N.; Ibnu Rasid, E.N.; Zolkifli, N.A.; Sastu@Zakaria, U.R.; Mohamed Yusoff, N.; Zulkapli, A.; Japri, N.; Lee, J.C.; Muhammad, H. Herbal-Based Formulation Containing Eurycoma longifolia and Labisia pumila Aqueous Extracts: Safe for Consumption? Pharmaceuticals 2021, 14, 142. https://doi.org/10.3390/ph14020142
Teh BP, Ahmad N, Ibnu Rasid EN, Zolkifli NA, Sastu@Zakaria UR, Mohamed Yusoff N, Zulkapli A, Japri N, Lee JC, Muhammad H. Herbal-Based Formulation Containing Eurycoma longifolia and Labisia pumila Aqueous Extracts: Safe for Consumption? Pharmaceuticals. 2021; 14(2):142. https://doi.org/10.3390/ph14020142
Chicago/Turabian StyleTeh, Bee Ping, Norzahirah Ahmad, Elda Nurafnie Ibnu Rasid, Nor Azlina Zolkifli, Umi Rubiah Sastu@Zakaria, Norliyana Mohamed Yusoff, Azlina Zulkapli, Norfarahana Japri, June Chelyn Lee, and Hussin Muhammad. 2021. "Herbal-Based Formulation Containing Eurycoma longifolia and Labisia pumila Aqueous Extracts: Safe for Consumption?" Pharmaceuticals 14, no. 2: 142. https://doi.org/10.3390/ph14020142
APA StyleTeh, B. P., Ahmad, N., Ibnu Rasid, E. N., Zolkifli, N. A., Sastu@Zakaria, U. R., Mohamed Yusoff, N., Zulkapli, A., Japri, N., Lee, J. C., & Muhammad, H. (2021). Herbal-Based Formulation Containing Eurycoma longifolia and Labisia pumila Aqueous Extracts: Safe for Consumption? Pharmaceuticals, 14(2), 142. https://doi.org/10.3390/ph14020142