Acute Pharmacological Effects of Oral and Intranasal Mephedrone: An Observational Study in Humans
Abstract
:1. Introduction
2. Results
2.1. Physiological Effects
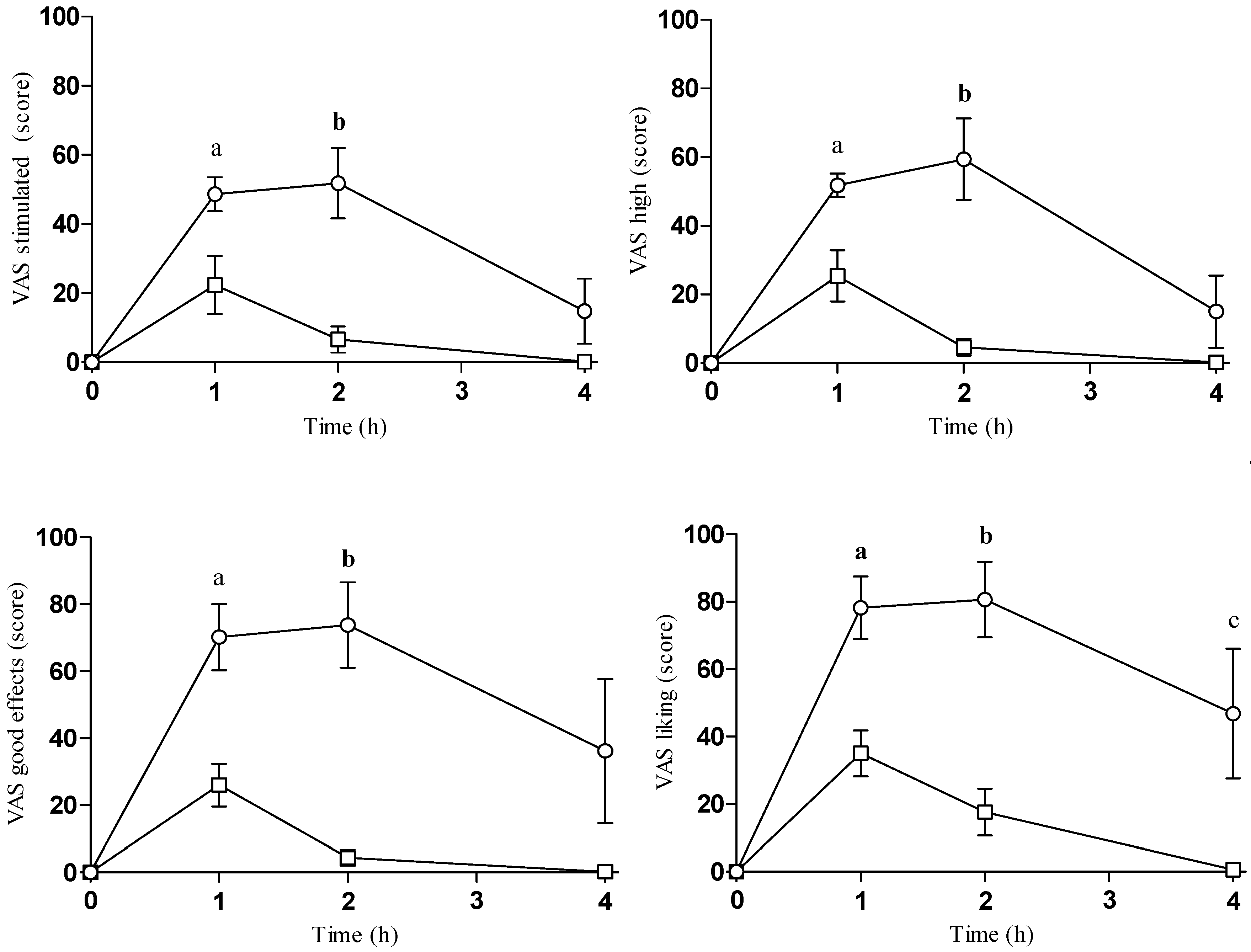
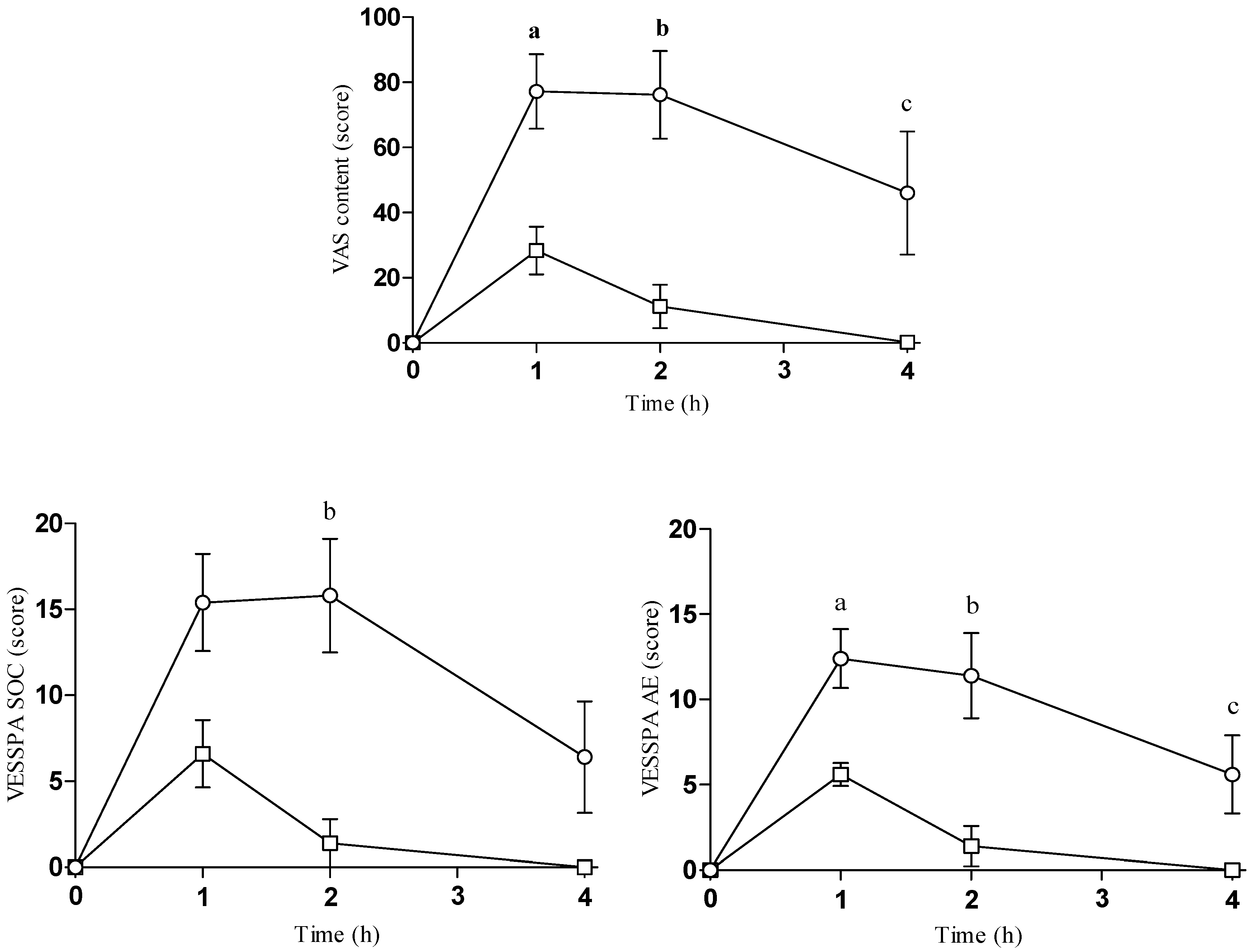
2.2. Subjective Effects
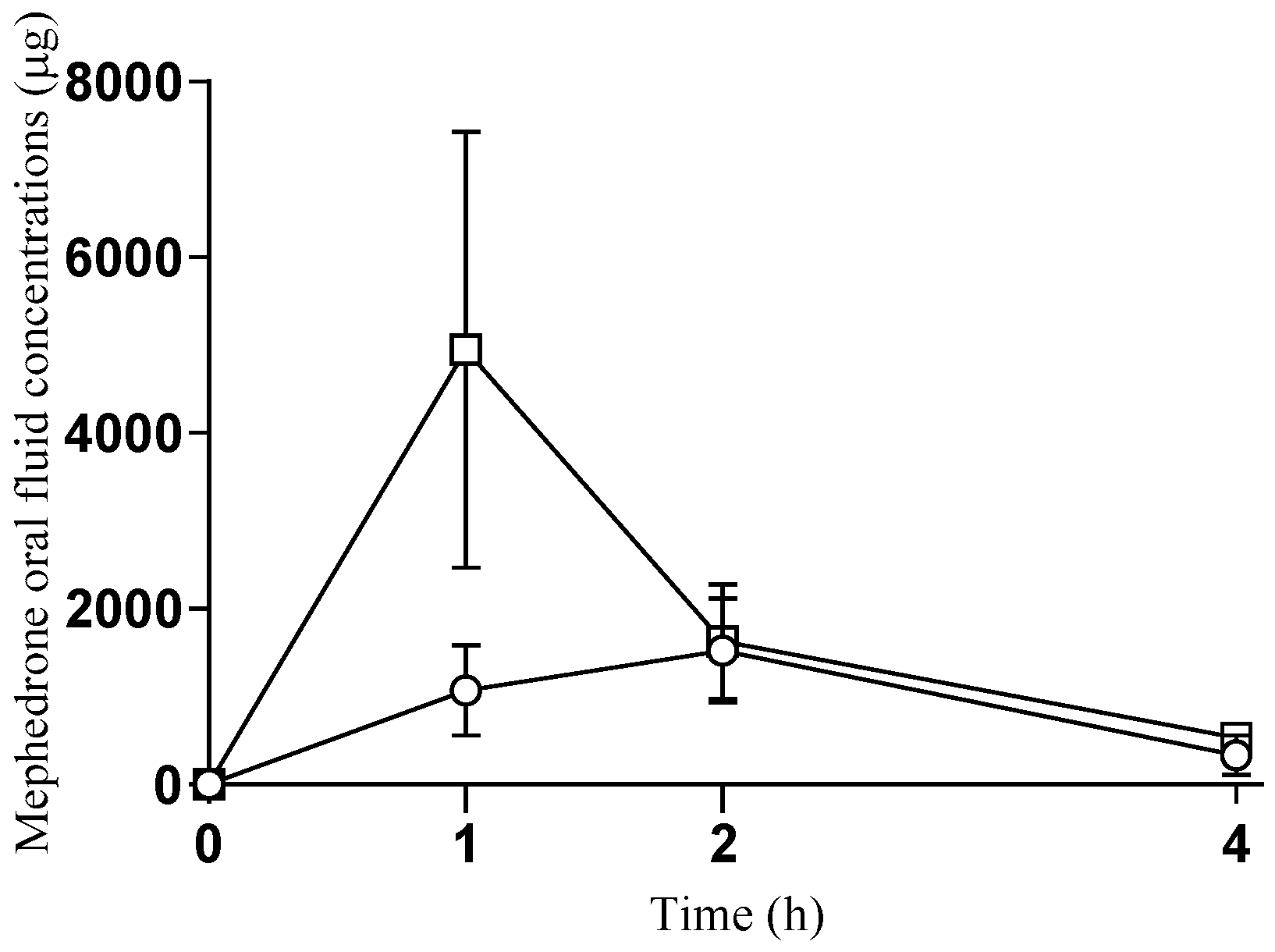
2.3. Oral Fluid Concentrations of Mephedrone
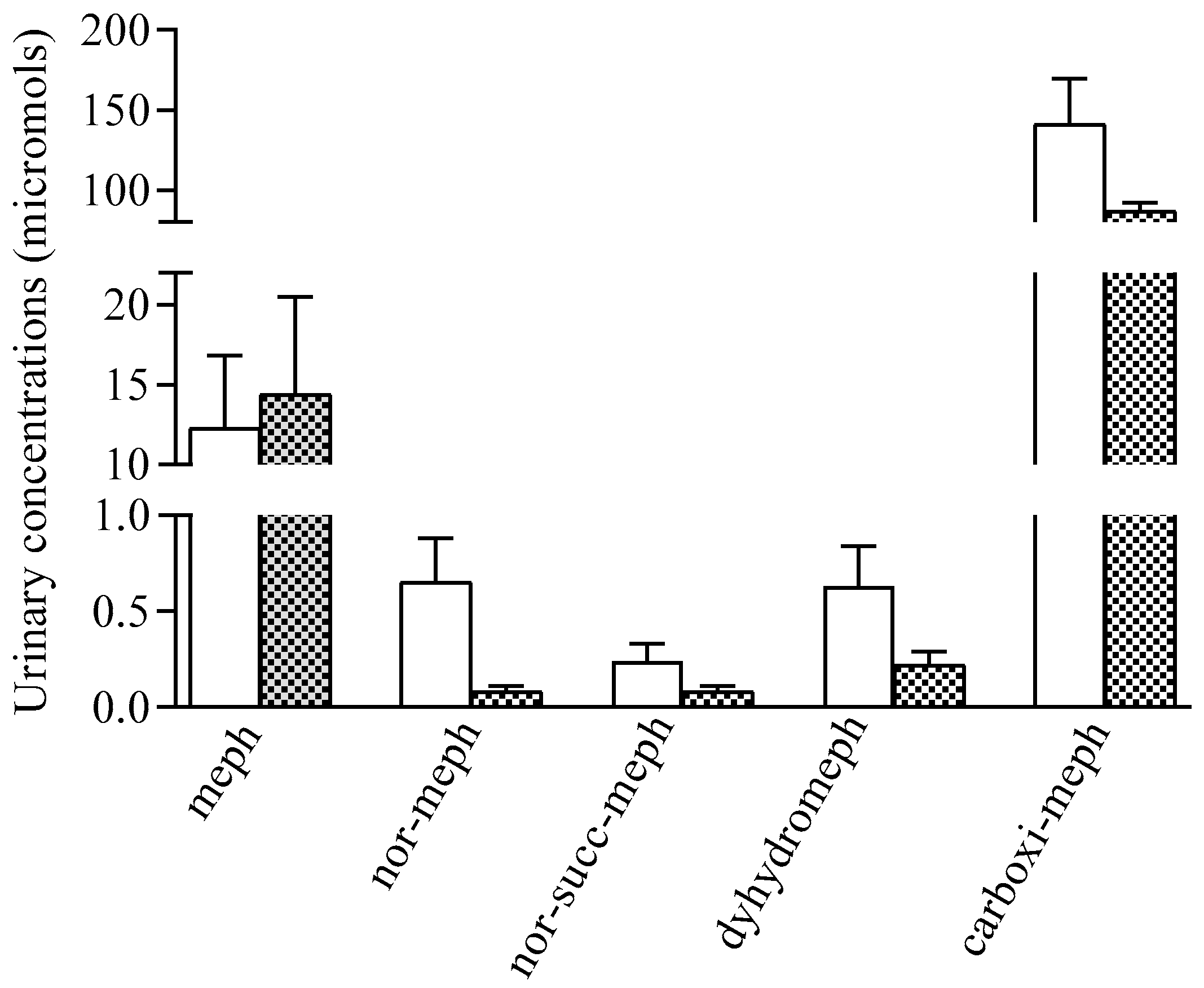
2.4. Urinary Concentrations of Mephedrone and Metabolites
3. Discussion
4. Materials and Methods
4.1. Participants
4.2. Design and Treatments
4.3. Procedures
4.4. Physiological Effects
4.5. Subjective Effects
4.6. Urinary Concentrations of Mephedrone and Metabolites
4.7. Oral Fluid Concentrations of Mephedrone
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Winstock, A.R.; Mitcheson, L.R.; Deluca, P.; Davey, Z.; Corazza, O.; Schifano, F. Mephedrone, new kid for the chop? Addiction 2011, 106, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Mephedrone. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/mephedrone (accessed on 20 November 2020).
- Papaseit, E.; Moltó, J.; Muga, R.; Torrens, M.; de la Torre, R.; Farré, M. Clinical Pharmacology of the Synthetic Cathinone Mephedrone. Curr. Top Behav. Neurosci. 2017, 32, 313–331. [Google Scholar] [PubMed]
- Kehr, J.; Ichinose, F.; Yoshitake, S.; Goiny, M.; Sievertsson, T.; Nyberg, F.; Yoshitake, T. Mephedrone, compared to MDMA (ecstasy) and amphetamine, rapidly increases both dopamine and serotonin levels in nucleus accumbens of awake rats. Br. J. Pharmacol. 2011, 164, 1949–1958. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simmler, L.; Buser, T.; Donzelli, M.; Schramm, Y.; Dieu, L.H.; Huwyler, J.; Chaboz, S.; Hoener, M.C.; Liechti, M.E. Pharmacological characterization of designer cathinones in vitro. Br. J. Pharmacol. 2013, 168, 458–470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Troya, J.; Martínez de Gándara, A.; Ryan, P.; Cuevas, G.; Pardo, V. Mephedrone and chemsex: When it stops being a party and becomes a fatal problem. Int. J. STD AIDS 2019, 30, 1028–1030. [Google Scholar] [CrossRef] [PubMed]
- Lea, T.; Reynolds, R.; De Wit, J. Mephedrone use among same-sex attracted young people in Sydney, Australia. Drug Alcohol Rev. 2011, 30, 438–440. [Google Scholar] [CrossRef]
- Mephedrone—An Update on Current Knowledge. Available online: https://www.drugsandalcohol.ie/12762/1/Mephedrone.pdf (accessed on 15 October 2020).
- Wood, D.M.; Dargan, P.I. Novel Psychoactive Substances. Classification, Pharmacology and Toxicology, 1st ed.; Academic Press: London, UK, 2013; pp. 1–440. [Google Scholar]
- Homman, L.; Seglert, J.; Morgan, M.J. An observational study on the sub-acute effects of mephedrone on mood, cognition, sleep and physical problems in regular mephedrone users. Psychopharmacology 2018, 235, 2609–2618. [Google Scholar] [CrossRef] [Green Version]
- Papaseit, E.; Olesti, E.; de la Torre, R.; Torrens, M.; Farre, M. Mephedrone Concentrations in Cases of Clinical Intoxication. Curr. Pharm. Des. 2017, 23, 5511–5522. [Google Scholar] [CrossRef]
- Busardò, F.P.; Kyriakou, C.; Napoletano, S.; Marinelli, E.; Zaami, S. Mephedrone related fatalities: A review. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 3777–3790. [Google Scholar]
- Cosbey, S.H.; Peters, K.L.; Quinn, A.; Bentley, A. Mephedrone (methylmethcathinone) in toxicology casework: A Northern Ireland perspective. J. Anal. Toxicol. 2013, 37, 74–82. [Google Scholar] [CrossRef] [Green Version]
- Mephedrone. Critical Review Report. Available online: https://www.who.int/medicines/areas/quality_safety/4_12_review.pdf (accessed on 15 October 2020).
- Busardò, F.P.; Kyriakou, C.; Tittarelli, R.; Mannocchi, G.; Pantano, F.; Santurro, A.; Zaami, S.; Baglìo, G. Assessment of the stability of mephedrone in ante-mortem and post-mortem blood specimens. Forensic Sci. Int. 2015, 256, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Olesti, E.; Farré, M.; Carbó, M.L.; Papaseit, E.; Perez-Mañá, C.; Torrens, M.; Yubero-Lahoz, S.; Pujadas, M.; Pozo, Ó.J.; de la Torre, R. Dose-Response Pharmacological Study of Mephedrone and Its Metabolites: Pharmacokinetics, Serotoninergic Effects, and Impact of CYP2D6 Genetic Variation. Clin. Pharmacol. Ther. 2019, 106, 596–604. [Google Scholar] [CrossRef] [PubMed]
- Olesti, E.; Pujadas, M.; Papaseit, E.; Pérez-Mañá, C.; Pozo, Ó.J.; Farré, M.; de la Torre, R. GC-MS Quantification Method for Mephedrone in Plasma and Urine: Application to Human Pharmacokinetics. J. Anal. Toxicol. 2017, 41, 100–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Czerwinska, J.; Parkin, M.C.; Dargan, P.I.; George, C.; Kicman, A.T.; Abbate, V. Stability of mephedrone and five of its phase I metabolites in human whole blood. Drug Test Anal. 2019, 11, 586–594. [Google Scholar] [CrossRef] [Green Version]
- Busardò, F.P.; Gottardi, M.; Pacifici, R.; Varì, M.R.; Tini, A.; Volpe, A.R.; Giorgetti, R.; Pichini, S. Nails Analysis for Drugs Used in the Context of Chemsex: A Pilot Study. J. Anal. Toxicol. 2020, 44, 69–74. [Google Scholar] [CrossRef]
- Martin, M.; Muller, J.F.; Turner, K.; Duez, M.; Cirimele, V. Evidence of mephedrone chronic abuse through hair analysis using GC/MS. Forensic Sci. Int. 2012, 218, 44–48. [Google Scholar] [CrossRef]
- Mayer, F.P.; Cintulova, D.; Pittrich, D.A.; Wimmer, L.; Luethi, D.; Holy, M.; Jaentsch, K.; Tischberger, S.; Gmeiner, G.; Hoener, M.C.; et al. Stereochemistry of phase-1 metabolites of mephedrone determines their effectiveness as releasers at the serotonin transporter. Neuropharmacology 2019, 148, 199–209. [Google Scholar] [CrossRef] [Green Version]
- Wright, M.J., Jr.; Vandewater, S.A.; Angrish, D.; Dickerson, T.J.; Taffe, M.A. Mephedrone (4-methylmethcathinone) and d-methamphetamine improve visuospatial associative memory, but not spatial working memory, in rhesus macaques. Br. J. Pharmacol. 2012, 167, 1342–1352. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Clemente, J.; López-Arnau, R.; Carbó, M.; Pubill, D.; Camarasa, J.; Escubedo, E. Mephedronepharmacokinetics after intravenous and oral administration in rats: Relation to pharmacodynamics. Psychopharmacology 2013, 229, 295–306. [Google Scholar] [CrossRef] [Green Version]
- Aarde, S.M.; Angrish, D.; Barlow, D.J.; Wright, M.J., Jr.; Vandewater, S.A.; Creehan, K.M.; Houseknecht, K.L.; Dickerson, T.J.; Taffe, M.A. Mephedrone (4-methylmethcathinone) supports intravenous self-administration in Sprague-Dawley and Wistar rats. Addict Biol. 2013, 18, 786–799. [Google Scholar] [CrossRef] [Green Version]
- Pedersen, A.J.; Reitzel, L.A.; Johansen, S.S.; Linnet, K. In vitro metabolism studies on mephedrone and analysis of forensic cases. Drug Test Anal. 2013, 5, 430–438. [Google Scholar] [CrossRef] [PubMed]
- Meyer, M.R.; Wilhelm, J.; Peters, F.T.; Maurer, H.H. Beta-keto amphetamines: Studies on the metabolism of the designer drug mephedrone and toxicological detection of mephedrone, butylone, and methylone in urine using gas chromatography-mass spectrometry. Anal. Bioanal Chem. 2010, 397, 1225–1233. [Google Scholar] [CrossRef] [PubMed]
- Khreit, O.I.; Grant, M.H.; Zhang, T.; Henderson, C.; Watson, D.G.; Sutcliffe, O.B. Elucidation of the Phase I and Phase II metabolic pathways of (±)-4’-methylmethcathinone (4-MMC) and (±)-4’-(trifluoromethyl)methcathinone (4-TFMMC) in rat liver hepatocytes using LC-MS and LC-MS. J. Pharm. Biomed. Anal. 2013, 72, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Pozo, Ó.J.; Ibáñez, M.; Sancho, J.V.; Lahoz-Beneytez, J.; Farré, M.; Papaseit, E.; de la Torre, R.; Hernández, F. Mass spectrometric evaluation of mephedrone in vivo human metabolism: Identification of phase I and phase II metabolites, including a novel succinyl conjugate. Drug Metab. Dispos. 2015, 43, 248–257. [Google Scholar] [CrossRef] [Green Version]
- Papaseit, E.; Pérez-Mañá, C.; Mateus, J.A.; Pujadas, M.; Fonseca, F.; Torrens, M.; Olesti, E.; de la Torre, R.; Farré, M. Human Pharmacology of Mephedrone in Comparison with MDMA. Neuropsychopharmacology 2016, 41, 2704–2713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papaseit, E.; Pérez-Mañá, C.; de Sousa FernandesPerna, E.B.; Olesti, E.; Mateus, J.; Kuypers, K.P.; Theunissen, E.L.; Fonseca, F.; Torrens, M.; Ramaekers, J.G.; et al. Mephedrone and Alcohol Interactions in Humans. Front. Pharmacol. 2020, 10, 1588. [Google Scholar] [CrossRef]
- De Sousa Fernandes Perna, E.B.; Papaseit, E.; Pérez-Mañá, C.; Mateus, J.; Theunissen, E.L.; Kuypers, K.; de la Torre, R.; Farré, M.; Ramaekers, J.G. Neurocognitive performance following acute mephedrone administration, with and without alcohol. J. Psychopharmacol. 2016, 30, 1305–1312. [Google Scholar] [CrossRef]
- Czerwinska, J.; Jang, M.; Costa, C.; Parkin, M.C.; George, C.; Kicman, A.T.; Bailey, M.J.; Dargan, P.I.; Abbate, V. Detection of mephedrone and its metabolites in fingerprints from a controlled human administration study by liquid chromatography-tandem mass spectrometry and paper spray-mass spectrometry. Analyst 2020, 145, 3038–3048. [Google Scholar] [CrossRef]
- Czerwinska, J.; Parkin, M.C.; Cilibrizzi, A.; George, C.; Kicman, A.T.; Dargan, P.I.; Abbate, V. Pharmacokinetics of Mephedrone Enantiomers in Whole Blood after a Controlled Intranasal Administration to Healthy Human Volunteers. Pharmaceuticals 2020, 14, 5. [Google Scholar] [CrossRef]
- Jones, L.; Reed, P.; Parrott, A. Mephedrone and 3,4-methylenedioxy-methamphetamine: Comparative psychobiological effects as reported by recreational polydrug users. J. Psychopharmacol. 2016, 30, 1313–1320. [Google Scholar] [CrossRef]
- Harris, D.S.; Boxenbaum, H.; Everhart, E.T.; Sequeira, G.; Mendelson, J.E.; Jones, R.T. The bioavailability of intranasal and smoked methamphetamine. Clin. Pharmacol. Ther. 2003, 74, 475–486. [Google Scholar] [CrossRef] [PubMed]
- Hart, C.L.; Gunderson, E.W.; Perez, A.; Kirkpatrick, M.G.; Thurmond, A.; Comer, S.D.; Foltin, R.W. Acute physiological and behavioral effects of intranasal methamphetamine in humans. Neuropsychopharmacology 2008, 33, 1847–1855. [Google Scholar] [CrossRef] [PubMed]
- Hanson, L.R.; Frey, W.H., 2nd. Intranasal delivery bypasses the blood-brain barrier to target therapeutic agents to the central nervous system and treat neurodegenerative disease. BMC Neurosci. 2008, 9 (Suppl. 3). [Google Scholar] [CrossRef] [PubMed] [Green Version]
- La Maida, N.; Di Trana, A.; Giorgetti, R.; Tagliabracci, A.; Busardò, F.P.; Huestis, M.A. A Review of Synthetic Cathinone-Related Fatalities From 2017 to 2020. Ther. Drug Monit. 2021, 43, 52–68. [Google Scholar] [CrossRef] [PubMed]
- Papaseit, E.; Farré, M.; Pérez-Mañá, C.; Torrens, M.; Ventura, M.; Pujadas, M.; de la Torre, R.; González, D. Acute Pharmacological Effects of 2C-B in Humans: An Observational Study. Front. Pharmacol. 2018, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papaseit, E.; Olesti, E.; Pérez-Mañá, C.; Torrens, M.; Grifell, M.; Ventura, M.; Pozo, O.J.; de Sousa Fernandes Perna, E.B.; Ramaekers, J.G.; de la Torre, R.; et al. Acute Effects of 2C-E in Humans: An Observational Study. Front. Pharmacol. 2020, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- González, D.; Torrens, M.; Farré, M. Acute Effects of the Novel Psychoactive Drug 2C-B on Emotions. BioMed Res. Int. 2015, 2015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lamas, X.; Farré, M.; Llorente, M.; Camí, J. Spanish version of the 49-item short form of the Addiction Research Center Inventory (ARCI). Drug Alcohol Depend. 1994, 35, 203–209. [Google Scholar] [CrossRef]
- Martínez-Riera, R.; Pérez-Mañá, C.; Papaseit, E.; Fonseca, F.; de la Torre, R.; Pizarro, N.; Torrens, M.; Farré, M. Soy Isoflavone Extract Does Not Increase the Intoxicating Effects of Acute Alcohol Ingestion in Human Volunteers. Front. Pharmacol. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Olesti, E.; Pascual, J.A.; Ventura, M.; Papaseit, E.; Farré, M.; de la Torre, R.; Pozo, O.J. LC-MS/MS method for the quantification of new psychoactive substances and evaluation of their urinary detection in humans for doping control analysis. Drug Test Anal. 2020, 12, 785–797. [Google Scholar] [CrossRef] [PubMed]
- Olesti, E.; Farré, M.; Papaseit, E.; Krotonoulas, A.; Pujadas, M.; de la Torre, R.; Pozo, Ó.J. Pharmacokinetics of Mephedrone and Its Metabolites in Human by LC-MS/MS. AAPS J. 2017, 19, 1767–1778. [Google Scholar] [CrossRef] [PubMed]
| Effects | Parameter | Mean ± SD | T-Student | ANOVA | T-Cpoints | |||
|---|---|---|---|---|---|---|---|---|
| Oral | Intranasal | t | p-Value | F | p-Value | |||
| Temperature | Emax | 0.4 ± 0.6 | −0.2 ± 0.2 | 2.477 | 0.038 | |||
| AUC0–4 | 0.8 ± 1.1 | −0.5 ± 0.4 | 2.271 | 0.071 | ||||
| T-C | 3.356 | 0.036 | ||||||
| Intensity | Emax | 48 ± 13 | 25 ± 17 | 2.376 | 0.045 | |||
| AUC0–4 | 114 ± 57 | 37 ± 30 | 2.700 | 0.027 | ||||
| T-C | 3.940 | 0.020 | b | |||||
| Stimulated | Emax | 56 ± 17 | 22 ± 19 | 2.976 | 0.018 | |||
| AUC0–4 | 141 ± 56 | 32 ± 32 | 3.775 | 0.005 | ||||
| T-C | 6.828 | 0.002 | a, b | |||||
| High | Emax | 65 ± 15 | 25 ± 17 | 3.952 | 0.004 | |||
| AUC0–4 | 156 ± 60 | 33 ± 24 | 4.238 | 0.003 | ||||
| T-C | 8.645 | <0.001 | a, b | |||||
| Good effects | Emax | 79 ± 24 | 26 ± 14 | 4.168 | 0.003 | |||
| AUC0–4 | 217 ± 101 | 32 ± 22 | 3.954 | 0.004 | ||||
| T-C | 7.120 | 0.001 | a, b | |||||
| Liking | Emax | 83 ± 21 | 35 ± 15 | 4.110 | 0.003 | |||
| AUC0–4 | 246 ± 94 | 62 ± 34 | 4.114 | 0.003 | ||||
| T-C | 7.330 | 0.001 | a, b, c | |||||
| Content | Emax | 79 ± 25 | 28 ± 16 | 3.737 | 0.006 | |||
| AUC0–4 | 238 ± 108 | 45 ± 37 | 3.766 | 0.005 | ||||
| T-C | 8.210 | 0.001 | a, b, c | |||||
| ARCI-MBG | Emax | 12 ± 1.7 | 6.4 ± 2.4 | 4.575 | 0.002 | |||
| AUC0–4 | 333 ± 14 | 12 ± 6.7 | 3.048 | 0.016 | ||||
| T-C | 1.448 | 0.254 | ||||||
| VESSPA-SOC | Emax | 17 ± 6.0 | 6.6 ± 4.4 | 2.999 | 0.017 | |||
| AUC0–4 | 46 ± 24 | 8.7 ± 8.7 | 3.237 | 0.012 | ||||
| T-C | 8.901 | <0.001 | b | |||||
| VESSPA-ACT | Emax | 13 ± 4.0 | 5.6 ± 1.5 | 3.679 | 0.006 | |||
| AUC0–4 | 35 ± 16 | 7.7 ± 4.9 | 3.589 | 0.007 | ||||
| T-C | 8.266 | 0.027 | a, b, c | |||||
| VESSPA-PS | Emax | 1.8 ± 1.1 | 0.4 ± 0.9 | 2.214 | 0.058 | |||
| AUC0–4 | 2.6 ± 1.7 | 0.4 ± 0.9 | 2.549 | 0.034 | ||||
| T-C | 1.567 | 0.223 | ||||||
| Pharmacokinetic Parameters | Cmax (ng/mL) | AUC0–4 (ng/mL h−1) | Tmax (h) |
|---|---|---|---|
| Oral | 1571 ± 1367 | 3684 ± 3443 | 2 (1–2) |
| Intranasal | 4950 ± 5545 | 7917 ± 7717 | 1 (1–1) |
| p-value | 0.296 | 0.373 | 0.083 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papaseit, E.; Olesti, E.; Pérez-Mañá, C.; Torrens, M.; Fonseca, F.; Grifell, M.; Ventura, M.; de la Torre, R.; Farré, M. Acute Pharmacological Effects of Oral and Intranasal Mephedrone: An Observational Study in Humans. Pharmaceuticals 2021, 14, 100. https://doi.org/10.3390/ph14020100
Papaseit E, Olesti E, Pérez-Mañá C, Torrens M, Fonseca F, Grifell M, Ventura M, de la Torre R, Farré M. Acute Pharmacological Effects of Oral and Intranasal Mephedrone: An Observational Study in Humans. Pharmaceuticals. 2021; 14(2):100. https://doi.org/10.3390/ph14020100
Chicago/Turabian StylePapaseit, Esther, Eulalia Olesti, Clara Pérez-Mañá, Marta Torrens, Francina Fonseca, Marc Grifell, Mireia Ventura, Rafael de la Torre, and Magí Farré. 2021. "Acute Pharmacological Effects of Oral and Intranasal Mephedrone: An Observational Study in Humans" Pharmaceuticals 14, no. 2: 100. https://doi.org/10.3390/ph14020100
APA StylePapaseit, E., Olesti, E., Pérez-Mañá, C., Torrens, M., Fonseca, F., Grifell, M., Ventura, M., de la Torre, R., & Farré, M. (2021). Acute Pharmacological Effects of Oral and Intranasal Mephedrone: An Observational Study in Humans. Pharmaceuticals, 14(2), 100. https://doi.org/10.3390/ph14020100







