Growth Inhibitory Efficacy of Chinese Herbs in a Cellular Model for Triple-Negative Breast Cancer
Abstract
1. Introduction
2. Experimental Model
2.1. Mechanistic Assays
2.2. Test Agents
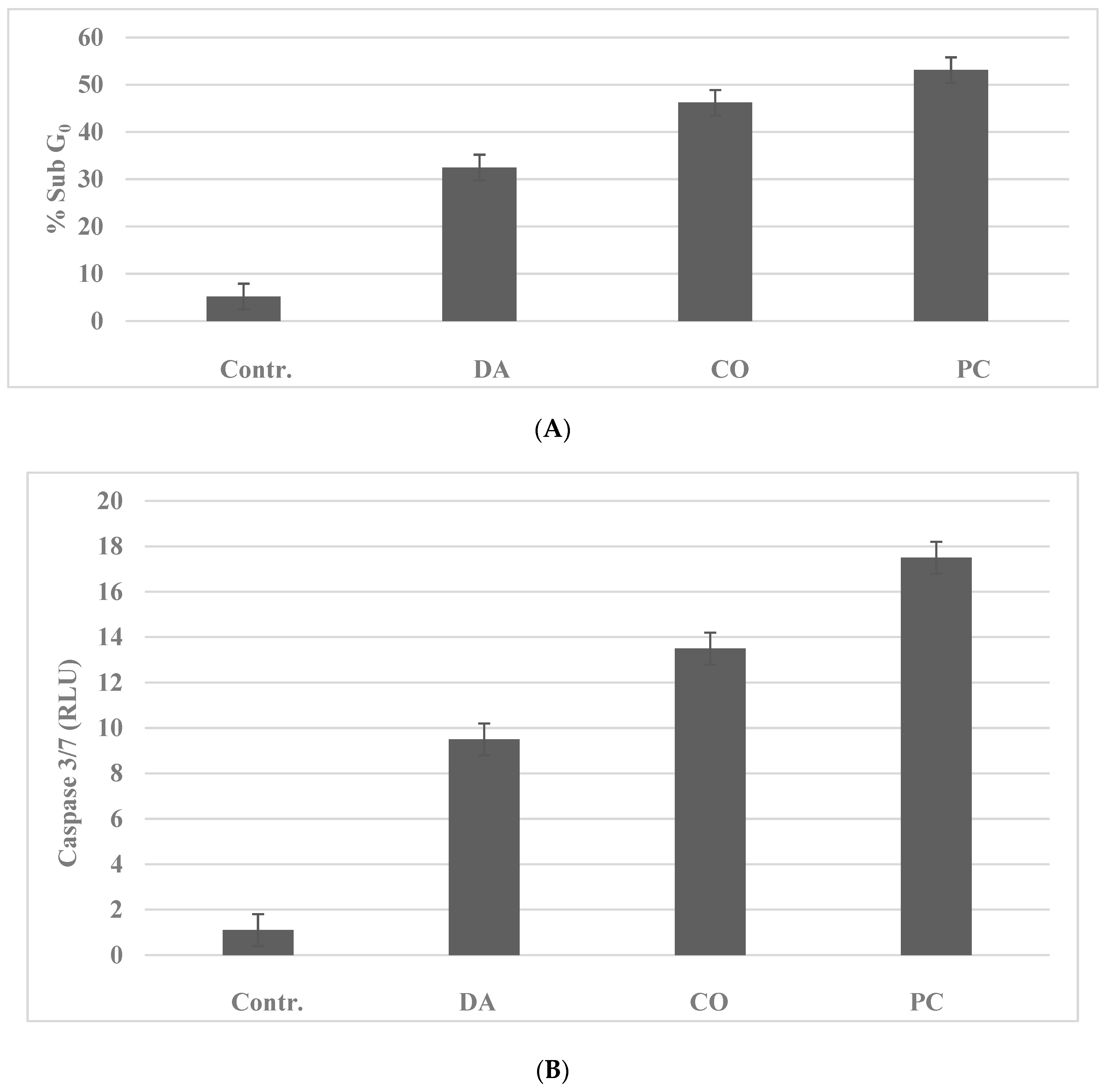
2.3. Anti-Proliferative Effects of Chinese Herbs
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- American Cancer Society. Facts & Figures 2021; American Cancer Society: Atlanta, GA, USA, 2021. [Google Scholar]
- Anders, C.K.; Carey, L.A. Biology, metastatic patterns and treatment of patients with triple-negative breast cancer. Clin. Breast Cancer 2009, 9 (Suppl. S2), S73–S81. [Google Scholar] [CrossRef] [PubMed]
- Sorlie, T.; Perou, C.M.; Tibshirani, R.; Aas, T.; Geisler, S.; Johnsen, H.; Hastie, T.; Eisen, M.B.; Van De Rijn, M.; Jeffrey, S.S.; et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc. Natl. Acad. Sci. USA 2001, 98, 10869–10874. [Google Scholar] [CrossRef] [PubMed]
- Lou, J.; Manning, B.D.; Cantley, J.C. Targeting PI3K/AKT pathway in human cancer: Rationale and promise. Cancer Cell 2003, 4, 257–262. [Google Scholar]
- Dinh, P.; Soutirou, C.; Piccart, M.J. The evolution of treatment strategies: Aiming at the target. Breast 2007, 16 (Suppl. S2), S10–S16. [Google Scholar] [CrossRef]
- Dean, M.; Fojo, T.; Bates, S. Tumor cells and drug resistance. Nat. Rev. Cancer 2005, 5, 275–284. [Google Scholar] [CrossRef]
- Kelloff, G.J.; Boone, C.W.; Steele, V.E.; Crowell, J.B.; Lubet, R.; Doody, L.A.; Greenwald, P. Development of breast cancer chemopreventive drugs. J. Cell. Biochem. 1993, 53, 2–13. [Google Scholar] [CrossRef]
- Steinmetz, K.A.; Potter, J.D. Vegetables, fruits and cancer. I. Epidemiology. Cancer Causes Control 1991, 2, 325–357. [Google Scholar] [CrossRef]
- Nagaprashantha, L.D.; Adhikari, R.; Singhal, J.; Chikara, S.; Awasthi, S.; Horne, D.; Singhal, S.S. Translational opportunities for broad-spectrum natural phytochemicals and targeted agent combinations in breast cancer. Int. J. Cancer 2018, 142, 658–670. [Google Scholar] [CrossRef]
- Mokbel, K.; Mokbel, K. Chemorpvention of breast cancer with vitamins and micronutrients: A concise review. In Vivo 2019, 33, 983–997. [Google Scholar] [CrossRef]
- Telang, N.T.; Li, G.; Sepkovic, D.W.; Bradlow, H.L.; Wong, G.Y.C. Anti-proliferative effects of Chinese herb Cornus officinalis in a cell culture model for estrogen receptor positive clinical breast cancer. Mol. Med. Rep. 2012, 5, 22–28. [Google Scholar] [CrossRef][Green Version]
- Telang NLi, G.; Katdare, M.; Sepkovic, D.W.; Bradlow, H.L.; Wong, G. Inhibitory effects of Chinese nutritional herbs in isogenic breast carcinoma cells with modulated estrogen receptor function. Oncol. Lett. 2016, 12, 3949–3957. [Google Scholar] [CrossRef]
- Telang, N.T.; Li, G.; Katdare, M.; Sepkovic, D.W.; Bradlow, H.L.; Wong, G.Y.C. The nutritional herb Epimedium grandiflorum inhibits the growth in a model for the Luminal A molecular subtype of breast cancer. Oncol. Lett. 2017, 13, 2477–2482. [Google Scholar] [CrossRef]
- Telang, N.T.; Nair, H.B.; Wong, G.Y.C. Growth inhibitory efficacy of Cornus officinalis in a cell culture model for triple-negative breast cancer. Oncol. Lett. 2019, 17, 5261–5266. [Google Scholar] [CrossRef]
- Telang, N.T.; Nair, H.B.; Wong, G.Y.C. Growth inhibitory efficacy of the nutritional herb Psoralea corylifolia in a model for triple-negative breast cancer. Int. J. Funct. Nutr. 2021, 2, 8. [Google Scholar] [CrossRef]
- Telang, N.; Nair, H.B.; Wong, G.Y.C. Growth inhibition by the Chinese nutritional herb Dipsacus asperoides in triple-negative breast cancer. Arch. Breast Cancer 2021, 9. in press. [Google Scholar]
- Tindle, H.A.; Davis, R.B.; Phillips, R.S.; Eisenburg, D.M. Trends in the use of complementary and alternative medicine by US adults: 1997–2002. Altern. Ther. Health Med. 2005, 11, 42–49. [Google Scholar]
- Molassiotis, A.; Scott, J.A.; Kearney, N.; Pud, D.; Magri, M.; Selvekerova, S.; Bruyns, I.; Fernadez-Ortega, P.; Panteli, V.; Margulies, A.; et al. Complementary and alternative medicine use in breast cancer patients in Europe. Support. Care Cancer 2006, 14, 260–267. [Google Scholar] [CrossRef]
- Heilyer, L.K.; Chin, S.; Chui, B.K. The use of complementary and alternative medicines among patients with locally advanced breast cancer: A descriptive study. BMC Cancer 2006, 6, 39–46. [Google Scholar] [CrossRef]
- Ye, J.; Jia, Y.; Ji, K.E.; Saunders, A.J.; Xue, K.; Ji, J.; Mason, M.D.; Jiang, W.G. Traditional Chinese medicine in the prevention and treatment of cancer and cancer metastasis. Oncol. Lett. 2015, 10, 1240–1250. [Google Scholar] [CrossRef]
- Neve, R.M.; Chin, K.; Fridlyand, J.; Yeh, J.; Baehner, F.L.; Fevr, T.; Clark, L.; Bayani, N.; Coppe, J.P.; Tong, F.; et al. A collection of breast cancer cell lines for the study of functionally distinct cancer subtypes. Cancer Cell 2006, 10, 515–527. [Google Scholar] [CrossRef]
- Subik, K.; Lee, J.-F.; Baxter, L.; Strzepak, T.; Costello, D.; Crowley, P.; Xing, L.; Hung, M.C.; Bonfiglio, T.; Hicks, D.G.; et al. The expression patterns of ER, PR, HER-2, CK 5/6, EGFR, Ki 67 and AR by immune-histochemical analysis in breast cancer cell lines. Breast Cancer 2010, 4, 35–41. [Google Scholar]
- Telang, N. Natural phytochemicals as testable therapeutic alternatives for HER-2-enriched breast cancer (Review). World Acad. Sci. J. 2020, 2, 19. [Google Scholar] [CrossRef]
- Castro, N.P.; Rangel, M.C.; Merchant, A.S.; Mac Kinnion, G.; Cuttitta, F.; Salomon, D.S.; Kim, Y.S. Sulphoraphane suppresses the growth of triple-negative breast cancer stem-like cells in vitro and in vivo. Cancer Prev. Res. 2019, 12, 147–158. [Google Scholar] [CrossRef]
- Freedman, V.H.; Shiu, S. Cellular tumorigenicity in nude mice: Correlation with cell growth in semisolid medium. Cell 1974, 3, 355–359. [Google Scholar] [CrossRef]
- Burkhart, D.L.; Sage, J. Cellular mechanisms of tumor suppression by the retinoblastoma gene. Nat. Rev. Cancer 2008, 8, 671–682. [Google Scholar] [CrossRef]
- Otto, T.; Sicinski, P. Cell cycle proteins as promising agents in cancer therapy. Nat. Rev. Cancer 2017, 17, 93–115. [Google Scholar] [CrossRef]
- Cox, L.A.; Chen, G.; Lee, E.Y. Tumor suppressor genes and their role in breast cancer. Breast Cancer Res. Treat. 1994, 32, 19–38. [Google Scholar] [CrossRef]
- Bosco, E.E.; Knudson, E.S. RB in breast cancer: At the crossroads of tumorigenesis and treatment. Cell Cycle 2007, 6, 667–671. [Google Scholar] [CrossRef]
- Sherr, C.J.; Roberts, J.M. CDK inhibitors: Positive and negative regulators of G1-phase progression. Genes Dev. 1999, 13, 1501–1512. [Google Scholar] [CrossRef]
- Boer, K. Impact of palbociclib combinations on treatment of advanced estrogen receptor positive/human epidermal growth factor receptor-2 negative breast cancer. Oncol. Targets Ther. 2016, 11, 6119–6125. [Google Scholar] [CrossRef]
- Klein, M.E.; Kovatcheva, M.; Davis, L.E.; Tap, W.D.; Koff, A. CDK 4/6 inhibitors: The mechanism of action may not be as simple as once thought. Cancer Cell 2018, 34, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Pernas, S.; Tolaney, S.M.; Winer, E.P.; Goel, S. CDK-4/6 inhibition in breast cancer: Current practice and future direction. Ther. Adv. Med. Oncol. 2018, 10, 1758835918786451. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Gao, J.; Wang, M.; Li, M. Potential prospect of CDK4/6inhibitros in triple-negative breast cancer. Cancer Manag. Res. 2021, 13, 5223–5237. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Xu, K.; Gao, F.; Huang, J.; Guan, X. Clinical considerations of CDK4/6 inhibitors in triple-negative breast cancer. Biochem. Biophys. Acta Rev. Cancer 2021, 1876, 188590. [Google Scholar] [CrossRef]
- Asghar, U.S.; Barr, A.R.; Cutts, R.; Beany, M.; Babina, I.; Sampath, D.; Giltnane, J.; Lacap, J.A.; Crocker, L.; Young, A.; et al. Single cell dynamics determines response to CDK 4/6 inhibition in triple-negative breast cancer. Clin. Cancer Res. 2017, 23, 5561–5572. [Google Scholar] [CrossRef]
- Li, T.; Xiong, Y.; Wang, Q.; Chen, F.; Zeng, Y.; Yu, X.; Wang, Y.; Zhou, F.; Zhou, Y. Ribociclib (LEE 011) suppresses cell proliferation and induces apoptosis of MDA-MB-231 cells by inhibiting CDK 4/6-cyclin D-Rb-E2F pathway. Artif. Cells Nanomed. Biotechnol. 2019, 47, 4001–4011. [Google Scholar] [CrossRef]
- Collison, E.A.; Trejo, C.L.; Silvo, J.M.; Gu, S.; Karkola, J.E.; Heiser, L.M.; Charles, R.P.; Rabinovich, B.A.; Hann, B.; Dankort, D.; et al. A central role for RAF-MEK-ERK signaling in the genesis of pancreatic ductal adenocarcinoma. Cancer Discov. 2012, 2, 685–693. [Google Scholar] [CrossRef]
- Mirzoeva, O.K.; Das, D.; Heiser, L.M.; Bhattacharya, S.; Sivak, D.; Gendelman, R.; Bayani, N.; Wang, N.J.; Neve, R.M.; Guan, Y.; et al. Basal subtype and MAPK/ERK kinase (MEK)-phosphoinositide 3-kinase feedback signaling determines susceptibility of breast cancer cells to MEK inhibition. Cancer Res. 2009, 69, 565–572. [Google Scholar] [CrossRef]
- Keton, A.B.; Saltor, E.A.; Piazza, G.A. The RAS-effector interaction as a drug target. Cancer Res. 2017, 77, 221–226. [Google Scholar] [CrossRef]
- Nussinov, R.; Tsai, C.-J.; Jang, H. Oncogenic RAS isoform signaling specificity at the membrane. Cancer Res. 2017, 78, 593–602. [Google Scholar] [CrossRef]
- Yager, R.; Solit, D.B. Overcoming adaptive resistance to KRAS inhibitors through vertical pathway targeting. Clin. Cancer Res. 2020, 26, 1538–1540. [Google Scholar] [CrossRef]
- Jaglanian, A.; Tsiani, T. Rosemary extract inhibits proliferation, survival, AKT and mTOR in triple-negative breast cancer cells. Int. J. Mol. Sci. 2020, 21, 810. [Google Scholar] [CrossRef]
- Wang, R.; Lu, X.; Yu, R. Lycopene inhibits epithelial-mesenchymal transition and promotes apoptosis in oral cancer via PI3K/AKT/m TOR signal pathway. Drug Des. Dev. Ther. 2020, 14, 2461–2471. [Google Scholar] [CrossRef]
- Cotter, T.G.; Lenon, S.V.; Glynn, J.G.; Martin, S.J. Cell death via apoptosis and its relationship to growth, development and differentiation of both tumor and normal cells. Anticancer Res. 1990, 10, 1153–1159. [Google Scholar]
- Stewart, B.W. Mechanism(s) of apoptosis: Integration of genetic, biochemical and cellular indicators. J. Natl. Cancer Inst. 1994, 86, 1286–1296. [Google Scholar] [CrossRef]
- Thompson, C.B. Apoptosis in the pathogenesis and treatment of disease. Science 1995, 267, 1456–1462. [Google Scholar] [CrossRef]
- Metcalf, A.; Streuli, C. Epithelial apoptosis. Bioassays 1997, 19, 711–720. [Google Scholar] [CrossRef]
- Hudis, C.A.; Gianni, L. Triple-negative breast cancer: An unmet medical need. Oncologist 2011, 16 (Suppl. S1), S1–S11. [Google Scholar] [CrossRef]
- Kim, S.H.; Singh, S.V. Role of Kruppel-like-factor-4/p21CIP1 axis in breast cancer stem-like cell inhibition by benzyl isothiocyanate. Cancer Prev. Res. 2019, 12, 125–134. [Google Scholar] [CrossRef]
- Cohen, I.; Tagliaferri, M.; Tripathy, D. Traditional Chinese medicine in the treatment of breast cancer. Semin. Oncol. 2002, 6, 653–674. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, Q.; Yu, L.; Zhu, J.; Cao, Y.; Gao, X. The signaling pathways and targets of traditional Chinese medicine and natural medicine in triple-negative breast cancer. J. Ethnopharmacol. 2021, 264, 113249. [Google Scholar] [CrossRef]
- Dou, J.-W.; Shang, R.-G.; Lei, X.-Q.; Li, K.-L.; Guo, Z.-Z.; Ye, K.; Yang, X.-J.; Li, Y.-W.; Zhou, Y.-Y.; Yao, J.; et al. Total saponins of Bolbostemma paniculatum (maxim.) Franquet exert anti-tumor activity against MDA-MB-231 human breast cancer cells via inhibiting PI3K/Akt/mTOR pathway. BMC Complement. Altern. Med. 2019, 19, 304. [Google Scholar] [CrossRef]
- Liu, L.; Yan, J.; Cao, Y.; Yan, Y.; Shen, X.; Yu, B.; Tao, L.; Wang, S. Proliferation, migration and invasion in triple-negative breast cancer cells are suppressed by berbamine via the PI3K/Akt/MDM2/p53 and PI3K/Akt/mTOR signaling pathways. Oncol. Lett. 2021, 21, 70. [Google Scholar] [CrossRef]
- Hong, Y.; Fan, D. Ginsenoside Rk1 induces cell cycle arrest and apoptosis in MDA-MB-231 triple-negative breast cancer cells. Toxicology 2019, 418, 22–31. [Google Scholar] [CrossRef]
- Zeng, Y.; Mao, J.; Wang, X.; Yin, B.; Shen, Z.; Di, C.; Gu, W.; Wu, M. Mechanism for ginsenoside Rh2-induced apoptosis of triple-negative breast cancer MDA-MB-231 cells. Clin. Exp. Obstet. Gynecol. 2020, 47, 99–104. [Google Scholar]
- Nakhjavani, M.; Palethorpe, H.M.; Tomita, Y.; Smith, E.; Price, T.J.; Yool, A.J.; Pei, J.V.; Townsend, A.R.; Hardingham, J.E. Stereoselective anti-cancer activities of ginsenoside Rg3 on triple-negative breast cancer models. Pharmaceuticals 2019, 12, 117. [Google Scholar] [CrossRef]
- Jin, Y.; Huynh, D.T.N.; Myung, C.-S.; Heo, K.-S. Ginsenoside Rh1 prevents migration and invasion through mitochondrial ROS-mediated inhibition of STAT3/NFkB signaling in MDA-MB-231 cells. Int. J. Mol. Sci. 2021, 22, 10458. [Google Scholar] [CrossRef]
- Peng, B.; He, R.; Xu, Q.; Yang, Y.; Hu, Q.; Hou, H.; Liu, X.; Li, J. Ginsenoside 20(S)-protopanaxadiol inhibits triple-negative metastasis in vivo by targeting EGFR-mediated MAPK pathway. Pharmacol. Res. 2019, 142, 1–13. [Google Scholar] [CrossRef]
- Nakhjavani, M.; Smith, E.; Palethorpe, H.M.; Tomita, Y.; Yeo, K.; Price, T.J.; Townsend, A.R.; Hardingham, J.E. Anti-cancer effects of an optimized combination of Ginsenoside Rg3 epimers on triple-negative breast cancer models. Pharmaceuticals 2021, 14, 633. [Google Scholar] [CrossRef]
- Wang, P.; Song, D.; Wan, D.; Li, L.; Mei, W.; Li, X.; Han, L.; Zhu, X.; Yang, L.; Cai, Y.; et al. Ginsenoside panaxatriol reverses TNBC paclitaxel resistance by inhibiting the IRAK1/NFkB and ERK pathways. Biochem. Biophys. Mol. Biol. 2020, 8, e9281. [Google Scholar]
- Liu, S.; Huang, J.; Gao, F.; Yin, Z.; Zhang, R. Ginsenoside RG1 augments doxorubicin-induced apoptotic cell death in MDA-MB-231 breast cancer cell lines. J. Biochem. Mol. Toxicol. 2021, e22945. [Google Scholar] [CrossRef] [PubMed]
- Bruna, A.; Rouda, O.M.; Greenwood, A.; Batra, A.S.; Callari, M.; Batra, R.N.; Pogrebniak, K.; Sandoval, J.; Cassidy, J.W.; Tufegdzic-Vidakovic, A.; et al. A biobank of breast cancer explants with preserved intra-tumor heterogeneity to screen anti-cancer compounds. Cell 2016, 167, 260–274.e22. [Google Scholar] [CrossRef] [PubMed]
- Sachs, N.; de Ligt, J.; Kopper, O.; Gogola, E.; Bounova, G.; Weeber, F.; Balgobind, A.V.; Wind, K.; Gracanin, A.; Begthel, H.; et al. A living biobank of breast cancer organoids captures disease heterogeneity. Cell 2018, 172, 373–386.e10. [Google Scholar] [CrossRef] [PubMed]
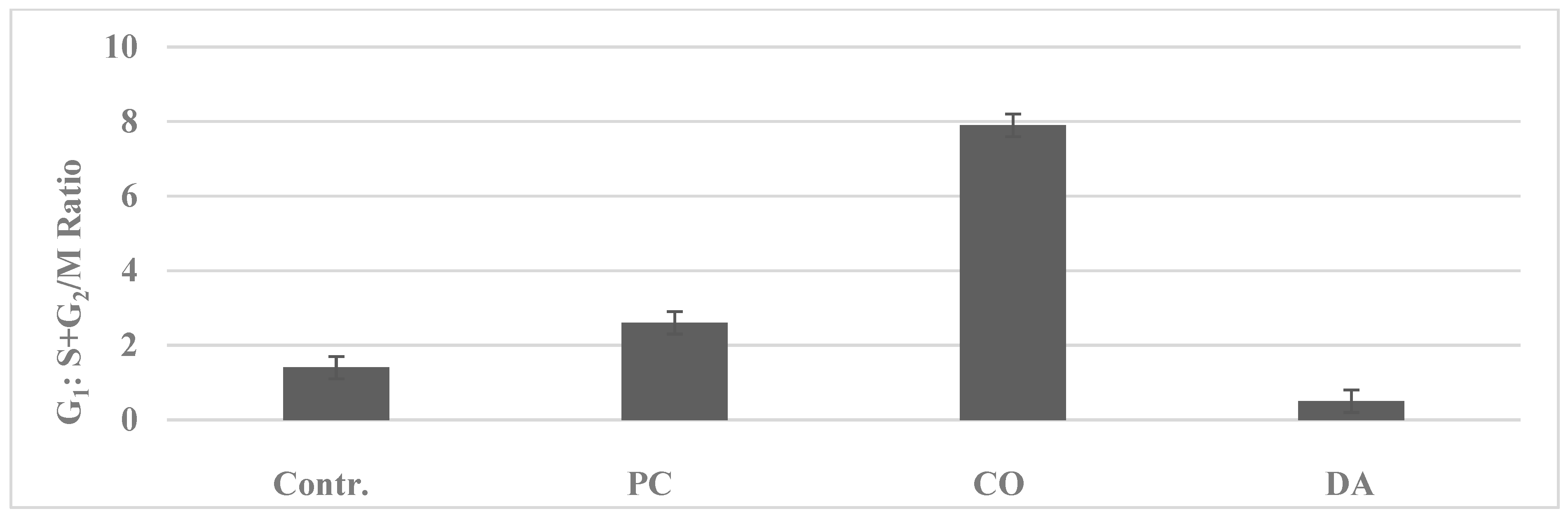
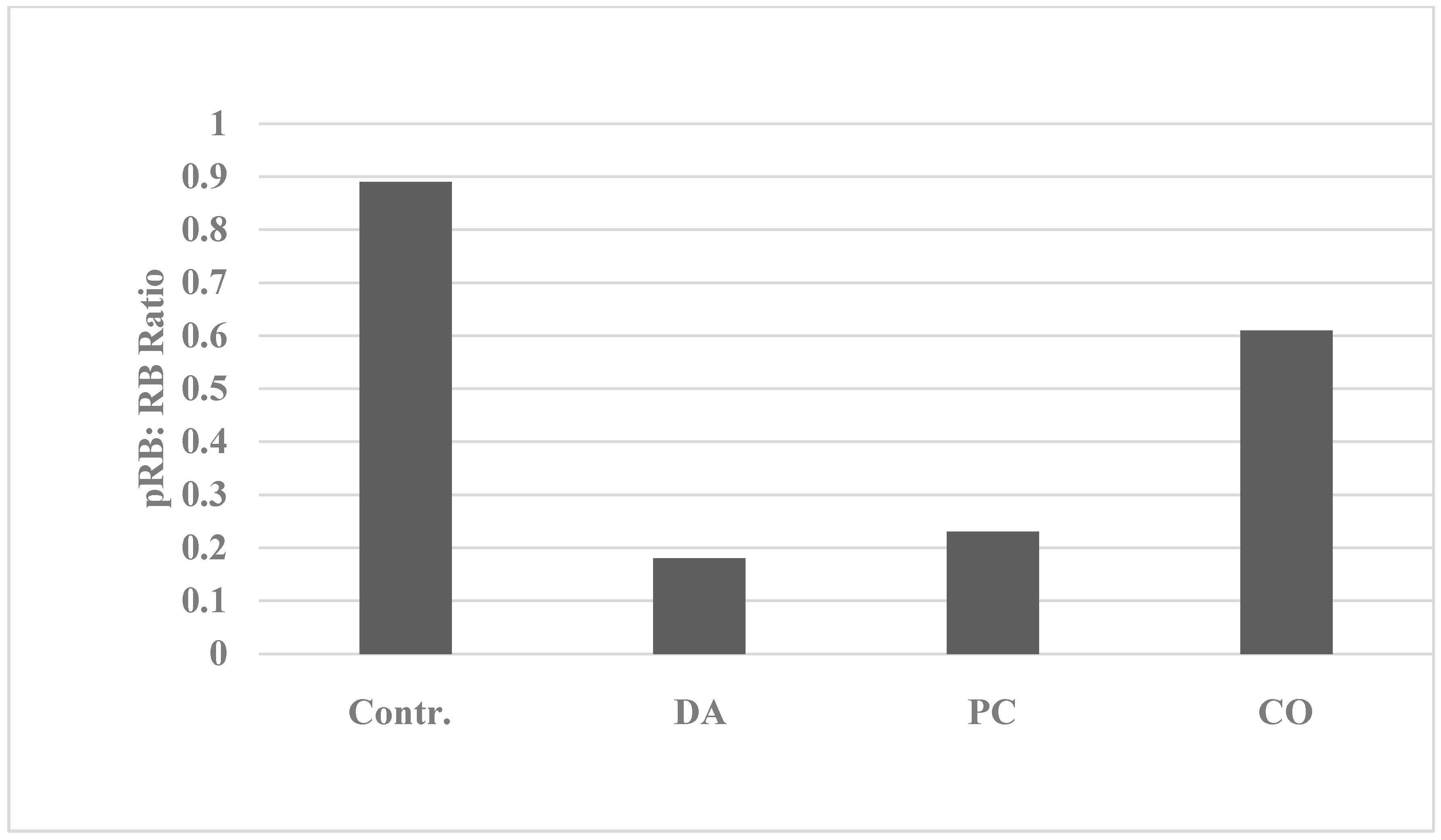
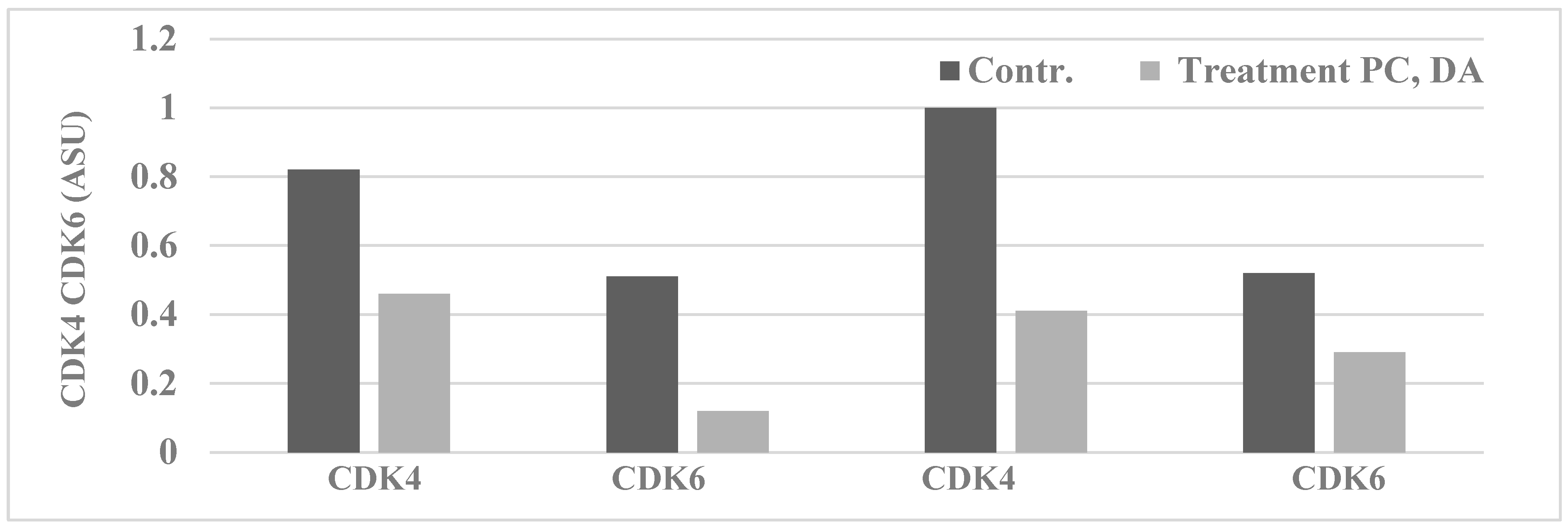
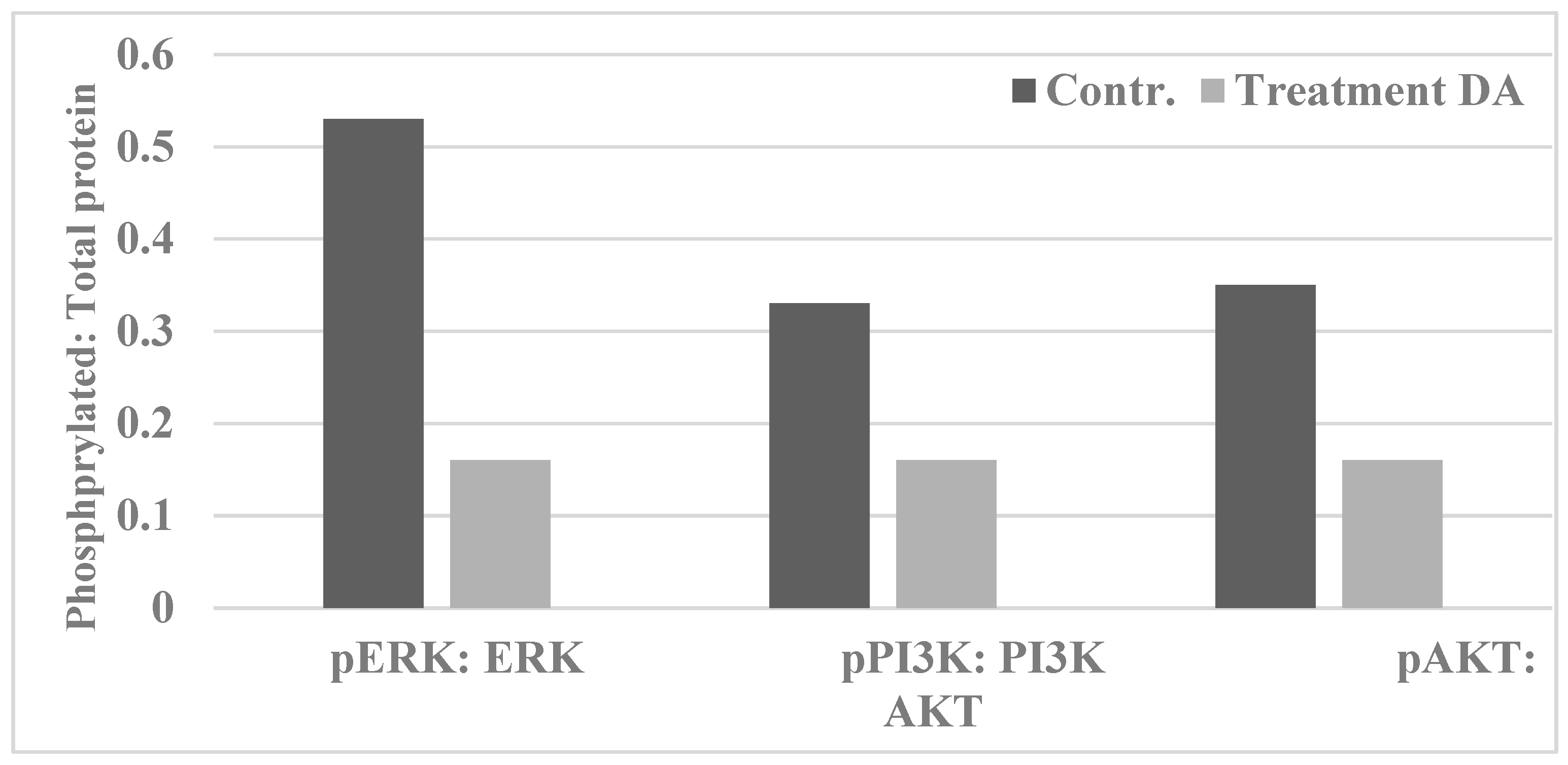
| Proliferation End Point | Cellular Model | p-Value | Relative to 184-B5 | |
|---|---|---|---|---|
| 184-B5 | MDA-MB-231 | |||
| PDT (h) a | 34.1 ± 1.7 | 15.0 ± 2.2 | 0.040 | −56.0% |
| Sat. Den. (×105) b | 23.7 ± 1.3 | 32.9 ± 2.3 | 0.048 | +38.8% |
| G1: S + G2/M c | 1.8 ± 0.3 | 0.6 ± 0.3 | 0.030 | −66.7% |
| AI Colony Number d | 1.2 ± 1.0 | 750.0 ± 76.0 | <0.001 | +624× |
| Herb | Origin | Bio-Active Agent | Reference |
|---|---|---|---|
| Cornus officinalis (CO) | fruit | anthocyanins | [11,14] |
| Cuscuta sinensis (CS) | seed | flavonoids | [12,19], personal communication |
| Dipsacus asperoides (DA) | root | saponins | [12], personal communication |
| Drynaria firtunie (DF) | bark | flavonoids | [12,19,24], personal communication |
| Epimedium grandiflorum (EG) | Leaf, stem | icariin, icaritin, prenylflavone | [12,13] |
| Eucommia ulmoides (EU) | bark | lignans, tanins, saponins | [12,19] |
| Lycium barbarum (LB) | bark | lignans, tanins, saponins | [12,19], personal communication |
| Ligustrum lucidum (LL) | fruit | terpenoids | [12] |
| Psoralea corylifolia (PC) | seed | Coumarins, flavonoids, meroterpenes | [15] |
| Smilax glabra (SG) | bark | Falvonoids, saponins | [24], personal communication |
| Taraxacum mangolicum (TM) | leaf | flavonoids | [24], personal communocation |
| Viola yeodoensis (VY) | leaf | terpenoids | [24], personal communication |
| Herb | Inhibitory Concentration (IC50 µg/mL) a |
|---|---|
| CO | 1.0 ± 0.3 |
| PC | 6.0 ± 1.1 |
| DA | 15.0 ± 3.7 |
| LB | 17.7 ± 4.5 |
| VY | 18.0 ± 4.5 |
| EU | 20.9 ± 5.2 |
| LL | 87.6 ± 21.7 |
| CS | 90.4 ± 22.4 |
| EG | 102.4 ± 25.6 |
| DF | 650.0 ± 24.4 |
| TM | 800.0 ± 20.7 |
| SG | >1000 |
| Herb | Inhibitory Concentration (IC90 µg/mL) a |
|---|---|
| CO | 5.0 ± 1.5 |
| PC | 20.0 ± 3.5 |
| DA | 30.0 ± 4.0 |
| LB | 32.0 ± 4.0 |
| EU | 38.0 ± 5.0 |
| VY | 200.0 ± 17.5 |
| LL | 274.0 ± 24.0 |
| CS | 671.0 ± 26.0 |
| DF | 1000.0 ± 13.6 |
| EG, SG, TM | >1000 |
| Treatment | AI Colony Number a | p-Value | % Inhibition |
|---|---|---|---|
| Control | 750 ± 76 | ---- | |
| DA | 55 ± 6 | 0.001 | 92.7 |
| PC | 69 ± 7 | 0.001 | 90.8 |
| CO | 120 ± 12 | 0.001 | 84.0 |
| LL | 339 ± 34 | 0.042 | 54.8 |
| DF | 372 ± 38 | 0.042 | 50.4 |
| SG | 373 ± 37 | 0.042 | 50.3 |
| LB | 373 ± 36 | 0.042 | 50.3 |
| VY | 375 ± 36 | 0.042 | 50.3 |
| EU | 396 ± 40 | 0.046 | 47.2 |
| TM | 396 ± 38 | 0.046 | 47.2 |
| CS | 649 ± 66 | NS | 13.5 |
| EG | 716 ± 73 | NS | 4.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Telang, N.T.; Nair, H.B.; Wong, G.Y.C. Growth Inhibitory Efficacy of Chinese Herbs in a Cellular Model for Triple-Negative Breast Cancer. Pharmaceuticals 2021, 14, 1318. https://doi.org/10.3390/ph14121318
Telang NT, Nair HB, Wong GYC. Growth Inhibitory Efficacy of Chinese Herbs in a Cellular Model for Triple-Negative Breast Cancer. Pharmaceuticals. 2021; 14(12):1318. https://doi.org/10.3390/ph14121318
Chicago/Turabian StyleTelang, Nitin T., Hareesh B. Nair, and George Y. C. Wong. 2021. "Growth Inhibitory Efficacy of Chinese Herbs in a Cellular Model for Triple-Negative Breast Cancer" Pharmaceuticals 14, no. 12: 1318. https://doi.org/10.3390/ph14121318
APA StyleTelang, N. T., Nair, H. B., & Wong, G. Y. C. (2021). Growth Inhibitory Efficacy of Chinese Herbs in a Cellular Model for Triple-Negative Breast Cancer. Pharmaceuticals, 14(12), 1318. https://doi.org/10.3390/ph14121318






