Bioactivity Potential of Aesculus hippocastanum L. Flower: Phytochemical Profile, Antiradical Capacity and Protective Effects on Human Plasma Components under Oxidative/Nitrative Stress In Vitro
Abstract
:1. Introduction
2. Results and Discussion
2.1. Qualitative UHPLC–PDA–ESI–TQ–MS/MS Profiling
2.2. Quantitative HPLC–PDA Analysis
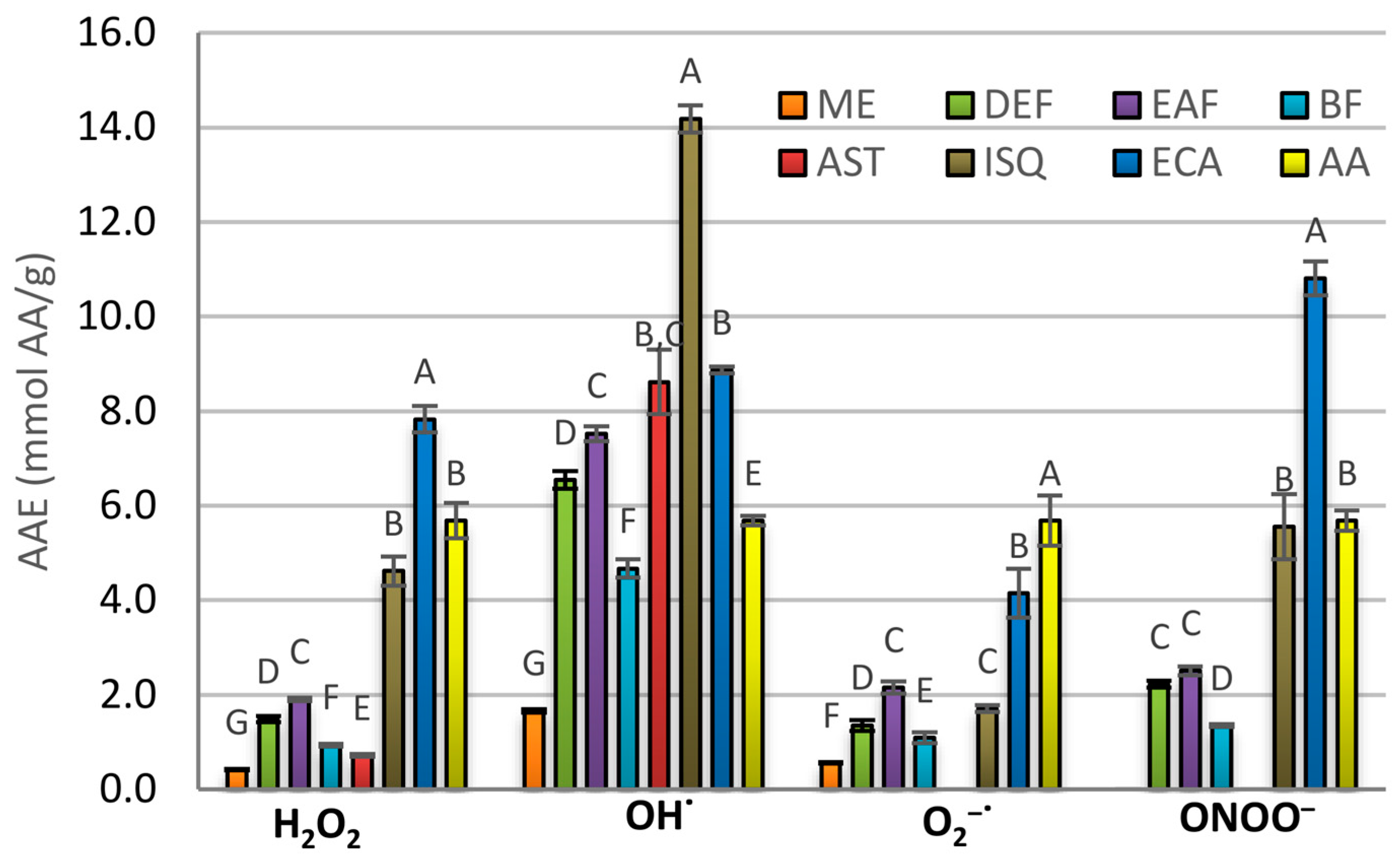
2.3. Antioxidant Activity in Chemical Models
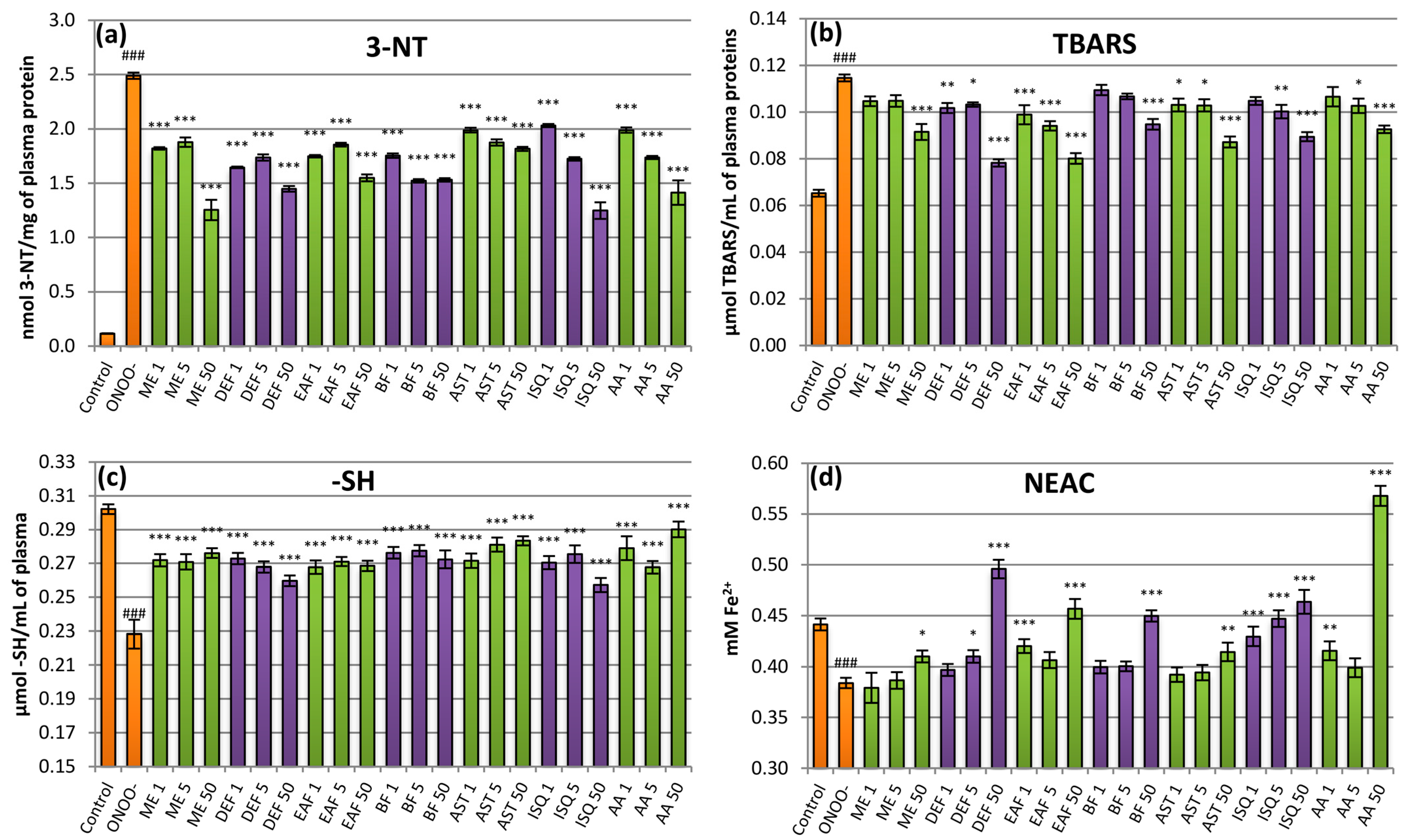
2.4. Antioxidant Activity in Plasma Model
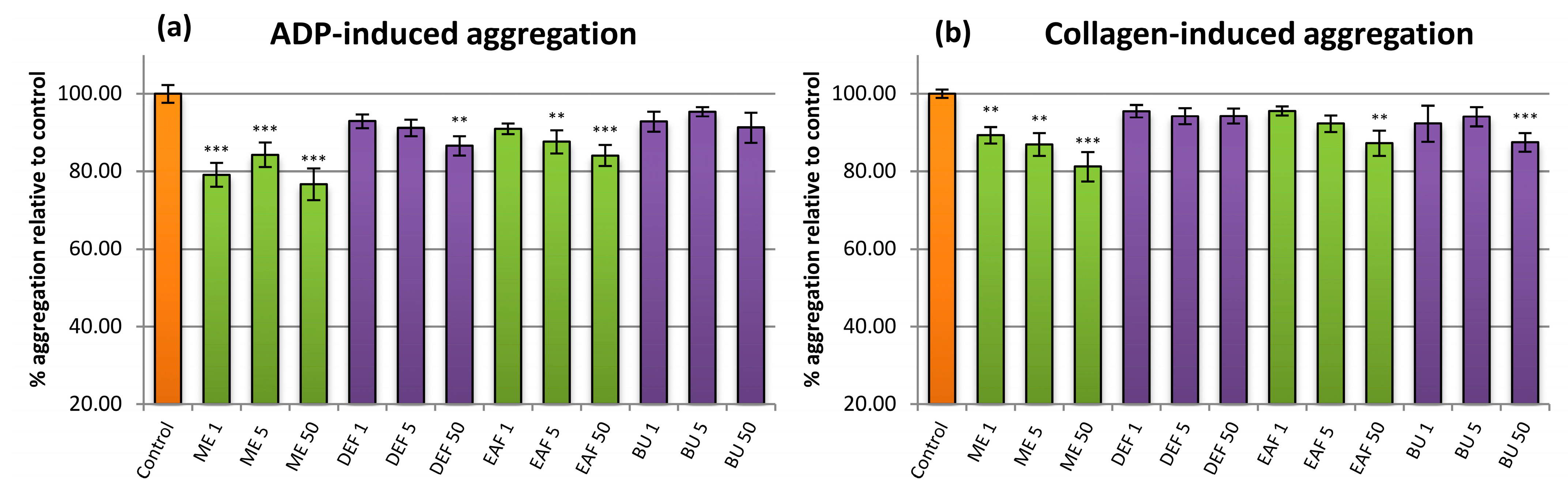
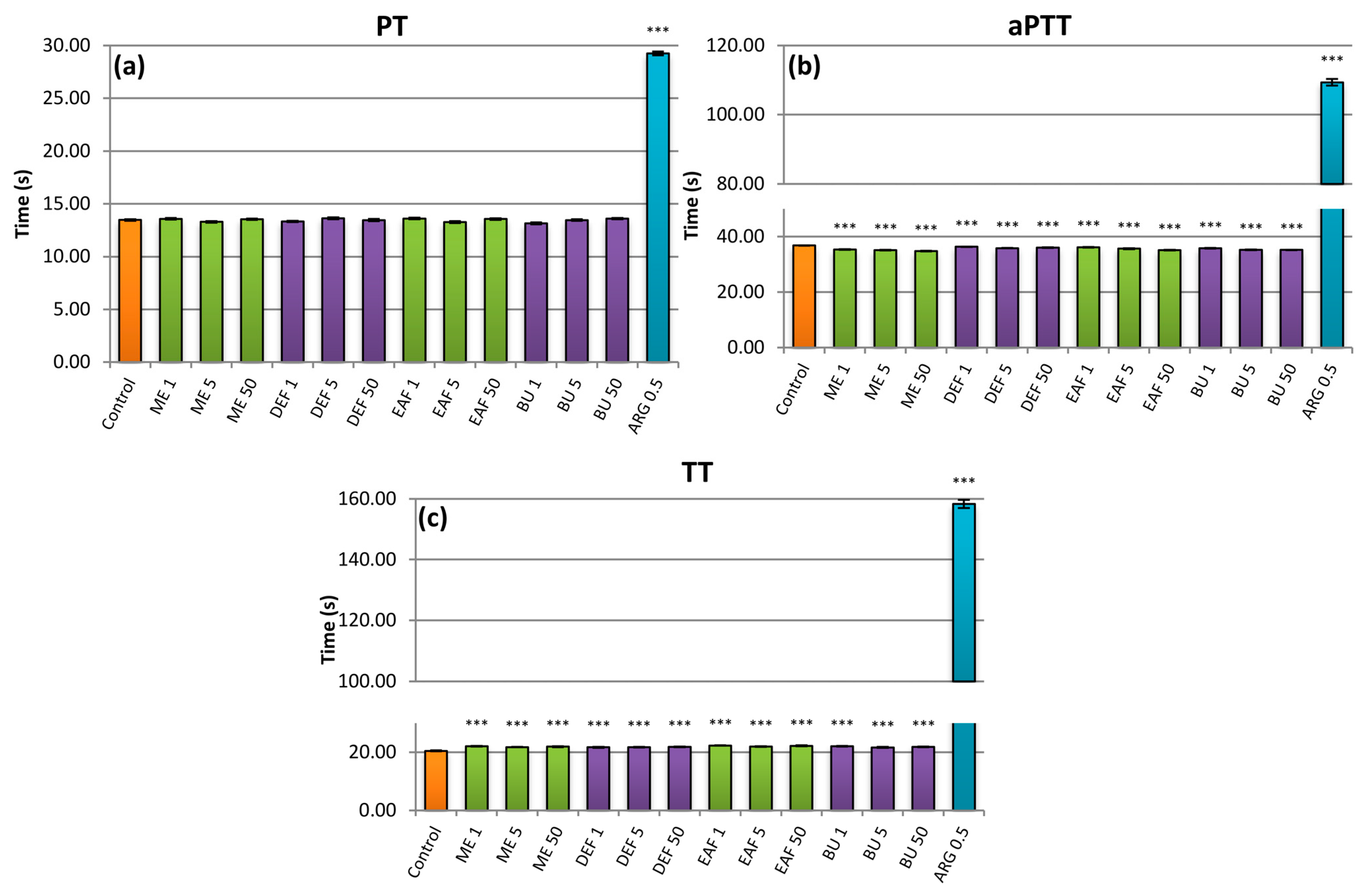
2.5. Influence on Plasma Haemostasis Parameters
3. Materials and Methods
3.1. General
3.2. Plant Material
3.3. Preparation of the Extracts
3.4. Qualitative UHPLC–PDA–ESI–TQ–MS/MS Profiling
3.5. Quantitative HPLC–PDA Analysis
3.6. Antioxidant Activity in Chemical Models
3.7. Preparation of Plasma Samples
3.8. Antioxidant Activity in Human Plasma Model
3.9. Influence on Plasma Hemostasis Parameters
3.10. Statistical analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, Z.; Li, S.; Lian, X.; Temple, A.; State, S.F.A. An overview of genus Aesculus L.: Ethnobotany, phytochemistry, and pharmacological activities. Pharm. Crop. 2010, 1, 24–51. [Google Scholar] [CrossRef] [Green Version]
- Lamer-Zarawska, E.; Kowal-Gierczak, B.; Niedworok, J. Fitoterapia i Leki Roślinne; Wydawnictwo Lekarskie PZWL: Warszawa, Poland, 2007. [Google Scholar]
- Dudek-Makuch, M.; Studzińska-Sroka, E. Horse Chestnut—Efficacy and safety in chronic venous insufficiency: An overview. Braz. J. Pharmacogn. 2015, 25, 533–541. [Google Scholar] [CrossRef] [Green Version]
- Owczarek, A.; Kolodziejczyk-Czepas, J.; Woźniak-Serwata, J.; Magiera, A.; Kobiela, N.; Wąsowicz, K.; Olszewska, M.A. Potential activity mechanisms of aesculus hippocastanum bark: Antioxidant effects in chemical and biological in vitro models. Antioxidants 2021, 10, 995. [Google Scholar] [CrossRef] [PubMed]
- Dudek-Makuch, M.; Matławska, I. Flavonoids from the flowers of Aesculus hippocastanum. Acta Pol. Pharm. 2011, 68, 403–408. [Google Scholar]
- Dudek-Makuch, M.; Studzinska-Sroka, E.; Korybalska, K.; Czepulis, N.; Luczak, J.; Rutkowski, R.; Marczak, L.; Dlugaszewska, J.; Grabowska, K.; Stobiecki, M.; et al. Biological activity of Aesculus hippocastanum flower extracts on vascular endothelial cells cultured in vitro. Phytochem. Lett. 2019, 30, 367–375. [Google Scholar] [CrossRef]
- Guzik, B.; Chwała, M.; Matusik, P.; Ludew, D.; Skiba, D.; Wilk, G.; Mrowiecki, W.; Batko, B.; Cencora, A.; Kapelak, B.; et al. Mechanisms of increased vascular superoxide production in human varicose veins. Pol. Arch. Med. Wewn. 2011, 121, 279–286. [Google Scholar] [CrossRef] [Green Version]
- Krzyściak, W.; Kózka, M. Generation of reactive oxygen species by a sufficient, insufficient and varicose vein wall. Acta Biochim. Pol. 2011, 58, 89–94. [Google Scholar] [CrossRef] [Green Version]
- Gwozdzinski, L.; Pieniazek, A.; Bernasinska-Slomczewska, J.; Hikisz, P.; Gwozdzinski, K. Alterations in the Plasma and Red Blood Cell Properties in Patients with Varicose Vein: A Pilot Study. Cardiol. Res. Pract. 2021, 2021, 5569961. [Google Scholar] [CrossRef]
- Glowinski, J.; Glowinski, S. Generation of reactive oxygen metabolites by the varicose vein wall. Eur. J. Vasc. Endovasc. Surg. 2002, 23, 550–555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karatepe, O.; Unal, O.; Ugurlucan, M.; Kemik, A.; Karahan, S.; Aksoy, M.; Kurtoglu, M. The impact of valvular oxidative stress on the development of venous stasis ulcer valvular oxidative stress and venous ulcers. Angiology 2010, 61, 283–288. [Google Scholar] [CrossRef]
- Lichota, A.; Gwozdzinski, L.; Gwozdzinski, K. Therapeutic potential of natural compounds in inflammation and chronic venous insufficiency. Eur. J. Med. Chem. 2019, 176, 68–91. [Google Scholar] [CrossRef]
- Poredos, P. Involvement of varicose veins in superficial venous thrombosis. Anat. Physiol. 2016, 6, 13–15. [Google Scholar] [CrossRef]
- Leite, P.M.; Martins, M.A.P.; Castilho, R.O. Review on mechanisms and interactions in concomitant use of herbs and warfarin therapy. Biomed. Pharmacother. 2016, 83, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Goel, N. Phenolic acids: Natural versatile molecules with promising therapeutic applications. Biotechnol. Rep. 2019, 24, e00370. [Google Scholar] [CrossRef]
- Kite, C.; Larsson, S.; Veitch, N.C.; Porter, E.A.; Ding, N.; Simmonds, M.S.J.; Gardens, R.B.; Tw, S.; Kingdom, U. Acyl spermidines in inflorescence extracts of elder. J. Agric. Food Chem. 2013, 61, 3501–3508. [Google Scholar] [CrossRef] [PubMed]
- Oszmiański, J.; Kalisz, S.; Aneta, W. The content of phenolic compounds in leaf tissues of white (Aesculus hippocastanum L.) and red horse chestnut (Aesculus carea H.) colonized by the horse chestnut leaf miner (Cameraria ohridella Deschka & Dimić). Molecules 2014, 19, 14625–14636. [Google Scholar] [CrossRef] [Green Version]
- Owczarek, A.; Kłys, A.; Olszewska, M.A. A validated 1H qNMR method for direct and simultaneous quantification of esculin, fraxin and (–)-epicatechin in Hippocastani cortex. Talanta 2019, 192, 263–269. [Google Scholar] [CrossRef]
- Santos-Buelga, C.; Kolodziej, H.; Treutter, D. Procyanidin trimers possessing a doubly linked structure from Aesculus hippocastanum. Phytochemistry 1995, 38, 499–504. [Google Scholar] [CrossRef]
- Morimoto, S.; Nonaka, G.I.; Nishioka, I. Tannins and related compounds. LIX. Aesculitannins, novel proanthocyanidins with doubly-bonded structures from Aesculus hippocastanum L. Chem. Pharm. Bull. 1987, 35, 4717–4729. [Google Scholar] [CrossRef] [Green Version]
- Kapusta, I.; Janda, B.; Szajwaj, B.; Stochmal, A.; Piacente, S.; Pizza, C.; Franceschi, F.; Franz, C.; Oleszek, W. Flavonoids in horse chestnut (Aesculus hippocastanum) seeds and powdered waste water byproducts. J. Agric. Food Chem. 2007, 55, 8485–8490. [Google Scholar] [CrossRef]
- Hübner, G.; Wray, V.; Nahrstedt, A. Flavonol oligosaccharides from the seeds of Aesculus hippocastanum. Planta Med. 1999, 65, 636–642. [Google Scholar] [CrossRef]
- Dudek-Makuch, M.; Matławska, I. Coumarins in horse chestnut flowers: Isolation and quantification by UPLC method. Acta Pol. Pharm. Drug Res. 2013, 70, 517–522. [Google Scholar]
- Marchelak, A.; Owczarek, A.; Matczak, M.; Pawlak, A.; Kolodziejczyk-Czepas, J.; Nowak, P.; Olszewska, M.A. Bioactivity potential of Prunus spinosa L. flower extracts: Phytochemical profiling, cellular safety, pro-inflammatory enzymes inhibition and protective effects against oxidative stress in vitro. Front. Pharmacol. 2017, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bijak, M.; Kolodziejczyk-Czepas, J.; Ponczek, M.B.; Saluk, J.; Nowak, P. Protective effects of grape seed extract against oxidative and nitrative damage of plasma proteins. Int. J. Biol. Macromol. 2012, 51, 183–187. [Google Scholar] [CrossRef]
- Bijak, M.; Bobrowski, M.; Borowiecka, M.; Podsędek, A.; Golański, J. Nowak Anticoagulant effect of polyphenols-rich extracts from black chokeberry and grape seeds. Fitoterapia 2011, 82, 811–817. [Google Scholar] [CrossRef] [PubMed]
- Hollman, P.C.H. Absorption, bioavailability, and metabolism of flavonoids. Pharm. Biol. 2004, 42, 74–83. [Google Scholar] [CrossRef]
- Saribal, D.; Kanber, E.M.; Hocaoglu-Emre, F.S.; Akyolcu, M.C. Effects of the oxidative stress and genetic changes in varicose vein patients. Phlebology 2019, 34, 406–413. [Google Scholar] [CrossRef]
- Panieri, E.; Santoro, M.M. ROS signaling and redox biology in endothelial cells. Cell. Mol. Life Sci. 2015, 72, 3281–3303. [Google Scholar] [CrossRef]
- Parcheta, M.; Świsłocka, R.; Orzechowska, S.; Akimowicz, M.; Choińska, R.; Lewandowski, W. Recent developments in effective antioxidants: The structure and antioxidant properties. Materials 2021, 14, 1984. [Google Scholar] [CrossRef]
- Guzik, T.J.; Korbut, R.; Adamek-Guzik, T. Nitric oxide and superoxide in inflammation and immune regulation. J. Physiol. Pharmacol. 2003, 54, 469–487. [Google Scholar]
- Bergamini, C.; Gambetti, S.; Dondi, A.; Cervellati, C. Oxygen, Reactive Oxygen Species and Tissue Damage. Curr. Pharm. Des. 2005, 10, 1611–1626. [Google Scholar] [CrossRef] [PubMed]
- Krzyściak, W.; Kowalska, J.; Kózka, M.; Papiez, M.A.; Kwiatek, W.M. Iron content (PIXE) in competent and incompetent veins is related to the vein wall morphology and tissue antioxidant enzymes. Bioelectrochemistry 2012, 87, 114–123. [Google Scholar] [CrossRef]
- Condezo-Hoyos, L.; Rubio, M.; Arribas, S.M.; España-Caparrós, G.; Rodríguez-Rodríguez, P.; Mujica-Pacheco, E.; González, M.C. A plasma oxidative stress global index in early stages of chronic venous insufficiency. J. Vasc. Surg. 2013, 57, 205–213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horecka, A.; Biernacka, J.; Hordyjewska, A.; Dąbrowski, W.; Terlecki, P.; Zubilewicz, T.; Musik, I.; Kurzepa, J. Antioxidative mechanism in the course of varicose veins. Phlebology 2018, 33, 464–469. [Google Scholar] [CrossRef] [PubMed]
- Riazanov, D.Y.; Mamunchak, O.V. Dynamics of endothelial dysfunction indices before and after operation in patients with varicose disease of the lower extremities. Zaporozhye Med. J. 2019, 21, 499–503. [Google Scholar] [CrossRef]
- Pryor, W.A.; Cueto, R.; Jin, X.; Koppenol, W.H.; Nguschwemlein, M.; Squadrito, G.L.; Uppu, P.L.; Uppu, R.M. A practical method for preparing peroxynitrite solutions of low ionic-strength and free of hydrogen-peroxide. Free Radic. Biol. Med. 1995, 18, 75–83. [Google Scholar] [CrossRef]
- Antignani, P.L. Medical Treatment of Chronic Venous Disease. SM J. Pharmacol. Ther. 2017, 3, 1015. [Google Scholar]
- Chamara, A.M.R.; Thiripuranathar, G. Assessement of haemostatic activity of medicinal palnts using in vitro methods: A concise review. IOSR J. Pharm. Biol. Sci. 2020, 15, 26–34. [Google Scholar]
- Wang, S. Bin; Jang, J.Y.; Chae, Y.H.; Min, J.H.; Baek, J.Y.; Kim, M.; Park, Y.; Hwang, G.S.; Ryu, J.S.; Chang, T.S. Kaempferol suppresses collagen-induced platelet activation by inhibiting NADPH oxidase and protecting SHP-2 from oxidative inactivation. Free Radic. Biol. Med. 2015, 83, 41–53. [Google Scholar] [CrossRef]
- Masselli, E.; Pozzi, G.; Vaccarezza, M.; Mirandola, P.; Galli, D.; Vitale, M.; Carubbi, C.; Gobbi, G. ROS in platelet biology: Functional aspects and methodological insights. Int. J. Mol. Sci. 2020, 21, 4866. [Google Scholar] [CrossRef]
- Palta, S.; Saroa, R.; Palta, A. Overview of the coagulation system. Indian J. Anaesth. 2014, 58, 515–523. [Google Scholar] [CrossRef] [PubMed]
- Bijak, M.; Nowak, P.; Borowiecka, M.; Ponczek, M.B.; Zbikowska, H.M.; Wachowicz, B. Protective effects of (-)-epicatechin against nitrative modifications of fibrinogen. Thromb. Res. 2012, 130, e123–e128. [Google Scholar] [CrossRef] [PubMed]
- Kolodziejczyk, J.; Olas, B.; Wachowicz, B.; Szajwaj, B.; Stochmal, A.; Oleszek, W. Clovamide-rich extract from Trifolium pallidum reduces oxidative stress-induced damage to blood platelets and plasma. J. Physiol. Biochem. 2011, 67, 391–399. [Google Scholar] [CrossRef] [PubMed]

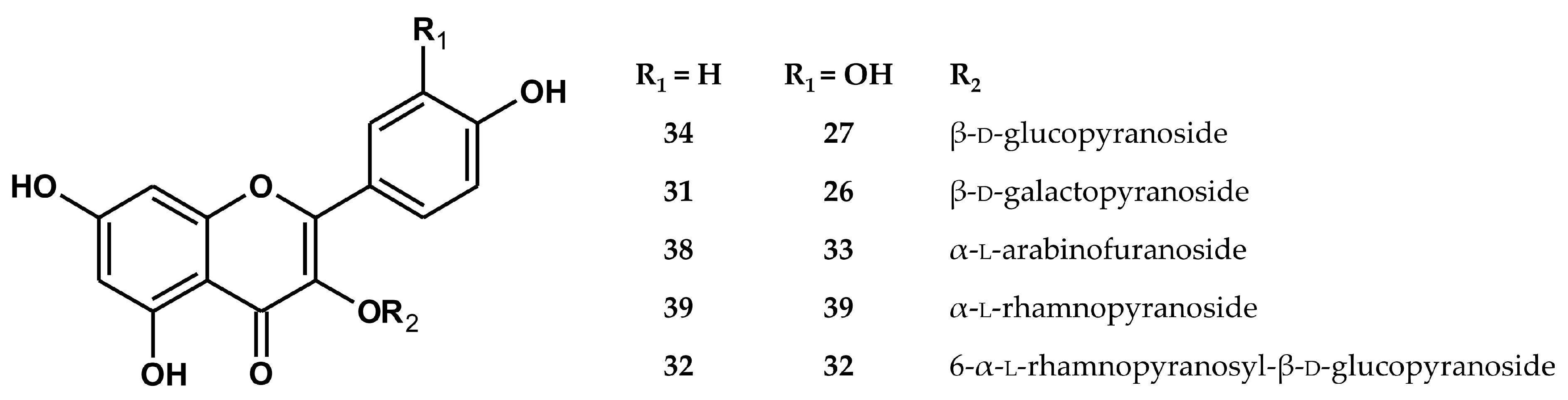
| No | Analyte | Rt (min) | UV λmax (nm) | [M−H]– | Fragmentary Ions |
|---|---|---|---|---|---|
| 1 | gallic acid a,b,c | 2.43 | 270 | 169 | 131 |
| 2 | protocatechuic acid a,b,c | 4.87 | 258, 293 | 153 | 109 |
| 3 | chlorogenic acid a,b,c | 5.85 | 324 | 353 | 191, 179 |
| 4 | coumaric acid derivative I b,c | 7.32 | 311 | 487 | 427, 163, 145, 119 |
| 5 | p-hydroxybenzoic acid a,b,c | 7.65 | 252 | 137 | |
| 6 | coumaroylquinic acid b,c | 7.87 | 329 | 337 | 163, 191 |
| 7 | coumaric acid derivative II b,c | 8.76 | 268, 314 | 487 | 427, 163, 145, 119 |
| 8 | cryptochlorogenic acid a,b,c | 9.24 | 322 | 353 | 179, 191 |
| 9 | coumaric acid derivative III b,c | 9.58 | 305 | 487 | 427 |
| 10 | caffeic acid a,b,c | 9.72 | 319 | 179 | 135 |
| 11 | Fraxin a,b | 10.03 | 304 | 369 | |
| 12 | procyanidin B2 a,b | 10.37 | 277 | 577 | 425, 289 |
| 13 | coumaric acid derivative IV b,c | 10.46 | 314 | 487 | 265, 163, 145, 119 |
| 14 | unidentified | 10.85 | 331 | 151 | |
| 15 | (−)-epicatechin a,b | 11.17 | 278 | 289 | 245, 205 |
| 16 | procyanidin trimer b | 12.48 | 278 | 863 | 447, 289 |
| 17 | p-coumaric acid a,b,c | 13.18 | 308 | 163 | 119 |
| 18 | quercetin dihexoside b | 13.55 | 263, 351 | 625 | 301, 445 |
| 19 | coumaric acid isomer b | 13.94 | 298 | 163 | |
| 20 | quercetin hexoside pentoside b | 14.70 | 266, 353 | 595 | 301 |
| 21 | procyanidin dimer A b | 15.03 | 276 | 575 | 449, 289 |
| 22 | kaempferol dihexoside | 15.16 | 264, 349 | 609 | 429, 285 |
| 23 | procyanidin dimer A b | 15.26 | 275 | 575 (245) | |
| 24 | quercetin 3-O-sophoroside b,c | 15.64 | 264, 347 | 609 | 300 |
| 25 | quercetin 3-O-rutinoside (rutin) a | 15.92 | 262, 355 | 609 | 301 |
| 26 | quercetin 3-O-β-d-galactopyranoside (hiperoside) a,b | 16.23 | 260, 354 | 463 | 301 |
| 27 | quercetin 3-O-β-d-glucopyranoside (isoquercetin) a | 16.57 | 253, 353 | 463 | 301 |
| 28 | kaempferol hexoside pentoside b | 16.66 | 260, 351 | 579 | 285 |
| 29 | procyanidin A2 a,b | 16.79 | 277 | 575 | 423, 289 |
| 30 | kaempferol hexoside rhamnoside b,c | 17.01 | 263, 351 | 593 | 285 |
| 31 | kaempferol 3-O-β-d-galactopyranoside (trifolin) a,b,c | 17.81 | 263, 348 | 447 | 285 |
| 32 | kaempferol 3-O-rutinoside a | 17.89 | 264, 347 | 593 | 285 |
| 33 | quercetin 3-O-α-l-arabinofuranoside (avicularin) a | 18.26 | 257, 353 | 433 | 301 |
| 34 | kaempferol 3-O-β-d-glucopyranoside (astragalin) a | 18.6 | 264, 347 | 447 | 285 |
| 35 | quercetin 3-O-α-l-rhamnopyranoside (quercitrin) a,b | 18.79 | 260, 349 | 447 | 301 |
| 36 | kaempferol pentoside b,c | 19.43 | 264, 324 | 417 | 285 |
| 37 | kaempferol acetylhexoside b,c | 20.23 | 264, 347 | 489 | 285 |
| 38 | kaempferol 3-O-α-l-arabinofuranoside (juglanin) a | 20.52 | 264, 347 | 417 | 285 |
| 39 | kaempferol 3-O-α-l-rhamnopyranoside (afzelin) a | 21.25 | 263, 342 | 431 | 285 |
| 40 | tricaffeoyl spermidine b,c | 22.57 | 322 | 630 | 468, 306 |
| 41 | quercetin a | 24.44 | 258, 370 | 301 | 151, 273 |
| 42 | dicaffeoyl-feruloyl spermidine b,c | 24.75 | 321 | 644 | 508, 372 |
| 43 | kaempferol a | 26.91 | 262, 366 | 285 | 131 |
| No a | Analyte | Content (mg/g) | |||
|---|---|---|---|---|---|
| ME | DEF | EAF | BF | ||
| 2 | protocatechuic acid b | 0.50 ± 0.07 A | 15.00 ± 0.40 B | 0.97 ± 0.11 A | n.d. |
| 3 | chlorogenic acid b | 0.52 ± 0.01 A | n.d. | n.d. | 2.68 ± 0.04 B |
| 4 | coumaric acid derivative I c | n.d. | n.d. | n.d. | 0.56 ± 0.01 |
| 5 | p-hydroxybenzoic acid b | n.d. | 5.85 ± 0.13 | n.d. | n.d. |
| 7 | coumaric acid derivative II c | n.d. | n.d. | n.d. | 0.56 ± 0.01 |
| 13 | coumaric acid derivative IV c | n.d. | n.d. | n.d. | 0.61 ± 0.02 |
| 15 | (−)-epicatechin b | 0.87 ± 0.03 A | 4.45 ± 0.13 B | 15.69 ± 0.89 C | n.d. |
| 16 | procyanidin trimer d | 2.11 ± 0.06 A | n.d. | 16.66 ± 0.05 B | n.d. |
| 17 | p-coumaric acid b | n.d. | 8.81 ± 0.14 | n.d. | n.d. |
| 18 | quercetin 3-O-sophoroside e | n.d. | n.d. | n.d. | 2.53 ± 0.05 |
| 19 | coumaric acid isomer c | n.d. | 0.91 ± 0.1 | n.d. | n.d. |
| 20 | quercetin hexoside pentoside e | n.d. | n.d. | n.d. | 3.74 ± 0.13 |
| 22 | kaempferol dihexoside | n.d. | n.d. | n.d. | 6.60 ± 0.09 |
| 23 | procyanidin dimer A d | 1.88 ± 0.03 A | n.d. | 4.52 ± 0.26 B | n.d. |
| 25 | quercetin 3-O-rutinoside (rutin) b | 1.64 ± 0.05 A | n.d. | 2.55 ± 0.02 B | 13.21 ± 0.15 C |
| 27 | quercetin 3-O-β-d-glucopyranoside (isoquercetin) b | 3.89 ± 0.04 B | 4.63 ± 0.07 C | 27.66 ± 0.15 D | 2.26 ± 0.04 A |
| 28 | kaempferol hexoside pentoside f | 2.32 ± 0.04 A | n.d. | n.d. | 15.62 ± 0.09 A |
| 29 | procyanidin A2 b | 2.35 ± 0.05 A | 12.25 ± 0.26 B | 20.77 ± 0.68 C | n.d. |
| 30 | kaempferol hexoside rhamnoside f | n.d. | n.d. | 2.08 ± 0.03 A | 6.27 ± 0.12 B |
| 31 | kaempferol 3-O-β-d-galactopyranoside (trifolin) g | 1.52 ± 0.02 A | 2.95 ± 0.11 B | n.d. | n.d. |
| 32 | kaempferol 3-O-rutinoside b | 16.31 ± 0.12 B | 2.00 ± 0.01 A | 54.77 ± 0.78 C | 81.97 ± 0.59 D |
| 33 | quercetin 3-O-α-l-arabinofuranoside (avicularin) b | 0.95 ± 0.02 A | 10.32 ± 0.23 C | 3.61 ± 0.15 B | n.d. |
| 34 | kaempferol 3-O-β-d-glucopyranoside (astragalin) b | 29.38 ± 0.22 B | 81.98 ± 1.93 C | 208.23 ± 1.95 D | 4.92 ± 0.05 A |
| 35 | quercetin 3-O-α-l-rhamnopyranoside (quercitrin) b | 0.83 ± 0.01 A | 6.49 ± 0.15 C | 5.89 ± 0.11 B | n.d. |
| 36 | kaempferol pentoside h | n.d. | 2.85 ± 0.05 B | 1.41 ± 0.01 A | n.d. |
| 37 | kaempferol acetylhexoside g | 2.37 ± 0.03 B | 1.82 ± 0.07 A | 16.64 ± 0.20 D | 4.92 ± 0.05 C |
| 38 | kaempferol 3-O-α-l-arabinofuranoside (juglanin) b | 2.96 ± 0.03 A | 53.89 ± 1.00 C | 8.01 ± 0.03 B | n.d. |
| 39 | kaempferol 3-O-α-l-rhamnopyranoside (afzelin) b | 3.00 ± 0.02 A | 52.33 ± 1.07 C | 15.59 ± 0.28 B | n.d. |
| 40 | tricaffeoyl spermidine i | 0.22 ± 0.01 | n.d. | 1.24 ± 0.05 | n.d. |
| 41 | quercetin b | n.d. | 8.01 ± 0.17 | n.d. | n.d. |
| 42 | dicaffeoyl-feruloyl spermidine i | 0.23 ± 0.01 | n.d. | 1.04 ± 0.07 | n.d. |
| 43 | kaempferol b | 0.39 ± 0.01 A | 32.93 ± 0.58 C | 4.70 ± 0.17 B | n.d. |
| Total phenolic acid derivatives | 1.46 ± 0.05 | 30.57 ± 0.54 | 3.25 ± 0.01 | 4.41 ± 0.06 | |
| Total flavanols | 7.22 ± 0.11 | 16.70 ± 0.35 | 57.64 ± 1.19 | n.d. | |
| Total flavonoids | 65.58 ± 0.44 | 260.20 ± 4.80 | 353.16 ± 1.06 | 142.04 ± 1.25 | |
| Total phenolics | 74.26 ± 0.57 | 307.46 ± 5.55 | 414.06 ± 1.27 | 146.45 ± 1.30 | |
| Analyte | SC50 [µg/mL] | |||
|---|---|---|---|---|
| H2O2 | OH• | O2−• | ONOO− | |
| MED | 157.78 ± 5.79 G | 532.57 ± 13.37 G | 59.09 ± 1.55 E | >400 |
| DEF | 44.20 ± 1.98 D | 134.93 ± 3.79 D | 24.18 ± 2.07 C | 173.42 ± 5.19 |
| EAF | 34.64 ± 0.55 C | 117.33 ± 2.41 C | 15.22 ± 0.94 B | 154.02 ± 5.64 |
| BF | 70.44 ± 2.42 E | 189.11 ± 7.85 F | 30.04 ± 3.07 D | 286.19 ± 6.68 |
| Astragalin | 91.86 ± 3.01 F | 102.46 ± 8.09 B,C | >500 | >400 |
| Isoquercitrin | 14.28 ± 0.71 B | 62.22 ± 2.72 A | 19.21 ± 0.83 B | 69.48 ± 1.88 A |
| (−)-Epicatechin | 8.41 ± 0.30 A | 99.53 ± 0.85 B | 7.89 ± 0.99 | |
| Ascorbic acid | 11.59 ± 0.81 B | 151.33 ± 2.98 E | 5.76 ± 0.96 A | 67.91 ± 2.58 A |
| Time (min) | Solvent A (%) | Solvent B (%) | Solvent C (%) |
|---|---|---|---|
| 0.0–1.0 | 92 | 1 | 7 |
| 1.0–20.0 | 92–68 | 1–25 | 7 |
| 20.0–25.0 | 68–18 | 25–75 | 7 |
| 25.0–30.0 | 18 | 75 | 7 |
| 30.1–35.0 | 92 | 1 | 7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Owczarek, A.; Kołodziejczyk-Czepas, J.; Marczuk, P.; Siwek, J.; Wąsowicz, K.; Olszewska, M.A. Bioactivity Potential of Aesculus hippocastanum L. Flower: Phytochemical Profile, Antiradical Capacity and Protective Effects on Human Plasma Components under Oxidative/Nitrative Stress In Vitro. Pharmaceuticals 2021, 14, 1301. https://doi.org/10.3390/ph14121301
Owczarek A, Kołodziejczyk-Czepas J, Marczuk P, Siwek J, Wąsowicz K, Olszewska MA. Bioactivity Potential of Aesculus hippocastanum L. Flower: Phytochemical Profile, Antiradical Capacity and Protective Effects on Human Plasma Components under Oxidative/Nitrative Stress In Vitro. Pharmaceuticals. 2021; 14(12):1301. https://doi.org/10.3390/ph14121301
Chicago/Turabian StyleOwczarek, Aleksandra, Joanna Kołodziejczyk-Czepas, Paulina Marczuk, Julia Siwek, Katarzyna Wąsowicz, and Monika Anna Olszewska. 2021. "Bioactivity Potential of Aesculus hippocastanum L. Flower: Phytochemical Profile, Antiradical Capacity and Protective Effects on Human Plasma Components under Oxidative/Nitrative Stress In Vitro" Pharmaceuticals 14, no. 12: 1301. https://doi.org/10.3390/ph14121301
APA StyleOwczarek, A., Kołodziejczyk-Czepas, J., Marczuk, P., Siwek, J., Wąsowicz, K., & Olszewska, M. A. (2021). Bioactivity Potential of Aesculus hippocastanum L. Flower: Phytochemical Profile, Antiradical Capacity and Protective Effects on Human Plasma Components under Oxidative/Nitrative Stress In Vitro. Pharmaceuticals, 14(12), 1301. https://doi.org/10.3390/ph14121301






