Chiral Flavonoids as Antitumor Agents
Abstract
1. Introduction
2. Flavonoids
2.1. Natural Chiral Flavonoids with Antitumor Activity
2.2. Synthetic Chiral Flavonoids with Antitumor Activity
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Kopustinskiene, D.M.; Jakstas, V.; Savickas, A.; Bernatoniene, J. Flavonoids as Anticancer Agents. Nutrients 2020, 12, 457. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Vasan, N.; Baselga, J.; Hyman, D.M. A view on drug resistance in cancer. Nature 2019, 575, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Abotaleb, M.; Samuel, S.M.; Varghese, E.; Varghese, S.; Kubatka, P.; Liskova, A.; Büsselberg, D. Flavonoids in Cancer and Apoptosis. Cancers 2018, 11, 28. [Google Scholar] [CrossRef] [PubMed]
- Kinghorn, A.D.; Chin, Y.-W.; Swanson, S.M. Discovery of natural product anticancer agents from biodiverse organisms. Curr. Opin. Drug Discov. Dev. 2009, 12, 189–196. [Google Scholar]
- Agrawal, A. Pharmacological Activities of Flavonoids: A Review. Int. J. Pharm. Sci. Nanotechnol. 2011, 4, 1394–1398. [Google Scholar] [CrossRef]
- Patil, V.M.; Masand, N. Chapter 12—Anticancer Potential of Flavonoids: Chemistry, Biological Activities, and Future Perspectives. In Studies in Natural Products Chemistry; Atta-ur, R., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 401–430. [Google Scholar]
- Xiang, Y.; Liu, S.; Yang, J.; Wang, Z.; Zhang, H.; Gui, C. Investigation of the interactions between flavonoids and human organic anion transporting polypeptide 1B1 using fluorescent substrate and 3D-QSAR analysis. Biochim. Biophys. Acta Biomembr. 2020, 1862, 183210. [Google Scholar] [CrossRef]
- Martins, B.T.; Correia da Silva, M.; Pinto, M.; Cidade, H.; Kijjoa, A. Marine natural flavonoids: Chemistry and biological activities. Nat. Prod. Res. 2019, 33, 3260–3272. [Google Scholar] [CrossRef]
- Mutha, R.E.; Tatiya, A.U.; Surana, S.J. Flavonoids as natural phenolic compounds and their role in therapeutics: An overview. Future J. Pharm. Sci. 2021, 7, 25. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Zhou, X.; Wang, T.; Wang, G.; Cao, F. Regulation of flavonoid metabolism in ginkgo leaves in response to different day-night temperature combinations. Plant Physiol. Biochem. 2020, 147, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Pastore, C.; Dal Santo, S.; Zenoni, S.; Movahed, N.; Allegro, G.; Valentini, G.; Filippetti, I.; Tornielli, G.B. Whole Plant Temperature Manipulation Affects Flavonoid Metabolism and the Transcriptome of Grapevine Berries. Front. Plant Sci. 2017, 8, 929. [Google Scholar] [CrossRef]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef]
- Ren, F.; Nian, Y.; Perussello, C.A. Effect of storage, food processing and novel extraction technologies on onions flavonoid content: A review. Food Res. Int. 2019, 132, 108953. [Google Scholar] [CrossRef] [PubMed]
- Tuenter, E.; Creylman, J.; Verheyen, G.; Pieters, L.; Van Miert, S. Development of a classification model for the antigenotoxic activity of flavonoids. Bioorganic Chem. 2020, 98, 103705. [Google Scholar] [CrossRef]
- Miadoková, E. Isoflavonoids—An overview of their biological activities and potential health benefits. Interdiscip. Toxicol. 2009, 2, 211–218. [Google Scholar] [CrossRef]
- Silva, L.; Shahidi, F.; Coimbra, M.A. Dried Fruits Phytochemicals and Health Effects; John Wiley & Sons: Oxford, UK, 2013. [Google Scholar]
- Křížová, L.; Dadáková, K.; Kašparovská, J.; Kašparovský, T. Isoflavones. Molecules 2019, 24, 1076. [Google Scholar] [CrossRef] [PubMed]
- Tungmunnithum, D.; Thongboonyou, A.; Pholboon, A.; Yangsabai, A. Flavonoids and Other Phenolic Compounds from Medicinal Plants for Pharmaceutical and Medical Aspects: An Overview. Medicines 2018, 5, 93. [Google Scholar] [CrossRef] [PubMed]
- Ullah, A.; Munir, S.; Badshah, S.L.; Khan, N.; Ghani, L.; Poulson, B.G.; Emwas, A.-H.; Jaremko, M. Important Flavonoids and Their Role as a Therapeutic Agent. Molecules 2020, 25, 5243. [Google Scholar] [CrossRef] [PubMed]
- Waheed Janabi, A.H.; Kamboh, A.A.; Saeed, M.; Lu, X.; BiBi, J.; Majeed, F.; Naveed, M.; Mughal, M.J.; Korejo, N.A.; Kamboh, R.; et al. Flavonoid-rich foods (FRF): A promising nutraceutical approach against lifespan-shortening diseases. Iran. J. Basic Med. Sci. 2020, 23, 140–153. [Google Scholar]
- Valavanidis, A.; Vlachogianni, T. Chapter 8—Plant Polyphenols: Recent Advances in Epidemiological Research and Other Studies on Cancer Prevention. In Studies in Natural Products Chemistry; Atta-ur, R., Ed.; Elsevier: Amsterdam, The Netherlands, 2013; pp. 269–295. [Google Scholar]
- Alseekh, S.; Perez de Souza, L.; Benina, M.; Fernie, A.R. The style and substance of plant flavonoid decoration; towards defining both structure and function. Phytochemistry 2020, 174, 112347. [Google Scholar] [CrossRef] [PubMed]
- Treml, J.; Šmejkal, K. Flavonoids as Potent Scavengers of Hydroxyl Radicals. Compr. Rev. Food Sci. Food Saf. 2016, 15, 720–738. [Google Scholar] [CrossRef]
- Rehman, M.U.; Tahir, M.; Khan, A.Q.; Khan, R.; Lateef, A.; Oday, O.H.; Qamar, W.; Ali, F.; Sultana, S. Chrysin suppresses renal carcinogenesis via amelioration of hyperproliferation, oxidative stress and inflammation: Plausible role of NF-κB. Toxicol. Lett. 2013, 216, 146–158. [Google Scholar] [CrossRef]
- Forni, C.; Rossi, M.; Borromeo, I.; Feriotto, G.; Platamone, G.; Tabolacci, C.; Mischiati, C.; Beninati, S. Flavonoids: A Myth or a Reality for Cancer Therapy? Molecules 2021, 26, 3583. [Google Scholar] [CrossRef]
- Zhao, J.; Yang, J.; Xie, Y. Improvement strategies for the oral bioavailability of poorly water-soluble flavonoids: An overview. Int. J. Pharm. 2019, 570, 118642. [Google Scholar] [CrossRef] [PubMed]
- Duodu, K.G.; Awika, J.M. Chapter 8—Phytochemical-Related Health-Promoting Attributes of Sorghum and Millets. In Sorghum and Millets, 2nd ed.; Taylor, J.R.N., Duodu, K.G., Eds.; AACC International Press: Washington, DC, USA, 2019; pp. 225–258. [Google Scholar]
- Cirmi, S.; Ferlazzo, N.; Lombardo, G.E.; Maugeri, A.; Calapai, G.; Gangemi, S.; Navarra, M. Chemopreventive Agents and Inhibitors of Cancer Hallmarks: May Citrus Offer New Perspectives? Nutrients 2016, 8, 698. [Google Scholar] [CrossRef]
- Brand, W.; Shao, J.; Hoek-van den Hil, E.F.; van Elk, K.N.; Spenkelink, B.; de Haan, L.H.; Rein, M.J.; Dionisi, F.; Williamson, G.; van Bladeren, P.J.; et al. Stereoselective conjugation, transport and bioactivity of s- and R-hesperetin enantiomers in vitro. J. Agric. Food Chem. 2010, 58, 6119–6125. [Google Scholar] [CrossRef]
- Curti, V.; Di Lorenzo, A.; Rossi, D.; Martino, E.; Capelli, E.; Collina, S.; Daglia, M. Enantioselective Modulatory Effects of Naringenin Enantiomers on the Expression Levels of miR-17-3p Involved in Endogenous Antioxidant Defenses. Nutrients 2017, 9, 215. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Lee, D.-H.; Jang, H.; Park, S.-Y.; Seol, J.-W. Naringenin exerts anticancer effects by inducing tumor cell death and inhibiting angiogenesis in malignant melanoma. Int. J. Med. Sci. 2020, 17, 3049–3057. [Google Scholar] [CrossRef] [PubMed]
- Arul, D.; Subramanian, P. Naringenin (citrus flavonone) induces growth inhibition, cell cycle arrest and apoptosis in human hepatocellular carcinoma cells. Pathol. Oncol. Res. 2013, 19, 763–770. [Google Scholar] [CrossRef]
- Bao, L.; Liu, F.; Guo, H.B.; Li, Y.; Tan, B.B.; Zhang, W.X.; Peng, Y.H. Naringenin inhibits proliferation, migration, and invasion as well as induces apoptosis of gastric cancer SGC7901 cell line by downregulation of AKT pathway. Tumour Biol. 2016, 37, 11365–11374. [Google Scholar] [CrossRef]
- Chang, H.-L.; Chang, Y.-M.; Lai, S.-C.; Chen, K.-M.; Wang, K.-C.; Chiu, T.-T.; Chang, F.-H.; Hsu, L.-S. Naringenin inhibits migration of lung cancer cells via the inhibition of matrix metalloproteinases-2 and -9. Exp. Ther. Med. 2017, 13, 739–744. [Google Scholar] [CrossRef]
- Devi, K.P.; Rajavel, T.; Nabavi, S.F.; Setzer, W.N.; Ahmadi, A.; Mansouri, K.; Nabavi, S.M. Hesperidin: A promising anticancer agent from nature. Ind. Crop. Prod. 2015, 76, 582–589. [Google Scholar] [CrossRef]
- Erdem Guzel, E.; Tektemur, N.K. Hesperetin may alleviate the development of doxorubicin-induced pulmonary toxicity by decreasing oxidative stress and apoptosis in male rats. Tissue Cell 2021, 73, 101667. [Google Scholar] [CrossRef]
- Ferreira de Oliveira, J.M.P.; Santos, C.; Fernandes, E. Therapeutic potential of hesperidin and its aglycone hesperetin: Cell cycle regulation and apoptosis induction in cancer models. Phytomedicine 2020, 73, 152887. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, Y.; Li, Y.; Zhang, L.; Yu, S. Preclinical Investigation of Alpinetin in the Treatment of Cancer-Induced Cachexia via Activating PPARγ. Front. Pharmacol. 2021, 12, 687491. [Google Scholar] [CrossRef]
- Zhao, X.; Guo, X.; Shen, J.; Hua, D. Alpinetin inhibits proliferation and migration of ovarian cancer cells via suppression of STAT3 signaling. Mol. Med. Rep. 2018, 18, 4030–4036. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Tang, B.; Wang, J.; Sui, H.; Jin, X.; Wang, L.; Wang, Z. Antiproliferative effect of alpinetin in BxPC-3 pancreatic cancer cells. Int. J. Mol. Med. 2012, 29, 607–612. [Google Scholar] [CrossRef]
- Zhang, T.; Guo, S.; Zhu, X.; Qiu, J.; Deng, G.; Qiu, C. Alpinetin inhibits breast cancer growth by ROS/NF-κB/HIF-1α axis. J. Cell. Mol. Med. 2020, 24, 8430–8440. [Google Scholar] [CrossRef] [PubMed]
- Saquib, Q.; Ahmed, S.; Ahmad, M.S.; Al-Rehaily, A.J.; Siddiqui, M.A.; Faisal, M.; Ahmad, J.; Alsaleh, A.N.; Alatar, A.A.; Al-Khedhairy, A.A. Anticancer efficacies of persicogenin and homoeriodictyol isolated from Rhus retinorrhoea. Process Biochem. 2020, 95, 186–196. [Google Scholar] [CrossRef]
- Li, W.X.; Cui, C.B.; Cai, B.; Wang, H.Y.; Yao, X.S. Flavonoids from Vitex trifolia L. inhibit cell cycle progression at G2/M phase and induce apoptosis in mammalian cancer cells. J. Asian Nat. Prod. Res. 2005, 7, 615–626. [Google Scholar] [CrossRef]
- Suksamrarn, A.; Chotipong, A.; Suavansri, T.; Boongird, S.; Timsuksai, P.; Vimuttipong, P.; Chuaynugul, A. Antimycobacterial activity and cytotoxicity of flavonoids from the flowers of Chromolaena odorata. Arch. Pharm. Res. 2004, 27, 507–511. [Google Scholar] [CrossRef]
- Han, N.; Huang, T.; Wang, Y.C.; Yin, J.; Kadota, S. Flavanone glycosides from Viscum coloratum and their inhibitory effects on osteoclast formation. Chem. Biodivers. 2011, 8, 1682–1688. [Google Scholar] [CrossRef]
- Singhal, S.S.; Singhal, S.; Singhal, P.; Singhal, J.; Horne, D.; Awasthi, S. Didymin: An orally active citrus flavonoid for targeting neuroblastoma. Oncotarget 2017, 8, 29428–29441. [Google Scholar] [CrossRef]
- Hung, J.-Y.; Hsu, Y.-L.; Ko, Y.-C.; Tsai, Y.-M.; Yang, C.-J.; Huang, M.-S.; Kuo, P.-L. Didymin, a dietary flavonoid glycoside from citrus fruits, induces Fas-mediated apoptotic pathway in human non-small-cell lung cancer cells in vitro and in vivo. Lung Cancer 2010, 68, 366–374. [Google Scholar] [CrossRef]
- Yao, Q.; Lin, M.T.; Zhu, Y.D.; Xu, H.L.; Zhao, Y.Z. Recent Trends in Potential Therapeutic Applications of the Dietary Flavonoid Didymin. Molecules 2018, 23, 2547. [Google Scholar] [CrossRef] [PubMed]
- Hsu, Y.L.; Hsieh, C.J.; Tsai, E.M.; Hung, J.Y.; Chang, W.A.; Hou, M.F.; Kuo, P.L. Didymin reverses phthalate ester-associated breast cancer aggravation in the breast cancer tumor microenvironment. Oncol. Lett. 2016, 11, 1035–1042. [Google Scholar] [CrossRef]
- Babaei, F.; Moafizad, A.; Darvishvand, Z.; Mirzababaei, M.; Hosseinzadeh, H.; Nassiri-Asl, M. Review of the effects of vitexin in oxidative stress-related diseases. Food Sci. Nutr. 2020, 8, 2569–2580. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Jiang, Q.; Liu, H.; Luo, S. Vitexin induces apoptosis through mitochondrial pathway and PI3K/Akt/mTOR signaling in human non-small cell lung cancer A549 cells. Biol. Res. 2019, 52, 7. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, M.; Cho, H.J.; Paul, S.; Jakhar, R.; Khan, I.; Lee, S.-J.; Kim, B.-Y.; Krishnan, M.; Khaket, T.P.; Lee, H.G.; et al. Vitexin induces apoptosis by suppressing autophagy in multi-drug resistant colorectal cancer cells. Oncotarget 2017, 9, 3278–3291. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Zhan, S.; Wang, Y.; Zhou, G.; Liang, H.; Chen, X.; Shen, H. Baicalin, the major component of traditional Chinese medicine Scutellaria baicalensis induces colon cancer cell apoptosis through inhibition of oncomiRNAs. Sci. Rep. 2018, 8, 14477. [Google Scholar] [CrossRef] [PubMed]
- Gong, W.; Zhao, Z.; Liu, B.; Lu, L.; Dong, J. Exploring the chemopreventive properties and perspectives of baicalin and its aglycone baicalein in solid tumors. Eur. J. Med. Chem. 2017, 126, 844–852. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Meena, A.; Luqman, S. Baicalin mediated regulation of key signaling pathways in cancer. Pharmacol. Res. 2021, 164, 105387. [Google Scholar] [CrossRef]
- Wang, N.; Tang, L.J.; Zhu, G.Q.; Peng, D.Y.; Wang, L.; Sun, F.N.; Li, Q.L. Apoptosis induced by baicalin involving up-regulation of P53 and bax in MCF-7 cells. J. Asian Nat. Prod. Res. 2008, 10, 1129–1135. [Google Scholar] [CrossRef]
- Wan, D.; Ouyang, H. Baicalin induces apoptosis in human osteosarcoma cell through ROS-mediated mitochondrial pathway. Nat. Prod. Res. 2018, 32, 1996–2000. [Google Scholar] [CrossRef]
- Sui, X.; Han, X.; Chen, P.; Wu, Q.; Feng, J.; Duan, T.; Chen, X.; Pan, T.; Yan, L.; Jin, T.; et al. Baicalin Induces Apoptosis and Suppresses the Cell Cycle Progression of Lung Cancer Cells Through Downregulating Akt/mTOR Signaling Pathway. Front. Mol. Biosci. 2021, 7, 602282. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Bai, H.; Sa, Y.; Zhu, P.; Liu, P. Inhibiting EMT, stemness and cell cycle involved in baicalin-induced growth inhibition and apoptosis in colorectal cancer cells. J. Cancer 2020, 11, 2303–2317. [Google Scholar] [CrossRef]
- Yano, H.; Mizoguchi, A.; Fukuda, K.; Haramaki, M.; Ogasawara, S.; Momosaki, S.; Kojiro, M. The herbal medicine sho-saiko-to inhibits proliferation of cancer cell lines by inducing apoptosis and arrest at the G0/G1 phase. Cancer Res. 1994, 54, 448–454. [Google Scholar] [PubMed]
- Shi, J.; Wu, G.; Zou, X.; Jiang, K. Enteral Baicalin, a Flavone Glycoside, Reduces Indicators of Cardiac Surgery-Associated Acute Kidney Injury in Rats. Cardiorenal Med. 2019, 9, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Ohkoshi, E.; Umemura, N. Induced overexpression of CD44 associated with resistance to apoptosis on DNA damage response in human head and neck squamous cell carcinoma cells. Int. J. Oncol. 2017, 50, 387–395. [Google Scholar] [CrossRef]
- Yu, Y.; Pei, M.; Li, L. Baicalin induces apoptosis in hepatic cancer cells in vitro and suppresses tumor growth in vivo. Int. J. Clin. Exp. Med. 2015, 8, 8958–8967. [Google Scholar]
- Chen, J.; Li, Z.; Chen, A.Y.; Ye, X.; Luo, H.; Rankin, G.O.; Chen, Y.C. Inhibitory effect of baicalin and baicalein on ovarian cancer cells. Int. J. Mol. Sci. 2013, 14, 6012–6025. [Google Scholar] [CrossRef]
- Yang, C.S.; Wang, H. Cancer Preventive Activities of Tea Catechins. Molecules 2016, 21, 1679. [Google Scholar] [CrossRef]
- George, V.C.; Dellaire, G.; Rupasinghe, H.P.V. Plant flavonoids in cancer chemoprevention: Role in genome stability. J. Nutr. Biochem. 2017, 45, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Min, K.-J.; Kwon, T.K. Anticancer effects and molecular mechanisms of epigallocatechin-3-gallate. Integr. Med. Res. 2014, 3, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Pojero, F.; Poma, P.; Spanò, V.; Montalbano, A.; Barraja, P.; Notarbartolo, M. Targeting multiple myeloma with natural polyphenols. Eur. J. Med. Chem. 2019, 180, 465–485. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.-H.; Hsieh, C.-H.; Tsai, S.-Y.; Wang, C.-Y.; Wang, C.-C. Anticancer effects of epigallocatechin-3-gallate nanoemulsion on lung cancer cells through the activation of AMP-activated protein kinase signaling pathway. Sci. Rep. 2020, 10, 5163. [Google Scholar] [CrossRef]
- Bae, J.; Kumazoe, M.; Yamashita, S.; Tachibana, H. Hydrogen sulphide donors selectively potentiate a green tea polyphenol EGCG-induced apoptosis of multiple myeloma cells. Sci. Rep. 2017, 7, 6665. [Google Scholar] [CrossRef]
- James, K.D.; Kennett, M.J.; Lambert, J.D. Potential role of the mitochondria as a target for the hepatotoxic effects of (−)-epigallocatechin-3-gallate in mice. Food Chem. Toxicol. 2018, 111, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Md Nesran, Z.N.; Shafie, N.H.; Md Tohid, S.F.; Norhaizan, M.E.; Ismail, A. Iron Chelation Properties of Green Tea Epigallocatechin-3-Gallate (EGCG) in Colorectal Cancer Cells: Analysis on Tfr/Fth Regulations and Molecular Docking. Evid.-Based Complementary Altern. Med. 2020, 2020, 7958041. [Google Scholar] [CrossRef]
- Tsai, Y.-J.; Chen, B.-H. Preparation of catechin extracts and nanoemulsions from green tea leaf waste and their inhibition effect on prostate cancer cell PC-3. Int. J. Nanomed. 2016, 11, 1907–1926. [Google Scholar]
- Li, J.; Hu, L.; Zhou, T.; Gong, X.; Jiang, R.; Li, H.; Kuang, G.; Wan, J.; Li, H. Taxifolin inhibits breast cancer cells proliferation, migration and invasion by promoting mesenchymal to epithelial transition via β-catenin signaling. Life Sci. 2019, 232, 116617. [Google Scholar] [CrossRef]
- Razak, S.; Afsar, T.; Ullah, A.; Almajwal, A.; Alkholief, M.; Alshamsan, A.; Jahan, S. Taxifolin, a natural flavonoid interacts with cell cycle regulators causes cell cycle arrest and causes tumor regression by activating Wnt/β-catenin signaling pathway. BMC Cancer 2018, 18, 1043. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Zhu, X.; Wang, Q.; Li, X.; Wang, E.; Zhao, Q.; Wang, Q.; Cao, H. The anti-tumor effect of taxifolin on lung cancer via suppressing stemness and epithelial-mesenchymal transition in vitro and oncogenesis in nude mice. Ann. Transl. Med. 2020, 8, 590. [Google Scholar] [CrossRef]
- Yang, C.-L.; Lin, Y.-S.; Liu, K.-F.; Peng, W.-H.; Hsu, C.-M. Hepatoprotective Mechanisms of Taxifolin on Carbon Tetrachloride-Induced Acute Liver Injury in Mice. Nutrients 2019, 11, 2655. [Google Scholar] [CrossRef]
- Butt, S.S.; Khan, K.; Badshah, Y.; Rafiq, M.; Shabbir, M. Evaluation of pro-apoptotic potential of taxifolin against liver cancer. PeerJ 2021, 9, e11276. [Google Scholar] [CrossRef]
- Bijak, M. Silybin, a Major Bioactive Component of Milk Thistle (Silybum marianum L. Gaernt.)-Chemistry, Bioavailability, and Metabolism. Molecules 2017, 22, 1942. [Google Scholar] [CrossRef]
- Tuli, H.S.; Mittal, S.; Aggarwal, D.; Parashar, G.; Parashar, N.C.; Upadhyay, S.K.; Barwal, T.S.; Jain, A.; Kaur, G.; Savla, R.; et al. Path of Silibinin from diet to medicine: A dietary polyphenolic flavonoid having potential anti-cancer therapeutic significance. Semin. Cancer Biol. 2021, 73, 196–218. [Google Scholar] [CrossRef] [PubMed]
- Mukhtar, S.; Zeng, X.; Qamer, S.; Saad, M.; Mubarik, M.S.; Mahmoud, A.H.; Mohammed, O.B. Hepatoprotective activity of silymarin encapsulation against hepatic damage in albino rats. Saudi J. Biol. Sci. 2021, 28, 717–723. [Google Scholar] [CrossRef] [PubMed]
- Si, L.; Liu, W.; Hayashi, T.; Ji, Y.; Fu, J.; Nie, Y.; Mizuno, K.; Hattori, S.; Onodera, S.; Ikejima, T. Silibinin-induced apoptosis of breast cancer cells involves mitochondrial impairment. Arch. Biochem. Biophys. 2019, 671, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Prasad, R.R.; Paudel, S.; Raina, K.; Agarwal, R. Silibinin and non-melanoma skin cancers. J. Tradit. Complementary Med. 2020, 10, 236–244. [Google Scholar] [CrossRef]
- Sherman, B.; Hernandez, A.M.; Alhado, M.; Menge, L.; Price, R.S. Silibinin Differentially Decreases the Aggressive Cancer Phenotype in an In Vitro Model of Obesity and Prostate Cancer. Nutr. Cancer 2020, 72, 333–342. [Google Scholar] [CrossRef]
- Raina, K.; Kumar, S.; Dhar, D.; Agarwal, R. Silibinin and colorectal cancer chemoprevention: A comprehensive review on mechanisms and efficacy. J. Biomed. Res. 2016, 30, 452–465. [Google Scholar]
- Jahanafrooz, Z.; Motamed, N.; Rinner, B.; Mokhtarzadeh, A.; Baradaran, B. Silibinin to improve cancer therapeutic, as an apoptotic inducer, autophagy modulator, cell cycle inhibitor, and microRNAs regulator. Life Sci. 2018, 213, 236–247. [Google Scholar] [CrossRef]
- Pashaei-Asl, F.; Pashaei-Asl, R.; Khodadadi, K.; Akbarzadeh, A.; Ebrahimie, E.; Pashaiasl, M. Enhancement of anticancer activity by silibinin and paclitaxel combination on the ovarian cancer. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1483–1487. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ge, Y.; Ping, X.; Yu, M.; Lou, D.; Shi, W. Synergistic apoptotic effects of silibinin in enhancing paclitaxel toxicity in human gastric cancer cell lines. Mol. Med. Rep. 2018, 18, 1835–1841. [Google Scholar] [CrossRef]
- Li, W.G.; Wang, H.Q. Inhibitory effects of Silibinin combined with doxorubicin in hepatocellular carcinoma; an in vivo study. J. Buon 2016, 21, 917–924. [Google Scholar] [PubMed]
- Bukowski, K.; Kciuk, M.; Kontek, R. Mechanisms of Multidrug Resistance in Cancer Chemotherapy. Int. J. Mol. Sci. 2020, 21, 3233. [Google Scholar] [CrossRef]
- Molavi, O.; Narimani, F.; Asiaee, F.; Sharifi, S.; Tarhriz, V.; Shayanfar, A.; Hejazi, M.; Lai, R. Silibinin sensitizes chemo-resistant breast cancer cells to chemotherapy. Pharm. Biol. 2017, 55, 729–739. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xie, X.; Hou, X.; Shen, J.; Shi, J.; Chen, H.; He, Y.; Wang, Z.; Feng, N. Functional oral nanoparticles for delivering silibinin and cryptotanshinone against breast cancer lung metastasis. J. Nanobiotechnology 2020, 18, 83. [Google Scholar] [CrossRef]
- Lampe, J.W. Emerging research on equol and cancer. J. Nutr. 2010, 140, 1369S–1372S. [Google Scholar] [CrossRef]
- De la Parra, C.; Otero-Franqui, E.; Martinez-Montemayor, M.; Dharmawardhane, S. The soy isoflavone equol may increase cancer malignancy via up-regulation of eukaryotic protein synthesis initiation factor eIF4G. J. Biol. Chem. 2012, 287, 41640–41650. [Google Scholar] [CrossRef]
- Brown, N.M.; Belles, C.A.; Lindley, S.L.; Zimmer-Nechemias, L.D.; Zhao, X.; Witte, D.P.; Kim, M.-O.; Setchell, K.D.R. The chemopreventive action of equol enantiomers in a chemically induced animal model of breast cancer. Carcinogenesis 2010, 31, 886–893. [Google Scholar] [CrossRef]
- Lund, T.D.; Blake, C.; Bu, L.; Hamaker, A.N.; Lephart, E.D. Equol an isoflavonoid: Potential for improved prostate health, in vitro and in vivo evidence. Reprod. Biol. Endocrinol. 2011, 9, 4. [Google Scholar] [CrossRef]
- Choi, E.J.; Kim, T. Equol induced apoptosis via cell cycle arrest in human breast cancer MDA-MB-453 but not MCF-7 cells. Mol. Med. Rep. 2008, 1, 239–244. [Google Scholar]
- Choi, E.J.; Ahn, W.S.; Bae, S.M. Equol induces apoptosis through cytochrome c-mediated caspases cascade in human breast cancer MDA-MB-453 cells. Chem. Biol. Interact. 2009, 177, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Setchell, K.D.; Clerici, C.; Lephart, E.D.; Cole, S.J.; Heenan, C.; Castellani, D.; Wolfe, B.E.; Nechemias-Zimmer, L.; Brown, N.M.; Lund, T.D.; et al. S-equol, a potent ligand for estrogen receptor beta, is the exclusive enantiomeric form of the soy isoflavone metabolite produced by human intestinal bacterial flora. Am. J. Clin. Nutr. 2005, 81, 1072–1079. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Wu, F.-H.; Wang, P.; Ke, J.-P.; Wan, X.-C.; Qiu, M.-H.; Bao, G.-H. Flavoalkaloids with a Pyrrolidinone Ring from Chinese Ancient Cultivated Tea Xi-Gui. J. Agric. Food Chem. 2018, 66, 7948–7957. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Zhang, Y.; Liu, Y.-C.; Liu, Y.; Luo, S.-H.; Huang, C.-S.; Li, S.-H. Leucoflavonine, a new bioactive racemic flavoalkaloid from the leaves of Leucosceptrum canum. Bioorganic Med. Chem. 2019, 27, 442–446. [Google Scholar] [CrossRef]
- Ur Rashid, M.; Alamzeb, M.; Ali, S.; Ullah, Z.; Shah, Z.A.; Naz, I.; Khan, M.R. The chemistry and pharmacology of alkaloids and allied nitrogen compounds from Artemisia species: A review. Phytother. Res. 2019, 33, 2661–2684. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.K.; Bharate, S.B.; Vishwakarma, R.A. Cyclin-dependent kinase inhibition by flavoalkaloids. Mini Rev. Med. Chem. 2012, 12, 632–649. [Google Scholar] [CrossRef] [PubMed]
- Beutler, J.A.; Cardellina, J.H., 2nd; McMahon, J.B.; Boyd, M.R.; Cragg, G.M. Anti-HIV and cytotoxic alkaloids from Buchenavia capitata. J. Nat. Prod. 1992, 55, 207–213. [Google Scholar] [CrossRef]
- Li, F.-F.; Sun, Q.; Wang, D.; Liu, S.; Lin, B.; Liu, C.-T.; Li, L.-Z.; Huang, X.-X.; Song, S.-J. Chiral Separation of Cytotoxic Flavan Derivatives from Daphne giraldii. J. Nat. Prod. 2016, 79, 2236–2242. [Google Scholar] [CrossRef]
- Andrushko, V.; Andrushko, N. Stereoselective Synthesis of Drugs and Natural Products; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Adly, F.G.; Ghanem, A.; Nag, A. Enantiomerically Pure Compounds by Enantioselective Synthetic Chiral Metal Complexes. In Asymmetric Synthesis of Drugs and Natural Products; CRC Press: Boca Raton, FL, USA, 2018; pp. 75–131. [Google Scholar]
- He, L.; Liang, Z.; Yu, G.; Li, X.; Chen, X.; Zhou, Z.; Ren, Z. Green and Efficient Resolution of Racemic Ofloxacin Using Tartaric Acid Derivatives via Forming Cocrystal in Aqueous Solution. Cryst. Growth Des. 2018, 18, 5008–5020. [Google Scholar] [CrossRef]
- Pinto, M.M.M.; Fernandes, C.; Tiritan, M.E. Chiral Separations in Preparative Scale: A Medicinal Chemistry Point of View. Molecules 2020, 25, 1931. [Google Scholar] [CrossRef] [PubMed]
- Yáñez, J.A.; Andrews, P.K.; Davies, N.M. Methods of analysis and separation of chiral flavonoids. J. Chromatogr. B 2007, 848, 159–181. [Google Scholar] [CrossRef]
- Lee, S.H.; Lee, K.R.; Cho, E.A.; Jung, S.H. Enantioseparation of Physiologically Active Some Flavonoids by Liquid Chromatography-Electrospray-Tandem Mass Spectrometry Based on Noncovalent Interactions with β-Cyclodextrin. Bull. Korean Chem. Soc. 2011, 32, 4415–4418. [Google Scholar] [CrossRef][Green Version]
- Liu, L.-X.; Huang, W.-J.; Xie, Q.-X.; Wu, B.; Yu, C.-B.; Zhou, Y.-G. Dynamic Kinetic Resolution of Flavonoids via Asymmetric Allylic Alkylation: Construction of Two Contiguous Stereogenic Centers on Nucleophiles. Am. Chem. Soc. Catal. 2021, 11, 12859–12863. [Google Scholar] [CrossRef]
- Chen, Q.-H. Review of Classics in Stereoselective Synthesis. J. Nat. Prod. 2011, 74, 1670. [Google Scholar] [CrossRef]
- Ji, J.; Mould, D.R.; Blum, K.A.; Ruppert, A.S.; Poi, M.; Zhao, Y.; Johnson, A.J.; Byrd, J.C.; Grever, M.R.; Phelps, M.A. A pharmacokinetic/pharmacodynamic model of tumor lysis syndrome in chronic lymphocytic leukemia patients treated with flavopiridol. Clin. Cancer Res. 2013, 19, 1269–1280. [Google Scholar] [CrossRef]
- Pajtás, D.; Kónya, K.; Kiss-Szikszai, A.; Džubák, P.; Pethő, Z.; Varga, Z.; Panyi, G.; Patonay, T. Optimization of the Synthesis of Flavone–Amino Acid and Flavone–Dipeptide Hybrids via Buchwald–Hartwig Reaction. J. Org. Chem. 2017, 82, 4578–4587. [Google Scholar] [CrossRef]
- Ibrahim, N.; Bonnet, P.; Brion, J.-D.; Peyrat, J.-F.; Bignon, J.; Levaique, H.; Josselin, B.; Robert, T.; Colas, P.; Bach, S.; et al. Identification of a new series of flavopiridol-like structures as kinase inhibitors with high cytotoxic potency. Eur. J. Med. Chem. 2020, 199, 112355. [Google Scholar] [CrossRef]
- Bisol, Â.; de Campos, P.S.; Lamers, M.L. Flavonoids as anticancer therapies: A systematic review of clinical trials. Phytother. Res. 2020, 34, 568–582. [Google Scholar] [CrossRef]
- Zocchi, L.; Wu, S.C.; Wu, J.; Hayama, K.L.; Benavente, C.A. The cyclin-dependent kinase inhibitor flavopiridol (alvocidib) inhibits metastasis of human osteosarcoma cells. Oncotarget 2018, 9, 23505–23518. [Google Scholar] [CrossRef]
- Cassaday, R.D.; Goy, A.; Advani, S.; Chawla, P.; Nachankar, R.; Gandhi, M.; Gopal, A.K. A phase II, single-arm, open-label, multicenter study to evaluate the efficacy and safety of P276-00, a cyclin-dependent kinase inhibitor, in patients with relapsed or refractory mantle cell lymphoma. Clin. Lymphoma Myeloma Leuk. 2015, 15, 392–397. [Google Scholar] [CrossRef]
- Ray, B.; Mehrotra, R. Nucleic acid binding mechanism of flavone derivative, riviciclib: Structural analysis to unveil anticancer potential. J. Photochem. Photobiol. B Biol. 2020, 211, 111990. [Google Scholar] [CrossRef]
- Joshi, K.S.; Rathos, M.J.; Joshi, R.D.; Sivakumar, M.; Mascarenhas, M.; Kamble, S.; Lal, B.; Sharma, S. In vitro antitumor properties of a novel cyclin-dependent kinase inhibitor, P276-00. Mol. Cancer Ther. 2007, 6, 918–925. [Google Scholar] [CrossRef]
- Galijatovic, A.; Otake, Y.; Walle, U.K.; Walle, T. Induction of UDP-glucuronosyltransferase UGT1A1 by the flavonoid chrysin in Caco-2 cells—Potential role in carcinogen bioinactivation. Pharm. Res. 2001, 18, 374–379. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.; Liu, D.; Jiang, Z.; Li, C.; Chen, L.; Xia, Y.; Liu, D.; Yao, Q.; Wang, D. Chrysin Induced Cell Apoptosis and Inhibited Invasion Through Regulation of TET1 Expression in Gastric Cancer Cells. OncoTargets Ther. 2020, 13, 3277–3287. [Google Scholar] [CrossRef] [PubMed]
- Brechbuhl, H.M.; Kachadourian, R.; Min, E.; Chan, D.; Day, B.J. Chrysin enhances doxorubicin-induced cytotoxicity in human lung epithelial cancer cell lines: The role of glutathione. Toxicol. Appl. Pharmacol. 2012, 258, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Wu, Y.; Hu, B.; Tao, L.; Chen, X.; Hoffelt, D.; Hu, F. Chrysin Inhibits Melanoma Tumor Metastasis via Interfering with the FOXM1/β-Catenin Signaling. J. Agric. Food Chem. 2020, 68, 9358–9367. [Google Scholar]
- Samarghandian, S.; Afshari, J.T.; Davoodi, S. Chrysin reduces proliferation and induces apoptosis in the human prostate cancer cell line pc-3. Clinics 2011, 66, 1073–1079. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.S.; Halagowder, D.; Devaraj, S.N. Methylated chrysin induces co-ordinated attenuation of the canonical Wnt and NF-kB signaling pathway and upregulates apoptotic gene expression in the early hepatocarcinogenesis rat model. Chem.-Biol. Interact. 2011, 193, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Phan, T.; Yu, X.-M.; Kunnimalaiyaan, M.; Chen, H. Antiproliferative Effect of Chrysin on Anaplastic Thyroid Cancer. J. Surg. Res. 2011, 170, 84–88. [Google Scholar] [CrossRef]
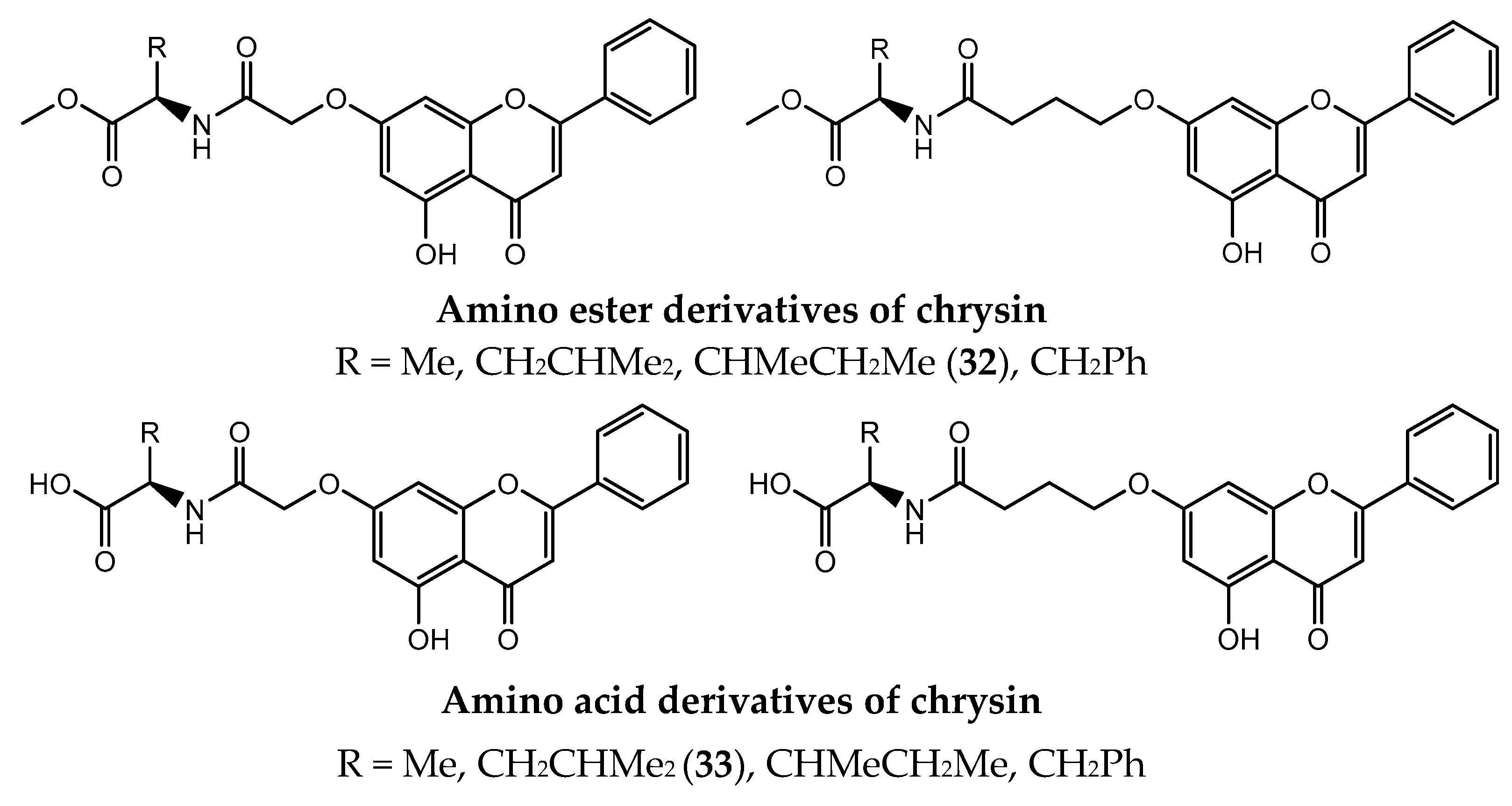
- Song, X.; Liu, Y.; Ma, J.; He, J.; Zheng, X.; Lei, X.; Jiang, G.; Zhang, L. Synthesis of novel amino acid derivatives containing chrysin as anti-tumor agents against human gastric carcinoma MGC-803 cells. Med. Chem. Res. 2015, 24, 1789–1798. [Google Scholar] [CrossRef]
- Liu, D.; Li, Y.; Shen, H.; Li, Y.; He, J.; Zhang, Q.; Liu, Y. Synthesis and anti-tumor activities of novel 7-O-amino acids chrysin derivatives. Chin. Herb. Med. 2018, 10, 323–330. [Google Scholar] [CrossRef]
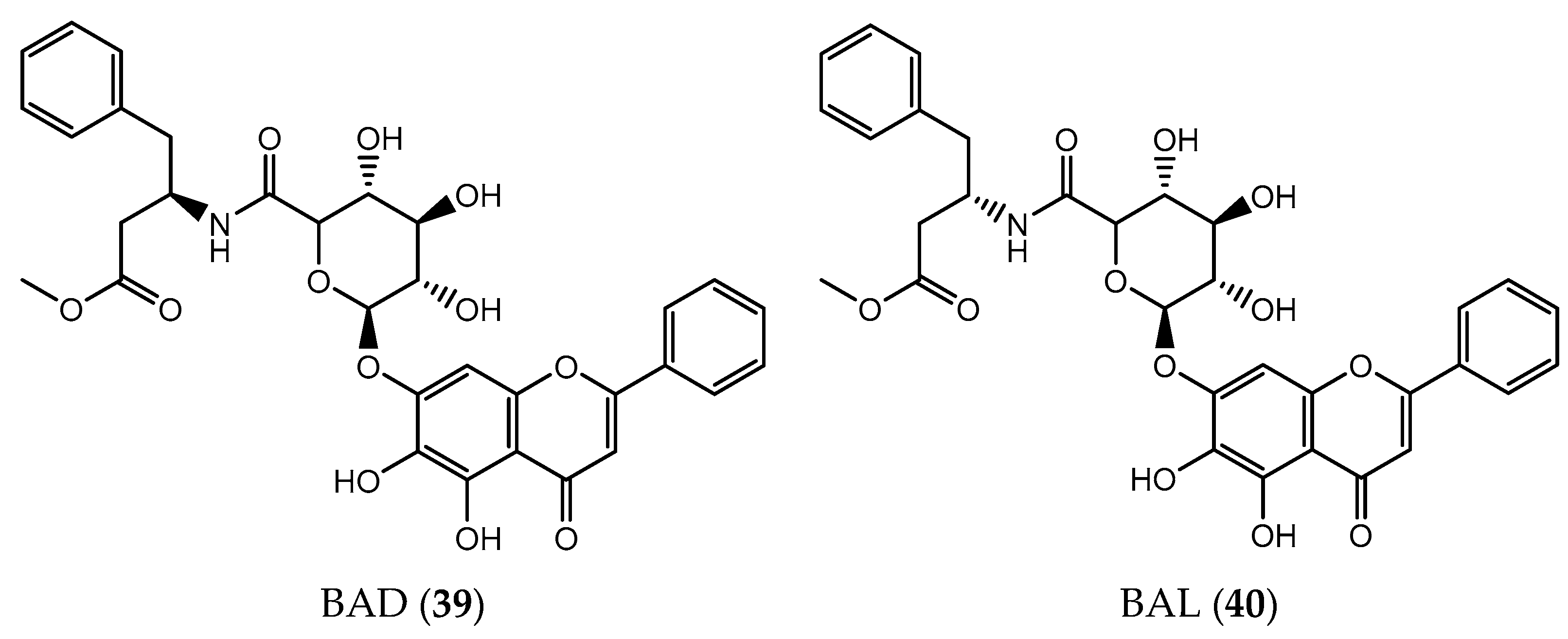
- Xu, Q.; Deng, H.; Li, X.; Quan, Z.-S. Application of Amino Acids in the Structural Modification of Natural Products: A Review. Front. Chem. 2021, 9, 650569. [Google Scholar] [CrossRef]
- Liu, Y.-M.; Li, Y.; Liu, R.-F.; Xiao, J.; Zhou, B.-N.; Zhang, Q.-Z.; Song, J.-X. Synthesis, characterization and preliminary biological evaluation of chrysin amino acid derivatives that induce apoptosis and EGFR downregulation. J. Asian Nat. Prod. Res. 2019, 23, 39–54. [Google Scholar] [CrossRef]
- Nguyen, L.A.; He, H.; Pham-Huy, C. Chiral drugs: An overview. Int. J. Biomed. Sci. 2006, 2, 85–100. [Google Scholar]
- Brooks, W.H.; Guida, W.C.; Daniel, K.G. The significance of chirality in drug design and development. Curr. Top. Med. Chem. 2011, 11, 760–770. [Google Scholar] [CrossRef]
- Li, Y.; Yao, J.; Han, C.; Yang, J.; Chaudhry, M.T.; Wang, S.; Liu, H.; Yin, Y. Quercetin, Inflammation and Immunity. Nutrients 2016, 8, 167. [Google Scholar] [CrossRef]
- Xu, D.; Hu, M.J.; Wang, Y.Q.; Cui, Y.L. Antioxidant Activities of Quercetin and Its Complexes for Medicinal Application. Molecules 2019, 24, 1123. [Google Scholar] [CrossRef]
- Vafadar, A.; Shabaninejad, Z.; Movahedpour, A.; Fallahi, F.; Taghavipour, M.; Ghasemi, Y.; Akbari, M.; Shafiee, A.; Hajighadimi, S.; Moradizarmehri, S.; et al. Quercetin and cancer: New insights into its therapeutic effects on ovarian cancer cells. Cell Biosci. 2020, 10, 32. [Google Scholar] [CrossRef] [PubMed]
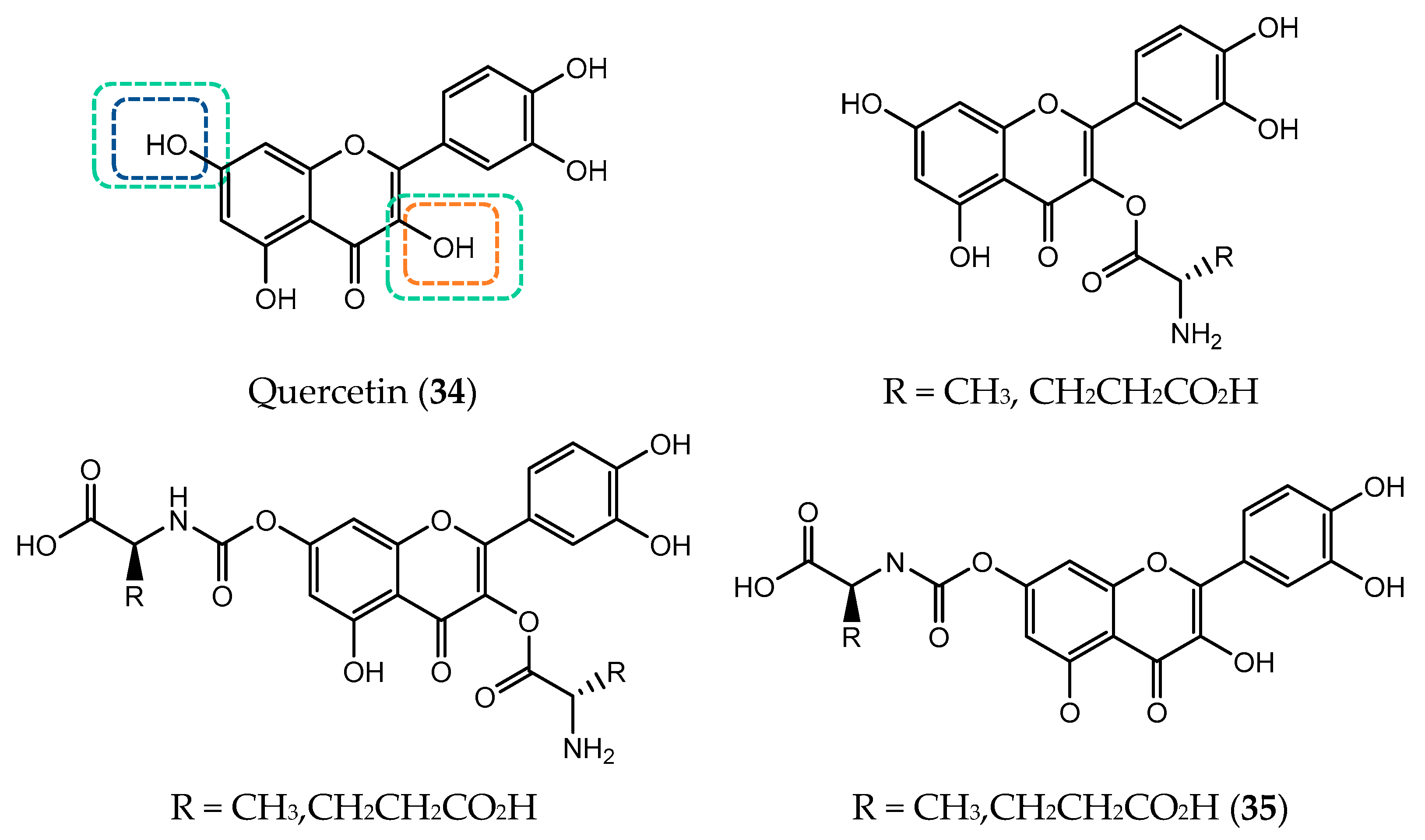
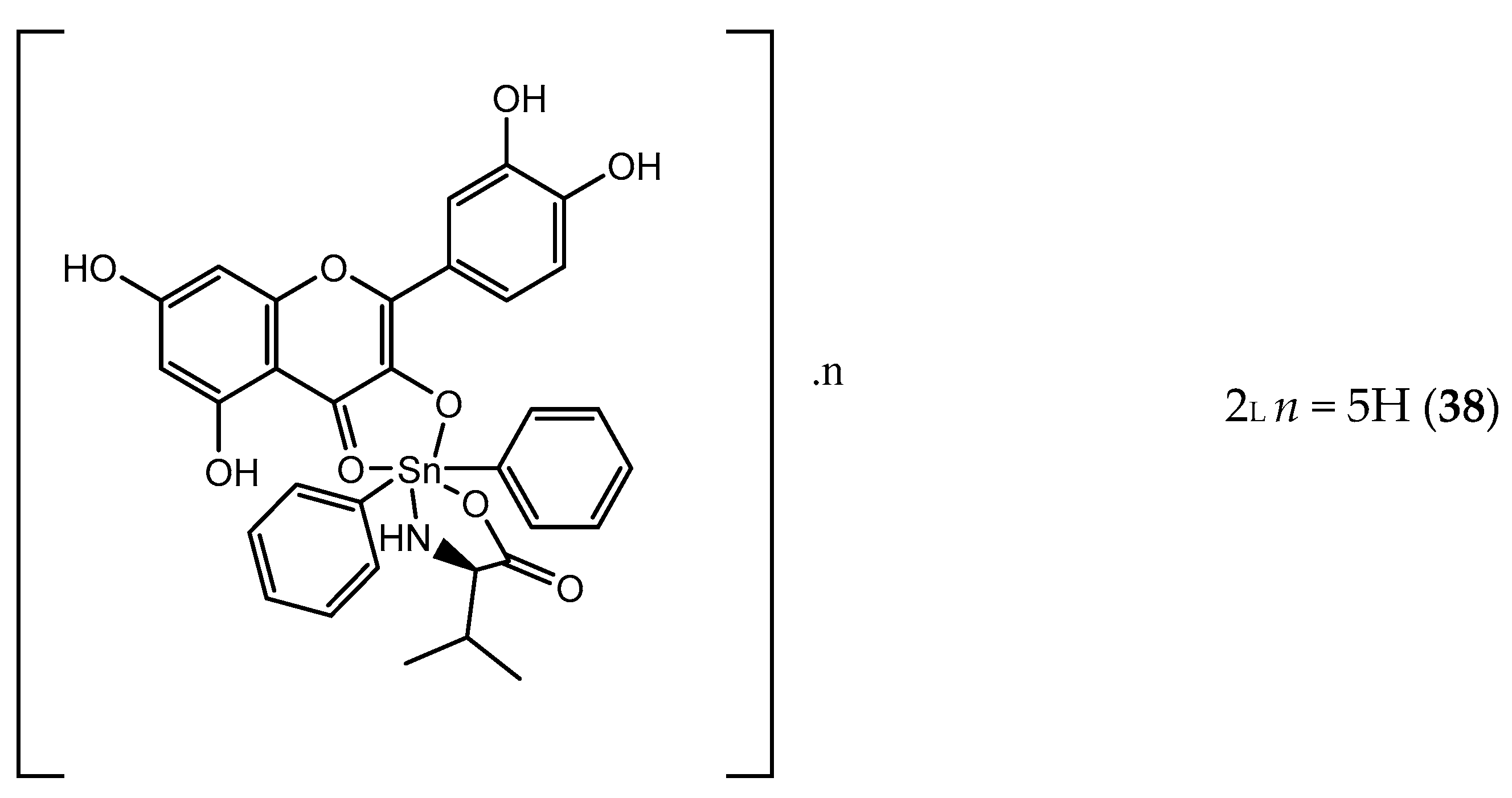
- Parveen, S.; Tabassum, S.; Arjmand, F. Human Topoisomerase I mediated cytotoxicity profile of l-valine-quercetin diorganotin(IV) antitumor drug entities. J. Organomet. Chem. 2016, 823, 23–33. [Google Scholar] [CrossRef]
- Kim, M.K.; Choo, H.; Chong, Y. Water-soluble and cleavable quercetin-amino acid conjugates as safe modulators for P-glycoprotein-based multidrug resistance. J. Med. Chem. 2014, 57, 7216–7233. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.K.; Kim, Y.; Choo, H.; Chong, Y. Quercetin-glutamic acid conjugate with a non-hydrolysable linker; a novel scaffold for multidrug resistance reversal agents through inhibition of P-glycoprotein. Bioorganic Med. Chem. 2017, 25, 1219–1226. [Google Scholar] [CrossRef]
- Arjmand, F. Tin Complexes, Antitumor Activity. In Encyclopedia of Metalloproteins; Kretsinger, R.H., Uversky, V.N., Permyakov, E.A., Eds.; Springer: New York, NY, USA, 2013; pp. 2224–2233. [Google Scholar]
- De Sousa, G.F.; Valdés-Martínez, J.; Pérez, G.E.; Toscano, R.A.; Abras, A.; Filgueiras, C.A.L. Heptacoordination in Organotin(IV) Complexes: Spectroscopic and Structural Studies of 2,6–Diacetylpyridine bis(thiosemicarbazone)di–n–butyltin(IV) Chloride Nitromethane Solvate, [nBu2Sn(H2daptsc)]Cl2•MeNO2 and of 2,6–Diacetylpyridine bis(semicarbazone)dimethyltin(IV) trans–Tetrachlorodimethylstannate(IV), [Me2Sn(H2dapsc)][Me2 SnCl4]. J. Braz. Chem. Soc. 2002, 13, 565–569. [Google Scholar]
- Ali, S.; Saira, S. Anticarcinogenicity and Toxicity of Organotin(IV) Complexes: A Review. Iran. J. Sci. Technol. Trans. A Sci. 2016, 42, 505–524. [Google Scholar] [CrossRef]
- Zhou, Q.; Yu, L.S.; Zeng, S. Stereoselectivity of chiral drug transport: A focus on enantiomer-transporter interaction. Drug Metab. Rev. 2014, 46, 283–290. [Google Scholar] [CrossRef]
- Hou, Y.; Pi, C.; Feng, X.; Wang, Y.; Fu, S.; Zhang, X.; Zhao, L.; Wei, Y. Antitumor Activity In Vivo and Vitro of New Chiral Derivatives of Baicalin and Induced Apoptosis via the PI3K/Akt Signaling Pathway. Mol. Ther. Oncolytics 2020, 19, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Leung, W.-H.; Mak, W.-L.; Chan, E.; Lam, T.; Lee, W.-S.; Kwong, H.-L.; Yeung, L.-L. Palladium-based Kinetic Resolution of Racemic Tosylaziridines. Synlett 2002, 2002, 1688–1690. [Google Scholar] [CrossRef]
- Yang, Z.; Xiao, F.; Zhang, Y.; Wu, Z.; Zheng, X. Asymmetric Synthesis of Chiral Flavan-3-Ols. Nat. Prod. Res. 2019, 33, 2995–3010. [Google Scholar] [CrossRef]
- Heravi, M.M.; Lashaki, T.B.; Fattahi, B.; Zadsirjan, V. Application of asymmetric Sharpless aminohydroxylation in total synthesis of natural products and some synthetic complex bio-active molecules. RSC Adv. 2018, 8, 6634–6659. [Google Scholar] [CrossRef]
- Li, L.; Chan, T.H. Enantioselective synthesis of epigallocatechin-3-gallate (EGCG), the active polyphenol component from green tea. Org. Lett. 2001, 3, 739–741. [Google Scholar] [CrossRef]
- Rensburg, H.; Van Heerden, P.; Ferreira, D. Enantioselective synthesis of flavonoids. Part 3.1trans- and cis-Flavan-3-ol methyl ether acetates. J. Chem. Soc. Perkin Trans. 1997, 1, 3415–3422. [Google Scholar] [CrossRef]
- Anderson, J.C.; Headley, C.; Stapleton, P.D.; Taylor, P.W. Asymmetric total synthesis of B-ring modified (−)-epicatechin gallate analogues and their modulation of beta-lactam resistance in Staphylococcus aureus. Tetrahedron 2005, 61, 7703–7711. [Google Scholar] [CrossRef]
- Takashi, H.; Ken, O.; Keisuke, S. General and Convenient Approach to Flavan-3-ols: Stereoselective Synthesis of (−)-Gallocatechin. Chem. Lett. 2006, 35, 1006–1007. [Google Scholar]
- Liu, Y.; Li, X.; Lin, G.; Xiang, Z.; Xiang, J.; Zhao, M.; Chen, J.; Yang, Z. Synthesis of catechins via thiourea/AuCl3-catalyzed cycloalkylation of aryl epoxides. J. Org. Chem. 2008, 73, 4625–4629. [Google Scholar] [CrossRef]
- Ohmori, K.; Yano, T.; Suzuki, K. General synthesis of epi-series catechins and their 3-gallates: Reverse polarity strategy. Org. Biomol. Chem. 2010, 8, 2693–2696. [Google Scholar] [CrossRef] [PubMed]
- Stadlbauer, S.; Ohmori, K.; Hattori, F.; Suzuki, K. A new synthetic strategy for catechin-class polyphenols: Concise synthesis of (−)-epicatechin and its 3-O-gallate. Chem. Commun. 2012, 48, 8425–8427. [Google Scholar] [CrossRef] [PubMed]
- Abe, H.; Itaya, S.; Sasaki, K.; Kobayashi, T.; Ito, H. Enantioselective Total Synthesis of the Proposed Structure of Furan-Containing Polyketide. Chem. Pharm. Bull. 2016, 64, 772–777. [Google Scholar] [CrossRef][Green Version]
- Hirooka, Y.; Nitta, M.; Furuta, T.; Kan, T. Efficient Synthesis of Optically Active Gallocatechin3-gallate Derivatives via 6-endo Cyclization. Synlett 2008, 2008, 3234–3238. [Google Scholar]
- Anderson, J.C.; Grounds, H.; Reeves, S.; Taylor, P.W. Improved synthesis of structural analogues of (−)-epicatechin gallate for modulation of staphylococcal β-lactam resistance. Tetrahedron 2014, 70, 3485–3490. [Google Scholar] [CrossRef]
- Charris, J.; Domínguez, J.; Lobo, G.; Riggione, F. Synthesis of some Thiochromone Derivatives and Activity Against Plasmodium falciparum In-vitro. Pharm. Pharmacol. Commun. 1999, 5, 107–110. [Google Scholar] [CrossRef]
- Meng, L.; Ngai, K.Y.; Chang, X.; Lin, Z.; Wang, J. Cu(I)-Catalyzed Enantioselective Alkynylation of Thiochromones. Org. Lett. 2020, 22, 1155–1159. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, T.; Watanabe, S.-I.; Mori, E.; Kadomoto, R.; Tanimura, S.; Kohno, M. Synthesis and structure-activity relationships of thioflavone derivatives as specific inhibitors of the ERK-MAP kinase signaling pathway. Bioorg. Med. Chem. 2004, 12, 2397–2407. [Google Scholar] [CrossRef]
- Meng, L.; Jin, M.Y.; Wang, J. Rh-Catalyzed Conjugate Addition of Arylzinc Chlorides to Thiochromones: A Highly Enantioselective Pathway for Accessing Chiral Thioflavanones. Org. Lett. 2016, 18, 4986–4989. [Google Scholar] [CrossRef] [PubMed]
- Rani, N.; Bharti, S.; Krishnamurthy, B.; Bhatia, J.; Sharma, C.; Kamal, M.A.; Ojha, S.; Arya, D.S. Pharmacological Properties and Therapeutic Potential of Naringenin: A Citrus Flavonoid of Pharmaceutical Promise. Curr. Pharm. Des. 2016, 22, 4341–4359. [Google Scholar] [CrossRef]
- Wang, Z.; Lu, W.; Li, Y.; Tang, B. Alpinetin promotes Bax translocation, induces apoptosis through the mitochondrial pathway and arrests human gastric cancer cells at the G2/M phase. Mol. Med. Rep. 2013, 7, 915–920. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Zhang, A.; Kuang, G.; Gong, X.; Jiang, R.; Lin, D.; Li, J.; Li, H.; Zhang, X.; Wan, J.; et al. Baicalin inhibits the metastasis of highly aggressive breast cancer cells by reversing epithelial-to-mesenchymal transition by targeting β-catenin signaling. Oncol. Rep. 2017, 38, 3599–3607. [Google Scholar] [CrossRef]
- Lee, C.-Y.; Chien, Y.-S.; Chiu, T.-H.; Huang, W.-W.; Lu, C.-C.; Chiang, J.-H.; Yang, J.-S. Apoptosis triggered by vitexin in U937 human leukemia cells via a mitochondrial signaling pathway. Oncol. Rep. 2012, 28, 1883–1888. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Li, D.; Chen, H.; Zhang, J.; Jin, X. Vitexin induces G2/M-phase arrest and apoptosis via Akt/mTOR signaling pathway in human glioblastoma cells. Mol. Med. Rep. 2018, 17, 4599–4604. [Google Scholar] [CrossRef]
- Wang, J.; Zheng, X.; Zeng, G.; Zhou, Y.; Yuan, H. Purified vitexin compound 1 inhibits growth and angiogenesis through activation of FOXO3a by inactivation of Akt in hepatocellular carcinoma. Int. J. Mol. Med. 2014, 33, 441–448. [Google Scholar] [CrossRef]
- Dou, J.; Wang, Z.; Ma, L.; Peng, B.; Mao, K.; Li, C.; Su, M.; Zhou, C.; Peng, G. Baicalein and baicalin inhibit colon cancer using two distinct fashions of apoptosis and senescence. Oncotarget 2018, 9, 20089–20102. [Google Scholar] [CrossRef] [PubMed]
- Chan, F.L.; Choi, H.L.; Chen, Z.Y.; Chan, P.S.F.; Huang, Y. Induction of apoptosis in prostate cancer cell lines by a flavonoid, baicalin. Cancer Lett. 2000, 160, 219–228. [Google Scholar] [CrossRef]
- Yan, X.; Rui, X.; Zhang, K. Baicalein inhibits the invasion of gastric cancer cells by suppressing the activity of the p38 signaling pathway. Oncol. Rep. 2015, 33, 737–743. [Google Scholar] [CrossRef]
- Liu, Y.; Hong, Z.; Chen, P.; Wang, J.; Zhou, Y.; Huang, J. Baicalin inhibits growth and induces apoptosis of human osteosarcoma cells by suppressing the AKT pathway. Oncol. Lett. 2019, 18, 3188–3194. [Google Scholar] [CrossRef]
- Decker, R.H.; Dai, Y.; Grant, S. The cyclin-dependent kinase inhibitor flavopiridol induces apoptosis in human leukemia cells (U937) through the mitochondrial rather than the receptor-mediated pathway. Cell Death Differ. 2001, 8, 715–724. [Google Scholar] [CrossRef][Green Version]
- Gojo, I.; Zhang, B.; Fenton, R.G. The cyclin-dependent kinase inhibitor flavopiridol induces apoptosis in multiple myeloma cells through transcriptional repression and down-regulation of Mcl-1. Clin. Cancer Res. 2002, 8, 3527–3538. [Google Scholar]
- Wu, T.; Qin, Z.; Tian, Y.; Wang, J.; Xu, C.; Li, Z.; Bian, J. Recent Developments in the Biology and Medicinal Chemistry of CDK9 Inhibitors: An Update. J. Med. Chem. 2020, 63, 13228–13257. [Google Scholar] [CrossRef]
- Das, A.; Baidya, R.; Chakraborty, T.; Samanta, A.K.; Roy, S. Pharmacological basis and new insights of taxifolin: A comprehensive review. Biomed. Pharmacother. 2021, 142, 112004. [Google Scholar] [CrossRef]
- Shafiei, S.S.; Solati-Hashjin, M.; Samadikuchaksaraei, A.; Kalantarinejad, R.; Asadi-Eydivand, M.; Abu Osman, N.A. Epigallocatechin Gallate/Layered Double Hydroxide Nanohybrids: Preparation, Characterization, and In Vitro Anti-Tumor Study. PLoS ONE 2015, 10, e0136530. [Google Scholar] [CrossRef]
- Shimizu, M.; Deguchi, A.; Joe, A.K.; McKoy, J.F.; Moriwaki, H.; Weinstein, I.B. EGCG inhibits activation of HER3 and expression of cyclooxygenase-2 in human colon cancer cells. J. Exp. Ther. Oncol. 2005, 5, 69–78. [Google Scholar]
- Rady, I.; Mohamed, H.; Rady, M.; Siddiqui, I.A.; Mukhtar, H. Cancer preventive and therapeutic effects of EGCG, the major polyphenol in green tea. Egypt. J. Basic Appl. Sci. 2018, 5, 1–23. [Google Scholar] [CrossRef]
- Braicu, C.; Gherman, C.D.; Irimie, A.; Berindan-Neagoe, I. Epigallocatechin-3-Gallate (EGCG) inhibits cell proliferation and migratory behaviour of triple negative breast cancer cells. J. Nanosci. Nanotechnol. 2013, 13, 632–637. [Google Scholar] [CrossRef] [PubMed]
- Magee, P.J.; Raschke, M.; Steiner, C.; Duffin, J.G.; Pool-Zobel, B.L.; Jokela, T.; Wahala, K.; Rowland, I.R. Equol: A comparison of the effects of the racemic compound with that of the purified S-enantiomer on the growth, invasion, and DNA integrity of breast and prostate cells in vitro. Nutr. Cancer 2006, 54, 232–242. [Google Scholar] [CrossRef] [PubMed]
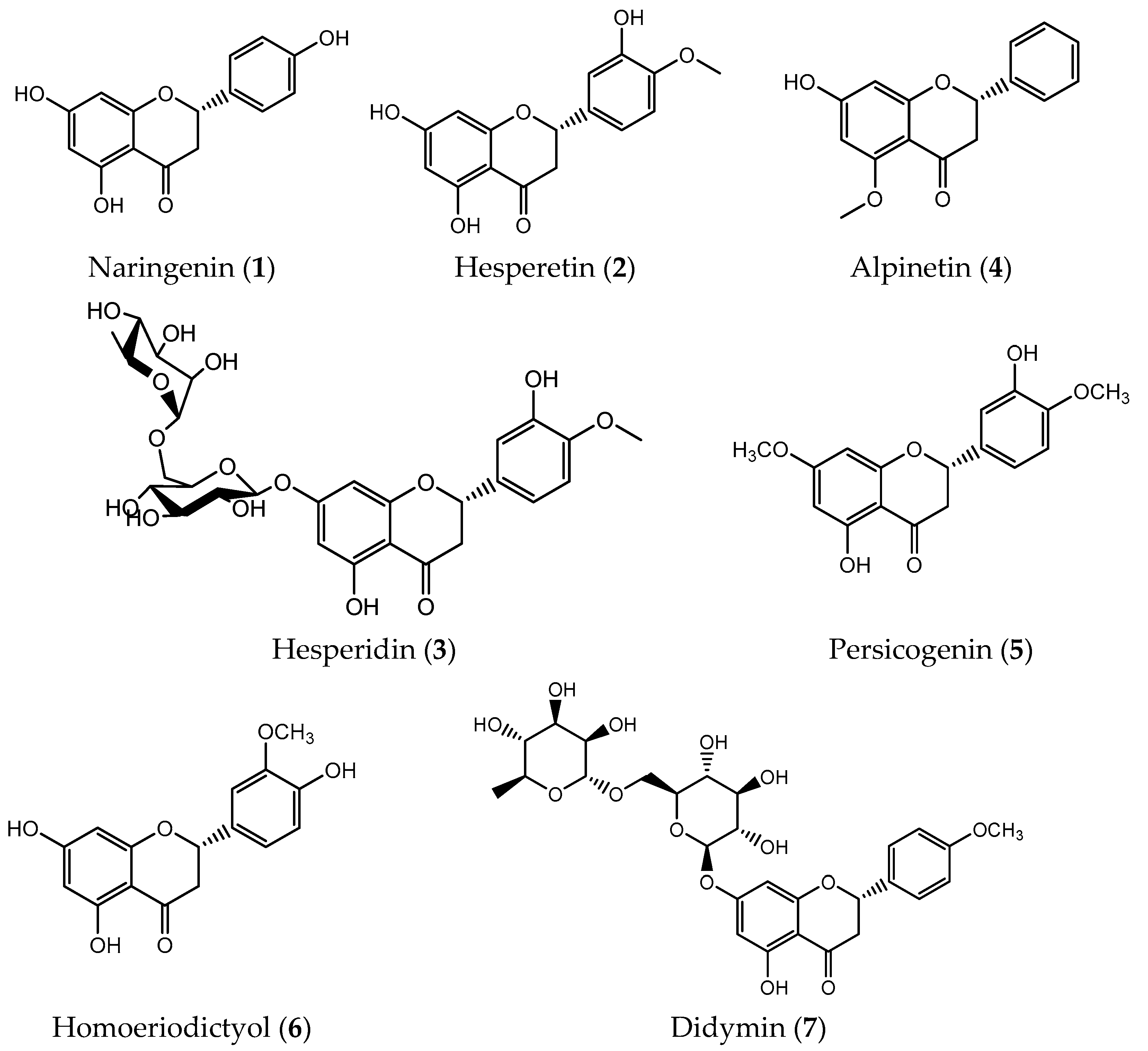


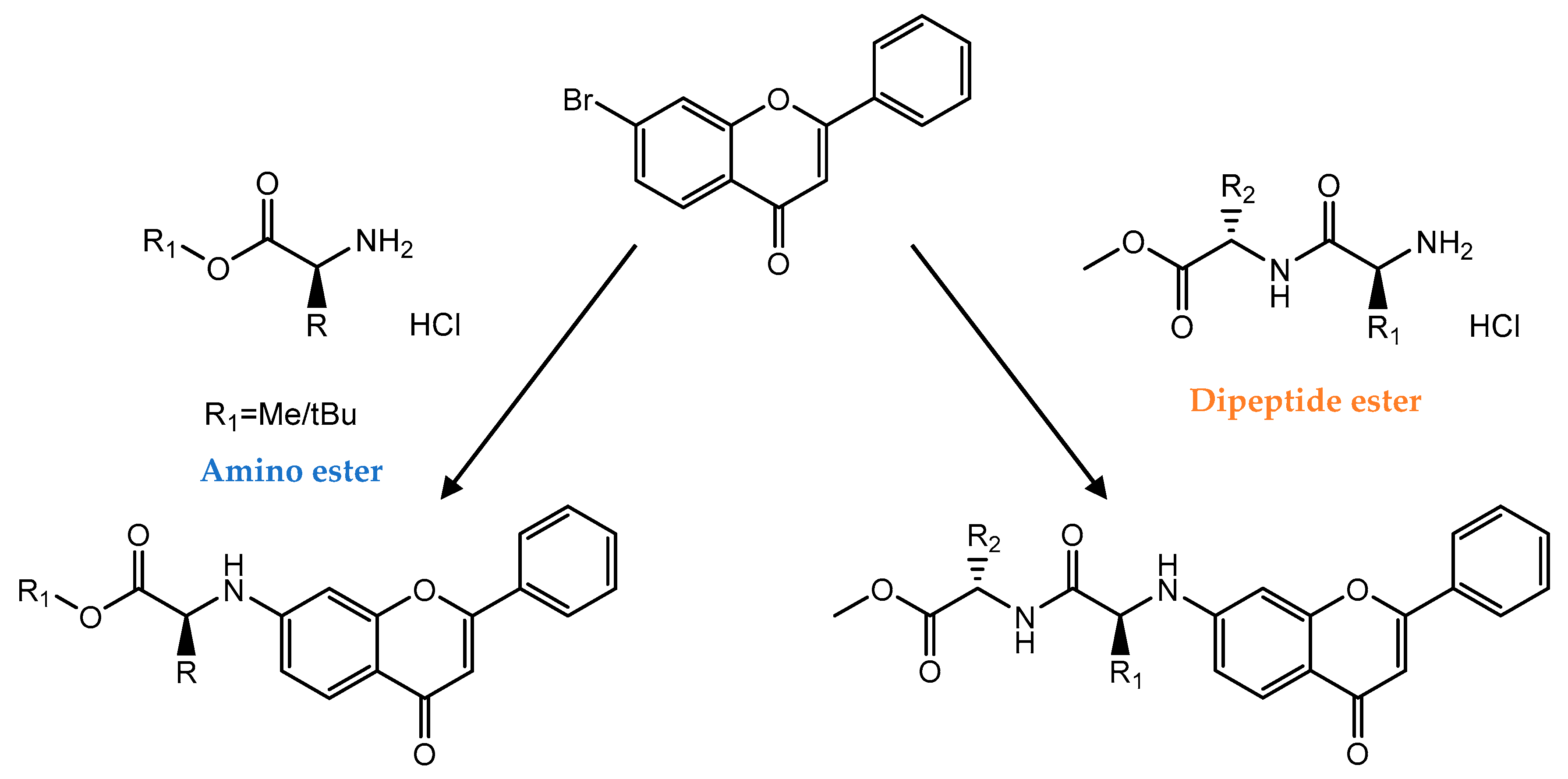
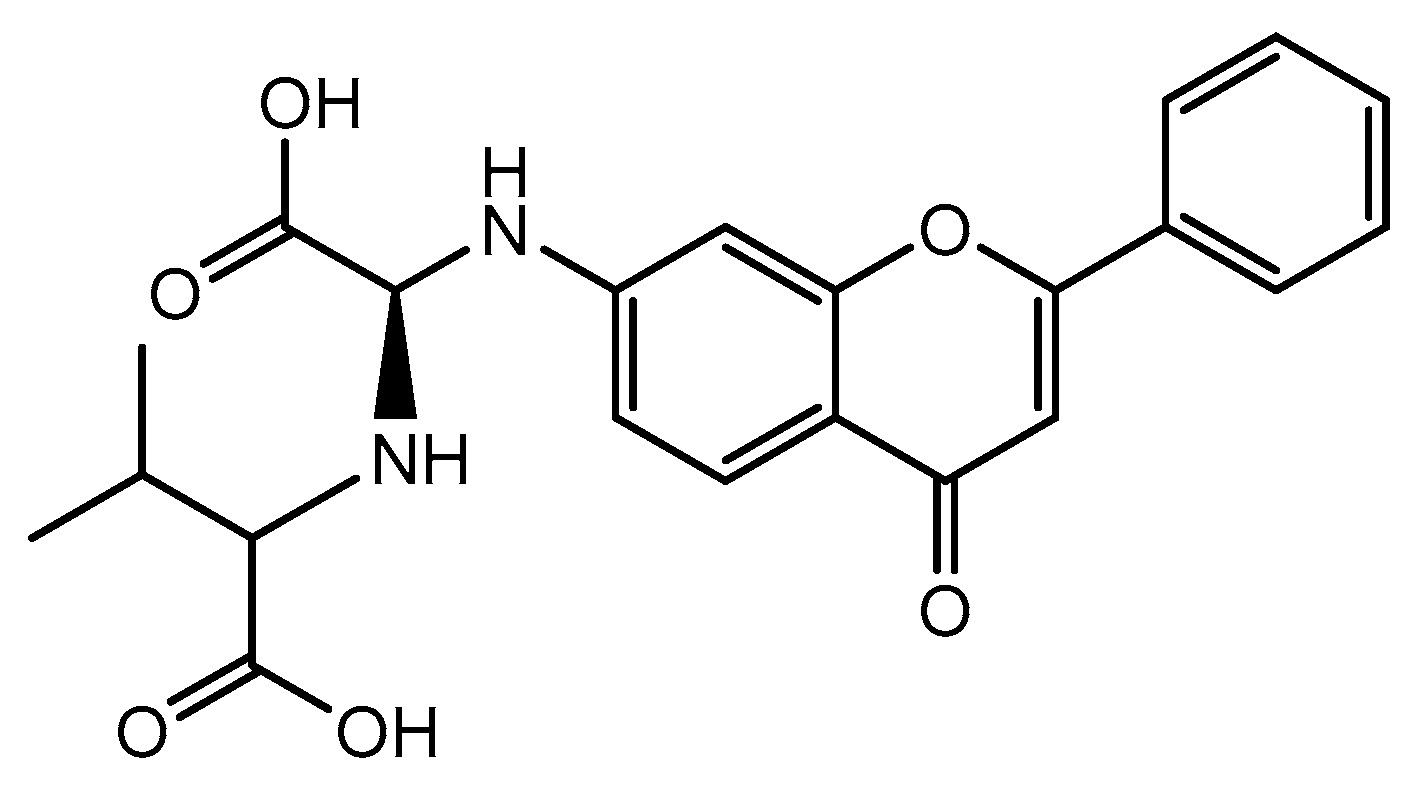

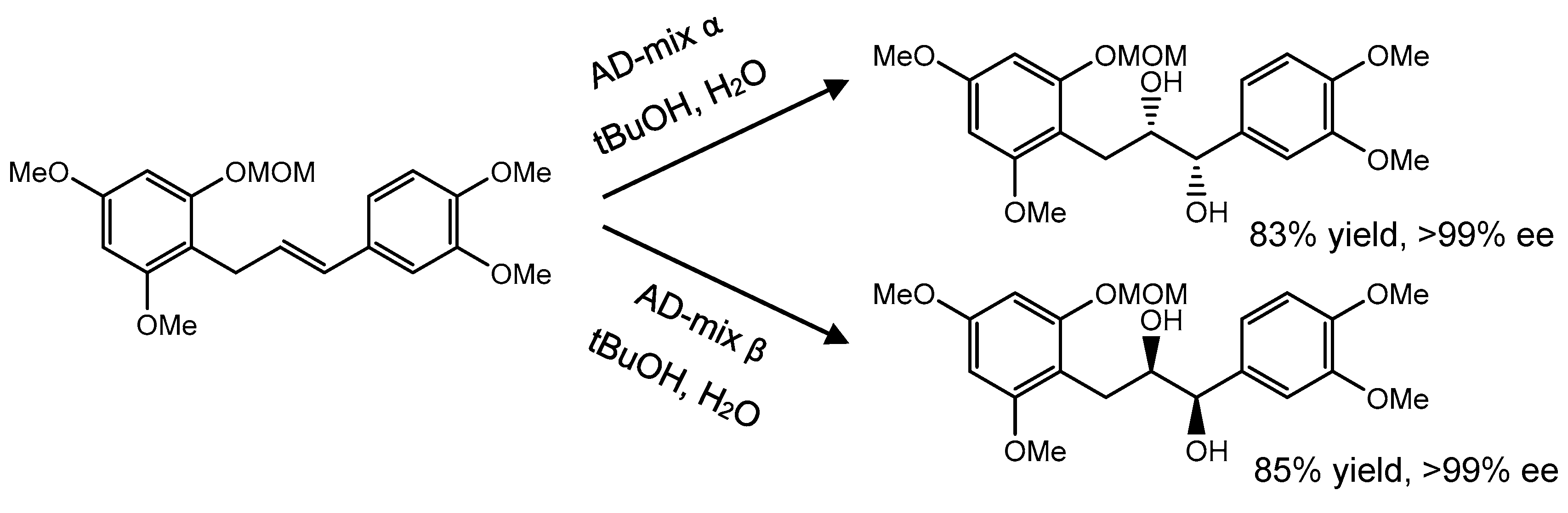
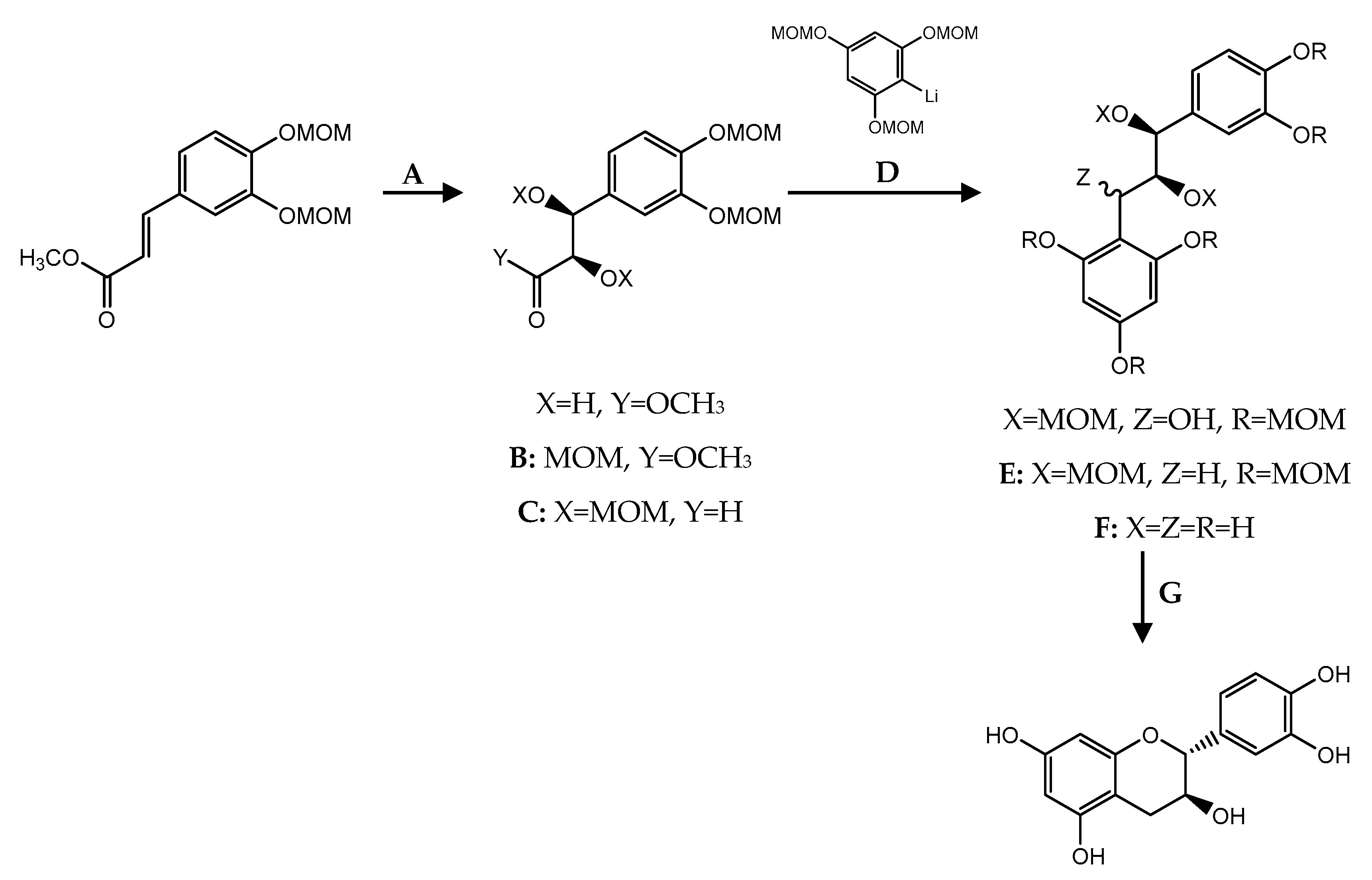
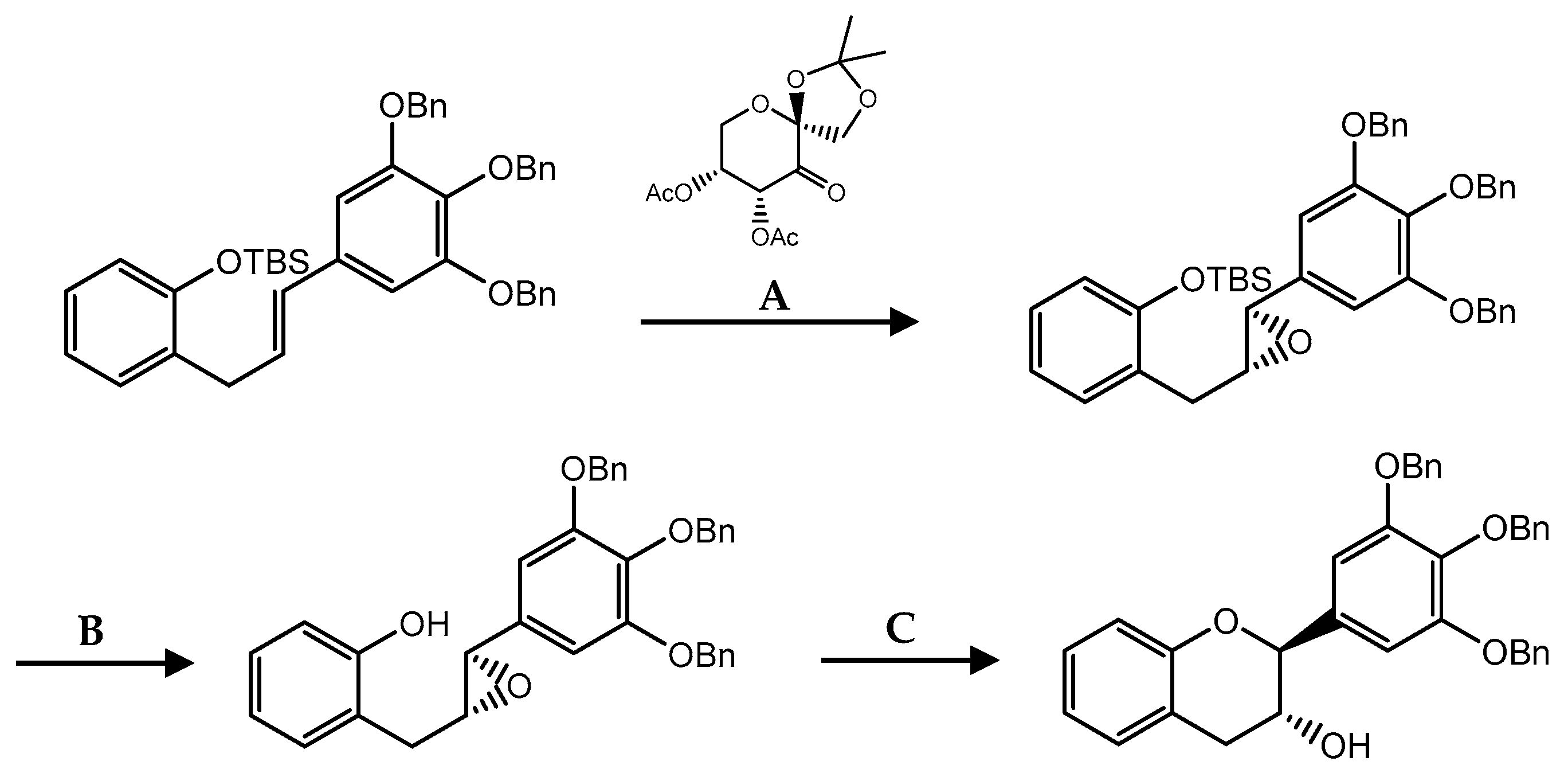
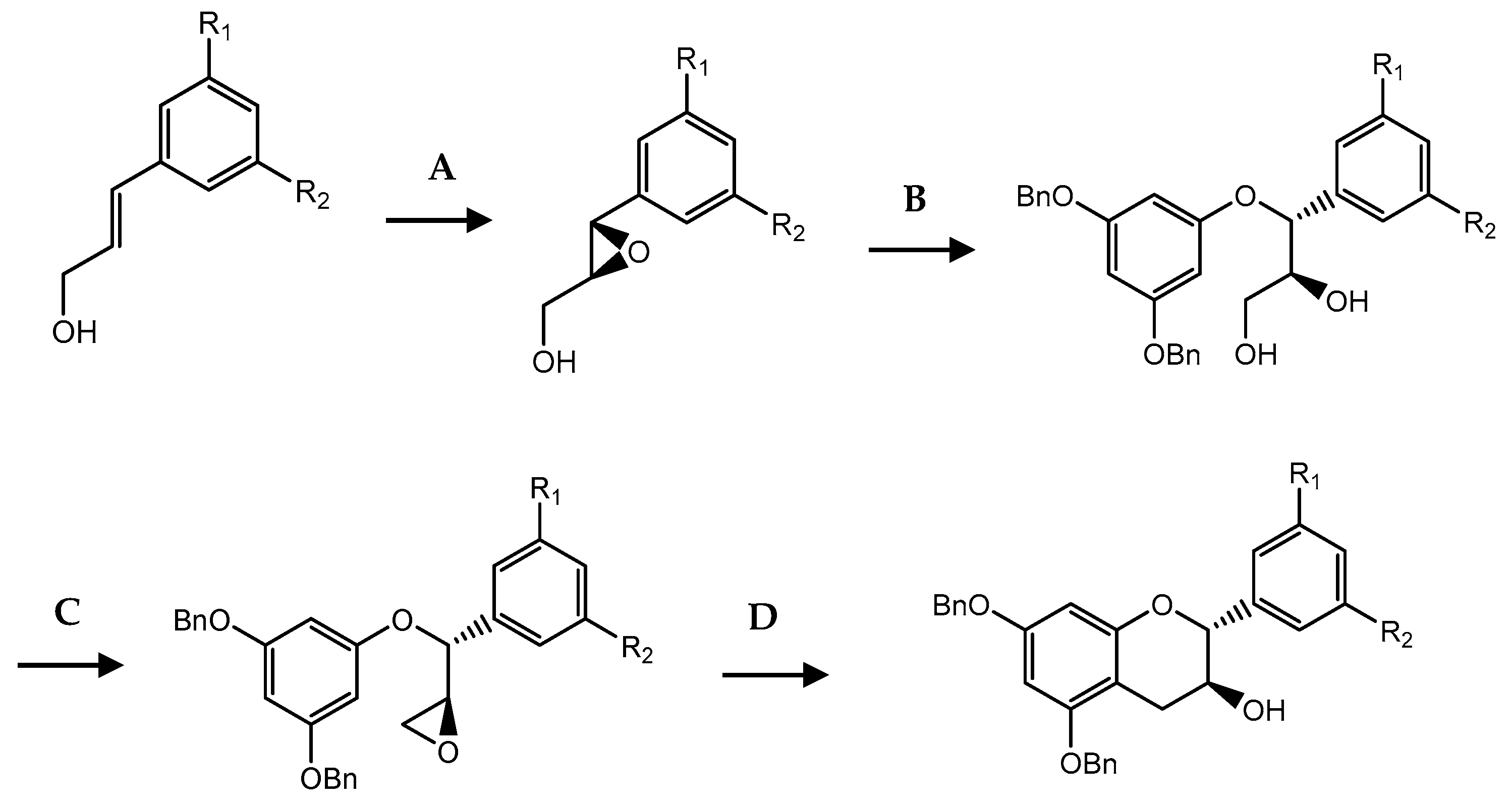

| C ring | Saturated | 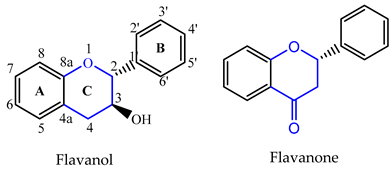 |
| Insaturated | 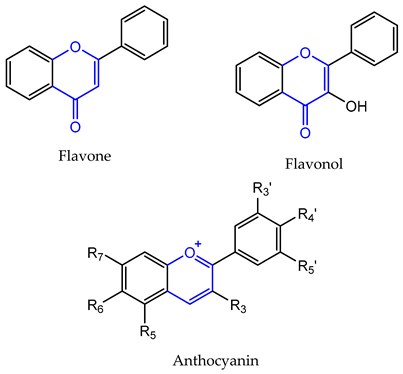 | |
| B ring |  | |
| Flavonoid Subclass | Name | Cancer Cells/Effects | Ref. |
|---|---|---|---|
| Flavanone | Naringenin (1) | hepatocellular carcinoma (IC50 = 100 µM) gastric cancer (IC50 = 10 µM) melanoma (IC50 = 3 µM) non-small-cell lung carcinoma (IC50 = 100 µM) | [35,165] |
| Hesperetin (2) | gastric (IC50 = 40 μM) breast (IC50 = 20 μM) prostate (IC50 = 90 μM) colon (IC50 = 100 μM) lung (IC50 = 40 μM) liver (IC50 = 87 μM) | [39] | |
| Alpinetin (4) | lung (IC50 = 25 μM) gastric (IC50 = 120 μM) ovarian (IC50 = 50 μM) pancreatic (IC50 = 60 μg/mL) | [40,41,42,166] | |
| Persicogenin (5) | human cervical cancer (IC50 = 500 μg/mL) breast carcinoma (IC50 = 500 μg/mL) human colon cancer (IC50 = 500 μg/mL) | [44] | |
| Homoeriodictyol (6) | human cervical cancer (IC50 = 500 μg/mL) breast carcinoma (IC50 = 500 μg/mL) human colon cancer (IC50 = 250 μg/mL) | [44] | |
| Didymin (7) | neuroblastoma (IC50 = 50 μM) lung (IC50 = 11.06 μM) | [50,52,167] | |
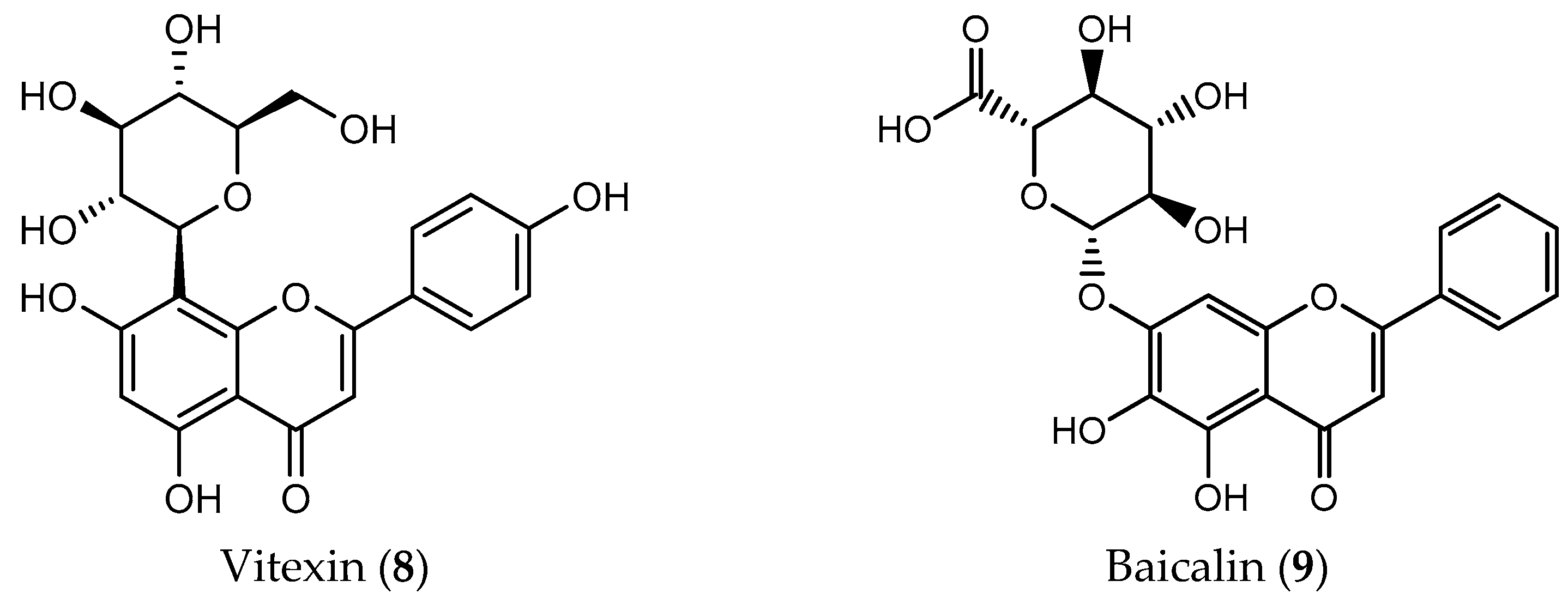
| Flavone | Vitexin (8) | leukemia (IC50 = 200 μM) glioblastoma (IC50 = 32 μM) hepatocellular carcinoma (IC50 = 5 μM) lung carcinoma (IC50 = 40 μM) | [53,168,169,170] |
| Baicalin (9) | breast (IC50 = 100 μM) colon (IC50 = 20 μM) prostate (IC50 = 150 μM) lung (IC50 = 80 μg/mL) gastric (IC50 = 80 μM) osteosarcoma (IC50 = 25 μM) | [60,167,171,172,173,174] | |
| Ficine (20) | CDK1 and CDK5 inhibition (IC50 = 0.04 µM) | [105] | |
| (−)-O-demthylbuchenavianine (21) | CDK1 inhibition (IC50 = 0.03 µM) CDK5 inhibition (IC50 = 0.05 µM) | [105] | |
| R-Leucoflavonine (23) | hepatocellular carcinoma (IC50 = 52.9 µM) | [103] | |
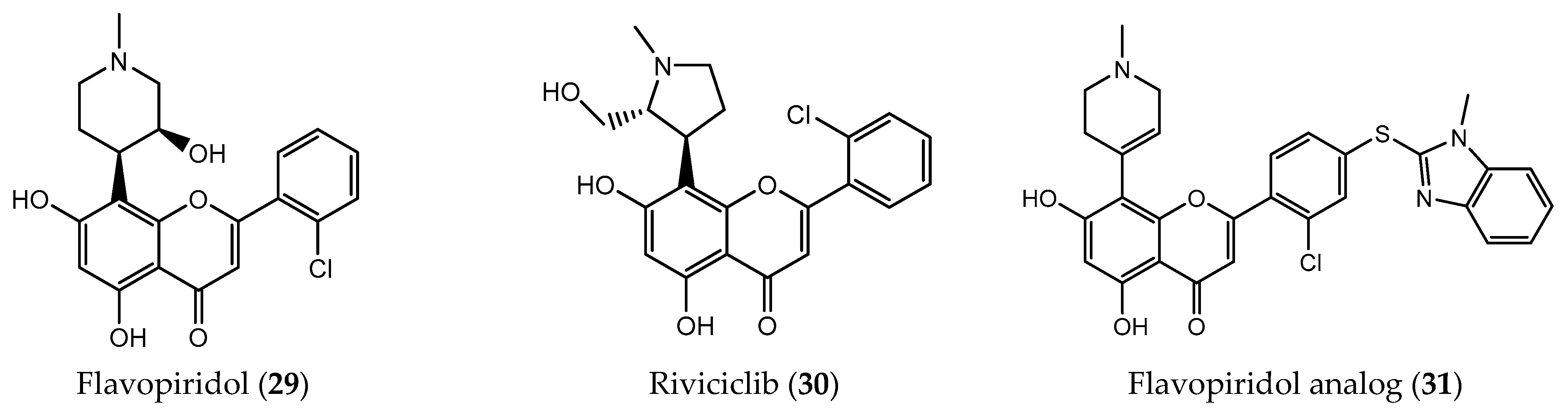
| Flavopiridol (29) | CDK1 inhibition (IC50 = 30 nM) CDK7 inhibition (IC50 = 10 nM) CDK9 inhibition (IC50 = 3 nM) colon-carcinoma (IC50 = 20 nM) breast cancer (IC50 = 75 nM) gastric adenocarcinoma (111 nM) | [175,176,177] | |
| Riviciclib (30) | CDK1 inhibition (IC50 = 79 nM) CDK9 inhibition (IC50 = 20 nM) | [177] | |
| 32 | gastric carcinoma (IC50 = 3.8 µM) | [131] | |
| 33 | breast (IC50 = 16.6 μM) | [134] | |
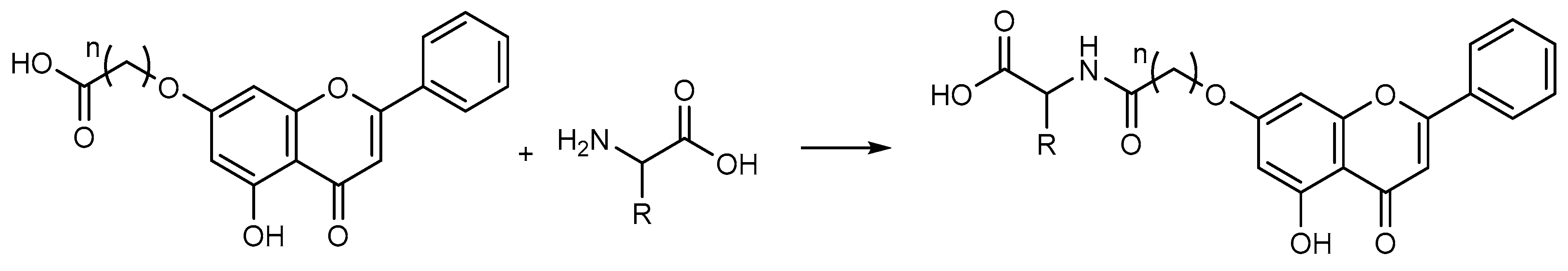
| Flavone−dipeptide hybrid L-Val-OH (41) | leukemia (IC50 = 9.2 µM) | [117] | |
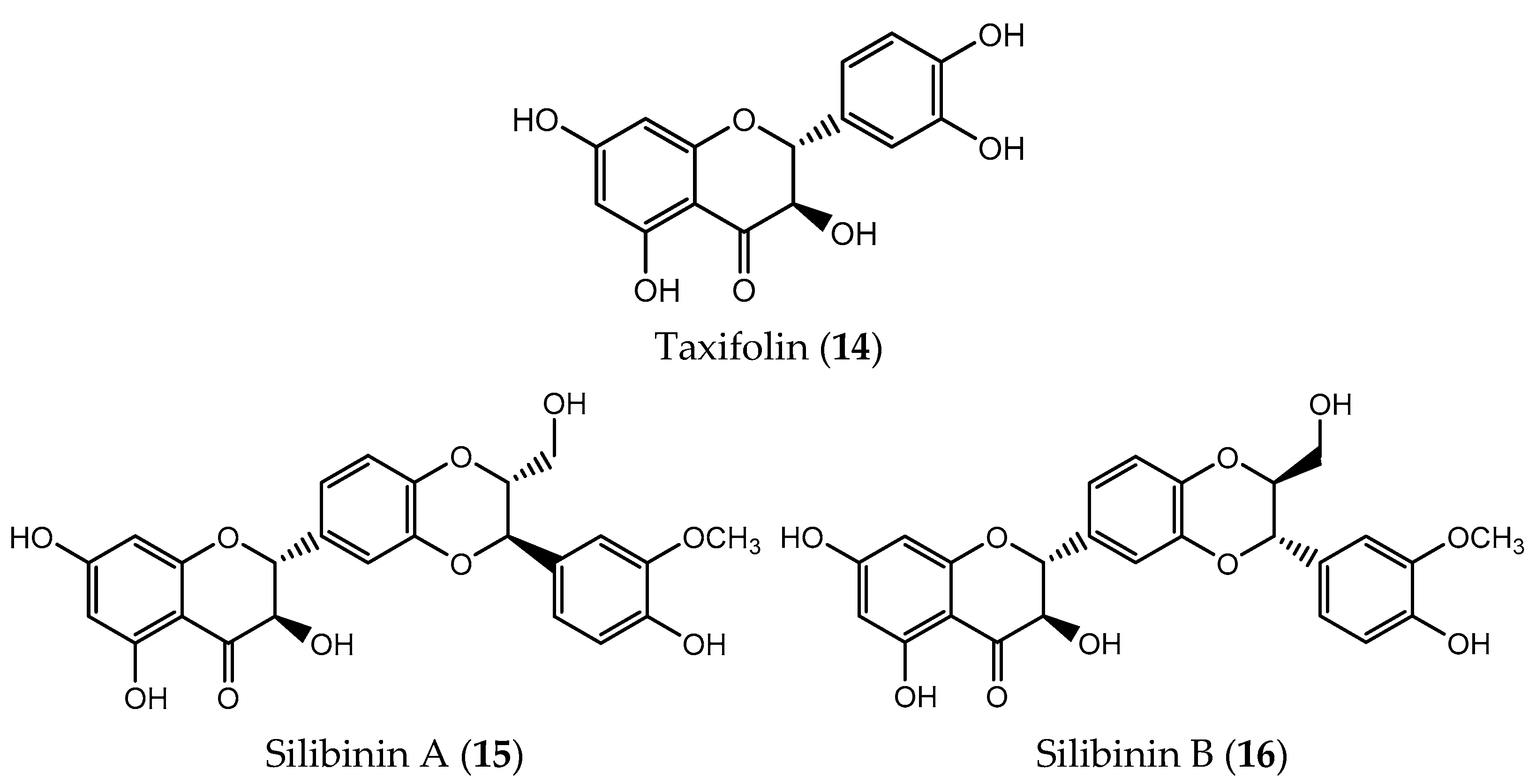
| Flavonol | Taxifolin (14) | colorectal (IC50 = 40 µM) breast (IC50 = 10 µM) lung (IC50 = 25 µM) skin (IC50 = 80 µM) | [178] |
| Quercetin−glutamic acid conjugate 7-O-Glu-Q (35) | MDR uterine sarcoma (IC50 = 0.14 μM) | [141] | |
| L-valinequercetin diorganotin(IV) (38) | cervix (GI50 < 10 μg/mL) breast (GI50 < 10 μg/mL) liver (GI50 < 10 μg/mL) pancreatic (GI50 < 10 μg/mL) | [140] | |
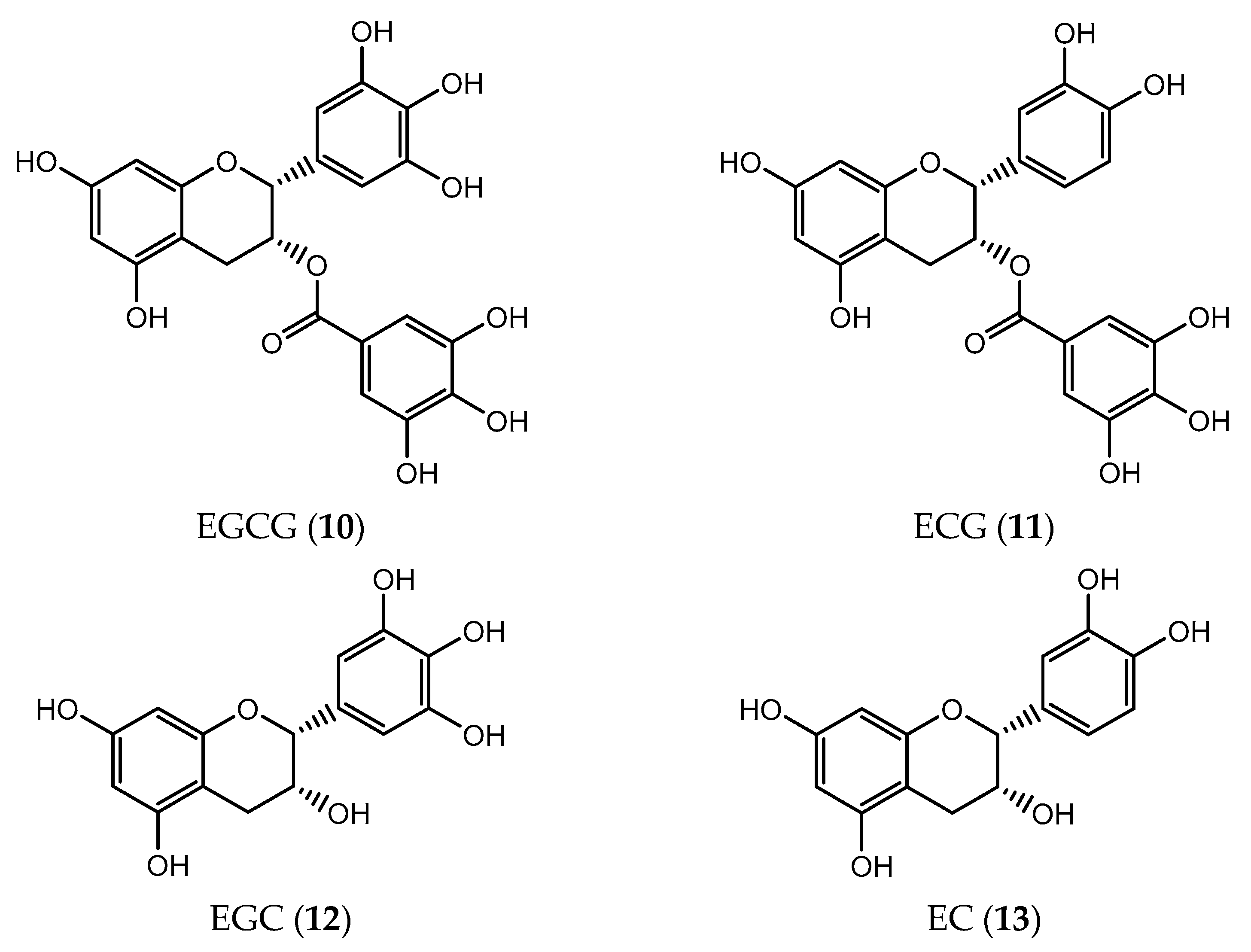
| Flavanol | (−)-epigallocatechin-3-gallate (EGCG) (10) | prostatic adenocarcinoma (IC50 = 39 µM) colon (IC50 = 3 µM) adrenal (IC50 = 20 µM) breast (IC50 = 20 µM) melanoma (IC50 = 7 µM) pancreatic (IC50 < 50 µM) | [179,180,181,182] |
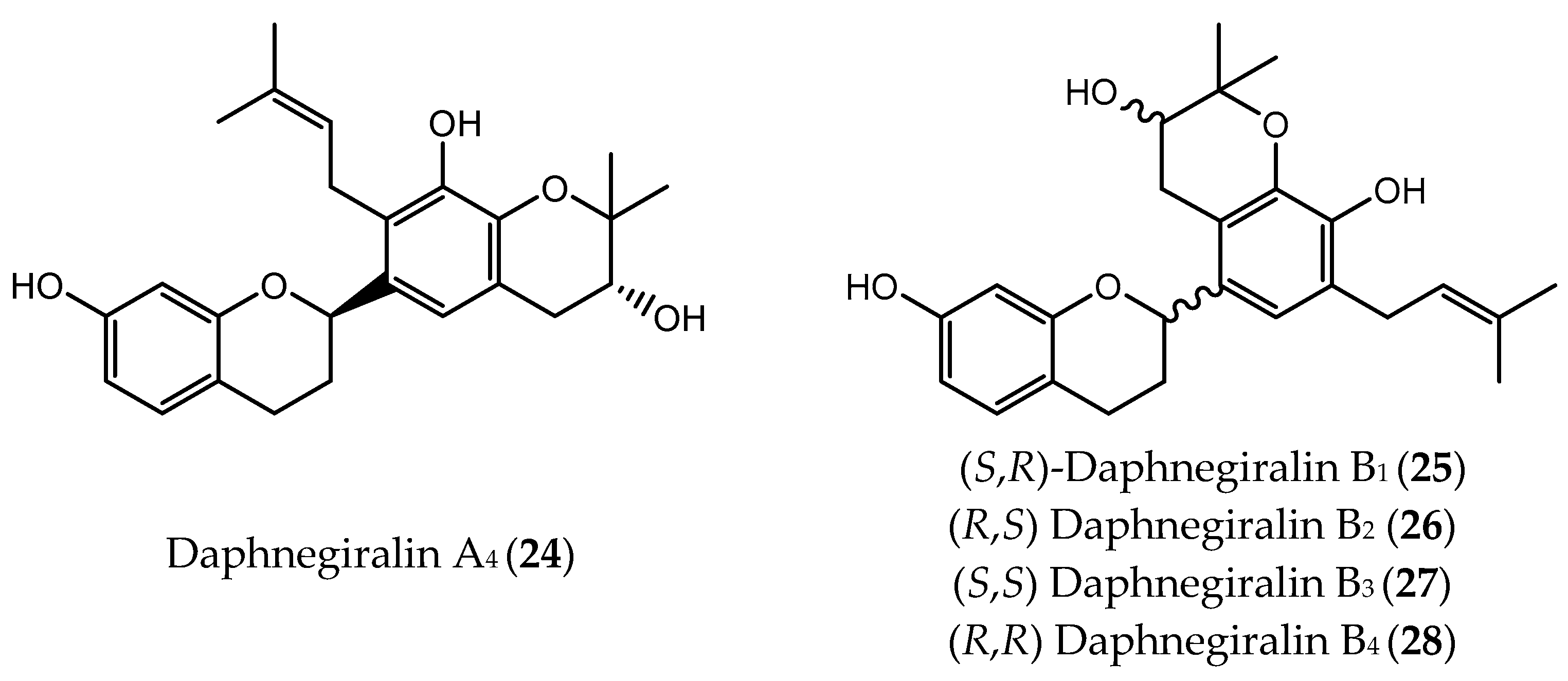
| Daphnegiralin A4 (24) | hepatocellular carcinoma (IC50 = 5.1 µM) | [107] | |
| Daphnegiralins B1: (2-S,2′-R) (25) Daphnegiralins B2: (2-R,2′-S) (26) | hepatocellular carcinoma (IC50 = 6.1 µM) | [107] | |
| Daphnegiralins B3: (2-S,2′-S) (27) Daphnegiralins B4: (2-R,2′-R) (28) | hepatocellular carcinoma (IC50 = 5.4 µM) | [107] | |
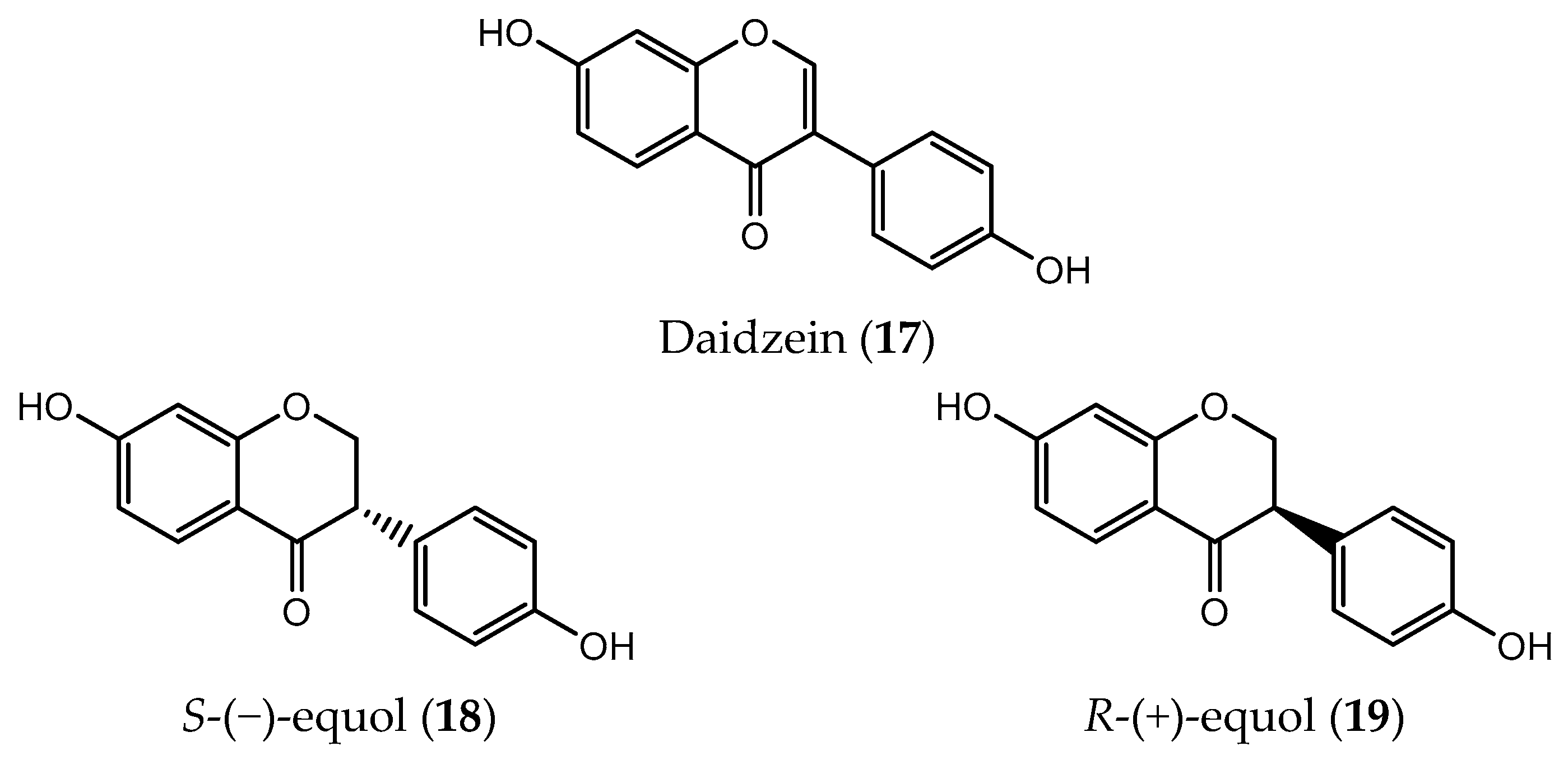
| Isoflavone | S-(−)-equol (18) | breast cancer (IC50 = 10 µM) prostate cancer (IC50 = 5 µM) | [97,183] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinto, C.; Cidade, H.; Pinto, M.; Tiritan, M.E. Chiral Flavonoids as Antitumor Agents. Pharmaceuticals 2021, 14, 1267. https://doi.org/10.3390/ph14121267
Pinto C, Cidade H, Pinto M, Tiritan ME. Chiral Flavonoids as Antitumor Agents. Pharmaceuticals. 2021; 14(12):1267. https://doi.org/10.3390/ph14121267
Chicago/Turabian StylePinto, Cláudia, Honorina Cidade, Madalena Pinto, and Maria Elizabeth Tiritan. 2021. "Chiral Flavonoids as Antitumor Agents" Pharmaceuticals 14, no. 12: 1267. https://doi.org/10.3390/ph14121267
APA StylePinto, C., Cidade, H., Pinto, M., & Tiritan, M. E. (2021). Chiral Flavonoids as Antitumor Agents. Pharmaceuticals, 14(12), 1267. https://doi.org/10.3390/ph14121267










