Exosome microRNAs in Metabolic Syndrome as Tools for the Early Monitoring of Diabetes and Possible Therapeutic Options
Abstract
:1. Introduction
2. Characteristics of Exosome-miRNAs
3. Dysregulation of Exosome-miRNAs in Diabetes
4. Mechanism of Exosome-miRNAs in Diabetes Progression
Exosome-miRNAs and Insulin Resistance
5. Exosome-miRNAs in Diabetes: Potential Clinical Applications
6. Challenging Tasks and Opportunities of Exosome miRNAs in Diabetes
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Alegre-Díaz, J.; Herrington, W.; López-Cervantes, M.; Gnatiuc, L.; Ramirez, R.; Hill, M.; Baigent, C.; McCarthy, M.; Lewington, S.; Collins, R.; et al. Diabetes and cause–specific mortality in Mexico City. N. Engl. J. Med. 2016, 375, 1961–1971. [Google Scholar] [CrossRef] [PubMed]
- Murphy–Chutorian, B.; Han, G.; Cohen, S.R. Dermatologic manifestations of diabetes mellitus: A review. Endocrinol. Metab. Clin. N. Am. 2013, 42, 869–898. [Google Scholar] [CrossRef] [PubMed]
- Cione, E.; Caroleo, M.C.; Cannataro, R.; Perri, M.; Pingitore, A.; Genchi, G. Vitamin A and Diabesity: New Insight for Drug Discovery. Mini Rev. Med. Chem. 2016, 16, 738–742. [Google Scholar] [CrossRef] [PubMed]
- King, H.; Aubert, R.E.; Herman, W.H. Global burden of diabetes, 1995–2025: Prevalence, numerical estimates, and projections. Diabetes Care 1998, 21, 1414–1431. [Google Scholar] [CrossRef]
- Cho, N.H.; Shaw, J.E.; Karuranga, S.; Huang, Y.; da Rocha Fernandes, J.D.; Ohlrogge, A.W.; Malanda, B. IDF diabetes atlas: Global estimates of diabetes prevalence for 2017 and projections for Diabetes. Res. Clin. Pract. 2018, 138, 271–281. [Google Scholar] [CrossRef]
- Wang, L.; Kong, L.; Wu, F.; Bai, Y.; Burton, R. Preventing chronic diseases in China. Lancet 2005, 366, 1821–1824. [Google Scholar] [CrossRef]
- Lysy, P.A.; Corritore, E.; Sokal, E.M. New insights into diabetes cell therapy. Curr. Diab. Rep. 2016, 16, 38. [Google Scholar] [CrossRef]
- Seo, N.; Akiyoshi, K.; Shiku, H. Exosome–mediated regulation of tumor immunology. Cancer Sci. 2018, 109, 2998–3004. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Yu, M.; Tian, W. Physiological and pathological impact of exosomes of adipose tissue. Cell Prolif. 2016, 49, 3–13. [Google Scholar] [CrossRef] [Green Version]
- Kowal, J.; Tkach, M.; Thery, C. Biogenesis and secretion of exosomes. Curr. Opin. Cell Biol. 2014, 29, 116–125. [Google Scholar] [CrossRef] [Green Version]
- Cione, E.; Lucente, M.; Gallelli, L.; De Sarro, G.; Luciani, F.; Caroleo, M.C. Innate Immunity and Human Milk MicroRNAs Content: A New Perspective for Premature Newborns. J. Compr. Ped. 2017, 8, e43359. [Google Scholar] [CrossRef] [Green Version]
- Perri, M.; Lucente, M.; Cannataro, R.; de Luca, I.F.; Gallelli, L.; Moro, G.; de Sarro, G.; Caroleo, M.C.; Cione, E. Variation in Immune-Related microRNAs Profile in Human Milk Amongst Lactating Women. MicroRNA 2018, 7, 107–114. [Google Scholar] [CrossRef]
- Zhang, H.; Deng, T.; Ge, S.; Liu, Y.; Bai, M.; Zhu, K.; Fan, Q.; Li, J.; Ning, T.; Tian, F.; et al. Exosome circRNA secreted from adipocytes promotes the growth of hepatocellular carcinoma by targeting deubiquitination–related. USPOncogene 2019, 38, 2844–2859. [Google Scholar] [CrossRef] [Green Version]
- Gallelli, L.; Cione, E.; Caroleo, M.C.; Carotenuto, M.; Lagana, P.; Siniscalchi, A.; Guidetti, V. MicroRNAs to Monitor Pain migraine and Drug Treatment. MicroRNA 2017, 6, 152–156. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, Y.; Wei, M.; Han, X.; Xu, T.; Cui, M. Advances in the study of exosomal lncRNAs in tumors and the selection of research methods. Biomed. Pharmacother. 2020, 123, 109716. [Google Scholar] [CrossRef]
- Mathieu, M.; Martin–Jaular, L.; Lavieu, G.; Thery, C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell–to–cell communication. Nat. Cell Biol. 2019, 21, 9–17. [Google Scholar] [CrossRef]
- Tingö, L.; Ahlberg, E.; Johansson, L.; Pedersen, S.A.; Chawla, K.; Sætrom, P.; Cione, E.; Simpson, M.R. Non-Coding RNAs in Human Breast Milk: A Systematic Review. Front. Immunol. 2021, 12, 725323. [Google Scholar] [CrossRef]
- Carrasco–Ramiro, F.; Peiro–Pastor, R.; Aguado, B. Human genomics projects and precision medicine. Gene Ther. 2017, 24, 551–561. [Google Scholar] [CrossRef]
- Wang, Y.; Nie, H.; He, X.; Liao, Z.; Zhou, Y.; Zhou, J.; Ou, C. The emerging role of super enhancer–derived non-coding RNAs in human cancer. Theranostics 2020, 10, 11049–11062. [Google Scholar] [CrossRef]
- Ou, C.; Sun, Z.; Li, X.; Ren, W.; Qin, Z.; Zhang, X.; Yuan, W.; Wang, J.; Yu, W.; Zhang, S.; et al. MiR–590–5p, a density–sensitive microRNA, inhibits tumorigenesis by targeting YAP1 in colorectal cancer. Cancer Lett. 2017, 399, 53–63. [Google Scholar] [CrossRef]
- Nie, H.; Wang, Y.; Liao, Z.; Zhou, J.; Ou, C. The function and mechanism of circular RNAs in gastrointestinal tumours. Cell Prolif. 2020, 53, e12815. [Google Scholar] [CrossRef] [PubMed]
- Ou, C.; Sun, Z.; He, X.; Li, X.; Fan, S.; Zheng, X.; Peng, Q.; Li, G.; Li, X.; Ma, J. Targeting YAP1/LINC00152/FSCN1 signaling axis prevents the progression of colorectal cancer. Adv. Sci. 2020, 7, 1901380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cortez, M.A.; Bueso–Ramos, C.; Ferdin, J.; Lopez–Berestein, G.; Sood, A.K.; Calin, G.A. MicroRNAs in body fluids–the mix of hormones and biomarkers. Nat. Rev. Clin. Oncol. 2011, 8, 467–477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valadi, H.; Ekstrom, K.; Bossios, A.; Sjostrand, M.; Lee, J.J.; Lotvall, J.O. Exosome–mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007, 9, 654–659. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.X.; Xie, P.; Li, Y.S.; Wen, T.; Yang, X.C. Osteoclast–derived miR–23a–5p–containing exosomes inhibit osteogenic differentiation by regulating Runx2. Cell Signal. 2020, 70, 109504. [Google Scholar] [CrossRef]
- Zhang, X.; Sai, B.; Wang, F.; Wang, L.; Wang, Y.; Zheng, L.; Li, G.; Tang, J.; Xiang, J. Hypoxic BMSC–derived exosomal miRNAs promote metastasis of lung cancer cells via STAT3–induced EMT. Mol. Cancer 2019, 18, 40. [Google Scholar] [CrossRef] [Green Version]
- Zhu, L.-P.; Tian, T.; Wang, J.-Y.; He, J.-N.; Chen, T.; Pan, M.; Xu, L.; Zhang, H.-X.; Qiu, X.-T.; Li, C.-C.; et al. Hypoxia–elicited mesenchymal stem cell–derived exosomes facilitates cardiac repair through miR–125b–mediated prevention of cell death in myocardial infarction. Theranostics 2018, 8, 6163–6177. [Google Scholar] [CrossRef]
- Xie, Y.; Dang, W.; Zhang, S.; Yue, W.; Yang, L.; Zhai, X.; Yan, Q.; Lu, J. The role of exosomal non-coding RNAs in cancer. Mol. Cancer 2019, 18, 37. [Google Scholar] [CrossRef] [Green Version]
- Zheng, H.; Zhan, Y.; Liu, S.; Lu, J.; Luo, J.; Feng, J.; Fan, S. The roles of tumor–derived exosomes in non–small cell lung cancer and their clinical implications. J. Exp. Clin. Cancer Res. 2018, 37, 226. [Google Scholar] [CrossRef]
- Trams, E.G.; Lauter, C.J.; Salem, N.J.; Heine, U. Exfoliation of membrane ecto–enzymes in the form of micro–vesicles. Biochim. Biophys. Acta 1981, 645, 63–70. [Google Scholar] [CrossRef]
- Johnstone, R.M.; Adam, M.; Hammond, J.R.; Orr, L.; Turbide, C. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes). J. Biol. Chem. 1987, 262, 9412–9420. [Google Scholar] [CrossRef]
- Raposo, G.; Nijman, H.W.; Stoorvogel, W.; Liejendekker, R.; Harding, C.V.; Melief, C.J.; Geuze, H.J. B lymphocytes secrete antigen–presenting vesicles. J. Exp. Med. 1996, 183, 1161–1172. [Google Scholar] [CrossRef]
- Hoshino, A.; Kim, H.S.; Bojmar, L.; Gyan, K.E.; Cioffi, M.; Hernandez, J.; Zambirinis, C.P.; Rodrigues, G.; Molina, H.; Heissel, S.; et al. Extracellular vesicle and particle biomarkers define multiple human cancers. Cell 2020, 182, 1044–1061. [Google Scholar] [CrossRef]
- Van der Pol, E.; Boing, A.N.; Harrison, P.; Sturk, A.; Nieuwland, R. Classification, functions, and clinical relevance of extracellular vesicles. Pharmacol. Rev. 2012, 64, 676–705. [Google Scholar] [CrossRef] [Green Version]
- Laulagnier, K.; Motta, C.; Hamdi, S.; Roy, S.; Fauvelle, F.; Pageaux, J.-F.; Kobayashi, T.; Salles, J.-P.; Perret, B.; Bonnerot, C.; et al. Mast cell– and dendritic cell–derived exosomes display a specific lipid composition and an unusual membrane organization. Biochem. J. 2004, 380, 161–171. [Google Scholar] [CrossRef]
- Conde–Vancells, J.; Rodriguez–Suarez, E.; Embade, N.; Gil, D.; Matthiesen, R.; Valle, M.; Elortza, F.; Lu, S.C.; Mato, J.M.; Falcon-Perez, J.M. Characterization and comprehensive proteome profiling of exosomes secreted by hepatocytes. J. Proteome Res. 2008, 7, 5157–5166. [Google Scholar] [CrossRef] [Green Version]
- Fei, F.; Joo, E.J.; Tarighat, S.S.; Schiffer, I.; Paz, H.; Fabbri, M.; Abdel-Azim, H.; Groffen, J.; Heisterkamp, N. B–cell precursor acute lymphoblastic leukemia and stromal cells communicate through galectin. Oncotarget 2015, 6, 11378–11394. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Zhang, S.; Yao, J.; Lowery, F.J.; Zhang, Q.; Huang, W.-C.; Li, P.; Li, M.; Wang, X.; Zhang, C.; et al. Microenvironment–induced PTEN loss by exosomal microRNA primes brain metastasis outgrowth. Nature 2015, 527, 100–104. [Google Scholar] [CrossRef]
- Bosque, A.; Dietz, L.; Gallego–Lleyda, A.; Sanclemente, M.; Iturralde, M.; Naval, J.; Alava, M.A.; Martinez-Lostao, L.; Martinez-Lostao, L.; Thierse, H.J.; et al. Comparative proteomics of exosomes secreted by tumoral Jurkat T cells and normal human T cell blasts unravels a potential tumorigenic role for valosin–containing protein. Oncotarget 2016, 7, 29287–29305. [Google Scholar] [CrossRef]
- Huang, X.; Yuan, T.; Tschannen, M.; Sun, Z.; Jacob, H.; Du, M.; Liang, M.; Dittmar, R.L.; Liu, Y.; Liang, M.; et al. Characterization of human plasma-derived exosomal RNAs by deep sequencing. BMC Genom. 2013, 14, 319. [Google Scholar] [CrossRef] [Green Version]
- Mittelbrunn, M.; Gutierrez-Vazquez, C.; Villarroya-Beltri, C.; Gonzalez, S.; Sanchez-Cabo, F.; Gonzalez, M.A.; Bernad, A.; Sanchez-Madrid, F. Unidirectional transfer of microRNA-loaded exosomes from T cells to antigen-presenting cells. Nat. Commun. 2011, 2, 282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang–Doran, I.; Zhang, C.Y.; Vidal–Puig, A. Extracellular vesicles: Novel mediators of cell communication in metabolic disease. Trends Endocrinol. Metab. 2017, 28, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Hewson, C.; Capraro, D.; Burdach, J.; Whitaker, N.; Morris, K.V. Extracellular vesicle associated long non-coding RNAs functionally enhance cell viability. Non-coding RNA Res. 2016, 1, 3–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Jong, O.G.; Verhaar, M.C.; Chen, Y.; Vader, P.; Gremmels, H.; Posthuma, G.; Schiffelers, R.M.; Gucek, M.; Van Balkom, B.W.M. Cellular stress conditions are reflected in the protein and RNA content of endothelial cell–derived exosomes. J. Extracell Vesicles. 2012, 1, 18396. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, D.; Chen, X.; Li, J.; Li, L.; Bian, Z.; Sun, F.; Lu, J.; Yin, Y.; Cai, X.; et al. Secreted monocytic miR–150 enhances targeted endothelial cell migration. Mol. Cell 2010, 39, 133–144. [Google Scholar] [CrossRef] [Green Version]
- Daneshgari, F.; Moore, C. Diabetic uropathy. Semin. Nephrol. 2006, 26, 182–185. [Google Scholar] [CrossRef]
- Mitrovic–Perisic, N.; Antic, S. Risk factors for coronary heart disease and actual diagnostic criteria for diabetes mellitus. Vojnosanitetski Pregled 2009, 66, 973–978. [Google Scholar] [CrossRef]
- Paul, P.; Chakraborty, A.; Sarkar, D.; Langthasa, M.; Rahman, M.; Bari, M.; Singha, R.S.; Malakar, A.K.; Chakraborty, S. Interplay between miRNAs and human diseases. J. Cell. Physiol. 2018, 233, 2007–2018. [Google Scholar] [CrossRef]
- Eriksson, J.G.; Laine, M.K. Insulin therapy in the elderly with type 2 diabetes. Minerva Endocrinol. 2015, 40, 283–295. [Google Scholar]
- Brennan, E.; Wang, B.; McClelland, A.; Mohan, M.; Marai, M.; Beuscart, O.; Derouiche, S.; Gray, S.; Pickering, R.; Tikellis, C.; et al. Protective effect of let–7 miRNA family in regulating inflammation in diabetes–associated atherosclerosis. Diabetes 2017, 66, 2266–2277. [Google Scholar] [CrossRef] [Green Version]
- Vickers, K.C.; Landstreet, S.R.; Levin, M.; Shoucri, B.M.; Toth, C.L.; Taylor, R.C.; Palmisano, B.T.; Tabet, F.; Cui, H.L.; Rye, K.-A.; et al. MicroRNA–223 coordinates cholesterol homeostasis. Proc. Natl. Acad. Sci. USA 2014, 111, 14518–14523. [Google Scholar] [CrossRef] [Green Version]
- Massart, J.; Sjogren, R.; Lundell, L.S.; Mudry, J.M.; Franck, N.; O’Gorman, D.J.; Egan, B.; Zierath, J.R.; Krook, A. Altered miR–29 expression in type 2 diabetes influences glucose and lipid metabolism in skeletal muscle. Diabetes 2017, 66, 1807–1818. [Google Scholar] [CrossRef] [Green Version]
- Santovito, D.; De Nardis, V.; Marcantonio, P.; Mandolini, C.; Paganelli, C.; Vitale, E.; Buttitta, F.; Bucci, M.; Mezzetti, A.; Consoli, A.; et al. Plasma exosome microRNA profiling unravels a new potential modulator of adiponectin pathway in diabetes: Effect of glycemic control. J. Clin. Endocrinol. Metab. 2014, 99, E1681–E1685. [Google Scholar] [CrossRef]
- Guay, C.; Menoud, V.; Rome, S.; Regazzi, R. Horizontal transfer of exosomal microRNAs transduce apoptotic signals between pancreatic beta–cells. Cell Commun. Signal. 2015, 13, 17. [Google Scholar] [CrossRef] [Green Version]
- Su, T.; Xiao, Y.; Xiao, Y.E.; Guo, Q.I.; Li, C.; Huang, Y.; Deng, Q.; Wen, J.; Zhou, F.; Luo, X.H. Bone marrow mesenchymal stem cells–derived exosomal MiR–29b–3p regulates aging–associated insulin resistance. ACS Nano 2019, 13, 2450–2462. [Google Scholar] [CrossRef]
- Fan, B.; Li, C.; Szalad, A.; Wang, L.; Pan, W.; Zhang, R.; Chopp, M.; Zhang, Z.G.; Liu, X.S. Mesenchymal stromal cell–derived exosomes ameliorate peripheral neuropathy in a mouse model of diabetes. Diabetologia 2020, 63, 431–443. [Google Scholar] [CrossRef]
- Nakano, M.; Nagaishi, K.; Konari, N.; Saito, Y.; Chikenji, T.; Mizue, Y.; Fujimiya, M. Bone marrow–derived mesenchymal stem cells improve diabetes–induced cognitive impairment by exosome transfer into damaged neurons and astrocytes. Sci. Rep. 2016, 6, 24805. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Zhang, C.; Liu, L.; Xi, A.; Chen, B.; Li, Y.; Du, J. Macrophage–derived miR–155–containing exosomes suppress fibroblast proliferation and promote fibroblast inflammation during cardiac injury. Mol. Ther. 2017, 25, 192–204. [Google Scholar] [CrossRef] [Green Version]
- Ashcroft, F.M.; Rorsman, P. Diabetes mellitus and the beta cell: The last ten years. Cell 2012, 148, 1160–1171. [Google Scholar] [CrossRef] [Green Version]
- Guay, C.; Jacovetti, C.; Nesca, V.; Motterle, A.; Tugay, K.; Regazzi, R. Emerging roles of non-coding RNAs in pancreatic beta–cell function and dysfunction. Diabetes Obes. Metab. 2012, 14 (Suppl. 3), 12–21. [Google Scholar] [CrossRef]
- Guay, C.; Regazzi, R. Circulating microRNAs as novel biomarkers for diabetes mellitus. Nat. Rev. Endocrinol. 2013, 9, 513–521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rottiers, V.; Naar, A.M. MicroRNAs in metabolism and metabolic disorders. Nat. Rev. Mol. Cell Biol. 2012, 13, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Perri, M.; Carollo, M.C.; Liu, N.; Gallelli, L.; de Sarro, G.; Kagechika, H.; Cione, E. 9-cis Retinoic acid modulates myotrophin expression and its miR in physiological and pathophysiological cell models. Exp. Cell Res. 2017, 354, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.; Jiang, H.; Wang, Z.; Wang, X.; Chen, H.; Shen, Z.; Xiao, L.; Guo, X.; Yang, T. Injury factors alter miRNAs profiles of exosomes derived from islets and circulation. Aging 2018, 10, 3986–3999. [Google Scholar] [CrossRef]
- Tsukita, S.; Yamada, T.; Takahashi, K.; Munakata, Y.; Hosaka, S.; Takahashi, H.; Gao, J.; Shirai, Y.; Kodama, S.; Asai, Y.; et al. MicroRNAs 106b and 222 improve hyperglycemia in a mouse model of insulin–deficient diabetes via pancreatic beta–cell proliferation. EBioMedicine 2017, 15, 163–172. [Google Scholar] [CrossRef] [Green Version]
- Xu, H.; Du, X.; Xu, J.; Zhang, Y.; Tian, Y.; Liu, G.; Wang, X.; Ma, M.; Du, W.; Liu, Y.; et al. Pancreatic beta-cell microRNA–26a alleviates type 2 diabetes by improving peripheral insulin sensitivity and preserving beta cell function. PLoS Biol. 2020, 18, e3000603. [Google Scholar] [CrossRef]
- Xu, G.; Thielen, L.A.; Chen, J.; Grayson, T.B.; Grimes, T.; Bridges, S.L.; Tse, H.M.; Smith, B.; Patel, R.; Li, P.; et al. Serum miR–204 is an early biomarker of type 1 diabetes–associated pancreatic beta–cell loss. Am. J. Physiol. Endocrinol. Metab. 2019, 317, E723–E730. [Google Scholar] [CrossRef]
- Samuel, V.T.; Shulman, G.I. Mechanisms for insulin resistance: Common threads and missing links. Cell 2012, 148, 852–871. [Google Scholar] [CrossRef] [Green Version]
- Stumvoll, M.; Goldstein, B.J.; van Haeften, T.W. Type 2 diabetes: Principles of pathogenesis and therapy. Lancet 2005, 365, 1333–1346. [Google Scholar] [CrossRef]
- Fernandez–Twinn, D.S.; Alfaradhi, M.Z.; Martin–Gronert, M.S.; Duque-Guimaraes, D.E.; Piekarz, A.; Ferland-McCollough, D.; Bushell, M.; Ozanne, S.E. Downregulation of IRS–1 in adipose tissue of offspring of obese mice is programmed cell–autonomously through post–transcriptional mechanisms. Mol. Metab. 2014, 3, 325–333. [Google Scholar] [CrossRef]
- Chen, Y.-H.; Heneidi, S.; Lee, J.-M.; Layman, L.C.; Stepp, D.W.; Gamboa, G.M.; Chen, B.-S.; Chazenbalk, G.; Azziz, R. miRNA–93 inhibits GLUT4 and is overexpressed in adipose tissue of polycystic ovary syndrome patients and women with insulin resistance. Diabetes 2013, 62, 2278–2286. [Google Scholar] [CrossRef] [Green Version]
- Xu, G.; Ji, C.; Song, G.; Zhao, C.; Shi, C.; Song, L.; Chen, L.; Yang, L.; Huang, F.; Pang, L.; et al. MiR–26b modulates insulin sensitivity in adipocytes by interrupting the PTEN/PI3K/AKT pathway. Int. J. Obes. 2015, 39, 1523–1530. [Google Scholar] [CrossRef]
- Thomou, T.; Mori, M.A.; Dreyfuss, J.M.; Konishi, M.; Sakaguchi, M.; Wolfrum, C.; Rao, T.N.; Winnay, J.N.; Garcia-Martin, R.; Grinspoon, S.K.; et al. Adipose–derived circulating miRNAs regulate gene expression in other tissues. Nature 2017, 542, 450–455. [Google Scholar] [CrossRef]
- Aswad, H.; Forterre, A.; Wiklander, O.P.B.; Vial, G.; Danty-Berger, E.; Lab, C.; Lamazière, A.; Meugnier, E.; Pesenti, S.; Ott, C.; et al. Exosomes participate in the alteration of muscle homeostasis during lipid–induced insulin resistance in mice. Diabetologia 2014, 57, 2155–2164. [Google Scholar] [CrossRef]
- Deng, Z.-B.; Poliakov, A.; Hardy, R.W.; Clements, R.; Liu, C.; Liu, Y.; Wang, J.; Xiang, X.; Zhang, S.; Zhuang, X.; et al. Adipose tissue exosome–like vesicles mediate activation of macrophage–induced insulin resistance. Diabetes 2009, 58, 2498–2505. [Google Scholar] [CrossRef] [Green Version]
- Karolina, D.S.; Arumugam, A.; Tavintharan, S.; Wong, M.T.K.; Lim, S.C.; Sum, C.F.; Jeyaseelan, K. MicroRNA 144 impairs insulin signaling by inhibiting the expression of insulin receptor substrate 1 in type 2 diabetes mellitus. PLoS ONE 2011, 6, e22839. [Google Scholar] [CrossRef]
- Guay, C.; Roggli, E.; Nesca, V.; Jacovetti, C.; Regazzi, R. Diabetes mellitus, a microRNA–related disease? Transl. Res. 2011, 157, 253–264. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.; Mohan, R.; Ozcan, S.; Tang, X. MicroRNA–30d induces insulin transcription factor MafA and insulin production by targeting mitogen–activated protein 4 kinase 4 (MAP4K4) in pancreatic beta–cells. J. Biol. Chem. 2012, 287, 31155–31164. [Google Scholar] [CrossRef] [Green Version]
- Katayama, M.; Wiklander, O.; Fritz, T.; Caidahl, K.; El-Andaloussi, S.; Zierath, J.R.; Zierath, A. Circulating exosomal miR–20b–5p is elevated in type 2 diabetes and could impair insulin action in human skeletal muscle. Diabetes 2019, 68, 515–526. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Zhang, B.; Zheng, W.; Kang, M.; Chen, Q.; Qin, W.; Li, C.; Zhang, Y.; Shao, Y.; Wu, Y. Exosomes derived from pancreatic cancer cells induce insulin resistance in C2C12 myotube cells through the PI3K/Akt/FoxO1 pathway. Sci. Rep. 2017, 7, 5384. [Google Scholar] [CrossRef] [Green Version]
- Karolina, D.S.; Tavintharan, S.; Arumugam, A.; Sepramaniam, S.; Pek, S.L.T.; Wong, M.T.K.; Lim, S.C.; Sum, C.F.; Jeyaseelan, K. Circulating miRNA profiles in patients with metabolic syndrome. J. Clin. Endocrinol. Metab. 2012, 97, E2271–E2276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cannataro, R.; Perri, M.; Gallelli, L.; Caroleo, M.C.; de Sarro, G.; Cione, E. Ketogenic Diet Acts on Body Remodeling and MicroRNAs Expression Profile. Microrna 2019, 8, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Cannataro, R.; Caroleo, M.C.; Fazio, A.; la Torre, C.; Plastina, P.; Gallelli, L.; Lauria, G.; Cione, E. Ketogenic Diet and microRNAs Linked to Antioxidant Biochemical Homeostasis. Antioxidants 2019, 8, 269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castano, C.; Kalko, S.; Novials, A.; Parrizas, M. Obesity–associated exosomal miRNAs modulate glucose and lipid metabolism in mice. Proc. Natl. Acad. Sci. USA 2018, 115, 12158–12163. [Google Scholar] [CrossRef] [Green Version]
- Ying, W.; Riopel, M.; Bandyopadhyay, G.; Dong, Y.; Birmingham, A.; Seo, J.B.; Ofrecio, J.M.; Wollam, J.; Hernandez-Carretero, A.; Fu, W.; et al. Adipose tissue macrophage–derived exosomal miRNAs can modulate in vivo and in vitro insulin sensitivity. Cell 2017, 171, 372–384. [Google Scholar] [CrossRef] [Green Version]
- Boyiadzis, M.; Whiteside, T.L. The emerging roles of tumor–derived exosomes in hematological malignancies. Leukemia 2017, 31, 1259–1268. [Google Scholar] [CrossRef]
- Eissa, S.; Matboli, M.; Bekhet, M.M. Clinical verification of a novel urinary microRNA panel: 133b, –342 and –30 as biomarkers for diabetic nephropathy identified by bioinformatics analysis. Biomed. Pharmacother. 2016, 83, 92–99. [Google Scholar] [CrossRef]
- Argyropoulos, C.; Wang, K.; McClarty, S.; Huang, D.; Bernardo, J.; Ellis, D.; Johnson, J. Urinary microRNA profiling in the nephropathy of type 1 diabetes. PLoS ONE 2013, 8, e54662. [Google Scholar] [CrossRef] [Green Version]
- Barutta, F.; Tricarico, M.; Corbelli, A.; Annaratone, L.; Pinach, S.; Grimaldi, S.; Gruden, G. Urinary exosomal microRNAs in incipient diabetic nephropathy. PLoS ONE 2013, 8, e73798. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, A.; Marban, E. Exosomes: Fundamental biology and roles in cardiovascular physiology. Annu. Rev. Physiol. 2016, 78, 67–83. [Google Scholar] [CrossRef] [Green Version]
- Eissa, S.; Matboli, M.; Aboushahba, R.; Bekhet, M.M.; Soliman, Y. Urinary exosomal microRNA panel unravels novel biomarkers for diagnosis of type 2 diabetic kidney disease. J. Diabetes Complicat. 2016, 30, 1585–1592. [Google Scholar] [CrossRef]
- Wan, S.; Wang, J.; Wu, J.; Song, J.; Zhang, C.Y.; Zhang, C.; Wang, C.; Wang, J.J. Increased serum miR–7 is a promising biomarker for type 2 diabetes mellitus and its microvascular complications. Diabetes Res. Clin. Pract. 2017, 130, 171–179. [Google Scholar] [CrossRef]
- De Gonzalo-Calvo, D.; Van Der Meer, R.W.; Rijzewijk, L.J.; Smit, J.W.A.; Revuelta, E.; Nasarre, L.; Escola-Gil, J.C.; Lamb, H.J.; Llorente-Cortes, V. Serum microRNA–1 and microRNA–133a levels reflect myocardial steatosis in uncomplicated type 2 diabetes. Sci. Rep. 2017, 7, 47. [Google Scholar] [CrossRef]
- Sidorkiewicz, I.; Niemira, M.; Maliszewska, K.; Erol, A.; Bielska, A.; Szalkowska, A.; Adamska-Patruno, E.; Szczerbinski, L.; Gorska, M.; Kretowski, A. Circulating miRNAs as a predictive biomarker of the progression from prediabetes to diabetes: Outcomes of a 5–year prospective observational study. J. Clin. Med. 2020, 9, 2184. [Google Scholar] [CrossRef]
- Deng, L.; Huang, Y.; Li, L.; Chen, H.; Su, J. Serum miR–29a/b expression in gestational diabetes mellitus and its influence on prognosis evaluation. J. Int. Med. Res. 2020, 48, 300060520954763. [Google Scholar] [CrossRef]
- Li, M.; Ke, Q.F.; Tao, S.C.; Guo, S.C.; Rui, B.Y.; Guo, Y.P. Fabrication of hydroxyapatite/chitosan composite hydrogels loaded with exosomes derived from miR–126–3p overexpressed synovial mesenchymal stem cells for diabetic chronic wound healing. J. Mater. Chem. B 2016, 4, 6830–6841. [Google Scholar] [CrossRef]
- Shi, R.; Zhao, L.; Cai, W.; Wei, M.; Zhou, X.; Yang, G.; Yuan, L. Maternal exosomes in diabetes contribute to the cardiac development deficiency. Biochem. Biophys. Res. Commun. 2017, 483, 602–608. [Google Scholar] [CrossRef]
- He, X.; Li, S.; Yu, B.; Kuang, G.; Wu, Y.; Zhang, M.; He, Y.; Ou, C.; Cao, P. Up–regulation of LINC00467 promotes the tumorigenesis in colorectal cancer. J. Cancer 2019, 10, 6405–6413. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.K.; Lee, J.; Simpson, R.J.; Lotvall, J.; Gho, Y.S. Expedia: A community web resource for prokaryotic and eukaryotic extracellular vesicles research. Semin. Cell Dev. Biol. 2015, 40, 4–7. [Google Scholar] [CrossRef]
- Mathivanan, S.; Fahner, C.J.; Reid, G.E.; Simpson, R.J. ExoCarta 2012: Database of exosomal proteins, RNA and lipids. Nucleic Acids Res. 2012, 40, D1241–D1244. [Google Scholar] [CrossRef] [Green Version]
- Pathan, M.; Fonseka, P.; Chitti, S.V.; Kang, T.; Sanwlani, R.; van Deun, J.; Hendrix, A.; Mathivanan, S. Vesiclepedia 2019: A compendium of RNA, proteins, lipids and metabolites in extracellular vesicles. Nucleic Acids Res. 2019, 47, D516–D519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, T.; Zhang, Q.; Zhang, J.; Li, C.; Miao, Y.-R.; Lei, Q.; Li, Q.; Guo, A.-Y. EVmiRNA: A database of miRNA profiling in extracellular vesicles. Nucleic Acids Res. 2019, 47, D89–D93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maity, S.; Bhat, A.H.; Giri, K.; Ambatipudi, K. BoMiProt: A database of bovine milk proteins. J Proteom. 2020, 215, 103648. [Google Scholar] [CrossRef] [PubMed]
- Li, J.R.; Tong, C.Y.; Sung, T.J.; Kang, T.Y.; Zhou, X.J.; Liu, C.C. CMEP: A database for circulating microRNA expression profiling. Bioinformatics 2019, 35, 3127–3132. [Google Scholar] [CrossRef]
- Fan, Y.; Habib, M.; Xia, J. Xeno–miRNet: A comprehensive database and analytics platform to explore xeno–miRNAs and their potential targets. PeerJ. 2018, 6, e5650. [Google Scholar] [CrossRef]
- Li, S.; Li, Y.; Chen, B.; Zhao, J.; Yu, S.; Tang, Y.; Zheng, Q.; Li, Y.; Wang, P.; He, X.; et al. exoRBase: A database of circRNA, lncRNA and mRNA in human blood exosomes. Nucleic Acids Res. 2018, 46, D106–D112. [Google Scholar] [CrossRef] [Green Version]
- Fang, S.; Zhang, L.; Guo, J.; Niu, Y.; Wu, Y.; Li, H.; Zhao, L.; Li, X.; Teng, X.; Sun, X.; et al. NONCODEV5: A comprehensive annotation database for long non-coding RNAs. Nucleic Acids Res. 2018, 46, D308–D314. [Google Scholar] [CrossRef]
- Russo, F.; Di Bella, S.; Nigita, G.; Macca, V.; Laganà, A.; Giugno, R.; Pulvirenti, A.; Ferro, A. miRandola: Extracellular circulating microRNAs database. PLoS ONE 2012, 7, e47786. [Google Scholar] [CrossRef]
- Masud, M.K.; Na, J.; Younus, M.; Hossain, S.A.; Bando, Y.; Shiddiky, M.J.A.; Yamauchi, Y. Superparamagnetic nanoarchitectures for disease–specific biomarker detection. Chem. Soc. Rev. 2019, 48, 5717–5751. [Google Scholar] [CrossRef]
- Soda, N.; Rehm, B.H.A.; Sonar, P.; Nguyen, N.T.; Shiddiky, M.J.A. Advanced liquid biopsy technologies for circulating biomarker detection. J Mater Chem. B 2019, 7, 6670–6704. [Google Scholar] [CrossRef]
- Nair, S.; Ormazabal, V.; Lappas, M.; McIntyre, H.D.; Salomon, C. Extracellular vesicles and their potential role inducing changes in maternal insulin sensitivity during gestational diabetes mellitus. Am. J. Reprod. Immunol. 2021, 85, e13361. [Google Scholar] [CrossRef]
- Santos, K.A.D.; Santos, I.C.C.D.; Silva, C.S.; Ribeiro, H.G.; Domingos, I.D.F.; Silbiger, V.N. Circulating exosomal miRNAs as biomarkers for the diagnosis and prognosis of colorectal cancer. Int. J. Mol. Sci. 2020, 22, 346. [Google Scholar] [CrossRef]
- Davis, M.E.; Chen, Z.G. Shin DIABETES. Nanoparticle therapeutics: An emerging treatment modality for cancer. Nat. Rev. Drug. Discov. 2008, 7, 771–782. [Google Scholar] [CrossRef]
- Kowal, J.; Arras, G.; Colombo, M.; Jouve, M.; Morath, J.P.; Primdal-Bengtson, B.; Dingli, F.; Loew, D.; Tkach, M.; Théry, C. Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc. Natl. Acad. Sci. USA 2016, 113, E968–E977. [Google Scholar] [CrossRef] [Green Version]
- Roy, B.; Ghose, S.; Biswas, S. Therapeutic strategies for miRNA delivery to reduce hepatocellular carcinoma. Semin. Cell Dev. Biol. 2021, in press. [Google Scholar] [CrossRef]
- Su, T.; Zhang, P.; Zhao, F.; Zhang, S. Exosomal microRNAs mediating crosstalk between cancer cells with cancer–associated fibroblasts and tumor–associated macrophages in the tumor microenvironment. Front. Oncol. 2021, 11, 631703. [Google Scholar] [CrossRef]
- Cione, E.; Zambrini, A.S.; Cannataro, R. MicroRNAs and Extracellular Vesicles in Milk: RNA-Based Micronutrients? J. Nutr. 2021, 151, 1378–1379. [Google Scholar] [CrossRef]
- Baden, L.R.; el Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef]
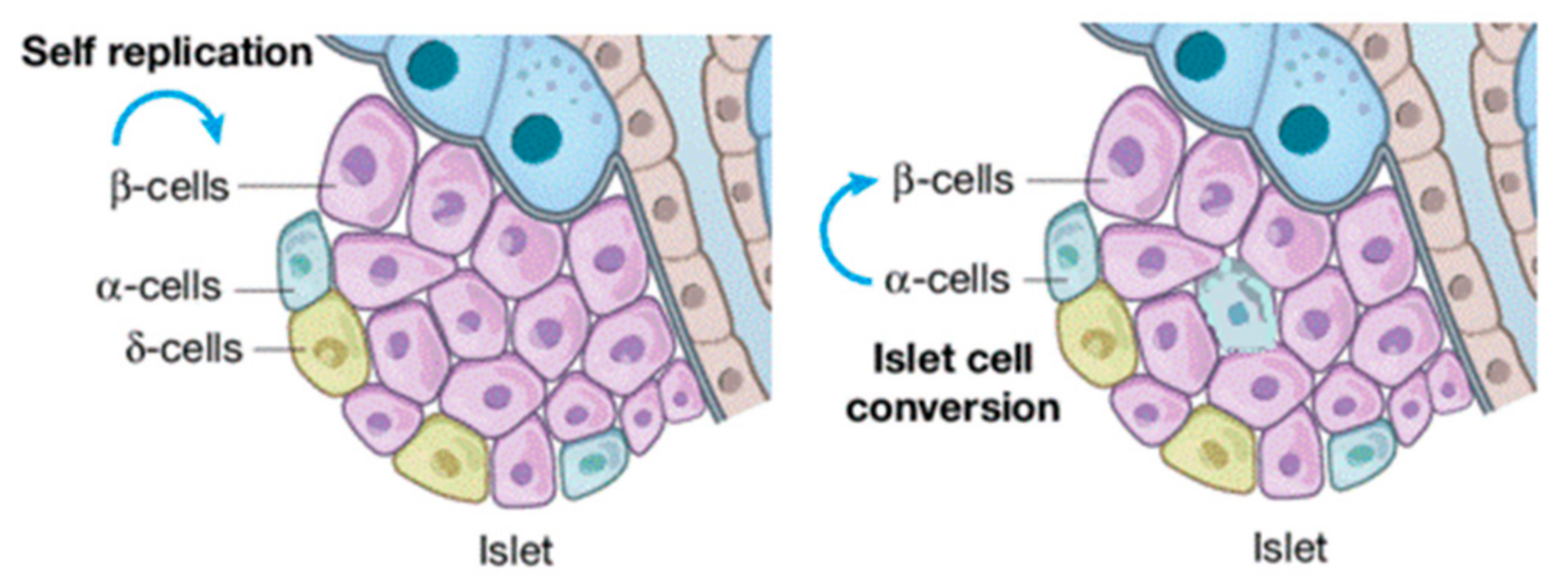
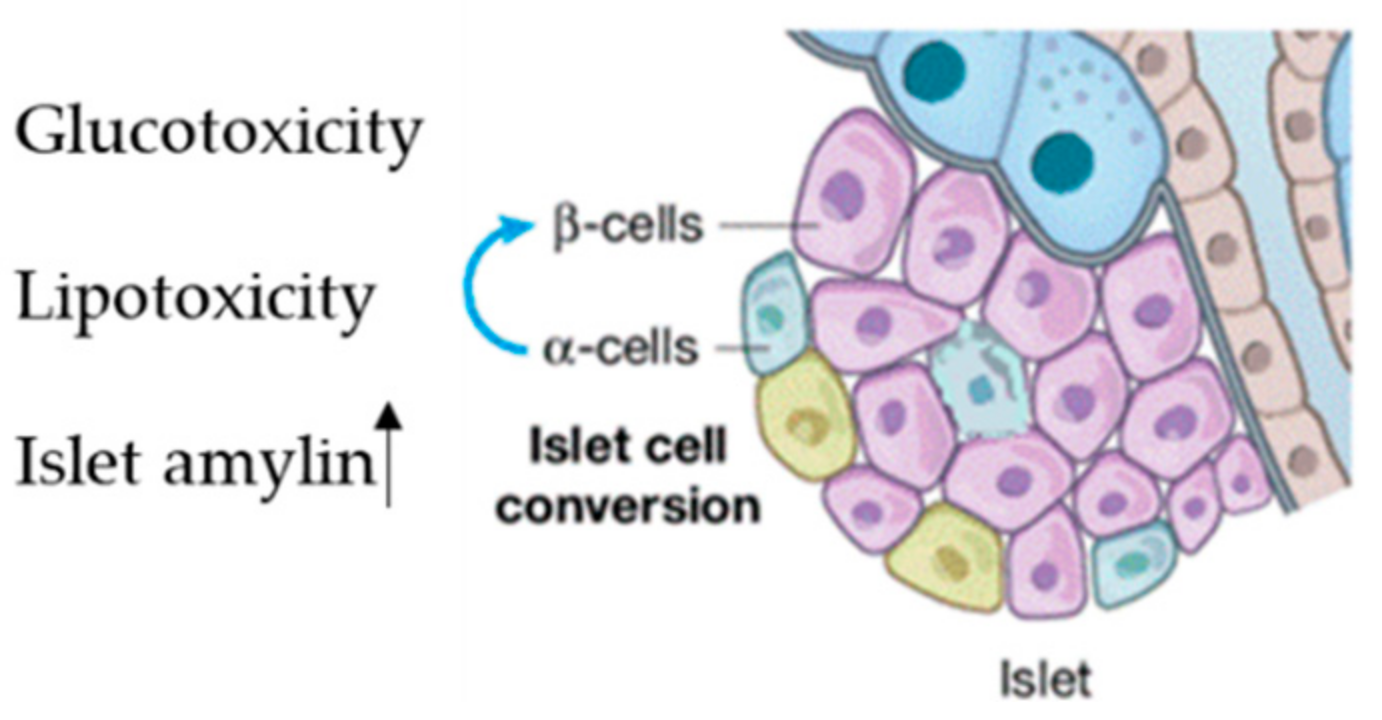

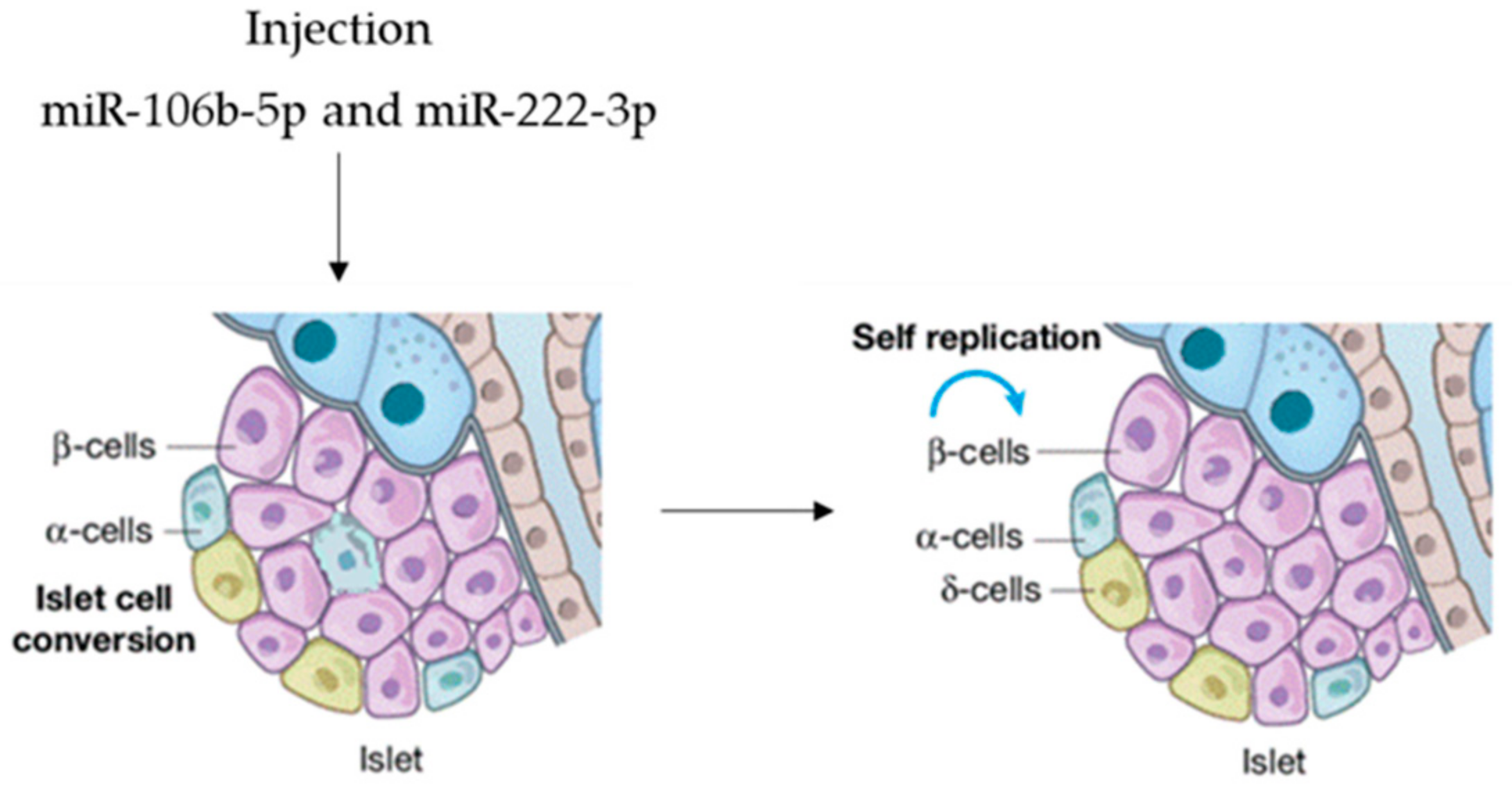
| Exosome-miRNA | Cell Type Origin | Action Site | References |
|---|---|---|---|
| Let-7 | Immune cells, Endothelial cells | Liver cells, Pancreatic β-cells | [50] |
| miR-29 | Pancreatic β-cells, Adipocytes, Hepatocytes | Liver cells, Pancreatic β-cells | [52] |
| miR-223 | Pre-adipocytes, Adipocytes, Macrophages | Liver cells, Pancreatic β-cells | [51] |
| miR-103 | Adipocytes, BM-derived stromal cells | Liver cells, Pancreatic β-cells | [53] |
| miR-375-3p | Pancreatic β-cells | Pancreatic β-cells | [63,64] |
| miR-106b-5p | BM-MSC | Pancreatic β-cells | [65] |
| miR-222-3p | BM-MSC | Pancreatic β-cells | [65] |
| miR-26a | Adipocytes, Hepatocytes, Vascular endothelial cells | Pancreatic β-cells | [66] |
| miR-20b-5p | Adipocytes, Hepatocytes | Skeletal muscle cells | [79] |
| miR-27a/b-3p | Endothelial cells, Adipocytes, Hepatocytes Glomerular mesangial cells | Liver cells Adipocytes | [82,83] |
| miR-320a | Adipocytes, Hepatocytes, Macrophages, Neutrophils | Liver cells, Adipocytes, Myocytes | [81,82] |
| miR-23a | Macrophages, Endothelial cells, Adipocytes | Liver cells | [81] |
| miR-197 | Endothelial cells, Adypocytes | Liver cells | [81] |
| miR-509-3p | Adipocytes, Macrophages | Liver cells | [81] |
| miR-29b-3p | BM-MSC | Liver cells, Adipocytes | [84,85] |
| miR-122 | Hepatocytes | Liver cells, Adipocytes | [84,85] |
| miR-192 | Hepatocytes | Liver cells, Adipocytes | [84,85] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cione, E.; Cannataro, R.; Gallelli, L.; De Sarro, G.; Caroleo, M.C. Exosome microRNAs in Metabolic Syndrome as Tools for the Early Monitoring of Diabetes and Possible Therapeutic Options. Pharmaceuticals 2021, 14, 1257. https://doi.org/10.3390/ph14121257
Cione E, Cannataro R, Gallelli L, De Sarro G, Caroleo MC. Exosome microRNAs in Metabolic Syndrome as Tools for the Early Monitoring of Diabetes and Possible Therapeutic Options. Pharmaceuticals. 2021; 14(12):1257. https://doi.org/10.3390/ph14121257
Chicago/Turabian StyleCione, Erika, Roberto Cannataro, Luca Gallelli, Giovambattista De Sarro, and Maria Cristina Caroleo. 2021. "Exosome microRNAs in Metabolic Syndrome as Tools for the Early Monitoring of Diabetes and Possible Therapeutic Options" Pharmaceuticals 14, no. 12: 1257. https://doi.org/10.3390/ph14121257
APA StyleCione, E., Cannataro, R., Gallelli, L., De Sarro, G., & Caroleo, M. C. (2021). Exosome microRNAs in Metabolic Syndrome as Tools for the Early Monitoring of Diabetes and Possible Therapeutic Options. Pharmaceuticals, 14(12), 1257. https://doi.org/10.3390/ph14121257









