The Effect of Curcumin Differs on Individual Cognitive Domains across Different Patient Populations: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
1.1. Curcumin in Cognitive Decline Animal Studies
1.2. Curcumin in Cognitive Epidemiological Studies and Clinical Trials
1.3. Individual Cognitive Domains and Gastrointestinal Adverse Events
2. Results
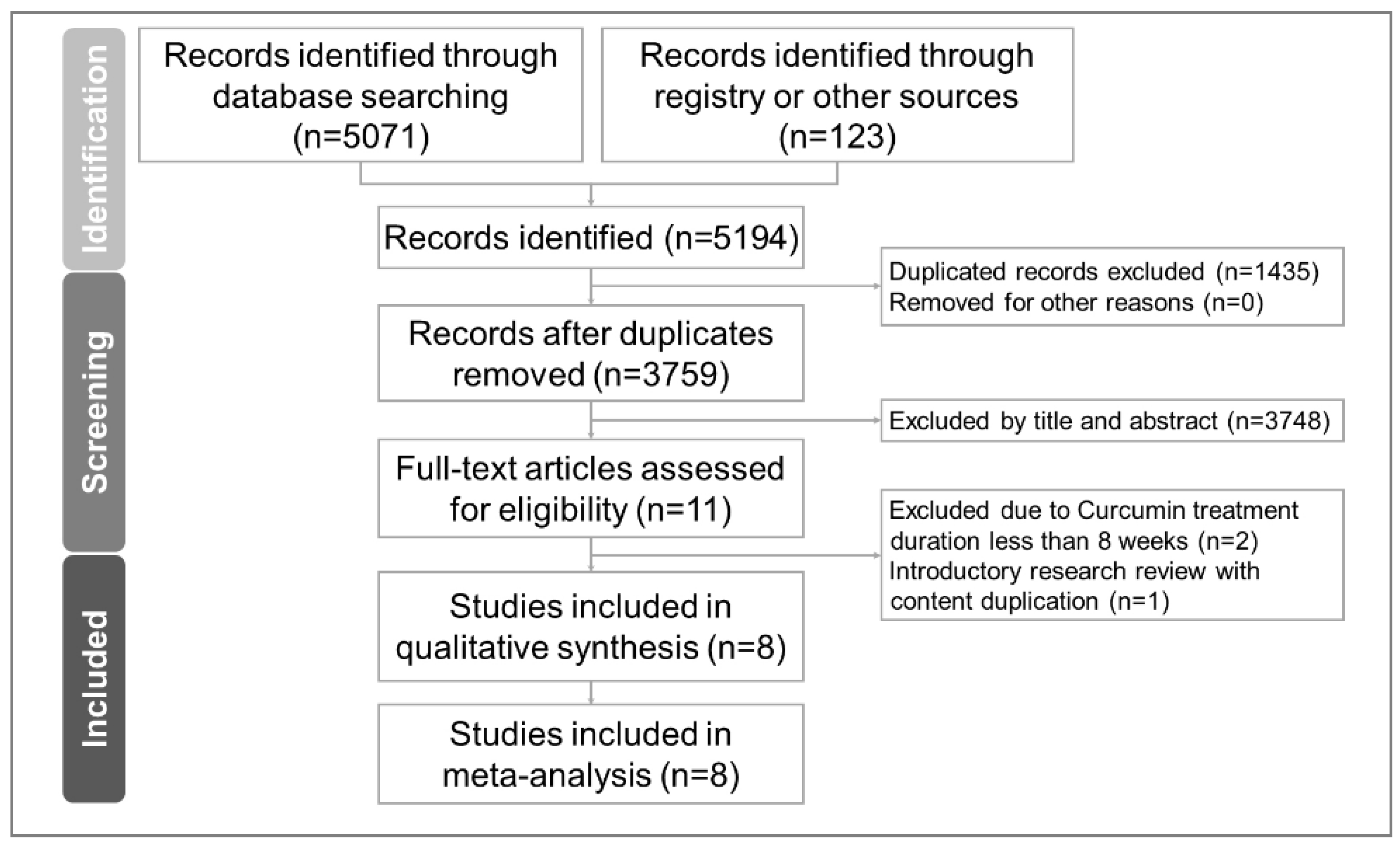
2.1. Study Selection
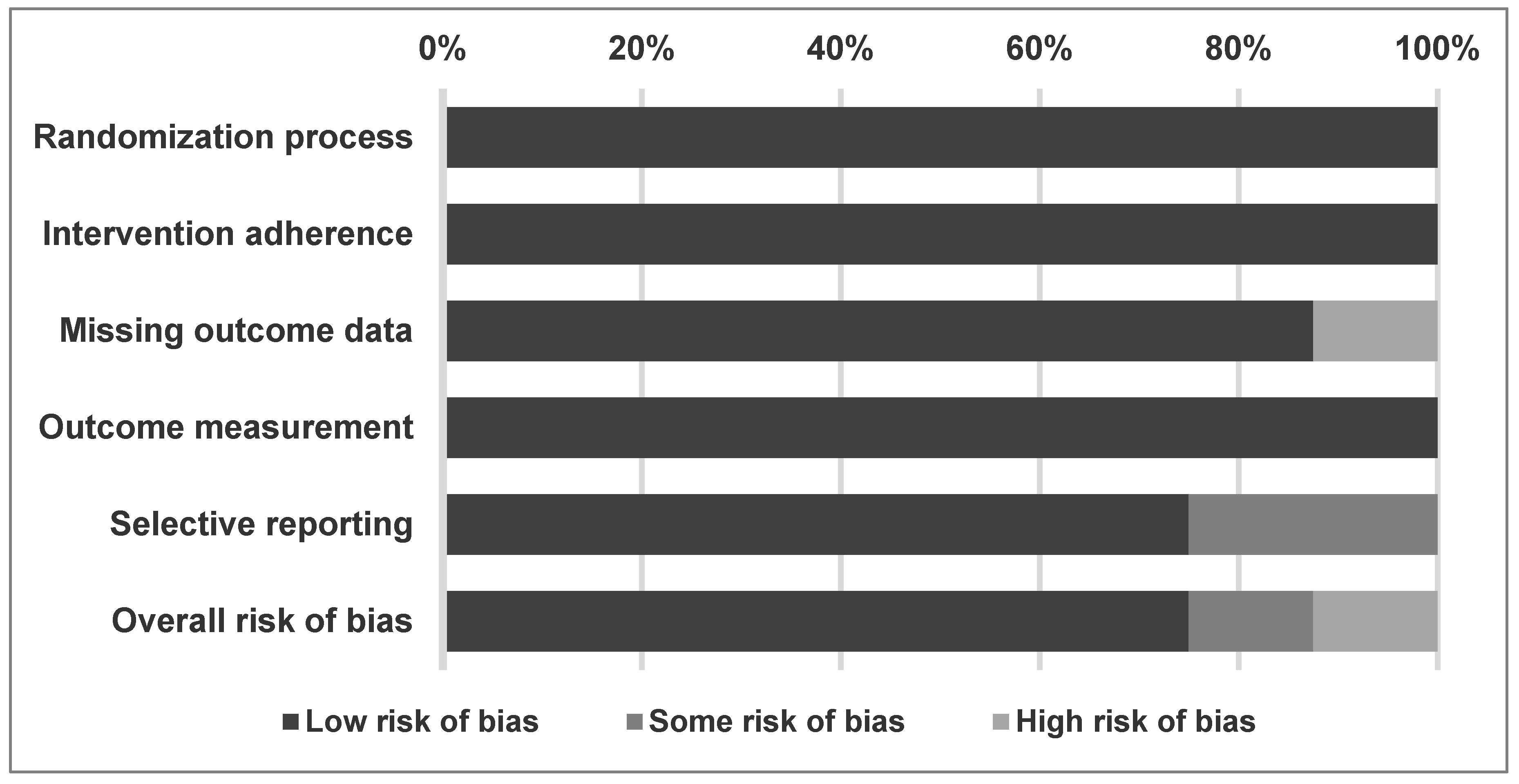
2.2. Methodological Quality of Included Studies
2.3. Primary Outcome
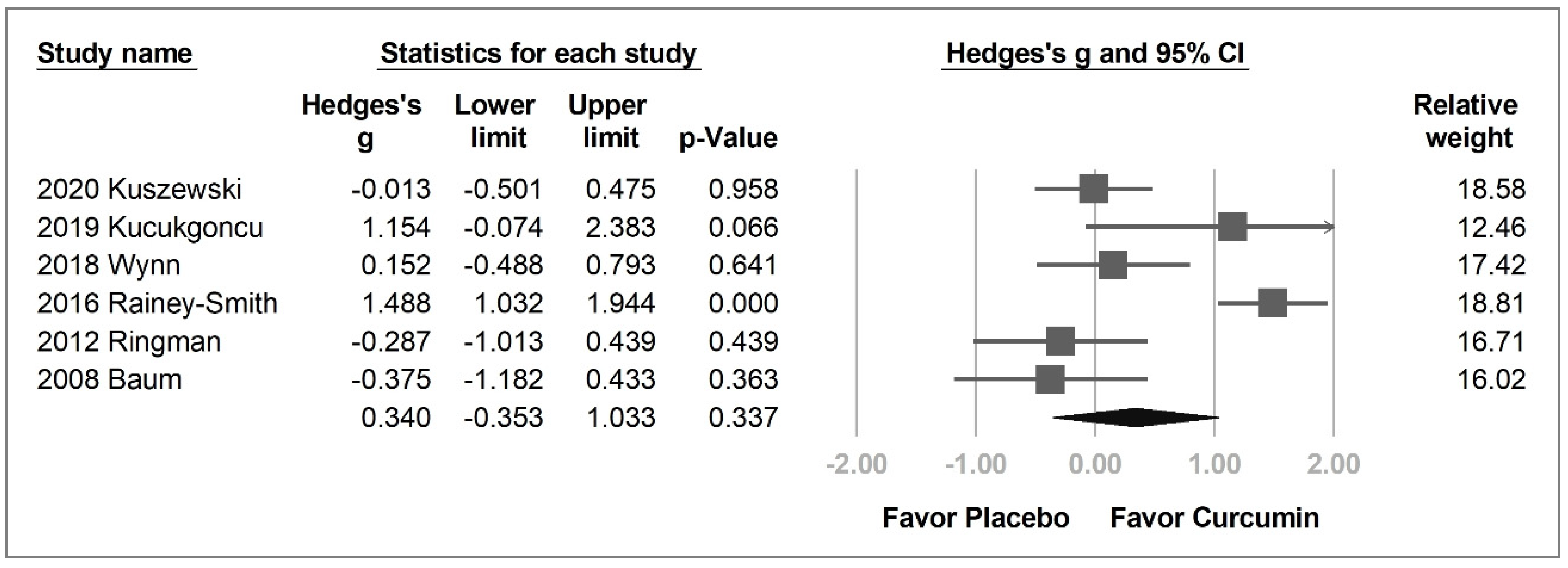
2.3.1. Overall Cognitive Function
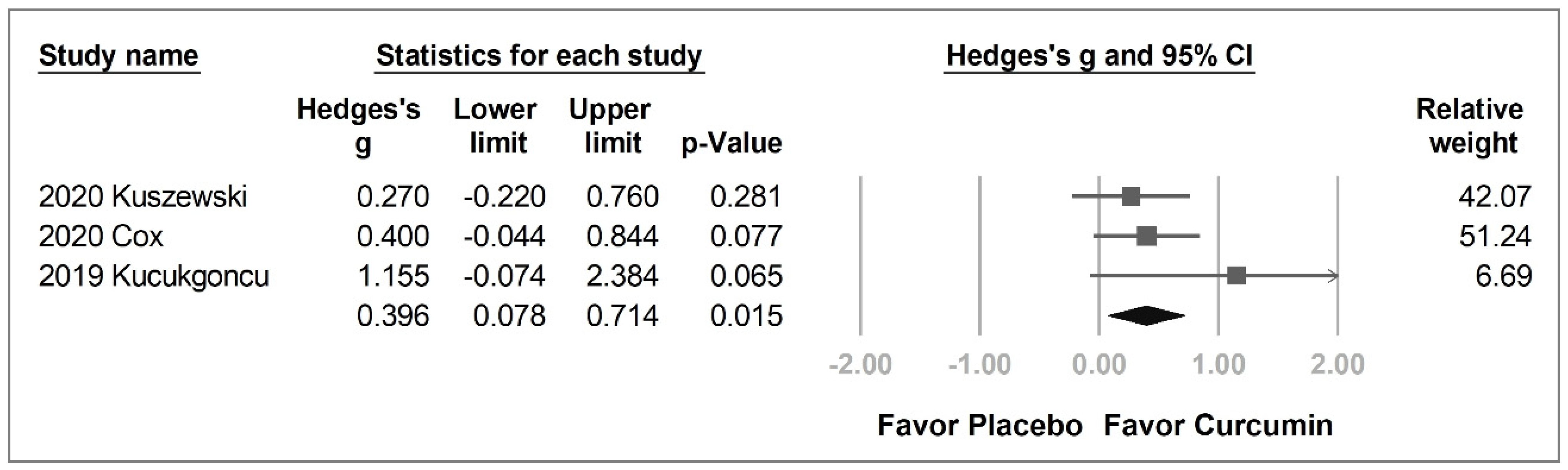
2.3.2. Individual Cognitive Domains
2.4. Secondary Outcomes
2.5. Publication Bias
3. Discussion
3.1. Main Results
3.2. Possible Mechanisms of the Improved Cognitive Function by Curcumin
3.3. Evidence Summary of Multisystem Health Benefits of Curcumin
3.4. Effects of Curcumin on Different Cognitive Domains
3.5. Adverse Gastrointestinal Effects of Curcumin
3.6. Different Formulations and Ingredients
3.7. Limitations
4. Materials and Methods
4.1. General Guidelines
4.2. Database Searches and Identification of Eligible Papers
4.3. Inclusion and Exclusion Criteria
4.4. Methodological Quality Appraisal
4.5. Primary Outcomes (Changes in Cognitive Function)
4.6. Secondary Outcomes (Withdrawal Rates and Adverse Event Rates)
4.7. Data Extraction and Management
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shehzad, A.; Rehman, G.; Lee, Y.S. Curcumin in inflammatory diseases. Biofactors 2013, 39, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Parada, E.; Buendia, I.; Navarro, E.; Avendaño, C.; Egea, J.; López, M.G. Microglial HO-1 induction by curcumin provides antioxidant, antineuroinflammatory, and glioprotective effects. Mol. Nutr. Food Res. 2015, 59, 1690–1700. [Google Scholar] [CrossRef] [PubMed]
- Sarker, M.R.; Franks, S.F. Efficacy of curcumin for age-associated cognitive decline: A narrative review of preclinical and clinical studies. Geroscience 2018, 40, 73–95. [Google Scholar] [CrossRef] [PubMed]
- Kocaadam, B.; Şanlier, N. Curcumin, an active component of turmeric (Curcuma longa), and its effects on health. Crit. Rev. Food Sci. Nutr. 2017, 57, 2889–2895. [Google Scholar] [CrossRef]
- Tayyem, R.F.; Heath, D.D.; Al-Delaimy, W.K.; Rock, C.L. Curcumin content of turmeric and curry powders. Nutr. Cancer 2006, 55, 126–131. [Google Scholar] [CrossRef]
- Moore, T.L.; Bowley, B.; Shultz, P.; Calderazzo, S.; Shobin, E.; Killiany, R.J.; Rosene, D.L.; Moss, M.B. Chronic curcumin treatment improves spatial working memory but not recognition memory in middle-aged rhesus monkeys. Geroscience 2017, 39, 571–584. [Google Scholar] [CrossRef] [Green Version]
- Bassani, T.B.; Turnes, J.M.; Moura, E.L.R.; Bonato, J.M.; Cóppola-Segovia, V.; Zanata, S.M.; Oliveira, R.; Vital, M. Effects of curcumin on short-term spatial and recognition memory, adult neurogenesis and neuroinflammation in a streptozotocin-induced rat model of dementia of Alzheimer’s type. Behav. Brain Res. 2017, 335, 41–54. [Google Scholar] [CrossRef]
- Noorafshan, A.; Karimi, F.; Karbalay-Doust, S.; Kamali, A.M. Using curcumin to prevent structural and behavioral changes of medial prefrontal cortex induced by sleep deprivation in rats. Excli J. 2017, 16, 510–520. [Google Scholar] [CrossRef]
- Ng, T.P.; Chiam, P.C.; Lee, T.; Chua, H.C.; Lim, L.; Kua, E.H. Curry consumption and cognitive function in the elderly. Am. J. Epidemiol. 2006, 164, 898–906. [Google Scholar] [CrossRef] [Green Version]
- Ringman, J.M.; Frautschy, S.A.; Teng, E.; Begum, A.N.; Bardens, J.; Beigi, M.; Gylys, K.H.; Badmaev, V.; Heath, D.D.; Apostolova, L.G.; et al. Oral curcumin for Alzheimer’s disease: Tolerability and efficacy in a 24-week randomized, double blind, placebo-controlled study. Alzheimers Res. Ther. 2012, 4, 43. [Google Scholar] [CrossRef] [Green Version]
- Rainey-Smith, S.R.; Brown, B.M.; Sohrabi, H.R.; Shah, T.; Goozee, K.G.; Gupta, V.B.; Martins, R.N. Curcumin and cognition: A randomised, placebo-controlled, double-blind study of community-dwelling older adults. Br. J. Nutr. 2016, 115, 2106–2113. [Google Scholar] [CrossRef]
- Zhu, L.N.; Mei, X.; Zhang, Z.G.; Xie, Y.P.; Lang, F. Curcumin intervention for cognitive function in different types of people: A systematic review and meta-analysis. Phytother. Res. 2019, 33, 524–533. [Google Scholar] [CrossRef] [Green Version]
- Baum, L.; Lam, C.W.; Cheung, S.K.; Kwok, T.; Lui, V.; Tsoh, J.; Lam, L.; Leung, V.; Hui, E.; Ng, C.; et al. Six-month randomized, placebo-controlled, double-blind, pilot clinical trial of curcumin in patients with Alzheimer disease. J. Clin. Psychopharmacol. 2008, 28, 110–113. [Google Scholar] [CrossRef] [Green Version]
- Cox, K.H.; Pipingas, A.; Scholey, A.B. Investigation of the effects of solid lipid curcumin on cognition and mood in a healthy older population. J. Psychopharmacol. 2015, 29, 642–651. [Google Scholar] [CrossRef]
- Small, G.W.; Siddarth, P.; Li, Z.; Miller, K.J.; Ercoli, L.; Emerson, N.D.; Martinez, J.; Wong, K.P.; Liu, J.; Merrill, D.A.; et al. Memory and Brain Amyloid and Tau Effects of a Bioavailable Form of Curcumin in Non-Demented Adults: A Double-Blind, Placebo-Controlled 18-Month Trial. Am. J. Geriatr. Psychiatry 2018, 26, 266–277. [Google Scholar] [CrossRef]
- Wynn, J.K.; Green, M.F.; Hellemann, G.; Karunaratne, K.; Davis, M.C.; Marder, S.R. The effects of curcumin on brain-derived neurotrophic factor and cognition in schizophrenia: A randomized controlled study. Schizophr. Res. 2018, 195, 572–573. [Google Scholar] [CrossRef]
- Funahashi, S. Working Memory in the Prefrontal Cortex. Brain Sci. 2017, 7, 49. [Google Scholar] [CrossRef]
- Murman, D.L. The Impact of Age on Cognition. Semin. Hear. 2015, 36, 111–121. [Google Scholar] [CrossRef]
- Kirova, A.M.; Bays, R.B.; Lagalwar, S. Working memory and executive function decline across normal aging, mild cognitive impairment, and Alzheimer’s disease. Biomed. Res. Int. 2015, 2015, 748212. [Google Scholar] [CrossRef] [Green Version]
- Bowie, C.R.; Harvey, P.D. Cognitive deficits and functional outcome in schizophrenia. Neuropsychiatr. Dis. Treat. 2006, 2, 531–536. [Google Scholar] [CrossRef] [Green Version]
- Soleimani, V.; Sahebkar, A.; Hosseinzadeh, H. Turmeric (Curcuma longa) and its major constituent (curcumin) as nontoxic and safe substances: Review. Phytother. Res. 2018, 32, 985–995. [Google Scholar] [CrossRef]
- Turmeric. In Drugs and Lactation Database (LactMed); National Library of Medicine (US): Bethesda, MD, USA, 2006.
- Sharma, R.A.; Euden, S.A.; Platton, S.L.; Cooke, D.N.; Shafayat, A.; Hewitt, H.R.; Marczylo, T.H.; Morgan, B.; Hemingway, D.; Plummer, S.M.; et al. Phase I clinical trial of oral curcumin: Biomarkers of systemic activity and compliance. Clin. Cancer Res. 2004, 10, 6847–6854. [Google Scholar] [CrossRef] [Green Version]
- Lee, M.S.; Wahlqvist, M.L.; Chou, Y.C.; Fang, W.H.; Lee, J.T.; Kuan, J.C.; Liu, H.Y.; Lu, T.M.; Xiu, L.; Hsu, C.C.; et al. Turmeric improves post-prandial working memory in pre-diabetes independent of insulin. Asia Pac. J. Clin. Nutr. 2014, 23, 581–591. [Google Scholar] [CrossRef]
- Ross, S.M. Curcuma longa (Theracumin®): A Bioavailable Form of Curcumin and Its Cognitive Benefits. Holist. Nurs. Pract 2018, 32, 217–220. [Google Scholar] [CrossRef]
- Kucukgoncu, S.; Guloksuz, S.; Tek, C. Effects of Curcumin on Cognitive Functioning and Inflammatory State in Schizophrenia: A Double-Blind, Placebo-Controlled Pilot Trial. J. Clin. Psychopharmacol. 2019, 39, 182–184. [Google Scholar] [CrossRef]
- Cox, K.H.M.; White, D.J.; Pipingas, A.; Poorun, K.; Scholey, A. Further Evidence of Benefits to Mood and Working Memory from Lipidated Curcumin in Healthy Older People: A 12-Week, Double-Blind, Placebo-Controlled, Partial Replication Study. Nutrients 2020, 12, 1678. [Google Scholar] [CrossRef]
- Kuszewski, J.C.; Howe, P.R.C.; Wong, R.H.X. Evaluation of Cognitive Performance following Fish-Oil and Curcumin Supplementation in Middle-Aged and Older Adults with Overweight or Obesity. J. Nutr. 2020, 150, 3190–3199. [Google Scholar] [CrossRef]
- Curcumin in Patients With Mild to Moderate Alzheimer’s Disease. ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/ct2/show/nct00099710 (accessed on 13 August 2021).
- Australian New Zealand Clinical Trials Registry. Biocurcumax from Curry Spice Turmeric in Retaining Cognitive Function. Available online: https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12611000437965 (accessed on 13 August 2021).
- 18-Month Study of Memory Effects of Curcumin. Study Results. ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/ct2/show/results/NCT01383161 (accessed on 13 August 2021).
- Curcumin as a Novel Treatment to Improve Cognitive Dysfunction in Schizophrenia. ClinicalTrials.Gov. Available online: https://ClinicalTrials.gov/show/NCT02104752 (accessed on 13 August 2021).
- A Pilot Trial of Curcumin Effects on Cognition in Schizophrenia. ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/ct2/show/NCT02476708 (accessed on 13 August 2021).
- Australian New Zealand Clinical Trials Registry. Investigation of the Effects of Longvida Curcumin on Cognitive Function, Mood and Biomarkers of Health. Available online: https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=370499 (accessed on 13 August 2021).
- Australian New Zealand Clinical Trials Registry. Cardiometabolic and cognitive benefits of omega-3 polyunsaturated fatty acids and curcumin supplementation in older, sedentary and overweight/obese adults. Available online: https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=370788 (accessed on 13 August 2021).
- Bristow, T.; Jih, C.S.; Slabich, A.; Gunn, J. Standardization and adult norms for the sequential subtracting tasks of serial 3’s and 7’s. Appl. Neuropsychol. Adult 2016, 23, 372–378. [Google Scholar] [CrossRef]
- Kaufman, D.; Geyer, H.; Milstein, M. Kaufman’s Clinical Neurology for Psychiatrists. In Dementia; Elsevier: Amsterdam, The Netherlands, 2017; pp. 105–149. [Google Scholar]
- Kuszewski, J.C.; Wong, R.H.X.; Howe, P.R.C. Can Curcumin Counteract Cognitive Decline? Clinical Trial Evidence and Rationale for Combining ω-3 Fatty Acids with Curcumin. Adv. Nutr. 2018, 9, 105–113. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Green, S.E. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0. Available online: https://training.cochrane.org/handbook/archive/v5.1/ (accessed on 13 August 2021).
- De Berardis, D.; Campanella, D.; Gambi, F.; La Rovere, R.; Carano, A.; Conti, C.M.; Sivestrini, C.; Serroni, N.; Piersanti, D.; Di Giuseppe, B.; et al. The role of C-reactive protein in mood disorders. Int. J. Immunopathol. Pharmacol. 2006, 19, 721–725. [Google Scholar] [CrossRef] [Green Version]
- Orsolini, L.; Sarchione, F.; Vellante, F.; Fornaro, M.; Matarazzo, I.; Martinotti, G.; Valchera, A.; Di Nicola, M.; Carano, A.; Di Giannantonio, M.; et al. Protein-C Reactive as Biomarker Predictor of Schizophrenia Phases of Illness? A Systematic Review. Curr. Neuropharmacol. 2018, 16, 583–606. [Google Scholar] [CrossRef] [PubMed]
- Gagliardi, S.; Morasso, C.; Stivaktakis, P.; Pandini, C.; Tinelli, V.; Tsatsakis, A.; Prosperi, D.; Hickey, M.; Corsi, F.; Cereda, C. Curcumin Formulations and Trials: What’s New in Neurological Diseases. Molecules 2020, 25, 5389. [Google Scholar] [CrossRef] [PubMed]
- Kandezi, N.; Mohammadi, M.; Ghaffari, M.; Gholami, M.; Motaghinejad, M.; Safari, S. Novel Insight to Neuroprotective Potential of Curcumin: A Mechanistic Review of Possible Involvement of Mitochondrial Biogenesis and PI3/Akt/ GSK3 or PI3/Akt/CREB/BDNF Signaling Pathways. Int. J. Mol. Cell Med. 2020, 9, 1–32. [Google Scholar] [CrossRef] [PubMed]
- Ashtary-Larky, D.; Rezaei Kelishadi, M.; Bagheri, R.; Moosavian, S.P.; Wong, A.; Davoodi, S.H.; Khalili, P.; Dutheil, F.; Suzuki, K.; Asbaghi, O. The Effects of Nano-Curcumin Supplementation on Risk Factors for Cardiovascular Disease: A GRADE-Assessed Systematic Review and Meta-Analysis of Clinical Trials. Antioxidants 2021, 10, 1015. [Google Scholar] [CrossRef]
- Chandan, S.; Mohan, B.P.; Chandan, O.C.; Ahmad, R.; Challa, A.; Tummala, H.; Singh, S.; Dhawan, P.; Ponnada, S.; Singh, A.B.; et al. Curcumin use in ulcerative colitis: Is it ready for prime time? A systematic review and meta-analysis of clinical trials. Ann. Gastroenterol. 2020, 33, 53–58. [Google Scholar] [CrossRef]
- Suhett, L.G.; de Miranda Monteiro Santos, R.; Silveira, B.K.S.; Leal, A.C.G.; de Brito, A.D.M.; de Novaes, J.F.; Lucia, C.M.D. Effects of curcumin supplementation on sport and physical exercise: A systematic review. Crit. Rev. Food Sci. Nutr. 2021, 61, 946–958. [Google Scholar] [CrossRef]
- Fernández-Lázaro, D.; Mielgo-Ayuso, J.; Seco Calvo, J.; Córdova Martínez, A.; Caballero García, A.; Fernandez-Lazaro, C.I. Modulation of Exercise-Induced Muscle Damage, Inflammation, and Oxidative Markers by Curcumin Supplementation in a Physically Active Population: A Systematic Review. Nutrients 2020, 12, 501. [Google Scholar] [CrossRef] [Green Version]
- Mansouri, K.; Rasoulpoor, S.; Daneshkhah, A.; Abolfathi, S.; Salari, N.; Mohammadi, M.; Rasoulpoor, S.; Shabani, S. Clinical effects of curcumin in enhancing cancer therapy: A systematic review. BMC Cancer 2020, 20, 791. [Google Scholar] [CrossRef]
- Howe, P.R.C.; Kuszewski, J.C.; Wong, R.H.X. Curcumin for Cognition-Does the Path Lie in the Cerebral Circulation? Adv. Nutr. 2019, 10, 182. [Google Scholar] [CrossRef]
- Naeimi, R.; Safarpour, F.; Hashemian, M.; Tashakorian, H.; Ahmadian, S.R.; Ashrafpour, M.; Ghasemi-Kasman, M. Curcumin-loaded nanoparticles ameliorate glial activation and improve myelin repair in lyolecithin-induced focal demyelination model of rat corpus callosum. Neurosci. Lett. 2018, 674, 1–10. [Google Scholar] [CrossRef]
- Wang, Y.F.; Gu, Y.T.; Qin, G.H.; Zhong, L.; Meng, Y.N. Curcumin ameliorates the permeability of the blood-brain barrier during hypoxia by upregulating heme oxygenase-1 expression in brain microvascular endothelial cells. J. Mol. Neurosci. 2013, 51, 344–351. [Google Scholar] [CrossRef]
- Wang, Y.; Luo, J.; Li, S.Y. Nano-Curcumin Simultaneously Protects the Blood-Brain Barrier and Reduces M1 Microglial Activation During Cerebral Ischemia-Reperfusion Injury. ACS Appl. Mater Interfaces 2019, 11, 3763–3770. [Google Scholar] [CrossRef]
- Gorelick, P.B.; Scuteri, A.; Black, S.E.; Decarli, C.; Greenberg, S.M.; Iadecola, C.; Launer, L.J.; Laurent, S.; Lopez, O.L.; Nyenhuis, D.; et al. Vascular contributions to cognitive impairment and dementia: A statement for healthcare professionals from the american heart association/american stroke association. Stroke 2011, 42, 2672–2713. [Google Scholar] [CrossRef]
- Watanabe, K.; Funahashi, S. A dual-task paradigm for behavioral and neurobiological studies in nonhuman primates. J. Neurosci. Methods 2015, 246, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, K.; Funahashi, S. Neural mechanisms of dual-task interference and cognitive capacity limitation in the prefrontal cortex. Nat. Neurosci. 2014, 17, 601–611. [Google Scholar] [CrossRef] [Green Version]
- Noorafshan, A.; Abdollahifar, M.A.; Asadi-Golshan, R.; Rashidian-Rashidabadi, A.; Karbalay-Doust, S. Curcumin and sertraline prevent the reduction of the number of neurons and glial cells and the volume of rats’ medial prefrontal cortex induced by stress. Acta Neurobiol. Exp. (Wars) 2014, 74, 44–53. [Google Scholar]
- Noorafshan, A.; Asadi-Golshan, R.; Abdollahifar, M.A.; Karbalay-Doust, S. Protective role of curcumin against sulfite-induced structural changes in rats’ medial prefrontal cortex. Nutr. Neurosci. 2015, 18, 248–255. [Google Scholar] [CrossRef]
- Magistro, D.; Takeuchi, H.; Nejad, K.K.; Taki, Y.; Sekiguchi, A.; Nouchi, R.; Kotozaki, Y.; Nakagawa, S.; Miyauchi, C.M.; Iizuka, K.; et al. The Relationship between Processing Speed and Regional White Matter Volume in Healthy Young People. PLoS ONE 2015, 10, e0136386. [Google Scholar] [CrossRef] [Green Version]
- Nilsson, J.; Thomas, A.J.; O’Brien, J.T.; Gallagher, P. White matter and cognitive decline in aging: A focus on processing speed and variability. J. Int. Neuropsychol. Soc. 2014, 20, 262–267. [Google Scholar] [CrossRef] [Green Version]
- Abel, S.; Vavasour, I.; Lee, L.E.; Johnson, P.; Ackermans, N.; Chan, J.; Dvorak, A.; Schabas, A.; Wiggermann, V.; Tam, R.; et al. Myelin Damage in Normal Appearing White Matter Contributes to Impaired Cognitive Processing Speed in Multiple Sclerosis. J. Neuroimaging 2020, 30, 205–211. [Google Scholar] [CrossRef]
- Kerchner, G.A.; Racine, C.A.; Hale, S.; Wilheim, R.; Laluz, V.; Miller, B.L.; Kramer, J.H. Cognitive processing speed in older adults: Relationship with white matter integrity. PLoS ONE 2012, 7, e50425. [Google Scholar] [CrossRef] [Green Version]
- Bartzokis, G. Age-related myelin breakdown: A developmental model of cognitive decline and Alzheimer’s disease. Neurobiol. Aging 2004, 25, 5–18. [Google Scholar] [CrossRef]
- O’Sullivan, M.; Jones, D.K.; Summers, P.E.; Morris, R.G.; Williams, S.C.; Markus, H.S. Evidence for cortical “disconnection” as a mechanism of age-related cognitive decline. Neurology 2001, 57, 632–638. [Google Scholar] [CrossRef] [Green Version]
- Daverey, A.; Agrawal, S.K. Curcumin Protects against White Matter Injury through NF-κB and Nrf2 Cross Talk. J. Neurotrauma 2020, 37, 1255–1265. [Google Scholar] [CrossRef]
- GRAS Notice (GRN) No. 822. U.S. Food & Drug Administration. GRAS Notice Inventory. Available online: https://www.fda.gov/media/132575/download (accessed on 13 August 2021).
- NTP Toxicology and Carcinogenesis Studies of Turmeric Oleoresin (CAS No. 8024-37-1) (Major Component 79%–85% Curcumin, CAS No. 458-37-7) in F344/N Rats and B6C3F1 Mice (Feed Studies). Natl. Toxicol. Program Tech. Rep. Ser. 1993, 427, 1–275.
- Atsumi, T.; Fujisawa, S.; Tonosaki, K. Relationship between intracellular ROS production and membrane mobility in curcumin- and tetrahydrocurcumin-treated human gingival fibroblasts and human submandibular gland carcinoma cells. Oral Dis. 2005, 11, 236–242. [Google Scholar] [CrossRef]
- Sandur, S.K.; Ichikawa, H.; Pandey, M.K.; Kunnumakkara, A.B.; Sung, B.; Sethi, G.; Aggarwal, B.B. Role of pro-oxidants and antioxidants in the anti-inflammatory and apoptotic effects of curcumin (diferuloylmethane). Free Radic. Biol. Med. 2007, 43, 568–580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McNally, S.J.; Harrison, E.M.; Ross, J.A.; Garden, O.J.; Wigmore, S.J. Curcumin induces heme oxygenase 1 through generation of reactive oxygen species, p38 activation and phosphatase inhibition. Int. J. Mol. Med. 2007, 19, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.K. Intention-to-treat concept: A review. Perspect. Clin. Res. 2011, 2, 109–112. [Google Scholar] [CrossRef] [PubMed]
- AustralianVitamins.com. Blackmores Brain Active. Available online: https://www.australianvitamins.com/product/blackmores-vitamins-brain-active (accessed on 24 November 2021).
- Mane Kancor®. Curcumin. Available online: https://manekancor.com/natural-colors-c-capture-pigments/curcumin (accessed on 24 November 2021).
- Arjuna Natural®. Biocurcumax® (aka BCM-95® or Curcugreen®). Available online: https://arjunanatural.com/bcm-95-bioavailable-curcumin/ (accessed on 24 November 2021).
- Sabinsa®. Curcumin C3 Complex®. Available online: https://curcuminoids.com/ (accessed on 24 November 2021).
- Verdure Sciences®. Longvida® SLCP™ (solid lipid curcumin particles). Available online: https://vs-corp.com/longvida/ (accessed on 24 November 2021).
- Theravalues®. Theracurmin®. Available online: https://theravalues.com/english/products/ (accessed on 24 November 2021).
- Katsidoni, V.; Alexiou, P.; Fotiadou, M.; Pelecanou, M.; Sagnou, M.; Panagis, G. Curcumin, demethoxycurcumin and bisdemethoxycurcumin differentially inhibit morphine’s rewarding effect in rats. Psychopharmacology 2014, 231, 4467–4478. [Google Scholar] [CrossRef]
- Anand, P.; Thomas, S.G.; Kunnumakkara, A.B.; Sundaram, C.; Harikumar, K.B.; Sung, B.; Tharakan, S.T.; Misra, K.; Priyadarsini, I.K.; Rajasekharan, K.N.; et al. Biological activities of curcumin and its analogues (Congeners) made by man and Mother Nature. Biochem. Pharmacol. 2008, 76, 1590–1611. [Google Scholar] [CrossRef]
- Agrawal, S.S.; Gullaiya, S.; Dubey, V.; Singh, V.; Kumar, A.; Nagar, A.; Tiwari, P. Neurodegenerative Shielding by Curcumin and Its Derivatives on Brain Lesions Induced by 6-OHDA Model of Parkinson’s Disease in Albino Wistar Rats. Cardiovasc. Psychiatry Neurol. 2012, 2012, 942981. [Google Scholar] [CrossRef]
- Yin, F.; Sancheti, H.; Patil, I.; Cadenas, E. Energy metabolism and inflammation in brain aging and Alzheimer’s disease. Free Radic. Biol. Med. 2016, 100, 108–122. [Google Scholar] [CrossRef] [Green Version]
- Ozben, T.; Ozben, S. Neuro-inflammation and anti-inflammatory treatment options for Alzheimer’s disease. Clin. Biochem. 2019, 72, 87–89. [Google Scholar] [CrossRef]
- Dauwan, M.; Begemann, M.J.H.; Slot, M.I.E.; Lee, E.H.M.; Scheltens, P.; Sommer, I.E.C. Physical exercise improves quality of life, depressive symptoms, and cognition across chronic brain disorders: A transdiagnostic systematic review and meta-analysis of randomized controlled trials. J. Neurol. 2021, 268, 1222–1246. [Google Scholar] [CrossRef] [Green Version]
- Sterne, J.A.C.; Savovic, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [Green Version]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Shea, B.J.; Reeves, B.C.; Wells, G.; Thuku, M.; Hamel, C.; Moran, J.; Moher, D.; Tugwell, P.; Welch, V.; Kristjansson, E.; et al. AMSTAR 2: A critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ 2017, 358, j4008. [Google Scholar] [CrossRef] [Green Version]
- Tsai, I.-C.; Chang, K.-V. The Effect of Curcumin Differs on Individual Cognitive Domains across Different Patient Populations: A Systematic Review and Meta-Analysis. Available online: https://inplasy.com/inplasy-2021-9-0085/ (accessed on 24 September 2021).
- Voulgaropoulou, S.D.; van Amelsvoort, T.; Prickaerts, J.; Vingerhoets, C. The effect of curcumin on cognition in Alzheimer’s disease and healthy aging: A systematic review of pre-clinical and clinical studies. Brain Res. 2019, 1725, 146476. [Google Scholar] [CrossRef]
- How Long Does It Take for Turmeric to Work? The Tumeric. Available online: https://theturmeric.co/blogs/the-root/how-long-does-it-take-for-turmeric-to-work (accessed on 13 August 2021).
- Meixner, M. Turmeric Dosage: How Much Should You Take Per Day? Healthline. Available online: https://www.healthline.com/nutrition/turmeric-dosage (accessed on 13 August 2021).
- Harvey, P.D. Domains of cognition and their assessment. Dialogues Clin. Neurosci. 2019, 21, 227–237. [Google Scholar] [CrossRef]
- Deeks, J.J.; Higgins, J.P.T.; Altman, D.G. Chapter 10: Analysing Data and Undertaking Meta-Analyses. Cochrane Handbook for Systematic Reviews of Interventions. Version 6.2. Available online: https://training.cochrane.org/handbook/current/chapter-10 (accessed on 13 August 2021).
- Higgins, J.P.T.; Li, T.; Deeks, J.J. Chapter 6: Choosing Effect Measures and Computing Estimates of Effect. Cochrane Handbook for Systematic Reviews of Interventions. Version 6.2. Available online: https://training.cochrane.org/handbook/current/chapter-06 (accessed on 13 August 2021).
- Higgins, J.P.T.; Eldridge, S.; Li, T. Chapter 23: Including Variants on Randomized Trials. Cochrane Handbook for Systematic Reviews of Interventions. Version 6.2. Available online: https://training.cochrane.org/handbook/current/chapter-23 (accessed on 13 August 2021).
- Borenstein, M.; Hedges, L.V.; Higgins, J.P.T.; Rothstein, H.R. Fixed-Effect versus Random-Effects Models. In Introduction to Meta-Analysis; Borenstein, M., Ed.; Wiley: Hoboken, NJ, USA, 2009; pp. 77–86. [Google Scholar]
- Hedges, L.V. Distribution theory for Glass’s estimator of effect size and related estimators. J. Educ. Stat. 1981, 6, 107–128. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef] [Green Version]
- Page, M.J.; Higgins, J.P.T.; Sterne, J.A.C. Chapter 13: Assessing Risk of Bias due to Missing Results in a Synthesis. Cochrane Handbook for Systematic Reviews of Interventions. Version 6.2. Available online: https://training.cochrane.org/handbook/current/chapter-13 (accessed on 13 August 2021).
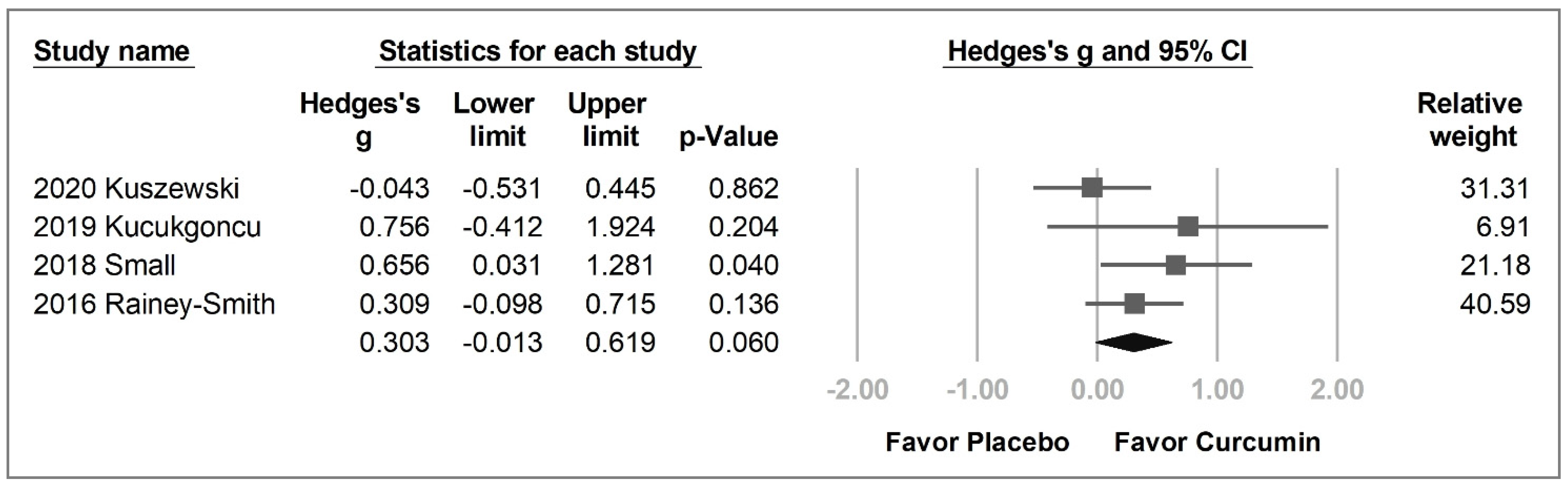
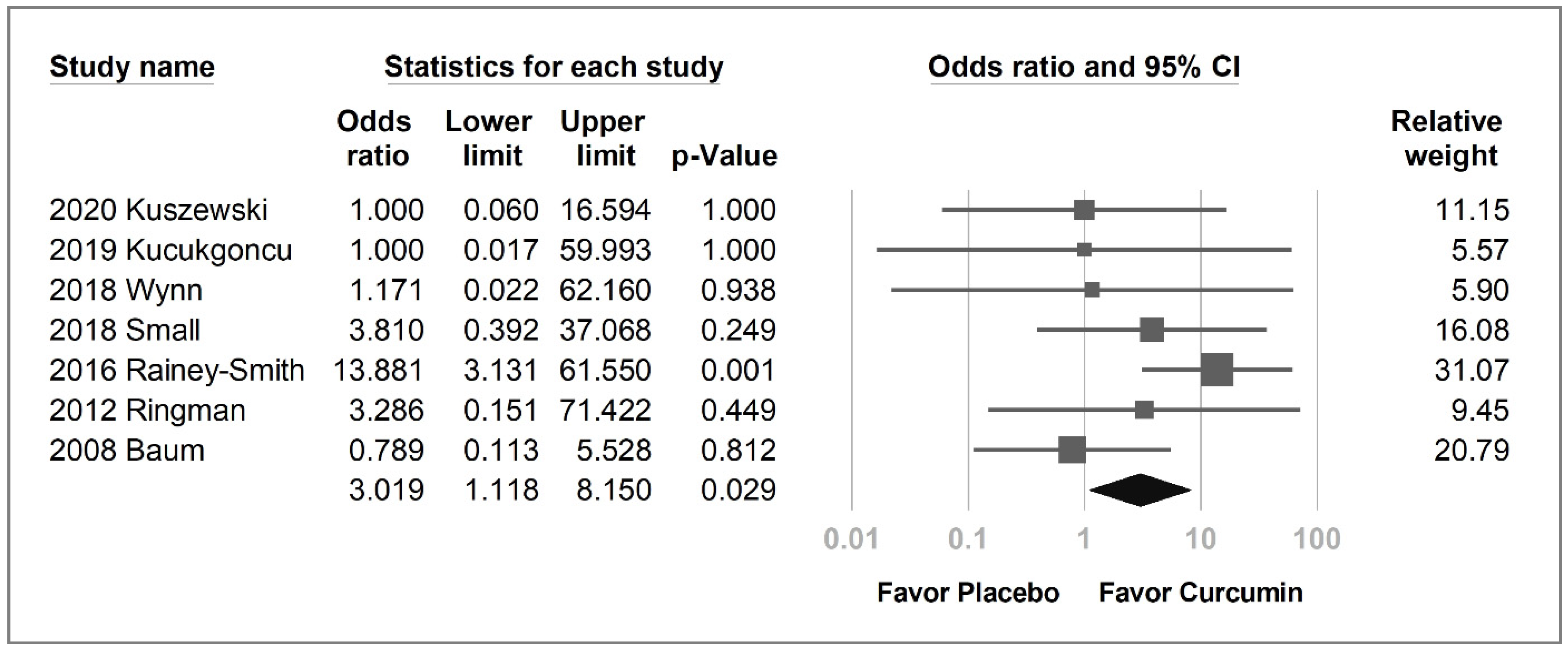
| Study | Kuszewski et al. | Cox et al. | Kucukgoncu et al. | Wynn et al. | Small et al. | Rainey-Smith et al. | Ringman et al. | Baum et al. |
|---|---|---|---|---|---|---|---|---|
| Reference/ Registry (if available) | [35,38] | [27,34] | [26,33] | [16,32] | [15,31] | [11,30] | [10,29] | [13] |
| Year | 2020 | 2020 | 2019 | 2018 | 2018 | 2016 | 2012 | 2008 |
| Location | Australia | Australia | United States | United States | United States | Australia | United States | China |
| Funding/grant | Blackmores Institute * | Verdure Sciences * | The State of Connecticut | Not mentioned | NIH grants USDE contract Foundations Professorships | McCusker Alzheimer’s Research Foundation Hollywood Private Hospital Research Foundation | J. D. F. Alzheimer’s Disease Foundation Institute for the Study of Aging | CUHK Bupa Foundation |
| Design | RCT, double-blind | RCT, double-blind | RCT, double-blind | RCT, double-blind | RCT, double-blind | RCT, double-blind | RCT, double-blind | RCT, double-blind |
| Allocation conceal | Independent investigator | External staff | Not mentioned | Not mentioned | Research pharmacy | Not mentioned | Research pharmacy | Not mentioned |
| Randomization | Minimization method | Stratified | Not mentioned | Not mentioned | Randomization table | Not mentioned | Block randomization | Stratified |
| Study duration | 16 weeks | 12 weeks | 8 weeks | 8 weeks | 18 months | 12 months | 24 weeks | 6 months |
| Subjects | Overweight older adults | Healthy older adults | Schizophrenia | Schizophrenia | Non-demented older adults | Older adults | Alzheimer’s disease | Alzheimer’s disease |
| Curcumin product | Brain Active® (Longvida®) | Longvida® | Theracumin® | Theracumin® | Theracumin® | Biocurcumax® | Curcumin C3 complex® | Powder or capsule |
| Curcumin manufacturer | Blackmores | Verdure Sciences | Theravalues | Theravalues | Theravalues | Arjuna Natural | Sabinsa | Kancor Flavors Arjuna Natural |
| Curcumin arms (N) | 160 mg/d curcumin (31) Placebo (32) | 80 mg/d curcumin (42) Placebo (43) (12 weeks: 39/40) | 180 mg/d curcumin (6) Placebo (6) (8 weeks: 5/5) | 360 mg/d curcumin (17) Placebo (19) | 180 mg/d curcumin (21) Placebo (19) | 1.32 g/d curcuminoids (39) Placebo (57) | 2 g/d curcuminoids (10) 4 g/d curcuminoids (9) Placebo (11) | 1 g/d curcuminoids (8) 4 g/d curcuminoids (11) Placebo (8) |
| Age (years) | 160 mg/d: 65.7 ± 1.4 Placebo: 65.8 ± 1.4 | 80 mg/d: 67.8 ± 6.0 Placebo: 68.4 ± 6.7 | 41.3 ± 12.7 | 360 mg/d: 50.1 ± 9.6 Placebo: 50.9 ± 10.6 | 180 mg/d: 63.1 ± 8.4 Placebo: 62.9 ± 9.4 | 66 ± 6.6 | 2 g/d: 76.7 ± 5.6 4 g/d: 75.3 ± 6.9 Placebo: 70.2 ± 12.4 | 1 g/d: 69.0 ± 10.9 4 g/d: 73.4 ± 6.6 Placebo: 77.8 ± 7.7 |
| Male % | 160 mg/d: 48% Placebo: 44% | 80 mg/d: 50% Placebo: 48.84% | Total: 75% | 360 mg/d: 64.7% Placebo: 100% | 180 mg/d: 43% Placebo: 47% | 1.32 g/d: 33.3% Placebo: 26.3% | 2 g/d: 33% 4 g/d: 30% Placebo: 45% | 1 g/d: 12.5% 4 g/d: 27.3% Placebo: 37.5% |
| Cognition domains | NIH toolbox+ | E-prime 2.0 | MCCB | MCCB | Customized | Customized | ADAS-Cog & MMSE | MMSE |
| Overall | Overall performance | N/A | Composite score | MCCB T-score | N/A | MoCA Non-computerized and Computerized composite scores | ADAS-Cog MMSE | MMSE |
| Working memory | Working memory | Serial 7 subtraction Serial 3 subtraction vMWM | Working memory | N/A | N/A | N/A | N/A | N/A |
| Processing speed | Processing speed | N/A | Processing speed | N/A | Trail making test part A | Wechsler digit symbol scale | N/A | N/A |
| Language | Language | N/A | N/A | N/A | N/A | COWAT | N/A | N/A |
| Episodic memory /visual learning | Episodic memory | N/A | Visual learning | N/A | BVMT-R recall BVMT-R delay | N/A | N/A | N/A |
| Verbal memory | Verbal memory | DATT recognition accuracy DATT response time | Verbal learning | N/A | BSRT CLTR BSRT total BSRT long-term storage | RAVLT list A trial 1–5 total RAVLT short-term recall RAVLT delayed recall | N/A | N/A |
| Cognitive flexibility/problem solving | Cognitive flexibility | Arrow flankers test | Attention-vigilance Problem solving | N/A | N/A | N/A | N/A | N/A |
| Social cognition | N/A | N/A | Social cognition | N/A | N/A | N/A | N/A | N/A |
| Fluid cognition | Fluid cognition | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Kuszewski et al. | Cox et al. | Kucukgoncu et al. | Wynn et al. | Small et al. | Rainey-Smith et al. | Ringman et al. | Baum et al. | |
|---|---|---|---|---|---|---|---|---|
| Reference/ Registry (if available) | [35,38] | [27,34] | [26,33] | [16,32] | [15,31] | [11,30] | [10,29] | [13] |
| Year | 2020 | 2020 | 2019 | 2018 | 2018 | 2016 | 2012 | 2008 |
| Randomization process | L | L | L | L | L | L | L | L |
| Intervention adherence | L | L | L | L | L | L | L | L |
| Missing outcome data | L | L | L | L | L | H 3 | L | L |
| Outcome measurement | L | L | L | L | L | L | L | L |
| Selective reporting | L | L | L | L 1 | S 2 | S 4 | L | L |
| Overall RoB | L | L | L | L 1 | S | H | L | L |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsai, I.-C.; Hsu, C.-W.; Chang, C.-H.; Tseng, P.-T.; Chang, K.-V. The Effect of Curcumin Differs on Individual Cognitive Domains across Different Patient Populations: A Systematic Review and Meta-Analysis. Pharmaceuticals 2021, 14, 1235. https://doi.org/10.3390/ph14121235
Tsai I-C, Hsu C-W, Chang C-H, Tseng P-T, Chang K-V. The Effect of Curcumin Differs on Individual Cognitive Domains across Different Patient Populations: A Systematic Review and Meta-Analysis. Pharmaceuticals. 2021; 14(12):1235. https://doi.org/10.3390/ph14121235
Chicago/Turabian StyleTsai, I-Chen, Chih-Wei Hsu, Chun-Hung Chang, Ping-Tao Tseng, and Ke-Vin Chang. 2021. "The Effect of Curcumin Differs on Individual Cognitive Domains across Different Patient Populations: A Systematic Review and Meta-Analysis" Pharmaceuticals 14, no. 12: 1235. https://doi.org/10.3390/ph14121235
APA StyleTsai, I.-C., Hsu, C.-W., Chang, C.-H., Tseng, P.-T., & Chang, K.-V. (2021). The Effect of Curcumin Differs on Individual Cognitive Domains across Different Patient Populations: A Systematic Review and Meta-Analysis. Pharmaceuticals, 14(12), 1235. https://doi.org/10.3390/ph14121235








