Inhibitory Effect of Chlorogenic Acid Analogues Comprising Pyridine and Pyrimidine on α-MSH-Stimulated Melanogenesis and Stability of Acyl Analogues in Methanol
Abstract
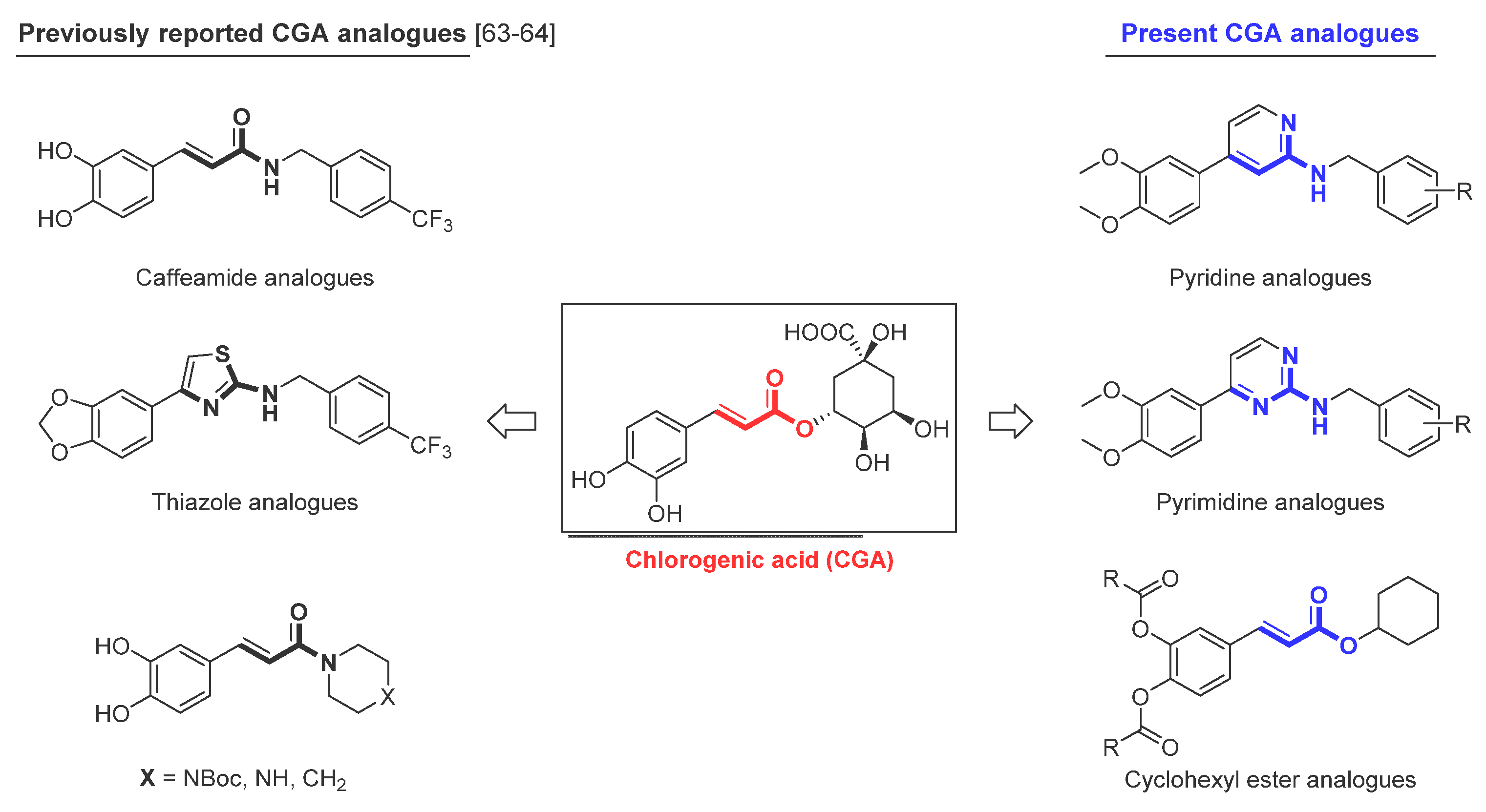
:1. Introduction
2. Results
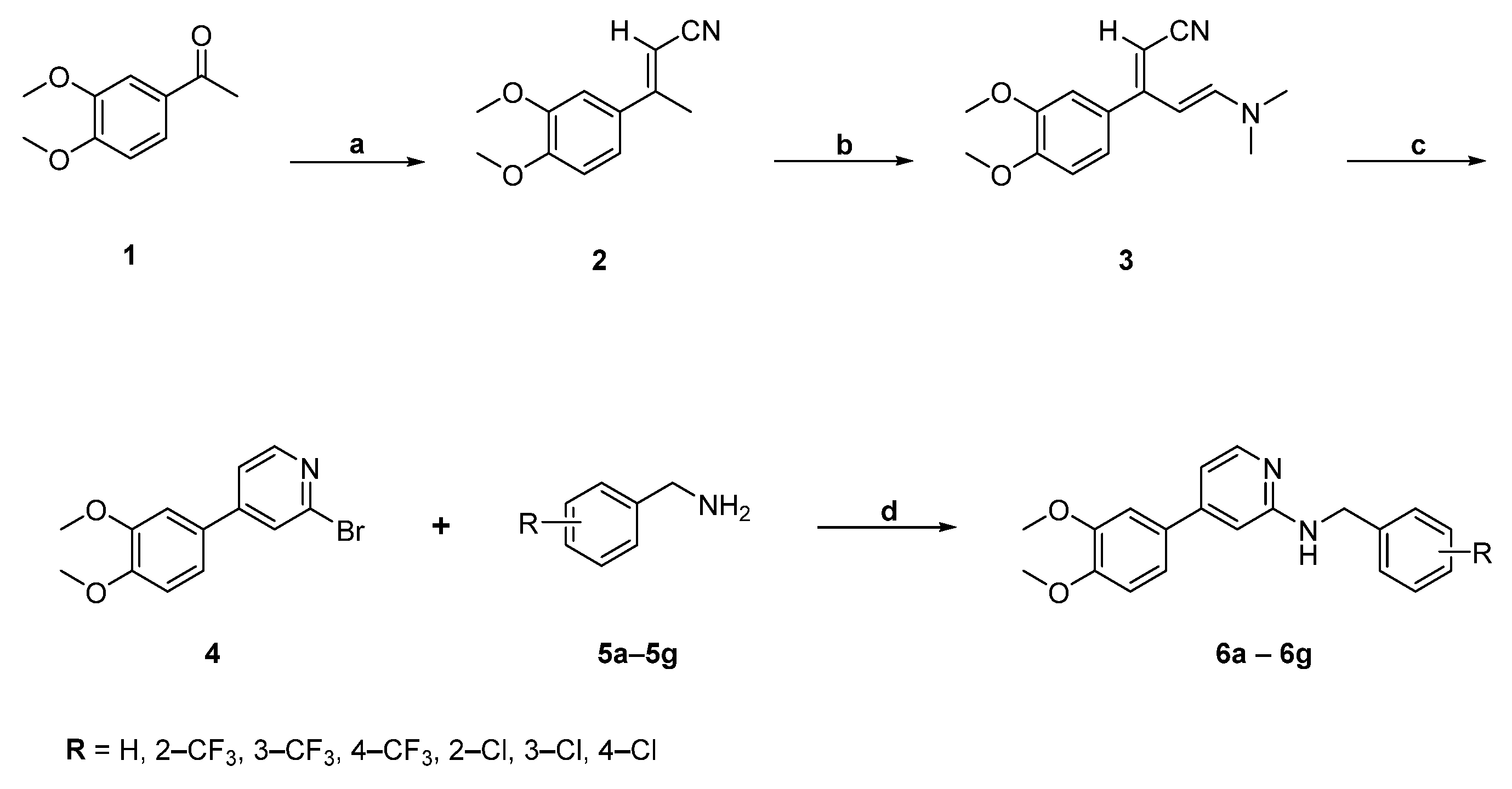
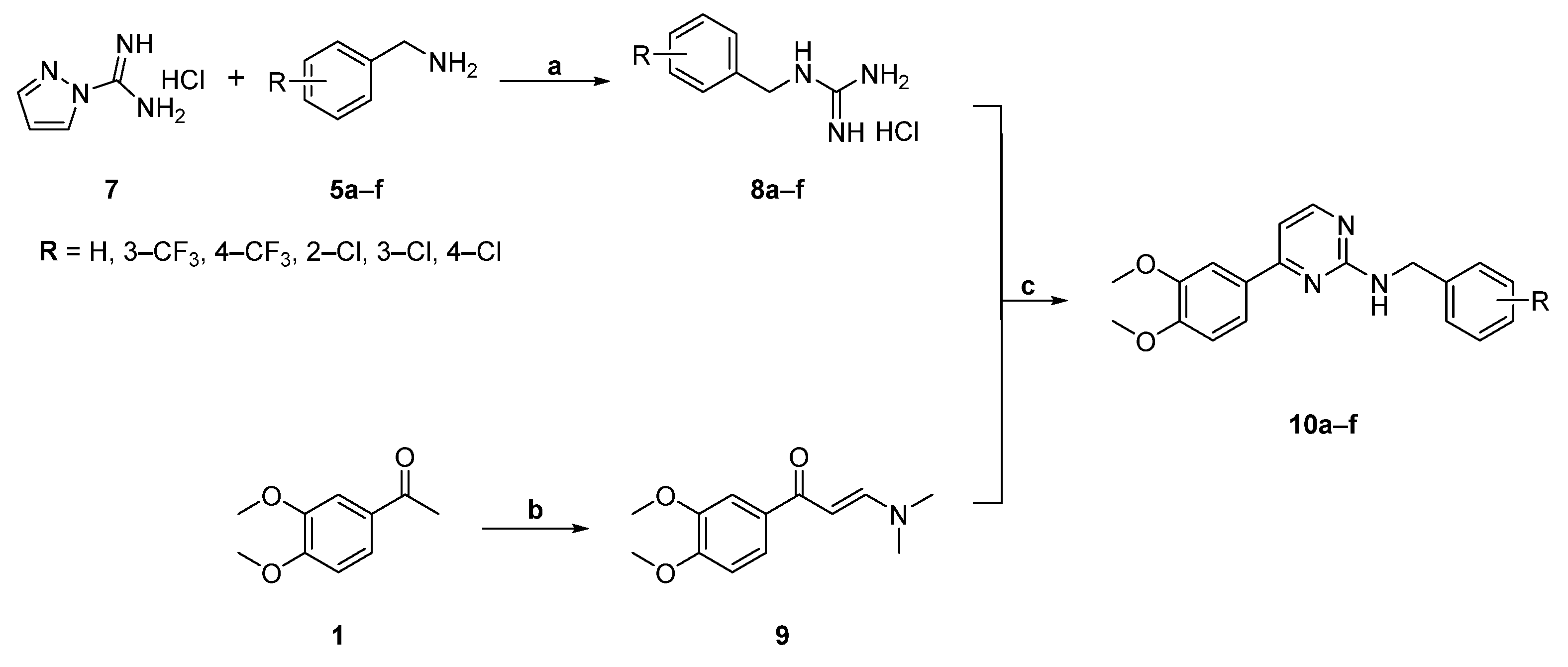
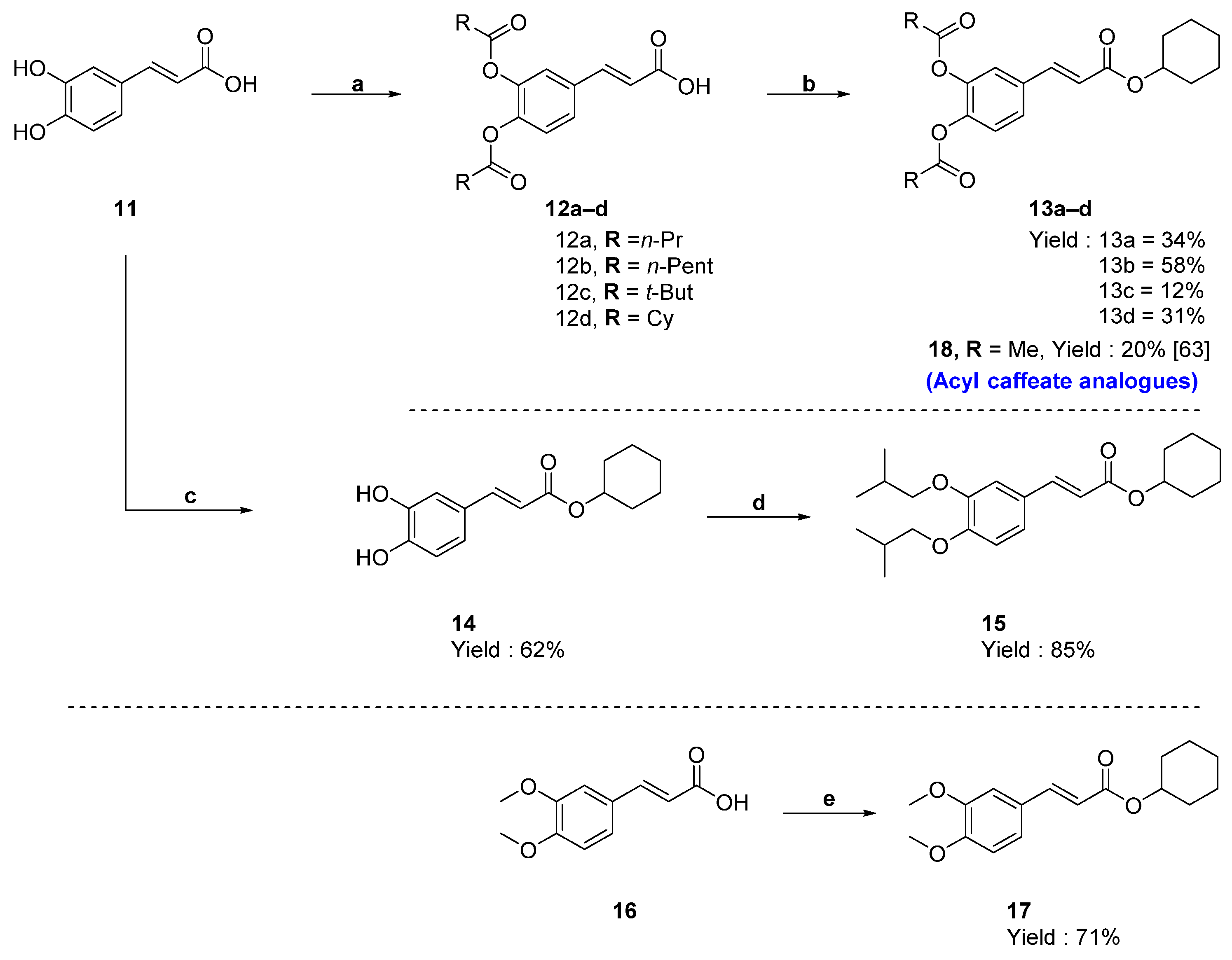
2.1. Chemistry
2.2. Biological Results
3. Discussion
4. Materials and Methods
4.1. General Information
4.2. General Procedure for the Synthesis of Pyridine Analogues (6a–g) of CGA
4.3. Synthesis of (E)-1-(3,4-Dimethoxyphenyl)-3-(dimethylamino)prop-2-en-1-one (9)
4.4. General Procedure for the Synthesis of Pyrimidine Analogues (10a–f) of CGA
4.5. Synthesis of O-Acylated Derivatives of Acrylic Acid (12a–12d)
4.6. Synthesis of Cyclohexyl Ester Analogues 13a–d of CGA
4.7. Synthesis of (E)-Cyclohexyl 3-(3,4-dihydroxyphenyl)acrylate (14)
4.8. Synthesis of (E)-Cyclohexyl 3-(3,4-diisobutoxyphenyl)acrylate (15)
4.9. Synthesis of (E)-Cyclohexyl 3-(3,4-dimethoxyphenyl)acrylate (17)
4.10. Biological Assay
4.10.1. Melanin Measurement
4.10.2. MTT Assay
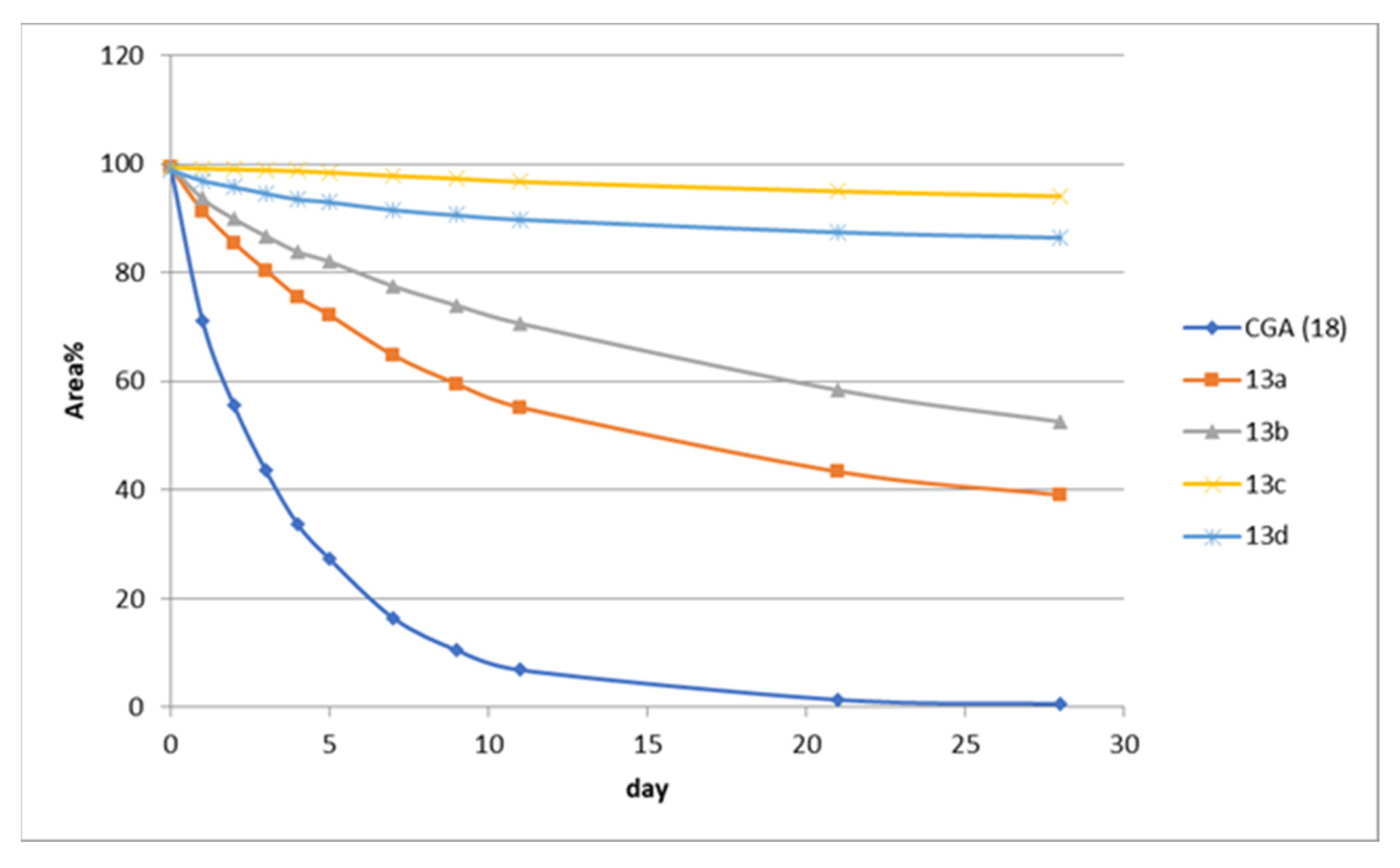
4.11. Stability of CGA Acyl Analogues in Methanol Solution
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pillaiyar, T.; Namasivayam, V.; Manickam, M.; Jung, S.-H. Inhibitors of Melanogenesis: An Updated Review. J. Med. Chem. 2018, 61, 7395–7418. [Google Scholar] [CrossRef]
- Pillaiyar, T.; Manickam, M.; Namasivayam, V. Skin whitening agents: Medicinal chemistry perspective of tyrosinase inhibitors. J. Enzym. Inhib. Med. Chem. 2017, 32, 403–425. [Google Scholar] [CrossRef] [Green Version]
- Imokawa, G.; Ishida, K. Inhibitors of Intracellular Signaling Pathways that Lead to Stimulated Epidermal Pigmentation: Perspective of Anti-Pigmenting Agents. Int. J. Mol. Sci. 2014, 15, 8293–8315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Videira, I.F.S.; Lima Moura, D.F.; Vasconcelos Magina, S.B.L.M. Mechanisms regulating melanogenesis. An. Bras. Dermatol. 2013, 88, 76–83. [Google Scholar] [CrossRef] [Green Version]
- Gillbro, J.M.; Olsson, M.J. The melanogenesis and mechanisms of skin-lightening agents–existing and new approaches. Int. J. Cosmet. Sci. 2011, 33, 210–221. [Google Scholar] [CrossRef]
- Obaid, R.J.; Mughal, E.U.; Naeem, N.; Sadiq, A.; Alsantali, R.I.; Jassas, R.S.; Moussa, Z.; Ahmed, S.A. Natural and synthetic flavonoid derivatives as new potential tyrosinase inhibitors: A systematic review. RSC Adv. 2021, 11, 22159–22198. [Google Scholar] [CrossRef]
- Li, J.; Feng, L.; Liu, L.; Wang, F.; Ouyang, L.; Zhang, L.; Hu, X.; Wang, G. Recent advances in the design and discovery of synthetic tyrosinase inhibitors. Eur. J. Med. Chem. 2021, 224, 113744. [Google Scholar] [CrossRef]
- Yuan, Y.; Jin, W.; Nazir, Y.; Fercher, C.; Blaskovich, M.A.T.; Cooper, M.A.; Barnard, R.T.; Ziora, Z.M. Tyrosinase inhibitors as potential antibacterial agents. Eur. J. Med. Chem. 2020, 187, 111892. [Google Scholar] [CrossRef] [PubMed]
- Lai, X.; Wichers, H.J.; Soler-Lopez, M.; Dijkstra, B.W. Structure and Function of Human Tyrosinase and Tyrosinase-Related Proteins. Chem. Eur. J. 2018, 24, 47–55. [Google Scholar] [CrossRef]
- Hasegawa, K.; Fujiwara, R.; Sato, K.; Shin, J.; Kim, S.J.; Kim, M.; Kang, H.Y. Possible involvement of keratinocyte growth factor in the persistence of hyperpigmentation in both human facial solar lentigines and melasma. Ann. Dermatol. 2015, 27, 626–629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Savoye, I.; Olsen, C.M.; Whiteman, D.C.; Bijon, A.; Wald, L.; Dartois, L.; Clavel-Chapelon, F.; Boutron-Ruault, M.C.; Kvaskoff, M. Patterns of Ultraviolet Radiation Exposure and Skin Cancer Risk: The E3N-SunExp Study. J. Epidemiol. 2018, 28, 27–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ortonne, J.P.; Bissett, D.L. Latest insights into skin hyperpigmentation. J. Investig. Dermatol. Symp. Proc. 2008, 13, 10–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bastiaens, M.; Hoefnagel, J.; Westendorp, R.; Vermeer, B.J.; Bavinck, J.N.B. Solar lentigines are strongly related to sun exposure in contrast to ephelides. Pigment Cell Res. 2004, 17, 225–229. [Google Scholar] [CrossRef]
- Niu, C.; Aisa, H.A. Upregulation of Melanogenesis and Tyrosinase Activity: Potential Agents for Vitiligo. Molecules 2017, 22, 1303. [Google Scholar] [CrossRef] [Green Version]
- De Gruijl, F.R. Skin cancer and solar UV radiation. Eur. J. Cancer 1999, 35, 2003–2009. [Google Scholar] [CrossRef]
- Neagu, E.; Radu, G.L.; Albu, C.; Paun, G. Antioxidant activity, acetylcholinesterase and tyrosinase inhibitory potential of Pulmonaria officinalis and Centarium umbellatum extracts. Saudi J. Biol. Sci. 2018, 25, 578–585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, H.; Newman, M.; Lardelli, M. The zebrafish orthologue of familial Alzheimer’s disease gene PRESENILIN 2 is required for normal adult melanotic skin pigmentation. PLoS ONE 2018, 13, e0206155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lavezzo, M.M.; Sakata, V.M.; Morita, C.; Rodriguez, E.E.C.; Abdallah, S.F.; da Silva, F.T.G.; Hirata, C.E.; Yamamoto, J.H. Vogt-Koyanagi-Harada disease: Review of a rare autoimmune disease targeting antigens of melanocytes. Orphanet J. Rare Dis. 2016, 11, 29. [Google Scholar] [CrossRef] [Green Version]
- Available online: https://cosmetics.specialchem.com/news/industry-news/skin-lightening-products-market-to-reach-usd23-bn-by-2020-global-industry-analysts (accessed on 16 February 2005).
- Iraji, A.; Panahi, Z.; Edraki, N.; Khoshneviszadeh, M.; Khoshneviszadeh, M. Design, synthesis, in vitro and in silico studies of novel Schiff base derivatives of 2-hydroxy-4-methoxybenzamide as tyrosinase inhibitors. Drug Dev. Res. 2021, 82, 533–542. [Google Scholar] [CrossRef]
- Hosseinpoor, H.; Moghadam Farid, S.; Iraji, A.; Askari, S.; Edraki, N.; Hosseini, S.; Jamshidzadeh, A.; Larijani, B.; Attarroshan, M.; Pirhadi, S.; et al. Anti-melanogenesis and anti-tyrosinase properties of aryl-substituted acetamides of phenoxy methyl triazole conjugated with thiosemicarbazide: Design, synthesis and biological evaluations. Bioorg. Chem. 2021, 114, 104979. [Google Scholar] [CrossRef] [PubMed]
- Gao, D.; Kim, J.H.; Kim, C.T.; Jeong, W.S.; Kim, H.M.; Sim, J.; Kang, J.S. Evaluation of Anti-Melanogenesis Activity of Enriched Pueraria lobata Stem Extracts and Characterization of Its Phytochemical Components Using HPLC–PDA–ESI–MS/MS. Int. J. Mol. Sci. 2021, 22, 8105. [Google Scholar] [CrossRef] [PubMed]
- Durai, P.; Ko, Y.-J.; Kim, J.-C.; Pan, C.-H.; Park, K. Identification of Tyrosinase Inhibitors and Their Structure-Activity Relationships via Evolutionary Chemical Binding Similarity and Structure-Based Methods. Molecules 2021, 26, 566. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Mai, Y.; Shi, H.; Liao, B.; Wang, F. Design, Synthesis, Biological Evaluation and Inhibition Mechanism of 3-/4-Alkoxy Phenylethylidenethiosemicarbazides as New, Potent and Safe Tyrosinase Inhibitors. Chem. Pharm. Bull. 2020, 68, 369–379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hosseinpoor, H.; Iraji, A.; Edraki, N.; Pirhadi, S.; Attarroshan, M.; Khoshneviszadeh, M.; Khoshneviszadeh, M. A Series of Benzylidenes Linked to Hydrazine-1-carbothioamide as Tyrosinase Inhibitors: Synthesis, Biological Evaluation and Structure–Activity Relationship. Chem. Biodivers. 2020, 17, e2000285. [Google Scholar] [CrossRef]
- Dettori, M.A.; Fabbri, D.; Dessì, A.; Dallocchio, R.; Carta, P.; Honisch, C.; Ruzza, P.; Farina, D.; Migheli, R.; Serra, P.A.; et al. Synthesis and Studies of the Inhibitory Effect of Hydroxylated Phenylpropanoids and Biphenols Derivatives on Tyrosinase and Laccase Enzymes. Molecules 2020, 25, 2709. [Google Scholar] [CrossRef]
- Ullah, S.; Kang, D.; Lee, S.; Ikram, M.; Park, C.; Park, Y.; Yoon, S.; Chun, P.; Moon, H.R. Synthesis of cinnamic amide derivatives and their anti-melanogenic effect in α-MSH-stimulated B16F10 melanoma cells. Eur. J. Med. Chem. 2019, 161, 78–92. [Google Scholar] [CrossRef]
- Hałdys, K.; Latajka, R. Thiosemicarbazones with tyrosinase inhibitory activity. Med. Chem. Comm. 2019, 10, 378–389. [Google Scholar] [CrossRef]
- Arepalli, S.K.; Lee, C.; Jung, J.K.; Kim, Y.; Lee, K.; Lee, H. Synthesis of N-arylindazole-3-carboxamide and N-benzoylindazole derivatives and their evaluation against α-MSH-stimulated melanogenesis. Bioorg. Med. Chem. Lett. 2019, 29, 2604–2608. [Google Scholar] [CrossRef]
- He, M.; Fan, M.; Liu, W.; Li, Y.; Wang, G. Design, synthesis, molecular modeling, and biological evaluation of novel kojic acid derivatives containing bioactive heterocycle moiety as inhibitors of tyrosinase and antibrowning agents. Food Chem. 2021, 362, 130241. [Google Scholar] [CrossRef]
- Cardoso, R.; Valente, R.; Souza da Costa, C.H.; da, S. Gonçalves Vianez, J.L.; Santana da Costa, K.; de Molfetta, F.A.; Nahum Alves, C. Analysis of Kojic Acid Derivatives as Competitive Inhibitors of Tyrosinase: A Molecular Modeling Approach. Molecules 2021, 26, 2875. [Google Scholar] [CrossRef]
- Ashooriha, M.; Khoshneviszadeh, M.; Khoshneviszadeh, M.; Rafiei, A.; Kardan, M.; Yazdian-Robati, R.; Emami, S. Kojic acid–natural product conjugates as mushroom tyrosinase inhibitors. Eur. J. Med. Chem. 2020, 201, 112480. [Google Scholar] [CrossRef]
- Hałdys, K.; Goldeman, W.; Anger-Góra, N.; Rossowska, J.; Latajka, R. Monosubstituted acetophenone thiosemicarbazones as potent inhibitors of tyrosinase: Synthesis, inhibitory studies, and molecular docking. Pharmaceuticals 2021, 14, 74. [Google Scholar] [CrossRef] [PubMed]
- Ketata, E.; Elleuch, H.; Neifar, A.; Mihoubi, W.; Ayadi, W.; Marrakchi, N.; Rezgui, F.; Gargouri, A. Anti-melanogenesis potential of a new series of Morita-Baylis-Hillman adducts in B16F10 melanoma cell line. Bioorg. Chem. 2019, 84, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Sepehri, N.; Iraji, A.; Yavari, A.; Asgari, M.S.; Zamani, S.; Hosseini, S.; Bahadorikhalili, S.; Pirhadi, S.; Larijani, B.; Khoshneviszadeh, M.; et al. The natural-based optimization of kojic acid conjugated to different thio-quinazolinones as potential anti-melanogenesis agents with tyrosinase inhibitory activity. Bioorg. Med. Chem. 2021, 36, 116044. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, P.G.; Dige, N.C.; Vanjare, B.D.; Raza, H.; Hassan, M.; Seo, S.-Y.; Kim, C.-H.; Lee, K.H. Facile synthesis of new quinazolinone benzamides as potent tyrosinase inhibitors: Comparative spectroscopic and molecular docking studies. J. Mol. Struct. 2019, 1198, 126915. [Google Scholar] [CrossRef]
- Tang, K.; Jiang, Y.; Zhang, H.; Huang, W.; Xie, Y.; Deng, C.; Xu, H.; Song, X.; Xu, H. Design, synthesis of Cinnamyl-paeonol derivatives with 1, 3-Dioxypropyl as link arm and screening of tyrosinase inhibition activity in vitro. Bioorg. Chem. 2021, 106, 104512. [Google Scholar] [CrossRef]
- Gaikwad, N.; Nanduri, S.; Madhavi, Y.V. Cinnamamide: An insight into the pharmacological advances and structure–activity relationships. Eur. J. Med. Chem. 2019, 181, 111561. [Google Scholar] [CrossRef] [PubMed]
- Lončar, B.; Perin, N.; Mioč, M.; Boček, I.; Grgić, L.; Kralj, M.; Tomić, S.; Stojković, M.R.; Hranjec, M. Novel amino substituted tetracyclic imidazo[4,5-b]pyridine derivatives: Design, synthesis, antiproliferative activity and DNA/RNA binding study. Eur. J. Med. Chem. 2021, 217, 113342. [Google Scholar] [CrossRef]
- Lee, S.; Kwon, N.H.; Seo, B.; Lee, J.Y.; Cho, H.Y.; Kim, K.; Kim, H.S.; Jung, K.; Jeon, Y.H.; Kim, S.; et al. Discovery of novel potent migrastatic Thiazolo[5,4-b]pyridines targeting Lysyl-tRNA synthetase (KRS) for treatment of Cancer metastasis. Eur. J. Med. Chem. 2021, 218, 113405. [Google Scholar] [CrossRef] [PubMed]
- Krajčovičová, S.; Jorda, R.; Vanda, D.; Soural, M.; Kryštof, V. 1,4,6-Trisubstituted imidazo[4,5-c]pyridines as inhibitors of Bruton’s tyrosine kinase. Eur. J. Med. Chem. 2021, 211, 113094. [Google Scholar] [CrossRef] [PubMed]
- Sahu, M.; Siddiqui, N. A review on biological importance of pyrimidines in the new era. J. Pharm. Pharm. Sci. 2016, 8, 8–21. [Google Scholar]
- Kaur, R.; Chaudhary, S.; Kumar, K.; Gupta, M.K.; Rawal, R.K. Recent synthetic and medicinal perspectives of dihydropyrimidinones: A review. Eur. J. Med. Chem. 2017, 132, 108–134. [Google Scholar] [CrossRef] [PubMed]
- Rani, J.; Kumar, S.; Saini, M.; Mundlia, J.; Verma, P.K. Biological potential of pyrimidine derivatives in a new era. Res. Chem. Intermed. 2016, 42, 6777–6804. [Google Scholar] [CrossRef]
- Gheibi, N.; Taherkhani, N.; Ahmadi, A.; Haghbeen, K.; Ilghari, D. Characterization of inhibitory effects of the potential therapeutic inhibitors, benzoic acid and pyridine derivatives, on the monophenolase and diphenolase activities of tyrosinase. Iran. J. Med. Sci. 2015, 18, 122–129. [Google Scholar]
- Choi, J.; Park, S.-J.; Jee, J.-G. Analogues of ethionamide, a drug used for multidrug-resistant tuberculosis, exhibit potent inhibition of tyrosinase. Eur. J. Med. Chem. 2015, 106, 157–166. [Google Scholar] [CrossRef]
- Bellei, B.; Pitisci, A.; Migliano, E.; Cardinali, G.; Picardo, M. Pyridinyl imidazole compounds interfere with melanosomes sorting through the inhibition of Cyclin G-associated Kinase, a regulator of cathepsins maturation. Cell. Signal. 2014, 26, 716–723. [Google Scholar] [CrossRef]
- Hseu, Y.-C.; Chen, X.-Z.; Vudhya Gowrisankar, Y.; Yen, H.-R.; Chuang, J.-Y.; Yang, H.-L. The Skin-Whitening Effects of Ectoine via the Suppression of α-MSH-Stimulated Melanogenesis and the Activation of Antioxidant Nrf2 Pathways in UVA-Irradiated Keratinocytes. Antioxidants 2020, 9, 63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mirmortazavi, S.S.; Farvandi, M.; Ghafouri, H.; Mohammadi, A.; Shourian, M. Evaluation of novel pyrimidine derivatives as a new class of mushroom tyrosinase inhibitor. Drug Des. Devel. Ther. 2019, 13, 2169–2178. [Google Scholar] [CrossRef] [Green Version]
- Chung, Y.C.; Kim, M.-J.; Kang, E.Y.; Kim, Y.B.; Kim, B.S.; Park, S.-M.; Hyun, C.-G. Anti-Melanogenic Effects of Hydroxyectoine via MITF Inhibition by JNK, p38, and AKT Pathways in B16F10 Melanoma Cells. Nat. Prod. Commun. 2019, 14. [Google Scholar] [CrossRef]
- Lu, H.; Tian, Z.; Cui, Y.; Liu, Z.; Ma, X. Chlorogenic acid: A comprehensive review of the dietary sources, processing effects, bioavailability, beneficial properties, mechanisms of action, and future directions. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3130–3158. [Google Scholar] [CrossRef]
- Kim, H.H.; Kim, J.K.; Kim, J.; Jung, S.-H.; Lee, K. Characterization of Caffeoylquinic Acids from Lepisorus thunbergianus and Their Melanogenesis Inhibitory Activity. ACS Omega 2020, 5, 30946–30955. [Google Scholar] [CrossRef]
- Li, H.-R.; Habasi, M.; Xie, L.-Z.; Aisa, H.A. Effect of Chlorogenic Acid on Melanogenesis of B16 Melanoma Cells. Molecules 2014, 19, 12940–12948. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, R.; Sharma, A.; Iqbal, M.S.; Srivastava, J.K. Therapeutic promises of chlorogenic acid with special emphasis on its anti-obesity property. Curr. Mol. Pharmacol. 2020, 13, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Tajik, N.; Tajik, M.; Mack, I.; Enck, P. The potential effects of chlorogenic acid, the main phenolic components in coffee, on health: A comprehensive review of the literature. Eur. J. Nutr. 2017, 56, 2215–2244. [Google Scholar] [CrossRef] [PubMed]
- Plazas, M.; Andújar, I.; Vilanova, S.; Hurtado, M.; Gramazio, P.; Herraiz, F.J.; Prohens, J. Breeding for Chlorogenic Acid Content in Eggplant: Interest and Prospects. Not. Bot. Horti Agrobot. Cluj-Napoca 2013, 41, 26–35. [Google Scholar] [CrossRef] [Green Version]
- Shi, H.; Dong, L.; Jiang, J.; Zhao, J.; Zhao, G.; Dang, X.; Lu, X.; Jia, M. Chlorogenic acid reduces liver inflammation and fibrosis through inhibition of toll-like receptor 4 signaling pathway. Toxicology 2013, 303, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Yun, N.; Kang, J.-W.; Lee, S.-M. Protective effects of chlorogenic acid against ischemia/reperfusion injury in rat liver: Molecular evidence of its antioxidant and anti-inflammatory properties. J. Nutr. Biochem. 2012, 23, 1249–1255. [Google Scholar] [CrossRef]
- Zhang, X.; Huang, H.; Yang, T.; Ye, Y.; Shan, J.; Yin, Z.; Luo, L. Chlorogenic acid protects mice against lipopolysaccharide-induced acute lung injury. Injury 2010, 41, 746–752. [Google Scholar] [CrossRef] [PubMed]
- Anqi, Z.; Xin, L.; Shaomi, Z.; Chi, L.; Shu, W.; Qinxiu, Z.; Junning, Z.; Linjiang, S. Chlorogenic acid induces apoptosis, inhibits metastasis and improves antitumor immunity in breast cancer via the NF-κB signaling pathway. Oncol. Rep. 2021, 45, 717–727. [Google Scholar]
- Luigi, S.; Alessia, S.; Michela, I.; Angela, R.; Annamaria, S.; Emilio, C.; Severina, P.; Michelina, C.; Silvio, N. Chlorogenic acid activates ERK1/2 and inhibits proliferation of osteosarcoma cells. J. Cell. Physiol. 2020, 235, 3741–3752. [Google Scholar]
- Alessia, S.; Angela, R.; Spina, A.; Silvio, N.; Luigi, S. Chlorogenic Acid Enhances Doxorubicin-Mediated Cytotoxic Effect in Osteosarcoma Cells. Int. J. Mol. Sci. 2021, 22, 8586. [Google Scholar]
- Jo, H.; Choi, M.; Sim, J.; Viji, M.; Li, S.; Lee, Y.H.; Kim, Y.; Seo, S.Y.; Zhou, Y.; Lee, K.; et al. Synthesis and biological evaluation of caffeic acid derivatives as potent inhibitors of α-MSH-stimulated melanogenesis. Bioorg. Med. Chem. Lett. 2017, 27, 3374–3377. [Google Scholar] [CrossRef] [PubMed]
- Jo, H.; Zhou, Y.; Viji, M.; Choi, M.; Lim, J.Y.; Sim, J.; Rhee, J.; Kim, Y.; Seo, S.Y.; Kim, W.J.; et al. Synthesis, biological evaluation, and metabolic stability of chlorogenic acid derivatives possessing thiazole as potent inhibitors of α-MSH-stimulated melanogenesis. Bioorg. Med. Chem. Lett. 2017, 27, 4854–4857. [Google Scholar] [CrossRef]
- Sim, J.; Viji, M.; Rhee, J.; Jo, H.; Cho, S.J.; Park, Y.; Seo, S.Y.; Jung, K.Y.; Lee, H.; Jung, J.K. γ-Functionalization of α,β-Unsaturated Nitriles under Mild Conditions: Versatile Synthesis of 4-Aryl-2-Bromopyridines. Adv. Synth. Catal. 2019, 361, 5458–5465. [Google Scholar] [CrossRef]
- An, T.; Kang, B.; Kang, S.; Pac, J.; Youk, J.; Lin, D.; Lee, Y. Guanidine cyclic diimides and their polymers. Chem. Commun. 2019, 55, 10222–10225. [Google Scholar] [CrossRef]
- Lingel, A.; Sendzik, M.; Huang, Y.; Shultz, M.D.; Cantwell, J.; Dillon, M.P.; Fu, X.; Fuller, J.; Gabriel, T.; Gu, J.; et al. Structure-Guided Design of EED Binders Allosterically Inhibiting the Epigenetic Polycomb Repressive Complex 2 (PRC2) Methyltransferase. J. Med. Chem. 2017, 60, 415–427. [Google Scholar] [CrossRef] [PubMed]
- Thanigaimalai, P.; Lee, K.C.; Bang, S.C.; Lee, J.H.; Yun, C.Y.; Roh, E.; Hwang, B.Y.; Kim, Y.; Jung, S.H. Inhibitory effect of novel tetrahydropyrimidine-2(1H)-thiones on melanogenesis. Bioorg. Med. Chem. 2010, 18, 1135–1142. [Google Scholar] [CrossRef]
| Compounds | R | IC50 (μM) a,c |
|---|---|---|
| Kojic acid b | - | 54.0 ± 1.5 |
| Arbutin b | - | 380.0 ± 9.5 |
| 6a | H | 17.0 ± 4.0 |
| 6b | 4-Cl | 4.5 ± 0.7 |
| 6c | 3-Cl | 5.0 ± 1.4 |
| 6d | 2-Cl | 8.0 ± 1.0 |
| 6e | 4-CF3 | 8.5 ± 0.7 |
| 6f | 3-CF3 | 2.5 ± 0.7 |
| 6g | 2-CF3 | 7.5 ± 0.7 |
| 10a | H | >100 |
| 10b | 4-Cl | 54.0 ± 6.0 |
| 10c | 3-Cl | 32.0 ± 2.0 |
| 10d | 2-Cl | 40.0 ± 8.0 |
| 10e | 4-CF3 | >100 |
| 10f | 3-CF3 | 20.0 ± 1.0 |
| 13a | n-Pr | 1.0 ± 0.1 |
| 13b | n-Pent | 1.3 ± 0.4 |
| 13c | t-But | 1.9 ± 0.1 |
| 13d | Cy | 2.3 ± 0.2 |
| 15 | - | >100 |
| 17 | - | >100 |
| Acyl Analogues | |||||
|---|---|---|---|---|---|
| Days | 18 | 13a | 13b | 13c | 13d |
| 0 | 99.5029 | 99.3294 | 99.8006 | 99.5492 | 98.9750 |
| 1 | 71.1918 | 91.3520 | 93.7356 | 99.3034 | 96.9973 |
| 2 | 55.5162 | 85.3800 | 89.9637 | 99.1518 | 95.8267 |
| 3 | 43.5699 | 80.3685 | 86.8066 | 99.0038 | 94.6557 |
| 4 | 33.7154 | 75.4921 | 83.9188 | 98.8061 | 93.5713 |
| 5 | 27.3752 | 72.147 | 82.1086 | 98.5395 | 93.0573 |
| 7 | 16.5047 | 64.7751 | 77.5962 | 97.9332 | 91.6229 |
| 9 | 10.5572 | 59.4842 | 74.0389 | 97.4173 | 90.6724 |
| 11 | 6.9200 | 55.1819 | 70.6843 | 96.8033 | 89.8768 |
| 21 | 1.4408 | 43.3505 | 58.4127 | 95.0243 | 87.5571 |
| 28 | 0.6625 | 39.0229 | 52.5500 | 94.0833 | 86.5501 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sim, J.; Lanka, S.; Jo, J.-W.; Chaudhary, C.L.; Vishwanath, M.; Jung, C.-H.; Lee, Y.-H.; Kim, E.-Y.; Kim, Y.-S.; Hyun, S.-S.; et al. Inhibitory Effect of Chlorogenic Acid Analogues Comprising Pyridine and Pyrimidine on α-MSH-Stimulated Melanogenesis and Stability of Acyl Analogues in Methanol. Pharmaceuticals 2021, 14, 1176. https://doi.org/10.3390/ph14111176
Sim J, Lanka S, Jo J-W, Chaudhary CL, Vishwanath M, Jung C-H, Lee Y-H, Kim E-Y, Kim Y-S, Hyun S-S, et al. Inhibitory Effect of Chlorogenic Acid Analogues Comprising Pyridine and Pyrimidine on α-MSH-Stimulated Melanogenesis and Stability of Acyl Analogues in Methanol. Pharmaceuticals. 2021; 14(11):1176. https://doi.org/10.3390/ph14111176
Chicago/Turabian StyleSim, Jaeuk, Srinu Lanka, Jeong-Woong Jo, Chhabi Lal Chaudhary, Manjunatha Vishwanath, Chan-Hyun Jung, Young-Hee Lee, Eun-Yeong Kim, Young-Soo Kim, Soon-Sil Hyun, and et al. 2021. "Inhibitory Effect of Chlorogenic Acid Analogues Comprising Pyridine and Pyrimidine on α-MSH-Stimulated Melanogenesis and Stability of Acyl Analogues in Methanol" Pharmaceuticals 14, no. 11: 1176. https://doi.org/10.3390/ph14111176
APA StyleSim, J., Lanka, S., Jo, J.-W., Chaudhary, C. L., Vishwanath, M., Jung, C.-H., Lee, Y.-H., Kim, E.-Y., Kim, Y.-S., Hyun, S.-S., Lee, H.-S., Lee, K., Seo, S.-Y., Viji, M., & Jung, J.-K. (2021). Inhibitory Effect of Chlorogenic Acid Analogues Comprising Pyridine and Pyrimidine on α-MSH-Stimulated Melanogenesis and Stability of Acyl Analogues in Methanol. Pharmaceuticals, 14(11), 1176. https://doi.org/10.3390/ph14111176






