Lipase Assisted (S)-Ketoprofen Resolution from Commercially Available Racemic Mixture
Abstract
1. Introduction
2. Results
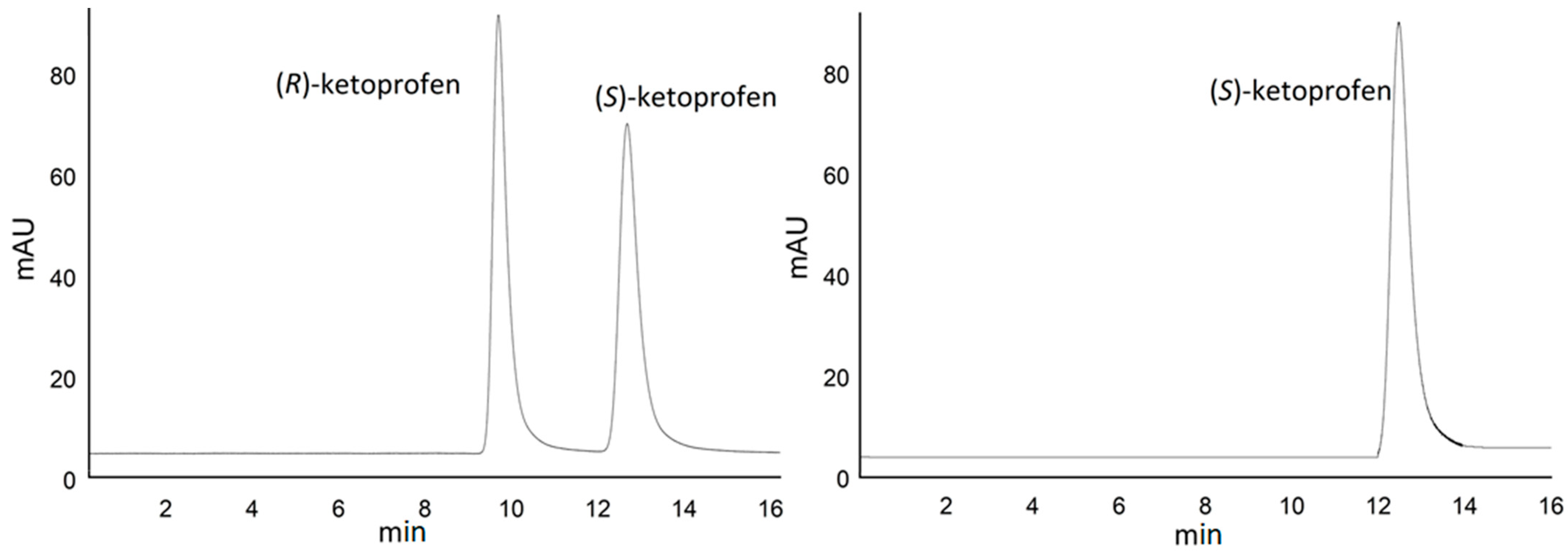
2.1. Ketoprofen Extraction and Characterization
2.2. Biocatalyst Selection
2.3. Solvent and Alcohol Selection
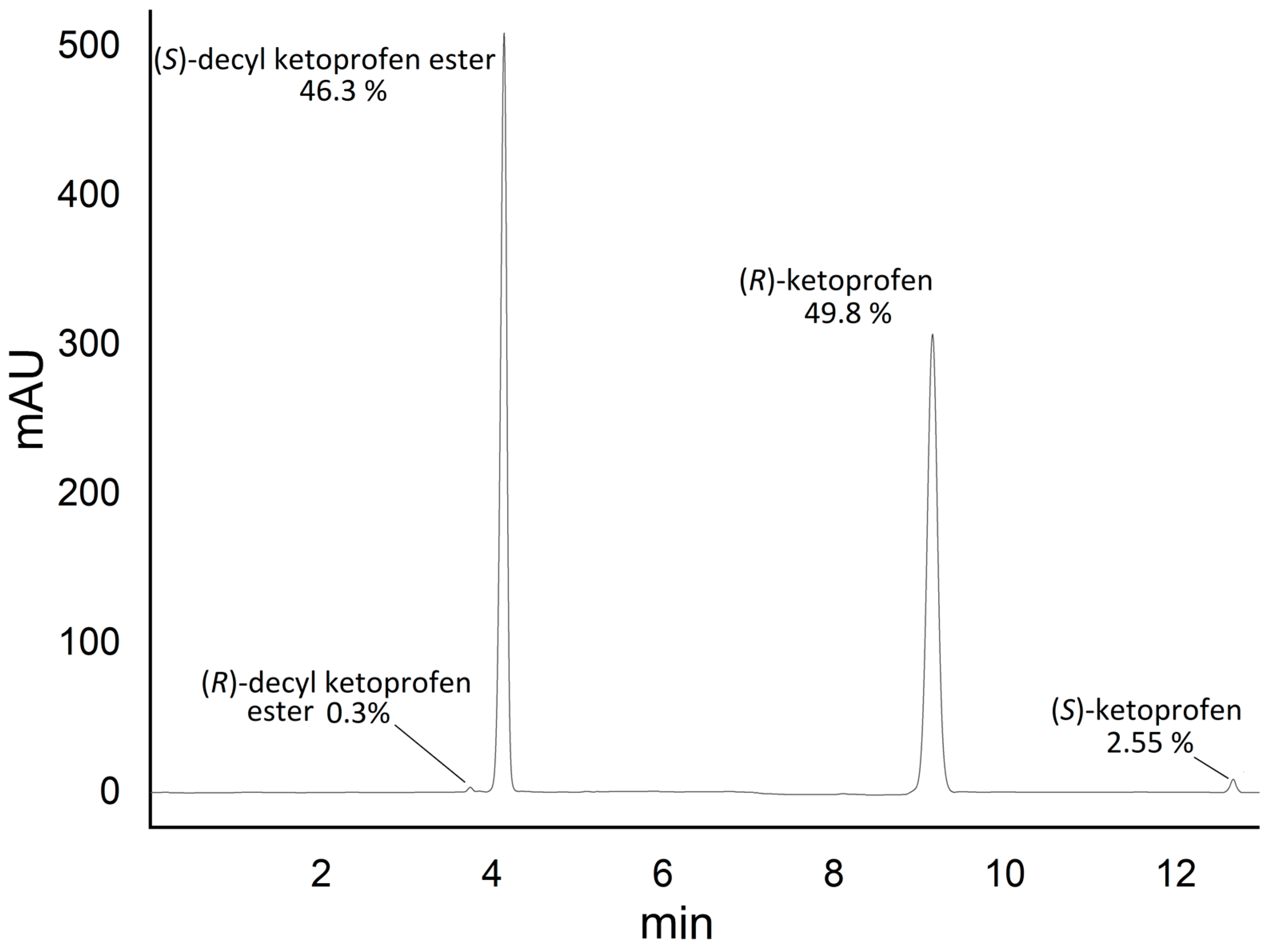
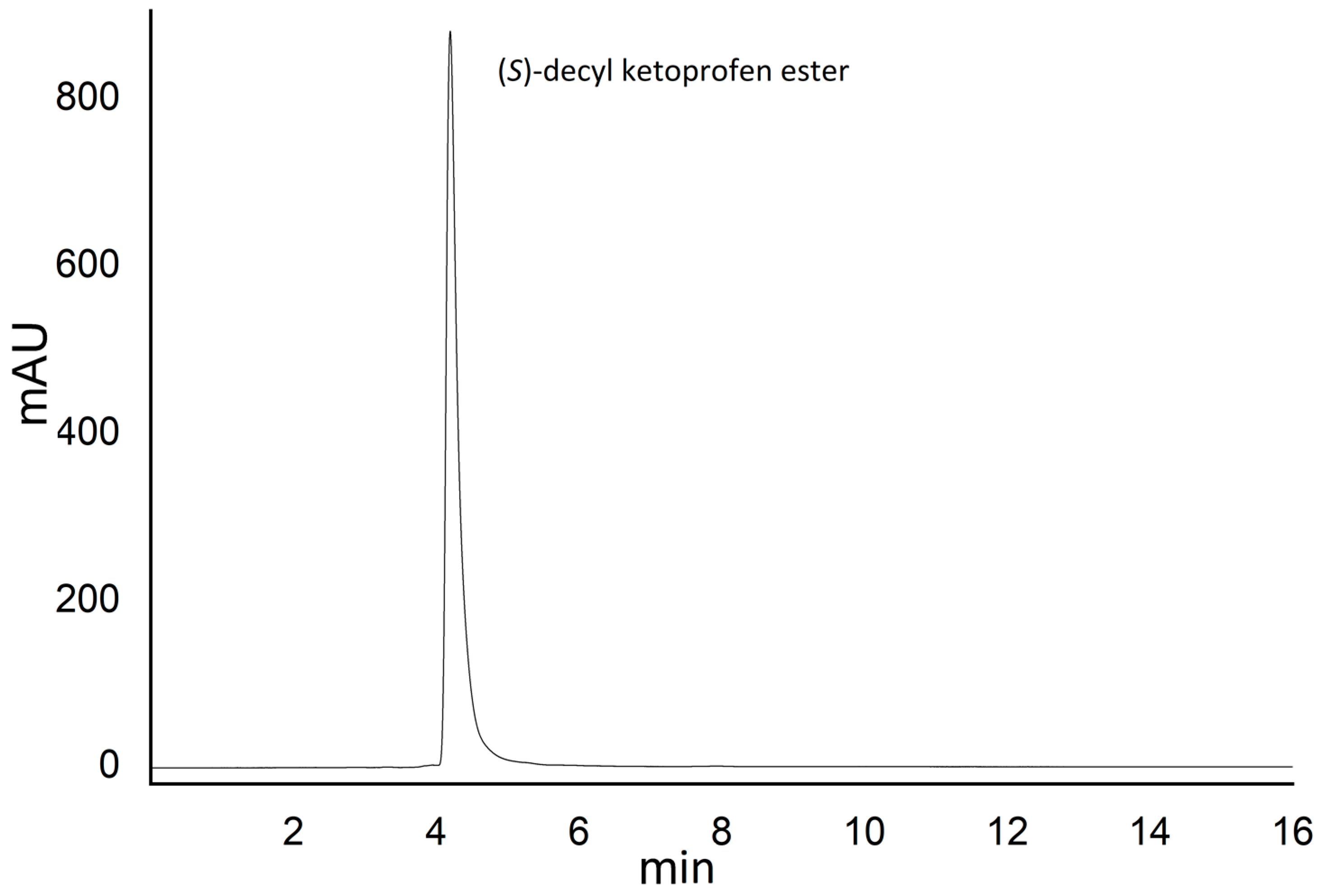
2.4. (S)-Decyl Ketoprofen Ester Isolation
2.5. (R)-Ketoprofen Racemization
2.6. (S)-Decyl Ketoprofen Hydrolysis
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Ketoprofen Extraction
4.3. High-Performance Liquid Chromatography
4.4. Enzymatic Esterification of Ketoprofen
4.5. (R)-Ketoprofen Racemization
4.6. Enzymatic Hydrolysis of Ketoprofen Ester
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chavez-Flores, D.; Salvador, J.M. Facile conversion of racemic ibuprofen to (S)-ibuprofen. Tetrahedron Asymmetry 2012, 23, 237–239. [Google Scholar] [CrossRef]
- Chávez-Flores, D.; Salvador, J.M. Commercially viable resolution of ibuprofen. Biotechnol. J. 2009, 4, 1222–1224. [Google Scholar] [CrossRef]
- Sardella, R.; Ianni, F.; Di Michele, A.; Di Capua, A.; Carotti, A.; Anzini, M.; Natalini, B. Enantioresolution and stereochemical characterization of two chiral sulfoxides endowed with COX-2 inhibitory activity. Chirality 2017, 29, 536–540. [Google Scholar] [CrossRef] [PubMed]
- Anthonsen, T.; Hoff, B.H. Resolution of derivatives of 1,2-propanediol with lipase B from Candida antarctica: Effect of substrate structure, medium, water activity and acyl donor on enantiomeric ratio. Chem. Phys. Lipids 1998, 93, 199–207. [Google Scholar] [CrossRef]
- Liu, Y.-Y.; Xu, J.-H.; Hu, Y. Enhancing effect of Tween-80 on lipase performance in enantioselective hydrolysis of ketoprofen ester. J. Mol. Catal. B Enzym. 2000, 10, 523–529. [Google Scholar] [CrossRef]
- Fernandes, C.; Palmeira, A.; Ramos, I.I.; Carneiro, C.; Afonso, C.; Tiritan, M.E.; Cidade, H.; Pinto, P.; Saraiva, M.L.M.; Reis, S.; et al. Chiral Derivatives of Xanthones: Investigation of the Effect of Enantioselectivity on Inhibition of Cyclooxygenases (COX-1 and COX-2) and Binding Interaction with Human Serum Albumin. Pharmaceuticals 2017, 10, 50. [Google Scholar] [CrossRef]
- Ríos-Quintana, R.; Estrada-Hernández, L.O. Descripción y Cuantificación de Riesgos Atribuidos a Analgésicos Antiinflamatorios No Esteroides No Selectivos Consumidos Por La Población Mexicana. Med. Interna México 2018, 34, 173–187. [Google Scholar] [CrossRef]
- Nugrahani, I.; Parwati, R. Challenges and Progress in Nonsteroidal Anti-Inflammatory Drugs Co-Crystal Development. Molecules 2021, 26, 4185. [Google Scholar] [CrossRef]
- De Loera-González, M.A.; Sánchez–Rodríguez, S.H.; Castro-Pastrana, L.I.; Flores-de la Torre, J.A.; López-Luna, A. Ecofarmacovigilancia. Rev. CENIC Cienc. Biológicas 2016, 47, 12–16. [Google Scholar]
- Matysiak, S.; Kula, J.; Błaszczyk, A. Chiral amide derivatives of ricinoleic acid and 3-hydroxynonanoic acid synthesis and cytotoxic activity. Med. Chem. Res. 2019, 28, 948–958. [Google Scholar] [CrossRef]
- Huras, B.; Zakrzewski, J.; Krawczyk, M.; Bombińska, D.; Cieniecka-Rosłonkiewicz, A.; Michalczyk, A. Synthesis and fungicidal activity of 2-methylalkyl isonicotinates and nicotinates. Med. Chem. Res. 2017, 26, 509–517. [Google Scholar] [CrossRef]
- Bui, C.; Rosenau, T.; Hettegger, H. Polysaccharide- and β-Cyclodextrin-Based Chiral Selectors for Enantiomer Resolution: Recent Developments and Applications. Molecules 2021, 26, 4322. [Google Scholar] [CrossRef] [PubMed]
- Ong, A.; Kamaruddin, A.; Bhatia, S.; Long, W.; Lim, S.; Kumari, R. Performance of free Candida antarctica lipase B in the enantioselective esterification of (R)-ketoprofen. Enzym. Microb. Technol. 2006, 39, 924–929. [Google Scholar] [CrossRef]
- Mwamwitwa, K.W.; Kaibere, R.M.; Fimbo, A.M.; Sabitii, W.; Ntinginya, N.E.; Mmbaga, B.T.; Shewiyo, D.H.; Shearer, M.C.; Smith, A.D.; Kaale, E.A. A retrospective cross-sectional study to determine chirality status of registered medicines in Tanzania. Sci. Rep. 2020, 10, 1–11. [Google Scholar] [CrossRef]
- Yuan, X.; Wang, L.; Liu, G.; Dai, G.; Tang, K. Resolution of (R, S)-ibuprofen catalyzed by immobilized Novozym40086 in organic phase. Chirality 2019, 31, 445–456. [Google Scholar] [CrossRef]
- Huang, K.; Jiao, F.; Liu, S.; Li, X.; Huang, D. Enantioselective Extraction of Ketoprofen Enantiomers Using Ester Alcohol (R, R)-Di-Tartarates or (S, S)-Di-Tartarates as Chiral Selector. Lat. Am. Appl. Res 2006, 36, 187–191. [Google Scholar]
- Mokhtari, Z.; Hekmatdoost, A.; Nourian, M. Antioxidant efficacy of vitamin D. J. Parathyroid. Diesase 2017, 5, 11–16. [Google Scholar]
- Foster, R.; Jamali, F.; Russell, A.; Alballa, S. Pharmacokinetics of ketoprofen enantiomers in healthy subjects following single and multiple doses. J. Pharm. Sci. 1988, 77, 70–73. [Google Scholar] [CrossRef]
- Calcaterra, A.; D’Acquarica, I. The market of chiral drugs: Chiral switches versus de novo enantiomerically pure compounds. J. Pharm. Biomed. Anal. 2018, 147, 323–340. [Google Scholar] [CrossRef]
- Alkatheeri, N.A.; Wasfi, I.A.; Lambert, M. Pharmacokinetics and metabolism of ketoprofen after intravenous and intramuscular administration in camels. J. Veter-Pharmacol. Ther. 1999, 22, 127–135. [Google Scholar] [CrossRef]
- Kantor, T.G. Ketoprofen: A Review of Its Pharmacologic and Clinical Properties. Pharmacother. J. Hum. Pharmacol. Drug Ther. 1986, 6, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Siodmiak, T.; Rumiński, J.K.; Marszall, M.P. Application of Lipases from Candida rugosa in the Enantioselective Esterification of (R,S)-Ibuprofen. Curr. Org. Chem. 2012, 16, 972–977. [Google Scholar] [CrossRef][Green Version]
- Nguyen, H.C.; Huong, D.T.M.; Juan, H.-Y.; Su, C.-H.; Chien, C.-C. Liquid Lipase-Catalyzed Esterification of Oleic Acid with Methanol for Biodiesel Production in the Presence of Superabsorbent Polymer: Optimization by Using Response Surface Methodology. Energies 2018, 11, 1085. [Google Scholar] [CrossRef]
- Akanbi, T.O.; Barrow, C.J. Lipase-Produced Hydroxytyrosyl Eicosapentaenoate is an Excellent Antioxidant for the Stabilization of Omega-3 Bulk Oils, Emulsions and Microcapsules. Molecules 2018, 23, 275. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Nie, K.; Wang, F.; Deng, L. Optimization of the Lipase-Catalyzed Selective Amidation of Phenylglycinol. Front. Bioeng. Biotechnol. 2020, 7, 486. [Google Scholar] [CrossRef]
- Dovale-Rosabal, G.; Rodríguez, A.; Espinosa, A.; Barriga, A.; Aubourg, S. Synthesis of EPA- and DHA-Enriched Structured Acylglycerols at the sn-2 Position Starting from Commercial Salmon Oil by Enzymatic Lipase Catalysis under Supercritical Conditions. Molecules 2021, 26, 3094. [Google Scholar] [CrossRef]
- Wu, W.-X.; Liu, Z. Novozym 435-Catalyzed Synthesis of Well-Defined Hyperbranched Aliphatic Poly(β-thioether ester). Molecules 2020, 25, 687. [Google Scholar] [CrossRef]
- Ramnath, L.; Sithole, B.; Govinden, R. Identification of lipolytic enzymes isolated from bacteria indigenous to Eucalyptus wood species for application in the pulping industry. Biotechnol. Rep. 2017, 15, 114–124. [Google Scholar] [CrossRef]
- Henke, E.; Schuster, S.; Yang, H.; Bornscheuer, U.T. Lipase-Catalyzed Resolution of Ibuprofen. In Biocatalysis; Springer: Berlin, Germany, 2000; pp. 107–112. [Google Scholar]
- Liu, J.; Zhang, Y.; Qiu, L.H.; Yang, F.; Ye, L.; Xia, Y. Kinetic resolution of ketoprofen ester catalyzed by lipase from a mutant of CBS 5791. J. Ind. Microbiol. Biotechnol. 2004, 31, 495–499. [Google Scholar] [CrossRef] [PubMed]
- Cernia, E.; Delfini, M.; Di Cocco, E.; Palocci, C.; Soro, S. Investigation of lipase-catalysed hydrolysis of naproxen methyl ester: Use of NMR spectroscopy methods to study substrate-enzyme interaction. Bioorganic Chem. 2002, 30, 276–284. [Google Scholar] [CrossRef]
- Lin, C.-N.; Tsai, S.-W. Dynamic kinetic resolution of suprofen thioester via coupled trioctylamine and lipase catalysis. Biotechnol. Bioeng. 2000, 69, 31–38. [Google Scholar] [CrossRef]
- Antona, N.D.; Lombardi, P.; Nicolosi, G.; Salvo, G. Large scale preparation of enantiopure S-ketoprofen by biocatalysed kinetic resolution. Process. Biochem. 2002, 38, 373–377. [Google Scholar] [CrossRef]
- Allen, A.E.; MacMillan, D.W.C. Asymmetric Synthesis of (S)-Ketoprofen. Synfacts 2011, 2011, 0697. [Google Scholar] [CrossRef][Green Version]
- Yao, Y.; Zou, X.; Wang, Y.; Yang, H.; Ren, Z.; Guan, Z. Palladium-Catalyzed Asymmetric Markovnikov Hydroxycarbonylation and Hydroalkoxycarbonylation of Vinyl Arenes: Synthesis of 2-Arylpropanoic Acids. Angew. Chem. Int. Ed. 2021, 202107856. [Google Scholar] [CrossRef]
- Calmes, M.; Daunis, J.; Jacquier, R.; Natt, F. Asymmetric synthesis of ketoprofen: A surprising base catalyst effect during asymmetric addition of pantolactone to methyl (3-benzoylphenyl) ketene. Tetrahedron 1994, 50, 6875–6880. [Google Scholar] [CrossRef]
- Ramminger, C.; Zim, D.; Lando, V.R.; Fassina, V.; Monteiro, A.L. Transition-metal catalyzed synthesis of Ketoprofen. J. Braz. Chem. Soc. 2000, 11, 105–111. [Google Scholar] [CrossRef]
- Yuchun, X.; Huizhou, L.; Jiayong, C. Kinetics of base catalyzed racemization of ibuprofen enantiomers. Int. J. Pharm. 2000, 196, 21–26. [Google Scholar] [CrossRef]


| Enz. % | Temp. °C | Candida rugosa L. | Mucor javanicus L. | Porcine pancreatic L. | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| c% | ee | E | c% | ee | E | c% | ee | E | ||
| 20 | 30 | 10.6 | 52.3 | 24 | 11.8 | 50.9 | 19 | 9.9 | 59.4 | 22 |
| 20 | 40 | 22.4 | 63.5 | 30 | 19.4 | 64.8 | 40 | 14.2 | 66.7 | 53 |
| 20 | 50 | 24.7 | 76.3 | 28 | 24.1 | 70.4 | 88 | 17.7 | 56.2 | 72 |
| 20 | 60 | 25.3 | 42.5 | 17 | 21.9 | 43.0 | 35 | 12.8 | 42.3 | 31 |
| 40 | 30 | 17.9 | 77.9 | 26 | 12.4 | 54.4 | 19 | 12.8 | 62.2 | 25 |
| 40 | 40 | 34.8 | 89.2 | 54 | 22.9 | 72.9 | 97 | 18.8 | 72.1 | 60 |
| 40 | 50 | 37.8 | 92.4 | 46 | 34.6 | 74.9 | 29 | 19.5 | 66.9 | 66 |
| 40 | 60 | 36.9 | 36.4 | 24 | 25.2 | 48.1 | 27 | 14.2 | 41.9 | 37 |
| 60 | 30 | 39.7 | 40.8 | 83 | 15.0 | 50.7 | 56 | 13.8 | 55.4 | 54 |
| 60 | 40 | 43.8 | 87.2 | 165 | 25.8 | 72.9 | 87 | 23.9 | 58.6 | 62 |
| 60 | 50 | 42.0 | 74.8 | 114 | 34.9 | 71.4 | 101 | 22.2 | 45.8 | 60 |
| 60 | 60 | 41.4 | 32.4 | 23 | 24.5 | 68.2 | 87 | 12.0 | 41.6 | 43 |
| Alcohol/Solvent | Hexane | Cyclohexane | Isooctane | |
|---|---|---|---|---|
| n-decanol | c % | 27.07 | 43.8 | 46.65 |
| ee | 23.29 | 87.20 | 99 | |
| E | 17.64 | 165 | 185 | |
| n-octanol | c % | 28.22 | 18.43 | 26.20 |
| ee | 18.52 | 16.61 | 23.92 | |
| E | 4.76 | 53.87 | 26.46 | |
| n-butanol | c % | 4.22 | 3.64 | 17.48 |
| ee | 0.98 | 0.78 | 13.56 | |
| E | 1.2 | 1.53 | 22.67 | |
| methanol | c % | 7.19 | 2.88 | 8.22 |
| ee | 1.16 | 0.49 | 8.02 | |
| E | 1.35 | 0.71 | 3.75 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Estrada-Valenzuela, D.; Ramos-Sánchez, V.H.; Zaragoza-Galán, G.; Espinoza-Hicks, J.C.; Bugarin, A.; Chávez-Flores, D. Lipase Assisted (S)-Ketoprofen Resolution from Commercially Available Racemic Mixture. Pharmaceuticals 2021, 14, 996. https://doi.org/10.3390/ph14100996
Estrada-Valenzuela D, Ramos-Sánchez VH, Zaragoza-Galán G, Espinoza-Hicks JC, Bugarin A, Chávez-Flores D. Lipase Assisted (S)-Ketoprofen Resolution from Commercially Available Racemic Mixture. Pharmaceuticals. 2021; 14(10):996. https://doi.org/10.3390/ph14100996
Chicago/Turabian StyleEstrada-Valenzuela, Daniela, Víctor H. Ramos-Sánchez, Gerardo Zaragoza-Galán, Jose C. Espinoza-Hicks, Alejandro Bugarin, and David Chávez-Flores. 2021. "Lipase Assisted (S)-Ketoprofen Resolution from Commercially Available Racemic Mixture" Pharmaceuticals 14, no. 10: 996. https://doi.org/10.3390/ph14100996
APA StyleEstrada-Valenzuela, D., Ramos-Sánchez, V. H., Zaragoza-Galán, G., Espinoza-Hicks, J. C., Bugarin, A., & Chávez-Flores, D. (2021). Lipase Assisted (S)-Ketoprofen Resolution from Commercially Available Racemic Mixture. Pharmaceuticals, 14(10), 996. https://doi.org/10.3390/ph14100996







